Abstract
The recently identified human thiamine pyrophosphate transporter (hTPPT; product of the SLC44A4 gene) is responsible for absorption of the microbiota-generated TPP in the large intestine. The hTPPT is highly expressed in the colon, but not in other regions of the intestinal tract and is localized exclusively at the apical membrane domain of epithelia. The hTPPT protein is predicted to have multiple TM domains with a number of putative N-glycosylation sites, but it is not known if the protein is actually glycosylated, and if so at which site, and their role in the functionality of the transporter. Using several approaches including inhibiting de novo N-glycosylation in human colonic epithelial NCM460 cells with tunicamycin as well as enzymatic de-glycosylation, we show that the hTPPT protein is, indeed, a glycoprotein. Glycosylation of hTPPT was shown, by mean of site-directed mutagenesis, to occur at Asn69, Asn155, Asn197, Asn393, and Asn416. However, only N-glycosylation at Asn69, Asn155, and Asn393 appeared to be important for transporter functionality possibly through an effect on protein conformation and/or interaction with its ligand (but not through changes in expression at the cell membrane as determined by live cell confocal imaging). Results of this study showed, for the first time, that the hTPPT is glycosylated and that N-linked glycosylation occurs at multiple sites with some of them being important for function. The results also provide an indirect support for a membrane topology for hTPPT with 10 transmembrane domains as predicted by the TMHMM transmembrane helixes prediction program.
Keywords: Thiamine pyrophosphate, glycosylation, transport, uptake, mutation
Graphical abstract
1. INTRODUCTION
Thiamine (vitamin B1) is indispensable for oxidative energy (sugar) metabolism and ATP production in the mitochondria via its role as a cofactor (mainly in the form of thiamine pyrophosphate; TPP) for multiple enzymes (transketolase, pyruvate dehydrogenase, alpha-ketoglutarate dehydrogenase, and branched-chain ketoacid dehydrogenase) [reviewed in 1, 2, 3]. The vitamin is also important in reducing cellular oxidative stress and maintaining the normal cellular redox state [4, 5, 6]. Recent studies have attributed additional roles to other forms of the vitamin, namely to thiamine triphosphate, in regulating the function of membrane chloride channels and in acting as a phosphate group donor to proteins [7]. With such important metabolic roles, it is not surprising that low intracellular levels of this micro-nutrient leads to impairment in a variety of cellular metabolic events including oxidative energy metabolism, management of oxidative stress [4, 5, 6, 8], and mitochondrial function/structure [9]. Deficiency of thiamine in humans leads to distinct abnormalities that include beriberi and Wernicke’s encephalopathy. Such a deficiency represents a significant nutritional problem in both developed and underdeveloped countries, and occurs in a variety of conditions including chronic alcoholism, diabetes mellitus, and inflammatory bowel disease [reviewed in 1, 10–14]. In contrast to the negative effects of thiamine deficiency, optimizing thiamine body homeostasis has the potential to prevent diabetes-associated nephropathy, retinopathy and related tissue damage [15, 16].
Humans and other mammals cannot synthesize thiamine endogenously, and thus, must obtain the vitamin from exogenous sources via intestinal absorption. The intestine is exposed to two sources of thiamine: a dietary source and a bacterial source [the latter is in reference to the thiamine generated by the large intestinal microbiota; refs. 17–20]. The dietary source of thiamine is processed and absorbed in the small intestine; the latter occurs via a specific carrier-mediated process [21–23] that involves the human thiamine transporter -1 and -2 [products of the SLC19A2 and SLC19A3 genes, respectively; refs. 24, 25]. A significant amount of the thiamine generated by the microbiota of the large intestine exists in the phosphorylated form of TPP [17]. Recent studies from our laboratory have shown that human colonocytes possess a highly efficient and specific carrier-mediated mechanism for TPP uptake [26]. We have also cloned the colonic TPP uptake system and showed the transporter to be the product of the SLC44A4 gene [27]. The ORF of human TPP transporter encodes a protein of 710 amino acids, having a predicted molecular mass of around 79 kDa, is highly conserved among mammals, and is predicted to have multiple TM domains with a number of putative glycosylation sites. Expression of the human TPP transporter was found to be restricted to the colon and is negligible in other parts of the GI tract [27]; also, the protein was found to be expressed exclusively at the apical membrane domain of epithelia [27].
Currently there is little known about the structure-function relationship of the colonic TPP transport system. We are interested in addressing this issue, and in this study we have focused on determining the glycosylation status of the protein, which of its putative N-glycosylation sites are actually glycosylated, and on the role of N-glycosylation in its function. Our results showed that the hTPPT protein is N-glycosylated at Asn69, Asn155, Asn197, Asn393, and Asn416; however, only glycosylation at Asn69, Asn155, and Asn393 appear to be important for functionality of the membrane transporter.
2. MATERIALS AND METHODS
2.1. Materials
Custom-made3H-labeled thiamine pyrophosphate ([3H]-TPP; specific activity, 1.0 Ci/mmol; radiochemical purity, 98.3%) was obtained from Moravek Biochemicals, Inc., (Brea, CA). Unlabeled TPP and all other chemicals and reagents used in the study were obtained from commercial vendors and were of analytical/molecular biology grade.
