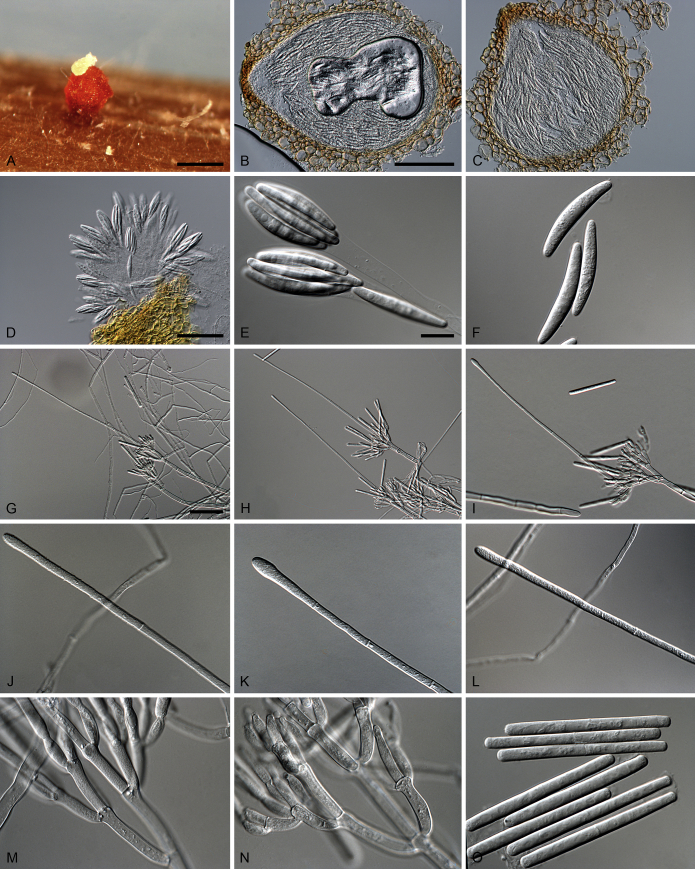
Abstract
Species in the genus Calonectria (Hypocreales) represent an important group of plant pathogenic fungi that cause serious losses to plant crops in tropical and subtropical climates. Calonectria leaf blight is currently one of the main impediments to Eucalyptus cultivation in Brazil, and various species of Calonectria have been associated with this disease. Since most previous identifications were solely based on morphological characters, much of the published literature needs to be re-evaluated. The aim of this study was thus to identify and determine the phylogenetic relationships among species that occur in the Eucalyptus growing regions of Brazil by using partial sequences of the β-tubulin, calmodulin, translation elongation factor 1-α and histone H3 gene regions. Based on extensive collections from soil and infected eucalypt leaf samples from plantations, phylogenetic inference revealed the Ca. pteridis complex to be the most common species complex present in Eucalyptus plantations in Brazil. By elucidating taxa in the Ca. pteridis, Ca. cylindrospora and Ca. candelabra species complexes, 20 novel Calonectria species were identified, and a new name in Calonectria provided for Cylindrocladium macrosporum as Ca. pseudopteridis.
Key words: Cylindrocladium, Calonectria leaf blight, Damping-off, Diversity, Taxonomy
Taxonomic novelties: New species: Calonectria brassiana R.F. Alfenas, L. Lombard & Crous; Ca. duoramosa R.F. Alfenas, L. Lombard & Crous; Ca. eucalypticola R.F. Alfenas, L. Lombard & Crous; Ca. glaebicola R.F. Alfenas, L. Lombard & Crous; Ca. maranhensis R.F. Alfenas, L. Lombard & Crous; Ca. metrosideri R.F. Alfenas, O.L. Pereira, Crous & A.C. Alfenas; Ca. multinaviculata R.F. Alfenas, L. Lombard & Crous; Ca. nemuricola R.F. Alfenas, L. Lombard & Crous; Ca. paraensis R.F. Alfenas, L. Lombard & Crous; Ca. piauiensis R.F. Alfenas, L. Lombard & Crous; Ca. propaginicola R.F. Alfenas, L. Lombard & Crous; Ca. pseudobrassicae R.F. Alfenas, L. Lombard & Crous; Ca. pseudocerciana R.F. Alfenas, L. Lombard & Crous; Ca. pseudohodgesii R.F. Alfenas, L. Lombard & Crous; Ca. pseudometrosideri R.F. Alfenas, L. Lombard & Crous; Ca. pseudospathulata R.F. Alfenas, L. Lombard & Crous; Ca. pseudovata R.F. Alfenas, L. Lombard & Crous; Ca. quinqueramosa R.F. Alfenas, L. Lombard & Crous; Ca. robigophila R.F. Alfenas, L. Lombard & Crous; Ca. silvicola R.F. Alfenas, L. Lombard & Crous; Ca. telluricola R.F. Alfenas, L. Lombard & Crous
New name: Ca. pseudopteridis (Sherb.) R.F. Alfenas, L. Lombard & Crous
Introduction
Calonectria species (asexual morph previously known as Cylindrocladium (Cy.)) are widely distributed around the world and cause diseases on a broad range of host plants in tropical and subtropical climates (Crous, 2002, Lombard et al., 2010a, Vitale et al., 2013). In Brazil, Calonectria species have been reported as pathogens of numerous important agronomic crops, such as potatoes (Solanum tuberosum; Dianese et al. 1986), soybeans (Glycine max; Bolkan et al. 1980), acerola (Malpighia glabra; Silva et al. 2001), mango (Mangifera indica; Tozetto & Ribeiro 1996), Eugenia spp. (Poltronieri et al. 2011), and several ornamentals (Reis et al. 2004). Thus far however, the majority of the reports from Brazil focused on forestry crops, such as Pinus and Acacia (Hodges and May, 1972, Hodges et al., 1973, Alfenas, 1986, Dianese et al., 1986, Novaes et al., 2012, Alfenas et al., 2013a, Alfenas et al., 2013b) and in particular on the epidemiology and disease control of Calonectria spp. associated with diseases of Eucalyptus in commercial plantations and nurseries (Blum et al., 1992, Mafia et al., 2008, Mafia et al., 2009, Graça et al., 2009, Ferreira et al., 2012, Alfenas et al., 2013c).
Based on the increasing global market for paper and wood pulp, and renewable energy, commercial Eucalyptus plantations in Brazil have expanded towards the warm and humid regions of northern and north-eastern Brazil where Calonectria leaf blight (CLB) has become the primary fungal leaf disease of this crop (Alfenas et al., 2009, Alfenas et al., 2013c). Other prominent diseases associated with Calonectria species on Eucalyptus in Brazil include damping-off, cutting rot and root rot (Alfenas and Ferreira, 1979, Alfenas et al., 1979, Alfenas, 1986, Alfenas et al., 2009). Calonectria leaf blight was first observed in commercial plantation trees of E. grandis in 1970, with more than 80 % of the trees showing severe defoliation (Alfenas & Ferreira 1979). Three Calonectria species were identified as the causal agents, which included Calonectria cylindrospora (= Cylindrocladium scoparium; see Lombard et al., 2015a, Lombard et al., 2015b), Ca. ilicicola (= Cy. parasiticum) and Ca. pyrochroa (= Cy. ilicicola). Additional species also reported to cause CLB and damping-off of Eucalyptus in Brazil include Ca. ovata (= Cy. ovatum), Ca. candelabra (= Cy. candelabrum; see Lombard et al., 2015a, Lombard et al., 2015b), and Ca. brassicae (= Cy. gracile) (Alfenas et al., 1979, Almeida and Bolkan, 1981, Alfenas, 1986, El-Gholl et al., 1993, Crous et al., 1998). However, these Calonectria species have been identified based solely on morphological characters of the asexual morphs (conidial dimensions and vesicle shape; Alfenas 1986, Ferreira 1989, Alfenas et al. 2009), which could have resulted in incorrect identifications.
In the 1990's, Eucalyptus leaf blight and defoliation caused by Ca. pteridis (= Cy. pteridis), in south-eastern Bahia and Pará provinces resulted in severe defoliation of E. grandis trees in these regions (Ferreira et al. 1995). Since then, Ca. pteridis has become the most common species reported from commercial plantations, primarily on E. camaldulensis, E. cloeziana, E. grandis, E. saligna, E. tereticornis, E. urophylla and hybrid E. grandis × E. urophylla (Alfenas et al. 2009). For most Eucalyptus species, the disease is characterised by spots that are initially small, circular or elongated and pale grey to pale brown, progressing and extending throughout the leaf blade, resulting in leaf drop and in some cases severe defoliation (Alfenas and Ferreira, 1979, Alfenas et al., 1979). It is believed that defoliation caused by CLB decreases timber volume due to the reduced photosynthetic area (Ferreira et al., 1995, Berger et al., 2007, Alfenas et al., 2009) and that weed growth is promoted due to light in the understory, which further subjects the trees to competition from weeds (Alfenas et al. 2009).
Planting of resistant genotypes is the most effective and economical method to control this disease in the field (Alfenas et al., 2009, Fonseca et al., 2010). However, selecting resistant genotypes has proven difficult since several Calonectria species appear to be associated with CLB. Pathogenicity trials done by Rehn et al. (2004) showed that several Calonectria species isolated from soil can be highly aggressive to Eucalyptus, but hardly any information is presently available on the diversity of Calonectria species occurring in soil in eucalypt plantations in Brazil.
Although morphological characters provide valuable information for species discrimination in Calonectria, incorporation of a polyphasic identification process with multi-gene DNA sequence data has elucidated various previously unknown species complexes (Crous et al., 2004b, Crous et al., 2006, Lombard et al., 2010b, Lombard et al., 2010c, Chen et al., 2011, Lombard et al., 2015a, Lombard et al., 2015b). For some of these species complexes, cryptic members can only be accurately identified on the basis of DNA sequence data. Except for a few recent studies (Alfenas et al., 2013a, Alfenas et al., 2013b), most previous reports of Calonectria species in Brazil need to be re-evaluated. Therefore, the aims of the present study were to conduct extensive surveys of soils and trees in various commercial Eucalyptus plantations in Brazil, cultivate as many isolates as possible, and subject them to DNA sequence analyses, to determine which morphological groups are dominant, and establish their distribution in Brazil.
Material and methods
Sampling and isolation
Samples of Eucalyptus leaves showing characteristic symptoms of CLB were collected in the main eucalypt growing regions of Brazil. Since the clonal plantations are established in different Management Operational Units (MOU), according to the characteristics of the soil and climatic conditions, a sample of 30 leaves per infected clone/species was collected. A random soil sample (400 g in the 0–20 cm layer) was also collected for each MOU and another from the surrounding native vegetation (Table 1). Additionally, diseased Azadirachta indica and Eucalyptus cuttings were collected from nurseries in the states of Minas Gerais (Viçosa) and Pará (Santana). The symptomatic plant material were kept in paper bags and the soil samples in plastic bags and transported to the Forest Pathology Laboratory/Bioagro of the Universidade Federal de Viçosa. All the collected plant materials were incubated in moist chambers at room temperature (25 °C ± 3 °C) for up to 14 d and inspected daily for fungal sporulation. The collected soil samples were baited with mature leaf discs of castor bean (Ricinus communis) and eucalypt twig segments as described by Gonçalves et al. (2001). Direct isolations were made onto malt extract agar (2 % w/v; MEA; Vetec, Brazil) and incubated for 7 d at 25 °C under continuous near-ultraviolet light. From these primary isolations, single conidial cultures were prepared on MEA and deposited in the culture collection of the CBS-KNAW Fungal Biodiversity Centre (CBS), Utrecht, The Netherlands, the working collections of Pedro W. Crous (CPC) maintained at CBS, and Acelino C. Alfenas (LPF) maintained at the Forest Pathology Laboratory/Bioagro, Universidade Federal de Viçosa, Brazil.
Table 1.
Collection details and GenBank assessions of Calonectria isolates included in this study.
| Species | Isolate nr.1 | Substrate | Locality | Collector | GenBank assession2 |
|||
|---|---|---|---|---|---|---|---|---|
| tub2 | cmdA | his3 | tef1 | |||||
| Calonectria brachiatica | CBS 111478; CMW 30981; CPC 1921 | Soil | Brazil | A.C. Alfenas | DQ190611 | GQ267383 | DQ190719 | FJ918568 |
| CBS 123699; CMW 25303 | Pinus tecunumanii | Buga, Colombia | M.J. Wingfield | FJ716708 | GQ267365 | FJ716712 | GQ267295 | |
| CBS 123700; CMW 25298 | Pinus maximinoi | Buga, Colombia | M.J. Wingfield | FJ696388 | GQ267366 | FJ696396 | GQ267296 | |
| CBS134665; LPF305 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395933 | KM396020 | KM396103 | KM395846 | |
| CBS134666; LPF298 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395934 | KM396021 | KM396104 | KM395847 | |
| Ca. brasiliensis | CBS 230.51; IMI 299576; CPC 2390 | Eucalyptus sp. | Brazil | R. Ciferri | GQ267241 | GQ267421 | GQ267259 | GQ267328 |
| CBS 114257; CMW 32949; CPC 1944 | Eucalyptus sp. | Brazil | A.C. Alfenas | GQ267242 | GQ267422 | GQ267260 | GQ267329 | |
| Ca. brassiana | CBS 134855; LPF378 | Soil (E. brassiana plantation) | Teresina, Piauí, Brazil | R.F. Alfenas | KM395969 | KM396056 | KM396139 | KM395882 |
| CBS 134856; LPF379 | Soil (E. brassiana plantation) | Teresina, Piauí, Brazil | R.F. Alfenas | KM395970 | KM396057 | KM396140 | KM395883 | |
| CBS 134857; LPF380 | Soil (E. brassiana plantation) | Teresina, Piauí, Brazil | R.F. Alfenas | KM395971 | KM396058 | KM396141 | KM395884 | |
| Ca. brassicae (= Cy. clavatum) | CBS 111869; CPC 2409; PC 551197 | Argyreia splendens | Indonesia | F. Bugnicourt | AF232857 | GQ267382 | DQ190720 | FJ918567 |
| CBS 143.72; ATCC 22833; IMI 164057 | Pinus caribaea | Itabira, Minas Gerais, Brazil | C.S. Hodges | KM395988 | KM396075 | – | KM395901 | |
| CBS 134657; LPF236 | Soil (Eucalyptus plantation) | Mucuri, Bahia, Brazil | E. Zauza | KM395918 | KM396005 | KM396088 | KM395831 | |
| CBS 134658; LPF234 | Soil (Eucalyptus plantation) | Mucuri, Bahia, Brazil | E. Zauza | KM395919 | KM396006 | KM396089 | KM395832 | |
| CBS 134659; LPF216 | Soil | Salinas, Minas Gerais, Brazil | D.B. Pinho | KM395920 | KM396007 | KM396090 | KM395833 | |
| CBS 134660; LPF493 | Soil | Salinas, Minas Gerais, Brazil | D.B. Pinho | KM395921 | KM396008 | KM396091 | KM395834 | |
| LPF235 | Soil (Eucalyptus plantation) | Mucuri, Bahia, Brazil | E. Zauza | KM395922 | KM396009 | KM396092 | KM395835 | |
| LPF237 | Soil (Eucalyptus plantation) | Mucuri, Bahia, Brazil | E. Zauza | KM395923 | KM396010 | KM396093 | KM395836 | |
| Ca. candelabra | CMW 31000; CPC 1675 | Eucalyptus sp. | Amazonas, Brazil | A.C. Alfenas | FJ972426 | GQ267367 | FJ972476 | FJ972525 |
| CMW 31001; CPC 1679 | Eucalyptus sp. | Amazonas, Brazil | A.C. Alfenas | GQ421779 | GQ267368 | GQ267246 | GQ267298 | |
| Ca. cerciana | CBS 123693; CMW 25309 | Eucalyptus hybrid | Zhanjiang Prov., CERC nursery, China | M.J. Wingfield & X.D. Zhou | FJ918510 | GQ267369 | FJ918528 | FJ918559 |
| CBS 123695; CMW 25290 | Eucalyptus hybrid | Zhanjiang Prov., CERC nursery, China | M.J. Wingfield & X.D. Zhou | FJ918511 | GQ267370 | FJ918529 | FJ918560 | |
| Ca. clavata | CBS 114557; ATCC 66389; CPC 2536 | Callistemon viminalis | USA | C.P. Seymour & E.L. Barnard | AF333396 | GQ267377 | DQ190623 | GQ267305 |
| CBS 114666; CMW 30994; CPC 2537 | Root debris in peat | USA | D. Ferrin & N.E. El-Gholl | DQ190549 | GQ267378 | DQ190624 | GQ267306 | |
| Ca. colombiana | CBS 115127; CPC 1160 | Soil | La Selva, Colombia | M.J. Wingfield | FJ972423 | GQ267455 | FJ972442 | FJ972492 |
| CBS 115638; CPC 1161 | Soil | La Selva, Colombia | M.J. Wingfield | FJ972422 | GQ267456 | FJ972441 | FJ972491 | |
| Ca. colombiensis | CBS 112220; CPC 723 | Soil | La Selva, Colombia | M.J. Wingfield | GQ267207 | AY725748 | AY725662 | AY725711 |
| CBS 112221; CPC 724 | Soil | La Selva, Colombia | M.J. Wingfield | AY725620 | AY725749 | AY725663 | AY725712 | |
| Ca. cylindrospora | CBS 110666 | Rosa sp. | USA | N.E. El-Gholl | FJ918509 | GQ267423 | FJ918527 | FJ918557 |
| Ca. densa | CBS 125249; CMW 31184 | Soil | Las Golondrinas, Pichincha, Ecuador | M.J. Wingfield | GQ267230 | GQ267442 | GQ267279 | GQ267350 |
| CBS 125261; CMW 31182 | Soil | Las Golondrinas, Pichincha, Ecuador | M.J. Wingfield | GQ267232 | GQ267444 | GQ267281 | GQ267352 | |
| Ca. duoramosa | CBS 134656; LPF434 | Soil (tropical rainforest) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395940 | KM396027 | KM396110 | KM395853 |
| LPF453 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395941 | KM396028 | KM396111 | KM395854 | |
| Ca. ecuadoriae | CBS 111394; CPC 1628 | Soil | Ecuador | M.J. Wingfield | DQ190599 | GQ267376 | DQ190704 | GQ267304 |
| CBS 111406; CPC 1635 | Soil | Ecuador | M.J. Wingfield | DQ190600 | GQ267375 | DQ190705 | GQ267303 | |
| Ca. eucalypticola | CBS 134846; LPF121 | Eucalyptus sp. (leaf) | Eunápolis, Bahia, Brazil | A.C. Alfenas | KM395963 | KM396050 | KM396133 | KM395876 |
| CBS 134847; LPF124 | Eucalyptus sp. (seeding) | Santa Bárbara, Minas Gerais, Brazil | A.C. Alfenas | KM395964 | KM396051 | KM396134 | KM395877 | |
| CBS 134848; LPF451 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395965 | KM396052 | KM396135 | KM395878 | |
| Ca. glaebicola | CBS 134852; LPF406 | Soil (Eucalyptus plantation) | Martinho Campos, Minas Gerais, Brazil | A.C. Alfenas | KM395966 | KM396053 | KM396136 | KM395879 |
| CBS 134853; LPF407 | Eucalyptus sp. (leaf) | Bico do Papagaio, Tocantins, Brazil | R.F. Alfenas | KM395967 | KM396054 | KM396137 | KM395880 | |
| CBS 134854; LPF408 | Eucalyptus sp. (leaf) | Bico do Papagaio, Tocantins, Brazil | R.F. Alfenas | KM395968 | KM396055 | KM396138 | KM395881 | |
| Ca. gordoniae | CBS 112142; CPC 3136; ATCC 201837 | Gordonia liasanthus | USA | D. Chiappini | AF449449 | GQ267381 | DQ190708 | GQ267309 |
| Ca. gracilipes | CBS 111141 | Soil | La Selva, Colombia | M.J. Wingfield | DQ190566 | GQ267385 | DQ190644 | GQ267311 |
| CBS 115674 | Soil | La Selva, Colombia | M.J. Wingfield | AF333406 | GQ267384 | DQ190645 | GQ267310 | |
| Ca. gracilis | CBS 111284 | Soil | Brazil | P.W. Crous | DQ190567 | GQ267408 | DQ190647 | GQ267324 |
| CBS 111807 | Manilkara zapota | Belém, Pará, Brazil | M. Aragaki | AF232858 | GQ267407 | DQ190646 | GQ267323 | |
| Ca. hodgesii | CBS 133608; LPF244 | Piptadenia gonoacantha | Viçosa, Minas Gerais, Brazil | R.F. Alfenas | KC491227 | KC491221 | – | KC491224 |
| CBS 133609; LPF245 | Anadenanthera peregrina | Viçosa, Minas Gerais, Brazil | R.F. Alfenas | KC491228 | KC491222 | – | KC491225 | |
| CBS 133610; LPF261 | Azadirachta indica | Viçosa, Minas Gerais, Brazil | R.F. Alfenas | KC491229 | KC491223 | – | KC491226 | |
| Ca. humicola | CBS 125251 | Soil | Las Golondrinas, Pichincha, Ecuador | M.J. Wingfield | GQ267233 | GQ267445 | GQ267282 | GQ267353 |
| CBS 125269 | Soil | Las Golondrinas, Pichincha, Ecuador | L. Lombard | GQ267235 | GQ267447 | GQ267284 | GQ267355 | |
| Ca. insularis | CBS 114558; CPC 768 | Soil | Tamatave, Madagascar | P.W. Crous | AF210861 | GQ267389 | FJ918526 | FJ918556 |
| CBS 114559; CPC 954 | Soil | Tamatave, Madagascar | P.W. Crous | AF210862 | GQ267390 | FJ918525 | FJ918555 | |
| Ca. leucothoës | CBS 109166; ATCC 64824; CPC 2385 | Leucothoë axillaris | USA | N.E. El-Gholl | FJ918508 | GQ267392 | FJ918523 | FJ918553 |
| Ca. pseudopteridis | CBS 163.28 | Washingtonia robusta | USA | C.D. Sherbakoff | – | KM396076 | – | KM395902 |
| Ca. maranhensis | CBS 134811; LPF142 | Eucalyptus sp. (leaf) | Açailândia, Maranhão, Brazil | A.C. Alfenas | KM395948 | KM396035 | KM396118 | KM395861 |
| CBS 134812; LPF143 | Eucalyptus sp. (leaf) | Açailândia, Maranhão, Brazil | A.C. Alfenas | KM395949 | KM396036 | KM396119 | KM395862 | |
| CBS 134825; LPF370 | Soil (Eucalyptus plantation) | Imperatriz, Maranhão, Brazil | R.F. Alfenas | KM395950 | KM396037 | KM396120 | KM395863 | |
| CBS 134828; LPF441 | Soil (Eucalyptus plantation) | Urbano Santos, Maranhão, Brazil | E. Zauza | KM395951 | KM396038 | KM396121 | KM395864 | |
| CBS 134829; LPF443 | Soil (Eucalyptus plantation) | Urbano Santos, Maranhão, Brazil | E. Zauza | KM395952 | KM396039 | KM396122 | KM395865 | |
