Abstract
The pitch canker pathogen Fusarium circinatum has caused devastation to Pinus spp. in natural forests and non-natives in commercially managed plantations. This has drawn attention to the potential importance of Fusarium species as pathogens of forest trees. In this study, we explored the diversity of Fusarium species associated with diseased Pinus patula, P. tecunumanii, P. kesiya and P. maximinoi in Colombian plantations and nurseries. Plants displaying symptoms associated with a F. circinatum-like infection (i.e., stem cankers and branch die-back on trees in plantations and root or collar rot of seedlings) were sampled. A total of 57 isolates were collected and characterised based on DNA sequence data for the translation elongation factor 1-α and β-tubulin gene regions. Phylogenetic analyses of these data allowed for the identification of more than 10 Fusarium species. These included F. circinatum, F. oxysporum, species within the Fusarium solani species complex and seven novel species in the Fusarium fujikuroi species complex (formerly the Gibberella fujikuroi species complex), five of which are described here as new. Selected isolates of the new species were tested for their pathogenicity on Pinus patula and compared with that of F. circinatum. Of these, F. marasasianum, F. parvisorum and F. sororula displayed levels of pathogenicity to P. patula that were comparable with that of F. circinatum. These apparently emerging pathogens thus pose a significant risk to forestry in Colombia and other parts of the world.
Key words: Fusarium, Morphology, Pathogenicity, Phylogenetics, Pinus kesiya, P. maximinoi, P. patula, P. tecunumanii
Taxonomic novelties: New species: F. fracticaudum Herron, Marinc. & M.J. Wingf.; F. marasasianum Herron, Marinc. & M.J. Wingf.; Fusarium parvisorum Herron, Marinc. & M.J. Wingf.; F. pininemorale Herron, Marinc. & M.J. Wingf.; F. sororula Herron, Marinc. & M.J. Wingf.
Introduction
Over the last two decades, the incidence of plant diseases in forest ecosystems has increased dramatically (Orwig, 2002, Fisher et al., 2012). This is primarily due to anthropogenic activities (e.g., Anagnostakis, 2001, Wingfield et al., 2001, Wingfield et al., 2008b, Garnas et al., 2012) and the disruption of forest ecosystems (Liebhold et al., 1995, Jactel et al., 2009). The disease levels are particularly increasing where native ecosystems have been disrupted by the planting of extensive areas to forest monocultures, especially exotic species (Chou, 1991, Bradshaw et al., 2000, Wingfield et al., 2001, Scholtof, 2006, Jactel et al., 2009). For example, in the Southern Hemisphere, large areas are planted with monocultures of exotic Pinus or Eucalyptus spp. (Wingfield 2003), which are typically located within or near natural woodlands and forests (Richardson et al., 1994, Ayala et al., 2005, Sano et al., 2010, Silva et al., 2011). In such areas where native and commercial forestry ecosystems co-occur, the risks associated with new plant diseases are significantly increased, particularly where trees in the two ecosystems are related (Perkins and Matlack, 2002, Tommerup et al., 2003, Wingfield et al., 2010, Blitzer et al., 2012).
The forests in Colombia, together with those in Brazil, Peru, Bolivia and Venezuela make up approx. 84 % of South America's total forested area (FAO 2012). Of the ca. 60.5 M ha of forests in Colombia, only 405 000 ha represent commercially managed plantations (FAO, 2005, FAO, 2010). Pinus spp. represent approximately 35 % of the commercially planted species in this country (IDEAM 2009). Although commercial forestry in Colombia is relatively young, a number of diseases and insect pests that damage Pinus spp. have been reported. Rodas (1998) recorded 30 different native species of defoliating insects occurring on exotic plantation species in the Andean region of Colombia. More recently, Fusarium circinatum, the causal agent of pitch canker, was also reported from diseased seedlings and established Pinus spp. in Colombia (Steenkamp et al. 2012). As time passes, the number of emerging pests and pathogens will likely increase, particularly as native organisms adapt to infest/infect non-native trees and where new organisms are accidentally introduced into the country.
Many Fusarium spp. have a global distribution and are economically important as producers of toxic secondary metabolites and infective agents of plants, animals and humans (Leslie & Summerell 2006). Notable examples include Fusarium poae, F. verticillioides and members of the F. solani species complex (FSSC), F. oxysporum species complex (FOSC) and the F. graminearum species complex (FGSC) (Matuo and Snyder, 1973, Marasas, 2001, Nucci and Anaissie, 2002, Pietro et al., 2003, Zhang et al., 2006, Streit et al., 2012). Although most cultivated plants are host to one or more pathogens in this genus (Leslie & Summerell 2006), the only Fusarium sp. known to severely affect Pinus spp. is F. circinatum (Wingfield et al. 2008a). In general, however, limited information is available regarding the diversity of Fusarium spp. associated with commercially propagated Pinus spp. or the possible diseases they cause in this setting.
Steenkamp et al. (2012) explored the presence of the pitch canker fungus on Pinus spp. in Colombia but also found a number of other Fusarium spp. that were frequently and/or consistently encountered (unpubl. data). All of these other fungi were also isolated from either Pinus seedlings or established plantation trees, showing symptoms typical of infection with F. circinatum. On seedlings, the symptoms included wilt, root and collar rot, and on established trees they included stem cankers and branch and tip die-back (Steenkamp et al. 2012). Knowledge regarding the identity and pathogenicity of these isolates is important in order to realistically quantify the risks they pose to Pinus-based plantation forestry in Colombia and other parts of the world. They could also represent threats to Pinus spp. where these trees grow naturally, as has been the case with F. circinatum in native forests in the United States (Gordon et al., 2001, Gordon, 2006, Wingfield et al., 2008a).
The aim of this study was, firstly, to identify the Fusarium spp. associated with diseased P. patula seedlings and with P. patula, P. tecunumanii, P. kesiya and P. maximinoi trees in plantations showing symptoms of pitch canker in Colombia. This was accomplished using conventional morphology and culture-based approaches together with the DNA sequence information for portions of the genes encoding translation elongation factor 1-α (tef1) and β-tubulin (tub2). Descriptions were provided for the new Fusarium spp. recognised. A second aim was to evaluate the pathogenicity of the identified fungi to Pinus and to determine whether they could have been responsible for the symptoms observed.
Materials and methods
Isolates
The Fusarium isolates used in this study were collected from a number of different locations and Pinus spp. in Colombia (Table 1). These included Pinus kesiya, P. maximinoi, P. patula and P. tecunumanii trees exhibiting canker-like infections in plantations in or near Calima, Aguaclara, La Cumbre (Valle de Cauca), Angela Maria (Risaralda), El Darién (Valle del Cauca), El Guasimo (Antioquia), Campania, Riosucio (Caldas), and Volconda (Valle de Cauca). Isolates were also obtained from symptomatic (i.e., wilting, root rot, root collar and stem discolouration) P. patula seedlings collected in nurseries [Bandeja (Valle de Cauce), Canaleta (Valle de Cauca) and Peňas Negra (Valle de Cauca)].
Table 1.
Host and geographic origin of the Fusarium isolates used in this study.
| Fusarium species1 | Accession number2 | Pinus species3 | Area in Colombia | Provenance | GPS co-ordinates |
|---|---|---|---|---|---|
| Fusarium sp. | CMW 25516; FCC 5428 | P. patula (T) | Angela Maria (Santa Rosa) | Risaralda | 75°36′21″ W 4°49′18″ N |
| Fusarium sp. | Colombia 18 | P. patula (T) | Angela Maria (Santa Rosa) | Risaralda | 75°36′21″ W 4°49′18″ N |
| F. circinatum | CMW 25239; FCC 5379 | P. tecunumanii (T) | Calima, El Darién | Valle del Cauca | 76°26′03″ W 3°56′57″ N |
| CMW 25240; FCC 5380 | P. tecunumanii (T) | Calima, El Darién | Valle del Cauca | 76°26′03″ W 3°56′57″ N | |
| CMW 25251; FCC 5391 | P. maximinoi (T) | Calima, El Darién | Valle del Cauca | 76°26′03″ W 3°56′57″ N | |
| CMW 25255; FCC 5395 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25256; FCC 5396 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25257; FCC 5397 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25258; FCC 5398 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25259; FCC 5399 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25260; FCC 5400 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25262; FCC 5402 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25263; FCC 5403 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25264; FCC 5404 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25265; FCC 5405 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25266; FCC 5406 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25271; FCC 5411 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25272; FCC 5412 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25273; FCC 5413 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25274; FCC 5414 | P. patula (S) | Vivero, Peňas Negras | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25518; FCC 5430 | P. kesiya (T) | Aguaclara, La Cumbre | Valle del Cauca | 76°1′33″ W 3°44′33″ N | |
| CMW 25519; FCC 5431 | P. patula (T) | Angela Maria (Santa Rosa) | Risaralda | 75°36′21″ W 4°49′18″ N | |
| CMW 25520; FCC 5432 | P. patula (T) | El Guasimo (Santa Rosa de Osos) | Antioquia | 75°26′30″ W 6°52′04″ N | |
| F. falciforme* | CMW 25507; FCC 5419 | P. maximinoi (S) | Vivero Canaleta | Valle del Cauca | 76°29′49″ W 3°51′45″ N |
| CMW 25514; FCC 5426 | P. tecunumanii (T) | La Suiza, Restrepo | Valle del Cauca | 76°29′33″ W 3°50′55″ N | |
| F. fracticaudum sp. nov. | CMW 25237; FCC 5377; CBS 137233 | P. tecunumanii (T) | Calima, El Darién | Valle del Cauca | 76°26′03″ W 3°56′57″ N |
| CMW 25238; FCC 5378 | P. tecunumanii (T) | Calima, El Darién | Valle del Cauca | 76°26′03″ W 3°56′57″ N | |
| CMW 25241; FCC 5381 | P. maximinoi (T) | Calima, El Darién | Valle del Cauca | 76°26′03″ W 3°56′57″ N | |
| CMW 25242; FCC 5382 | P. maximinoi (T) | Calima, El Darién | Valle del Cauca | 76°26′03″ W 3°56′57″ N | |
| CMW 25245; FCC 5385; CBS 137234 | P. maximinoi (T) | Angela Maria (Santa Rosa) | Risaralda | 75°36′21″ W 4°49′18″ N | |
| CMW 25249; FCC 5389 | P. maximinoi (T) | Angela Maria (Santa Rosa) | Risaralda | 75°36′21″ W 4°49′18″ N | |
| CMW 25250; FCC 5390 | P. maximinoi (T) | Angela Maria (Santa Rosa) | Risaralda | 75°36′21″ W 4°49′18″ N | |
| CMW 25511; FCC 5423 | P. tecunumanii (T) | Volconda (Calima El Darién) | Valle del Cauca | 76°25′06″ W 4°01′47″ N | |
| F. keratoplasticum* | CMW 25505; FCC 5417 | P. tecunumanii (S) | Vivero Bandeja | Valle del Cauca | 76°29′49″ W 3°51′45″ N |
| CMW 25515; FCC 5427 | P. tecunumanii (T) | La Suiza, Restrepo | Valle del Cauca | 76°29′33″ W 3°50′55″ N | |
| F. marasasianum sp. nov. | CMW 25246; FCC 5386 | P. tecunumanii (T) | Calima, El Darién | Valle del Cauca | 76°26′03″ W 3°56′57″ N |
| CMW 25248; FCC 5388 | Pinus sp. (T) | Colombia | n/a | n/a | |
| CMW 25252; FCC 5392 | Pinus sp. (T) | Colombia | n/a | n/a | |
| CMW 25253; FCC 5393; CBS 137237 | Pinus sp. (T) | Colombia | n/a | n/a | |
| CMW 25261; FCC 5401; CBS 137238 | P. patula (S) | Vivero, Peňas Negra | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25512; FCC 5424 | P. tecunumanii (T) | Volconda (Calima, El Darién) | Valle del Cauca | 76°25′06″ W 4°01′47″ N | |
| F. parvisorum sp. nov. | CMW 25267; FCC 5407; CBS 137236 | P. patula (S) | Vivero, Peňas Negra | Valle del Cauca | 76°29′49″ W 3°51′45″ N |
| CMW 25268; FCC 5408; CBS 137235 | P. patula (S) | Vivero, Peňas Negra | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| CMW 25269; FCC 5409 | P. patula (S) | Vivero, Peňas Negra | Valle del Cauca | 76°29′49″ W 3°51′45″ N | |
| F. pininemorale sp. nov. | CMW 25243; FCC 5383; CBS 137240 | P. tecunumanii (T) | Angela Maria (Santa Rosa) | Risaralda | 75°36′21″ W 4°49′18″ N |
| CMW 25244; FCC 5384; | P. tecunumanii (T) | Angela Maria (Santa Rosa) | Risaralda | 75°36′21″ W 4°49′18″ N | |
| CBS 137239 | |||||
| CMW 25247; FCC 5387 | P. tecunumanii (T) | Calima, El Darién | Valle del Cauca | 76°26′03″ W 3°56′57″ N | |
| FSSC 5* | CMW 25509; FCC 5421 | P. maximinoi (S) | Vivero Canaleta | Valle del Cauca | 76°29′49″ W 3°51′45″ N |
| FSSC 20* | CMW 25506; FCC 5418 | P. maximinoi (S) | Vivero Canaleta | Valle del Cauca | 76°29′49″ W 3°51′45″ N |
| F. sororula sp. nov. | CMW 25254; FCC 5394; CBS 137241 | Pinus sp. (T) | Colombia | n/a | n/a |
| CMW 25513; FCC 5425 | P. tecunumanii (T) | Angela Maria (Santa Rosa) | Risaralda | 75°36′21″ W 4°49′18″ N | |
| CMW 25517; FCC 5429 | P. patula (T) | Campania, Riosucio | Caldas | 75°49′18″ W 5°21′45″ N | |
| Colombia 8 | P. patula (T) | Volconda (Calima, El Darién) | Valle del Cauca | 76°25′06″ W 4°01′47″ N | |
| Colombia 19 | P. patula (T) | Angela Maria (Santa Rosa) | Risaralda | 75°36′21″ W 4°49′18″ N | |
| CMW 40578; CBS 137242 | P. patula (T) | Angela Maria (Santa Rosa) | Risaralda | 75°36′21″ W 4°49′18″ N | |
| F. oxysporum | CMW 25503; FCC 5415 | P. tecunumanii (S) | Vivero Eras | Valle del Cauca | 76°29′49″ W 3°51′45″ N |
| CMW 25504; FCC 5416 | P. tecunumanii (S) | Vivero Bandeja | Valle del Cauca | 76°29′49″ W 3°51′45″ N |
Five novel Fusarium species were described in this study. The FSSC species and lineages (indicated with *) were recognised according to O'Donnell et al. (2008) and Short et al. (2013). n/a = not available.
