Abstract
Purpose
To develop methods to delineate the relationship between endothelial cell toxicity and tissue fixation (toxicity/fixation) using sodium hydroxymethylglycinate (SMG), a formaldehyde releaser, and riboflavin-UVA (CXL) for therapeutic tissue cross-linking of the cornea.
Methods
Eleven (11) fresh cadaveric rabbit heads were used for ex vivo corneal cross-linking simulation. Following epithelial debridement, the tissue was exposed to 1/4 Max (9.765mM) or 1/3Max (13.02mM) SMG at pH 8.5 for 30min or riboflavin-UVA (CXL). The contralateral cornea served as a paired control. Post-exposure, cross-linking efficacy was determined by thermal denaturation temperature (Tm) and endothelial damage was assessed using calcein AM and ethidium homodimer staining (Live/Dead Kit). Confocal laser scanning fluorescence microscopy was used to generate live/dead cell counts following a standardized algorithm.
Results
The ΔTm following CXL, 1/3 SMG, and 1/4 SMG was 2.19±0.91°C, 1.33±0.49 °C, and 1.10 ±0.46 °C, respectively. Endothelial cell damage was expressed as the percent of dead cells/live + dead cells counted per high powered field. The values were 2.95±1.74% (control) and 8.86±11.10% (CXL) [p=0.390]; 0.98±0.20% (control) and 19.53±32.22% (1/3max SMG) [p=0.426]; and 2.70±2.37% (control) and 2.84±2.24% (1/4 max SMG) [p=0.938];. The values for endothelial toxicity were then indexed over the shift in Tm in order to yield a toxicity/fixation index. The values were as follows: 2.70 for CXL, 13.95 for 1/3 max, and 0.13 for 1/4 max.
Conclusions
Quarter max (1/4 Max = 9.765mM) SMG effectively cross-linked tissue and was non-toxic to endothelial cells. Thus, SMG is potentially a compound that could achieve both desired effects.
Keywords: cornea, endothelium, sodium hydroxymethylglycinate, tissue cross-linking, keratoconus
Introduction
CXL has revolutionized corneal therapeutics.1-3 Although interest and use continues to expand, CXL remains an investigational procedure in the United States and many questions remain regarding CXL from a clinical and basic science perspective. The use of ultraviolet light in the cross-linking process has been a concern from the outset of the U.S. clinical trials, which underscores a potential hazard of using the current cross-linking method. It also highlights a potential benefit of alternative topical methods for cross-linking. That is, ultraviolet light may not be necessary. UV light exposure to the eye has been implicated in a number of deleterious effects, including damage to the lens4 and retina5 and as such, the longterm effects are difficult to predict. Regarding corneal cytotoxicity, although damage to stromal keratocytes is known to occur, the effects on corneal endothelial cells has been debated. Increased endothelial damage with CXL has been reported for both in vivo and in vitro studies.6,7 In a clinical study, the application of CXL to human corneas less than 400µm resulted in some toxicity to the endothelium.8,9 Most keratoconic patients are young and because human corneal endothelial cells do not have regenerative capacity,10 endothelial preservation is critical. Providing corneal stabilization without exposing patients to UV light could remove a potential hazard of the treatment.
Sodium hydroxymethylglycinate (SMG) is a small water soluble compound (MW=127) and is one of a group of formaldehyde releasers (FARs), recently introduced for therapeutic tissue cross-linking (TXL) of the cornea. FARs are used as preservatives in a wide array of popular cosmetic and personal care products 11 such as skin care products, body wash, fingernail polish and shampoo. FARs have also been employed in the textile industry as cross-linking agents to impart anti-wrinkle properties to clothing.12 In a recent study, we reported SMG to be highly effective as a corneal stiffening agent in ex vivo rabbit eyes with an in vitro toxicity threshold on par with previously studied higher order nitroalcohols, and was less than 0.1mM.13 The current study is an extension of that previous work in which we evaluate the efficacy and safety of corneal cross-linking with SMG, comparing it with CXL in an ex-vivo rabbit model. Our intent was to identify an optimal concentration of chemical agent that can effectively cross-link collagen with minimal to no effect on endothelial cell viability. These studies lay the groundwork for future studies using live animals.
Methods and Materials
Chemicals
Sodium hydroxymethylglycinate (SMG), hydroxypropyl methyl cellulose (HPMC, 15 centipoise), dextran (high molecular weight = 425-575,000 Da), sodium bicarbonate and ethylenediaminetetraacetic acid (EDTA) were obtained from Sigma-Aldrich Corp. (St. Louis, MO). Riboflavin-5-phosphate was obtained from MP Biomedicals (Santa Ana, CA). Dulbecco's phosphate buffered saline (DPBS) solution (MgCl2 & CaCl2 free) was obtained from Life Technologies (Carlsbad, CA). All chemical solutions and buffers were prepared fresh using Millipore water (double distilled, de-ionized water, ρ = 18.2 MΩcm at 25 °C) on the day of cross-linking.