| Ca. metrosideri | CBS 133603; LPF101 | Metrosideros polymorpha | Viçosa, Minas Gerais, Brazil | R.F. Alfenas | KC294313 | KC294304 | KC294307 | KC294310 |
| CBS 133604; LPF103 | Metrosideros polymorpha | Viçosa, Minas Gerais, Brazil | R.F. Alfenas | KC294314 | KC294305 | KC294308 | KC294311 | |
| CBS 133605; LPF104 | Metrosideros polymorpha | Viçosa, Minas Gerais, Brazil | R.F. Alfenas | KC294315 | KC294306 | KC294309 | KC294312 | |
| Ca. multinaviculata | CBS 134858; LPF233 | Soil (Eucalyptus plantation) | Mucuri, Bahia, Brazil | E. Zauza | KM395985 | KM396072 | KM396155 | KM395898 |
| CBS 134859; LPF418 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395986 | KM396073 | KM396156 | KM395899 | |
| CBS 134862; LPF472 | Soil (Eucalyptus plantation) | Mucuri, Bahia, Brazil | E. Zauza | KM395987 | KM396074 | KM396157 | KM395900 | |
| Ca. multiphialidica | CBS 112678 | Soil | Cameroon | Abadie | AY725628 | AY725761 | AY725673 | AY725723 |
| Ca. naviculata | CBS 101121 | Leaf litter | João Pessoa, Brazil | R.F. Castañeda | GQ267211 | GQ267399 | GQ267252 | GQ267317 |
| CBS 116080 | Soil | Amazonas, Brazil | M.J. Wingfield | AF333409 | GQ267398 | GQ267251 | GQ267316 | |
| Ca. nemuricola | CBS 134837; LPF085 | Soil (tropical rainforest) | Araponga, Minas Gerais, Brazil | A.C. Alfenas & P.W. Crous | KM395979 | KM396066 | KM396149 | KM395892 |
| CBS 134838; LPF090 | Soil (tropical rainforest) | Araponga, Minas Gerais, Brazil | A.C. Alfenas & P.W. Crous | KM395980 | KM396067 | KM396150 | KM395893 | |
| CBS 134839; LPF094 | Soil (tropical rainforest) | Araponga, Minas Gerais, Brazil | A.C. Alfenas & P.W. Crous | KM395981 | KM396068 | KM396151 | KM395894 | |
| Ca. orientalis | CBS 125259 | Soil | Teso East, Indonesia | M.J. Wingfield | GQ267237 | GQ267449 | GQ267286 | GQ267357 |
| CBS 125260 | Soil | Lagan, Indonesia | M.J. Wingfield | GQ267236 | GQ267448 | GQ267285 | GQ267356 | |
| LPF032 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395910 | KM395996 | – | KM395822 | |
| LPF300 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395911 | KM395997 | – | KM395823 | |
| LPF301 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395912 | KM395998 | – | KM395824 | |
| LPF435 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395913 | KM395999 | – | KM395825 | |
| Ca. ovata | CBS 111299 | E. tereticornis | Tucuruí, Pará, Brazil | P.W. Crous | GQ267212 | GQ267400 | GQ267253 | GQ267318 |
| CBS 111307 | E. tereticornis | Tucuruí, Pará, Brazil | P.W. Crous | AF210868 | GQ267401 | GQ267254 | GQ267319 | |
| Ca. paraensis | CBS 134669; LPF430 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395924 | KM396011 | KM396094 | KM395837 |
| LPF306 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395925 | KM396012 | KM396095 | KM395838 | |
| LPF308 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395926 | KM396013 | KM396096 | KM395839 | |
| LPF309 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395927 | KM396014 | KM396097 | KM395840 | |
| LPF429 | Soil (tropical rainforest) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395928 | KM396015 | KM396098 | KM395841 | |
| Ca. pauciramosa | CMW 5683 | E. grandis | Knysna, South Africa | P.W. Crous | FJ918514 | GQ267405 | FJ918531 | FJ918565 |
| CMW 30823 | Soil | Tzaneen, South Africa | S. de Buisson | FJ918515 | GQ267404 | FJ918532 | FJ918566 | |
| Ca. piauiensis | CBS 134849; LPF291 | Soil (tropical rainforest) | Serra das Confusões, Piauí | O.L. Pereira | KM395972 | KM396059 | KM396142 | KM395885 |
| CBS 134850; LPF377 | Soil (Eucalyptus plantation) | Teresina, Piauí, Brazil | R.F. Alfenas | KM395973 | KM396060 | KM396143 | KM395886 | |
| CBS 134851; LPF381 | Soil (tropical rainforest) | Teresina, Piauí, Brazil | R.F. Alfenas | KM395974 | KM396061 | KM396144 | KM395887 | |
| Ca. pini | CBS 123698 | Pinus patula | Buga, Colombia | C.A. Rodas | GQ267224 | GQ267436 | GQ267273 | GQ267344 |
| CBS 125253 | Pinus patula | Buga, Colombia | C.A. Rodas | GQ267225 | GQ267437 | GQ267274 | GQ267345 | |
| Ca. polizzii | CBS 125270 | Callistemon citrinus | Sicily, Messina, Italy | G. Polizzi | FJ972417 | GQ267461 | FJ972436 | FJ972486 |
| CBS 125271 | Arbustus unedo | Sicily, Messina, Italy | G. Polizzi | FJ972418 | GQ267462 | FJ972437 | FJ972487 | |
| Ca. propaginicola | CBS 134815; LPF220 | Eucalyptus sp. (seeding) | Santana, Pará, Brazil | A.C. Alfenas | KM395953 | KM396040 | KM396123 | KM395866 |
| CBS 134816; LPF222 | Eucalyptus sp. (seeding) | Santana, Pará, Brazil | A.C. Alfenas | KM395954 | KM396041 | KM396124 | KM395867 | |
| CBS 134817; LPF223 | Eucalyptus sp. (seeding) | Santana, Pará, Brazil | A.C. Alfenas | KM395955 | KM396042 | KM396125 | KM395868 | |
| CBS 134820; LPF287 | Used planting substrate | Santana, Pará, Brazil | A.C. Alfenas | KM395956 | KM396043 | KM396126 | KM395869 | |
| CBS 134821; LPF289 | Used planting substrate | Santana, Pará, Brazil | A.C. Alfenas | KM395957 | KM396044 | KM396127 | KM395870 | |
| LPF218 | Eucalyptus sp. (seeding) | Santana, Pará, Brazil | A.C. Alfenas | KM395958 | KM396045 | KM396128 | KM395871 | |
| LPF221 | Eucalyptus sp. (seeding) | Santana, Pará, Brazil | A.C. Alfenas | KM395959 | KM396046 | KM396129 | KM395872 | |
| Ca. pseudobrassicae | CBS 134661; LPF260 | Soil (Eucalyptus plantation) | Santana, Pará, Brazil | A.C. Alfenas | KM395935 | KM396022 | KM396105 | KM395848 |
| CBS 134662; LPF280 | Soil (Eucalyptus plantation) | Santana, Pará, Brazil | A.C. Alfenas | KM395936 | KM396023 | KM396106 | KM395849 | |
| Ca. pseudocerciana | CBS 134822; LPF365 | Eucalyptus sp. (seeding) | Santana, Pará, Brazil | A.C. Alfenas | KM395960 | KM396047 | KM396130 | KM395873 |
| CBS 134823; LPF366 | Eucalyptus sp. (seeding) | Santana, Pará, Brazil | A.C. Alfenas | KM395961 | KM396048 | KM396131 | KM395874 | |
| CBS 134824; LPF367 | Eucalyptus sp. (seeding) | Santana, Pará, Brazil | A.C. Alfenas | KM395962 | KM396049 | KM396132 | KM395875 | |
| Ca. pseudohodgesii | CBS 134813; LPF205 | Eucalyptus sp. (seeding) | Viçosa, Minas Gerais, Brazil | R.F. Alfenas | KM395903 | KM395989 | KM396077 | KM395815 |
| CBS 134814; LPF206 | Eucalyptus sp. (seeding) | Viçosa, Minas Gerais, Brazil | R.F. Alfenas | KM395904 | KM395990 | KM396078 | KM395816 | |
| CBS 134818; LPF262 | Azadirachta indica (leaf) | Viçosa, Minas Gerais, Brazil | R.F. Alfenas | KM395905 | KM395991 | KM396079 | KM395817 | |
| CBS 134819; LPF265 | Azadirachta indica (leaf) | Viçosa, Minas Gerais, Brazil | R.F. Alfenas | KM395906 | KM395992 | KM396080 | KM395818 | |
| Ca. pseudometrosideri | CBS 134843; LPF100 | Metrosideros polymorpha | Viçosa, Minas Gerais, Brazil | A.C. Alfenas | KM395907 | KM395993 | KM396081 | KM395819 |
| CBS 134844; LPF147 | Eucalyptus sp. (leaf) | Açailândia, Maranhão, Brazil | R.F. Alfenas | KM395908 | KM395994 | KM396082 | KM395820 | |
| CBS 134845; LPF210 | Soil (Eucalyptus plantation) | Maceió, Alagoas, Brazil | M.M. Coutinho | KM395909 | KM395995 | KM396083 | KM395821 | |
| Ca. pseudonaviculata | CBS 114417; CPC 10926 | Buxus sempervirens | West Auckland, New Zealand | C. Crepel | GQ267214 | GQ267409 | GQ267258 | GQ267325 |
| CBS 116251; CPC 3399 | Buxus sempervirens | West Auckland, New Zealand | C.R. MacDiarmid | AF449455 | KM396000 | – | KM395826 | |
| Ca. pseudoscoparia | CBS 125255 | E. grandis | Pichincha, Ecuador | M.J. Wingfield | GQ267227 | GQ267439 | GQ267276 | GQ267347 |
| CBS 125257 | E. grandis | Pichincha, Ecuador | M.J. Wingfield | GQ267229 | GQ267441 | GQ267278 | GQ267349 | |
| Ca. pseudospathulata | CBS 134840; LPF066 | Soil (tropical rainforest) | Araponga, Minas Gerais, Brazil | A.C. Alfenas & P.W. Crous | KM395982 | KM396069 | KM396152 | KM395895 |
| CBS 134841; LPF072 | Soil (tropical rainforest) | Araponga, Minas Gerais, Brazil | A.C. Alfenas & P.W. Crous | KM395983 | KM396070 | KM396153 | KM395896 | |
| CBS 134842; LPF087 | Soil (tropical rainforest) | Araponga, Minas Gerais, Brazil | A.C. Alfenas & P.W. Crous | KM395984 | KM396071 | KM396154 | KM395897 | |
| Ca. pseudovata | CBS 134674; LPF267 | Soil (Eucalyptus plantation) | Santana, Pará, Brazil | A.C. Alfenas | KM395945 | KM396032 | KM396115 | KM395858 |
| CBS 134675; LPF285 | Soil (Eucalyptus plantation) | Santana, Pará, Brazil | A.C. Alfenas | KM395946 | KM396033 | KM396116 | KM395859 | |
| LPF286 | Soil (Eucalyptus plantation) | Santana, Pará, Brazil | A.C. Alfenas | KM395947 | KM396034 | KM396117 | KM395860 | |
| Ca. pseudospathiphylli | CBS 109165; CPC 1623 | Soil | Ecuador | M.J. Wingfield | FJ918513 | GQ267412 | AF348241 | FJ918562 |
| Ca. pteridis | CBS 111793; ATCC 34395; CPC 2372 | Arachnoides adiantiformis | USA | P.W. Crous | DQ190578 | GQ267413 | DQ190679 | FJ918563 |
| CBS 111871; CPC 2443 | Pinus sp. | Spain | T.L. Krugner | DQ190579 | GQ267414 | DQ190681 | FJ918564 | |
| CBS 134670; LPF410 | Eucalyptus sp. (leaf) | Imperatriz, Maranhão, Brazil | R.F. Alfenas | KM395914 | KM396001 | KM396084 | KM395827 | |
| CBS 134671; LPF422 | Eucalyptus sp. (leaf) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395915 | KM396002 | KM396085 | KM395828 | |
| CBS 134672; LPF201 | Eucalyptus sp. (leaf) | Imperatriz, Maranhão, Brazil | R.F. Alfenas | KM395916 | KM396003 | KM396086 | KM395829 | |
| CBS 134673; LPF202 | Eucalyptus sp. (leaf) | Imperatriz, Maranhão, Brazil | R.F. Alfenas | KM395917 | KM396004 | KM396087 | KM395830 | |
| Ca. quinqueramosa | CBS 134654; LPF065 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395942 | KM396029 | KM396112 | KM395855 |
| CBS 134655; LPF281 | Soil (Eucalyptus plantation) | Santana, Pará, Brazil | A.C. Alfenas | KM395943 | KM396030 | KM396113 | KM395856 | |
| CBS 134863; LPF302 | Soil (Eucalyptus plantation) | Monte Dourado, Pará, Brazil | R.F. Alfenas | KM395944 | KM396031 | KM396114 | KM395857 | |
| Ca. robigophila | CBS 134652; LPF192 | Eucalyptus sp. (leaf) | Açailândia, Maranhão, Brazil | R.F. Alfenas | KM395937 | KM396024 | KM396107 | KM395850 |
| CBS 134653; LPF193 | Eucalyptus sp. (leaf) | Açailândia, Maranhão, Brazil | R.F. Alfenas | KM395938 | KM396025 | KM396108 | KM395851 | |
| LPF190 | Eucalyptus sp. (leaf) | Açailândia, Maranhão, Brazil | R.F. Alfenas | KM395939 | KM396026 | KM396109 | KM395852 | |
| Ca. silvicola | CBS 134836; LPF079 | Soil (tropical rainforest) | Araponga, Minas Gerais, Brazil | A.C. Alfenas & P.W. Crous | KM395975 | KM396062 | KM396145 | KM395888 |
| CBS 135237; LPF081 | Soil (tropical rainforest) | Araponga, Minas Gerais, Brazil | A.C. Alfenas & P.W. Crous | KM395978 | KM396065 | KM396148 | KM395891 | |
| CPC 18741; LPF071 | Soil (tropical rainforest) | Araponga, Minas Gerais, Brazil | A.C. Alfenas & P.W. Crous | KM395976 | KM396063 | KM396146 | KM395889 | |
| CPC 18766; LPF096 | Soil (tropical rainforest) | Mucuri, Bahia, Brazil | E. Zauza | KM395977 | KM396064 | KM396147 | KM395890 | |
| Ca. spathiphylli | CBS 114540; ATCC 44730; CPC 2378 | Spathiphyllum sp. | USA | S. A. Alfieri | AF348214 | GQ267424 | AF348230 | GQ267330 |
| CBS 116168; CPC 789 | Spathiphyllum sp. | Switzerland | L. Petrini | FJ918512 | GQ267425 | FJ918530 | FJ918561 | |
| Ca. spathulata | CBS 555.92 | Eucalyptus viminalis | Brazil | N.E. El-Gholl | AF308463 | GQ267426 | FJ918524 | FJ918554 |
| CBS 112689 | Araucaria angustifolia | São Paulo, Brazil | C.S. Hodges | GQ267215 | GQ267427 | GQ267261 | GQ267331 | |
| Ca. sulawesiensis | CBS 125248 | Eucalyptus sp. | Sulawesi, Indonesia | M.J. Wingfield | GQ267223 | GQ267435 | GQ267272 | GQ267343 |
| CBS 125277 | Eucalyptus sp. | Sulawesi, Indonesia | M.J. Wingfield | GQ267222 | GQ267434 | GQ267271 | GQ267342 | |
| Ca. telluricola | CBS 134663; LPF214 | Soil (tropical rainforest) | Salinas, Minas Gerais, Brazil | D.B. Pinho | KM395929 | KM396016 | KM396099 | KM395842 |
| CBS 134664; LPF217 | Soil (tropical rainforest) | Mucuri, Bahia, Brazil | E. Zauza | KM395930 | KM396017 | KM396100 | KM395843 | |
| CBS 134667; LPF263 | Soil (Eucalyptus plantation) | Mucuri, Bahia, Brazil | E. Zauza | KM395931 | KM396018 | KM396101 | KM395844 | |
| CBS 134668; LPF254 | Soil (Eucalyptus plantation) | Mucuri, Bahia, Brazil | E. Zauza | KM395932 | KM396019 | KM396102 | KM395845 | |
| Ca. variabilis | CBS 112691; CPC 2506 | Theobroma grandiflorum | Brazil | F. Carneiro | GQ267240 | GQ267458 | GQ267264 | GQ267335 |
| CBS 114677; CPC 2436 | Schefflera morototoni | Brazil | F. C. de Albuquerque | AF333424 | GQ267457 | GQ267263 | GQ267334 | |
| Ca. zuluensis | CBS 125268 | E. grandis | Kwa-Zulu Natal, Kwambonambi, South Africa | L. Lombard | FJ972414 | GQ267459 | FJ972433 | FJ972483 |
| CBS 125272 | E. grandis | Kwa-Zulu Natal, Kwambonambi, South Africa | L. Lombard | FJ972415 | GQ267460 | FJ972434 | FJ972484 | |
ATCC: American Type Culture Collection, Virginia, U.S.A., CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands, CMW: collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa, CPC: Pedro W. Crous working collection housed at CBS, IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham lane, U.K., LPF: Laboratório de Patologia Florestal, Universidade Federal de Viçosa, Viçosa, Brazil. Ex-type strains indicated in bold.
tub2 = β-tubulin, cmdA = calmodulin, his3 = histone H3, tef1 = translation elongation factor-1α; sequences generated in this study indicated in italics.
DNA sequencing and phylogenetic analyses
Genomic DNA was isolated from 7-d-old fungal mycelium grown on MEA following the protocol of the Wizard® Genomic DNA Purification (Promega Corporation, WI, USA) kit. For amplification of gene regions, the DreamTaq™ Master Mix (MBI Fermentas, Vilnius, Lithuania) was used, following the manufacturer's protocol. Initially, partial gene sequences of the translation elongation factor 1-α (tef1) were determined for all isolates collected using the primers EF1-728F (O'Donnell et al. 1998) and EF-2 (Carbone & Kohn 1999) following the protocol and conditions outlined by Crous et al. (2004b). Subsequently, partial fragments of β-tubulin (tub2), calmodulin (cmdA) and histone 3 (his3), were determined following the protocols and primers outlined by Crous et al. (2004b) and Groenewald et al. (2013). DNA sequencing reactions were performed using the BigDye® Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems Life Technologies, Carlsbad, CA, USA) following the protocol provided by the manufacturer. To ensure the integrity of the sequences, amplicons were sequenced in both directions using the same primers used for amplification. Purified sequence reactions were run on an ABI Prism 3730xl DNA Sequencer (Life Technologies, Carlsbad, CA, USA). The quality of the electropherograms generated were evaluated using Sequence Scanner Software v. 1.0 (Applied Biosystems) and PHPH (http://www.biomol.unb.br/phph/). Consensus sequences were determined using Seqman (DNAStar Inc., Madison, Wisconsin, USA). All sequences were manually corrected and the arrangement of nucleotides in ambiguous positions was corrected using comparisons of the sequences generated from both the forward and reverse primers. In addition to the sequences generated in this study, other sequences were obtained from NCBI's GenBank nucleotide database (www.ncbi.nlm.nih.gov) and added to the DNA sequence datasets generated in this study (Table 1).
Sequence datasets for the four loci were aligned in MAFFT v. 7.0 (Katoh & Standley 2013), and manually corrected where necessary using MEGA v. 5 (Tamura et al. 2011). Single nucleotide polymorphisms (SNP's) were determined for each gene region with the aid of DnaSP v. 5.00.06 (Librado & Rozas 2009). The best evolutionary model of nucleotide substitution for each gene region was selected according to Akaike Information Criterion (AIC) using MrModeltest v. 2.3 (Nylander 2004) and incorporated into the analyses.
An initial phylogenetic analysis was done for the aligned tef1 data set which included 1019 taxa, including outgroup, using MrBayes v. 3.1.2 (Ronquist & Huelsenbeck 2003) to identify the possible groups present in our samples. Based on this, 85 Calonectria isolates were selected for further study and divided into four separate datasets representing the (1) Ca. brassicae and Ca. pteridis complex, (2) Ca. cylindrospora complex, (3) Ca. candelabra complex and (4) Ca. naviculata complex, to reduce the number of gaps in the alignments and consequently improve the resolution of the analyses. To determine whether the four gene regions determined were congruent, congruence index trees (De Vienne et al. 2007) and a 70 % reciprocal bootstrap method (Gueidan et al. 2007) were applied to each gene region used.
Phylogenetic analyses were based on both Bayesian Inference (BI) and Maximum Parsimony (MP). For BI, the best evolutionary model of nucleotide substitution for each gene region was incorporated into the analyses. Analyses in MrBayes v. 3.2.1 (Ronquist & Huelsenbeck 2003) used the Markov Chain Monte Carlo (MCMC) algorithm and employed two sets of four chains started in parallel from a random tree topology with the heating parameter set at 0.3. The MCMC analysis ran until the average standard deviation of split frequencies came below 0.01, with trees saved every 1 000 generations. The first 25 % of saved trees were discarded as the “burn-in” phase and posterior probabilities (PP) determined from the remaining trees.
MP analyses were performed in PAUP (Phylogenetic Analysis Using Parsimony, v. 4.0b10; Swofford 2003) with phylogenetic relationships estimated by heuristic searches with 1 000 random addition sequences. The tree-bisection-reconnection option was used, with the branch swapping option set to “best trees” only. All characters were weighted equally and alignment gaps treated as fifth state. Measures calculated for parsimony included tree length (TL), consistency index (CI), retention index (RI) and rescaled consistence index (RC). Bootstrap analyses (Hillis & Bull 1993) were based on 1 000 replications. All resulting trees were illustrated using Geneious v. 5.5.4 (Drummond et al. 2011). Sequences derived in this study were deposited in GenBank (Table 1) and the alignments in TreeBASE (www.treebase.org/treebase/index.html).