CMW: Culture collection at the FABI, University of Pretoria, South Africa. FCC, original numbers of the Fusarium culture collection at FABI, University of Pretoria, South Africa. CBS, Culture collection at the CBS-KNAW Fungal diversity Centre, Utrecht, Netherlands. Isolate numbers in boldface represent ex-type cultures.
Letters in brackets indicate whether the isolates came from seedlings (S) or mature trees (T).
Diseased plant tissue was surface-disinfected for 1 min in a solution containing 1.5 % (v/v) sodium hypochlorite, rinsed with sterile distilled water, immersed in 70 % (v/v) ethanol for 1 min and air-dried. Small pieces of tissue, cut from the leading edges of lesions, were plated directly onto half-strength potato dextrose agar medium (½ PDA) and Fusarium selective medium (FSM, Nash & Snyder 1962). Following incubation at 27.5 °C, isolates resembling Fusarium were transferred to fresh PDA and grown for 7 d at 23 °C, after which pure cultures were prepared. This was done by washing the conidia from the mycelium using a 2.5 % (v/v) Tween 60 (Sigma–Aldrich, St Louis, Missouri, USA) solution and spreading 1 mL of the spore suspension across the surface of water agar medium (WA; 20 g/L PDA; Biolab Diagnostics). Following incubation at 16 °C for 2 d, single germinating conidia were transferred to fresh PDA and incubated for 7 d at 23 °C. All of the cultures collected for this study are maintained in the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, South Africa and representative isolates representing novel species were deposited in the culture collection of the CBS-KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands (CBS). Dried cultures of novel species were deposited in the fungarium of the Agricultural Research Council (ARC), Pretoria, South Africa (PREM).
DNA extraction, PCR amplification and sequencing
Fungal DNA was extracted from 7-d-old cultures using a modified CTAB (hexadecyltrimethylammonium bromide) method (Steenkamp et al. 1999) and mycelium was scraped directly from the surface of the growth media. Specific regions of tef1 and tub2 were amplified with a Bio-Rad iCycler (Bio-Rad, California, USA) using, respectively, primers EF-1 and EF-2 (O'Donnell et al., 1998, Geiser et al., 2004) and primers T1 and T2 (O'Donnell & Cigelnik 1997). Each amplification reaction contained 2–4 ng/μL DNA, 0.25 μM of each primer, 200 μM dNTPs (Fermentas, Nunningen, Germany), 2.5 mM MgCl2, 0.04 U/μL of Supertherm Taq polymerase and reaction buffer with KCl (10×) (Southern Cross Biotechnology, Cape Town, South Africa). The PCR started with an initial denaturation step at 95 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 54 °C (tub2) or 56 °C (tef1) for 45 s and 72 °C for 1 min. A final extension step at 72 °C for 10 min was used to conclude the PCR.
Amplified PCR products were purified using polyethylene glycol (Steenkamp et al. 2006) or G50 Sephadex columns (Sigma, Steinheim, Germany). The purified samples were then sequenced in both directions using the original PCR primers, an ABI PRISM BigDye® Terminator v. 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, California) and an ABI PRISM® 3100 Genetic Analyzer (Applied Biosystems). Electropherogrammes were examined and manually corrected where necessary using Chromas Lite v. 2.1.1 (Technelysium, Australia) and BioEdit v. 7.2.5 (Hall 1999). The tef1 nucleotide sequences were compared to those in the Fusarium-ID identification database (Geiser et al. 2004; http://isolate.fusariumdb.org) using the basic local alignment search tool (BLAST) search algorithm (Altschul et al. 1990).
Multiple sequence alignments were generated with MAFFT v. 7.0 (http://mafft.cbrc.jp/alignment/software/) with the L-INS-i option selected (Katoh et al., 2002, Katoh et al., 2005, Katoh and Toh, 2008, Katoh and Standley, 2013) and corrected manually where necessary. The datasets constructed for tef1 and tub2 contained all the sequences generated in this study and the recognised species and phylogenetic lineages in the F. fujikuroi complex (FFSC, previously known as the Gibberella fujikuroi species complex) (Geiser et al. 2013), as well as the outgroup species F. oxysporum (Table 2). To infer phylogenies, the tef1 and tub2 datasets were analysed separately, as well as combined as previously described (O'Donnell et al., 1998, O'Donnell et al., 2000, Geiser et al., 2005). MrBayes v. 3.2.1 (Heulsenbeck et al. 2001) and PhyML v. 3.0 (Guindon et al. 2010) were used to generate phylogenies based on Bayesian inference (BI) and Maximum Likelihood (ML), respectively. The best-fit parameters, as indicated by jModelTest v. 2.1.3 (Guindon and Gascuel, 2003, Posada, 2008, Darriba et al., 2012), for ML analyses of the tef1 and tub2 datasets included gamma correction (G) to account for among site rate variation and the TIM2ef and TIM2 (Posada 2003) models, respectively. BI analysis of these datasets utilised the General Time Reversible (GTR) model (Tavare 1986) with G. Bayesian inference and ML analysis of the combined dataset also utilised GTR+G. The ML branch support was estimated using bootstrap analyses based on 1 000 pseudoreplicates and model parameters as described above. The BI analyses were based on 6 M generations using one cold and three heated chains, and Bayesian posterior probabilities were calculated after discarding a burn-in corresponding to approximately 75 000 generations post-stationarity. The BI-based analysis of the combined dataset utilised separate model parameters for each gene (Heulsenbeck et al. 2001). Phylogenetic trees were viewed and edited using MEGA v. 5 (Tamura et al. 2011). All novel sequences were deposited in GenBank (see Table 2 for accession numbers), while the alignments and phylogenetic trees were deposited in Tree BASE.
Table 2.
The species names and their GenBank accession numbers for all the Fusarium isolates included in the phylogenetic analyses.
| Species1 | Host/substrate | Origin | Culture collection2 | GenBank accession3 |
|
|---|---|---|---|---|---|
| tub2 | tef1 | ||||
| Fusarium acutatum | – | India | NRRL 13308 | U34431a | AF160276b |
| F. ananatum | Ananas comosus | England | NRRL 22945 | U34420a | AF160297a |
| F. anthophilum | Hippeastrum sp. | Germany | NRRL 13602 | U61541a | AF160292a |
| F. bactridioides | Cronartium conigenum | USA | NRRL 20476 | U34434a | AF160290a |
| F. begoniae | Begonia elatior | Germany | NRRL 25300 | U61543a | AF160293a |
| F. brevicatenulatum | Striga asiatica | Madagascar | NRRL 25446 | U61545a | AF160265a |
| F. bulbicola | Nerine bowdenii | Netherlands | NRRL 13618 | U61546a | AF160294a |
| F. circinatum | Pinus radiata | USA | NRRL 25331 | U61547a | AF160295a |
| F. concentricum | Musa sapientum | Costa Rica | NRRL 25181 | U61548a | AF160282a |
| F. denticulatum | Ipomoea batatas | USA | NRRL 25302 | U61550a | AF160269a |
| F. dlaminii | Soil | South Africa | n/a | n/aj,k | n/aj,k |
| F. fracticaudum* | Pinus maximinoi | Colombia | CBS 137233 | KJ541051 | KJ541059 |
| Pinus maximinoi | Colombia | CBS 137234 | KJ541048 | KJ541058 | |
| F. fractiflexum | Cymbidium sp. | Japan | NRRL 28852 | AF160315c | AF160288c |
| F. fujikuroi | Oryza sativa | Taiwan | NRRL 13566 | U34415a | AF160279a |
| F. globosum | Zea mays | Central America | NRRL 26131 | U61557a | AF160285a,l |
| F. guttiforme | Ananas comosus | South America | NRRL 22945 | U34446a | AF160297a, l |
| F. inflexum | Vicia faba | Germany | NRRL 20433 | U34435a | AF008479a |
| F. konzum | Andropogon gerardii | North America | MRC 8854 | EU220234j | EU220235j |
| F. lactis | Ficus carica | USA | NRRL 25200 | U61551a | AF160272a |
| F. lyarnte | Soil | Australia | F19374 | EF107122f | EF107118f |
| F. mangiferae | Mangifera indica | India | NRRL 25226 | U61561a | AF160281a |
| F. marasasianum* | Pinus patula | Colombia | CBS 137238 | KJ541054 | KJ541063 |
| Pinus patula | Colombia | CBS 137237 | KJ541052 | KJ541062 | |
| F. mexicanum | Mangifera indica | Mexico | NRRL 53147 | GU737494e | GU737282e |
| F. musae | Musa sp. | Honduras | MUCL 52574 | FN545368h | FN552086h |
| F. napiforme | Pennisetum typhoides | South Africa | NRRL 13604 | U34428a | AF160266a |
| F. nygamai | Sorghum bicolor | Australia | NRRL 13448 | U34426a | AF160273a |
| F. oxysporum | Pseudotsuga menziesii | USA | NRRL 22902 | U34424a | AF160312a |
| F. phyllophilum | Dracaena deremensis | Italy | NRRL 13617 | U34432a | AF160274a |
| F. parvisorum* | Pinus patula | Colombia | CBS 137236 | KJ541055 | KJ541060 |
| Pinus patula | Colombia | CBS 137235 | KJ541056 | KJ541061 | |
| F. pininemorale* | Pinus tecunumanii | Colombia | CBS 137240 | KJ541049 | KJ541064 |
| Pinus tecunumanii | Colombia | CBS 137239 | KJ541050 | KJ541065 | |
| F. proliferatum | Cattleya sp. | Germany | NRRL 22944 | U34416a | AF160280a |
| F. pseudoanthophilum | Zea mays | Zimbabwe | NRRL 25206 | U61553a | AF160264a |
| F. pseudocircinatum | Solanum sp. | Zimbabwe | NRRL 22946 | U34427a | AF160271a |
| F. pseudonygamai | Pennisetum typhoides | Ghana | NRRL 13592 | U34421a | AF160263a |
| F. ramigenum | Ficus carica | Nigeria | NRRL 25208 | U61554a | AF160267a |
| F. sacchari | Saccharum officinarum | USA | NRRL 13999 | U34414a | AF160278a |
| F. sororula* | Pinus patula | Colombia | CBS 137242 | KJ541057 | KJ541067 |
| Pinus patula | Colombia | CBS 137241 | KJ541053 | KJ541066 | |
| F. sterilihyphosum | Mangifera indica | India | MRC 2802 | AF160316a | AF160300a |
| F. subglutinans | Zea mays | USA | NRRL 22016 | U34417a | AF160289a |
| F. succisae | Succisa pratensis | Germany | NRRL 13613 | U34419a | AF160291a |
| F. temperatum | Zea mays | Belgium | MUCL 52450 | HM067695g | HM067687g |
| F. thapsinum | Sorghum bicolor | South Africa | NRRL 22045 | U34418a | AF160270a |
| F. tupiense | Mangifera indica | Brazil | CML 262 | DQ445781i | DQ452859i |
| F. udum | – | Germany | NRRL 22949 | U34433a | AF160275a |
| F. verticillioides | Zea mays | Germany | NRRL 22172 | U34413a | AF160262a |
| F. werrikimbe | Sorghum leiocladum | Australia | F19350 | EF107133f | EF107131f |
| F. xylarioides | Coffea sp. | Ivory Coast | NRRL 25486 | AY707118d | AY707136d |
| Fusarium sp. | Striga hermonthica | Madagascar | NRRL 26061 | AF160319a | AF160303a |
| – | Niger | NRRL 26152 | AF160349a | AF160306a | |
| Sorghum bicolor seed | Tanzania | NRRL 26064 | AF160346a | AF160302a | |
| Zea mays | Central America | NRRL 25221 | U61560a | AF160268a | |
| Striga hermonthica | Africa | NRRL 26793 | AF160324a | AF160309a | |
| Oryza sativa | Southeast Asia | NRRL 25615 | AF160320a | AF160304a | |
| Soil | Australia | NRRL 25807 | U61542a | AF160305a | |
| – | – | NRRL 25195 | U61558a | AF160298a | |
| Ipomoea batatas | Central America | NRRL 25346 | U61564a | AF160296a | |
| Ornamental reed | South Africa | NRRL 26756 | AF160322a | AF160307a | |
| Ornamental reed | South Africa | NRRL 26757 | AF160323a | AF160308a | |
| Palm | – | NRRL 25204 | U61559a | AF160299a | |
| Bidens pilosa | South America | NRRL 29124 | AF160326a | AF160311a | |
| Zea mays | Central America | NRRL 25622 | DQ448031a | AF160301a | |
| Triticum sp. | South Asia | NRRL 25309 | U61563a | AF160284a | |
| Oryza sativa | Southeast Asia | NRRL 25303 | U61562a | AF160283a | |
| Soil | Papua New Guinea | NRRL 26427 | AF160313a | AF160286a | |
n/a = not available.
