Abstract
One of the defining characteristics of adolescence in humans is a large shift in the timing and structure of sleep. Some of these changes are easily observable at the behavioral level, such as a shift in sleep patterns from a relatively morning to a relatively evening chronotype. However, there are equally large changes in the underlying architecture of sleep, including a > 60% decrease in slow brain wave activity, which may reflect cortical pruning. In this review we examine the developmental forces driving adolescent sleep patterns using a cross-species comparison. We find that behavioral and physiological sleep parameters change during adolescence in non-human mammalian species, ranging from primates to rodents, in a manner that is often hormone-dependent. However, the overt appearance of these changes is species-specific, with polyphasic sleepers, such as rodents, showing a phase-advance in sleep timing and consolidation of daily sleep/wake rhythms. Using the classic two-process model of sleep regulation, we demonstrate via a series of simulations that many of the species-specific characteristics of adolescent sleep patterns can be explained by a universal decrease in the build-up and dissipation of sleep pressure. Moreover, and counterintuitively, we find that these changes do not necessitate a large decrease in overall sleep need, fitting the adolescent sleep literature. We compare these results to our previous review detailing evidence for adolescent changes in the regulation of sleep by the circadian timekeeping system (Hagenauer and Lee, 2012), and suggest that both processes may be responsible for adolescent sleep patterns.
Keywords: Adolescent, Puberty, Sleep, Chronotype, Circadian, Homeostasis, Two-process model, Hormone, Development, Rodent
Introduction
Sleep is arguably the primary activity of the developing brain (Dahl, 1998). During infancy, a time of rapid synaptogenesis and brain growth, individuals spend on average 13–16 h a day asleep. This average does not drop below12 h until the age of four when cortical synaptic density plateaus. At ten years of age children still sleep for 10 h a day (Jenni and Carskadon, 2007). If sleep is critically tied to brain development, then characterizing sleep during adolescence should be of particular interest. Adolescence is not only a time of transition from a developing brain and body to adulthood, but also a complex and sensitive period that includes large hormonal fluctuations, permanent brain reorganization, and rapid growth (Sisk and Zehr, 2005; Spear, 2000). If the relationship between sleep and development is not merely correlational but essentially linked, then sleep deprivation during adolescence may permanently alter the developmental trajectory of the brain and behavior (Shaffery et al., 2006). Despite this possibility, many modern social and technological features compel teenagers in developed countries to be overwhelmingly and chronically sleep deprived (Andrade and Menna-Barreto, 2002; Giannotti and Cortesi, 2002; Gradisar et al., 2011; Thorleifsdottir et al., 2002; Yang et al., 2005).
For this reason, there has been growing scientific interest in the unique changes that characterize adolescent sleep patterns, with an eye on public health applications. As there have been several excellent recent reviews on this topic (Carskadon 2010; Colrain and Baker, 2011; Feinberg and Campbell, 2010), we will focus on a less discussed topic: similarities and differences in the manner that sleep patterns change during development across species. If we presume that many of the physiological events associated with puberty and adolescence are not events specific to humans but instead represent common changes necessary for achieving sexual maturity and independent life across mammalian species (Sisk and Zehr, 2005; Spear, 2000), many of the changes in sleep observed during adolescence in humans should be observable in common laboratory species. Indeed, using model species allows us to examine the hormonal and neural causes of adolescent sleep patterns in greater depth, as well as to characterize their developmental trajectory when isolated from social constraint and the influences of artificial lighting and modern technology.
Puberty & adolescence: humans vs. laboratory species
Before comparing adolescent sleep patterns across species, it is important to understand that the developmental progression of puberty and adolescence differs between humans and traditional laboratory species. Colloquially the terms “puberty” and “adolescence” are used interchangeably, but scientifically they refer to separate concepts (Sisk and Zehr, 2005). Traditionally, puberty is defined as the process leading to the attainment of sexual maturation (Spear, 2000), beginning with the activation of the hypothalamic-pituitary gonadal axis and ending with reproductive competency (Plant, 1994; Sisk and Foster, 2004). In humans, puberty is initiated following eight or more years of gonadal “quiescence”, and includes both an increased secretion of hormones from the gonads (gonadarche), such as estrogens and androgens, which drive genital and breast development (Plant, 1994), and an increased secretion of androgens from the adrenals (adrenarche), which initiate the development of pubic hair (Campbell, 2006). Some mammalian species, such as non-human primates, show a similar developmental sequence to humans (Plant, 1994), including adrenarche and pre-pubertal gonadal quiescence, but rodents typically do not (Ojeda and Urbanski, 1994), and external secondary sex characteristics are typically species-specific. Thus, it is sometimes difficult to say with certainty how the progression of particular pubertal events in laboratory species relates to standardized stages in humans. That said, the general timing of puberty in laboratory species can still be delineated by secondary sex characteristic development, including reproductive cyclicity and vaginal patency in females, and penile and testicular development in males (Ojeda and Urbanski, 1994).
Defining equivalent stages of adolescence in rodents and humans is even more complicated. Whereas puberty is defined by reproductive development, adolescence is defined as the period of social, emotional and cognitive transition between childhood and adulthood (Sisk and Zehr, 2005). Adolescence encompasses puberty, and in humans the definition frequently spans the entire second decade of life, including 3–6 years beyond the attainment of full sexual maturity. In animal studies, the term “adolescence” is traditionally used specifically to refer to research focusing on the neural and behavioral changes accompanying the transition from juvenile dependence into the relative independence of adulthood (Spear, 2000). This transition includes both the hormone-dependent and hormone-independent remodeling of cortical and limbic circuitry necessary for adult decision-making, cognition and social interaction (Sisk and Zehr, 2005). Since independent life in the laboratory begins at artificial weaning, some researchers use weaning to define the beginning of adolescence, allowing overlap with life events associated with young childhood in humans. Other researchers assume adolescence begins with the initiation of puberty. The end of rodent adolescence can be argued as anywhere between the time of attaining reproductive competence to the attainment of adult body size and social interactions. With these considerations in mind, we have presented a simplified diagram of human vs. rodent development in relationship to some of the changes in sleep patterns observed in each species (Fig. 1).
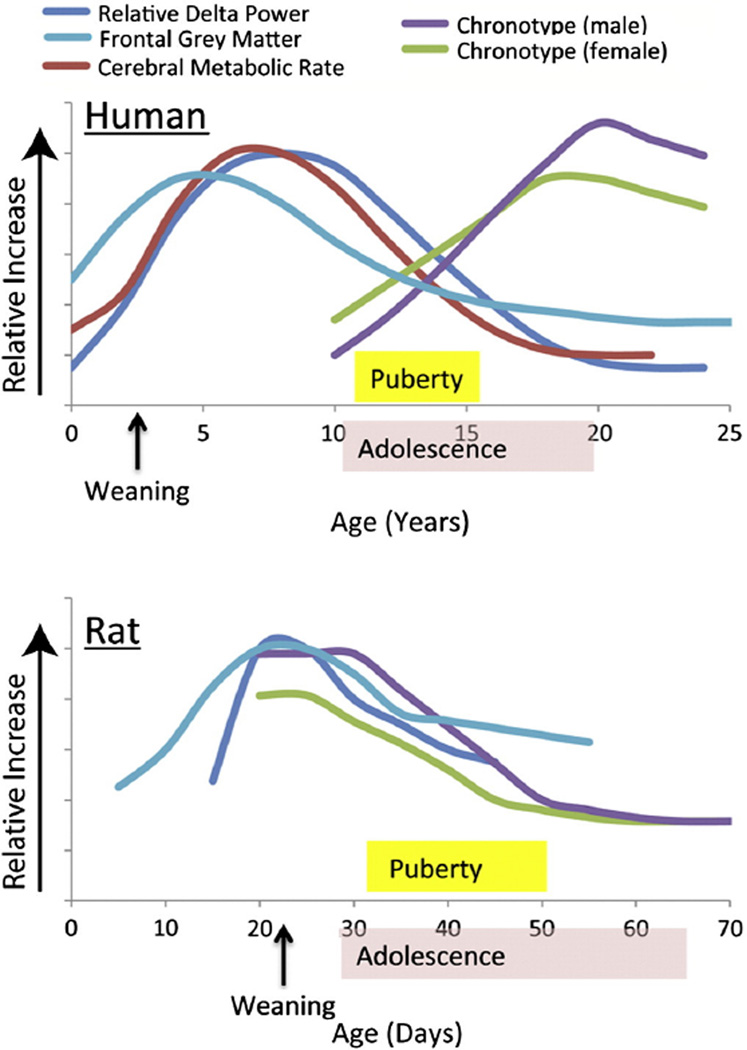
Fig. 1.