All procedures were performed according to the Association for Research in Vision and Ophthalmology Statement on the use of animals in Ophthalmic and Vision Research.
TXL with SMG
We performed chemical TXL with SMG using a similar method to a prior study.13 Six intact cadaveric rabbit heads with clear corneas were obtained from the local abattoir within 1 hour post-mortem. The central corneal thickness was measured with an ultrasound pachymeter (Pachmate, DGH Technologies, PA, USA). A single drop of proparacaine (0.5%), was applied to the corneal surface followed by epithelial removal using a crescent blade. An 8-mm Hessburg-Barron corneal vacuum reservoir (JEDMED, St. Louis, MO, USA) was then attached to the corneal surface. SMG solutions at a concentration equivalent to one quarter or one third the maximum allowed value (max=39.06mM or 0.5%, 1/4 max=9.76mM, 1/3 max=13.02mM) were dissolved in a buffer solution containing 0.1 M NaHCO3 (max=maximum allowed concentration in cosmetics and other personal care products as dictated by the European committee on cosmetics standards). The final solution of SMG was titrated to the desired pH 8.5 using a 0.1 N HCl solution. SMG solution was applied every 5 min over a 30-minute period. In other words, the cross-linking solution was changed out every 5 min with fresh cross-linking solution in order to augment formaldehyde delivery to the tissues. The control contralateral eye was treated identically with vehicle after epithelial removal. Immediately after treatment, the eye was irrigated with balanced salt solution. A central 8-mm corneal trephination was then performed. In order to include the only treated region and to ensure that we were considering effects related to the exposed/treated region (that is, to avoid transitional zones), we performed a 6-mm corneal punch (JEDMED, St. Louis, MO, USA) on the 8mm treated button region, carefully, with the endothelium positioned facing upward.
CXL
Five (5) intact cadaveric rabbit heads were used to conduct CXL with similar methods.14 The central corneal thickness was measured with an ultrasound pachymeter. A single drop of proparacaine (0.5%) was applied. This was followed by corneal epithelial removal of a central 8-mm region using a crescent blade. The solution containing 0.1% riboflavin-5-phosphate solution in 1.1% HPMC was instilled on the debrided corneal surface for 5 minutes prior to UV treatment. UVA Irradiation was performed using the Optos XLink Corneal Collagen Cross-Linking System (Optos, Dunfermline, UK) (370 nm, 3 mW/cm2) for 30 minutes. During irradiation, riboflavin solution was instilled every 3 minutes. At the end of the procedure, the eye was washed with balanced salt solution. The control contralateral eye was treated identically but without irradiation. As described above, a central 6-mm corneal trephined button was taken from the 8mm area of epithelial debridement.
Ex Vivo Live-Dead Assay
In tissue culture plates, the 6.0mm corneal buttons (paired samples) were stained with 2 uM calcein-AM and 4 mM ethidium homodimer (The Live/Dead Kit, Molecular Probes Inc., Eugene, OR, U.S.A.). Corneal buttons were always placed endothelial side up to decrease mechanical damage of the corneal endothelium. The plates were then placed in a 37°C-, 5% CO2-humidified incubator for 45 minutes. Corneal buttons were immersed in PBS and then placed between two coverslips. These samples were then analyzed by scanning laser confocal microscopy (Leica; Deerfield, IL) equipped with a fluorescence light source for detecting green and red fluorescent regions. (Fluorovert; Leica).