Taxonomy
Single conidial isolates were grown on synthetic nutrient-poor agar (SNA; Nirenburg 1981) at 24 °C, following the protocol of Lombard et al. (2009). After 7 d of incubation, the morphological characteristics of the asexual morphs were determined by mounting fungal structures in clear lactic acid and 30 measurements at ×1 000 magnification were determined for each isolate using a Zeiss Axioscope 2 microscope with interference contrast (DIC) optics. Additionally, crosses were made as described by Lombard et al., 2010b, Lombard et al., 2010c in all possible combinations based on the identities determined by DNA sequence analysis of the tef1 gene region. Isolates were crossed with themselves as controls, thus making it possible to distinguish between heterothallic and homothallic mating systems of the isolates. The plates were stacked in plastic containers and incubated at room temperature (25°C ± 3 °C) for 6–8 wk. Crosses were regarded as successful when isolate combinations produced ascomata extruding viable ascospores. Morphological characteristics of the sexual morphs were determined by mounting ascomata in tissue freezing medium (Leica Biosystems, Nussloch, Germany) and cutting sections with a Leica CM1100 cryostate (Leica Biosystems, Nussloch, Germany). The 10 μm sections were mounted in 85 % lactic acid and 3 % KOH. The 95 % confidence levels were determined and extremes of conidial and ascospore measurements are given in parentheses. For other structures only extremes are presented. Colony morphology was assessed using 7-d-old cultures on MEA and oatmeal agar (OA) incubated at 24 °C and the colour charts of Rayner (1970). All descriptions, illustrations and nomenclatural data were deposited in MycoBank (Crous et al. 2004a).
Results
Sampling and isolation
A total of 1 017 isolates were obtained of which 646 were from Eucalyptus leaves displaying symptoms of CLB in plantations, 320 isolates were baited from soils collected within the commercial Eucalyptus plantations, 13 isolates were obtained from Eucalyptus seedlings and two from A. indica seedlings in the nursery, and 36 isolates were obtained from the surrounding native vegetation. Eighty-five of these isolates were selected for further study (Table 1) based on preliminary phylogenetic analysis of the tef1 gene region (results not shown).
Phylogenetic analyses
Approximately 500–550 bases were determined for the his3, tef1 and tub2 gene regions and 650 bases for the cmdA gene region. The preliminary tef1 sequence analysis, which included 1 019 taxa as well as outgroup taxa (Ca. colombiensis CBS 112220 & CBS 112221), showed that the majority of the collected isolates from Eucalyptus with CLB symptoms belonged to the Ca. pteridis species complex (484 of 545 isolates; results not shown). The remaining isolates were divided among the Ca. brassicae, Ca. cylindrospora, Ca. naviculata and Ca. candelabra species complexes.
For the Bayesian analyses, the evolutionary model selected for each gene region for each dataset is presented in Table 2. The Bayesian consensus trees for each of the datasets confirmed the tree topologies obtained from the MP analyses, and therefore, only the Bayesian consensus trees are presented with bootstrap support values (BS) and posterior probabilities (PP) shown for well-supported nodes. Congruency tests revealed no conflicts in tree topologies for the four gene regions used in each of the four separate datasets and were therefore combined.
Table 2.
Evolutionary substitution models determined for each gene region used in the Baysian phylogenetic inference.
| Calonectria complex | Evolution model |
|||
|---|---|---|---|---|
| tef1 | tub2 | cmdA | his3 | |
| Ca. brassicae and Ca. pteridis complexes | HKY + G | HKY + G | GTR + G | GTR + I + G |
| Ca. cylindrospora complex | HKY + G | HKY + I + G | HKY + I + G | GTR + I + G |
| Ca. naviculata complex | HKY + I | HKY + I | HKY + I | GTR + I |
| Ca. candelabra complex | GTR + G | GTR + G | GTR + I + G | HKY + G |
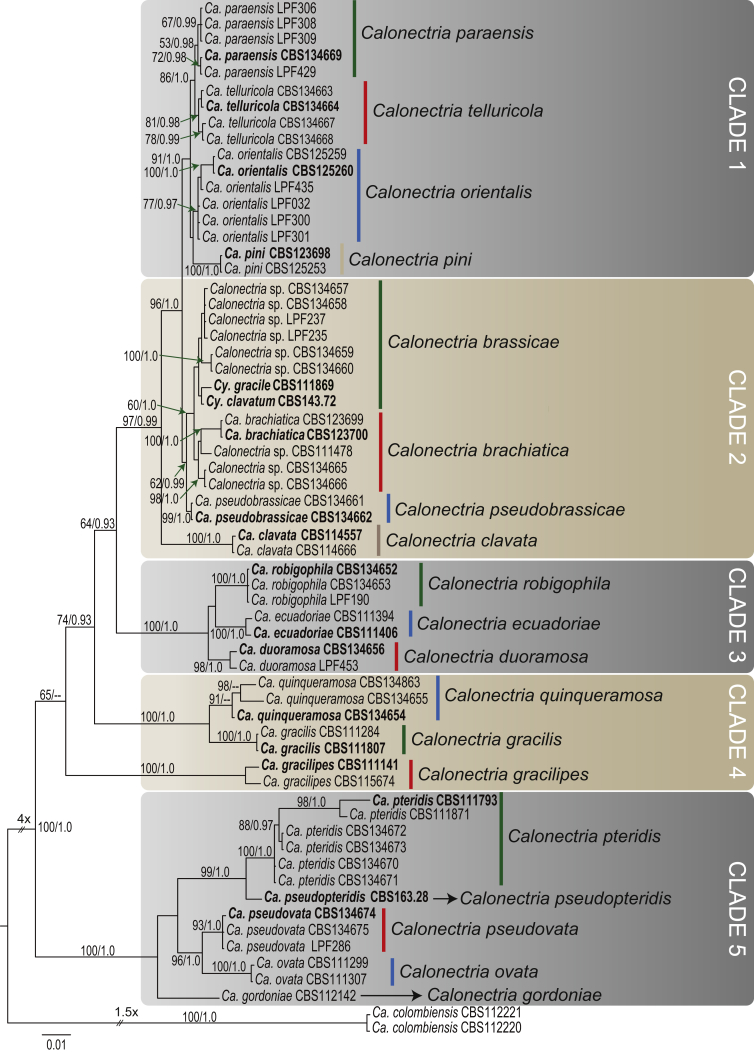
The combined dataset for the Ca. brassicae and Ca. pteridis complexes included 61 ingroup taxa, with Ca. colombiensis (CBS 112220 & CBS 112221) as the outgroup taxon. The sequence dataset consisted of 1 958 characters, including alignment gaps. Of these, 1 289 were constant, 35 were parsimony-uninformative and 634 parsimony-informative. The MP analysis yielded 1 000 trees (TL = 1 304, CI = 0.685, RI = 0.902, RC = 0.618). The BI analysis lasted 1 625 000 generations and the consensus tree (Fig. 1) and posterior probabilities (PP) were calculated from 2 439 trees. In the tree, five main clades could be resolved. Clade 1 included four smaller clades, of which two of the clades represented Ca. orientalis (ex-type CBS 125260; BS = 77, PP = 0.97) and Ca. pini (ex-type CBS 123698; BS = 100, PP = 1.0), respectively. The remaining two clades (BS = 53, PP = 0.98 and BS = 81, PP = 0.98, respectively), which include CBS 134669, LPF 306, LPF308, LPF309, LPF429 in one clade, and CBS 134663, CBS 134664, CBS 134667, CBS 134668 in the other, appear to represent two distinct lineages. Clade 2 consisted of four smaller clades, of which three represented known Calonectria species. The clade representing Ca. brassicae, which included the ex-types of Cy. gracile (CBS 111869) and Cy. clavatum (CBS 134.71), could not be resolved in this study. Similarly, the clade representing Ca. brachiatica (ex-type CBS 123700) could not be resolved. Two isolates (CBS 134661, CBS 1346620) formed a basal sister clade (BS = 100, PP = 1.0) to the clades representing Ca. brassicae and Ca. brachiatica, possibly indicating a previously unrecognised lineage. Clade 3 included three well-supported sister clades, one of which (BS = 100, PP = 1.0) represents Ca. ecuadoriae (ex-type CBS 111406). The other two clades, one incorporating CBS 134652, CBS 134653 and LPF190 (BS = 100, PP = 1.0) and the other CBS 134656 and LPF453 (BS = 98, PP = 1.0), each represent novel lineages. Isolates CBS 134654, CBS 134655 and CBS 134863, in Clade 4, clustered together in a clade (BS = 91, PP < 0.95) closely related to but separate from the clade (BS = 100, PP = 1.0) representing Ca. gracilis (ex-type CBS 111807), representing a novel lineage. In Clade 5, three isolates obtained from Eucalyptus leaves (CBS 134674, CBS 134675, LPF286) formed a sister clade (BS = 93, PP = 1.0) closely related to but separate from the clade (BS = 100, PP = 1.0) representing Ca. ovata (CBS 111299 and CBS 111307). Four representative isolates (CBS 134670 – CBS 134673) collected during this study, clustered within the clade (BS = 100, PP = 1.0) representing Ca. pteridis (ex-type CBS 111793). The ex-type of Cy. macrosporum (CBS 163.28) formed a distinct basal lineage to the Ca. pteridis clade, indicating that this species was incorrectly synonymised under Ca. pteridis (Crous 2002).
Fig. 1.
Consensus phylogram of 2 439 trees resulting from a Bayesian analysis of the combined four gene sequence alignment of the Calonectria brassicae and Ca. pteridis complexes. Accession numbers in bold represent ex-type strains. Bayesian posterior probabilities and Maximum Parsimony bootstrap support values are indicated at the nodes and the scale bar represents the number of expected changes per site. The tree was rooted to Ca. colombiensis (CBS 112220, CBS 112221).
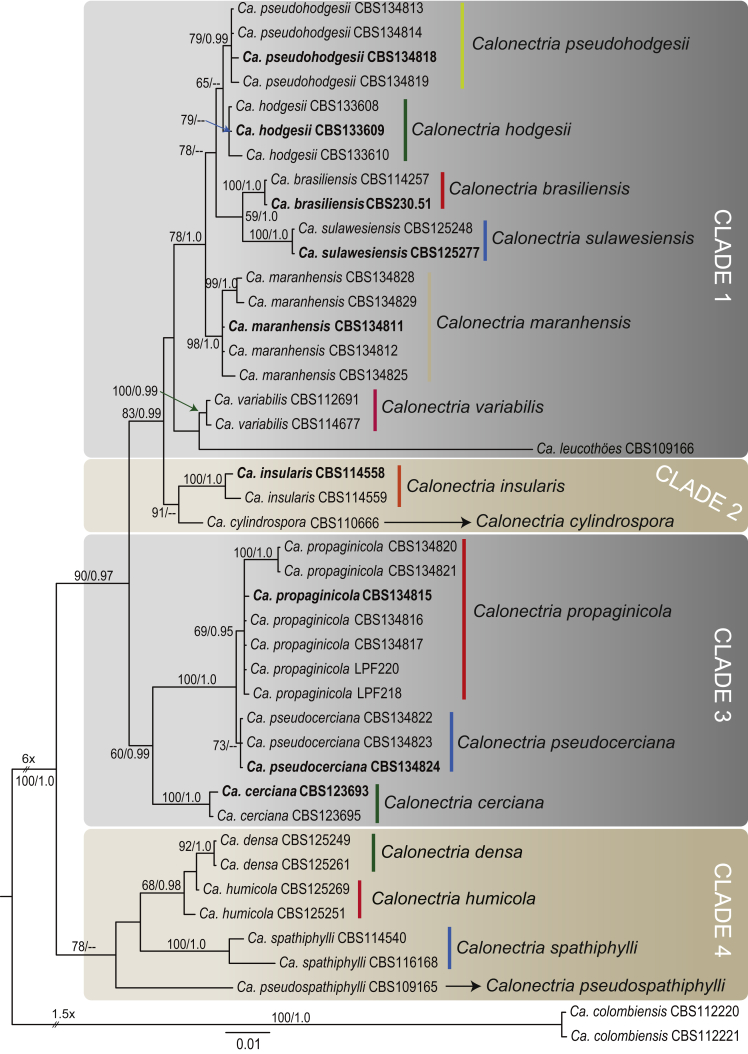
The combined dataset for the Ca. cylindrospora species complex included 41 ingroup taxa, with Ca. colombiensis (CBS 112220 & CBS 112221) as the outgroup taxon. The sequence dataset consisted of 1 975 characters, including alignment gaps. Of these, 1 355 were constant, 85 were parsimony-uninformative and 535 parsimony-informative. The MP analysis yielded 1 000 trees (TL = 1 002, CI = 0.767, RI = 0.891, RC = 0.684). The BI analysis lasted 985 000 generations and the consensus tree (Fig. 2) and posterior probabilities (PP) were calculated from 1 480 trees. In the tree, four main clades could be resolved with the isolates collected in this study clustering in Clades 1 & 3. In Clade 1, isolates obtained during this study clustered in two smaller clades, one of which formed a distinct basal clade (BS = 98, PP = 1.0) to the clades representing Ca. hodgesii (ex-type CBS 133609), Ca. brasiliensis (ex-type CBS 230.51) and Ca. sulawesiensis (ex-type CBS 125277), representing a distinct lineage. The remaining isolates (CBS 134813, CBS 134814, CBS 134818, CBS 134819) formed a clade (BS = 79, PP = 0.99) closely related to, but distinct from, the Ca. hodgesii clade, also representing a previously unrecognised lineage. In Clade 3, the newly collected isolates also clustered in two well-supported but distinct clades (containing CBS 134815; BS = 69, PP = 0.95 & containing CBS 134824; BS = 73, PP < 0.95, respectively) with Ca. cerciana (ex-type CBS 123693) forming a basal clade to both these lineages.
Fig. 2.
Consensus phylogram of 1 480 trees resulting from a Bayesian analysis of the combined four gene sequence alignment of the Calonectria cylindrospora complex. Accession numbers in bold represent ex-type strains. Bayesian posterior probabilities and Maximum Parsimony bootstrap support values are indicated at the nodes and the scale bar represents the number of expected changes per site. The tree was rooted to Ca. colombiensis (CBS 112220, CBS 112221).
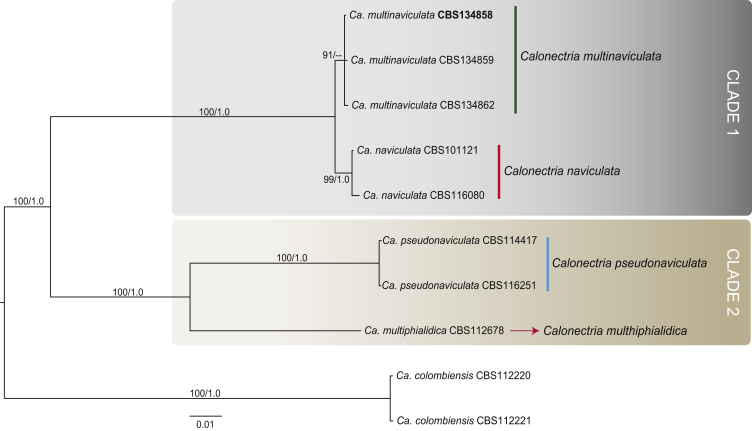
The combined dataset for the Ca. naviculata species complex included eight ingroup taxa, with Ca. colombiensis (CBS 112220 & CBS 112221) as the outgroup taxon. The sequence dataset consisted of 1 994 characters, including alignment gaps. Of these, 1 431 were constant, 86 were parsimony-uninformative and 477 parsimony-informative. The MP analysis yielded 1 000 trees (TL = 764, CI = 0.919, RI = 0.931, RC = 0.855). The BI analysis lasted 275 000 generations and the consensus tree (Fig. 3) and posterior probabilities (PP) were calculated from 15 trees. Only three isolates (CBS 134858, CBS 134859, CBS 134862), collected in this study, grouped in this dataset. They formed a well-supported clade (BS = 91, PP < 0.95) closely related to, but distinct from, the clade representing Ca. naviculata (ex-type CBS 101121).
Fig. 3.
Consensus phylogram of 15 trees resulting from a Bayesian analysis of the combined four gene sequence alignment of the Calonectria naviculata complex. Accession numbers in bold represent ex-type strains. Bayesian posterior probabilities and Maximum Parsimony bootstrap support values are indicated at the nodes and the scale bar represents the number of expected changes per site. The tree was rooted to Ca. colombiensis (CBS 112220, CBS 112221).
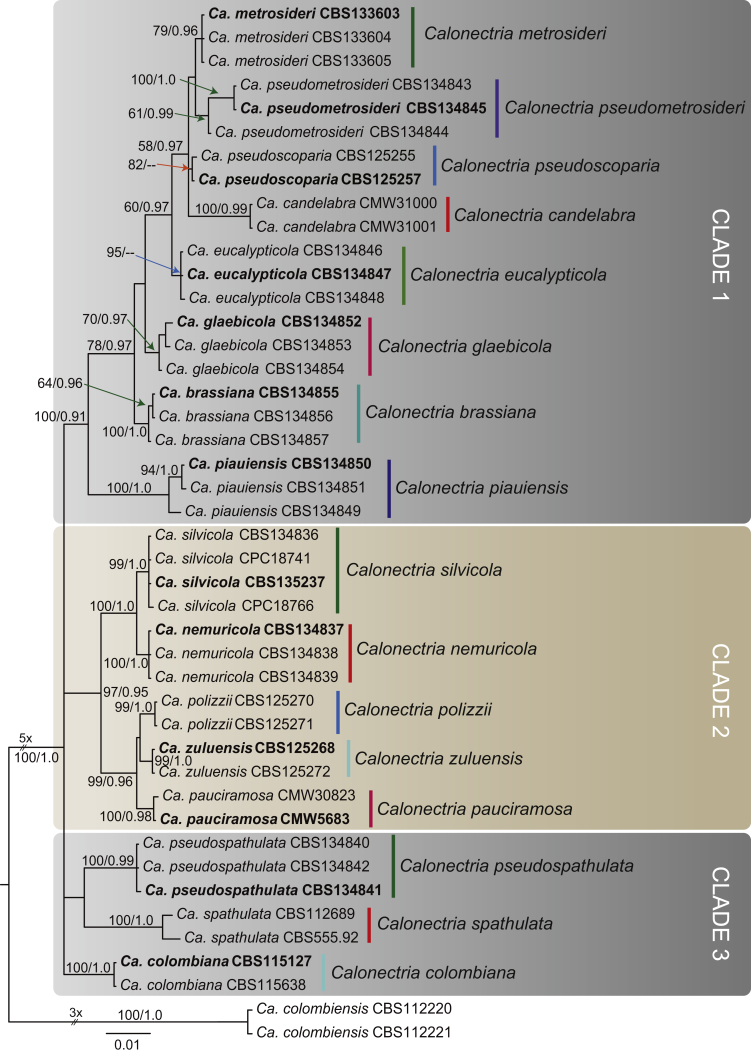
The combined dataset for the Ca. candelabra species complex included 42 ingroup taxa, with Ca. colombiensis (CBS 112220 & CBS 112221) as the outgroup taxon. The sequence dataset consisted of 1 930 characters, including alignment gaps. Of these, 1 448 were constant, six were parsimony-uninformative and 476 parsimony-informative. The MP analysis yielded 1 000 trees (TL = 690, CI = 0.813, RI = 0.922, RC = 0.750). The BI analysis lasted 1 565 000 generations and the consensus tree (Fig. 4) and posterior probabilities (PP) were calculated from 2 348 trees. In the tree, three main clades are resolved, with each clade incorporating isolates collected in this study. In Clade 1, the newly collected isolates clustered into five well-supported clades. The first of these clades (containing CBS 134845; BS = 61, PP = 0.99) is closely related but separate from the Ca. metrosideri clade (ex-type CBS 133603). The remaining four clades of newly collected isolates formed basal sister clades to Ca. candelabra (CMW31000 & CMW31001), Ca. pseudoscoparia (ex-type CBS 125257) and Ca. metrosideri. In Clade 2, the isolates from the current study clustered into two separate well-supported clades (containing CBS 135237; BS = 99, PP = 1.0, and containing CBS 134837; BS = 100, PP = 1.0) sister to the clades of Ca. pauciramosa (ex-type CMW5683), Ca. polizzii (CBS 125270 & CBS 125271) and Ca. zuluensis (ex-type CBS 125268). The newly collected isolates in Clade 3 clustered together in a well-supported clade (containing CBS 134841; BS = 100, PP = 0.99) closely related to, but distinct from, the Ca. spathulata clade (CBS 555.92 & CBS 112689).
Fig. 4.
Consensus phylogram of 2 348 trees resulting from a Bayesian analysis of the combined four gene sequence alignment of the Calonectria candelabra complex. Accession numbers in bold represent ex-type strains. Bayesian posterior probabilities and Maximum Parsimony bootstrap support values are indicated at the nodes and the scale bar represents the number of expected changes per site. The tree was rooted to Ca. colombiensis (CBS 112220, CBS 112221).
Taxonomy
Morphological observations (Table 3) supported by phylogenetic inference showed that the majority of the strains collected in this study belonged to Ca. pteridis. The remaining isolates are shown to represent several distinct taxa that are provided with names in Calonectria. Furthermore, Ca. metrosideri is invalid, as Alfenas et al. (2013a) did not include collection and specimen details and it is, therefore, validated here. Calonectria pseudopteridis (= Cylindrocladium macrosporum) is resurrected to species rank based on phylogenetic inference.
Table 3.