A timeline illustrating the relationship between sleep variables, puberty, and brain development in humans and laboratory rats. (Top) An adaptation of a figure from Feinberg and Campbell (2010) and Feinberg et al. (1990) illustrating that the developmental timing of changes in delta power during slow wave sleep (dark blue) correlates with changes in frontal grey matter (synaptic density, light blue) and cerebral metabolic rate (red) in humans (x-axis = years, y-axis = the relative increase in each variable representing the developmental pattern of change). Adolescent changes in chronotype have been added to the model using data from Roenneberg et al. (2004, green = female, purple = male), progressing from morning-type (lower values) to evening-type (higher values). (Bottom) An illustration of the approximate developmental changes for the same variables in rats (x-axis = days, based on Bronzino et al., 1987; Gvilia et al., 2011; Hagenauer et al., 2011a; Jouvet-Mounier et al., 1970; Van Eden and Uylings, 1985). Note that delta power and frontal grey matter volume seem to peak at a similar age, followed by a change in chronotype, but unlike in humans chronotype shifts earlier instead of later. These trends should be considered tentative compared to the high degree of replicability supporting the human data.
Sleep is regulated by homeostatic “sleep pressure” and a daily circadian rhythm
Traditionally, sleep patterns are thought to derive from three primary components: an internal (endogenous) daily circadian timekeeping system, a homeostatic drive for sleep (“sleep pressure”) that builds up over the course of being awake and decreases during sleep, and other external constraints (which are collectively referred to as “masking”). Both internal components are known to be sensitive to sex (gonadal) hormones, such as estrogen and testosterone (Hagenauer and Lee, 2012; Mong et al., 2011), and thus could be affected by puberty.
While sleep is a “resting” behavior typified by reduced consciousness and response to external stimuli, it is characterized in the laboratory by electroencephalographic (EEG, “brain wave”) signatures with both slow and active properties (Dement and Kleitman, 1957). To examine sleep, electrodes are attached to the skin of the scalp and face to allow for the measurement of EEG, eye movements, and muscle tone. The EEG recordings are then analyzed for the distribution and power of brain waves of particular frequencies (Hz), ranging from slow to fast (delta: 0.5–4.5 Hz, theta: 4–8 Hz, alpha: 8–12 Hz, sigma: 12–15 Hz; beta: 15–30 Hz). Using these parameters, sleep is divided into two major states, “active” rapid eye movement sleep (REMS) and “restorative” non-REM sleep (NREMS). REMS is characterized by the low amplitude and mixed frequency waves typical of an active and awake brain, and is accompanied by rapid eye movements and muscle atonia (Aserinsky and Kleitman, 1953). NREM sleep is characterized by low muscle tone and low-frequency/high-amplitude waveforms known as “slow waves”. Slow wave activity (SWA: EEG delta power/min) and theta wave activity during NREM sleep correlate with sleep depth and are responsive to homeostatic sleep pressure: they increase as a function of previous wake duration, and dissipate across sleep episodes (Borbely et al., 1981; Daan et al., 1984; Dement and Kleitman, 1957). For this reason, NREM sleep is considered restorative.
In humans, NREMS has been historically further subdivided into Stages 1–4 based on the presence of increasing amounts of SWA, with Stages 3 and 4 now referred to collectively as slow wave sleep (SWS or N3). Stage 2 sleep is distinguished by the presence of “sleep spindles”, which are a stereotyped waveform in the sigma band (10–15 Hz; Dement and Kleitman, 1957; Moser et al., 2009). In laboratory rodents, NREMS is typically discussed as a single quantity without further subdivision, and the existence of sleep spindles is considered debatable (although see Yasenkov and Deboer, 2010).
In adult humans, sleep progresses via cycles (~90 min), beginning the night in NREMS, progressing deeper through Stage 2 to SWS, and finally initiating REMS. Depending on sleep duration, an individual may have five or more of these cycles before waking, with each successive cycle containing proportionally less SWS and proportionally more REMS (Dement and Kleitman, 1957). The sleep cycles in laboratory rodents are much shorter and take place on the order of minutes. In most species, sleep still predominantly occurs at a particular time of day (day for nocturnal species, night for diurnal species) but it does so stochastically, punctuated by episodes of wake (Kas and Edgar, 1999; Mistlberger, 2005; Phillips et al., 2010; Tobler and Borbeley, 1986). Despite this “polyphasic” pattern of sleeping, SWA still decreases during relatively consolidated periods of sleep, and builds up at times of day characterized by high amounts of waking activity, reflecting sleep pressure (Franken et al., 1991; Tobler and Borbeley, 1986; Tobler et al., 1992).
The daily rhythm in sleep is generated by the circadian timekeeping system, which alternately promotes wake or sleep depending on the time of day (Fleshner et al., 2011; Mistlberger, 2005; although see Edgar et al., 1993). In order for this system to tell time accurately, internally-generated rhythms are “entrained” by environmental time cues such as sunlight. Morning light shifts the circadian timekeeping system earlier (phase-advance) and evening light shifts it later (phase-delay), whereas light exposure at midday has little effect (Johnson, 1992; Roenneberg et al., 2003). Thus, the time at which the circadian system promotes sleep or wake can be influenced by internal factors (endogenous rhythm period and circadian light sensitivity) as well as external factors (artificial light exposure).
To better conceptualize how circadian timekeeping and the homeostatic drive for sleep interact to generate sleep–wake cycles, researchers often use a model of sleep–wake regulation known as the 2-process model (Daan et al., 1984). In this model, the homeostatic drive to sleep (Process S) is represented by a set of two exponential functions: a saturating build up of sleep pressure during waking (with the time constant τi), and a rapid decline during sleep (with the time constant τd), derived from measurements of SWA. The circadian component (Process C) is represented by a sinusoidal lower “wake threshold” (L) and upper “sleep threshold” (U, Fig. 2). In humans, it is theorized that the peaks for these two processes are staggered, such that sleep at the beginning of the night is primarily driven by homeostatic drive (S), and sleep at the end of the night is maintained by a circadian drive (C). This staggered phase relationship produces a consolidated sleep episode (Dijk and Czeisler, 1994). In contrast, in rodents, it is theorized that either a reduced threshold for sleep onset (U), or accelerated time constant for homeostatic build up and dissipation (τi and τd) produces a polyphasic sleep pattern, with a higher density of sleep happening during the peak circadian drive for sleep (Daan et al., 1984; Phillips et al., 2010; Tobler et al., 1992).
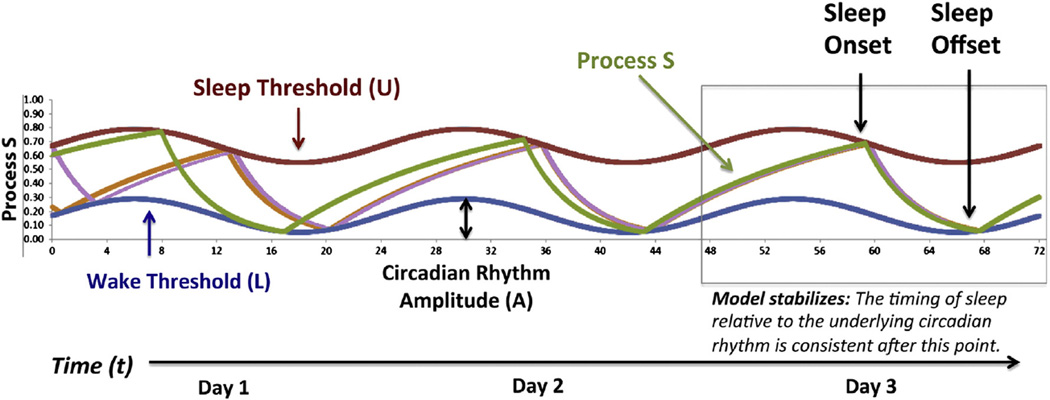
Fig. 2.
The 2-process model illustrates interactions between the circadian and homeostatic regulation of sleep. In this model, the homeostatic drive to sleep (Process S, green) is represented by a set of two exponential functions: a saturating build up of sleep pressure during waking (St + 1 = 1 − (1 − St) * e−Δt/τi, with a time constant of τi = 18.18 h and an instantaneous build up rate of 0.055/h) and a rapid decline during sleep (St + 1 = St * e−Δt/τd, with a time constant of τd = 4.2 h and an instantaneous break down rate of 0.238/h). These time constants were originally derived from observations of a physiological correlate of sleep pressure during human sleep deprivation experiments: the build-up and dissipation of slow wave activity (SWA; Daan et al., 1984). Thus, the y-axis of this figure, which spans from 0 to 1, ultimately represents the theoretical upper and lower asymptotes for SWA generation. In this figure, three days of simulated data are presented, with time (t, hrs) indicated by the x-axis. Using parameters optimized to fit the sleep patterns of young adults (Daan et al., 1984), Process S in the model is allowed to fluctuate between a sinusoidal lower “wake threshold” (L, blue, average value of 0.17) and upper “sleep threshold” (U, red, average value of 0.67), representing the circadian regulation of sleep (Process C) with a rhythm amplitude of A = 0.12. Thus, time awake is denoted by the rising slope of S, and time of sleep is denoted by the falling slope. In general, this model will progress towards a stable (ideal) timing of sleep and wake, even when initial levels of sleep pressure differ (S, orange and lavender lines).