Fluorescent images were captured using a Nikon A1 laser scanning confocal microscope on an Eclipse Ti stand (Nikon Instruments, Melville, NY) using a 10X/NA0.3 Plan Fluor Nikon objective for cell counting and 20X/NA0.75 Plan Apo Nikon objective for a higher magnification picture. Red and green fluorescence images were acquired sequentially to prevent bleed-through using standard lasers and emission filters: green (live cells, Calcein AM) was excited at 488 nm with the corresponding emission collected from 500-550 nm, red (dead cells, Ethidium homodimer-1) was excited at 561 nm with the corresponding emission collected from 570-620 nm. In order to further avoid taking images from the cut edges of the corneal button, a 4.5mm central area was selected from the 6mm button sample. Cells were counted using FIJI/ImageJ NIH software.15 The cellular damage was calculated as the percentage of dead cells (red cells) from the total number of cells (red cells + green cells): 100 × red cells / (red cells + green cells). The procedure used to estimate the number of dead and live cells was as follows: Images acquired in red and green channels were first processed by applying an intensity threshold to the image so that the background fluorescence was removed. The number of dead cells was estimated from the red channel images by counting objects with the “Analyze Particles” function in Fiji. The number of live cells was then estimated from the green channel images by measuring the total area with cells and dividing it by the area of a single cell. Ten cells were manually selected and measured in each image to determine an average cell area. The measured average cell area was approximately 300 um2. The total area of cell counting per cornea was 3.2mm2 which was about one fifth of the area of the central 4.5mm (15.89mm2) zone. Image collection, processing, and analysis were performed by Emilia Laura Munteanu, at the Confocal and Specialized Microscopy Shared Resource of the Herbert Irving Comprehensive Cancer Center at Columbia University. Following imaging the cornea button was incubated in Optisol for 24-72 hrs followed by DSC analysis.
Differential Scanning Calorimetry (DSC)
Corneal buttons were removed from Optisol. After removal of excess Optisol solution, tissue samples were transferred to preweighed 50-uL aluminum pans. Peak thermal denaturation temperature (Tm) of all 6-0mm corneal buttons was measured using a Perkin-Elmer DSC 6000 Autosampler (Waltham, MA, USA). As previously discussed the difference in thermal denaturation temperature (ΔTm) between the crosslinked sample and contralateral paired central sample was determined using the Pyris software (version 11.0; Perkin-Elmer, Waltham, MA, USA) as previously reported.13
Statistical analysis
Endothelial damage between cross-linked corneas and their paired controls was carried out using standard paired t-tests. The mean central corneal thickness and ΔTm among groups were also compared using Kruskal-Wallis test. The Statistical Package for the Social Sciences version 13.0 (SPSS Inc., Chicago, IL) was used for all statistical analyses. The significance value was P < 0.05.
Results
Central Corneal Thickness (CCT)
CCT measurements were taken prior to cross-linking in all samples. Limited information is available for post cross-linking pachymetry. CCT is an indicator of corneal health and as mentioned previously, clinical recommendations for corneal thickness minima for CXL is 400um. Since the epithelium is 30-50 um in thickness, corneal thickness at the time of CXL was below 400um. This may also in part explain the increased toxicity seen for CXL in this study.
The mean central corneal thickness (CCT) before the procedure was 396±32 μm. The mean CCT of control and CXL was 389±35 μm and 381±27 μm, respectively. The mean CCT was 410±33 μm (control) and 411±26 μm (1/4 Max, 9.765mM SMG). The mean CCT was 400±49 μm (control) and 390±40 μm (1/3Max, 13.02 mM SMG). The difference of mean CCT among groups was not statistically significant (Kruskal-Wallis test, p=0.385).
Cross-linking effects as determined by DSC (Figure 1)
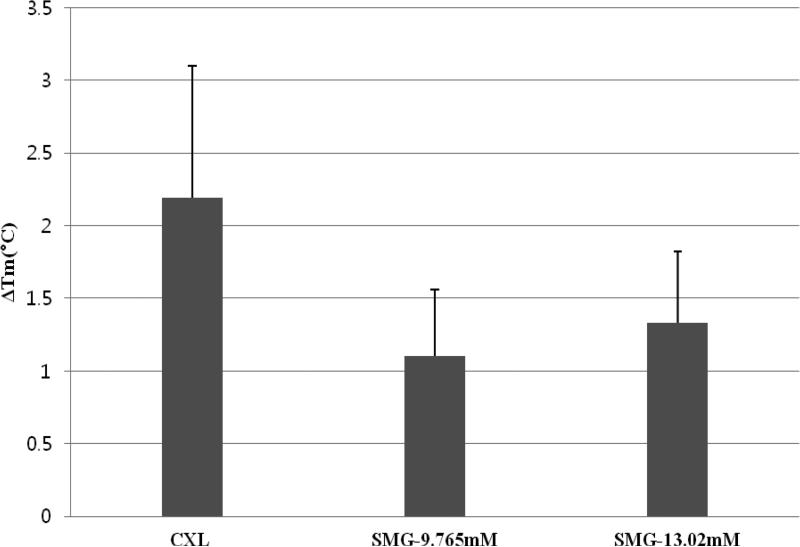
Figure 1.
Corneal cross-linking effect of CXL vs. TXL using SMG as determined by DSC. Peak thermal transition temperatures (Tm) were compared. Each sample was compared to its own control and the difference (or shift) in Tm was expressed as the ΔTm. The greatest effect was seen with CXL, followed by 1/3 max SMG, followed by 1/4 max SMG. When combined with the cell toxicity data, we can evaluate the balance between “toxicity and fixation”. Based on these results, we believe that this concentration would be an appropriate starting concentration for in vivo animal studies.