Morphological characterisitics of Calonectria spp. included in this study.
| Species | Perithecia |
Asci |
Ascospores |
Conidiogenous apparatus |
Stipe extention |
Vesicle |
Macroconidia |
Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (μm) | Shape | Size (μm) | Size (μm) | Septation | Size (μm) | Branches | Size (μm) | Diam (μm) | Shape | Size (μm) | Septation | Length/Diam ratio | ||
| Calonectria brassicae species complex | ||||||||||||||
| Ca. brachiatica | 40–81 × 35–84 | 5 | 134–318 × 4–5 | 5–7 | clavate | (37–)40–48(–50) × 4–6 | 1(–2) | 8.8 | Lombard et al. (2009) | |||||
| Ca. brassicae | 35–75 × 15–60 | 5 | 140–350 × 2.5–3 | 2–6 | clavate | (38–)40–55(–65) × 3.5–6 | 1 | 11.78 | Crous (2002) | |||||
| Ca. clavata | 360–630 × 290–500 | subglobose to ovoid | 53–155 × 10–22.5 | (30–)40–50(–54) × (4–)5–6(–6.5) | 1(–3) | 40–70 × 25–50 | 4 | 60–110 × 5–6 | 3–4 | narrowly clavate | (44–)50–70(–80) × 4–6 | 1(–3) | 13 | Crous (2002) |
| Ca. duoramosa | 20–60 × 30–50 | 2 | 175–310 × 3–5 | 4–6 | acicular to clavate | (35–)44–48(–55) × 3–5 | 1 | 11.38 | This study | |||||
| Ca. ecuadoriae | 30–100 × 30–100 | 7 | 200–300 × 2–3 | 3–5 | clavate | (45–)48–55(–65) × 4–5 | 1(–3) | 11.33 | Crous et al. (2006) | |||||
| Ca. gracilipes | 350–400 × 300–380 | subglobose to ovoid | 80–120 × 12–18 | (28–)33–40(–45) × (5–)6–7(–7.5) | 1 | 30–70 × 25–35 | 3 | 150–260 × 2.5–3 | 3–4 | clavate | (35–)40–48(–60) × 4–6 | 1 | 10 | Crous (2002) |
| Ca. gracilis | 350–400 × 330–380 | subglobose to ovoid | 75–100 × 8–15 | (27–)33–45(–50) × (4–)4.5–5(–6) | 1 | 60–100 × 30–70 | 4 | 160–350 × 2–3 | 2–11 | clavate | (40–)53–58(–65) × 3.5–5 | 1(–3) | 12.44 | Crous (2002) |
| Ca. orientalis | 54–174 × 67–92 | 5 | 90–218 × 5–10 | 5–10 | clavate to broadly clavate | (43–)46–50(–53) × 4–5 | 1 | 12 | Lombard et al. (2010c) | |||||
| Ca. paraensis | 45–55 × 60–75 | 2 | 120–195 × 3–5 | 4–6 | clavate | (35–)40–43(–50) × 3–6 | 1 | 8.85 | This study | |||||
| Ca. pini | 49–81 × 35–84 | 3 | 121–266 × 5–7 | 4–6 | clavate | (37–)40–48(–50) × 4–6 | 1 | 8.8 | Lombard et al. (2010c) | |||||
| Ca. pseudobrassicae | 50–115 × 60–100 | 3 | 190–300 × 3–5 | 3–5 | clavate | (30–)39–42(–48) × 4–6 | 1 | 8.04 | This study | |||||
| Ca. quinqueramosa | 160–400 × 115–250 | pyriform to subglobose | 50–105 × 10–25 | (25–)39–42(–50) × 5–7 | 1 | 30–60 × 35–65 | 5 | 170–340 × 2–4 | 3–5 | narrowly clavate to clavate | (45–)57–61(–70) × 4–6 | 1 | 11.57 | This study |
| Ca. robigophila | 15–60 × 30–70 | 6 | 125–225 × 3–4 | 4–5 | acicular to clavate | (45–)49–52(–60) × 3–5 | 1 | 12.6 | This study | |||||
| Ca. telluricola | 45–95 × 40–80 | 4 | 100–225 × 2–4 | 3–6 | clavate | (35–)40–42(–50) × 3–6 | 1 | 9.13 | This study | |||||
| Calonectria candelabra species complex | ||||||||||||||
| Ca. brassiana | 50–135 × 50–80 | 3 | 90–172 × 2–3 | 3–7 | ellipsoid to narrowly obpyriform | (35–)50–56(–65) × 3–5 | 1 | 12.91 | This study | |||||
| Ca. candelabra | 350–450 × 300–350 | subglobose to ovoid | 70–130 × 7–15 | (40–)45–50(–60) × 5–6 | 1 | 30–70 × 50–80 | 5 | 100–220 × 3–3.5 | 5–8 | ellipsoid to narrowly obpyriform | (45–)58–68(–80) × 4–5(–6) | 1 | 13.33 | Crous (2002) |
| Ca. colombiana | 270–410 × 175–285 | subglobose to ovoid | 87–162 × 12–18 | (28–)31–36(–40) × 3–5 | 1 | 38–115 × 35–91 | 4 | 143–173 × 5–7 | 8–12 | obpyriform to ellipsoidal | (33–)35–39(–40) × 3–4 | 1 | 12.33 | Lombard et al. (2010b) |
| Ca. eucalypticola | 45–75 × 35–62 | 3 | 145–170 × 2–4 | 5–7 | ellipsoid to obpyriform | (43–)49–52(–55) × 3–5 | 1 | 12.2 | This study | |||||
| Ca. glaebicola | 25–40 × 27–45 | 2 | 100–165 × 2–4 | 3–5 | ellipsoid to narrowly obpyriform | (45–)50–52(–55) × 3–5 | 1 | 12.06 | This study | |||||
| Ca. metrosideri | 60–75 × 40–65 | 4 | 90–170 × 2–4 | 5–9 | spathulate to obpyriform | (40–)44–46(–51) × 3–5 | 1 | 11.25 | Alfenas et al. (2013a) | |||||
| Ca. mossambicensis | 37–87 × 19–59 | 3 | 91–203 × 2–6 | 2–8 | obpyriform to ellipsoidal | (35–)38–46(–50) × 3–6 | 1 | 10.5 | Crous et al. (2013) | |||||
| Ca. nemuricola | 50–80 × 40–60 | 4 | 150–205 × 6–12 | 7–13 | obpyriform | (40–)44–46(–50) × 3–5 | 1 | 11.06 | This study | |||||
| Ca. pauciramosa | 250–400 × 170–300 | subglobose to ovoid | 70–140 × 8–25 | (30–)33–38(–40) × 6–7(–8) | 1 | 20–50 × 35–85 | 3 | 120–230 × 2–3 | 5–11 | obpyriform to ellipsoidal | (30–)45–55(–60) × (3.5–)4–5 | 1 | 12.5 | Schoch et al. (1999) |
| Ca. piauiensis | 35–80 × 20–60 | 2 | 95–130 × 2–3 | 3–7 | ellipsoid to narrowly obpyriform | (38–)47–52(–60) × 3–5 | 1 | 11.27 | This study | |||||
| Ca. polizzii | 28–51 × 27–57 | 3 | 111–167 × 5–6 | 6–9 | obpyriform to ellipsoidal | (31–)32–42(–49) × 3–5 | 1 | 9.25 | Lombard et al. (2010a) | |||||
| Ca. pseudometrosideri | 30–76 × 45–65 | 3 | 160–210 × 2–4 | 5–7 | ellipsoid to obpyriform | (40–)49–52(–60) × (3–)4.5(–5) | 1 | 11.34 | This study | |||||
| Ca. pseudoscoparia | 52–74 × 34–87 | 4 | 124–201 × 4–6 | 6–10 | obpyriform to ellipsoidal | (41–)45–51(–52) × 3–3 | 1 | 12 | Lombard et al. (2010b) | |||||
| Ca. pseudospathulata | 60–100 × 30–70 | 3 | 145–190 × 2–4 | 7–10 | obpyriform | (35–)41–44(–50) × 3–5 | 1 | 10.46 | This study | |||||
| Ca. silvicola | 45–105 × 35–90 | 3 | 130–195 × 3–4 | 7–10 | obpyriform | (30–)40–42(–50) × 3–5 | 1 | 9.17 | This study | |||||
| Ca. spathulata | 300–500 × 200–350 | subglobose to ovoid | 90–150 × 13–17 | (38–)45–55(–60) × (4.5–)5–6(–7) | (1)–3 | 60–100 × 30–70 | 3 | 150–300 × 3–4 | 6–10 | ellipsoid to obpyriform to clavate | (48–)75–90(–100) × (4–)5–6 | (1–)3(–6) | 13.33 | Crous 2002 |
| Ca. zuluensis | 292–394 × 170–285 | subglobose to ovoid | 92–140 × 10–16 | (26–)29–34(–38) × 4–5 | 1 | 37–70 × 35–67 | 3 | 110–171 × 5–8 | 6–10 | ellipsoid to obpyriform | (31–)34–38(–40) × 3–5 | 1 | 8 | Lombard et al. (2010a) |
| Calonectria cylindrospora species complex | ||||||||||||||
| Ca. brasiliensis | 81–103 × 58–90 | 3 | 204–266 × 6–7 | 7–11 | ellipsoid to obpyriform | (35–)36–40(–41) × 3–5 | 1 | 10.86 | Lombard et al. (2010a) | |||||
| Ca. cerciana | 62–113 × 70–98 | 4 | 148–222 × 5–6 | 8–13 | fusiform to obpyriform | (37–)41–46(–49) × 5–6 | 1 | 8.8 | Lombard et al. (2010d) | |||||
| Ca. cylindrospora | 280–520 × 280–400 | globose to subglobose | 75–100 × 8–15 | (24–)30–40(–49) × (4–)5–6(–8) | 1 | 60–100 × 60–110 | 6 | 150–200 × 3–4 | 6–8 | ellipsoid to pyriform or clavate | (40–)42–50(–66) × 3–4(–5) | 1 | 11.25 | Crous (2002) |
| Ca. densa | 49–78 × 63–123 | 4 | 149–192 × 5–6 | 10–12 | ovoid to ellipsoid to sphaeropedunculate | (47–)50–58(–62) × (5–)6 | 1 | 9 | Lombard et al. (2010d) | |||||
| Ca. hodgesii | 61–72 × 45–65 | 3 | 136–196 × 2–4 | 6–11 | pyriform to ellipsoidal or ovoid to sphaeropedunculate | (44–)49–51(–55) × 3–5 | 1 | 12.5 | Alfenas et al. (2013b) | |||||
| Ca. humicola | 43–71 × 42–49 | 3 | 126–157 × 4–5 | 10–12 | globose to ovoid to sphaeropedunculate | (45–)48–54(–56) × 4–5 | 1 | 10.2 | Alfenas et al. (2013b) | |||||
| Ca. insularis | 350–450 × 300–350 | subglobose to ovoid | 70–125 × 7–18 | (27–)30–36(–42) × 5–6(–7) | 1 | 45–90 × 45–80 | 6 | 110–250 × 4–5 | 4–13 | obpyriform to broadly ellipsoidal | (33–)40–50(–60) × 3.5–4 | 1 | 11.25 | Crous (2002) |
| Ca. leucothoës | 25–50 × 50–80 | 6 | 160–250 × 3–6 | 6–11.5 | ellipsoid to obpyriform | (45–)68–78(–97) × (4–)5–5.5(–6.5) | (1–)3(–6) | 14.6 | Crous (2002) | |||||
| Ca. maranhensis | 45–65 × 45–71 | 3 | 125–190 × 3–5 | 7–11 | ellipsoid, obpyriform to sphaeropedunculate | (50–)56–58(–65) × (3–)5(–6) | 1 | 11.85 | This study | |||||
| Ca. propaginicola | 40–75 × 31–85 | 4 | 130–250 × 2–5 | 5–12 | ellipsoid, obpyriform to sphaeropedunculate | (40–)48–51(–55) × 3–5 | 1 | 12.67 | This study | |||||
| Ca. pseudocerciana | 50–90 × 50–95 | 3 | 160–250 × 2–5 | 4–10 | clavate or ellipsoidal to obpyriform | (45–)53–55(–65) × (3–)4.5(–5) | 1 | 11.95 | This study | |||||
| Ca. pseudohodgesii | 50–90 × 40–95 | 3 | 130–190 × 2–5 | 7–12 | obpyriform to sphaeropendunculate | (35–)43–46(–55) × 3–5 | 1 | 11.95 | This study | |||||
| Ca. pseudospatiphylli | 350–550 × 300–500 | globose to subglobose | 90–150 × 7–25 | (30–)38–45(–55) × 5–6 | 1(–3) | 70–100 × 25–70 | 4 | 100–250 × 2.5–3.5 | 8–12 | sphaeropendunculate to ellipsoidal | (40–)47–55(–60) × 4–5 | 1(–3) | 13 | Crous (2002) |
| Ca. spathiphylli | 380–655 × 340–650 | subglobose to ovoid | 120–230 × 7–25 | (22–)40–52(–65) × (3–)4.5–5.5(–7) | 1(–3) | 60–150 × 40–90 | 4 | 170–260 × 3–4 | 8–15 | globoid or ellipsoid to obpyriform | (45–)46–80(–120) × (5–)6(–7) | 1(–3) | 11.67 | Crous (2002) |
| Ca. sulawesiensis | 43–81 × 41–79 | 5 | 113–262 × 5–7 | 5–7 | broadly clavate to ellipsoid | (41–)45–51(–54) × (3–)4(–6) | 1 | 12 | Lombard et al. (2010b) | |||||
| Ca. variabilis | 260–450 × 220–350 | globose to ovoid | 90–120 × 10–20 | (34–)38–50(–60) × 4–5(–6) | 1(–3) | 40–70 × 20–100 | 3 | 130–250 × 2–3 | 6–11 | sphaeropendunculate to ovoid or ellipsoid to clavate | (48–)68–77(–85) × 4–5(–7) | (1–)3(–4) | 14.6 | Crous (2002) |
| Calonectria pteridis species complex | ||||||||||||||
| Ca. gordoniae | 4 | 3–6 | narrowly clavate | (45–)62(–81) × 4–6 | 1 | 12.6 | Leahy et al. (2000) | |||||||
| Ca. ovata | 350–550 × 350–450 | globose to ovoid | 70–120 × 10–25 | (35–)55–70(–90) × (4–)5–6 | 1 | 30–55 × 20–45 | 3 | 185–230 × 2.5–4 | 8–14 | ovate | (50–)65–80(–110) × 4–6 | 1(–3) | 10.9 | Crous 2002 |
| Ca. pseudovata | 55–121 × 75–105 | 3 | 140–280 × 3–6 | 8–12 | ovate to ellipsoidal | (55–)67–70(–80) × (4–)5(–7) | 1 | 13.73 | This study | |||||
| Ca. pteridis | 300–500 × 280–350 | subglobose to ovoid | 70–120 × 10–25 | (30–)45–60(–75) × (4–)5–6(–7) | 1(–3) | 75–150 × 45–170 | 5 | 150–300 × 2.5–4 | 4–6 | clavate to narrowly ellipsoidal | (50–)70–100(–130) × (4–)5–6 | 1(–3) | 14.91 | Crous 2002 |
| Calonectria naviculata species complex | ||||||||||||||
| Ca. multinaviculata | 30–65 × 40–70 | 3 | 75–140 × 2–5 | 4–7 | naviculate | (40–)44–49(–52) × (2–)3.5(–4) | 1 | 13.72 | This study | |||||
| Ca. multiphialidica | 70–150 × 70–150 | 8 | 170–300 × 4–5 | 8–16 | sphaeropedunculate to clavate | (45–)48–55(–65) × (4–)4.5(–5) | 1 | 11.78 | Crous et al., 2004a, Crous et al., 2004b | |||||
| Ca. naviculata | 350–450 × 350–400 | globose to ovoid | 70–100 × 8–12 | (20–)40–48(–52) × (3–)5–6(–6.5) | 3 | 45–90 × 25–100 | 4 | 150–200 × 3–4.5 | 5–11 | naviculate to ellipsoidal | (40–)42–50 × 3(–4) | 1 | 15 | Crous 2002 |
| Ca. pseudonaviculata | 30–60 × 30–45 | 4 | 120–180 × 3–4 | 4–8 | naviculate | (50–)55–65(–80) × 4–5(–6) | 1(–3) | 18 | Crous 2002 | |||||
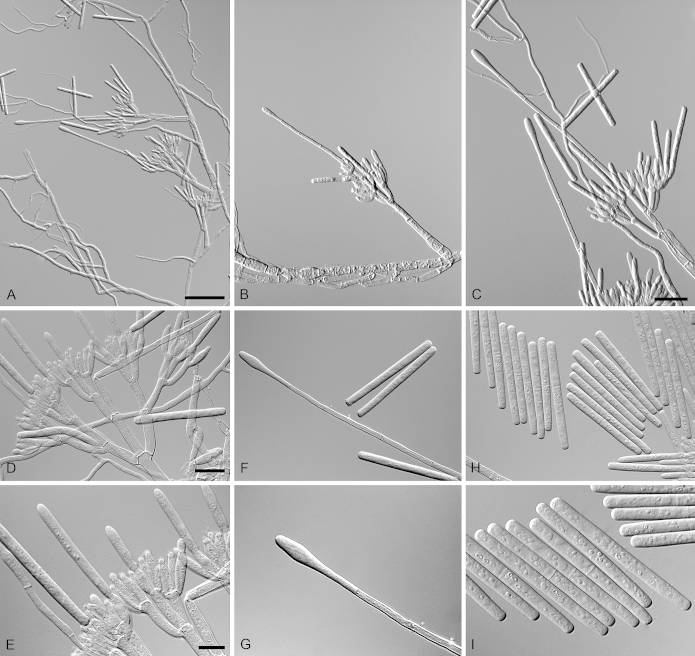
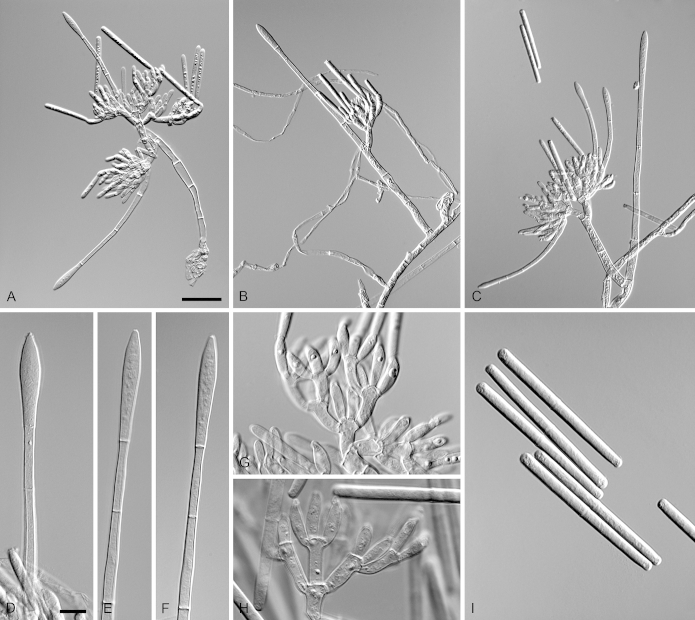
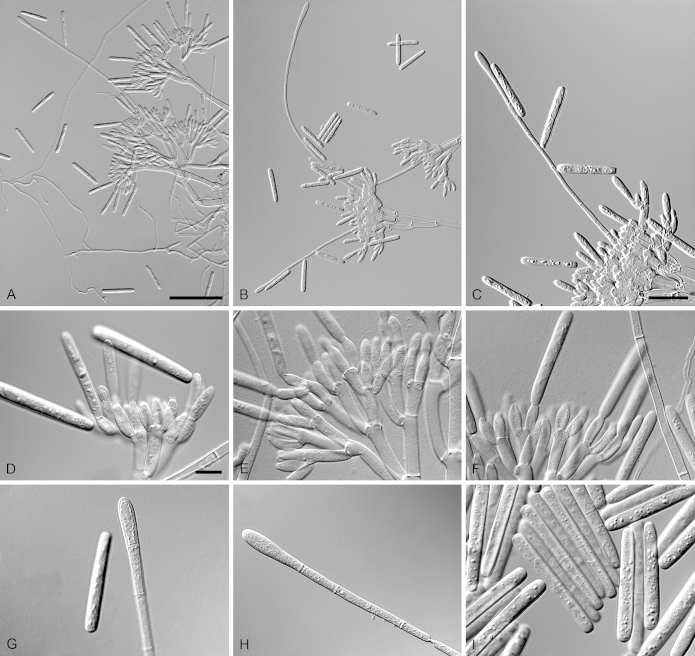
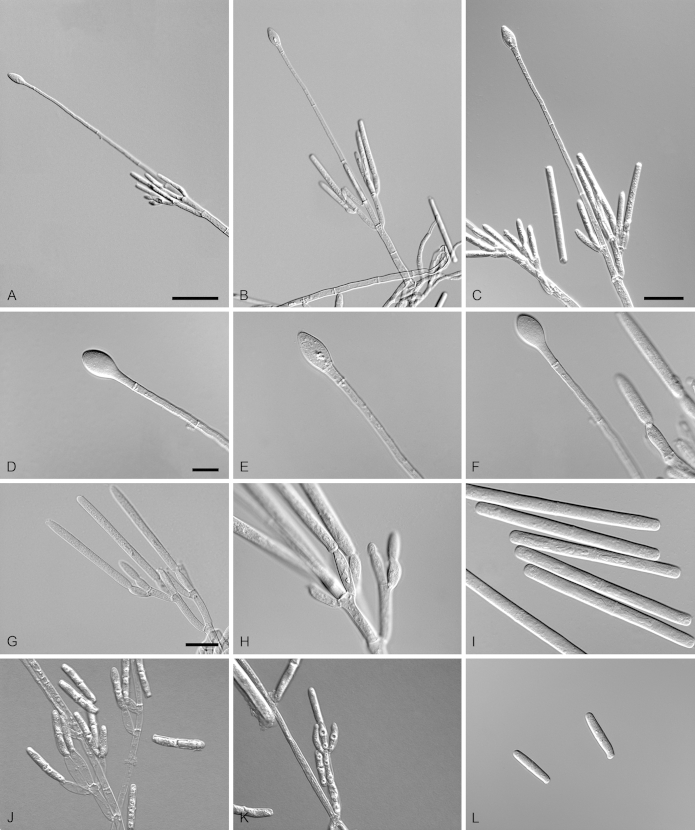
Calonectria brassiana R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810001. Fig. 5.
Fig. 5.
Calonectria brassiana (ex-type CBS 134855). A–C. Macroconidiophores. D–E. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. F–G. Ellipsoidal to narrowly obpyriform vesicles. H–I. Macroconidia. Scale bars: A = 50 μm (apply to B); C = 20 μm; D = 10 μm (apply to F, H); E = 10 μm (apply to G, I).
Etymology: Name refers to Eucalyptus brassiana, the plantation tree species associated with the soil from which this fungus was isolated.