Sleep pattern development in adolescent humans
For the sake of concise interspecies comparison, we will focus this review on the strongest insights provided from the human literature, as derived from extensive longitudinal naturalistic studies and smaller well-controlled laboratory studies. More detailed reviews can be found in Colrain and Baker (2011), Feinberg and Campbell (2010), and Carskadon (2011).
Sleep timing
Perhaps the best known characteristic of teenage sleep patterns is the tendency to stay up late. Teenagers maintain later (delayed) bedtimes than younger children, even when wake times are constrained by school or work (Crowley et al., 2007; Gradisar et al., 2011; Thorleifsdottir et al., 2002). This tendency has been attributed to many external influences, ranging from evening work schedules and increased academic responsibilities to late night television and social opportunities (Cain and Gradisar, 2010; Carskadon et al., 1989; Van den Bulck, 2004). Current evidence, however, demonstrates that social factors do not completely account for the adolescent shift towards delayed sleep patterns, otherwise known as evening chronotype. Indeed, the observation that adolescents develop a tendency towards evening-type behavior goes back as far as the early 20th century, when Terman and Hocking (1913a) noticed that adolescent boys in boarding school developed an increasing need to be woken by an alarm clock between the ages of 11–18 years in order to make it to their 9 a.m. classes. Terman and Hocking (1913b) suggested that early school hours may cause insufficient sleep in children.
Since then, researchers have found that a delay in the timing of sleep during adolescence, accompanied by a decrease in total sleep time due to socially-constrained wake times, is both a widespread and cross-cultural phenomenon. A delay in the timing of sleep during the second decade of life has been observed in over 20 countries in 6 continents, in cultures ranging from pre-industrial to modern (as reviewed in Carskadon, 2008; Gradisar et al., 2011). A recent meta-analysis of 13 studies from 9 countries indicated that weekend bedtimes are delayed by ~2.75 h between the ages of 11–18, even though overall bedtimes and wake times varied by culture (Gradisar et al., 2011). Retrospective longitudinal measures confirm Terman's observations: later sleep times in adolescents are not merely a modern phenomenon but existed before the advent of computers, internet, and cell phones (Carskadon, 2008; Roenneberg et al., 2004).
The developmental timing of this transition parallels pubertal development (Crowley et al., 2007; Russo et al., 2007; Thorleifsdottir et al., 2002; Yang et al., 2005). Home-based studies of adolescents show that delayed sleep phase correlates with secondary-sex development (Sadeh et al., 2009), and this correlation holds true for subjective ratings of chronotype and puberty even when grade level in school is held constant (Carskadon et al., 1993). If we assume that teenagers attending the same grade in school are exposed to a similar social environment, this evidence suggests that a biological component drives adolescent sleep patterns. Moreover, girls begin to show a delay in the timing of sleep one year earlier than boys, mimicking their younger pubertal onset, and show maximum eveningness at a younger age (15–19.5 years vs. 18–21 years in boys, (Fig. 1)). There is also a sex difference in the magnitude of the delay, with boys showing more extreme changes in chronotype across the adolescent period, leading to overall greater eveningness (Randler, 2011; Roenneberg et al., 2004). Altogether, this evidence suggests that the adolescent transition into a more evening chronotype is driven by pubertal hormones.
Sleep architecture
Dramatic changes in the architecture of sleep during adolescence have been noted since the 1970's (Feinberg et al., 1990). In particular, during adolescence there is a large decrease (40%) in both the total amount of slow-wave sleep (SWS) obtained each day, as well as the percentage of time spent in SWS during a nocturnal sleep episode (Baker et al., 2012; Carskadon and Acebo, 2002; Carskadon et al., 1980; Gaudreau et al., 2001; Jenni and Carskadon, 2004; Jenni et al., 2005; Kurth et al., 2010a,b; Tarokh et al., 2012). This decrease in the amount of SWS is accompanied by an equally large decrease in SWA and theta activity during both NREM and REM sleep (Baker et al., 2012; Buchmann et al., 2011; Campbell and Feinberg, 2009; Campbell et al., 2011, 2012; Gaudreau et al., 2001; Jenni and Carskadon, 2004; Jenni et al., 2005; Kurth et al., 2010a,b). During adolescence NREMS delta and theta power plummet by > 60% (Campbell et al., 2011; Feinberg and Campbell, 2010). The decline in theta power precedes the decline in delta power by several years, beginning in late childhood (between ages 6 and 9, Campbell and Feinberg, 2009) and starting to plateau at age 16 (Campbell et al., 2012). Delta power falls between ages 11 and 18 (Campbell et al., 2012), strongly paralleling the timing of pubertal maturation. Girls show an earlier decline in delta power than boys by 1.2 years, mirroring sex differences in the timing of puberty. Even after controlling for these sex differences, the decline in delta power strongly correlates with pubertal timing, especially with the development of pubic hair, suggesting a possible role for androgens in adolescent sleep (Campbell et al., 2012).
As slow-wave sleep diminishes during adolescence, Stage 2 sleep becomes increasingly prominent (Carskadon et al., 1980; Gaudreau et al., 2001; Jenni and Carskadon, 2004; Jenni et al., 2005; Kurth et al., 2010b; Tarokh et al., 2012). This growth in Stage 2 sleep is accompanied by an increase in the peak frequency for sleep spindles (Jenni and Carskadon, 2004; Kurth et al., 2010b) and a shift in the relative power associated with these frequencies (Buchmann et al., 2011; Gaudreau et al., 2001; Jenni and Carskadon, 2004; Kurth et al., 2010b). The power of waves in faster EEG frequency ranges also decreases during adolescence (beta waves: 15–30 Hz; Buchmann et al., 2011; Gaudreau et al., 2001; Jenni and Carskadon, 2004; Jenni et al., 2005; Kurth et al., 2010b), suggesting that the decline in delta and theta power during adolescence may represent a general change in the mechanisms underlying brain wave generation (Feinberg and Campbell, 2010; Feinberg et al., 1990). Whether these changes in Stage 2 sleep and faster EEG frequencies are as clearly associated with pubertal maturation as the decline in delta power remains to be discovered.
Sleep homeostasis
Although daytime sleepiness in adolescence is epidemic, the cause of this sleepiness is likely to derive primarily from social factors rather than an increased homeostatic drive for sleep. Many adolescents maintain schedules during the school year that result in insufficient and ill-timed sleep. Unlike adults, growing adolescents require on average as much as 9–10 h of sleep per night (Carskadon, 2011; Carskadon et al., 1980), but 45% report obtaining less than 8 h on school nights (National Sleep Foundation Sleep and Teens Task Force, 2000). Similar trends have been observed in multiple modern societies, including Korea, Brazil, and Italy (Andrade and Menna-Barreto, 2002; Giannotti and Cortesi, 2002; Gradisar et al., 2011; Yang et al., 2005). This chronic sleep deprivation is accompanied by 15–70% of adolescents reporting excessive sleepiness, including oversleeping, inadvertent napping, and sleeping during class (Gradisar et al., 2011; Thorleifsdottir et al., 2002). In one study it was found that 10th grade students on average could fall asleep within 5 min during a sleep latency test administered during the morning (8:30 a.m.), and 48% of these subjects showed at least one episode of immediate onset REMS, implying a level of daytime sleepiness akin to narcolepsy (Carskadon et al., 1998). Adolescent sleepiness is further reflected in multiple markers of sleep efficiency, including an increase in the percentage of time in bed spent sleeping and a decrease in night-time sleep latency and the number of awakenings after sleep onset (Feinberg et al., 2012; Gaudreau et al., 2001; Kurth et al., 2010b).
Whether a change in sleepiness develops naturally during adolescence under conditions free of sleep restriction is debatable. Controlled laboratory studies indicate that following a week or more of regular, sufficient sleep (10 h), adolescents are fully awake in the morning but exhibit a midday dip in their ability to stay awake (Carskadon et al., 1980; Carskadon and Acebo, 2002). Despite this dip, their wakefulness recovers, and by evening late adolescents are significantly more able to stay awake than well-rested pre-pubertal children (Taylor et al., 2005).