Figure 1 shows data obtained from the DSC analysis. The instrument has an expected degree of detection error of +/− 0.3°C. The ΔTm following CXL was 2.19±0.91°C as determined by DSC. The shift after 1/4 Max (9.765mM) SMG treatment was 1.10 ±0.46°C. The shift after 1/3Max (13.02 mM) SMG treatment was 1.33±0.49°C (Figure 1). The difference of ΔTm among groups was not significant (Kruskal-Wallis test, p=0.215).
Endothelial cell toxicity (Figure 2)
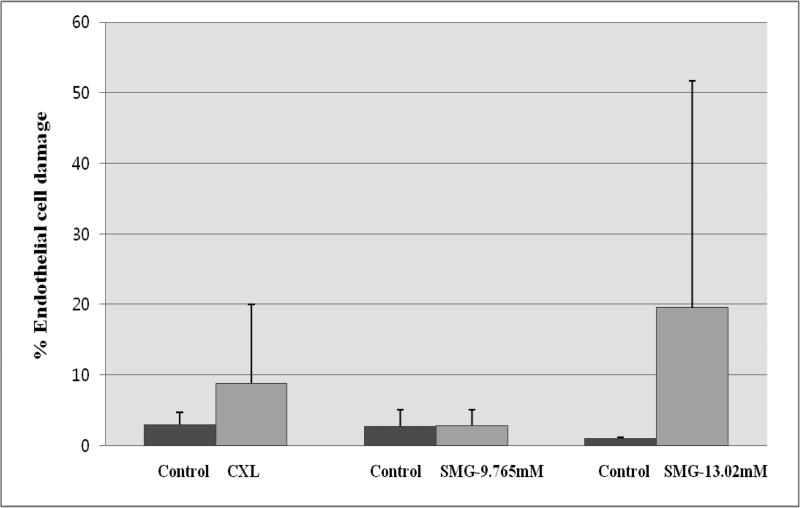
Figure 2.
Endothelial damage from CXL vs. TXL using SMG. Cells were imaged using a confocal fluorescence microscope following live/dead staining using calcein AM (red) and ethidium homodimer (green) [488ex/500-550em]. The method for counting was as described in Methods and Materials. Each condition has its own paired control. All three sets of controls exhibited low levels of dead stained endothelial cells (<3%). SMG at 1/4 max (9.765mM0 was found to induce the least amount of endothelial damage (<3%). 1/3 max SMG was the most toxic (~19%) but a significant sample variability was noted (~30%). CXL showed intermediate endothelial damage at ~8%.
Endothelial cell toxicity was evaluated following cross-linking by staining the tissue using live/dead staining followed by confocal fluorescence microscopy imaging. Representative examples of both CXL-treated (Figure 3) and 1/4 max SMG (Figure 4) are included. In addition, identification of dead endothelial cells in the correct location was carried out using tangential sequential image selection with 3-D reconstruction as shown in Figure 5. In CXL treated samples, the endothelial cell damage was 2.95±1.74% (control) and 8.86±11.10% (CXL). There was no significant difference for endothelial cell damage between the control and CXL samples (paired t-test, p=0.390). In 1/4 max treated SMG samples, the endothelial cell damage was 2.70±2.37% (control) and 2.84±2.24% (1/4 max, 9.765mM SMG). In 1/3 max treated SMG samples, the endothelial cell damage was 0.98±0.20% (control) and 19.53±32.22% (1/3 max, 13.02 mM SMG). The endothelial cell damage after SMG treatment at both concentrations was not significant when compared with control (paired t-test, p=0.938, p=0.426, respectively).
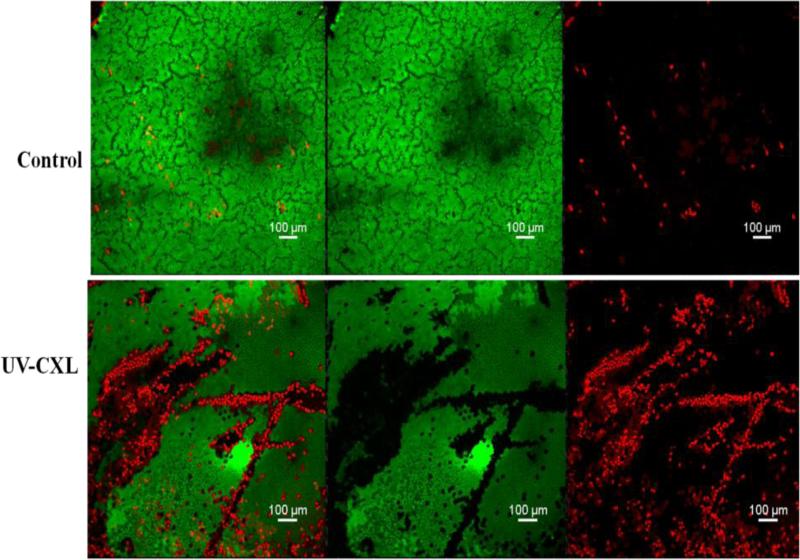
Figure 3.