2.2. Cell culture and transfection
The human colonic NCM460 cell line was obtained from INCELL (San Antonio, TX). The human retinal pigment epithelial ARPE19 cells and Madin-Darby canine kidney (MDCK) cells were purchased from American Type Culture Collection (Manassas, VA). Cells were cultured in Dulbecco’s Modified Eagles Medium (DMEM) (ARPE19 cells) or Minimal Essential Medium (MEM) (MDCK cells) media supplemented with 10% (vol/vol) FBS, penicillin (100,000 U/l), and streptomycin (10 mg/l). The NCM460 cells were maintained in Ham’s F-12 culture medium supplemented with 20% (vol/vol) FBS and antibiotics. Transient transfection was performed using Lipofectamine 2000 (Invitrogen) following manufacturer’s protocol.
2.3. Uptake assay
Initial rate of uptake (5 min) of ([3H]-TPP was examined at 37°C in Krebs-Ringer (K-R) buffer in cells maintained in 12-well plates as previously described [26, 27]. Protein concentrations of cell digest were measured in parallel wells using Dc protein assay kit (Bio-Rad, Hercules, CA). In transfected cells, uptake of TPP by the induced system was calculated by subtracting uptake by ARPE19 cells transiently transfected with hTPPT from uptake by cells transfected with empty vector; carrier-mediated TPP uptake was determined by subtracting the passive diffusion component from total uptake.
2.4. Tunicamycin and PNGase F treatment
NCM460 cells were treated with 2μg/ml tunicamycin for 48 hrs followed by TPP uptake and Western blot analysis. Total membrane protein (~100 μg) was prepared from NCM460 cells and then incubated with Peptide-N- Glycosidase F (PNGase F) (10 μg protein/μl of PNGase F; NEB Inc., MA) for 1 hr at 37° C followed by Western blot analysis.
2.5. Site-directed mutagenesis
Quick Change II Site-directed mutagenesis kit from Stratagen (La Jolla, CA) was used for mutating individual residues using full-length hTPPT (accession number KU175229) construct cloned into pFLAG-CMV-2 or YFP vector [27] and specific mutant primers (Table 1) following manufacturer’s instruction. The mutated clone was then identified by sequencing of the isolated plasmid DNA (Laragen, CA).
Table 1.
Primers used for site-directed mutagenesis of hTPPT.
| Mutation | Forward and Reverse Primers (5′-3′) |
|---|---|
| N29D | CGAGGCCCCATCAAGGACAGAAGCTGCACAGATGTCATC GATGACATCTGTGCAGCTTCTGTCCTTGATGGGGCCTCG |
| N69D | CAAGTCCTCTACCCCAGGGACTCTACTGGGGCCTAC GTAGGCCCCAGTAGAGTCCCTGGGGTAGAGGACTTG |
| N155D | CCAGGGGTACCCTGGGATATGACGGTGATCACAAGC GCTTGTGATCACCGTCATATCCCAGGGTACCCCTGG |
| N197D | GCGCTCCCAGGGATCACCGATGACACCACCATACAGCAG CTGCTGTATGGTGGTGTCATCGGTGATCCCTGGGAGCGC |
| N298D | CAGCTGGGTTTCACCACCGACCTCAGTGCCTACCAG CTGGTAGGCACTGAGGTCGGTGGTGAAACCCAGCTG |
| N393D | GTGCTCTGGGCATCCGACATCAGCTCCCCCGGC GCCGGGGGAGCTGATGTCGGATGCCCAGAGCAC |
| N409D | CCAATAAATACATCATGCGACCCCACGGCCCACCTTGTG CACAAGGTGGGCCGTGGGGTCGCATGATGTATTTATTGG |
| N416D | ACGGCCCACCTTGTGGACTCCTCGTGCCCAGGGCTG CAGCCCTGGGCACGAGGAGTCCACAAGGTGGGCCGT |
The mutated DNA codon is underlined.
2.6. Cell surface biotinylation and Western blotting
Whole cell lysates or plasma membrane proteins isolated with a cell surface protein isolation kit (Pierce Biotechnology, Rockford, IL) were prepared as described [27]. Proteins were separated in NuPAGE 4–12% Bis-Tris gradient minigels (Invitrogen), transferred onto immobilon polyvinylidene difluoride membrane (Fisher Scientific), and subjected to Western blot analysis. The primary antibodies were goat polyclonal anti-hTPPT antibodies [CTL4 (D-14) antibody, catalog # sc-68049; Santa Cruz Biotechnology, Santa Cruz, CA] at 1:200 dilution or monoclonal anti-FLAG M2 antibody (Sigma) at 1:1000 dilution. The secondary antibodies were anti-goat IRDye-800 antibody or anti-mouse IRDye-800 antibodies (LI-COR Bioscience, Lincoln, NE) at 1:30,000 dilutions. Immunoreactive bands were visualized using the Odyssey infrared imaging system (LI-COR Bioscience, Lincoln, NE).
2.7. Confocal imaging
hTPPT-YFP (WT) and mutant constructs were transiently transfected into MDCK cells and transfected cells were imaged after 48 hrs using an inverted Nikon C-1 confocal microscopy as described before [27–29].