This adolescent evening vigor has been interpreted as potentially reflecting a decrease in the build-up of the homeostatic drive for sleep across the day. This conclusion seems to fit well with the large decrease in SWA observed during this developmental period. As discussed earlier, SWA is homeostatically regulated, with levels during the first NREM period of a sleep episode reflecting the duration of prior waking. In that sense, the higher levels of SWA in pre-pubertal children might indicate that more intense sleep need is reached by bedtime, and the progress of SWA dissipation across the night towards plateau levels could suggest a biological imperative for longer sleep time (Gaudreau et al., 2001). However, the fact that a decrease in EEG activity occurs during adolescence across all frequencies (delta, theta, alpha, beta) and in multiple states, including REMS and waking (Baker et al., 2012; Buchmann et al., 2011; Gaudreau et al., 2001; Jenni and Carskadon, 2004), suggests that the adolescent decrease in SWA may more broadly reflect the process by which EEG waves are generated.
A popular theory put forward by Feinberg and Campbell (2010) and Feinberg et al. (1990) is that the decline in delta power during adolescence reflects cortical synaptic pruning, as EEG slow waves reflect synchronized synaptic activity centered in the frontal lobe. A hallmark of human brain development is a rapid proliferation of synaptic connections in early childhood, followed by a large overall decrease until maturation. This synaptic pruning, along with parallel increases in axon myelination, is believed to increase the efficiency of cortical networks, and is largely credited for many of the cognitive improvements gained during adolescence (Giedd et al., 2012). Feinberg et al. (1990) pointed out that the decline in delta power during adolescence closely parallels the time course of synaptic pruning and diminishing cerebral metabolic rate. This theory has held up well, with recent studies showing that the adolescent decline in delta power progresses from the occipital to frontal regions, paralleling the caudal to frontal progression of cortical development (Baker et al., 2012; Kurth et al., 2010b). A loss of synaptic strength during adolescence is also suggested by a decrease in the slope of EEG slow-waves (Kurth et al., 2010a). However, perhaps the most convincing evidence comes from a recent study that found a strong correlation between SWA and cortical grey matter volume measured by magnetic resonance imaging during adolescence (Buchmann et al., 2011). It is worth noting that sex differences in the developmental timing of delta power decline (Campbell et al., 2012) mimic sex differences in the decline in grey matter volume during adolescence (Giedd et al., 2012). Interestingly, the adolescent decline in delta power tightly correlates with pubertal development driven by androgens (Campbell et al., 2012), and recent studies link androgen receptor polymorphisms and adolescent cortical pruning (Raznahan et al., 2010), further suggesting a connection between the two phenomena, although it remains unclear whether the relevant androgens are of gonadal or adrenal origin (as theorized by Campbell, 2006, 2011).
Since adolescent decreases in EEG activity seem readily explainable by general cortical development, several authors have attempted to address the topic of adolescent homeostatic drive by instead examining the relative increase of delta and theta power across waking and decrease during sleep. The results from these studies have been inconsistent but suggestive. Methodologically, it is challenging to directly compare the studies' results because measures of relative change are highly sensitive to normalization procedures, especially since age-related differences in SWA are greater at sleep onset than at the end of the night. For example, children have a greater build-up of SWA across the day than older adolescents (Gaudreau et al., 2001) in comparison to levels at the end of the last sleep episode (Tobler et al., 1992). Since the build-up of homeostatic sleep pressure (Process S) follows a saturating exponential curve, further sleep deprivation in young children produces either a relatively smaller or equivalent slow wave response to that of older adolescents (Jenni et al., 2005; Kurth et al., 2010a). Using an exponential fit based on the 2-process model, Jenni et al. (2005) modeled these results as a lengthening of the time constant for SWA build-up across waking (τi) during adolescence, allowing SWA to plateau at a relatively higher upper asymptote (UA), but the results could also be interpreted as a lack of adolescent changes in the response to sleep deprivation (Kurth et al., 2010a).
Several laboratories have examined adolescent changes in the dissipation of Process S across the night. In these studies SWA or theta power was normalized using general means (either over the entire night or across the first 5 sleep episodes), which causes the exponential model fits to be mathematically constrained. Thus, a change in one variable (such as projected SWA at sleep onset) must theoretically be balanced by a change in the other variables (τd or the lower asymptote for SWA). In that sense, it is extremely suggestive that several studies imply adolescent changes in at least one parameter characterizing sleep homeostasis as modeled using SWA (Campbell et al., 2011; Gaudreau et al., 2001; trend in Tarokh et al., 2012; but see Jenni and Carskadon, 2004). Changes in the homeostatic regulation of theta power during adolescence are particularly clear (Campbell et al., 2011; Gaudreau et al., 2001), with older adolescents showing relatively less theta power at the beginning of the night, a slower exponential decline (τd), and higher lower asymptote (LA) at the end of the night. Collectively, these results suggest that the dynamics of homeostatic sleep pressure build-up and dissipation may change across adolescence. As will be explained later, these results do not necessarily imply a large decrease in the daily need for sleep, but may drive later sleep timing.
Sleep pattern development in laboratory animals
Sleep pattern development during adolescence in laboratory animals is less well-studied, but supports the assertion that other species show adolescent changes in sleep timing, architecture, and homeostasis, although these changes may not take the same form that they do in adolescent humans.
Sleep timing
A delay in the timing of rest and activity occurs during puberty in rhesus macaques resembling that of human adolescents (Golub et al., 1996, 2002). In contrast, in species with polyphasic sleep, puberty appears to be accompanied by a phase-advance in the timing of rest and activity and the development of more consolidated patterns of sleep and wakefulness (rat: Cambras and Diez-Noguera, 1988; Castro and Andrade, 2005; Diez-Noguera and Cambras, 1990; Hagenauer et al., 2011a; Ibuka, 1984; Joutsiniemi et al., 1991; Sieck et al., 1978: degu: Hagenauer et al., 2011b; Hummer et al., 2007; mice: Weinert and Waterhouse, 1999; marmoset: Melo et al., 2010). For example, in our laboratory, the daily rest/activity rhythms of juveniles and early pubertal rodents (rats, degus) are characterized by a crepuscular (bimodal “twilight”) pattern, with the majority of activity occurring near the end of the typical active phase for the species. As puberty progresses, daily activity shifts earlier (phase-advances), so that by adulthood most activities have consolidated at the beginning of the active phase for the species (Hagenauer et al., 2011a,b; McGinnis et al., 2007). A similar consolidation or phase advance of daily/rest activity rhythms during puberty may also occur in mice (Weinert and Waterhouse, 1999) and marmosets (Melo et al., 2010). Overall, these results indicate that a shift in the timing of rest and activity during puberty is likely to be a common phenomenon in mammals.
This shift in timing is related to pubertal hormones. Similar to humans, there is a large sex difference in the magnitude of the chronotype shift during puberty in rodents, with males showing a greater shift than females (Hagenauer et al., 2011a,b; although see Hummer et al., 2007), and the developmental timing of the shift parallels sex differences in the progression through puberty (Hagenauer et al., 2011a). A similar correlation with pubertal timing was found in rhesus macaques in a study comparing the activity rhythms of rhesus macaques with normal or delayed pubertal development (Golub et al., 2002). We recently found that prepubertally gonadectomized animals either completely lacked a shift in chronotype during the normal pubertal period (degus: Hagenauer et al., 2011b; Hummer et al., 2007) or showed a much diminished shift in chronotype (rats: Hagenauer et al., 2011a) which could be rescued by pubertal hormone replacement with either androgens or estrogens (M.H. Hagenauer, C.L. Fournier, A.M. Wehner, J. Eisman, B. Ajegba, and T.M. Lee, unpublished data). Rhythm consolidation is similarly related to increasing hormone levels, as consolidation is prevented by prepubertal gonadectomy (Hagenauer et al., 2011a,b), and mimicked by the induction of precocious puberty (Sieck et al., 1978).
Sleep architecture
Several studies in rodents have reported an increase in wakefulness during puberty, which is complemented by a decrease in NREM and REM sleep (Ibuka, 1984; Mirmiran et al., 1982; Sieck et al., 1976). These results may be due to hormonal increases, as they are observed following the induction of precocious puberty (Sieck et al., 1976), and activity levels are decreased by pre-pubertal gonadectomy (Hagenauer et al., 2011a) and increased by pubertal hormone treatment (M.H. Hagenauer, C.L. Fournier, A.M. Wehner, J. Eisman, B. Ajegba, and T.M. Lee, unpublished data). However, these changes may only be true for animals maintained in impoverished laboratory housing environments, as rats maintained under enriched conditions actually show an increase in NREM and REM sleep during puberty (Mirmiran et al., 1982).