Sexual morph not observed. Conidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 55–155 × 5–8 μm; stipe extensions septate, straight to flexuous, 90–172 μm long, 2–3 μm wide at the apical septum, terminating in ellipsoidal to narrowly obpyriform vesicles, 3–7 μm diam. Conidiogenous apparatus 50–80 μm long, 50–135 μm wide; primary branches aseptate, 20–30 × 4–6 μm, secondary branches aseptate, 15–25 × 3–6 μm, and tertiary branches aseptate, 10–17 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 9–15 × 3–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight to slightly curved, (35–)50–56(–65) × 3–5 μm (av. = 53 × 4 μm), L/W ratio = 12.91, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies buff on the surface and sepia in reverse; extensive white aerial mycelium with moderate sporulation on the aerial mycelium; chlamydospores sparse, occurring throughout the medium and forming microsclerotia. Colonies moderately fast growing (40–60 mm diam) on MEA and OA, after 7 d at 25 °C.
Material examined: Brazil, Piauí state, Teresina, from soil collected in Eucalyptus brassiana plantation, Jul. 2011, R.F. Alfenas (holotype CBS H-21376, culture ex-type CBS 134855 = LPF378), CBS 134856 = LPF379, CBS 134857 = LPF380.
Note: The macroconidia of Ca. brassiana are larger than those of Ca. eucalypticola, Ca. glaebicola, Ca. metrosideri, Ca. piauiensis, Ca. pseudoscoparia and Ca. pseudometrosideri, but smaller than those of Ca. candelabra (Table 3).
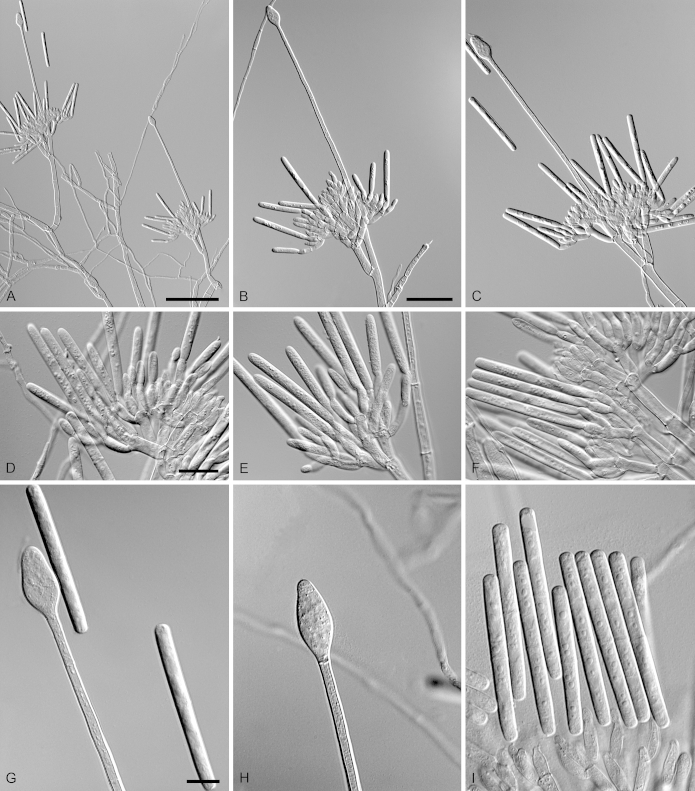
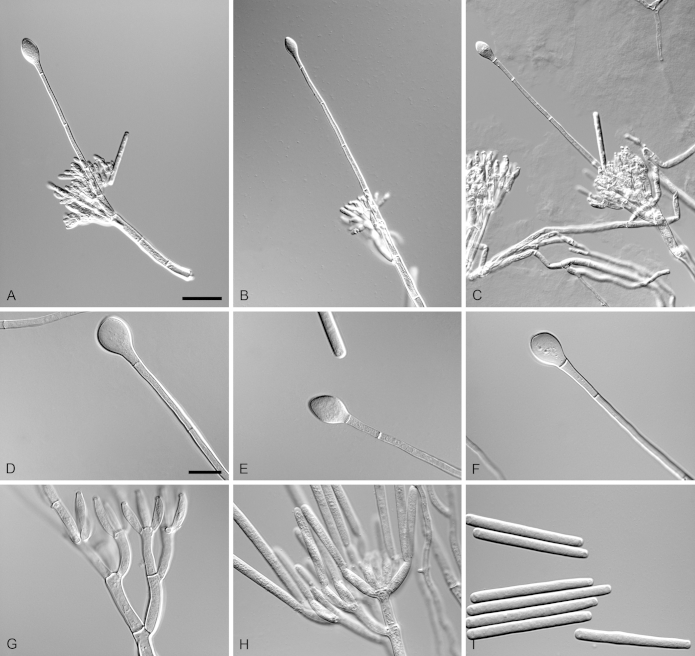
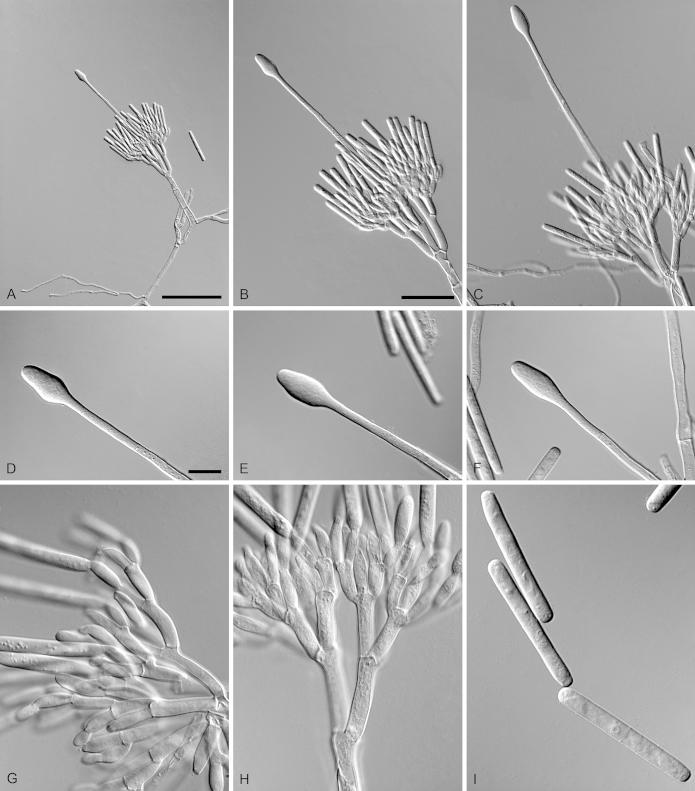
Calonectria duoramosa R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810002. Fig. 6.
Fig. 6.
Calonectria duoramosa (ex-type CBS 134656). A–C. Macroconidiophores. D–F. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. G–I. Clavate vesicles. J–K. Macroconidia. Scale bars: A = 50 μm; B = 50 μm (apply to C); D = 10 μm (apply to E–K).
Etymology: Name refers to the two levels of fertile branches formed in the conidiogenous apparatus of this fungus.
Sexual morph not observed. Conidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 45–95 × 4–7 μm; stipe extensions septate, straight to flexuous, 175–310 μm long, 3–5 μm wide at the apical septum, terminating in acicular to clavate vesicles, 4–6 μm diam. Conidiogenous apparatus 20–60 μm long, 30–50 μm wide; primary branches aseptate, 20–30 × 4–6 μm and secondary branches aseptate, 10–20 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 7–15 × 3–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (35–)44–48(–55) × 3–5 μm (av. = 46 × 4 μm), L/W ratio = 11.38, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies umber to fawn on the surface and dark brick in reverse; sparse aerial mycelium; chlamydospores sparse, occurring throughout the medium, with moderate to extensive sporulation on the aerial mycelium. Colonies slow growing (33–43 mm diam) on MEA, and fast growing (79–83 mm diam) on OA, after 7 d at 25 °C.
Materials examined: Brazil, Pará state, Monte Dourado, from soil collected in tropical rainforest, Aug. 2010, R.F. Alfenas (holotype CBS H-21380, culture ex-type CBS 134656 = LPF434); from soil collected in Eucalyptus plantation, Aug. 2010, R.F. Alfenas, culture LPF453.
Notes: Calonectria duoramosa characteristically forms only two levels of branching in its conidiogenous apparatus distinguishing it from Ca. ecuadoriae and Ca. robigophila. The macroconidia of Ca. duoramosa are also slightly smaller than those of Ca. ecuadoriae and Ca. robigophila (Table 3).
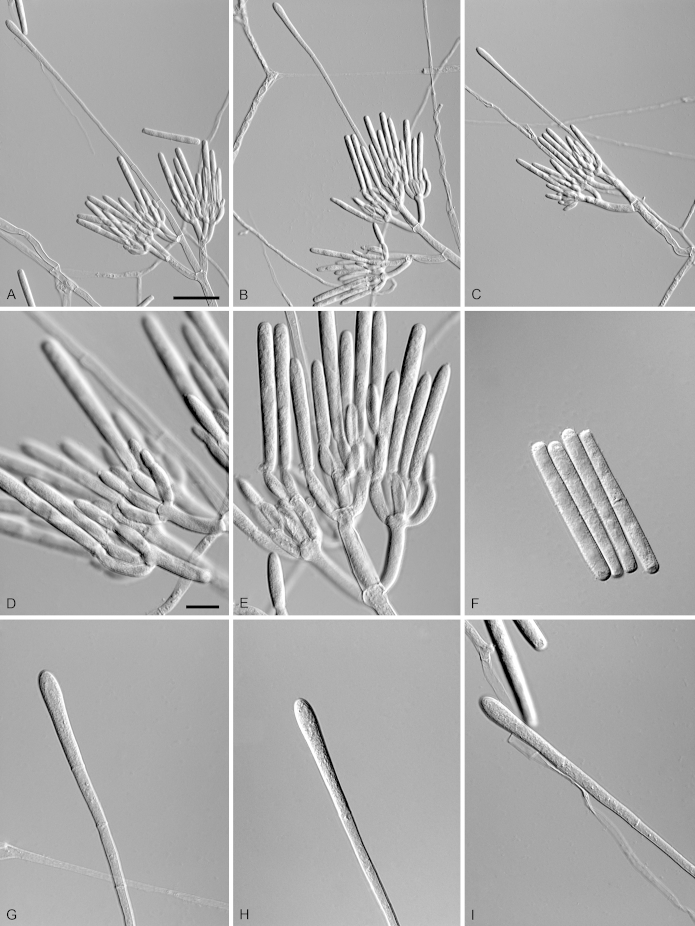
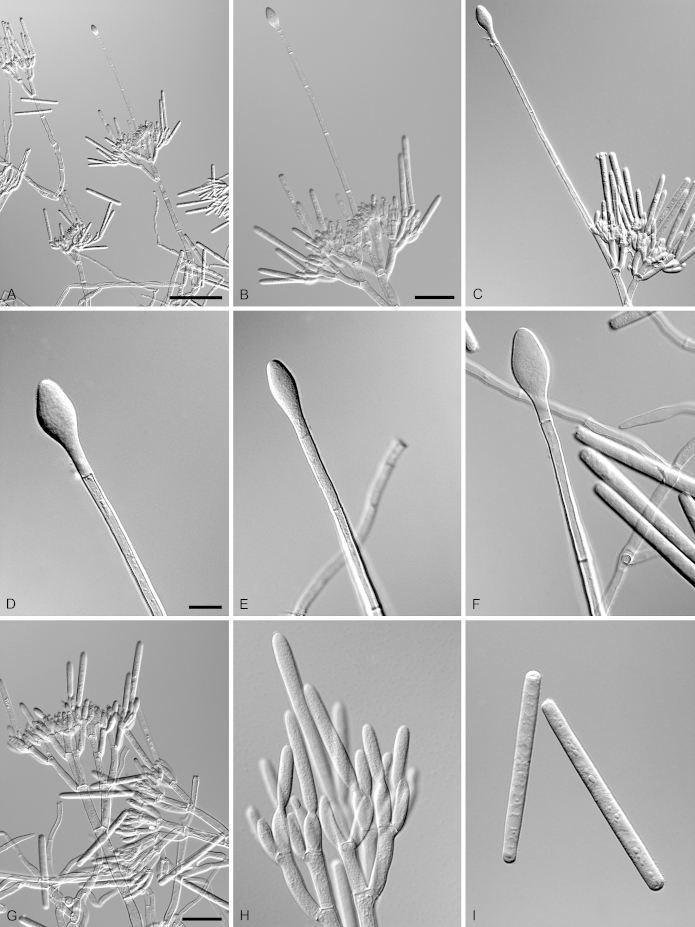
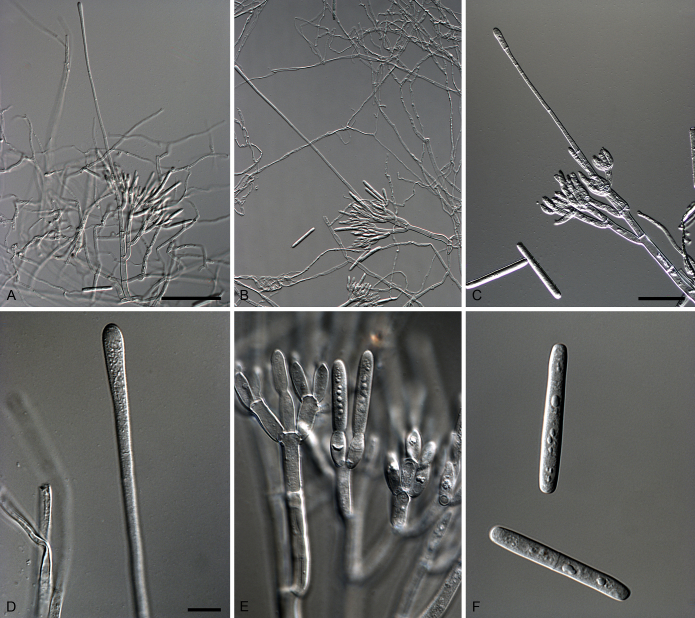
Calonectria eucalypticola R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810003. Fig. 7.
Fig. 7.
Calonectria eucalypticola (ex-type CBS 134847). A–C. Macroconidiophores. D. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. E–F. Ellipsoidal to obpyriform vesicles. G. Macroconidia. Scale bars: A = 50 μm (apply to B–C); D = 10 μm; E = 10 μm (apply to F); G = 10 μm.
Etymology: Name refers to the host genus, Eucalyptus, from which this fungus was first isolated.
Sexual morph not observed. Conidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 50–242 × 5–10 μm; stipe extensions septate, straight to flexuous, 145–170 μm long, 2–4 μm wide at the apical septum, terminating in ellipsoidal to obpyriform vesicles, 5–7 μm diam. Conidiogenous apparatus 35–62 μm long, 45–75 μm wide; primary branches aseptate, 20–25 × 4–6 μm; secondary branches aseptate, 16–19 × 3–5 μm, tertiary branches aseptate, 9–16 × 2–4 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 6–12 × 2–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight to slightly curved, (43–)49–52(–55) × 3–5 μm (av. = 50 × 4 μm), L/W ratio = 12.20, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies cinnamon to dark brick on the surface and sepia in reverse; moderate to extensive sporulation on the aerial mycelium, especially at the margins; chlamydospores moderate occurring throughout the medium forming microsclerotia. Colonies slow growing (40–45 mm diam) on MEA, and moderate growing (50–55 mm diam) on OA, after 7 d at 25 °C.
Materials examined: Brazil, Minas Gerais state, Santa Bárbara, from stem of Eucalyptus seedling, Dec. 2010, A.C. Alfenas (holotype CBS H-21359, culture ex-type CBS 134847 = LPF124); Bahia state, Eunápolis, from Eucalyptus leaf, Mar. 2012, A.C. Alfenas, CBS 134846 = LPF121; Pará state, Monte Dourado, from soil collected in Eucalyptus plantation, July 2012, R.F. Alfenas, CBS 134848 = LPF451.
Note: Calonectria eucalypticola can be distinguished from its closest relatives (Fig. 4) based on macroconidial dimensions and the number of fertile branches produced in the conidiogenous apparatus (Table 3).
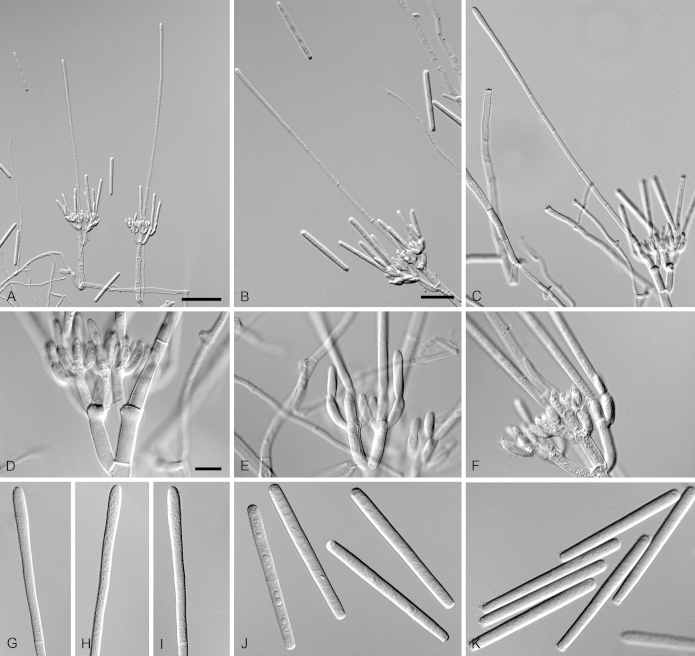
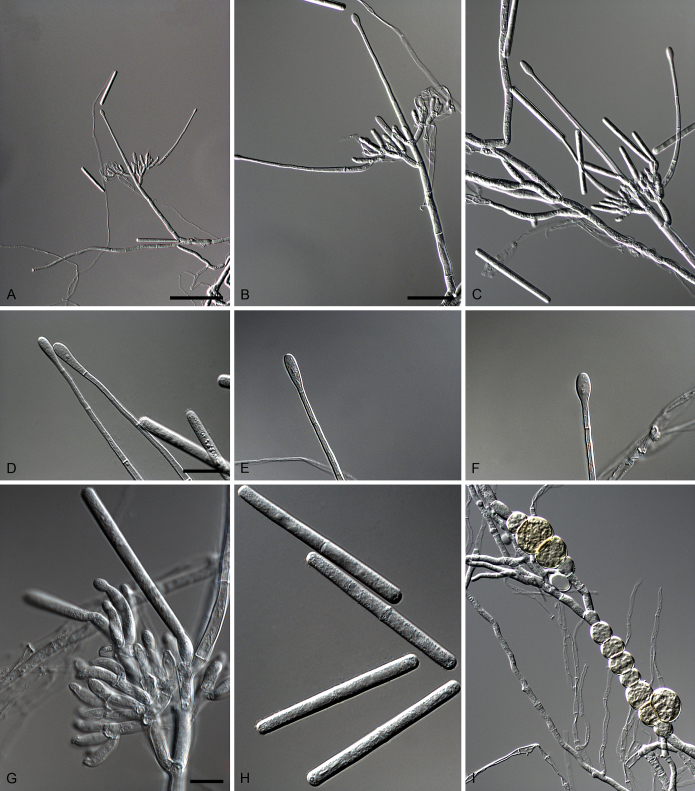
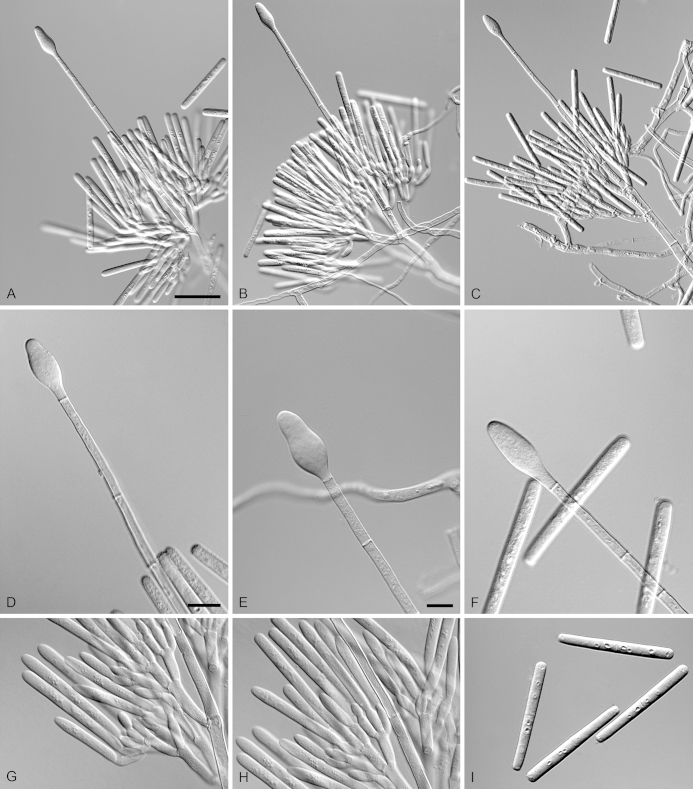
Calonectria glaebicola R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810004. Fig. 8.
Fig. 8.
Calonectria glaebicola (ex-type CBS 134852). A–C. Macroconidiophores. D–E. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. F–G. Ellipsoidal to narrowly obpyriform vesicles. H. Macroconidia. I. Chlamydospores. Scale bars: A = 50 μm (apply to B–C); D = 10 μm (apply to E–I).
Etymology: Name refers to soil, the substrate from which this fungus was first isolated.
Sexual morph not observed. Conidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 50–130 × 5–7 μm; stipe extensions septate, straight to flexuous, 100–165 μm long, 2–4 μm wide at the apical septum, terminating in ellipsoidal to narrowly obpyriform vesicles, 3–5 μm diam. Conidiogenous apparatus 27–45 μm long, 25–40 μm wide; primary branches aseptate, 14–22 × 3–5 μm, secondary branches aseptate, 11–15 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 5–13 × 3–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight to slightly curved, (45–)50–52(–55) × 3–5 μm (av. = 50 × 4 μm), L/W ratio = 12.06, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies buff on the surface and sepia to umber in reverse; extensive aerial mycelium with moderate sporulation on the aerial mycelium; chlamydospores sparse, occurring throughout the medium forming microsclerotia. Colonies moderate growing (45–60 mm diam) on MEA and OA, after 7 d at 25 °C.
Materials examined: Brazil, Minas Gerais state, Martinho Campos, from soil collected in Eucalyptus plantation, Jul. 2010; A.C. Alfenas (holotype CBS H-21378, culture ex-type CBS 134852 = LPF406); Tocantins, Bico do Papagaio, from Eucalyptus leaf, Aug. 2012, R.F. Alfenas, CBS 134853 = LPF407, CBS 134854 = LPF408.