2.8. Statistical Analysis
Data on uptake of TPP are presented as mean ± SE of at least three independent experiments and are expressed as pmol/milligram protein/5 min. Level of significance was determined by the Student’s t-test, and a p-value of < 0.05 was considered as being significant. Carrier-mediated 3H-TPP uptake was determined by subtracting the diffusion component (uptake in the presence of 1 mM TPP) from total uptake. Western blot and confocal imaging were all performed on two or more separate occasions, and representative blots/images are presented in this paper.
3. RESULTS
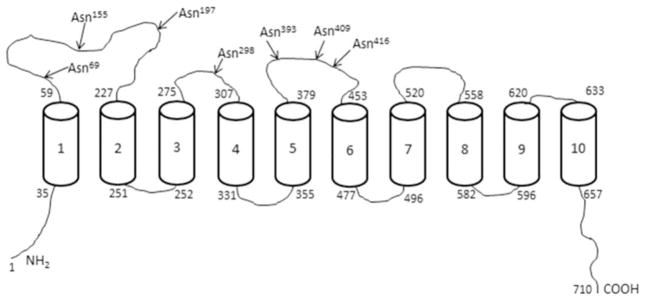
Topology prediction for membrane transporter can be achieved using various predictive algorithms, of which the SOSUI transmembrane helixes prediction program, i.e. SOSUI (http://bp.nuap.nagoya-u.ac.jp/sosui) and the TMHMM transmembrane helixes prediction program (http://www.cbs.dtu.dk/services/TMHMM-2.0) are most commonly used. Hydrophobicity analysis using the SOSUI transmembrane helixes program predicts the hTPPT protein to have 13 putative transmembrane domains (TMD), with an extracellular amino terminus and an intracellular carboxyl terminus, and is potentially N-glycosylated (http://www.cbs.dtu.dk/services/NetNGlyc) at Asn29. This topographical model has been reported by us previously [27] but has not been verified experimentally.
The TMHMM transmembrane helixes program predicts the presence of 10 TMD for hTPPT with both N- and C-terminal ends oriented inside (Fig. 1). Based on this topographical model, the hTPPT has seven putative N-linked glycosylation sites (N-X-S/T) located in the extracellular loops between TMD: Asn69, Asn155, Asn197 (a large loop between TMDs 1 and 2), Asn298 (a loop between TMDs 3 and 4), and Asn393, Asn409, Asn416 (a loop between TMDs 5 and 6). While Asn69, Asn197, Asn298, Asn393, and Asn416 sites are conserved across species (human, bovine, mouse and rat), the Asn155 potential glycosylation site (N-M-T) is replaced by Asp in the mouse and rat TPPT; on the other hand, the potential site Asn409 (N-P-T) exists only in human.
Fig. 1. Structural model of the hTPPT1.
According to TMHMM, hTPPT1 is predicted to have 10 TMD with both N- and C-terminal ends oriented in the cytoplasm. The arrows indicate the location of potential N-glycosylation sites by the NetNGlyc1.0 server.
Thus, both of the prominent transmembrane helixes prediction programs suggest that the hTPPT is glycosylated but there is no experimental evidence in support of that; also no information exists as to which site(s) is actually glycosylated and whether glycosylation influences function.
3.1. hTPPT is glycosylated in human colonic epithelial cells and glycosylation is important for transport function
Our previous studies with the human colonic epithelial NCM460 cells and human proximal colonic apical membrane (AM) preparations have shown that the colonic hTPPT protein is post-translationally modified [27]. This observation is based on the decreased electrophoretic mobility of the hTPPT protein band immuno-detected by Western Blot using specific anti-SLC44A4/hTPPT polyclonal antibodies. Indeed, the molecular mass of the prominent immunoreactive band was ~ 110 kDa for plasma-membrane associated hTPPT protein in NCM460 cells and ~90–110 kDa for the hTPPT in the proximal colonic AM. This experimentally determined molecular mass of hTPPT is higher than the predicted molecular mass of ~ 79 kDa estimated for the predominant colonic hTPPT isoform. Since the hTPPT polypeptide is predicted to have potential N-linked glycosylation sites(s), we hypothesized that the post-translational modification is occurring at these glycosylation sites. To test this hypothesis, we used two experimental approaches to assess the glycosylation status of the hTPPT protein in colonic epithelial cells. In the first approach, we inhibited the de novo N-glycosylation in the colonic epithelial NCM460 cells by tunicamycin, and analyzed the migration pattern of hTPPT. NCM460 cells were treated with tunicamycin (2 μg/ml) for 48 hrs followed by Western blot analysis of the cell lysate using specific anti-SLC44A4 polyclonal antibodies. As shown in Figure 2A, after the tunicamycin treatment: (i) the intensity of the ~ 110 kDa band [mature plasma-membrane associated hTPPT; 27] dramatically decreased, and (ii) it was associated with the appearance of new prominent immunoreactive band of lower molecular mass (~95–100 kDa). Importantly, there was no change in intensity of the ~75–79 kDa band (which is most likely non-glycosylated core protein), indicating that the tunicamycin treatment does not lead to a complete de-glycosylation of hTPPT. These data indicate that in colonic epithelial NCM460 cells the hTPPT protein is N-glycosylated and suggest that post-translational modifications other than N-linked glycosylation are also involved in protein maturation.
Fig. 2. The hTPPT is glycosylated in NCM460 cells and N-linked de-glycosylation affects TPP uptake.