Species for which type strains were included in the study are in boldface. The new species described in this study are indicated with *.
The abbreviations for the culture collections: CBS (Centraalbureau voor Schimmelcultures) Culture collection at the CBS-KNAW Fungal Biodiversity Centre, Utrecht, Netherlands; CML (Coleção Micológica de Lavras) Universidade Federal de Lavras, Lavras, Minas Gerais, Brazil; F (University of Sydney) Sydney, New South Wales, Australia; MRC (Medical Research Center) Tygerberg, Cape Town, South Africa; MUCL (Mycothèque de l'Université Catholique de Louvain), Louvain-la-Neuve, Belgium and NRRL (National Center for Agricultural Utilization Research) Peoria, Illinois, USA.
References for studies where DNA sequences were generated: aO'Donnell et al. 1998; bO'Donnell et al. 2000; cAoki et al. 2001; dGeiser et al. 2005; eOtero-Colina et al. 2010; fWalsh et al. 2010; gScauflaire et al. 2011; hVan Hove et al. 2011; iLima et al. 2012; jKvas et al. 2009, kMarasas et al. 1985, lNirenberg & O'Donnell 1998.
Morphology
The morphological characters of 10 isolates, two for each of the five purportedly novel species as determined by the phylogenetic analyses (see below), were studied. These isolates were as follows: F. fracticaudum (CMW 25237; CMW 25245), F. marasasianum (CMW 25253; CMW 25261), F. parvisorum (CMW 25267; CMW 25268), F. pininemorale (CMW 25243; CMW 25244) and F. sororula (CMW 25254; CMW 40578).
The morphological characteristics examined included those of the microconidia, macroconidia, and conidiophores. Measurements of microconidia and macroconidia were made using 7-d- and 14-d-old cultures grown on carnation leaf agar (CLA; 20 g/L agar Biolab Diagnostics, 5–6 carnation leaf pieces). Microscope slides were prepared for each isolate by mounting structures in 85 % (v/v) lactic acid (Sigma–Aldrich, St Louis, Missouri, USA) and 25–50 measurements were recorded for each characteristic. Microconidia and macroconidia sizes were recorded as minimum–maximum (average). Characteristics of the specimens were described based on the species descriptions of Leslie & Summerell (2006).
For all isolates, the colony reverse colour was observed on full-strength PDA after incubation at room temperature, in the dark and under near-UV light. Colony colours (surface and reverse) were described using the colour charts of Rayner (1970). Colony growth rates were assessed on full-strength PDA in 90 mm Petri plates at 10–35 °C at 5 °C intervals. Three plates were used for each culture and two measurements of colony diameter perpendicular to each were made during 8 d of incubation in the dark, after which averages were computed. Descriptions and nomenclature were deposited in MycoBank (Crous et al. 2004).
Pathogenicity
Two isolates of each of the five novel species were inoculated onto 6-mo-old P. patula seedlings in a glass house (Table 3). Isolate FCC 3579, which is a virulent strain of F. circinatum used in routine screening trials (Porter et al. 2009), was used for comparative purposes as a positive control. The inocula for the pathogenicity trial were prepared by growing the isolates on full-strength PDA for 10 d at 25 °C, after which spores were washed from the cultures using a sterile 15 % glycerol solution. These spore suspensions were filtered through cheese cloth and adjusted to a concentration of 5 × 104 spores/mL using a haemocytometer. Each isolate was inoculated on 16 seedlings by first cutting the growth tips from the tops of the seedlings, approximately 1 cm from the top, and then placing a 1 μL drop of the spore suspension onto the cut end using a pipette (Porter et al. 2009). The seedlings used for the negative controls were treated in the same manner, except that a 15 % glycerol solution replaced the spore suspension. The seedlings were arranged using a randomised block design and maintained in a greenhouse. After 6 wk, disease severity was evaluated by measuring the lesion lengths from the inoculation site to the leading edge of the lesions down the stems. The entire trial was repeated once.
Table 3.
The results of pathogenicity tests with Fusarium spp. on Pinus patula seedlings.
| Fusarium species1 | Isolate | Mean lesion length (mm)2 |
Standard error (combined) | ||
|---|---|---|---|---|---|
| Replicate 1 | Replicate 2 | Combined | |||
| F. circinatum | FCC 3579 | 49.00 (14) c | 52.53 (15) b | 50.83 (29) b | 1.76 |
| F. fracticaudum | CMW 25237 | 1.00 (15) f | 1.42 (14) f | 1.21 (29) f | 1.76 |
| CMW 25245 | 1.85 (14) f | 1.85 (13) f | 1.85 (27) f | 1.82 | |
| F. marasasianum | CMW 25253 | 1.86 (14) f | 1.71 (14) f | 1.75 (28) f | 1.79 |
| CMW 25261 | 48.33 (15) c | 41.13 (15) c | 44.73 (30) c | 1.73 | |
| F. parvisorum | CMW 25267 | 43.36 (14) d | 32.07 (15) d | 37.53 (29) d | 1.76 |