Note: Calonectria glaebicola is morphologically similar to its closest relatives (Fig. 4), from which it can be distinguished only by phylogenetic inference.
Calonectria maranhensis R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810005. Fig. 9.
Fig. 9.
Calonectria maranhensis (ex-type CBS 134811). A–C. Macroconidiophores. D–F. Ellipsoidal, obpyriform to sphaeropedunculate vesicles. G–H. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. I. Macroconidia. Scale bars: A = 20 μm (apply to B–C); D = 10 μm (apply to E–F); G = 10 μm (apply to H–I).
Etymology: Name refers to Maranhão state, Brazil, the region where this fungus was first collected.
Sexual morph not observed. Conidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 55–105 × 6–9 μm; stipe extensions septate, straight to flexuous, 125–190 μm long, 3–5 μm wide at the apical septum, terminating in ellipsoidal, obpyriform to sphaeropedunculate vesicles, 7–11 μm diam. Conidiogenous apparatus 45–71 μm long, 45–65 μm wide; primary branches aseptate, 20–45 × 3–6 μm; secondary branches aseptate, 15–20 × 3–5 μm, tertiary branches aseptate, 11–16 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 8–15 × 3–5 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight to slightly curved, (50–)56–58(–65) × (3–)5(–6) μm (av. = 57 × 5 μm), L/W ratio = 11.85, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies greyish sepia to dark brick on the surface and sepia to umber in reverse; extensive white aerial mycelium with moderate sporulation on the aerial mycelium; chlamydospores moderate to extensive occurring throughout the medium. Colonies moderately slow growing (50–55 mm diam) on MEA, and fast growing (80–85 mm diam) on OA, after 7 d at 25 °C.
Materials examined: Brazil, Maranhão state, Açailândia, from Eucalyptus leaf, May 2011, A.C. Alfenas (holotype CBS H-21360, culture ex-type CBS 134811 = LPF142), CBS 134812 = LPF143; Imperatriz, from soil in Eucalyptus plantation, May 2011, R.F. Alfenas, CBS 134825 = LPF370.
Note: Calonectria maranhensis can be distinguished from Ca. brasiliensis, Ca. hodgesii, Ca. sulawesiensis and Ca. variabilis by the dimensions of their macroconidia (Table 3).
Calonectria metrosideri R.F. Alfenas, O.L. Pereira, Crous & A.C. Alfenas, sp. nov. MycoBank MB810023.
≡ Calonectria metrosideri R.F. Alfenas, O.L. Pereira, Crous & A.C. Alfenas, Forest Pathology 43: 262. 2013. Nom. inval., Art 37.7.
See Alfenas et al. (2013a) for description and illustrations.
Material examined: Brazil, Minas Gerais state, Viçosa, Universidade Federal de Viçosa, forest nursery, isolated from leaf of Metrosideros polymorpha, Apr 2010, R.F. Alfenas (holotype CBS H-21146, culture ex-type CBS 133603).
Note: The original description of Ca. metrosideri is invalid, as no type specimen was designated. This issue is now addressed, and the name validly published.
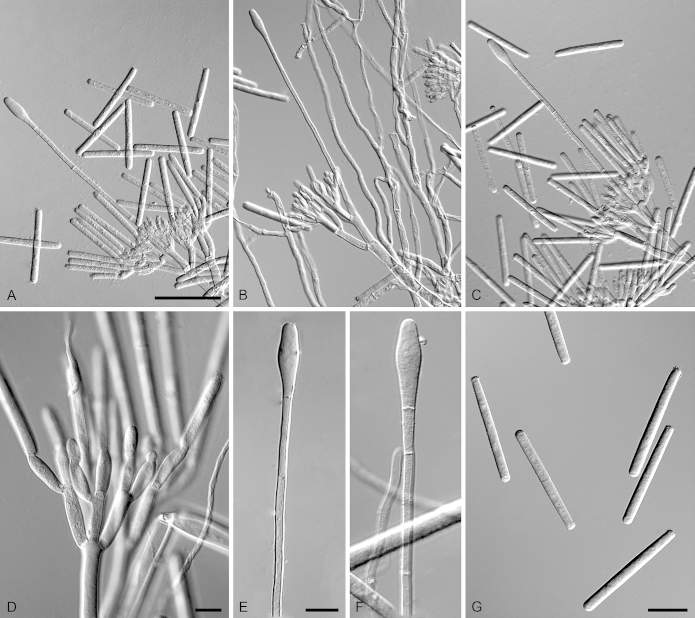
Calonectria multinaviculata R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810006. Fig. 10.
Fig. 10.
Calonectria multinaviculata (ex-type CBS 134858). A–C. Macroconidiophores. D–F. Naviculate vesicles. G–H. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. I. Macroconidia. Scale bars: A = 50 μm (apply to B–C); D = 10 μm (apply to E–I).
Entymology: Name refers to the multiple naviculate terminal vesicles formed by this fungus.
Sexual morph not observed. Conidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 45–90 × 5–7 μm; stipe extensions septate, straight to flexuous, 75–140 μm long, 2–5 μm wide at the apical septum, terminating in naviculate vesicles, 4–7 μm diam, abundant lateral stipe extension also present. Conidiogenous apparatus 30–65 μm long, 40–70 μm wide; primary branches aseptate, 19–22 × 3–6 μm, secondary branches aseptate, 9–18 × 3–6 μm, tertiary branches aseptate, 9–12 × 2–4 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 6–12 × 2–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight to slightly curved, (40–)44–49(–52) × (2–)3.5(–4) μm (av. = 46 × 3.5 μm), L/W ratio = 13.72, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies buff on the surface and sepia to umber in reverse; extensive white aerial mycelium with sparse to moderate sporulation on the aerial mycelium; chlamydospores not seen. Colonies moderately fast to fast growing (50–70 mm diam) on MEA and OA, after 7 d at 25 °C.
Materials examined: Brazil, Bahia state, Mucuri, from soil collected in Eucalyptus plantation, Aug. 2010; E. Zauza (holotype CBS 134858, preserved as metabolically inactive culture; culture ex-type CBS 134858 = LPF233), CBS 134862 = LPF472; Pará state, Monte Dourado, from soil collected in Eucalyptus plantation, July 2012, R.F. Alfenas, CBS 134859 = LPF418.
Note: Calonectria multinaviculata can be distinguished from Ca. naviculata by having fewer fertile branches in the conidiogenous apparatus, and having abundant lateral stipe extensions, which are absent in Ca. naviculata (Table 3).
Calonectria nemuricola R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810007. Fig. 11.
Fig. 11.
Calonectria nemuricola (ex-type CBS 134837). A–C. Macroconidiophores. D–F. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. G–H. Obpyriform vesicles. I. Macroconidia. Scale bars: A = 100 μm; B = 50 μm (apply to C); D = 10 μm (apply to E–F); G = 10 μm (apply to H–I).
Etymology: Name refers to a forest, the habitat this fungus was collected from.
Sexual morph not observed. Conidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 50–105 × 6–12 μm; stipe extensions septate, straight to flexuous, 150–205 μm long, 2–4 μm wide at the apical septum, terminating in obpyriform vesicles, 7–13 μm diam. Conidiogenous apparatus 40–60 μm long, 50–80 μm wide; primary branches aseptate, 19–25 × 3–7 μm, secondary branches aseptate, 11–18 × 3–5 μm, tertiary branches aseptate, 9–12 × 3–5 μm, additional branches rare, (–4), aseptate, 7–10 × 3–4 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 5–11 × 2–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight to slightly curved, (40–)44–46(–50) × 3–5 μm (av. = 45 × 4 μm), L/W ratio = 11.06, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies buff on the surface and sepia to umber in reverse; extensive white aerial mycelium with sparse to moderate sporulation on the aerial mycelium; chlamydospores sparse, occurring throughout the medium, forming microsclerotia; Colonies fast growing (55–80 mm diam) on MEA and OA, after 7 d at 25 °C.
Material examined: Brazil, Minas Gerais state, Araponga (Serra do Brigadeiro), from soil collected in tropical rainforest, Aug. 2010, A.C. Alfenas & P.W. Crous (holotype CBS H-21358, culture ex-type CBS 134837 = LPF085), CBS 134838 = LPF090, CBS 134839 = LPF094.
Note: The macroconidia of Ca. nemuricola are larger than those of Ca. mossambicensis, Ca. polizzii, Ca. silvicola and Ca. zuluensis, but smaller than those of Ca. pauciramosa (Table 3).
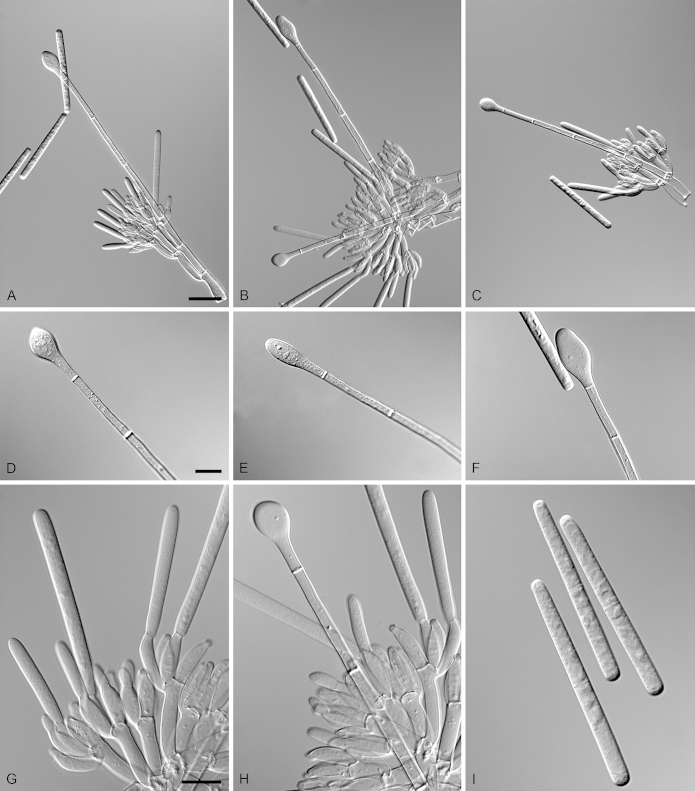
Calonectria paraensis R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810008. Fig. 12.
Fig. 12.
Calonectria paraensis (ex-type CBS 134669). A–C. Macroconidiophores. D–E. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. F. Macroconidia. G–I. Clavate vesicles. Scale bars: A = 50 μm (apply to B–C); D = 10 μm (apply to E–I).
Etymology: Name refers to the Pará state in Brazil where the fungus was collected.
Sexual morph not observed. Conidiophores consist of a stipe bearing a penicillate suites of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 52–110 × 5–7 μm; stipe extensions septate, straight to flexuous, 120–195 μm long, 3–5 μm wide at the apical septum, terminating in a clavate vesicle, 4–6 μm diam. Conidiogenous apparatus 45–55 μm long, 60–75 μm wide; primary branches aseptate, 18–24 × 4–6 μm, secondary branches aseptate, 14–23 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 7–11 × 2–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (35–)40–43(–50) × 3–6 μm (av. = 42 × 5 μm), L/W ratio = 8.85, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies buff on the surface and ochraceous to umber in reverse; extensive aerial mycelium; chlamydospores not seen; sparse sporulation on aerial mycelium. Colonies moderate growing (40–60 mm diam) on MEA, and fast growing (75–85 mm diam) on OA, after 7 d at 25 °C.
Material examined: Brazil, Pará state, Monte Dourado, from soil in Eucalyptus plantation, Aug 2011, R.F. Alfenas (holotype CBS H–21379, culture ex-type CBS 134669 = LPF430), LPF429.
Note: Calonectria paraensis is a new member of the Ca. brassicae complex, distinguished from Ca. pini and Ca. orientalis by the size and length/diam ratio of its macroconidia, numbers of branches per conidiophores, and vesicle diameter (Table 3).
Calonectria piauiensis R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810009. Fig. 13.
Fig. 13.
Calonectria piauiensis (ex-type CBS 134850). A–C. Macroconidiophores. D–F. Ellipsoidal to narrowly obpyriform vesicles. G. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. H. Macroconidia. I. Chlamydospores. Scale bars: A = 100 μm; B = 50 μm (apply to C, I); D = 10 μm (apply to E–F); G = 10 μm (apply to H).
Etymology: Name refers to Piauí state, Brazil, the region this fungus was isolated from.
Sexual morph not observed. Conidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 50–110 × 4–6 μm; stipe extensions septate, straight to flexuous, 95–130 μm long, 2–3 μm wide at the apical septum, terminating in ellipsoidal to narrowly obpyriform vesicles, 3–7 μm diam. Abundant lateral stipe extensions also present. Conidiogenous apparatus 20–60 μm long, 35–80 μm wide; primary branches aseptate, 12–20 × 3–5 μm, secondary branches aseptate, 8–10 × 3–4 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 6–12 × 3–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight to slightly curved, (38–)47–52(–60) × 3–5 μm (av. = 49 × 4.5 μm), L/W ratio = 11.27, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies buff on the surface and sepia in reverse; extensive white aerial mycelium with moderate sporulation on the aerial mycelium; chlamydospores sparse, occurring throughout the medium, forming microsclerotia. Colonies moderately fast growing (50–75 mm diam) after 7 d at 25 °C on MEA and OA.
Materials examined: Brazil, Piauí state, Teresina, from soil collected in Eucalyptus brassiana plantation, Jul. 2011, R.F. Alfenas (holotype CBS H-21375, culture ex-type CBS 134850 = LPF377), CBS 134851 = LPF381; Serra das Confusões, from soil and leaf litter collected in semi-arid vegetation, Jul. 2012, D.B. Pinho & O.L. Pereira, CBS 134849 = LPF291.
Note: Calonectria piauiensis can be distinguished from other closely related species in the Ca. candelabra complex by the abundant lateral stipe extensions produced on the conidiogenous apparatus not reported for other member species in the Ca. candelabra complex (Table 3).
Calonectria propaginicola R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810018. Fig. 14.
Fig. 14.
Calonectria propaginicola (ex-type CBS 134815). A–C. Macroconidiophores. D–F. Ellipsoidal, obpyriform to sphaeropedunculate vesicles. G–H. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. I. Macroconidia. Scale bars: A = 50 μm (apply to B–C); D = 10 μm (apply to E–I).
Etymology: Name refers to cuttings from which this fungus was first isolated.
Sexual morph not observed. Conidiophores containing a stipe bearing penicillate suites of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 52–180 × 6–8 μm; stipe extensions septate, straight to flexuous, 130–250 μm long, 2–5 μm wide at the apical septum, terminating in ellipsoidal, obpyriform to sphaeropedunculate vesicles, 5–12 μm diam. Conidiogenous apparatus 31–85 μm long, 40–75 μm wide; primary branches 0–1-septate, 18–30 × 3–7 μm; secondary branches aseptate, 10–22 × 3–6 μm, tertiary branches aseptate, 11–20 × 3–5 μm, and additional branches (–4), aseptate, 9–15 × 3–4 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 5–12 × 3–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight to slightly curved, (40–)48–51(–55) × 3–5 μm (av. = 49 × 4 μm), L/W ratio = 12.67, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies buff to pale umber on the surface and sepia in reverse; moderate to extensive aerial mycelium with extensive sporulation on the aerial mycelium, especially in the centre of the colony; chlamydospores moderate, occurring throughout the medium, forming microsclerotia. Colonies fast growing (65–70 mm diam) on MEA, and (80–85 mm diam) on OA, after 7 d at 25 °C.
Material examined: Brazil, Pará state, Santana, from Eucalyptus seedling, Apr. 2011, A.C. Alfenas (holotype CBS H-21366, culture ex-type CBS 134815 = LPF220), CBS 134816 = LPF222.
Note: Calonectria propaginicola can be distinguished from Ca. cerciana and Ca. pseudocerciana based on its terminal vesicle morphology, and length/diam ratio of the macroconidia.
Calonectria pseudobrassicae R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810010. Fig. 15.
Fig. 15.
Calonectria pseudobrassicae (ex-type CBS 134662). A–C. Macroconidiophores. D–F. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. G–H. Clavate vesicles. I. Macroconidia. Scale bars: A = 100 μm (apply to B); C = 50 μm; D = 10 μm (apply to E–I).
Etymology: Name refers to the fact that this species closely resembles Calonectria brassicae.
Sexual morph not observed. Conidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 50–125 × 5–8 μm; stipe extensions septate, straight to flexuous, 190–300 μm long, 3–5 μm wide at the apical septum, terminating in clavate vesicles, 5–6 μm diam. Conidiogenous apparatus 50–115 μm long, 60–100 μm wide; primary branches aseptate, 15–30 × 5–7 μm; secondary branches aseptate, 15–25 × 4–6 μm, tertiary branches aseptate, 10–20 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 7–15 × 3–5 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight to slightly curved, (30–)39–42(–48) × 4–6 μm (av. = 41 × 5 μm), L/W ratio = 8.04, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies light amber, forming a rosy buff concentric ring on the surface, and ochraceous to umber in reverse; extensive aerial mycelium; sparse sporulation on the aerial mycelium; chlamydospores not seen. Colonies moderately fast growing (58–61 mm diam) on MEA, and fast growing (80–84 mm diam) on OA, after 7 d at 25 °C.
Material examined: Brazil, Pará state, Santana, Apr. 2011, A.C. Alfenas (holotype CBS H-21371, culture ex-type CBS 134662 = LPF280), CBS 134661 = LPF260.
Note: Calonectria pseudobrassicae is morphologically distinguished from Ca. brassicae and Ca. brachiatica by the number of conidiophore branches and slightly smaller macroconidia (Table 3).
Calonectria pseudocerciana R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810011. Fig. 16.
Fig. 16.
Calonectria pseudocerciana (ex-type CBS 134824). A–C. Macroconidiophores. D–F. Obpyriform to sphaeropedunculate vesicles. G–H. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. I. Macroconidia. Scale bars: A = 50 μm (apply to B–C); D = 10 μm (apply to E–I).
Etymology: Name refers to the fact that this fungus closely resembles Calonectria cerciana.
Sexual morph not observed. Conidiophores consists of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 40–110 × 5–7 μm; stipe extensions septate, straight to flexuous, 130–190 μm long, 2–5 μm wide at the apical septum, terminating in obpyriform to sphaeropedunculate vesicles, 7–12 μm diam. Conidiogenous apparatus 40–95 μm long, 50–90 μm wide; primary branches 0–1-septate, 20–45 × 4–7 μm; secondary branches aseptate, 13–30 × 3–6 μm, tertiary branches aseptate, 8–18 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 5–15 × 3–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight to slightly curved, (35–)43–46(–55) × 3–5 μm (av. = 45 × 4 μm), L/W ratio = 10.6, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies pale buff on the surface and cinnamon to sepia in reverse; extensive white aerial mycelium; sparse sporulation on the aerial mycelium; chlamydospores sparse, occurring throughout the medium, forming microsclerotia. Colonies fast growing (65–70 mm diam) on MEA, and (70–80 mm diam) on OA, after 7 d at 25 °C.
Material examined: Brazil, Pará state, Santana, from stem of Eucalyptus seedling, Apr. 2011, A.C. Alfenas (holotype CBS H-21366, culture ex-type CBS 134824 = LPF367), CBS 134822 = LPF365.
Note: Calonectria pseudocerciana can be distinguished from Ca. cerciana and Ca. propaginicola based on the morphology of their terminal vesicle and length/diam ratio of the macroconidia.
Calonectria pseudohodgesii R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810012. Fig. 17.
Fig. 17.
Calonectria pseudohodgesii (ex-type CBS 134818). A–C. Macroconidiophores. D–F. Ellipsoidal to obpyriform vesicles. G–H. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. I. Macroconidia. Scale bars: A = 100 μm; B = 50 μm (apply to C); D = 10 μm (apply to E–F, H–I); G = 20 μm.
Etymology: Name refers to the fact that this fungus closely resembles Calonectria hodgesii.
Sexual morph not observed. Conidiophores containing a stipe bearing penicillate suites of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 35–160 × 5–8 μm; stipe extensions septate, straight to flexuous, 160–250 μm long, 2–5 μm wide at the apical septum, terminating in clavate (rarely), ellipsoidal to obpyriform vesicles, 4–10 μm diam (av. = 8 μm). Conidiogenous apparatus 50–90 μm long, 50–95 μm wide; primary branches aseptate, 20–35 × 4–7 μm; secondary branches aseptate, 15–30 × 4.5–6 μm, tertiary branches aseptate, 10–20 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 7–15 × 3–5 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight to slightly curved, (45–)53–55(–65) × (3–)4.5(–5) μm (av. = 54 × 4.5 μm), L/W ratio = 11.95, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia were not observed.
Culture characteristics: Colonies fawn to cinnamon, rosy buff at the margin on the surface, and sepia in reverse; extensive white aerial mycelium with extensive sporulation on the aerial mycelium; chlamydospores moderate to extensive occurring throughout the medium. Colonies moderately fast growing (60–65 mm diam) on MEA, and fast growing (80–85 mm diam) on OA, after 7 d at 25 °C.
Materials examined: Brazil, Minas Gerais state, Viçosa, on leaf of rooted Azadirachta indica cutting, Mar. 2011, R.F. Alfenas (holotype CBS H-21368, culture ex-type CBS 134818 = LPF262), CBS 134819 = LPF265; from stem of Eucalyptus seedling, Mar. 2011, R.F. Alfenas, CBS 134813 = LPF205, CBS 134814 = LPF206.
Note: The macroconidia of Ca. pseudohodgesii are larger than those of Ca. hodgesii and the stipe extensions of Ca. pseudohodgesii are also longer than those of Ca. hodgesii (Table 3).
Calonectria pseudometrosideri R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810013. Fig. 18.
Fig. 18.
Calonectria pseudometrosideri (ex-type CBS 134845). A–C. Macroconidiophores. D–F. Ellipsoidal to obpyriform vesicles. G–H. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. I. Macroconidia. Scale bars: A = 50 μm (apply to B, G); D = 10 μm (apply to E–F, H–I).
Etymology: Name refers to the fact that this fungus closely resembles Calonectria metrosideri.