Confocal micrographs (10×) of endothelium using calcein –AM (green, live) and ethidium homodimer-1 (red, dead) – stained corneas from control and CXL. The first two panels (top and bottom) show an overlay of dead (red) and green (live) cells. The middle two panels (top and bottom) show only green (live) cells. The right-most panels (top and bottom) show the dead (red) cells only. Areas of increased cell damage are shown in the CXL treated sample (three bottom panels).
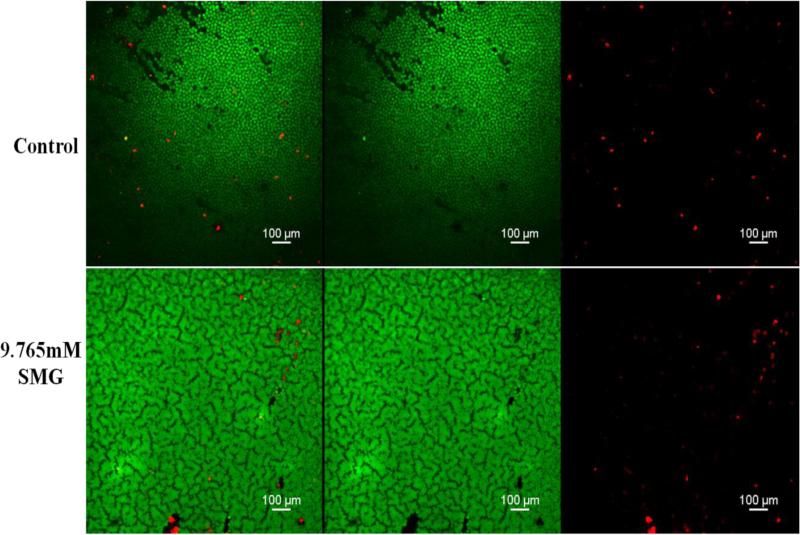
Figure 4.
Confocal micrographs (10×) of endothelium using calcein –AM (green, live) and Ethidium homodimer-1 (red, dead) – stained corneas from control and 1/4 Max (9.765mM) sodium hydroxymethylglycinate (SMG). The first two panels (top and bottom) show an overlay of dead (red) and green (live) cells. The middle two panels (top and bottom) show only green (live) cells. The right-most panels (top and bottom) show the dead (red) cells only. In contrast to figure 3 images, the endothelial layer is relatively spared following exposure to 1/4 max SMG.
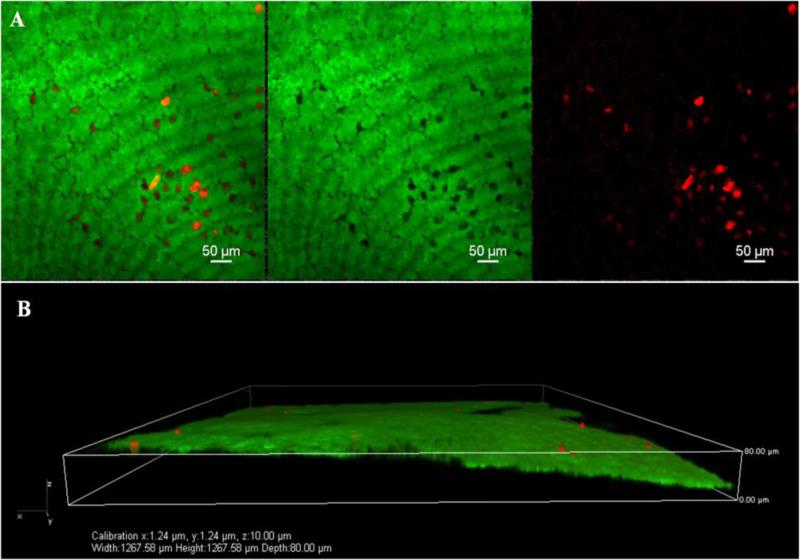
Figure 5.