| CMW 25269 | 50.33 (15) b | 52.79 (14) b | 51.52 (29) b | 1.76 | |
| F. pininemorale | CMW 25243 | 1.5 (14) f | 1.33 (15) f | 1.41 (29) f | 1.76 |
| CMW 25244 | 1.8 (15) f | 1.33 (15) f | 1.57 (30) f | 1.73 | |
| F. sororula | CMW 25254 | 12.38 (13) e | 12.80 (15) e | 12.61 (28) e | 1.79 |
| CMW 40578 | 59.86 (15) a | 54.73 (15) a | 57.30 (30) a | 1.73 | |
| n/a | Control | 1.46 (16) f | 1.18 (16) f | 1.31 (32) f | 1.68 |
Strain numbers in boldface indicate the ex-type strains.
Values in parentheses represent total number of measurements for each treatment from which the means were calculated. A one-way ANOVA (Analysis of Variance) indicated significance between all inoculum treatments. The observed F-value was 187.48 and the significance probability associated with the F-statistic was <0.0001. Individual means were compared and grouped according to the Duncan Multiple Range Test with a confidence level of 95 %. Means that were not significantly different are indicated with the same letter.
Analysis of Variation (ANOVA) was used to determine significant differences within and between treatments for the first pathogenicity test and the Duncan Multiple Range Test was used to compare treatment differences (Onofri 2006, Zaiontz 2013, www.real-statistics.com). After conclusion of the pathogenicity trial, Koch's postulates were confirmed with re-isolations from the diseased seedling tissues and using tef1 sequence data for a representative set of isolates to confirm that the inoculated fungi were indeed responsible for the observed lesions.
Results
Isolates
A total of 57 isolates resembling those of the genus Fusarium were recovered from the diseased plant material. All isolates were collected in Colombia either from P. patula seedlings in nurseries or from trees in established plantations of P. kesiya, P. maximinoi and P. tecunumanii (Table 1). All of the trees and seedlings sampled showed similar symptoms to trees and seedlings typically infected with F. circinatum (Wingfield et al. 2008a).
Sequence analysis
Comparison of the tef1 sequences against those in the NCBI database and the Fusarium Identification Database (Fusarium-ID) (http://isolate.fusariumdb.org/) revealed that 49 of the 57 Fusarium isolates examined in this study represented members of the FFSC. Of these, 21 isolates displayed 97–99 % tef1 sequence similarity to F. circinatum. The sequences for six isolates were 98–99 % similar to that of F. begoniae and those for 22 isolates were 97–99 % similar to that of F. sterilihyphosum. Among the remaining eight isolates, two shared 98–100 % tef1 sequence similarity with members of the FOSC while six shared 98–100 % tef1 sequence similarity to members of the FSSC.
Phylogenetic analysis
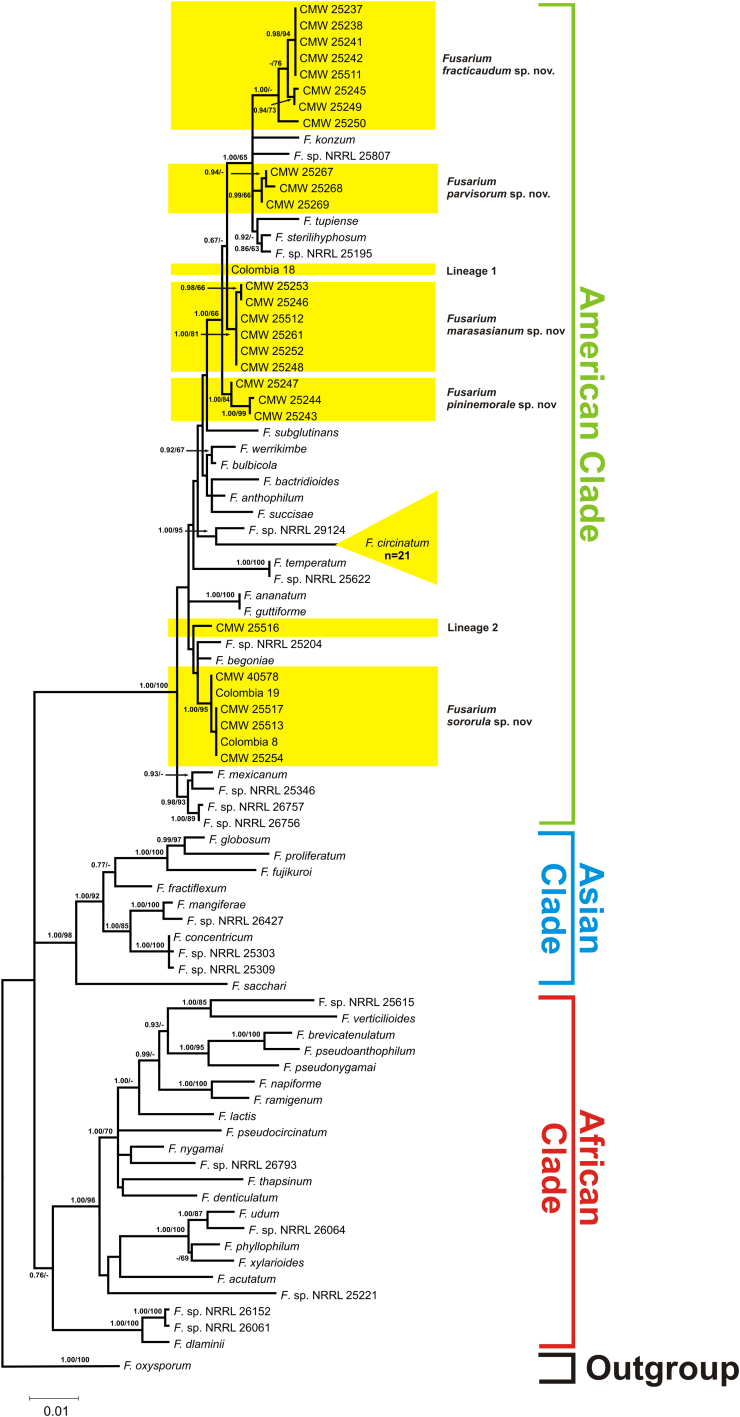
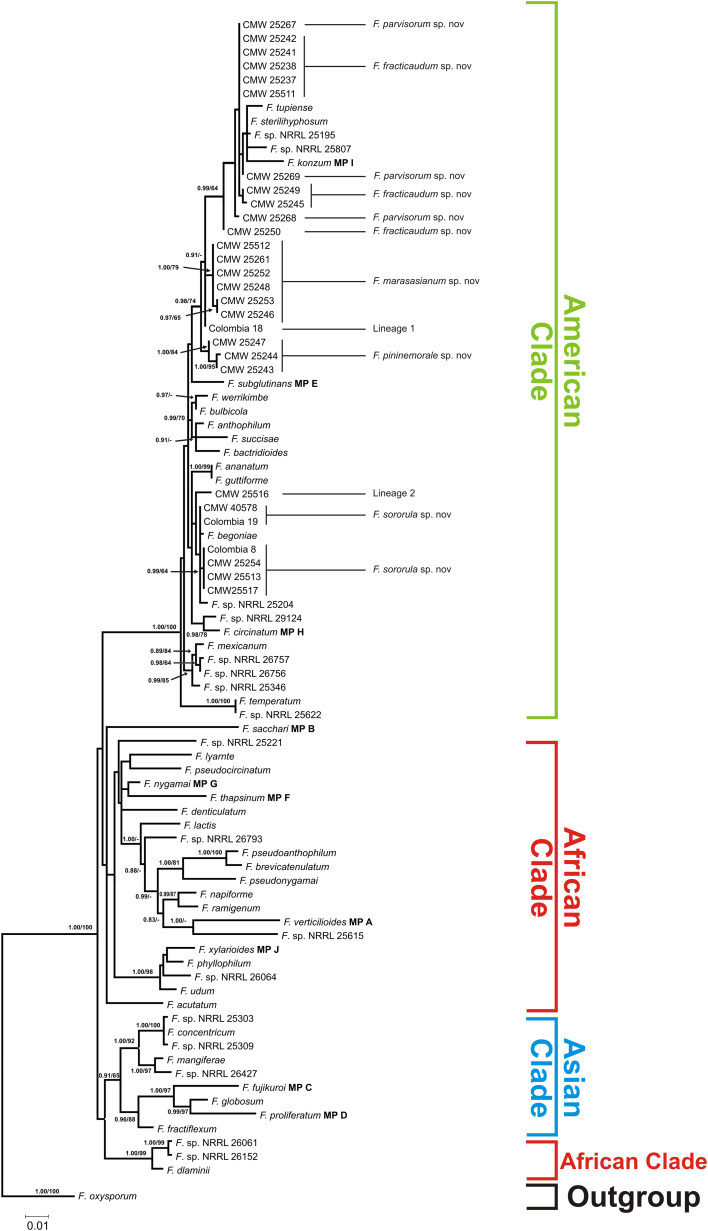
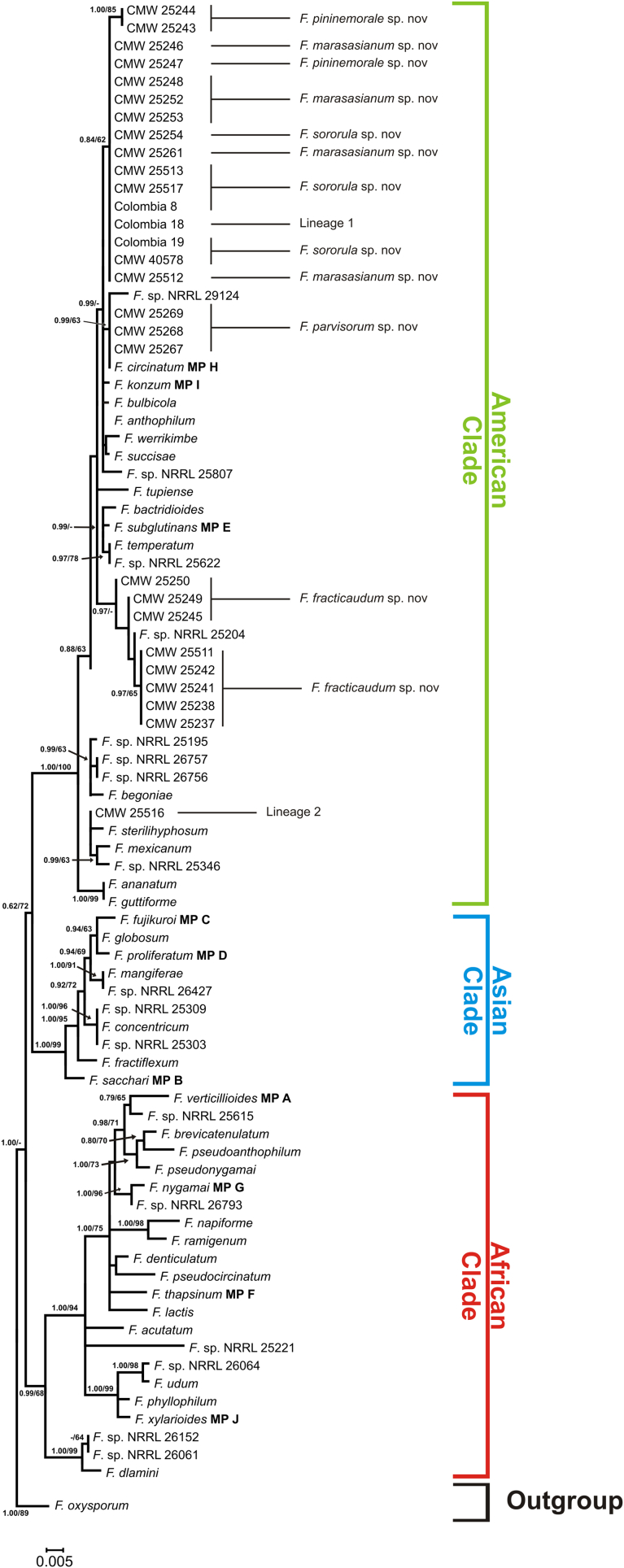
The aligned tef1 and tub2 datasets consisted of 675 and 552 nucleotides, respectively. Maximum Likelihood and BI analyses of these datasets generated trees (Fig. 1, Fig. 2, Fig. 3) with topologies resembling those previously recovered from these gene regions (O'Donnell et al., 1998, O'Donnell et al., 2000, Geiser et al., 2005) in which the FFSC is separated into three large clades (i.e., the so-called “American”, “Asian” and “African” clades). All of the 49 FFSC isolates examined in this study formed part of the “American” clade.
Fig. 1.
Maximum likelihood (ML) phylogeny of the Fusarium fujikuroi species complex (FFSC), including Fusarium isolates collected from Colombia, inferred from the combined tef1 and tub2 sequence data. The tree is rooted to F. oxysporum. A similar topology was generated using Bayesian inference (BI). The FFSC taxa are grouped into the so-called “American, “African” and “Asian” clades (O'Donnell et al. 1998). The blocks indicate the five novel species and two phylogenetic lineages identified in this study. Bootstrap support values (>60 %) for ML and Bayesian posterior probabilities (>0.6) are indicated at the internodes in the order BI/ML. Branches with bootstrap support values less than 60 % or posterior probability values less than 0.6 are indicated with a “-”. NRRL, ARS Culture collection Peoria, IL, USA.
Fig. 2.
Maximum likelihood phylogeny of the Fusarium fujikuroi species complex (FFSC), including the isolates collected from Colombia, inferred from the tef1 sequence data. The tree is rooted to F. oxysporum and a similar topology was obtained using Bayesian inference. Branch support, as well as clade and isolate information are indicated as detailed in the legend of Fig. 1.
Fig. 3.
Maximum likelihood phylogeny of the Fusarium fujikuroi species complex (FFSC), including the isolates collected from Colombia, inferred from the tub2 sequence data. The tree is rooted to F. oxysporum and a similar topology was obtained using Bayesian inference. Branch support, as well as clade and isolate information are indicated as detailed in the legend of Fig. 1.