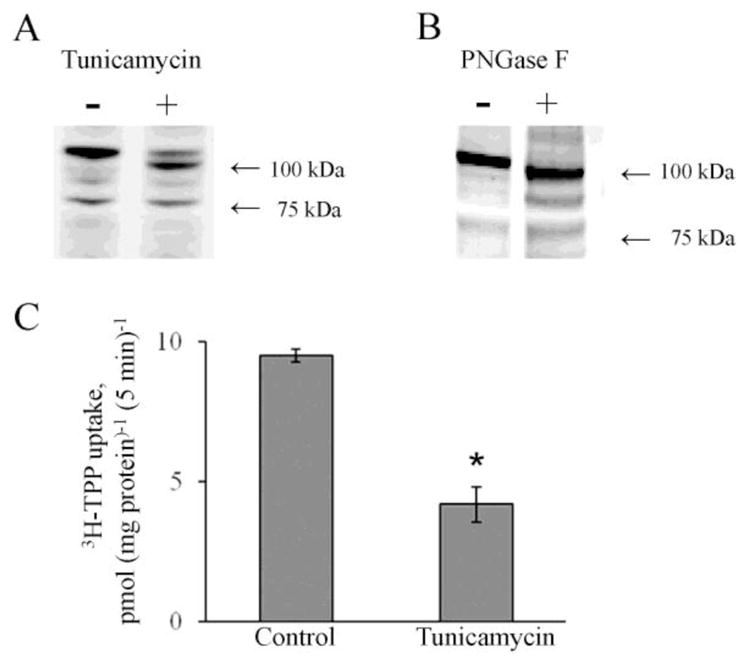
A & B, Western Blot analysis was performed after the tunicamycin treatment (2 μg/ml, 48 hrs) of NCM460 cells (A) and PNGaseF treatment of crude membrane protein fraction from NCM460 cells (B) as described in “Materials and Methods” using polyclonal anti-SLC44A4/hTPPT antibody. In both cases, a shift in an apparent molecular size of hTPPT is shown. The images are representatives of two independent experiments with similar results. C, Effect of tunicamycin on carrier-mediated TPP uptake by NCM460 cells. Cells were preincubated with tunicamycin (2 μg/ml, 48 hrs). [3H]-TPP (0.3 μM) was added to the incubation medium (K-R buffer of pH 7.4) at the onset of incubation, and uptake was measured after 5min of incubation. Control cells - no tunicamycin pretreatment. Data are mean ± S.E. of six to eight separate uptake determinations. *p < 0.01.
In the second approach, we used enzymatic de-glycosylation approach to assess the N-linked glycosylation status of hTPPT in NCM460 cells. A crude membrane protein fractions of NCM460 cells were treated with PNGase F, an amidase that cleaves between the innermost N-acetyl glucosamine and asparagine residue in high mannose, hybrid, and complex oligosaccharides [30], followed by Western Blot analysis. Western blot analysis (Fig. 2B) showed that in samples treated with PNGase F the hTPPT immunoreactive band of ~110 kDa (control cells) was shifted to a lower size band of ~100 kDa. These results further confirm the above observations with tunicamycin that the hTPPT is, in fact, N-glycosylated in colonic epithelial cells.
To determine the impact of N-linked glycosylation on functionality of the colonic hTPPT, we examined the effect of tunicamycin (2 μg/ml; for 48 hrs) treatment of colonic epithelial NCM460 cells on uptake of 3H-TPP (0.3 μM). The results (Fig. 2C) showed that tunicamycin treatment causes a significant (p < 0.01; ~56%) inhibition in carrier-mediated TPP uptake compared to untreated control cells.
3.2. Mutagenesis of the potential N-glycosylation sites and the effect on hTPPT function
To further verify the role of N-linked glycosylation in hTPPT function, we examined the contribution of the individual potential N-glycosylation sites in transport function. This was achieved by introducing mutations into the individual putative N-glycosylation sites and examining the effect of such modifications on the migration pattern of the hTPPT protein, and on its transport function. These experiments were performed using ARPE19 cells (after transient transfection with the hTPPT) because these cells are easier to transfect than NCM460 cells, and have been used in previous studies to characterize hTPPT [27]. First, we showed that the hTPPT expressed in ARPE19 cells is also the subject of post-translational modifications likely via N-glycosylation, as it was found in the case of NCM460 cells. This was done by mean of Western Blot analysis (using anti-SLC44A4/hTPPT polyclonal antibodies) of the total cell lysates and plasma membrane proteins that showed that the predominant plasma membrane associated form of hTPPT has an apparent molecular weight of ~85 kDa (Fig. 3). We then moved to mutate all the eight putative N-glycosylation sites predicted for the hTPPT by the above-described SOSUI and TMHMM topographical prediction models. Individual mutations were introduced resulting in the replacement of the individual asparagine residues with aspartate (an amino acid that has similar structure to asparagine but cannot be glycosylated) followed by examining the effect of the particular mutation on the migration pattern of hTPPT. In these studies, wild-type or the mutant forms of hTPPT (FLAG- tagged) were transiently expressed in ARPE19 cells, followed by cell lysates preparation and immunoblotting using anti-FLAG monoclonal antibodies. The results showed an increase in electrophoretic mobility of the prominent immunoreactive band of ~85 kDa (seen in control, i.e. cells transfected with the wild-type hTPPT) in cells transfected with the hTPPT mutated at Asn69, Asn155, Asn197, Asn393, and Asn416 (Fig. 4A). In contrast, in cells transfected with the protein mutated at Asn29, Asn298, Asn409, the hTPPT migrated at a molecular weight of ~85 kDa, i. e., the same as the control protein. These observations indicate that Asn69, Asn155, Asn197, Asn393, and Asn416 residues of hTPPT are glycosylated in ARPE19 cells. These data are consistent with the topographical model of hTPPT predicted by the TMHMM transmembrane helixes prediction program but not that predicted by the SOSUI program.