Another highly replicated finding is the emergence of a daily rhythm in REM sleep during puberty in rodents. Prior to puberty, NREM sleep exhibits a rhythm typical of adults, but the rhythm in REM sleep is either low-amplitude, non-existent, or phase-reversed from that typical of adults (Alfoldi et al., 1990; Gvilia et al., 2011; Ibuka, 1984; Perryman, 2010; Sieck et al., 1976; but see Frank and Heller, 1997). The emergence of a daily rhythm in REMS appears to be independent of pubertal hormones, as the induction of precocious puberty is insufficient to induce a daily REMS rhythm in rats (Sieck et al., 1976). As REMS is highly circadian modulated (Dijk and Czeisler, 1994), the development of a REMS rhythm during puberty in rats may indicate an overall increase in circadian output to sleep systems.
It is unclear whether other species show a decline in SWA during puberty similar to humans. Several studies indicate that delta power is still increasing following weaning in rodents (Gvilia et al., 2011; Jouvet-Mounier et al., 1970; but see Bronzino et al., 1987; Frank and Heller, 1997), and then either decreases or holds steady during puberty (Ahnaou et al., 2007; Bronzino et al., 1987). Complementing the human results, developmental trends in delta power appear somewhat similar to changes observed in frontal grey matter volume (Van Eden and Uylings, 1985), but overall the results remain weak and relatively uncharacterized. Since there is evidence that cortical development during adolescence is sensitive to environmental enrichment (Bock et al., 2008), it is possible that full cortical pruning and a decline in delta power akin to human adolescents may only consistently emerge in laboratory animals living in and interacting with more natural environments. In this case, more detailed studies of delta power during NREM sleep in rodents could help pinpoint developmental stages with robust cortical plasticity (and potential vulnerability) analogous to human adolescence.
Sleep homeostasis
Following weaning, rodents develop more resistance to sleep deprivation, both in terms of SWA build-up and observable behavior (Alfoldi et al., 1990). An informal comparison of data from pre-pubertal animals with published data from adults suggests that this trend continues during adolescence (Alfoldi et al., 1990). Recently, we confirmed that a resistance to sleep deprivation develops during adolescence in a slow-developing species of rodent (Perryman, 2010), indicating that these previous findings from rodents were not simply a product of rapid growth. Whether this reduced homeostatic sleep drive is caused by pubertal hormones remains unknown, but gonadal hormones have been shown to alter sleep homeostasis in studies of adult rodents (Mong et al., 2011).
Discussion
Both humans and laboratory animals undergo large changes in sleep patterns during adolescence, including changes in sleep timing, sleep architecture, and sleep homeostasis. In our previous review, we discussed in detail how these changes might relate to hormonally-driven development of the circadian timekeeping system (Hagenauer and Lee, 2012). There are several pieces of evidence that suggest that the phasing of circadian output shifts during adolescence, paralleling the shift in sleep timing. The strongest evidence comes from an examination of adolescent rhythms in the “night hormone” melatonin, which tightly reflects the phasing of output from the brain's master circadian regulator, the suprachiasmatic nucleus (Lewy and Sack, 1989). Daily melatonin rhythms reveal that adolescent humans continue to show a delayed circadian rhythm phase after several weeks of regulated schedules that allow for sufficient sleep, even under controlled laboratory conditions in which there is limited possibility for social influence (Carskadon et al., 1997, 2004). Since the circadian system possesses a bi-directional relationship with the hypothalamic-pituitary-gonadal axis (Kennaway, 2005; Sellix and Menaker, 2010; Turek and Van Cauter, 1994), and circadian physiology and rhythmic behavior are known to be highly sensitive to gonadal hormone exposure during adulthood and early development in laboratory rodents (Karatsoreos and Silver, 2007; Mong et al., 2011), we theorized that circadian physiology was likely to be affected by pubertal hormones. This theory was supported by growing evidence that multiple properties of the circadian system change during the time of adolescence in humans and animals, including the period of internally-generated rhythms (Carskadon, 2008; Carskadon et al., 1999; Hagenauer et al., 2011a; Hummer, 2006; Hummer et al., 2007; McGinnis et al., 2007) and sensitivity to environmental time cues (Borisenkov, 2011; Crowley and Carskadon, 2010; Hagenauer et al., 2009; Sharkey et al., 2011; Weinert and Kompauerova, 1998; Weinert et al., 1994). This evidence led us to conclude that the changes in sleep timing that occur during adolescence may represent the influence of pubertal hormones on the circadian system, potentially providing an evolutionary benefit of allowing young animals to occupy a distinct temporal niche from that of older, dominant individuals (Hagenauer and Lee, 2012).
In this review, we present a complementary hypothesis rooted in the sleep literature. Using the original two-process model (Daan et al., 1984), we have run a set of simulations that indicate that many of the species-specific characteristics of adolescent sleep patterns can be recreated by a universal decrease in the homeostatic drive to sleep (τi, τd). In species with monophasic sleep, such as humans and macaques, our model predicts that a lengthening of the time constants for the build-up and dissipation of sleep pressure (~45% increase in τi, τd) could produce a large (3–4 h) phase delay in sleep timing accompanied by a small decrease in overall sleep time (0.7–1 h; Fig. 3A & B). These changes in sleep homeostasis (τi, τd) would produce a relative decrease in sleep pressure (Process S: SWA and theta power) at sleep onset and a relative increase in sleep pressure at sleep offset (Fig. 3C). Moreover, if the circadian fluctuation in the model is more broadly interpreted to not only influence thresholds for waking and sleeping (U & L), but generate daily fluctuation in arousal levels, the model also may predict a paradoxical increase in daytime sleepiness (as originally proposed by Carskadon and Acebo, 2002) because it increases the desynchrony between sleep timing and the circadian drive to sleep. Overall, these predictions fit the human literature, which shows a clear phase delay in sleep timing, and a decrease in sleep pressure at sleep onset in adolescents as measured by percent time in SWS or SWA. A lengthening of the homeostatic time constants during adolescence has also been consistently observed (9–43%), but often has not reached statistical significance (Campbell et al., 2011; Gaudreau et al., 2001; Jenni et al., 2005; Tarokh et al., 2012). A similar model, in which only the time constant for the homeostatic build-up of sleep (τi) is lengthened (as proposed by Jenni et al., 2005), also produces a delay in sleep timing but at the cost of a large decrease in overall sleep time in a manner that does not fit adolescent sleep patterns (Fig. 3D).
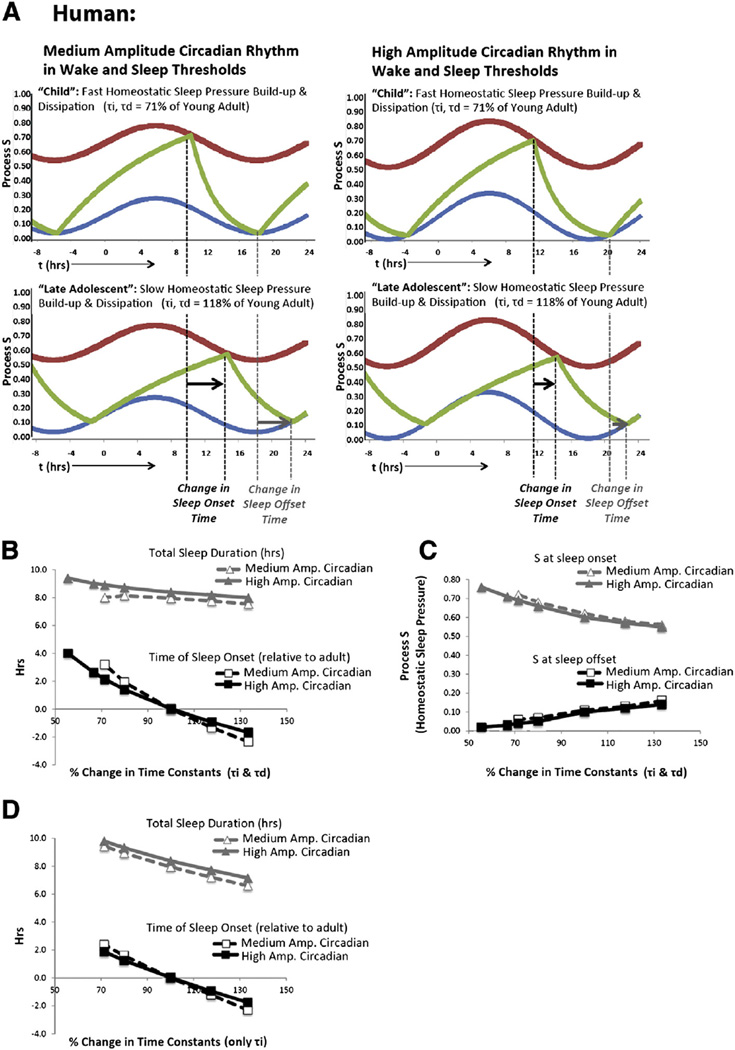
Fig. 3.