Analyses of the combined sequence dataset separated the isolates from Colombia into eight distinct groups. Of these, only one corresponded to a known species (i.e., F. circinatum). The remaining seven lineages appeared to represent novel species based on the fact that the isolates did not cluster with any known FFSC species. Because of the limited resolving power of most single-gene phylogenetic analyses of the FFSC, not all eight groups were recovered from the respective individual tef1 and tub2 phylogenies (Fig. 2, Fig. 3), although they were not incongruent with those supported by the combined dataset (Fig. 1). Application of a modified version of Nixon & Wheeler's (1990) phylogenetic species concept indicated that the seven lineages identified for the Colombian isolates could be recognised as distinct species. This species concept essentially defines species as diagnosable groups on phylogenetic trees, for example, and is commonly employed for taxonomic studies on the FFSC (O'Donnell et al., 1998, O'Donnell et al., 2000, Geiser et al., 2005). In this study, descriptions are provided for five lineages that included multiple representatives (see below). Lineages 1 and 2 were represented by inordinately few isolates to justify describing them at the present time.
In general, the results of the BLAST analyses were not mirrored in the phylogenies, because isolates that had sequences similar to those of F. sterilihyphosum did not group closely with this species and were rather scattered into five phylogenetic lineages throughout the American clade (Fig. 1). Also, isolates that had sequence similarity to F. begoniae formed part of a group that did not include this species. Isolates that had a 99–100 % sequence similarity with F. circinatum were the only isolates that grouped with the type strain of any species. This general lack of consistency between the results of BLAST and phylogenetic analyses highlights the limitations associated with using sequence similarity alone for diagnosing novel species (e.g., Kang et al., 2010, Hibbett et al., 2011, Boykin et al., 2012).
Taxonomy
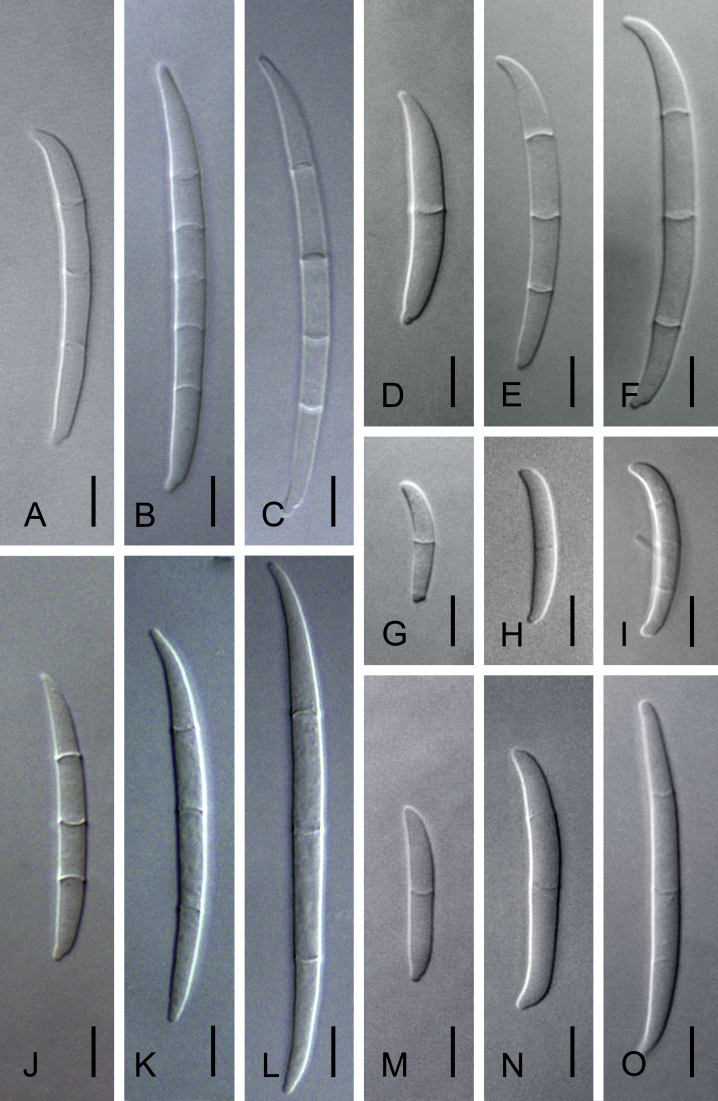
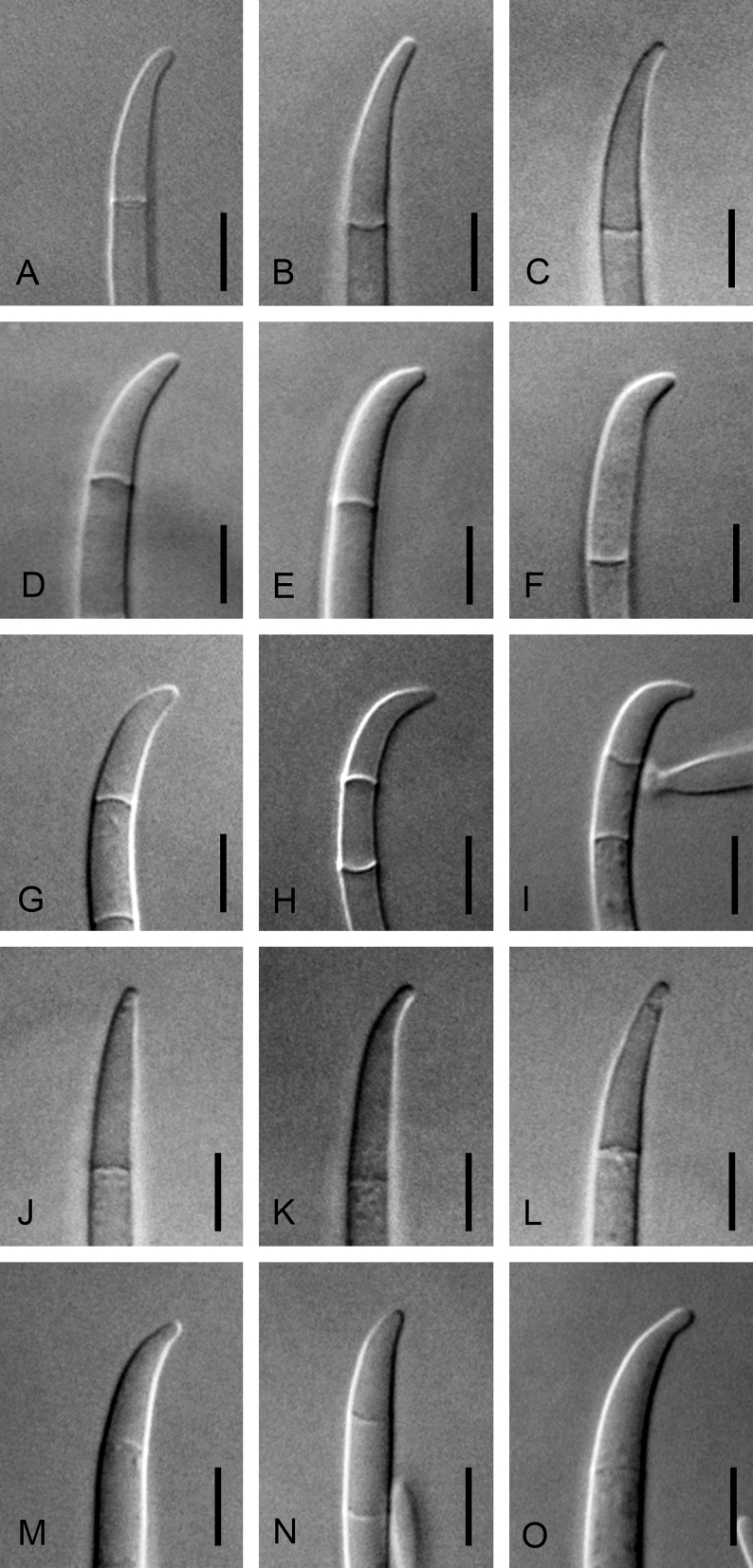
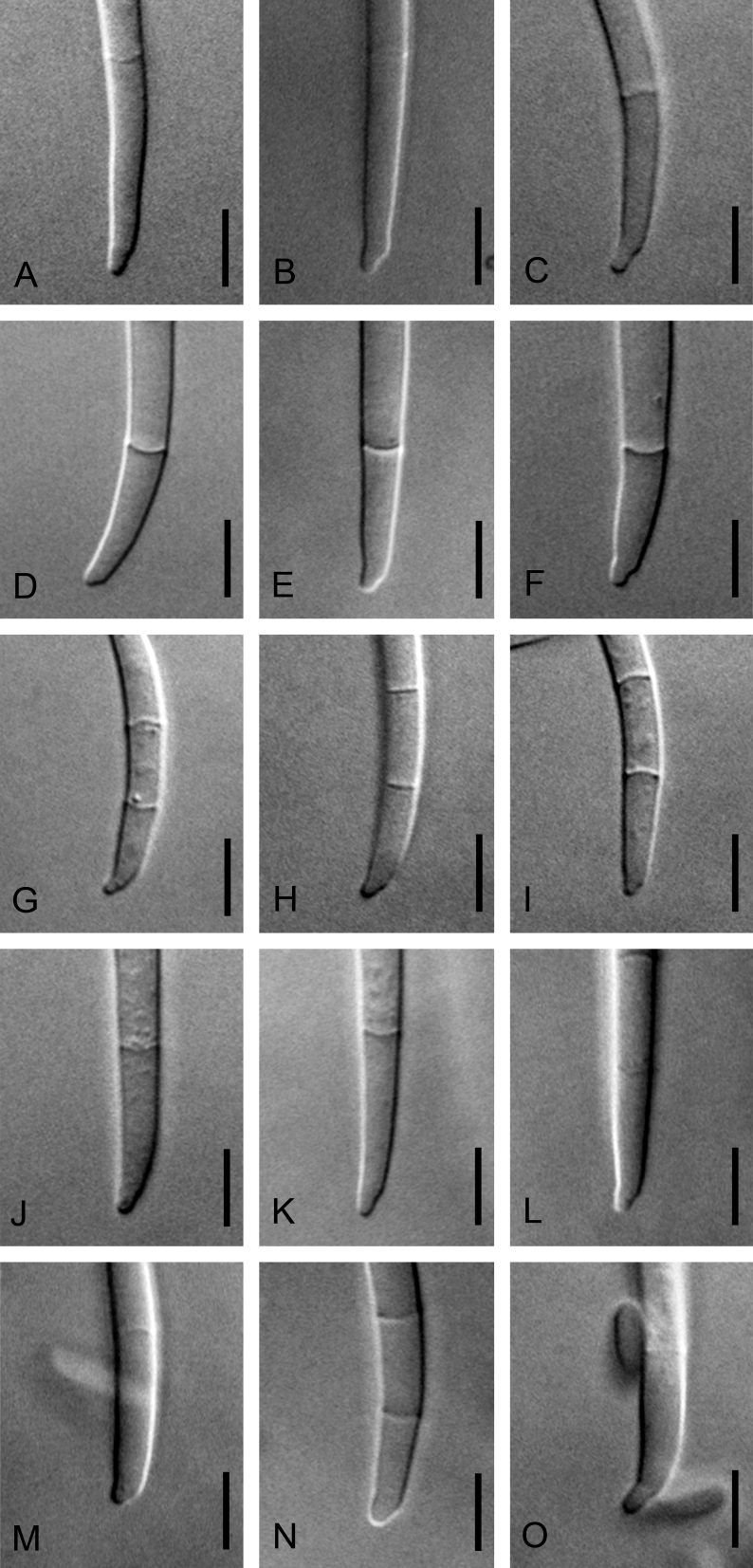
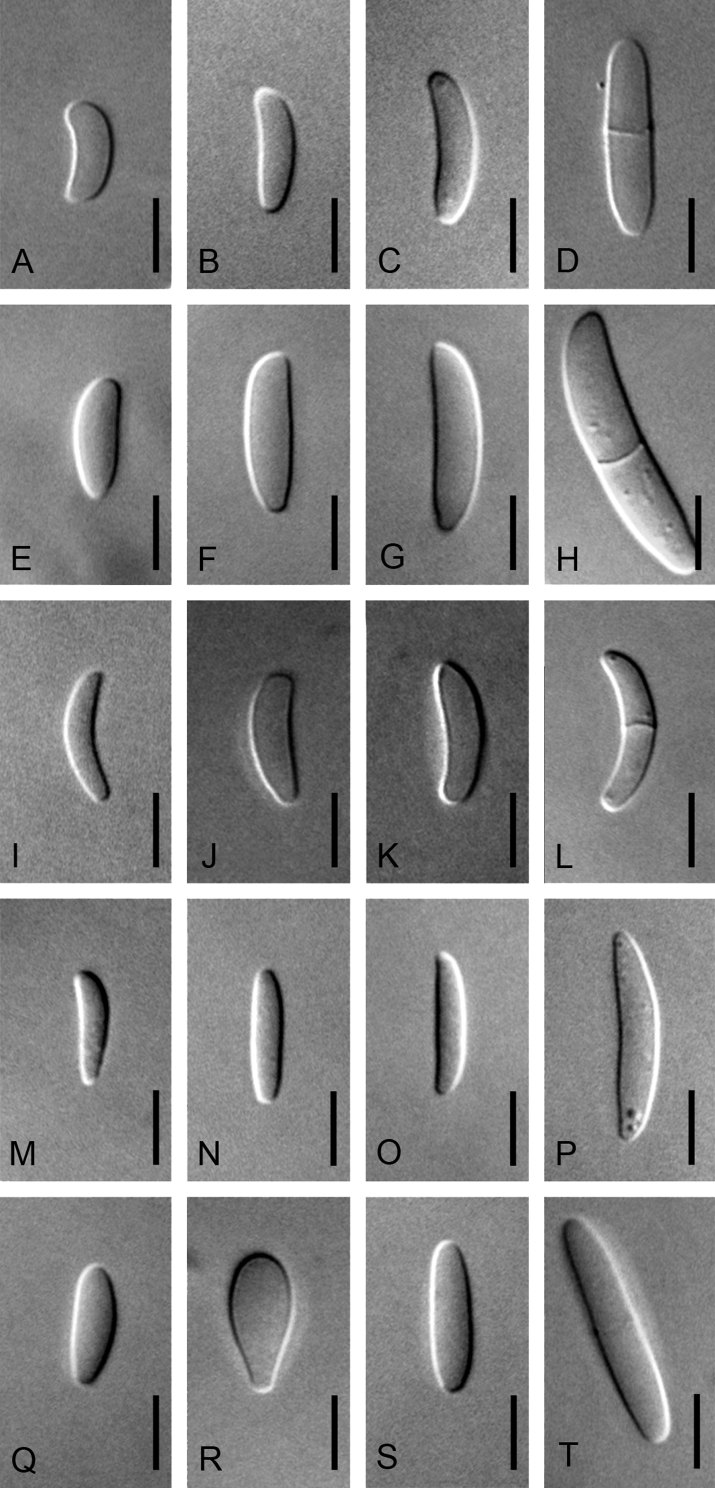
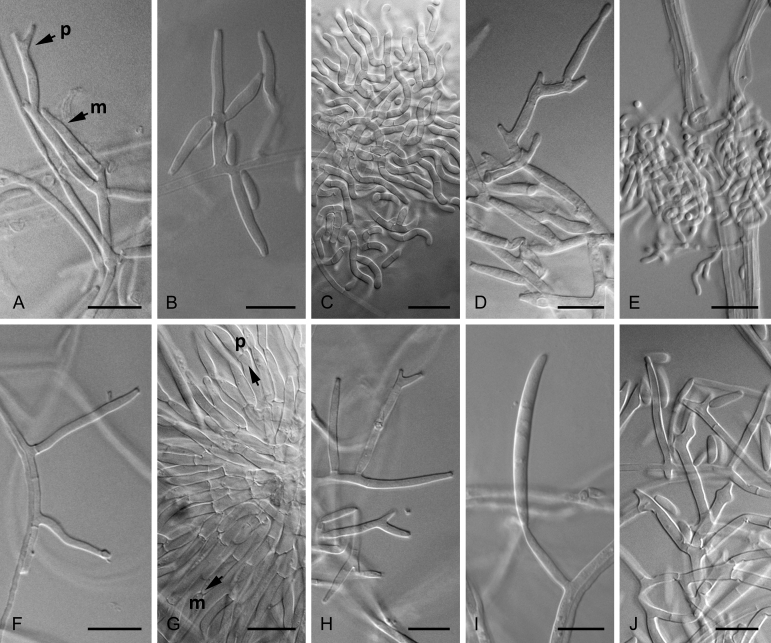
Morphological characters used to distinguish the five novel species included colony colour and conidial size, shape, septation and arrangement (Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8). Although the isolates shared an optimum growth temperature (i.e., 25 °C), there were differences in their average growth/d, which ranged from 6 to 15.4 mm/d (Table 4). The isolates also differed morphologically from F. sterilihyphosum, F. begonia and F. circinatum. Based on the results of both the phylogenetic and morphological analyses, five distinct novel species in the FFSC are described below.
Fig. 4.
Variation observed in size and shape of macroconidia produced by Fusarium fracticaudum sp. nov. (A–C), Fusarium marasasianum sp. nov. (D–F), Fusarium parvisorum sp. nov. (G–I), Fusarium pininemorale sp. nov. (J–L) and Fusarium sororula sp. nov. (M–O). Scale bar = 5 μm.
Fig. 5.
Variation observed in apical cells produced by Fusarium fracticaudum sp. nov. (A–C), Fusarium marasasianum sp. nov. (D–F), Fusarium parvisorum sp. nov. (G–I), Fusarium pininemorale sp. nov. (J–L) and Fusarium sororula sp. nov. (M–O). Scale bar = 5 μm.
Fig. 6.
Variation observed in basal foot cells produced by Fusarium fracticaudum sp. nov. (A–C), Fusarium marasasianum sp. nov. (D–F), Fusarium parvisorum sp. nov. (G–I), Fusarium pininemorale sp. nov. (J–L) and Fusarium sororula sp. nov. (M–O). Scale bar = 5 μm.
Fig. 7.
Variation observed in the size and shape of microconidia produced by Fusarium fracticaudum sp. nov. (A–D), Fusarium parvisorum sp. nov. (E–H), Fusarium marasasianum sp. nov. (I–L), Fusarium pininemorale sp. nov. (M–P) and Fusarium sororula sp. nov. (Q–T). Scale bar = 5 μm.
Fig. 8.
Monophialidic (m) and polyphialidic (p) conidiogenous cells, as well as circinate hyphae of the species described in this study. Microconidia produced by Fusarium fracticaudum sp. nov. on mono- and polyphialides (A, B), circinate hyphae and microconidia produced by Fusarium parvisorum sp. nov. (C, D) and Fusarium marasasianum sp. nov. (E, F), macroconidia borne on mono- and polyphialides (G) and microconidia borne on monophialides (H) produced by Fusarium pininemorale sp. nov., and the condiogenous cells of Fusarium sororula sp. nov. bearing macroconidia (I) and microconidia (J). Scale bar: A, B, D, F–J = 10 μm, C, E = 25 μm.