Fig. 3. Plasma membrane expression of hTPPT in ARPE19 cells transiently transfected with hTPPT.
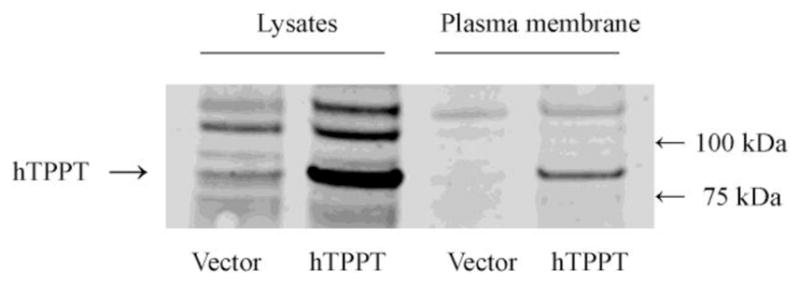
ARPE19 cells were labeled with EZ-Link Sulfo-NHS-SS-Biotin, and biotinylated proteins were isolated with NeutrAvidin-agarose followed by Western blot analysis using the polyclonal anti-SLC44A4/hTPPT antibodies. Control cells, transiently transfected with the vector. The image is representative of two separate sets of experiments with similar results.
Fig. 4. The effect of mutations in the putative N-glycosylation sites of hTPPT on the migration pattern of the hTPPT protein and its transport function.
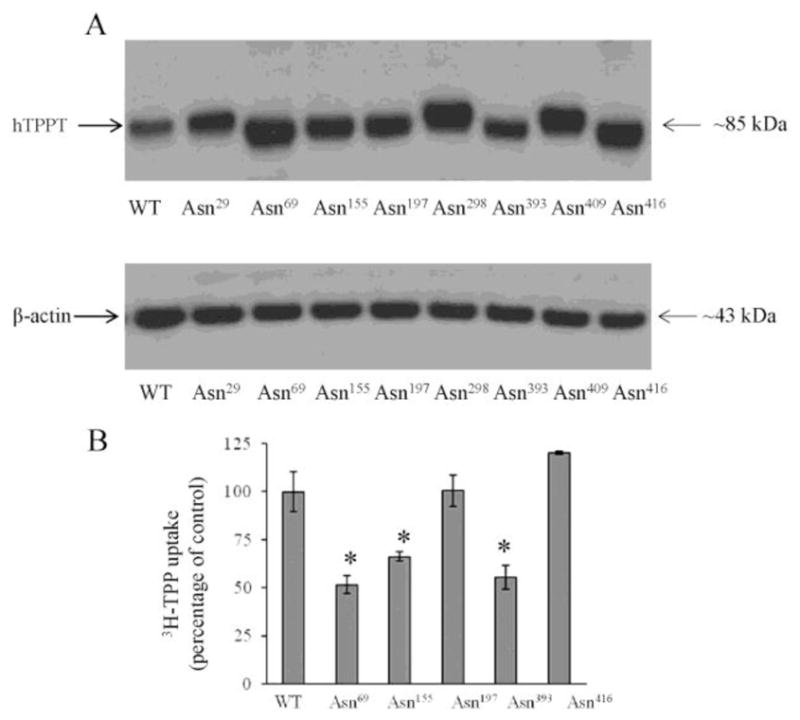
A, Western blot analysis of cell lysates prepared from ARPE19 cells transiently transfected with the wild-type (WT) or the mutant forms of hTPPT (FLAG - tagged everywhere) was performed using monoclonal anti-FLAG antibody (upper panel) or monoclonal anti-β-actin antibody (lower panel). The images are representatives of two independent experiments with similar results. B, Effect of mutations in N-linked glycosylation sites of hTPPT on carrier-mediated TPP uptake. ARPE19 cells transiently expressing mutants (or control, wild-type hTPPT, WT) were used for TPP uptake measurements. [3H]-TPP (0.3 μM) was added to the incubation medium (KR buffer of pH 7.4) at the onset of incubation, and uptake was measured after 5min of incubation. Carrier-mediated TPP uptake by the induced system was calculated as described in “Materials and Methods”. Data are mean ± S.E. of six to eight separate uptake determinations. *p < 0.01.
To examine the effect of mutations in N-linked glycosylation sites on the functionality of hTPPT, we measured carrier-mediated TPP uptake by the induced system in ARPE19 cells transiently expressing mutants as compared to wild-type hTPPT. The results (Fig. 4B) showed that mutating Asn69, Asn155, and Asn393 of the hTPPT protein led to a significant (p < 0.01) decrease in TPP uptake. In contrast, no change in TPP uptake was detected in cells transfected with the hTPPT mutated at Asn197 and Asn416. We also generated double mutants of hTPPT by disrupting, in pairs, three glycosylation sites observed to be important for transporter function, i.e. N69D/N155D, N69D/N393D, N155D/N393D and tested their effect on TPP uptake upon transient transfection of ARPE19 cells. Among them, only the mutant N155D/N393D (data not shown) resulted in further reduction in TPP uptake compared to mutants in single glycosylation site, indicating that the effect of disrupting Asn155 and Asn393 glycosylation sites was cumulative.