A. Higher magnification (20×) confocal micrographs of endothelium using calcein –AM (green, live) and ethidium homodimer-1 (red, dead) – stained corneas from UV-CXL using 20X/NA0.75 Plan Apo Nikon objective. B. Tangential sequential image selection for 3-D reconstruction of image A. was carried out. The purpose of this image analysis maneuver was to confirm that the origin of the dead cells was indeed coming from the endothelial monolayer. This was confirmed.
Toxicity/Fixation
A simple index was developed in order to express the endothelial cell toxicity as a ratio to the efficacy of tissue fixation as determined by the shift in thermal denaturation Tm. For the toxicity value, the difference in toxicity percentages between treated and controls were used for the numerator, and the difference in maximal thermal denaturation Tm between treated and control (ΔTm) was used for the denominator. Using this simple fraction we create values that represent the “toxicity/fixation” balance of a given cross-linking treatment. For CXL, 1/4 max, and 1/3 max, they were 2.70, 0.13, and 13.95, respectively. This, then, is a relative value that can be used to compare different treatment approaches.
Discussion
The possibility of endothelial cell damage after CXL has been debated. In vitro and live animal studies have reported upon endothelial toxicity after CXL. In endothelial culture experiments with 500 μM riboflavin and UVA irradiance of 0.35 mW/cm2, a significant cytotoxic effect on porcine corneal endothelial cells was observed.16 In rabbits with the mean corneal thickness of 330μm, CXL (3mW/cm2 irradiance) resulted in complete loss of endothelium in the irradiated area.17 In rabbit corneas with a corneal thickness less than 400 um, the endothelial UVA dose reached a cytotoxic level using the standard surface UVA dose of 5.4 J/cm2 (3 mW/cm2).7 During a 4-year follow-up after CXL, the mean endothelial cell density remained unchanged in 17 progressive keratoconus patients who had a corneal thickness of at least 450μm at the thinnest point prior to treatment.18 In a different study however, in which a total of 38 eyes of 38 patients with progressive keratoconus were treated with a minimal corneal thickness of more than 400 um, the endothelial cell density (2373±580.7 cells/mm2) one year postoperatively when compared with baseline values (2799±282.2 cells/mm2) (p=0.07) was lower.19 Corneal thickness is an important factor in irradiation toxicity. CXL in cornea less than 400um may be harmful to the endothelium.8,9 Following CXL in thin corneas (corneal thickness less than 400 μm) and after epithelial removal, a significant endothelial cell density decrease during a 12 months follow-up period (preoperative: 2733 ± 180 cells/mm2, last follow-up visit: 2441 ± 400 cells/mm2, P <0 .01) was noted.8 Although CXL in corneas with thickness greater than 400μm appears to be safe to the endothelium under current guidelines, there is the possibility of localized endothelial damage, particularly if the UV irradiance source is not homogenous.9
Sodium Hydroxymethylglycinate (SMG) used in this study is a chemical preservative commonly used in cosmetics to prevent bacterial or fungal growth. SMG is also included in a commercially available eye drop to treat dry eyes in Europe (BluGelA, Sooft Italy). In our previous study, SMG was shown to induce a good corneal stiffening effect in an ex vivo model and SMG at pH 8.5 showed the greatest upward shift in Tm. In this study, we also used SMG at pH 8.5.13 TXL with SMG at a concentration of one quarter max (max=maximum allowed concentration in cosmetics and other personal care products as dictated by the European committee on cosmetics standards) showed a collagen crosslinking effect by DSC with no corneal endothelial cell toxicity when compared to the paired, untreated control. TXL with SMG could provide an alternative crosslinking method, particularly in cases of thinner corneas.
One limitation of our study was the following: our result showing CXL induced cytotoxicity was less than that reported by Wollensak et al. 16 Although the mean percentage of dead endothelial cells was greater than that of controls in CXL samples, the results did not show a statistically significant difference as compared with controls. It is possible that we may have underestimated endothelial toxicity based on observations related to delayed-type apoptosis in UV exposed corneal endothelial cells. We checked for endothelial cell damage 90 min. after the cross-linking (CXL or TXL) treatment. In a study in which live animals were killed at 4 hours following CXL, no apoptotic endothelial cells were observed. However, the authors noted that maximum cytotoxic damage was found at 24 hours following UVA irradiation. This is known as a “delayed type of apoptosis”.7,20 Thus, the number of dead endothelial cells in our study may have been an underestimation, since we could not account for “delayed apoptosis” under our experimental conditions.