Table 4.
The results of the growth studies conducted on F. fracticaudum, F. marasasianum, F. parvisorum, F. pininemorale and F. sororula.
| Species1 | Isolate number | Growth (mm) at various incubation temperatures after 8 d2 |
Growth/d 25 °C3 | |||||
|---|---|---|---|---|---|---|---|---|
| 10 °C | 15 °C | 20 °C | 25 °C | 30 °C | 35 °C | |||
| Fusarium fracticaudum | CMW 25237 | 20.83 | 32 | 51.83 | 46.33 | 20.83 | 0 | 6.89 |
| CMW 25245 | 11.96 | 36.5 | 56.65 | 67 | 13.33 | 0 | 9.09 | |
| F. marasasianum | CMW 25253 | 15.8 | 45.83 | 68.34 | 80 | 52.33 | 0 | 11.43 |
| CMW 25261 | 13.17 | 46.83 | 80 | 80 | 48.67 | 0 | 15.43 | |
| F. parvisorum | CMW 25267 | 15.83 | 41.67 | 71.83 | 80 | 52.17 | 0 | 11.43 |
| CMW 25268 | 15.97 | 43.83 | 75.5 | 80 | 44 | 0 | 13.33 | |
| F. pininemorale | CMW 25243 | 17 | 33.75 | 45.6 | 51.83 | 41.33 | 0 | 6 |
| CMW 25244 | 22.17 | 40.33 | 57.13 | 76.75 | 44 | 0 | 10.17 | |
| F. sororula | CMW 25254 | 14.66 | 31.33 | 48.8 | 62 | 40.66 | 0 | 7.48 |
| CMW 40578 | 11.33 | 44 | 66.83 | 80 | 47.16 | 0 | 11.43 | |
Strain numbers in boldface indicate the ex-type strains.
Agar plates are 80.0 mm diam.
Average growth per day was recorded at 25 °C, the optimum temperature for growth.
Fusarium fracticaudum Herron, Marinc. & M.J. Wingf., sp. nov. MycoBank MB809885. Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8.
Etymology: From fracti (Latin for broken or bent) and caudum (Latin for tail) to describe the “broken tail” of the skewed macroconidial foot cell.
Macroconidia abundant, elongate, straight, 38–63.5 × 2.5–4.5 μm (av. 47.6 × 3.3 μm), with 3–5 septa, apical cells tapering, curved, 9–15 μm long (av. 12.2 μm), basal cells distinctly notched to foot-shaped, 9–14.5 μm long (av. 11.8 μm). Microconidia abundant, fusiform to obovoid, occasionally curved, 8–13 × 1.5–3 μm (av. 9.9 × 2.3 μm), with 0–1 septum. Conidiogenous cells monophialidic or polyphialidic, 11–23.5 μm long, microconidia arranged in false heads.
Culture characteristics: Colonies showing optimal growth at 25 °C with an average growth rate of 6.9 mm/d (CMW 25237) and 9.1 mm/d (CMW 25245). Colony reverse in the dark more or less uniformly fulvous or in near-UV uniformly buff.
Habitat: Stem canker on mature Pinus maximinoi trees.
Distribution: Angela Maria (Santa Rosa) and Calima (Darien Valle) Colombia, South America.
Materials examined: Colombia, Angela Maria (Santa Rosa), Risaralda (75°36′21″ W and 4°49′18″ N), P. maximinoi, 2007, M.J. Wingfield & C.A. Rodas (holotype PREM 60895, ex-type culture CMW 25245 = CBS 137233); Calima (Darien Valle), Colombia (76°26′03″ W and 3°56′57″ N), P. maximinoi, 2007, M.J. Wingfield & C.A. Rodas, (paratype PREM 60894, living culture, CMW 25237 = CBS 137234).
Fusarium marasasianum Herron, Marinc. & M.J. Wingf. sp. nov. MycoBank MB809887. Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8.
Etymology: Named for the late Professor W.F.O. Marasas who dedicated the greater part of his professional life to the study of Fusarium spp. and mentored many students, including the authors of this study.
Macroconidia abundant, elongate, straight, 23.5–44.5 × 2.5–4 μm (av. 34.8 × 3.1 μm), with 0–3 septa, apical cells tapering, curved or hooked, 7–14 μm long (av. 10.4 μm), basal cells not well-developed, barely to distinctly notched or foot-shaped, 6.5–12 μm long (av. 9.2 μm). Microconidia scarce, fusiform to obovoid, 7.5–18 × 2–3.5 μm (av. 11.4 × 2.7 μm), with 0–1 septum. Conidiogenous cells monophialidic or polyphialidic, 9–27 × 2–3.5 μm long, microconidia arranged in false heads. Other characteristics include the presence of circinate hyphae.
Culture characteristics: Colonies showing optimal growth at 25 °C with an average growth rate of 11.4 mm/d (CMW 25253) and 15.4 mm/d (CMW 25261). Colony reverse in the dark unpigmented with spots of purple or in near UV light entirely dark purple but with less intensity.
Habitat: Diseased roots of Pinus patula seedlings.
Distribution: Vivero, Peňas Negra, Valle del Cauca, Colombia, South America.
Materials examined: Colombia, Vivero, Peňas Negra, Valle del Cauca, (76°29′49″ W and 3°51′45″ N), Pinus patula, 2007, M.J. Wingfield & C.A. Rodas (holotype PREM 60899, ex-type culture, CMW 25261 = CBS 137238); Pinus patula 2007, M.J. Wingfield & C.A. Rodas, (paratype PREM 60898, ex-paratype culture, CMW 25253 = CBS 137237).
Fusarium parvisorum Herron, Marinc. & M.J. Wingf., sp. nov. MycoBank MB809886. Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8.
Etymology: From parvi (Latin for small) and sorum (Latin for spore), describing the small macroconidia produced by this species.
Macroconidia not abundant, squat, straight, 12.5–29.5 × 1.5–3 μm (av. 19.1 × 2.3 μm), with 1–3 septa, apical cells hooked, 4.5–10.5 μm long (av. 7.1 μm), basal cells not well developed, barely to distinctly notched, 4.5–12 μm long (av. 7.6 μm). Microconidia not abundant, fusiform to obovoid, 7–13 × 1.5–3 μm (av. 9.7 × 2.5 μm), with 0–1 septum. Conidiogenous cells monophialidic or polyphialidic, 5.5–27 × 1.5–3 μm long, microconidia arranged in false heads. Other characteristics include the presence of circinate hyphae.
Culture characteristics: Colonies showing optimal growth at 25 °C with an average growth of 11.4 mm/d (CMW 25267) and 13.3 mm/d (CMW 25268). Colony reverse in the dark and near-UV light unpigmented.
Habitat: Diseased roots of Pinus patula seedlings.
Distribution: Vivero, Peňas Negra, Valle del Cauca, Colombia, South America.
Materials examined: Colombia, Vivero, Peňas Negra, Valle del Cauca, (76°29′49″ W and 3°51′45″ N), Pinus patula, 2007, M.J. Wingfield & C.A. Rodas (holotype PREM 60897, ex-type culture CMW 25267 = CBS 137236); P. patula, 2007, M.J. Wingfield & C.A. Rodas, (paratype PREM 60896, ex-paratype culture, CMW 25268 = CBS 137235).
Fusarium pininemorale Herron, Marinc. & M.J. Wingf., sp. nov. MycoBank MB809888. Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8.
Etymology: From pin (from pine), the host of this species and nemorale (from nemoralis which is Latin for a “collection” or “group”), thus describing the fact that this species was isolated from a group of pines or pine plantation.
Macroconidia abundant, elongate, straight, 35–52 × 2–3.5 μm (av. 42.2 × 2.9 μm), with 3–4 septa, apical cells tapering, curved, 8.5–14 μm long (av. 12.0 μm), basal cells foot-shaped, elongated foot shape, barely to distinctly notched, 9–14 μm long (av. 11.0 μm). Microconidia scarce, fusiform to obovoid, 5–16.5 × 1.5–3 μm (av. 10.1 × 2.2 μm), 0–1 septa. Conidiogenous cells monophialidic or polyphialidic, 6.5–32 × 2–3.5 μm long, microconidia arranged in false heads.
Culture characteristics: Colonies showing optimal growth at 25 °C with an average growth rate of 6 mm/d (CMW 25243) and 10.2 mm/d (CMW 25244). Colony reverse in the dark and near-UV light unpigmented.
Habitat: Stem canker on Pinus tecunumanii.
Distribution: Angela Maria (Santa Rosa), Risaralda, Colombia, South America.
Materials examined: Colombia, Angela Maria (Santa Rosa), Risaralda (75°36′21″ W and 4°49′18″ N), Pinus tecunumanii, 2007, M.J. Wingfield & C.A. Rodas, (holotype PREM 60901, ex-type culture, CMW 25243 = CBS 137240); Pinus tecunumanii, 2007, M.J. Wingfield & C.A. Rodas, (paratype PREM 60900, ex-paratype culture, CMW 25244 = CBS 137239).
Fusarium sororula Herron, Marinc. & M.J. Wingf., sp. nov. MycoBank MB809889. Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8.
Etymology: From soror- (Latin for sister) and sororula (diminutive: little sister). This name depicts the fact that this species produces small macroconidia similar to its sister species, F. parvisorum, also described in this study.
Macroconidia scarce, elongate, straight, 20–42.5 × 2–4 μm (av. 28.7 × 2.9 μm), with 1–3 septa, apical cells hooked, 7.5–12.5 μm long (av. 9.3 μm), basal cells foot-shaped, elongated foot shape, barely to distinctly notched, 7–12.5 μm long (av. 9.1 μm), some producing secondary conidia. Microconidia abundant, fusiform to obovoid or pyriform, 5.5–15.5 × 1.5–3 μm (avg. 8.1 × 2.2 μm), with 0–1 septum. Conidiogenous cells monophialidic or polyphialidic, 10.9–34 μm long, microconidia arranged in false heads.
Culture characteristics: Colonies showing optimal growth at 25 °C at an average growth rate of 7.5 mm per d (CMW 25254) and 11.4 mm per d (CMW 40578). Colony reverse in the dark with patches, sectors or entire area of purple or dark purple or in near-UV light with patches of partly covered with purple or dark purple.
Habitat: Stem canker on Pinus patula.
Distribution: Angela Maria (Santa Rosa), Risaralda, Colombia, South America.
Materials examined: Colombia, Angela Maria (Santa Rosa), Risaralda (75°36′21″ W and 4°49′18″ N), Pinus patula, 2007, M.J. Wingfield & C.A. Rodas (holotype PREM 60903, ex-type culture CMW 40578 = CBS 137242); Pinus patula, 2007, M.J. Wingfield & C.A. Rodas, (paratype PREM 60902, ex-paratype culture, CMW 25254 = CBS 137241).
Pathogenicity
In the pathogenicity trial, five of the 11 Fusarium isolates used to inoculate 6-m-old P. patula trees produced lesions that were significantly larger (P < 0.0001) than those recorded for the negative controls (Table 3). These included the two examined isolates of F. parvisorum (CMW 25269 and CMW 25267) and F. sororula (CMW 25254 and CMW 40578), as well as one isolate of F. marasasianum (CMW 25261). In most of these cases, the lesions produced were within the same size range as those observed for F. circinatum (Table 3). All of these fungi were re-isolated from the inoculated plants, fulfilling Koch's postulates, while no Fusarium spp. were isolated from control plants.
Discussion
In this study, more than 10 distinct Fusarium spp. were recovered from Pinus tissue showing symptoms of infection similar to those found for F. circinatum. These included the pitch canker fungus itself, the five newly described species F. fracticaudum, F. marasasianum, F. parvisorum, F. pininemorale, F. sororula, and two undescribed species in the FFSC and isolates belonging to the FOSC (Baayen et al. 2000) and FSSC (O'Donnell, 2000, Zhang et al., 2006). Of these, only F. circinatum was known as a primary pathogen having an established association with Pinus, prior to the present study (Nirenberg and O'Donnell, 1998, Gordon, 2006, Wingfield et al., 2008a). Given the small number of sites examined in this study and the recovery of five new species, an expanded survey of the fusaria associated with Pinus in Colombia and surrounding countries would likely yield even more novel taxa, of which some might represent significant threats to forestry worldwide.
The distribution of the Fusarium species examined in this study varied in terms of host and tissue type from which they were recovered. Like F. circinatum, we isolated F. marasasianum and FSSC spp. from nursery seedlings and from cankers on established plantation trees. Isolates of F. fracticaudum, F. pininemorale and F. sororula were isolated from plantation trees only and isolates of F. oxysporum and F. parvisorum only from nursery seedlings. Also, F. circinatum, F. fracticaudum, F. marasasianum, F. sororula and FSSC spp. were isolated from more than one Pinus spp., while F. pininemorale and FOSC spp. were restricted to P. tecunumanii. Apart from the two putative novel Fusarium spp. represented by single isolates, all species examined here were also recovered from more than one location in Colombia.