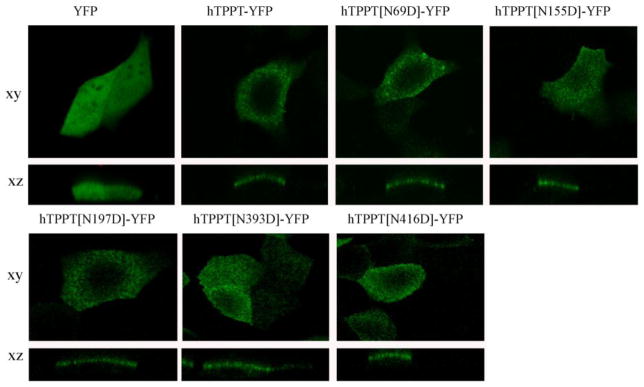
To examine whether the decrease in TPP uptake by cells with hTPPT disrupted N-glycosylation sites at Asn69, Asn155, and Asn393 was due to impaired expression of the protein at the cell membrane, we determined the cell surface expression level of hTPPT. This was done using live cell confocal imaging of transiently expressing MDCK cells with hTPPT-YFP (WT) and mutant constructs (hTPPT[N69D]-YFP, hTPPT[N155D]-YFP, and hTPPT[N393D]-YFP (cells were transfected with equal amount of DNA and imaged with similar laser intensity for all constructs). The results (Fig. 5) showed that cell surface expression of hTPPT was comparable in cells transfected with the different mutants and with the wild-type construct. Two additional controls were used, mutants (hTPPT[N197D]-YFP and hTPPT[N416D]-YFP) (glycosylated residue; no effect on uptake) and both showed comparable level of hTPPT expression at the cell membrane. These data indicate that lack of glycosylation of Asn69, Asn155, and Asn393 does not affect delivery of the transporter to the plasma membrane, and suggest that other mechanism(s) is involved in the decrease of transport function of hTPPT due to de-glycosylation.
Fig. 5. Effect of mutations in the putative N-glycosylation sites of hTPPT on the cell surface expression of hTPPT.
Lateral (xy) and axial (xz) images were obtained from MDCK cells transiently expressing YFP, wild-type (hTPPT-YFP) and mutant constructs after 48 hrs of post-transfection. Image shown represents 3 separate sets of experiments with comparable results.
4. DISCUSSION
Many proteins, including those at the cell surface, are known to be N-glycosylated, and this post-translation modification plays a role in protein functioning through regulation of protein folding, maintenance of protein stability, proper trafficking to subcellular compartments, and modulation of biological activity [31–33]. hTPPT, a transporter that plays an important role in the absorption of the microbiota-generated TPP in the large intestine, appears to be post-translationally modified since its predicted molecular size of ~ 79 kDa differs from its apparent molecular size of ~110 kDa detected on a Western blot [27]. Knowing that the hTPPT polypeptide has a variety of the putative N-linked glycosylation sites, we assessed the N-glycosylation status of hTPPT in colonic cells using two independent approaches: the de novo inhibition of N-glycosylation by tunicamycin, and, enzymatic removal of N-linked carbohydrate chains by PNGase F. The results of both approaches showed that the hTPPT is, indeed, N-glycosylated in colonic cells. The observations that the molecular weight of the N-de-glycosylated protein did not decrease to its expected de-glycosylated core protein of ~79 kDa suggest that other post-translational modifications might contribute to hTPPT maturation. O-linked glycosylation might be of particular interest since O-glycosylation has been reported to play a role in apical sorting of membrane transporter [34]. This issue, however, will be addressed in future investigations.
N-linked glycosylation is known to affect functionality of many transporters, including those involved in vitamin uptake [28, 35, 36]. Here, we found that the tunicamycin-induced inhibition of N-glycosylation of hTPPT resulted in impairment of TPP uptake by colonic NCM460 cells suggesting an important role for N-glycosylation in the function of the transporter. To understand which of the hTPPT putative N-glycosylation sites were actually glycosylated, and the contribution of that glycosylation on function, we mutated each of the putative N-glycosylation sites and analyzed the consequences of such mutations on migration pattern and function of hTPPT. We utilized the ARPE19 cells in the latter studies as they have better transfection efficiency then NCM460 cells and have been used previously in characterizing many features of the hTPPT. We observed that in ARPE19 cells, the mature plasma membrane associated form of hTPPT also has a decreased electro-mobility (as it was previously found in the case of NCM460 cells) and migrated as a band of ~85 kDa (band of ~110 kDa in NCM460 cells). This suggests that the hTPPT protein is glycosylated in both cell lines, but to a different extent in colonic and retinal cells, and points to tissue-specificity in protein maturation via glycosylation. Our studies also showed that while the Asn69, Asn155, Asn197, Asn393, and Asn416 residues of hTPPT are all glycosylated in ARPE19 cells, only the mutations at Asn69, Asn155, and Asn393 have functional consequences. Since mutations at the latter sites did not affect cell membrane expression of the mutated hTPPT, it is possible that glycosylation at these sites affects the conformation of the protein and/or its interaction with its ligand. Further studies are needed to address these issues.