Another possibility is related to the differences in vehicles used for riboflavin photochemical cross-linking. The report by Wollensak et al20 for endothelial cell toxicity following the CXL procedure from 2003 (in rabbit) used a traditional vehicle, which is 20% high molecular weight (HMW) dextran (T-500). We used a 1.1% HPMC solution. When compared to alternative vehicles such as methylcellulose (MC) and hydroxypropyl methylcellulose (HPMC), the HMW dextran creates less of a “thick ocular surface film that increases UV light absorption”. Thus, a thicker film produced by HPMC in our study could have “shielded” the endothelium, explaining our lower level of observed endothelial cell toxicity21.
In this study, we looked for acute toxic effects rather than a delayed type of apoptosis. Rabbit endothelium has a greater ability to regenerate than humans. We tried to stain and image as soon as possible after CXL or SMG treatment, eliminating the possibility of rabbit endothelial regeneration. In future in vivo studies, we will evaluate toxicity at 24 hours after treatment in order to observe any delayed type of apoptosis and will also pursue longer-term effects of toxicity after the cross-linking treatments.
Using confocal laser scanning fluorescence microscopy, we were able to see detailed images of the entire intact single layer of endothelium, as well as higher magnification pictures of fluorescent cells and three-dimensional reconstructions (Figure 5). We found confocal laser scanning fluorescence microscopy coupled with live-dead staining to provide a useful method to evaluate endothelial cell integrity. Confocal microscopy, particularly in conjunction with tissue autofluorescence and second harmonic generated signals22 have been experiencing increasing use in the evaluation of the cornea not only in diseases such as keratoconus23 but also following induced cross-linking.24 We have also conducted studies using these related techniques and will report upon those results at a later time (unpublished).
It seems clear that at the extremes of cross-linking conditions, cell toxicity and tissue fixation go “hand-in-hand.” That is, relatively high concentrations of cross-linking agents that induce significant biomechanical (or thermal stability) increased change also will induce widespread cytotoxicity. Similarly, very low levels of cross-linking reagents and/or photochemical reactions, may neither kill any cells, nor induce tissue stabilization. Clearly, there could be a wide range of “in between” effects such that either cell toxicity or tissue fixation is favored during a treatment application. We hope to use this type of experimental setup in order to find conditions that maximize induced biomechanical effects and minimize cell toxicity. In this study, we identified an optimal concentration of chemical agent (SMG) that could induce a collagen cross-linking effect without inducing endothelial cell damage. Treating with SMG at 9.765mM was safe when compared with paired controls as determined by cytotoxic effects to endothelial cells. In addition, a modest but consistent shift in Tm by DSC was shown. This study will lay the groundwork for future animal and human studies.
Acknowledgments
Supported in part by Research to Prevent Blindness and by National Institutes of Health Grants NCRR UL1RR024156, NEI P30 EY019007, and NEI R01EY020495 (dcp). A special thank you to Emilia Laura Munteanu, Ph.D., who performed image collection, processing, and analysis at the Confocal and Specialized Microscopy Shared Resource of the Herbert Irving Comprehensive Cancer Center at Columbia University, supported by NIH grant #P30 CA013696 (National Cancer Institute).
Footnotes
Conflicts of Interest
*Patent pending through Columbia University. SLT and DCP are named inventors as employees of Columbia University. No other conflicts.
Disclosure: SY Kim, None; N. Babar, None; A. Takaoka, None; M. Zyablitskaya, None; Takayuki Nagasaki, None; S.L. Trokel, None; D.C. Paik, None. *Patent pending through Columbia University.