The recovery of isolates residing in the FOSC and FSSC complexes was not unexpected as they are known to harbour plant pathogens (Baayen et al., 2000, O'Donnell et al., 2000, Zhang et al., 2006). For example, isolates from both complexes have been associated with diseased Pinus strobus seed and seedlings (Riffle and Strong, 1960, Enebak, 1988, Ocamb and Juzwik, 1995) and Pinus radiata seedlings in bare root nurseries (Dick & Dobbie 2002). However, the symptoms induced by these fusaria typically do not resemble those of the pitch canker fungus (Ocamb and Juzwik, 1995, Dick and Dobbie, 2002, Wingfield et al., 2008a). Their recovery from the Pinus tissues in this study is likely to be a consequence of the fact that members of these two species complexes are often saprobes with ubiquitous distributions (Burgess 1981).
Apart from FOSC and FSSC isolates, all of the Fusarium spp. included in this study form part of the “American” clade (sensu O'Donnell et al. 1998) of the FFSC. The emergence of this clade, together with the so-called “Asian” and “African” clades was initially suggested by O'Donnell et al. (1998) to be due to fragmentation of Gondwana during the upper Cretaceous through to the Paleocene. However, the same authors later reported that the complex emerged more recently (ca. 8.8 M yr ago) and that the apparent biogeographic clustering is probably due to long distance dispersal from South America to Africa and then to Asia in the late Miocene (O'Donnell et al. 2013). Nevertheless, the members of the respective clades are generally associated with hosts that have their origin in the specific geographic areas. For example, the “American” clade species F. circinatum and F. subglutinans are thought to have evolved with their hosts (i.e., Pinus and Zea spp., respectively) in Mexico and Central America (Gaut and Doebley, 1997, Iltis, 2000, Wikler and Gordon, 2000). These fungi were then introduced with their hosts to other regions as part of the global development and expansion of agriculture and forestry (Desjardins et al., 2000, Wingfield et al., 2008a). Following this view, it is possible that the new Fusarium spp. identified in this study also originated from Mexico and Central America, because these regions represent centres of origin for many Pinus spp. (Millar 1993).
An alternative hypothesis is that the new species recognised in this study are native on other host plant species in Colombia. This would then suggest that the Fusarium spp. have undergone host shifts to Pinus spp. from other hosts. This is plausible as the phenomenon of host jumping (Slippers et al. 2005) occurs frequently in environments where native ecosystems and exotic monoculture-based forestry or agriculture exist in close association (Burgess and Wingfield, 2004, Stenlid et al., 2011). Furthermore, these host jumps occur more readily when the host species are related. This has been shown, for example, for Chrysoporthe austroafricana (Gryzenhout et al. 2004), which is native to southern Africa and associated with native Myrtales (Heath et al. 2006), but can cause comparable (and often more severe) symptoms on exotic Eucalyptus spp. planted in intensively managed plantations (Nakabonge et al. 2006). Another example is myrtle rust caused by Puccinia psidii, which is native on many Myrtaceae in Latin America but has jumped to Eucalyptus spp. planted as exotics to establish forest plantations (Coutinho et al., 1998, Glen et al., 2007). Future studies should thus seek to understand the host range and centres of origin of the newly identified species, which would in turn reveal the potential risks these fusaria pose to conifers and other gymnosperms native to the Colombia.
Results of this study showed that the new species, F. marasasianum, F. parvisorum and F. sororula all have the ability to cause disease in P. patula seedlings. These fungi induced lesions in seedlings that were as large as or larger than those caused by a virulent isolate of F. circinatum. However, we observed some level of variation in pathogenicity and virulence between the isolates of the same species. Although such variation in the ability of isolates to cause disease is well documented for plant pathogenic Fusarium spp. (Burgess, 1981, Gordon and Okamoto, 1992, Appel and Gordon, 1995, Miedaner et al., 2001, Carter et al., 2002), an important aspect of our results is that Pinus spp. in Colombia are infected with additional Fusarium pathogens as aggressive as F. circinatum. This has significant implications for commercial forestry in Colombia and elsewhere where Pinus spp. are planted as non-natives or where they occur naturally. In general, the susceptibility of planting stock to these new pathogens will need to be evaluated, by following approaches similar to those used for F. circinatum (e.g., Roux et al., 2007, Mitchell et al., 2011). Suitable control strategies will also have to be developed, although this will require detailed knowledge regarding the distribution, host range and ecology of the newly recognised pathogens.
Studies such as this one where new Fusarium pathogens of Pinus spp. have been discovered are important, not only to diagnose new diseases, but to improve global quarantine measures and thus contain their potential spread to new areas. For example, strategies can now be developed to identify and track the possible movement of the apparently aggressive pathogens F. marasasianum, F. parvisorum and F. sororula in Colombia and possibly elsewhere in the world. Active monitoring of these areas is of particular importance for forest industries where rotation periods are especially long. This implies that they are exposed to pests and pathogens for extended periods of time and where problems emerge, the consequences can be dire. But even where early detection is achieved, the appearance of new tree diseases is difficult to treat or prevent. In the case of F. circinatum, 70 yr after its discovery, it has spread to more than ten countries on five different continents (reviewed by Steenkamp et al. 2012) and losses remain very serious in some areas.
Acknowledgements
We thank members of the Tree Protection Co-Operative Programme (TPCP), the National Research Foundation (NRF) and the THRIP Initiative of the Department of Trade and Industry, South Africa for financial support. Sappi and York timbers provided seedlings to conduct the pathogenicity trials for which we are grateful. We thank the staff of the DNA sequencing facility at the University of Pretoria for assistance with DNA sequencing and we are grateful to Dr Hugh Glen for suggesting names for the new Fusarium spp. This paper is dedicated to Prof. Wally F.O. Marasas (deceased), recognising his long-standing encouragement and his passion not only for Fusarium but also for the students and researchers studying these fungi.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
References
- Altschul S.F., Gish W., Miller W. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anagnostakis S.L. The effect of multiple importations of pests and pathogens on a native tree. Biological Invasions. 2001;3:245–254. [Google Scholar]
- Aoki T., O'Donnell K., Ichikawa K. Fusarium fractiflexum sp. nov. and two other species within the Gibberella fujikuroi species complex recently discovered in Japan that form aerial conidia in false heads. Mycoscience. 2001;42:461–478. [Google Scholar]
- Appel D.J., Gordon T.R. Intraspecific variation within populations of Fusarium oxysporum based on RFLP analysis of the Intergenic Spacer Region of the rDNA. Experimental Mycology. 1995;19:120–128. doi: 10.1006/emyc.1995.1014. [DOI] [PubMed] [Google Scholar]
- Ayala L., Avi P., Jaime K. Invasion of Pinus halepensis from plantations into adjacent natural habitats. Applied Vegetation Science. 2005;8:85–92. [Google Scholar]
- Baayen R.P., O'Donnell K., Bonants P.J.M. Gene genealogies and AFLP analyses in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae speciales causing wilt and rot diseases. Phytopathology. 2000;90:891–900. doi: 10.1094/PHYTO.2000.90.8.891. [DOI] [PubMed] [Google Scholar]
- Blitzer E.J., Dormann C.F., Holzschuh A. Spillover of functionally important organisms between managed and natural habitats. Agriculture Ecosystems and Environment. 2012;146:34–43. [Google Scholar]
- Boykin L.M., Armstrong K.F., Kubatko L. Species delimitation and global biosecurity. Evolutionary Bioinformatics. 2012;8:1–37. doi: 10.4137/EBO.S8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw R.E., Ganley R.J., Jones W.T. High levels of dothistromin toxin produced by the forest pathogen Dothistroma pini. Mycological Research. 2000;104:325–332. [Google Scholar]
- Burgess T.I. General ecology. In: Nelson P.E., Toussoun T.A., Cook R.J., editors. Fusarium: diseases, biology and taxonomy. Pennsylvania State University Press; University Park and London, USA: 1981. pp. 225–235. [Google Scholar]
- Burgess L.W., Wingfield M.J. Impact of fungal pathogens in natural forest ecosystems: a focus on Eucalyptus. In: Sivasithamparam K., Dixon K.W., Barrett R.L., editors. Microorganisms in plant conservation and biodiversity. Kluwer Academic Publishers; 2004. pp. 283–306. [Google Scholar]
- Carter J.P., Rezanoor H.N., Holden D. Variation in pathogenicity associated with the genetic diversity of Fusarium graminearum. European Journal of Plant Pathology. 2002;108:573–583. [Google Scholar]
- Chou C.K.S. Perspectives of disease threat in large-scale Pinus radiata monoculture – the New Zealand experience. European Journal of Forest Pathology. 1991;21:71–81. [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Coutinho T.A., Wingfield M.J., Alfenans A.C. Eucalyptus rust: a disease with the potential for serious international implications. Plant Disease. 1998;82:819–825. doi: 10.1094/PDIS.1998.82.7.819. [DOI] [PubMed] [Google Scholar]
- Darriba D., Taboada G.L., Doalla R. jModeltest 2: more models, new heuristics and parallel computing. Nature Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins A.E., Plattner R.D., Gordon T.R. Gibberella fujikuroi mating population A and Fusarium subglutinans from teosinte species and maize from Mexico and Central America. Mycological Research. 2000;104:856–872. [Google Scholar]
- Dick M.A., Dobbie K. Species of Fusarium on Pinus radiata in New Zealand. New Zealand Plant Protection. 2002;55:58–62. [Google Scholar]
- Enebak S.A. University of Minnesota; St. Paul, United States of America: 1988. Control of soilborne pathogens in forest tree nurseries. M.Sc thesis. [Google Scholar]
- FAO . 2005. Global forest resource assessment. Forest area statistics for Colombia. [Google Scholar]
- FAO . 2010. Global forest resource assessment. Forest area statistics for Colombia. [Google Scholar]
- FAO . 2012. State of forestry in the Latin American and Caribbean region: period 2010–2011. Latin American and Caribbean Forestry Commission, 27th session. [Google Scholar]
- Fisher M.C., Henk D.A., Briggs C.J. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484:186–223. doi: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnas J.R., Hurley B.P., Slippers B. Biological control of forest plantation pests in an interconnected world requires greater international focus. International Journal of Pest Management. 2012;58:211–223. [Google Scholar]
- Gaut B.S., Doebley J.F. DNA sequence evidence for the segmental allotetraploid origin of maize. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6809–6814. doi: 10.1073/pnas.94.13.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser D.M., Aoki T., Bacon C.W. One fungus, one name: defining the genus Fusarium in a scientifically robust way that preserves longstanding use. Phytopathology. 2013;103:400–408. doi: 10.1094/PHYTO-07-12-0150-LE. [DOI] [PubMed] [Google Scholar]
- Geiser D.M., Jiménez-Gasco M., Kang S. FUSARIUM-ID v. 1.0: a DNA sequence database for identifying Fusarium. European Journal of Plant Pathology. 2004;110:473–479. [Google Scholar]
- Geiser D.M., Ivey M.L.L., Hakiza G. Gibberella xylarioides (anamorph: Fusarium xylarioides), a causative agent of coffee wilt disease in Africa, is a previously unrecognized member of the G. fujikuroi species complex. Mycologia. 2005;97:191–201. doi: 10.3852/mycologia.97.1.191. [DOI] [PubMed] [Google Scholar]
- Glen M., Alfenas A.C., Zauza E.A.V. Puccinia psidii: a threat to the Australian environment and economy – a review. Australasian Plant Pathology. 2007;36:1–16. [Google Scholar]
- Gordon T.R. Pitch canker disease of pines. Phytopathology. 2006;96:657–659. doi: 10.1094/PHYTO-96-0657. [DOI] [PubMed] [Google Scholar]
- Gordon T.R., Okamoto D. Population structure and the relationship between pathogenic and nonpathogenic strains of Fusarium oxysporum. Phytopathology. 1992;82:73–77. [Google Scholar]
- Gordon T.R., Storer A.J., Wood D.L. The pitch canker epidemic in California. Plant Disease. 2001;85:1128–1139. doi: 10.1094/PDIS.2001.85.11.1128. [DOI] [PubMed] [Google Scholar]
- Gryzenhout M., Myburg H., Merwe N.A. van der. Chrysoporthe, a new genus to accommodate Cryphonectria cubensis. Studies in Mycology. 2004;50:119–142. [Google Scholar]
- Guindon S., Dufayard J., Lefort V. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Guindon S., Gascuel O. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Systematic Biology. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Heath R.N., Gryzenhout M., Roux J. Discovery of the canker pathogen Chrysoporthe austroafricana on native Syzygium spp. in South Africa. Plant Disease. 2006;90:433–438. doi: 10.1094/PD-90-0433. [DOI] [PubMed] [Google Scholar]