In addition to clarifying the glycosylation status of the hTPPT protein, determining which residues are actually glycosylated, and the consequence of that glycosylation on functionality, our studies also provide support for the membrane topology predicted by the TMHMM transmembrane helixes prediction program over the SOSUI program as the protein was found to be glycosylated at sites predicted to be in the extracellular domains of the membrane protein in the former but not the latter model. Similar topology and N-glycosylation status has been described for other members of the SLC44A family (37, 38, 39). In summary, our studies show that the hTPPT is a glycoprotein and that N-glycosylation is important for its function.
Highlights.
hTPPT is the glycosylated protein.
N-glycosylation occurs at Asn69, Asn155, Asn197, Asn393, and Asn416.
Glycosylation at Asn69, Asn155, and Asn393 is important for hTPPT functionality.
This study provides a support for a 10 TMD topology for the hTPPT.
Acknowledgments
This study was supported by grants from the National Institutes of Health (DK-56061and AA018071) and the Department of Veterans Affairs.
Footnotes
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berdanier CD. Advanced Nutrition Micronutrients. CRC Press; Boca Raton, FL: 1998. pp. 80–88. [Google Scholar]
- 2.Bettendorff L, Wins P. Thiamin diphosphate in biological chemistry: new aspects of thiamin metabolism, especially triphosphate derivatives acting other than as cofactors. FEBS J. 2009;276:2917–2925. doi: 10.1111/j.1742-4658.2009.07019.x. [DOI] [PubMed] [Google Scholar]
- 3.Singleton CK, Martin PR. Molecular mechanisms of thiamin utilization. Curr Mol Med. 2001;1:197–207. doi: 10.2174/1566524013363870. [DOI] [PubMed] [Google Scholar]
- 4.Calingasan NY, Chun WJ, Park LC, Uchida K, Gibson GE. Oxidative stress is associated with region-specific neuronal death during thiamin deficiency. J Neuropath Exp Neurol. 1999;58:946–958. doi: 10.1097/00005072-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Hazell AS, Butterworth RF. Update of cell damage mechanisms in thiamine deficiency: focus on oxidative stress, excitotoxicity, and inflammation. Alcohol. 2009;44:141–147. doi: 10.1093/alcalc/agn120. [DOI] [PubMed] [Google Scholar]
- 6.Portari GV, Marchini JS, Vannucchi H, Jordao AA. Antioxidant effect of thiamin on acutely alcoholized rats and lack of efficacy using thiamin or glucose to reduce blood alcohol content. Basic Clin Pharmacol Toxicol. 2008;103:482–486. doi: 10.1111/j.1742-7843.2008.00311.x. [DOI] [PubMed] [Google Scholar]
- 7.Bettendorff L, Lakaye B, Kohn G, Wins P. Thiamine triphosphate: a ubiquitous molecule in search of a physiological role. Metab Brain Dis. 2014;29:1069–1082. doi: 10.1007/s11011-014-9509-4. [DOI] [PubMed] [Google Scholar]
- 8.Gangolf M, Czerniecki J, Radermecker M, Detry O, Nisolle M, Jouan C, Martin D, Chantraine F, Lakaye B, Wins P, Grisar T, Bettendorff L. Thiamin status in humans and content of phosphorylated thiamin derivatives in biopsies and cultured cells. PLoS One. 2010;5:e13616. doi: 10.1371/journal.pone.0013616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bettendorff L, Goessens G, Sluse F, Wins P, Bureau M, Laschet J, Grisar T. Thiamin deficiency in cultured neuroblastoma cells: effect on mitochondrial function and peripheral benzodiazepine receptors. J Neurochem. 1995;64:2013–2021. doi: 10.1046/j.1471-4159.1995.64052013.x. [DOI] [PubMed] [Google Scholar]
- 10.Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J. 2011;437:357–372. doi: 10.1042/BJ20110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Said HM. Recent advances in transport of water-soluble vitamins in organs of the digestive system: a focus on the colon and the pancreas. Am J Physiol Gastrointest Liver Physiol. 2013;305:G601–G610. doi: 10.1152/ajpgi.00231.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Said HM. Water-soluble vitamins. World Rev Nutr Diet. 2015;111:30–37. doi: 10.1159/000362294. [DOI] [PubMed] [Google Scholar]
- 13.Tanphaichirt V. Modern Nutrition in Health and Disease. Lea and Febiger; New York, NY: 1994. pp. 359–375. [Google Scholar]
- 14.Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome and related neurological disorders due to alcoholism and malnutrition. Philadelphia, PA: Davis; 1989. [Google Scholar]
- 15.Babei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes. 2003;52:2110–2120. doi: 10.2337/diabetes.52.8.2110. [DOI] [PubMed] [Google Scholar]
- 16.Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Berfeld R, Giardino I, Brownlee M. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 17.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J, Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, MeloMinardi R, M’rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P MetaHIT Consortium. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurerrant NMB, Dutcher RA. Assay of vitamins B and G as influenced by coprophagy. J Biol Chem. 1932;98:225–235. [Google Scholar]