References
- 1.Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:149–160. doi: 10.1016/j.jcrs.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal V. Long-term results of cornea collagen cross-linking with riboflavin for keratoconus. Indian J Ophthalmol. 2013;61:433–434. doi: 10.4103/0301-4738.116072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asri D, Touboul D, Fournie P, et al. Corneal collagen crosslinking in progressive keratoconus: multicenter results from the French National Reference Center for Keratoconus. J Cataract Refract Surg. 2011;37:2137–2143. doi: 10.1016/j.jcrs.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Dillon J, Skonieczna M, Mandal K, et al. The photochemical attachment of the O-glucoside of 3-hydroxykynurenine to alpha-crystallin: a model for lenticular aging. Photochem Photobiol. 1999;69:248–253. [PubMed] [Google Scholar]
- 5.Roberts JE. Ultraviolet radiation as a risk factor for cataract and macular degeneration. Eye Contact Lens. 2011;37:246–249. doi: 10.1097/ICL.0b013e31821cbcc9. [DOI] [PubMed] [Google Scholar]
- 6.Wollensak G, Iomdina E, Dittert DD, et al. Wound healing in the rabbit cornea after corneal collagen cross-linking with riboflavin and UVA. Cornea. 2007;26:600–605. doi: 10.1097/ICO.0b013e318041f073. [DOI] [PubMed] [Google Scholar]
- 7.Wollensak G, Spoerl E, Wilsch M, et al. Endothelial cell damage after riboflavin-ultraviolet-A treatment in the rabbit. J Cataract Refract Surg. 2003;29:1786–1790. doi: 10.1016/s0886-3350(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 8.Kymionis GD, Portaliou DM, Diakonis VF, et al. Corneal collagen cross-linking with riboflavin and ultraviolet-A irradiation in patients with thin corneas. Am J Ophthalmol. 2012;153:24–28. doi: 10.1016/j.ajo.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 9.Spoerl E, Mrochen M, Sliney D, et al. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385–389. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- 10.Laing RA, Sandstrom M, Berrospi AR, et al. Morphological changes in corneal endothelial cells after penetrating keratoplasty. Am J Ophthalmol. 1976;82:459–464. doi: 10.1016/0002-9394(76)90495-5. [DOI] [PubMed] [Google Scholar]
- 11.de Groot A, White IR, Flyvholm MA, et al. Formaldehyde-releasers in cosmetics: relationship to formaldehyde contact allergy. Part 2. Patch test relationship to formaldehyde contact allergy, experimental provocation tests, amount of formaldehyde released, and assessment of risk to consumers allergic to formaldehyde. Contact Dermatitis. 2010;62:18–31. doi: 10.1111/j.1600-0536.2009.01631.x. [DOI] [PubMed] [Google Scholar]
- 12.de Groot AC, Le Coz CJ, Lensen GJ, et al. Formaldehyde-releasers: relationship to formaldehyde contact allergy. Part 2. Formaldehyde-releasers in clothes: durable press chemical finishes. Contact Dermatitis. 2010;63:1–9. doi: 10.1111/j.1600-0536.2010.01698.x. [DOI] [PubMed] [Google Scholar]
- 13.Babar N, Kim M, Cao K, et al. Cosmetic preservatives as therapeutic corneal and scleral tissue cross-linking agents. Invest Ophthalmol Vis Sci. 2015;56:1274–1282. doi: 10.1167/iovs.14-16035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 15.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wollensak G, Sporl E, Reber F, et al. Corneal endothelial cytotoxicity of riboflavin/UVA treatment in vitro. Ophthalmic Res. 2003;35:324–328. doi: 10.1159/000074071. [DOI] [PubMed] [Google Scholar]
- 17.Wollensak G, Iomdina E. Biomechanical and histological changes after corneal crosslinking with and without epithelial debridement. J Cataract Refract Surg. 2009;35:540–546. doi: 10.1016/j.jcrs.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 18.Alessio G, L'Abbate M, Furino C, et al. Confocal microscopy analysis of corneal changes after photorefractive keratectomy plus cross-linking for keratoconus: 4-year follow-up. Am J Ophthalmol. 2014;158:476–484. e471. doi: 10.1016/j.ajo.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Jordan C, Patel DV, Abeysekera N, et al. In vivo confocal microscopy analyses of corneal microstructural changes in a prospective study of collagen cross-linking in keratoconus. Ophthalmology. 2014;121:469–474. doi: 10.1016/j.ophtha.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Podskochy A, Gan L, Fagerholm P. Apoptosis in UV-exposed rabbit corneas. Cornea. 2000;19:99–103. doi: 10.1097/00003226-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Wollensak G, Aurich H, Wirbelauer C, et al. Significance of the riboflavin film in corneal collagen crosslinking. J Cataract Refract Surg. 2010;36:114–120. doi: 10.1016/j.jcrs.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 22.Wang BG, Konig K, Halbhuber KJ. Two-photon microscopy of deep intravital tissues and its merits in clinical research. J Microsc. 2010;238:1–20. doi: 10.1111/j.1365-2818.2009.03330.x. [DOI] [PubMed] [Google Scholar]
- 23.Morishige N, Shin-Gyou-Uchi R, Azumi H, et al. Quantitative analysis of collagen lamellae in the normal and keratoconic human cornea by second harmonic generation imaging microscopy. Invest Ophthalmol Vis Sci. 2014;55:8377–8385. doi: 10.1167/iovs.14-15348. [DOI] [PubMed] [Google Scholar]
- 24.Steven P, Hovakimyan M, Guthoff RF, et al. Imaging corneal crosslinking by autofluorescence 2-photon microscopy, second harmonic generation, and fluorescence lifetime measurements. J Cataract Refract Surg. 2010;36:2150–2159. doi: 10.1016/j.jcrs.2010.06.068. [DOI] [PubMed] [Google Scholar]