Sexual morph not observed. Conidiophores containing a stipe bearing penicillate suites of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 62–220 × 6–8 μm; stipe extensions septate, straight to flexuous, 160–210 μm long, 2–4 μm wide at the apical septum, terminating in ellipsoidal to obpyriform vesicles, 5–7 μm diam. Conidiogenous apparatus 30–76 μm long, 45–65 μm wide; primary branches 0(–1)-septate, 21–30 × 5–7 μm; secondary branches aseptate, 16–22 × 4–7 μm, tertiary branches aseptate, 10–17 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides elongated doliiform to reniform, hyaline, aseptate, 9–17 × 3–5 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight to slightly curved, (40–)49–52(–60) × (3–)4.5(–5) μm (av. = 51 × 4.5 μm), L/W ratio = 11.34, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies cinnamon to dark brick on the surface, and sepia in reverse; moderate aerial white mycelium with moderate to extensive sporulation on the aerial mycelium, especially at the margins; chlamydospores moderate to extensive, occurring throughout the medium forming microsclerotia. Colonies slow growing (35–40 mm diam) on MEA, and moderately slow growing (45–50 mm diam) on OA, after 7 d at 25 °C.
Materials examined: Brazil, Alagoas state, Maceió, from soil collected in Eucalyptus plantation, Apr. 2011, M.M. Coutinho (holotype CBS 134845, preserved as metabolically inactive culture, culture ex-type CBS 134845 = LPF210); Maranhão, Açailândia, from leaf of Eucalyptus sp., Aug 2012, A.C. Alfenas CBS 134844 = LPF147; Minas Gerais, Viçosa, from leaf of Metrosideros polymorpha, Mar. 2012, R.F. Alfenas, CBS 134843 = LPF100.
Note: Calonectria pseudometrosideri can be distinguished from Ca. metrosideri by their larger macroconidia and longer stipe extensions (Table 3).
Calonectria pseudopteridis (Sherb.) R.F. Alfenas, L. Lombard & Crous, nom. nov. MycoBank MB810024.
Basionym: Cylindrocladium macrosporum Sheb., Phytopathology 18: 219. 1928.
Notes: Sobers (1968) synonymised Cy. macrosporum under Ca. pteridis based on morphological similarities of the asexual morphs. This was further validated in the monographic studies of Crous & Wingfeld (1994) and Crous (2002). However, to our knowledge, the ex-type strain (CBS 163.28) of Cy. macrosporum has never been subjected to DNA sequence analysis. Phylogenetic inference in this study showed that the ex-type strain of Cy. macrosporum is closely related to, but distinct from, the ex-type strain (CBS 111793) of Ca. pteridis, and is therefore reinstated here as a separate species of Calonectria. As the name Ca. macrospora is already occupied, we provide a new name for this species.
Calonectria pseudospathulata R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810014. Fig. 19.
Fig. 19.
Calonectria pseudospathulata (ex-type CBS 134841). A–C. Macroconidiophores. D–F. Obpyriform vesicles. G–H. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. I. Macroconidia. Scale bars: A = 50 μm (apply to B–C); D = 10 μm (apply to G–I); E = 10 μm (apply to F).
Etymology: Name refers to the fact that this species closely resembles Calonectria spathulata.
Sexual morph not observed. Conidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 45–95 × 5–8 μm; stipe extensions septate, straight to flexuous, 145–190 μm long, 2–4 μm wide at the apical septum, terminating in obpyriform vesicles, 7–10 μm diam. Conidiogenous apparatus 30–70 μm long, 65–100 μm wide; primary branches aseptate, 15–25 × 4–7 μm, secondary branches aseptate, 12–20 × 4–5 μm, tertiary branches aseptate, 10–12 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 5–10 × 3–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight to slightly curved, (35–)41–44(–50) × 3–5 μm (av. = 43 × 4 μm), L/W ratio = 10.46, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies cinnamon to dark brick on the surface and sepia to umber in reverse; extensive aerial white mycelium with moderate sporulation on the aerial mycelium; chlamydospores moderately abundant, occurring throughout the medium, forming microsclerotia. Colonies slow to moderately slow growing (40–60 mm diam) on MEA and OA, after 7 d at 25 °C.
Material examined: Brazil, Minas Gerais state, Araponga (Serra do Brigadeiro), from soil collected in tropical rainforest, Aug. 2010, A.C. Alfenas & P.W. Crous (holotype CBS H-21356, living ex-type CBS 134841 = LPF072), CBS 134840 = LPF066, CBS 134842 = LPF087.
Note: The macroconidia of Ca. pseudospathulata are smaller than those of Ca. spathulata (Table 3).
Calonectria pseudovata R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810015. Fig. 20.
Fig. 20.
Calonectria pseudovata (ex-type CBS 134674). A–C. Macroconidiophores. D–F. Ovate to ellipsoidal vesicles. G–H. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. I. Macroconidia. J–K. Microconidiophores. L. Microconidia. Scale bars: A = 50 μm (apply to B); C = 50 μm; D = 10 μm (apply to E–F, H–I); G = 10 μm (apply to J–L).
Etymology: Name refers to the fact that the species closely resembles Calonectria ovata.
Sexual morph not observed. Conidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 35–105 × 5–7 μm; stipe extensions septate, straight to flexuous, 140–280 μm long, 3–6 μm wide at the apical septum, terminating in fusiform, ovate to ellipsoidal vesicles, 8–12 μm diam. Conidiogenous apparatus 55–121 μm long, 75–105 μm wide; primary branches 0–1-septate, 25–75 × 5–8 μm; secondary branches aseptate, 15–35 × 4–7 μm, tertiary branches aseptate, 15–30 × 4–6 μm, each terminal branch producing 2–6 phialides; phialides elongate doliiform to reniform, hyaline, aseptate, 10–25 × 3–5 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (55–)67–70(–80) × (4–)5(–7) μm (av. = 69 × 5 μm), L/W ratio = 13.73, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Microconidiophores comprise a stipe, a stipe extension and a penicillate or subverticillate arrangement of fertile branches. Stipe extension septate, thin-walled, terminating in an ellipsoidal to ovoid vesicle, 3–5 μm diam. Primary branches aseptate, 8–15 × 2–4 μm, secondary branches aseptate, 5–10 × 2–4 μm, terminating in 1–3 phialides; phialides elongate doliiform to reniform, straight to slightly curved, hyaline, aseptate, 7–15 × 2–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Microconidia cylindrical, straight to curved, rounded at apex, (10–)20–23(–30) × (3–)4(–6) μm (av. = 22 × 4 μm), L/W ratio = 5.38, 1-septate, held in fascicles by colourless slime. Megaconidia not observed.
Culture characteristics: Colonies ochraceous to rosy buff on the surface and umber in reverse; moderate to extensive aerial mycelium; sparse sporulation on the aerial mycelium; chlamydospores not seen. Colonies moderately fast growing (55–64 mm diam) on MEA and on OA, after 7 d at 25 °C.
Material examined: Brazil, Pará state, Santana, from soil in Eucalyptus plantation, Apr. 2011, A.C. Alfenas (holotype CBS H-21370, culture ex-type CBS 134674 = LPF267), CBS 134675 = LPF285, LPF286.
Notes: Calonectria pseudovata can be distinguished from Ca. ovata by the shape of the terminal vesicle and smaller macroconidia produced by Ca. pseudovata. The microconidia of Ca. pseudovata are also slightly smaller than those of Ca. ovata.
Calonectria quinqueramosa R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810016. Fig. 21.
Fig. 21.
Calonectria quinqueramosa (ex-type CBS 134654). A. Ascoma. B–C. Vertical section through ascomata, showing wall structure. D–E. Asci. F. Ascospores. G–I. Macroconidiophores. J–L. Narrowly clavate to clavate vesicles. M–N. Conidiogenous apparatus with conidiophore branches, doliiform to reniform phialides. O. Macroconidia. Scale bars: A = 500 μm; B = 100 μm (apply to C); D = 50 μm (apply to I); E = 20 μm (apply to F, J–O); G = 50 μm (apply to H).
Etymology: Name refers to the characteristic five branches formed in the conidiogenous apparatus of this fungus.
Ascomata perithecial, solitary or in groups, orange to red, becoming brown with age; in section apex and body orange to red, base red-brown, pyriform to sub-globose, 160–400 μm high, 115–250 μm diam, body turning dark red, and base dark red-brown (KOH+). Perithecial walls rough, consisting of two thick-walled layers: outside layer of textura globulosa, 25–85 μm wide; becoming more compressed towards inner layer of textura angularis, 10–30 μm wide; becoming thin-walled and hyaline towards the centre, outer layer cells 10–20 × 10–30 μm; inner cells 4–6 × 8–15 μm: perithecial base up to 135 μm wide; consisting of dark red, angular cells; merging with an erumpent stroma, cells of the outer wall layer continuing into the pseudoparenchymatous cells of the erumpent stroma. Asci 8-spored, clavate, 50–105 × 10–25 μm, tapering to a long thin stalk. Ascospores aggregated in the upper third of the ascus, hyaline, guttulate, fusoid with rounded ends, straight to curved, (1–)3-septate, slightly constricted at the septum, (25–)39–42(–50) × 5–7 μm (av. = 40 × 6 μm). Cultures were homothallic. Conidiophores consists of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 50–145 × 5–7 μm; stipe extensions septate, straight to flexuous, 170–340 μm long, 2–4 μm wide at the apical septum, terminating in narrowly clavate to clavate vesicles, 3–5 μm diam. Conidiogenous apparatus 30–60 μm long, 35–65 μm wide; primary branches aseptate, 10–35 × 3–6 μm; secondary branches aseptate, 10–30 × 3–5 μm; tertiary branches aseptate, 10–20 × 2–4 μm and additional branches (–5), aseptate, 10–15 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 6–18 × 2–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (45–)57–61(–70) × 4–6 μm (av. = 59 × 5 μm), L/W ratio = 11.57, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia were not observed.
Culture characteristics: Colonies umber to fawn on the surface and dark brick in reverse; sparse aerial mycelium; chlamydospores sparse, occurring throughout the medium, with moderate to extensive sporulation on the aerial mycelium. Colonies moderately fast growing (57–70 mm diam) on MEA, and fast growing (78–81 mm diam) on OA, after 7 d at 25 °C.
Materials examined: Brazil, Pará state, Monte Dourado, from soil in Eucalyptus plantation, May 2011, R.F. Alfenas (holotype CBS H-21355, culture ex-type CBS 134654 = LPF065), LPF302; Santana, from soil in Eucalyptus plantation, Apr. 2011, A.C. Alfenas, CBS 134655 = LPF281.
Note: Calonectria quinqueramosa can be distinguished from Ca. gracilis and Ca. gracilipes by the size of its ascospores and macroconidia, and by the number of fertile branches formed in the conidiogenous apparatus (Table 3).
Calonectria robigophila R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810017. Fig. 22.
Fig. 22.
Calonectria robigophila (ex-type CBS 134652). A–C. Macroconidiophores. D–F. Clavate vesicles. G–H. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. I. Macroconidia. Scale bars: A = 50 μm; B = 50 μm (apply to C); D = 10 μm (apply to I); E = 10 μm (apply to F–H).
Etymology: Name refers to Calonectria leaf blight, the disease this fungus is associated with.
Sexual morph not observed. Conidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 65–120 × 5–8 μm; stipe extensions septate, straight to flexuous, 125–225 μm long, 3–4 μm wide at the apical septum, terminating in acicular to clavate vesicles, 4–5 μm diam. Conidiogenous apparatus 15–60 μm long, 30–70 μm wide; primary branches aseptate, 18–35 × 4–7 μm; secondary branches aseptate, 10–20 × 3–5 μm; tertiary branches aseptate, 10–20 × 3–5 μm and additional branches (–6), aseptate, 10–15 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 5–10 × 3–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (45–)49–52(–60) × 3–5 μm (av. = 50 × 4 μm), L/W ratio = 12.6, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies umber to sienna on the surface, and umber to sepia in reverse; sparse to moderate aerial mycelium with moderate to extensive sporulation on the aerial mycelium; forming sparse chlamydospores occurring throughout the medium. Colonies slow growing (34–40 mm diam) on MEA, and fast growing (70–80 mm diam) OA, after 7 d at 25 °C.
Material examined: Brazil, Maranhão state, Açailândia, on leaves of Eucalyptus sp., May 2011, R.F. Alfenas (holotype CBS H-21361, living ex-type CBS 134652 = LPF192), LPF190, CBS 134653 = LPF193.
Notes: Calonectria robigophila can be distinguished from Ca. ecuadoriae by the dimensions and septation of its macroconidia. Furthermore, Ca. robigophila formed fewer fertile branches than reported for Ca. ecuadoriae (Crous et al. 2006, Table 3).
Calonectria silvicola R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810019. Fig. 23.
Fig. 23.
Calonectria silvicola (ex-type CBS 135237). A–C. Macroconidiophores. D–F. Obpyriform vesicles. G–H. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. I. Macroconidia. Scale bars A = 100 μm; B = 50 μm (apply to C); D = 10 μm (apply to E–I).
Etymology: Name refers to a forest, the habitat this fungus was isolated from.
Sexual morph not observed. Conidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 50–220 × 7–9 μm; stipe extensions septate, straight to flexuous, 130–195 μm long, 3–4 μm wide at the apical septum, terminating in obpyriform vesicles, 7–10 μm diam. Conidiogenous apparatus 35–90 μm long, 45–105 μm wide; primary branches aseptate, 20–30 × 3–6 μm, secondary branches aseptate, 13–26 × 3–6 μm, tertiary branches aseptate, 8–15 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 6–10 × 3–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight to slightly curved, (30–)40–42(–50) × 3–5 μm (av. = 41 × 4.5 μm), L/W ratio = 9.17, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies cinnamon to dark brick on the surface and sepia in reverse; moderate aerial mycelium with extensive sporulation on the aerial mycelium, especially at the centre; chlamydospores moderate to extensive, occurring throughout the medium, forming microsclerotia. Colonies slow growing (30–40 mm diam) on MEA, and moderately slow growing (45–50 mm diam) on OA, after 7 d at 25 °C.
Materials examined: Brazil, Bahia state, Mucuri, form soil collected in tropical rainforest, Aug. 2011, E. Zauza (holotype CBS H-21357, living ex-type CBS 135237 = LPF081); Minas Gerais state, Araponga, from soil collected in tropical rainforest, Aug. 2010, A.C. Alfenas & P.W. Crous, CBS 134836 = LPF079, CPC 18741 = LPF071, CPC 18766 = LPF096.
Note: The macroconidia of Ca. silvicola are larger than those of Ca. polizzii and Ca. zuluensis, but smaller than those of Ca. mossambicensis, Ca. nemuricola and Ca. pauciramosa (Table 3).
Calonectria telluricola R.F. Alfenas, L. Lombard & Crous, sp. nov. MycoBank MB810020. Fig. 24.
Fig. 24.
Calonectria telluricola (ex-type CBS 134664). A–C. Macroconidiophores. D. Clavate vesicle. E. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. F. Macroconidia. Scale bars: A = 100 μm (apply to B); C = 20 μm; D = 10 μm (apply to E–F).
Etymology: Name refers to soil, the substrate this fungus was isolated from.
Sexual morph not observed. Conidiophores consist of a stipe bearing penicillate suites of fertile branches, stipe extension, and terminal vesicle; stipe septate, hyaline, smooth, 55–125 × 5–7 μm; stipe extensions septate, straight to flexuous, 100–225 μm long, 2–4 μm wide at the apical septum, terminating in clavate vesicles, 3–6 μm diam. Conidiogenous apparatus 45–95 μm long, 40–80 μm wide; primary branches aseptate, 20–30 × 5–8 μm; secondary branches aseptate, 15–30 × 4–5 μm; tertiary branches aseptate, 10–20 × 5–6 μm, and additional branches (–4), aseptate, 10–15 × 3–4 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 7–11 × 3–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (35–)40–42(–50) × 3–6 μm (av. = 41 × 5 μm), L/W ratio = 9.13, 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies buff on the surface and ochraceous to umber in reverse; extensive aerial mycelium; chlamydospores not seen; sparse sporulation on the aerial mycelium. Colonies moderately fast growing (45–60 mm diam) at 25 °C on MEA, and fast growing (76–83 mm diam) on OA, after 7 d at 25 °C.
Materials examined: Brazil, Bahia state, Mucuri, from soil collected in tropical rainforest, Oct. 2011, E. Zauza (holotype CBS H–21365, culture ex-type CBS 134664 = LPF217); from soil collected in Eucalyptus plantations, Apr. 2011, E. Zauza, CBS 134667 = LPF263.
Note: Calonectria telluricola can be morphologically distinguished from other members of the Ca. brassicae complex by the length/diam ratio of its macroconidia, and number of conidiophore branches (Table 3).
Discussion
The present study represents the largest number of Calonectria isolates and species from Brazil ever subjected to DNA sequence analyses. Phylogenetic studies published on the genus Calonectria in recent years have substantially influenced its taxonomy (Lombard et al. 2010c). In the past five years alone, the phylogenetic species recognition concept (Taylor et al. 2000) has led to the description of at least 20 additional new species of Calonectria (Lombard et al., 2010b, Lombard et al., 2010c, Chen et al., 2011, Xu et al., 2012, Alfenas et al., 2013a, Alfenas et al., 2013b). In this study, a further 20 new Calonectria species are introduced from Brazil, with the name Ca. pseudopteridis being introduced for Cy. macrosporum, which is restored to species rank based on morphological characteristics and phylogenetic inference. The introduction of these novel Calonectria species supports the view that there are many more species in this genus to be discovered, particularly from the tropics and Southern Hemisphere (Crous et al., 2006, Lombard et al., 2010c).
Mendes & Urben (2014) list 23 Calonectria and 24 Cylindrocladium species in their list of fungi known from Brazil, although this is based on the old nomenclature and associated publications. However, recent literature (Crous, 2002, Lombard et al., 2009, Lombard et al., 2010c, Alfenas et al., 2013a, Alfenas et al., 2013b) provide DNA proof for at least 25 Calonectria species from Brazil, which include Ca. avesiculata, Ca, brasiliensis, Ca. brassicae, Ca. canadensis, Ca. cylindrospora, Ca. gracilis, Ca. hederae, Ca. hodgesii, Ca. hurae, Ca. ilicicola, Ca. indusiata, Ca. insularis, Ca. kyotensis, Ca. lauri, Ca. leguminum, Ca. metrosideri, Ca. naviculata, Ca. ovata, Ca. pauciramosa, Ca. penicilloides, Ca. pteridis, Ca. pyrochroa, Ca. spathiphylli, Ca. spathulata and Ca. variabilis. Additionally, Ca. rubropunctata (Silva & Minter 1995) and Ca. meliolae (Herbário Virtual da Flora e dos Fungos – http://inct.splink.org.br) have also been reported from Brazil, but we regard these taxa as dubious, pending their re-examination.
Most of the isolates obtained from Eucalyptus leaves displaying symptoms of CLB were identified as Ca. pteridis in this study. Calonectria pteridis is regarded as the most important causal agent of CLB of Eucalyptus throughout Brazil (Alfenas et al., 2004, Graça et al., 2009, Alfenas et al., 2013c) and was first reported in Brazil by Hodges & May (1972), causing needle blight of Pinus caribaea var. hondurensis. Thereafter, this fungal pathogen has been reported on various plant hosts in Brazil, mostly associated with leaf spot diseases of these respective hosts (Silva and Souza, 1981, Dianese et al., 1986, Silva, 1996, Trindade et al., 1998, Crous, 2002). Past taxonomic studies that included Ca. pteridis (Crous and Wingfeld, 1994, Crous et al., 1997, Crous, 2002, Crous et al., 2006) concluded that this species should be regarded as a species complex based on the morphological variation observed during these studies. However, phylogenetic inference in this study failed to identify any cryptic species among the hundreds of Ca. pteridis isolates obtained during this study. Only one new taxon, Ca. pseudovata, could be identified in the Ca. pteridis species complex, which in the past also included Ca. gordoniae and Ca. ovata (Crous et al., 1997, Crous et al., 2006, Lombard et al., 2010c). Calonectria pseudovata appears to have a limited distribution, as all isolates were obtained from soil collected in a commercial Eucalyptus plantation in the state of Pará, and its pathogenicity needs to be tested experimentally. Additionally, Ca. pseudopteridis is introduced for Cy. macrosporum, based on the phylogenetic inference in this study. This species was considered synonymous with Ca. pteridis (Sobers, 1968, Crous and Wingfeld, 1994, Crous, 2002) based on morphology only, but DNA sequence analysis revealed it to be distinct from that species. However, more isolates need to be (re)collected and strains from previous studies (Renard and Viennot-Bourgin, 1973, Renard and Quillec, 1979, Ahmad and Ahmad, 1982) need to be re-evaluated to determine the distribution and host range of Ca. pseudopteridis.
Species in the Ca. brassicae complex are characterised by having clavate vesicles and small (<60 μm), 1-septate macroconidia. Species known to belong to this complex include: Ca. brachiatica, Ca. brassicae, Ca. clavata, Ca. ecuadoriae, Ca. gracilipes, Ca. gracilis, Ca. orientalis and Ca. pini (Crous et al., 2006, Lombard et al., 2009, Lombard et al., 2010c). In the study by Crous et al. (2006) re-evaluating Calonectria species with clavate vesicles, only two new species could be resolved at that time. However, in this study, six new Calonectria species were delineated within this complex, with only Calonectria robigophila associated with CLB. The remaining species (Ca. duoramosa, Ca. paraensis, Ca. pseudobrassicae, Ca. quinqueramosa and Ca. telluricola) were all isolated from soil, and the extent of their pathogenicity to Eucalyptus still needs to be assessed. Only Ca. paraensis and Ca. telluricola were isolated from soils collected in tropical rainforests surrounding established Eucalyptus plantations. However, whether these species originated from these natural environments and were introduced into plantations through the movement of soil, still needs to be determined.
The Ca. cylindrospora complex is characterised by having 1-septate macroconidia and vesicles varying from pyriform to obpyriform or ovoid to ellipsoidal, and includes Ca. brasiliensis, C. cerciana, Ca. cylindrospora, Ca. hawksworthii, Ca. hodgesii, Ca. insularis, Ca. leucothöes, Ca. sulawesiensis and Ca. variabilis (Crous, 2002, Lombard et al., 2010c, Alfenas et al., 2013b). This complex has been extended in this study by the introduction of four new species (Ca. maranhensis, Ca. pseudocerciana, Ca. pseudohodgesii and Ca. propaginicola), based on phylogenetic inference and morphological features. Previous studies (Crous et al., 1993, Overmeyer et al., 1996, Schoch et al., 1999, Schoch et al., 2000) focussing on the taxonomy of the Ca. cylindrospora complex initially regarded these species as either Ca. cylindrospora (= Cy. scoparium) or Ca. candelabra (= Cy. candelabrum) based on their morphological similarities. However, Ca. cylindrospora has been circumscribed as having ellipsoidal to pyriform vesicles and Ca. candelabra having ellipsoidal to obpyriform vesicles (Crous et al. 1993). Of the four species described in this complex, only Ca. maranhensis was isolated from soil and Eucalyptus leaves displaying CLB collected in commercial plantations. The remaining three species were all isolated from Eucalyptus seedlings displaying symptoms of damping-off, with Ca. pseudohodgesii also isolated from Azadirachta indica leaves showing CLB symptoms, collected at the same nursery in Viçosa.