Modeling adolescent sleep in humans using the two-process model. To examine the theoretical effect of an adolescent change in each of the sleep parameters, we varied them around the initial values derived from young adults in Daan et al., 1984 (illustrated in Fig. 2). Slowing the homeostatic time constants (τi, τd) reliably produced a delay in the timing of sleep. To come to this conclusion, τi and τd were varied between 56% and 133% of their original values and only values that produced stable, monophasic sleep were seriously considered. Similar to Daan et al. (1984), we also explored the effect of varying other parameters on sleep patterns, including varying circadian amplitude by 67%–133% (between 0.08 and 0.16), and sleep threshold (U) by 50% of amplitude (data not shown). For all variable combinations, 16 simulations were constructed containing five days of data (1440 min/day). To examine the robustness of the relationship between sleep phasing and time of day, each of the 16 simulations began with one of eight possible initial levels of sleep pressure (Process S, varying from the lower wake threshold (L) to upper sleep threshold (U)) as well as a tendency for S to increase (awake) or decrease (asleep). As shown in Fig. 2, despite these varying starting points, invariably a stable sleep phase (with a 24 h period) developed in the human data (as indicated by a standard deviation of <0.3% in any 2 hour block), so sleep phasing was determined by examining the fifth day of the data set. A) Examples of modeled human sleep/wake data, following the conventions of Fig. 2. B) The time of sleep onset is relatively more advanced (positive) when sleep pressure builds up and dissipates quickly (smaller τi, τd compared to young adults in Daan et al., 1984), and relatively phase-delayed (negative) when sleep pressure builds up and dissipates slowly (larger τi, τd compared to young adults). We found that these changes were accompanied by age-appropriate sleep durations when the circadian rhythm in the wake and sleep threshold had an amplitude that was higher (A = 0.16, “High Amp”) than that previously identified for young adults (Daan et al., 1984, A = 0.12, “Med Amp”), whereas an amplitude similar to that of young adults allowed sleep timing to change in response to variation in the homeostatic time constants (τi, τd) while maintaining a relatively stable sleep duration of 7.5–8.3 h. Therefore, we would theorize that simulations that include a medium amplitude circadian rhythm might better predict changes in sleep timing in response to altered sleep homeostasis in adults. C) When homeostatic time constants are faster (τi, τd), the amount of sleep pressure (Process S) is higher at sleep onset and lower at sleep offset than when homeostatic time constants are slower (τi, τd). D) Models that only include changes in the homeostatic build-up (but not dissipation) of sleep pressure (as suggested by Jenni and Carskadon, 2005) produce large changes in both sleep onset time and sleep duration, and thus do not closely mimic adolescent sleep patterns.
For polyphasic species, such as laboratory rodents and marmosets, our model predicts an increased consolidation of sleep and wake episodes, accompanied by a small phase advance in the sleep/wake cycle (Fig. 4), similar to what has been observed in laboratory studies. A parallel increase in the amplitude of circadian output to sleep systems or in the sensitivity of the sleep system to circadian signals might allow for the emergence of daily rhythms in REM sleep and the further consolidation of daily rhythms in sleeping and waking, although the model still cannot fully explain the presence of juvenile crepuscularity.
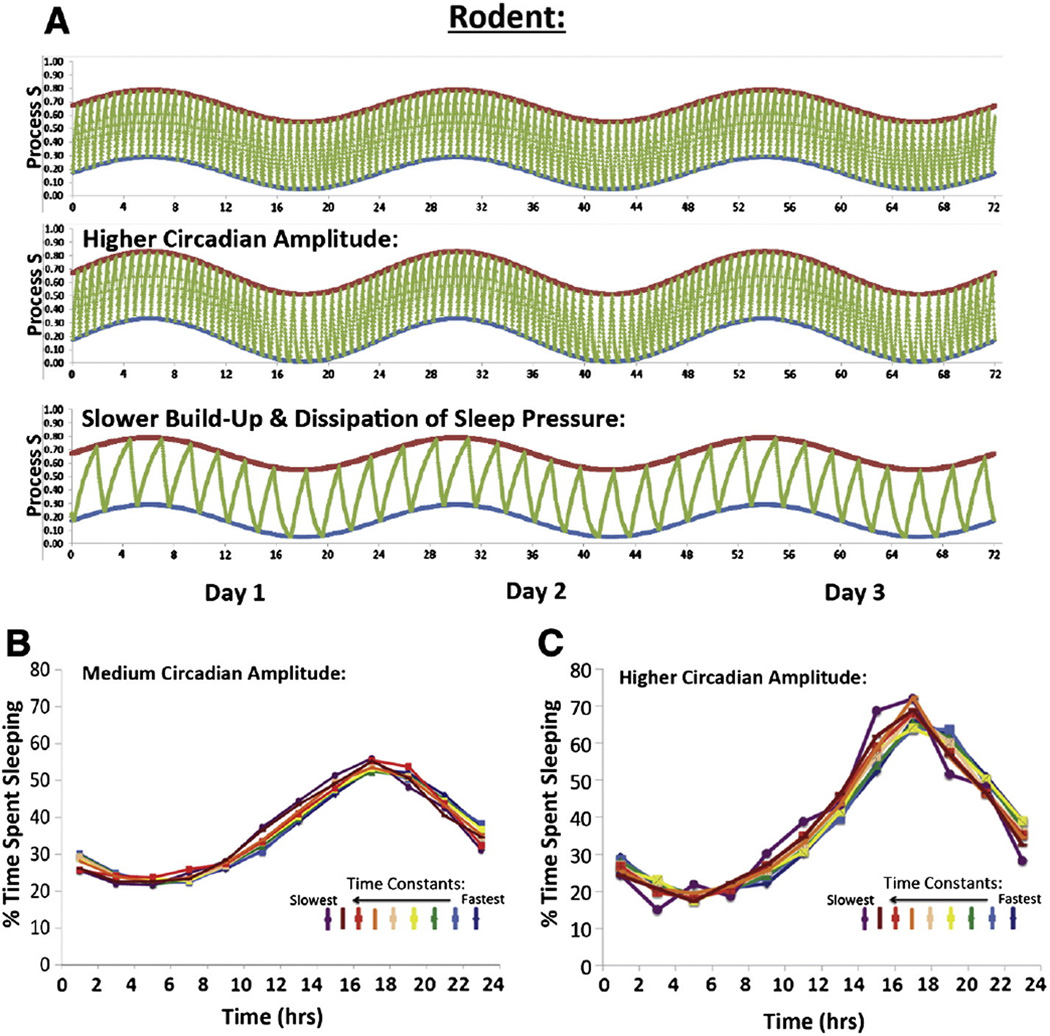
Fig. 4.
Modeling adolescent sleep in rodents using the two-process model. A) To model adolescent changes in the sleep patterns of polyphasic species, such as laboratory rodents, we increased the speed of homeostatic sleep pressure build up and dissipation (Phillips et al., 2010), and then ran a similar set of simulations as humans, but varying the time constants between 50% and 400% of τi = 0.215 h (an instantaneous build up rate of 1.65/h) and τd = 0.606 h (an instantaneous break down rate of 4.65/h). It should be noted that although the time constants for sleep pressure build up and dissipation have not been well-studied in developing rodents, models predict that a 160–200% increase in τ might occur in these small, fast-developing species simply due to brain growth (Phillips et al., 2010) and observations of behavioral and SWA suggest that the ability to tolerate sleep deprivation may increase by 3 to 12-fold (!) in rats between weaning and adulthood (Alfoldi et al., 1990). We found that a higher amplitude circadian rhythm in the sleep and wake threshold and slower time constants produced an obvious consolidation of sleep and wake. B) Slower time constants produced a small phase advance in the timing of sleep. Sleep data from the simulations was averaged over three days to produce estimates of daily rhythms in percentage time spent asleep, with data from the slowest time constants illustrated in red, and the fastest illustrated in blue. The phase advance in response to slower time constants is indicated by the leftward shift in the curves, as indicated by the rainbow appearance. C) These trends were more obvious in models that included a higher amplitude circadian rhythm in the sleep and wake threshold (A = 0.16). These models also showed a higher amplitude daily rhythm in sleep and wake, typical of mature rodents.