- 19.Gurerrant NM, Dutcher RA, Brown RA. Further studies concerning formation of B vitamins in digestive tract of rat. J Nutr. 1937;13:305–315. [Google Scholar]
- 20.Najjar VA, Holt LE. The biosynthesis of thiamine in man and its implications in human nutrition. JAMA. 1943;123:683–684. [Google Scholar]
- 21.Dudeja PK, Tyagi S, Kavilaveettil RJ, Gill R, Said HM. Mechanism of thiamine uptake by human jejunal brush-border membrane vesicles. Am J Physiol Cell Physiol. 2001;281:C786–C792. doi: 10.1152/ajpcell.2001.281.3.C786. [DOI] [PubMed] [Google Scholar]
- 22.Rindi G, Laforenza U. Thiamine intestinal transport and related issues: recent aspects. Proc Soc Exp Biol Med. 2000;224:246–55. doi: 10.1046/j.1525-1373.2000.22428.x. [DOI] [PubMed] [Google Scholar]
- 23.Said HM, Ortiz A, Kumar CK, Chatterjee N, Dudeja PK, Rubin S. Transport of thiamine in human intestine: mechanism and regulation in intestinal epithelial cell model Caco-2. Am J Physiol. 1999;227:C645–C651. doi: 10.1152/ajpcell.1999.277.4.C645. [DOI] [PubMed] [Google Scholar]
- 24.Reidling JC, Lambrecht N, Kassir M, Said HM. Impaired intestinal vitamin B1 (thiamin) uptake in thiamin transporter-2-deficient mice. Gastroenterology. 2010;138:1802–1809. doi: 10.1053/j.gastro.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Said HM, Balamurugan K, Subramanian VS, Marchant JS. Expression and functional contribution of hTHTR-2 in thiamin absorption in human intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G491–G498. doi: 10.1152/ajpgi.00361.2003. [DOI] [PubMed] [Google Scholar]
- 26.Nabokina SM, Said HM. A high-affinity and specific carrier-mediated mechanism for uptake of thiamine pyrophosphate by human colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2012;303:G389–G395. doi: 10.1152/ajpgi.00151.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nabokina SM, Inoue K, Subramanian VS, Valle JE, Yuasa H, Said HM. Molecular identification and functional characterization of the human colonic thiamine pyrophosphate transporter. J BiolChem. 2014;289:4405–4416. doi: 10.1074/jbc.M113.528257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subramanian VS, Marchant JS, Reidling JC, Said HM. N-Glycosylation is required for Na+-dependent vitamin C transporter functionality. Biochem Biophys Res Commun. 2008;374:123–127. doi: 10.1016/j.bbrc.2008.06.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subramanian VS, Marchant JS, Parker I, Said HM. Cell biology of the human thiamine transporter-1 (hTHTR1); Intracellular trafficking and membrane targeting mechanisms. J Biol Chem. 2003;278:3976–3984. doi: 10.1074/jbc.M210717200. [DOI] [PubMed] [Google Scholar]
- 30.Maley F, Trimble RB, Tarentino AL, Plummer TH., Jr Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal Biochem. 1989;180:195–204. doi: 10.1016/0003-2697(89)90115-2. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Maza R, Poyatos I, Lopez-Coreuera B, Nunez E, Gimenez C, Zafra F, Aragon C. The role of N-glycosylation in transport to the plasma membrane and sorting of the neuronal glycine transporter GLYT2. J Biol Chem. 2001;259:2168–2173. doi: 10.1074/jbc.M006774200. [DOI] [PubMed] [Google Scholar]
- 32.Vagin O, Kraut JA, Sachs G. Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am J Physiol Renal Physiol. 2009;296:F459–F469. doi: 10.1152/ajprenal.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F, Xu W, Hong M, Pan ZZ, Sinko PJ, Ma J, You G. The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4. Mol Pharmacol. 2005;67:868–876. doi: 10.1124/mol.104.007583. [DOI] [PubMed] [Google Scholar]
- 34.Potter BA, Hughey RP, Weisz OA. Role of N- and O-glycans in polarized biosynthetic sorting. Am J Physiol Cell Physiol. 2006;290:C1–C10. doi: 10.1152/ajpcell.00333.2005. [DOI] [PubMed] [Google Scholar]
- 35.Ghosal A, Subramanian VS, Said HM. Role of the putative N-glycosylation and PKC-phosphorylation sites of the human sodium-dependent multivitamin transporter (hSMVT) in function and regulation. Biochem Biophys Acta. 2011;1808:2073–2080. doi: 10.1016/j.bbamem.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unal ES, Zhao R, Gold A, Qiu ID. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT) Biochem Biophys Acta. 2008;1778:1407–1414. doi: 10.1016/j.bbamem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Regan S, Traiffort E, Ruat M, Cha N, Compaore D, Meunier FM. An electric lobe suppressor for a yeast choline transport mutation belongs to a new family of transporter-like proteins. Proc Natl Acad Sci U S A. 2000;97:1835–1840. doi: 10.1073/pnas.030339697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traiffort E, O’Regan S, Ruat M. The choline transporter-like family SLC44: properties and roles in human diseases. Mol Aspects Med. 2013;34:646–654. doi: 10.1016/j.mam.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Michel V, Bakovis M. The ubiquitous choline transporter SLC44A1. Central Nervous system. Agents Med Chem. 2012;12:70–81. doi: 10.2174/187152412800792733. [DOI] [PubMed] [Google Scholar]