- Heulsenbeck J.P., Ronquist F., Nielsen R. Bayesian inference of phylogeny and its impact on evolutionary biology. Science. 2001;294:2310–2314. doi: 10.1126/science.1065889. [DOI] [PubMed] [Google Scholar]
- Hibbett D.S., Ohman A., Glotzer D. Progress in molecular and morphological taxon discovery in fungi and options for formal classification of environmental sequences. Fungal Biology Reviews. 2011;25:38–47. [Google Scholar]
- Hove V.F., Waalwijk C., Logrieco A. Gibberella musae (Fusarium musae) sp. nov., a recently discovered species from bananas is sister to F. verticillioides. Mycologia. 2011;103:570–585. doi: 10.3852/10-038. [DOI] [PubMed] [Google Scholar]
- IDEAM (Instituto de Hidrologia, Meteorologia y Estudios Ambientales) 2009. Informe Anual sobre el estado del medio ambiente y los recursos naturales renovables en Colombia, Bosques-2009. 236 pp. [Google Scholar]
- Iltis H.H. Homeotic sexual translocations and the origin of maize (Zea mays, Poaceae): a new look at an old problem. Economic Botany. 2000;54:7–42. [Google Scholar]
- Jactel H., Nicoll B.C., Branco M. The influences of forest stand management on biotic and abiotic risks of damage. Annals of Forest Science. 2009;66:1–18. [Google Scholar]
- Kang S., Mansfield M.A., Park B. The promise and pitfalls of sequence-based identification of plant-pathogenic fungi and oomycetes. Phytopathology. 2010;100:732–737. doi: 10.1094/PHYTO-100-8-0732. [DOI] [PubMed] [Google Scholar]
- Katoh K., Kuma K., Toh H. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Research. 2005;33:511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Misawa K., Kuma K. MAFFT: a novel method for rapid multiple sequence alignment based on Fourier transform. Nucleic Acids Research. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Toh H. Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics. 2008;9:286–298. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- Kvas M., Marasas W.F.O., Wingfield B.D. Diversity and evolution of Fusarium species in the Gibberella fujikuroi complex. Fungal Diversity. 2009;34:1–21. [Google Scholar]
- Leslie J.F., Summerell B.A. Blackwell Professional; Ames, IA: 2006. The Fusarium laboratory manual. [Google Scholar]
- Liebhold A.M., Macdonald W.L., Bergdahl D. Invasion by exotic forest pests: a threat to forest ecosystems. Forest Science. 1995;30:1–52. [Google Scholar]
- Lima S., Pfenning L.H., Costa S.S. Fusarium tupiense sp. nov., a member of the Gibberella fujikuroi complex that causes mango malformation in Brazil. Mycologia. 2012;104:1408–1419. doi: 10.3852/12-052. [DOI] [PubMed] [Google Scholar]
- Marasas W.F.O. Discovery and occurrence of the fumonisins: a historical perspective. Environmental Health Perspectives. 2001;109:239–243. doi: 10.1289/ehp.01109s2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasas W.F.O., Nelson P.E., Toussoun T.A. Fusarium dlamini, a new species from Southern Africa. Mycologia. 1985;77:971–975. [Google Scholar]
- Matuo T., Snyder W.C. Use of morphology and mating populations in the identification of formae speciales in Fusarium solani. Phytopathology. 1973;63:562–565. [Google Scholar]
- Miedaner T., Schilling A.G., Geiger H.H. Molecular genetic diversity and variation for aggressiveness in populations of Fusarium graminearum and Fusarium culmorum sampled from wheat fields in different countries. Journal of Phytopathology. 2001;149:641–648. [Google Scholar]
- Millar C.I. Impact of the Eocene on the evolution of Pinus L. Annals of the Missouri Botanical Garden. 1993;80:471–498. [Google Scholar]
- Mitchell R.G., Steenkamp E.T., Coutinho T.A. The pitch canker fungus, Fusarium circinatum: implications for South African forestry. Southern Forests. 2011;73:1–13. [Google Scholar]
- Nakabonge G., Roux J., Gryzenhout M. Distribution of Chrysoporthe canker pathogens on Eucalyptus and Syzygium spp. in eastern and southern Africa. Plant Disease. 2006;90:734–740. doi: 10.1094/PD-90-0734. [DOI] [PubMed] [Google Scholar]
- Nash S.M., Snyder W.C. Quantitative estimates by plate counts of the propagules of the bean root rot Fusarium in field soils. Phytopathology. 1962;52:667–672. [Google Scholar]
- Nirenberg H.I., O'Donnell K. New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia. 1998;90:434–458. [Google Scholar]
- Nixon K.C., Wheeler Q.D. An amplification of the phylogenetic species concept. Cladistics. 1990;6:211–223. [Google Scholar]
- Nucci M., Anaissie E. Fusarium infections in immunocompromised patients. Clinical Microbiology Reviews. 2002;20:695–704. doi: 10.1128/CMR.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocamb C.M., Juzwik J. Fusarium species associated with rhizosphere soil and diseased roots of eastern white pine seedlings and associated nursery soils. Canadian Journal of Plant Pathology. 1995;17:325–330. [Google Scholar]
- O'Donnell K. Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia. 2000;92:919–938. [Google Scholar]
- O'Donnell K., Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- O'Donnell K., Cigelnik E., Nirenberg H.I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia. 1998;90:465–493. [Google Scholar]
- O'Donnell K., Nirenberg H.I., Aoki T. A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additionally phylogenetically distinct species. Mycoscience. 2000;41:61–78. [Google Scholar]
- O'Donnell K., Rooney A.P., Proctor R.H. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genetics and Biology. 2013;52:20–31. doi: 10.1016/j.fgb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- O'Donnell K., Sutton D.A., Fothergill A. Molecular phylogenetic diversity, multilocus haplotype nomenclature and in vitro antifungal resistance within the Fusarium solani species complex. Journal of Clinical Microbiology. 2008;46:2477–2490. doi: 10.1128/JCM.02371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onorfi A. Enhancing Excel capability to perform statistical analyses in agriculture applied research. In: International Association for statistical Computing, editor. Computational statistics and data analysis & statistical software newsletters. 2006. http://www.csdassn.org/softlist.cfm 15/02/2006. [Google Scholar]
- Orwig D.A. Ecosystem to regional impacts of introduced pests and pathogens: historical context, questions and issues. Journal of Biogeography. 2002;29:1471–1474. [Google Scholar]
- Otero-Colina G., Rodríguez-Alvarado G., Fernández-Pavía S.P. Identification and characterization of a novel etiological agent of mango malformation disease in Mexico, Fusarium mexicanum sp. nov. Phytopathology. 2010;100:1176–1184. doi: 10.1094/PHYTO-01-10-0029. [DOI] [PubMed] [Google Scholar]
- Perkins T.E., Matlack G.R. Human-generated pattern in commercial forests of southern Mississippi and consequences for the spread of pests and pathogens. Forest Ecology and Management. 2002;157:143–154. [Google Scholar]
- Pietro A.D., Madrid M.P., Caracuel Z. Fusarium oxysporum: exploring the molecular arsenal of a vascular wilt fungus. Molecular Plant Pathology. 2003;4:315–325. doi: 10.1046/j.1364-3703.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- Porter B., Wingfield M.J., Coutinho T.A. Susceptibility of South African native conifers to the pitch canker pathogen Fusarium circinatum. South African Journal of Botany. 2009;75:380–382. [Google Scholar]
- Posada D. Using MODELTEST and PAUP* to select a model of nucleotide substitution. Current Protocols in Bioinformatics. 2003 doi: 10.1002/0471250953.bi0605s00. Chapter 6: Unit 6.5. [DOI] [PubMed] [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Molecular Biology and Evolution. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Rayner R.W. CMI and British Mycological Society; Kew, Surrey, England: 1970. A mycological colour chart. [Google Scholar]
- Richardson D.M., Williams P.A., Hobbs R.J. Pine invasions in the Southern Hemisphere: determinants of spread and invadability. Journal of Biogeography. 1994;21:511–527. [Google Scholar]
- Riffle J.W., Strong F.C. Studies in White pine seedling root rot. Quarterly Bulletin. Michigan State University, Agricultural Experiment Station. 1960;42:845–853. [Google Scholar]
- Rodas P.C.A. Actas Congresso Internacional de plagas forestales, 18–21 August 1997, Pucon, IX Region. Corporacion Nacional Forestal; Santiago, Chile: 1998. Programma manejo de defoliadoras en plantaciones forestales en Colombia; pp. 141–155. [Google Scholar]
- Roux J., Eisenberg B., Kanzler A. Testing of selected South African Pinus hybrids and families for tolerance to the pitch canker pathogen, Fusarium circinatum. New Forests. 2007;33:109–123. [Google Scholar]
- Sano E., Rosa R., Brito J.L.S. Land cover mapping of the tropical savannah region in Brazil. Environmental Monitoring and Assessment. 2010;166:113–124. doi: 10.1007/s10661-009-0988-4. [DOI] [PubMed] [Google Scholar]
- Scauflaire J., Gourgue M., Munant F. Fusarium temperatum f. sp. nov. from maize, an emergent species closely related to Fusarium subglutinans. Mycologia. 2011;103:586–597. doi: 10.3852/10-135. [DOI] [PubMed] [Google Scholar]
- Scholthof K.B.G. The disease triangle: pathogens, the environment and society. Nature Reviews Microbiology. 2006;5:152–156. doi: 10.1038/nrmicro1596. [DOI] [PubMed] [Google Scholar]
- Short D.P.G., O'Donnell K., Thrane U. Phylogenetic relationships among members of the Fusarium solani species complex in human infections and the descriptions of F. keratoplasticum sp. nov. and F. petroliphilum stat nov. Fungal Genetics and Biology. 2013;53:59–70. doi: 10.1016/j.fgb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Silva P.H.M. da, Poggiani F., Sebbenn Can Eucalyptus invade native forest fragments close to commercial stands? Forest Ecology and Management. 2011;261:2075–2080. [Google Scholar]
- Slippers B., Stenlid J., Wingfield M.J. Emerging pathogens: fungal host jumps following anthropogenic introduction. Trends in Ecology and Evolution. 2005;20:420–421. doi: 10.1016/j.tree.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Steenkamp E.T., Rodas C.A., Kvas M. Fusarium circinatum and pitch canker of Pinus in Colombia. Australasian Plant Pathology. 2012;41:483–491. [Google Scholar]
- Steenkamp E.T., Wingfield B.D., Coutinho T.A. Differentiation of Fusarium subglutinans f. sp. pini by histone gene sequence data. Applied and Environmental Microbiology. 1999;65:3401–3406. doi: 10.1128/aem.65.8.3401-3406.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp E.T., Wright J., Baldauf S.L. The protistan origins of animals and fungi. Molecular Biology and Evolution. 2006;23:93–106. doi: 10.1093/molbev/msj011. [DOI] [PubMed] [Google Scholar]
- Stenlid J., Olivia J., Boberg J.B. Emerging diseases in European forest ecosystems and responses in society. Forests. 2011;2:486–504. [Google Scholar]
- Streit E., Schatzmayr G., Tassis P. Current situation of mycotoxin contamination and co-occurrence in animal feed – focus on Europe. Toxins. 2012;4:788–809. doi: 10.3390/toxins4100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N. MEGA 5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavare S. Some probabilistic and statistical problems in the analysis of DNA sequences. In: Miura R.M., editor. Some mathematical questions in biology – DNA sequence analysis. American Mathematical Society; USA: 1986. pp. 57–86. [Google Scholar]
- Tommerup I.C., Alfenas A.C., Old K.M. Guava rust in Brazil – a threat to Eucalyptus and other Myrtaceae. New Zealand Journal of Forestry Science. 2003;33:420–428. [Google Scholar]
- Walsh J.L., Laurence M.H., Liew E.C.Y. Fusarium: two endophytic novel species from tropical grasses of northern Australia. Fungal Diversity. 2010;44:149–159. [Google Scholar]
- Wikler K.R., Gordon T.R. An initial assessment of genetic relationships among populations of Fusarium circinatum in different parts of the world. Canadian Journal of Botany. 2000;78:709–717. [Google Scholar]
- Wingfield M.J. Increasing threat of diseases to exotic plantation forests in the southern hemisphere: lessons from Cryphonectria canker. Australasian Plant Pathology. 2003;32:133–139. [Google Scholar]
- Wingfield M.J., Hammerbacher A., Ganley R.J. Pitch canker caused by Fusarium circinatum – a growing threat to pine plantations and forests worldwide. Australasian Plant Pathology. 2008;37:319–334. [Google Scholar]
- Wingfield M.J., Slippers B., Hurley B.P. Eucalypt pests and diseases: growing threats to plantation productivity. Southern Forests. 2008;70:139–144. [Google Scholar]
- Wingfield M.J., Slippers B., Roux J. Worldwide movement of exotic forest fungi, especially in the tropics and the southern hemisphere. BioScience. 2001;51:135–140. [Google Scholar]
- Wingfield M.J., Slippers B., Wingfield B.D. Novel associations between pathogens, insects and tree species threaten world forests. New Zealand Journal of Forestry Science. 2010;40(Suppl.):S95–S103. [Google Scholar]
- Zaiontz C. 2014. Real statistics using Excel.www.real-statistics.com [Google Scholar]
- Zhang N., O'Donnell K., Sutton D.A. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. Journal of Clinical Microbiology. 2006;44:2186–2190. doi: 10.1128/JCM.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]