In total, we ran over 3400 simulated days of data but the results for our model should still be considered preliminary, as we have not determined whether the conclusions can be generalized to other models of polyphasic sleep, or whether they hold true when we only consider a physiological amount of variation in the sleep variables for each of the respective species. Despite these weaknesses, recently we found evidence that a shift in the timing of melatonin secretion and circadian gene expression in the suprachiasmatic nucleus does not occur in laboratory rodents during puberty, despite clear shifts in the timing of rest and activity (Liu T., Wehner A.M., Borjigin J., Hagenauer M.H., Lee T.M., unpublished data; Hagenauer M.H., Altshuler D.B., Wang S.S., Mossner J.M., Lee T.M., unpublished data). Although these data certainly do not rule out pubertal influences on the circadian system, which are strongly indicated by a variety of evidence (Hagenauer and Lee, 2012), they do support our homeostatic model, and raise the question of whether the delayed melatonin rhythms in human adolescents may represent an interaction between adolescent circadian development and human usage of artificial lighting, instead of a simple hormonally-driven circadian shift. Growing evidence suggests that adolescents may be relatively more sensitive to the circadian phase-shifting effects of evening light (for a full review see Hagenauer and Lee, 2012), and several studies have indicated that evening chronotype in adolescents is less severe in rural locations where there is less exposure to artificial light at night (Peixoto et al., 2009; Vollmer et al., 2012). In this case, it may be that a propensity towards evening chronotype develops in humans during adolescence due to hormonally-driven changes in sleep homeostasis, but this propensity is then aggravated by a further shift in circadian timekeeping due to a heightened sensitivity to evening light exposure.
In conclusion, we have demonstrated that the basic features of adolescent sleep patterns in a variety of species can be recreated in a model based on a decrease in the homeostatic drive to sleep (τi, τd). We hope that this model demonstrates the usefulness of cross-species comparisons, and will inspire further investigation into adolescent sleep in non-human species. Also, although many questions remain regarding the source of adolescent sleep patterns, it is clear that interventions that address either homeostatic drive or circadian phase are likely to help adolescents shift their sleep earlier to better adjust to societal needs. In particular, the homeostatic drive to sleep could be enhanced by the incorporation of greater physical activity into the school day, and the phase of circadian output could be shifted earlier by parental restrictions on screen time in the evenings (Cain and Gradisar, 2010) and a reduction of evening light exposure (Peixoto et al., 2009; Vollmer et al., 2012).
Acknowledgments
We would like to thank John Basler and Dr. Victoria Booth for their guidance and feedback regarding model construction. We would also like to thank Katherine Prater, Chelsea Fournier, Dr. Stephanie Crowley, and Dr. Jamie Perryman for their insightful feedback and editing. This research was supported by a grant from the National Science Foundation (TML, MHH — IBN-0952046) as well as an Endocrinology training grant from the National Institute of Health through the Department of Pediatrics at University of Michigan (MHH — T32-DK071212).
Footnotes
This article is part of a Special Issue “Puberty and Adolescence”.
References
- Ahnaou A, Nayak S, Heylen A, Ashton D, Drinkenburg WHIM. Sleep and EEG profile in neonatal hippocampal lesion model of schizophrenia. Physiol. Behav. 2007;92:461–467. doi: 10.1016/j.physbeh.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Alfoldi P, Tobler I, Borbely AA. Sleep regulation in rats during early development. Am. J. Physiol. 1990;258:R634–R644. doi: 10.1152/ajpregu.1990.258.3.R634. [DOI] [PubMed] [Google Scholar]
- Andrade M, Menna-Barreto L. Sleep patterns of high school students living in Sao Paulo, Brazil. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; 2002. pp. 118–131. [Google Scholar]
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118(3062):273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- Baker FC, Turlington SR, Colrain I. Developmental changes in the sleep electroencephalogram of adolescent boys and girls. J. Sleep Res. 2012;21:59–67. doi: 10.1111/j.1365-2869.2011.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Murmu RP, Ferdman N, Leshem M, Braun K. Refinement of dendritic and synaptic networks in the rodent anterior cingulate and orbitofrontal cortex: critical impact of early and late social experience. Dev. Neurobiol. 2008;68:685–695. doi: 10.1002/dneu.20622. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. EEG Clin. Neurophysiol. 1981;51:483–493. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- Borisenkov MF. The pattern of entrainment of the human sleep–wake rhythm by the natural photoperiod of the north. Chronobiol. Int. 2011;28:921–929. doi: 10.3109/07420528.2011.623978. [DOI] [PubMed] [Google Scholar]
- Bronzino JD, Siok CJ, Austin K, Austin-Lafrance RJ, Morgane PJ. Spectral analysis of the electroencephalogram in the developing rat. Brain Res. Dev. Brain Res. 1987;35:257–267. doi: 10.1016/0165-3806(87)90050-2. [DOI] [PubMed] [Google Scholar]
- Buchmann A, Ringli M, Kurth S, Schaerer M, Geiger A, Jenni OG, Huber R. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb. Cortex. 2011;21:607–615. doi: 10.1093/cercor/bhq129. [DOI] [PubMed] [Google Scholar]
- Cain N, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: a review. Sleep Med. 2010;11:735–742. doi: 10.1016/j.sleep.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Cambras T, Diez-Noguera A. Changes in motor activity during the development of the circadian rhythm in the rat. J. Interdiscip. Cycle Res. 1988;19:65–74. [Google Scholar]
- Campbell B. Adrenarche and the evolution of human life history. Am. J. Hum. Biol. 2006;18:569–589. doi: 10.1002/ajhb.20528. [DOI] [PubMed] [Google Scholar]
- Campbell B. Adrenarche in comparative perspective. Am. J. Hum. Biol. 2011;23:44–52. doi: 10.1002/ajhb.21111. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. PNAS. 2009;106(13):5177–5180. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Darchia N, Higgins LM, Dykan IV, Davis NM, de Bie E, Feinberg I. Adolescent changes in the homeostatic regulation of EEG activity in the delta and theta frequency bands during NREM sleep. Sleep. 2011;34(1):83–91. doi: 10.1093/sleep/34.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Grimm KJ, de Bie E, Feinberg I. Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. PNAS. 2012;109(15):5740–5743. doi: 10.1073/pnas.1120860109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA. Maturation of processes regulating sleep in adolescents. In: Marcus CL, Carroll JL, Donnelly DF, Loughlin GM, editors. Sleep in Children: Developmental Changes in Sleep Patterns. 2nd. Informa Healthcare; 2008. pp. 95–109. [Google Scholar]
- Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr. Clin. North Am. 2011;58:637–647. doi: 10.1016/j.pcl.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C. Regulation of sleepiness in adolescents: update, insights, and speculation. Sleep. 2002;25(6):606–614. doi: 10.1093/sleep/25.6.606. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2(4):453–460. doi: 10.1093/sleep/2.4.453. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Mancuso J, Rosekind MR. Impact of part-time employment on adolescent sleep patterns. Sleep Res. 1989;18:114. [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms in adolescent humans. J. Biol. Rhythms. 1997;12(3):278–289. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–881. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Labyak SE, Acebo C, Seifer R. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci. Lett. 1999;260:129–132. doi: 10.1016/s0304-3940(98)00971-9. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann. N.Y. Acad. Sci. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Castro ECV, Andrade MMM. Longitudinal study of the spectral composition of behavioral rhythms in the rat. Biol. Rhythm. Res. 2005;36:131–140. [Google Scholar]
- Colrain IM, Baker FC. Changes in sleep as a function of adolescent development. Neuropsychol. Rev. 2011;21:5–21. doi: 10.1007/s11065-010-9155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiol. Int. 2010;27:1469–1492. doi: 10.3109/07420528.2010.503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8:602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Daan S, Beersma DGM, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am. J. Physiol. 1984;246:R161–R178. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- Dahl RE. The development and disorders of sleep. Adv. Pediatr. 1998;45:73–90. [PubMed] [Google Scholar]
- Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. EEG Clin. Neurophysiol. 1957;9:673–690. doi: 10.1016/0013-4694(57)90088-3. [DOI] [PubMed] [Google Scholar]