The Ca. candelabra complex is characterised by having ellipsoidal to obpyriform vesicles and 1-septate macroconidia (Schoch et al., 1999, Crous, 2002, Lombard et al., 2010b). This complex includes Ca. candelabra, Ca. colombiana, Ca. metrosideri, Ca. mexicana, Ca. mossambicensis, Ca. pauciramosa, Ca. pseudoscoparia, Ca. polizzii, Ca. spathulata and Ca. zuluensis (Schoch et al., 1999, Lombard et al., 2010b Lombard et al., 2011, Crous et al., 2013, Alfenas et al., 2013a). Eight new species (Ca. brassiana, Ca. eucalypticola, Ca. glaebicola, Ca. nemuricola, Ca. paiuiensis, Ca. pseudospathulata and Ca. silvicola) are introduced in this complex from this study. Of these, Ca. eucalypticola was the only species isolated from Eucalyptus seedlings displaying symptoms of damping-off, and from soil and Eucalyptus leaves with CLB symptoms in commercial plantations. This suggests that this species could have been introduced into the plantation environment from infected seedlings supplied by the nursery. Calonectria nemuricola, Ca. pseudospathulata and Ca. silvicola were only isolated from soils collected in tropical rainforests neighbouring commercial Eucalyptus plantations, and therefore their pathogenicity on Eucalyptus still needs to be determined. Calonectria piauiensis was isolated from soils collected in both commercial plantations and neighbouring tropical rainforests, whereas Ca. brassiana was only isolated from soils collected in a commercial plantation of E. brassiana. Both Ca. glaebicola and Ca. pseudometrosideri were found in soils and on Eucalyptus leaves, with the latter also isolated from Metrosideros polymorpha. The Ca. candelabra complex represents an important pathogen complex, having been reported worldwide on numerous plant hosts, and being regarded as dominant in commercial forest nurseries (Schoch et al., 1999, Crous, 2002, Lombard et al., 2010b, Vitale et al., 2013). Members of this complex have been reported in regions where the climatic conditions differ significantly, supporting the view that these species can tolerate a wide range of environmental conditions (Crous, 2002, Lombard et al., 2010b, Lombard et al., 2010c). Calonectria multinaviculata is introduced here as a new species in the Ca. naviculata complex. This species was isolated from soil collected in commercial Eucalyptus plantations, and therefore nothing is known about its pathogenicity.
Considering the distribution and substrates from which the new species described here were collected, it is apparent that the majority of species were isolated from soils collected in commercial Eucalyptus plantations. It is still uncertain whether these soil-borne species were originally present in the soil or were introduced during the establishment of these plantations. Extensive pathogenicity trials are, however, needed to determine if these species present a potential risk to Eucalyptus plantations in Brazil. The highest species diversity found in Eucalyptus plantations appears to be in the Brazilian states of Pará, Minas Gerais and Bahia, with fewer species recorded from the states of Maranhão and Piauí, and only single species from the states of Alagoas and Tocantins. More extensive surveys are required for the remaining areas in Brazil to obtain a clearer view on the species diversity of Calonectria in Brazil.
Recently, Lombard et al. (2010c) divided the genus Calonectria into two groups, the Prolate- and Sphaero-Naviculate groups, which corresponded well with terminal vesicle morphology of the respective species. The Prolate Group includes the majority of plant pathogenic Calonectria species, which appear to have distinct biogeographic distributions. For example, the C. reteaudii complex has only been reported from Australia, China, Indonesia and New Zealand, while the C. brassicae complex has only been reported from South and Central America, now including C. orientalis which is newly reported from Brazil here. The remaining members of the Prolate Group appear to have broad geographic distributions (Schoch et al., 2001, Lombard et al., 2010c). All novel taxa treated in this study, belonged to the Prolate Group. In the Sphaero-Naviculate Group there were no obvious patterns of distribution and pathogenicity, and only vesicle morphology appeared consistent. However, the highest species diversity in this group appears to be in the Northern Hemisphere and Asia (Crous et al., 2004b, Lombard et al., 2015a, Lombard et al., 2015b).
Other than clear global distribution patterns in the Prolate- and Sphaero-Naviculate groups of Calonectria species, there also appears to be a movement of taxa from natural forests to commercial forest nurseries, and again from nurseries to commercial plantations. The next logical question would be to establish if these species are also exported along with plant material in international trade. While this could be feasible in South America, no evidence yet has been found supporting such movement of any of the new species described here to other continents. There are however ample examples of the global movement of other pathogen groups along with this host, such as Ceratocystis fimbriata (Ferreira et al. 2011), Teratosphaeria nubilosa (Teratosphaeria leaf Blight; Hunter et al., 2009, Quaedvlieg et al., 2014), and Chrysoporthe cubensis (Van der Merwe et al. 2013). Global trade in forest products and plant material remains a serious concern for biosecurity, as Calonectria species could represent a more serious threat when introduced into favourable climate zones, and hence could pose a serious problem for the establisment of Eucalyptus plantations elsewhere in the world.
Acknowledgements
We thank the technical staff, Arien van Iperen and Tonimara de Souza Cândido (cultures), Marjan Vermaas (photographic plates), Mieke Starink-Willemse (DNA isolation, amplification and sequencing) for their invaluable assistance. We also wish to thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG, Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES for financial support of the work. The authors also acknowledge the companies: Clonar Resistência a Doenças Florestais, Jari Celulose and Suzano Papel e Celulose. The first author is grateful to Dr J.Z. (Ewald) Groenewald for advice regarding DNA sequence analyses.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
Contributor Information
R.F. Alfenas, Email: ralfenas@clonareucalipto.com.br.
L. Lombard, Email: l.lombard@cbs.knaw.nl.
References
- Ahmad N., Ahmad S. Needle blight disease of pine caused by Cylindrocladium macrosporum. Malaysian Forester. 1982;45:84–86. [Google Scholar]
- Alfenas A.C. Fungos do gênero Cylindrocladium como patógenos florestais, no Brasil. Fitopatologia Brasileira. 1986;11:275–277. [Google Scholar]
- Alfenas A.C., Zauza E.A.V., Mafia R.G. 2nd. ed. Editora UFV; Viçosa, MG, Brazil: 2009. Clonagem e doenças do eucalipto. [Google Scholar]
- Alfenas A.C., Ferreira F.A. A mancha de folha do eucalipto no Brasil causada por três espécies de Cylindrocladium – Uma revisão da descrição da doença. Revista Árvore. 1979;3:47–56. [Google Scholar]
- Alfenas A.C., Matsuoka A.K., Ferreira F.A. Identificação, características culturais e patogenicidade de três espécies de Cylindrocladium, isoladas de manchas de folha de Eucalyptus spp. Fitopatologia Brasileira. 1979;4:445–459. [Google Scholar]
- Alfenas R.F., Pereira O.L., Ferreira M.A. Calonectria metrosideri, a highly aggressive pathogen causing leaf blight, root rot, and wilt of Metrosideros spp. in Brazil. Forest Pathology. 2013;43:257–265. [Google Scholar]
- Alfenas R.F., Pereira O.L., Jorge V.L. A new species of Calonectria causing leaf blight and cutting rot of three forest tree species in Brazil. Tropical Plant Pathology. 2013;38:513–521. [Google Scholar]
- Alfenas R.F., Pereira O.L., Freitas R.G. Mass spore production and inoculation of Calonectria pteridis on Eucalyptus spp. under different environmental conditions. Tropical Plant Pathology. 2013;38:406–413. [Google Scholar]
- Alfenas A.C., Zauza E.A., Mafia R.G. Editora UFV; Viçosa, MG, Brazil: 2004. Clonagem e doenças do eucalipto. [Google Scholar]
- Almeida O.C., Bolkan H.A. Ocorrência e distribuição do gênero Cylindrocladium no Distrito Federal. Fitopatologia Brasileira. 1981;6:223–228. [Google Scholar]
- Berger S., Sinha A.K., Roitsch T. Plant physiology meets phytopathology: plant primary metabolism and plant-pathogen interactions. Journal of Experimental Botany. 2007;58:4019–4026. doi: 10.1093/jxb/erm298. [DOI] [PubMed] [Google Scholar]
- Blum L.E.B., Dianese J.C., Costa C.L. Comparative pathology of Cylindrocladium clavatum and C. scoparium on Eucalyptus spp. and screening of Eucalyptus provenances for resistance to Cylindrocladium damping-off. Tropical Pest Management. 1992;38:155–159. [Google Scholar]
- Bolkan H.A., Dianese J.C., Ribeiro W.R.C. Disease caused by Cylindrocladium on potato tubers in Brazil. Plant Disease. 1980;64:225. [Google Scholar]
- Carbone I., Kohn L.M. A method for designing primer sets for the speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Chen S., Lombard L., Roux J. Novel species of Calonectria associated with Eucalyptus leaf blight in Southeast China. Persoonia. 2011;26:1–12. doi: 10.3767/003158511X555236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W. APS Press; St. Paul, Minnesota, USA: 2002. Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera. [Google Scholar]
- Crous P.W., Alfenas A.C., Junghans T.G. Variability within Calonectria ovata and its anamorph Cylindrocladium ovatum from Brazil. Sydowia. 1998;50:1–13. [Google Scholar]
- Crous P.W., Alfenas A.C., Wingfield M.J. Calonectria scoparia and Calonectria morganii sp. nov., and variation among isolates of their Cylindrocladium anamorphs. Mycological Research. 1993;97:701–708. [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Crous P.W., Groenewald J.Z., Risède J.-M. Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology. 2004;50:415–430. doi: 10.3114/sim.55.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Groenewald J.Z., Risède J.-M. Calonectria species and their Cylindrocladium anamorphs: species with clavate vesicles. Studies in Mycology. 2006;55:213–226. doi: 10.3114/sim.55.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Theron L., van Zyl W.H. Delineation of Cylindrocladium species with 1–3-septate conidia and clavate vesicles based on morphology and rDNA RFLPs. Mycologia Research. 1997;101:210–214. [Google Scholar]
- Crous P.W., Wingfield M.J. A monograph of Cylindrocladium, including anamorphs of Calonectria. Mycotaxon. 1994;51:341–435. [Google Scholar]
- Crous P.W., Wingfield M.J., Guarro J. Fungal Planet description sheets: 154–213. Persoonia. 2013;31:188–296. doi: 10.3767/003158513X675925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vienne D.M., Giraud T., Martin O.C. A congruence index for testing topological similarity between trees. Bioinformatics. 2007;23:3119–3124. doi: 10.1093/bioinformatics/btm500. [DOI] [PubMed] [Google Scholar]
- Dianese J.C., Ribeiro W.R.C., Urben A.F. Root rot of soybean caused by Cylindrocladium clavatum in central Brazil. Plant Disease. 1986;70:977–980. [Google Scholar]
- Drummond A.J., Ashton B., Buxton S. 2011. Geneious v. 5.4.http://www.geneious.com/ Available from: [Google Scholar]
- El-Gholl N.E., Alfenas A.C., Crous P.W. Description and pathogenicity of Cylindrocladium ovatum sp. nov. Canadian Journal of Botany. 1993;71:466–470. [Google Scholar]
- Ferreira F.A. SIF, UFV; Viçosa, MG: 1989. Patologia florestal – principais doenças florestais no Brasil. 570 pp. [Google Scholar]
- Ferreira M.A., Alfenas A.C., Binoti D.H.B. Slow sand filtration eradicates eucalypt clonal nursery plant pathogens from recycled irrigation water in Brazil. Tropical Plant Pathology. 2012;37:319–325. [Google Scholar]
- Ferreira F.A., Alfenas A.C., Moreira A.M. Mancha-de-pteridis doença foliar de eucalipto em áreas tropicais brasileiras. Fitopatologia Brasileira. 1995;20:107–110. [Google Scholar]
- Ferreira M.A., Harrington T.C., Alfenas A.C. Movement of genotypes of Ceratocystis fimbriata within and among Eucalyptus plantations in Brazil. Phytopathology. 2011;101:1005–1012. doi: 10.1094/PHYTO-01-11-0015. [DOI] [PubMed] [Google Scholar]
- Fonseca S.M., de Resende M.D.V., Alfenas A.C. Universidade Federal de Viçosa; Viçosa: 2010. Manual Prático de Melhoramento Genético do Eucalipto. [Google Scholar]
- Gonçalves R.C., Alfenas A.C., Maffia L.A. Evaluation of bioassays to quantify Cylindrocaldium inocula in soil. Mycoscience. 2001;42:261–264. [Google Scholar]
- Graça R.N., Alfenas A.C., Maffia L.A. Factors influencing infection of eucalypts by Cylindrocladium pteridis. Plant Pathology. 2009;58:971–981. [Google Scholar]
- Groenewald J.Z., Nakashima C., Nishikawa J. Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology. 2013;75:115–170. doi: 10.3114/sim0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueidan C., Roux C., Lutzoni F. Using multigene phylogeny analysis to assess generic delineation and character evolution in Verrucariaceae (Verrucariales, Ascomycota) Mycological Research. 2007;111:1145–1168. doi: 10.1016/j.mycres.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Hillis D.M., Bull J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology. 1993;42:182–192. [Google Scholar]
- Hodges C.S., May L.C. A root disease of pine, araucaria, and eucalyptus in Brazil caused by a new species of Cylindrocladium. Phytopathology. 1972;62:898–901. [Google Scholar]
- Hodges C.S., Reis M.S., May L.C. Duas enfermidades em plantações de essências florestais exóticas no Brasil. Brasil Florestal. 1973;6:5–12. [Google Scholar]
- Hunter G.C., Crous P.W., Carnegie A.J. Teratosphaeria nubilosa, a serious leaf disease pathogen of Eucalyptus spp. in native and introduced areas. Molecular Plant Pathology. 2009;10:1–14. doi: 10.1111/j.1364-3703.2008.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy R.M., Schubert T.S., El-Gholl N.E. Cylindrocladium gordoniae sp. nov. Mycotaxon. 2000;76:77–83. [Google Scholar]
- Librado P., Rozas J. DnaSP v. 5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Lombard L., Chen S.F., Mou X. New species, hyper-diversity and potential importance of Calonectria spp. from Eucalyptus in South China. Studies in Mycology. 2015;80:181–188. doi: 10.1016/j.simyco.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Crous P.W., Wingfeld B.D. Species concepts in Calonectria (Cylindrocladium) Studies in Mycology. 2010;66:15–30. doi: 10.3114/sim.2010.66.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Crous P.W., Wingfeld B.D. Multigene phylogeny and mating tests reveal three cryptic species related to Calonectria pauciramosa. Studies in Mycology. 2010;66:1–14. doi: 10.3114/sim.2010.66.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Crous P.W., Wingfeld B.D. Phylogeny and systematic of the genus Calonectria. Studies in Mycology. 2010;66:31–69. doi: 10.3114/sim.2010.66.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., van der Merwe N.A., Groenewald J.Z. Generic concepts in Nectriaceae. Studies in Mycology. 2015;80:189–245. doi: 10.1016/j.simyco.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Polizzi G., Guarnaccia V. Calonectria spp. causing leaf spot, crown and root rot of ornamental plants in Tunisia. Persoonia. 2011;27:73–79. doi: 10.3767/003158511X615086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Rodas C.A., Crous P.W. Cylindrocladium species associated with dying Pinus cuttings. Persoonia. 2009;23:41–47. doi: 10.3767/003158509X471052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Zhou X.D., Crous P.W., Wingfield B.D., Wingfield M.J. Calonectria species associated with cutting rot of Eucalyptus. Persoonia. 2010;24:1–11. doi: 10.3767/003158510X486568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafia R.G., Alfenas A.C., Ferreira E.M. Reuse of untreated irrigation water as a vehicle of inoculum of pathogens in eucalyptus clonal nursery. Tropical Plant Pathology. 2008;33:96–102. [Google Scholar]
- Mafia R.G., Alfenas A.C., Maffia L.A. Plant growth promoting rhizobacteria as agents in the biocontrol of eucalyptus mini-cutting rot. Tropical Plant Pathology. 2009;34:10–17. [Google Scholar]
- Mendes M.A.S., Urben A.F. Embrapa Recursos Genéticos e Biotecnologia; Brasília, DF: 2014. Fungos relatados em plantas no Brasil, Laboratório de Quarentena Vegetal.http://pragawall.cenargen.embrapa.br/aiqweb/michtml/fgbanco01.asp Available at: Accessed on August 2014. [Google Scholar]
- Nirenburg H.I. A simplified method for identifying Fusarium spp. occurring on wheat. Canadian Journal of Botany. 1981;59:1599–1609. [Google Scholar]
- Novaes Q.S., Souza V.C., Dias P.C. Toona ciliata, a new host of Cylindrocladium clavatum in Brazil. Summa Phytopathologica. 2012;38:251–252. [Google Scholar]
- Nylander J.A.A. Evolutionary Biology Centre, Uppsala University; 2004. MrModeltest v. 2. Programme distributed by the author. [Google Scholar]
- O'Donnell K., Kistler H.C., Cigelnik E. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:2044–2049. doi: 10.1073/pnas.95.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmeyer C., Lünnemann S., von Wallbrünn C. Genetic variability among isolates and sexual offspring of the plant pathogenic fungus Calonectria morganii on the basis of random amplifications of polymorphic DNA (RAPD) and restriction fragment length polymorphism (RFLP) Current Microbiology. 1996;33:249–255. doi: 10.1007/s002849900108. [DOI] [PubMed] [Google Scholar]
- Poltronieri L.S., Alfenas R.F., Verzignassi J.R. Leaf blight and defoliation of Eugenia spp. caused by Cylindrocladium candelabrum and C. spathiphylli in Brazil. Summa Phytopathologica. 2011;37:147–149. [Google Scholar]
- Quaedvlieg W., Binder M., Groenewald J.Z. Introducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia. 2014;33:1–40. doi: 10.3767/003158514X681981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner R.W. Commonwealth Mycological Institute; Kew, Surrey: 1970. A mycological colour chart. British Mycological Society. [Google Scholar]
- Rehn V.N.C., Menezes M., Rehn K.G. Isolation, morphological identification and pathogenicity of Cylindrocladium scoparium and C. clavatum isolates obtained from plants rhizosphere cultivated in Pernambuco State. Brazilian Journal of Microbiology. 2004;35:292–294. [Google Scholar]
- Reis A., Mafia R.G., Silva P.P. Cylindrocladium spathiphylli, causal agent of Spathiphyllum root and collar rot in the Federal District-Brazil. Fitopatologia Brasileira. 2004;29:102. [Google Scholar]
- Renard J.L., Quillec G. Diseases and anomalies of oil palm in the nursery. Oleagineux. 1979;34:331–337. [Google Scholar]
- Renard J.L., Viennot-Bourgin G. A new disease of the oil palm due to Cylindrocladium macrosporum. Oleagineux. 1973;28:443–445. [Google Scholar]
- Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Schoch C.L., Crous P.W., Wingfield B.D. The Cylindrocladium candelabrum species complex includes four distinct mating populations. Mycologia. 1999;91:286–298. [Google Scholar]
- Schoch C.L., Crous P.W., Wingfield M.J. Phylogeny of Calonectria and selected hypocrealean genera with cylindrical macroconidia. Studies in Mycology. 2000;45:45–62. [Google Scholar]
- Schoch C.L., Crous P.W., Wingfield B.D. Phylogeny of Calonectria based on comparisons of β-tubulin DNA sequences. Mycological Research. 2001;105:1045–1052. [Google Scholar]
- Silva G.S. Cylindrocladium pteridis, causal agent of leaf spot of buriti (Mauritia flexuosa) Fitopatologia Brasileira. 1996;21:523. [Google Scholar]
- Silva G.S., Cutrim F.A., Ferreira F.A. Mancha foliar e podridão de frutos da acerola causadas por Calonectria ilicicola. Fitopatologia Brasileira. 2001;26:101. [Google Scholar]
- Silva M., Minter D.W. Fungi from Brazil – recorded by Batista and co-workers. Mycological Papers. 1995;169:1–585. [Google Scholar]
- Silva G.S., Souza E.A.P. Mancha foliar do coqueiro causada por Cylindrocladium pteridis Wolf. Fitopatologia Brasileira. 1981;6:515–517. [Google Scholar]
- Sobers E.K. Morphology and host range of Cylindrocladium pteridis. Phytopathology. 1968;58:1265–1270. [Google Scholar]
- Swofford D.L. Computer program. Sinauer Associates; Sunderland, Massachusetts, USA: 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods). v4.0b10. [Google Scholar]
- Tamura K., Peterson D., Peterson N. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.W., Jacobson D.J., Kroken S.M. Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology. 2000;31:21–32. doi: 10.1006/fgbi.2000.1228. [DOI] [PubMed] [Google Scholar]
- Trindade D.R., Poltronieri L.S., Martins e Silva H. Occurrence of leaf spot on coconuts caused by Cylindrocladium pteridis in the state of Pará, Brazil. Fitopatologia Brasileira. 1998;23:412. [Google Scholar]
- Tozetto D.R., Ribeiro W.R.C. Leaf spot of mangoes caused by Cylindrocladium scoparium in central Brazil. Journal of Phytopathology. 1996;144:471–472. [Google Scholar]
- Van der Merwe N.A., Steenkamp E.T., Rodas C. Host switching between native and non-native trees in a population of the canker pathogen Chrysoporthe cubensis from Colombia. Plant Pathology. 2013;62:642–648. [Google Scholar]
- Vitale A., Crous P.W., Lombard L. Calonectria diseases on ornamental plants in Europe and the Mediterranean basin: an overview. Journal of Plant Pathology. 2013;95:463–476. [Google Scholar]
- Xu J.J., Qin S.Y., Hao Y.Y. A new species of Calonectria causing leaf disease of water lily in China. Mycotaxon. 2012;122:177–185. [Google Scholar]