- Diez-Noguera A, Cambras T. Sex differences in the development of the motor activity circadian rhythm in rats under constant light. Physiol. Behav. 1990;47:889–894. doi: 10.1016/0031-9384(90)90014-u. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci. Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys — evidence for opponent processes in sleep wake regulation. J. Neurosci. 1993;13(3):1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG. Sleep EEG changes during adolescence: an index of fundamental brain reorganization. Brain Cogn. 2010;72:56–65. doi: 10.1016/j.bandc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Thode HC, Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cerebral metabolic rate and synaptic density. J. Theor. Biol. 1990;142:149–161. doi: 10.1016/s0022-5193(05)80218-8. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Davis NM, de Bie E, Grimm KJ, Campbell IG. The maturational trajectories of NREM and REM sleep durations differ across adolescence on both school night and extended sleep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R533–R540. doi: 10.1152/ajpregu.00532.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Booth V, Forger DB, Diniz Behn CG. Circadian regulation of sleep–wake behavior in nocturnal rats requires multiple signals from suprachiasmatic nucleus. Philos. Trans. R. Soc. Lond. A. 2011;369:3855–3883. doi: 10.1098/rsta.2011.0085. [DOI] [PubMed] [Google Scholar]
- Frank MG, Heller HC. Development of diurnal organization of EEG slow-wave activity and slow-wave sleep in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997;273:R472–R478. doi: 10.1152/ajpregu.1997.273.2.R472. [DOI] [PubMed] [Google Scholar]
- Franken P, Tobler I, Borbely AA. Sleep homeostasis in the rat: simulation of the time course of EEG slow-wave activity. Neurosci. Lett. 1991;130:141–144. doi: 10.1016/0304-3940(91)90382-4. [DOI] [PubMed] [Google Scholar]
- Gaudreau H, Carrier J, Montplaisir J. Age-related modifications of NREM sleep EEG: from childhood to middle age. J. Sleep Res. 2001;10:165–172. doi: 10.1046/j.1365-2869.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F. Sleep patterns and daytime function in adolescence: an epidemiological survey of an Italian high school student sample. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; 2002. pp. 132–147. [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, Lenroot RK. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol. Sex Differ. 2012;3(19):1–9. doi: 10.1186/2042-6410-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Takeuchi PT, Hendrickx CL, Gershwin AG. Activity and attention in zinc-deprived adolescent monkeys. Am. J. Clin. Nutr. 1996;64(6):908–915. doi: 10.1093/ajcn/64.6.908. [DOI] [PubMed] [Google Scholar]
- Golub MS, Takeuchi PT, Hoban-Higgins TM. Nutrition and circadian activity offset in adolescent rhesus monkeys. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; 2002. pp. 50–68. [Google Scholar]
- Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12:110–118. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Gvilia I, Suntsova N, Angara B, McGinty D, Szymusiak R. Maturation of sleep homeostasis in developing rats: a role for preoptic area neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300:R885–R894. doi: 10.1152/ajpregu.00727.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Lee TM. The neuroendocrine control of the circadian system: Adolescent chronotype. Front. Neuroendocrinol. 2012;33:211–229. doi: 10.1016/j.yfrne.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev. Neurosci. 2009;31:276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, King AF, Possidente B, McGinnis MY, Lumia AR, Peckham EM, Lee TM. Changes in circadian rhythms during puberty in Rattus norvegicus: developmental time course and gonadal dependency. Horm. Behav. 2011a;60:46–57. doi: 10.1016/j.yhbeh.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Ku JH, Lee TM. Chronotype changes during puberty depend on gonadal hormones in the slow-developing rodent, Octodon degus. Horm. Behav. 2011b;60:37–45. doi: 10.1016/j.yhbeh.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummer DL. Dissertation in Fulfillment of a Doctor of Philosophy in Psychology. Ann Arbor: University of Michigan; 2006. Development and Sexual Differentiation of the Circadian System of Octodon Degus. [Google Scholar]
- Hummer DL, Jechura TJ, Mahoney MM, Lee TM. Gonadal hormone effects on entrained and free-running rhythms in the developing diurnal rodent, Octodon degus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R586–R597. doi: 10.1152/ajpregu.00043.2006. [DOI] [PubMed] [Google Scholar]
- Ibuka N. Ontogenesis of circadian sleep-wakefulness rhythms and developmental changes of sleep in the altricial rat and in the precocial guinea pig. Behav. Brain Res. 1984;11:185–196. doi: 10.1016/0166-4328(84)90210-9. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27(4):774–783. [PubMed] [Google Scholar]
- Jenni OG, Carskadon MA. Sleep behavior and sleep regulation from infancy through adolescence: normative aspects. Sleep Med. Clin. 2007;2:321–329. [Google Scholar]
- Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28(11):1446–1454. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- Johnson CH. Phase response curves: what can they tell us about circadian clocks? In: Hiroshige T, Honma K, editors. Circadian Clocks from Cell to Human. Sapporo: Hokkaido University Press; 1992. pp. 209–246. [Google Scholar]
- Joutsiniemi SL, Leinonen L, Laakso ML. Continuous recording of locomotor activity in groups of rats: postweaning maturation. Physiol. Behav. 1991;50:649–654. doi: 10.1016/0031-9384(91)90562-3. [DOI] [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev. Psychobiol. 1970;2(4):216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Silver R. Minireview: the neuroendocrinology of the suprachiasmatic nucleus as a conductor of body time in mammals. Endocrinology. 2007;148:5640–5647. doi: 10.1210/en.2007-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kas MJH, Edgar DM. Circadian timed wakefulness at dawn opposes compensatory sleep responses after sleep deprivation in Octodon degus. Sleep. 1999;22(8):1045–1053. doi: 10.1093/sleep/22.8.1045. [DOI] [PubMed] [Google Scholar]
- Kennaway DJ. The role of circadian rhythmicity in reproduction. Hum. Reprod. Update. 2005;11:91–101. doi: 10.1093/humupd/dmh054. [DOI] [PubMed] [Google Scholar]
- Kurth S, Jenni OG, Riedner BA, Tononi G, Carskadon MA, Huber R. Characteristics of sleep slow waves in children and adolescents. Sleep. 2010a;33(4):475–480. doi: 10.1093/sleep/33.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Ringli M, Geiger A, LeBourgeois M, Jenni OG, Huber R. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalographic study. J. Neurosci. 2010b;30(40):13211–13219. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiol. Int. 1989:93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figueira HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiol. Behav. 2007;92:1010–1018. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo PR, Belisio AS, Menezes AAL, Azevedo CVM. Influence of seasonality on circadian motor activity rhythm in common marmosets during puberty. Chronobiol. Int. 2010;27(7):1420–1437. doi: 10.3109/07420528.2010.501416. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Van den dungen H, Uylings HBM. Sleep patterns during rearing under different environmental conditions in juvenile rats. Brain Res. 1982;233:287–298. doi: 10.1016/0006-8993(82)91203-3. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res. Rev. 2005;49:429–454. doi: 10.1016/j.brainresrev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, Silver R. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J. Neurosci. 2011;31:16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser D, Anderer P, Gruber G, Parapatics S, Loretz E, Boeck M, Kloesch G, Heller E, Schmidt A, Danker-Hopfe H, Saletu B, Zeitlhofer J, Dorffner G. Sleep classification according to AASM and Rechtshaffen & Kales: effects on sleep scoring parameters. Sleep. 2009;32(2):139–149. doi: 10.1093/sleep/32.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation Sleep and Teens Task Force. Adolescent Sleep Needs and Patterns: Research Report and Resource Guide. Washington D.C: National Sleep Foundation; 2000. pp. 1–26. [Google Scholar]
- Ojeda SR, Urbanski HF. Puberty in the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven: 1994. pp. 363–409. [Google Scholar]
- Peixoto CAT, da Silva AGT, Carskadon MA, Louzada FM. Adolescents living in homes without electrical lighting have earlier sleep times. Behav. Sleep Med. 2009;7:73–80. doi: 10.1080/15402000902762311. [DOI] [PubMed] [Google Scholar]
- Perryman JI. Dissertation in Fulfillment of a Doctor of Philosophy in Neuroscience. Ann Arbor: University of Michigan; 2010. Circadian and Homeostatic Components of Sleep Across Sex and Development in a Diurnal Rodent, Octodon Degus. [Google Scholar]
- Phillips AJK, Robinson PA, Kedziora DJ, Abeysuriya RG. Mammalian sleep dynamics: how diverse features arise from a common physiological framework. PLOS Comput. Biol. 2010;6(6):1–9. doi: 10.1371/journal.pcbi.1000826. e1000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM. Puberty in primates. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven: 1994. pp. 453–485. [Google Scholar]
- Randler C. Gender differences in Morningness-Eveningness during adolescence. J. Genet. Psychol. 2011;172(3):302–303. doi: 10.1080/00221325.2010.535225. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, Addington A, Gogtay N, Rapoport JL, Giedd JN. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. PNAS. 2010;107(39):16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Daan S, Merrow M. The art of entrainment. J. Biol. Rhythms. 2003;18:183–194. doi: 10.1177/0748730403018003001. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Curr. Biol. 2004;14:R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Russo PM, Bruni O, Lucidi F, Ferri R, Violani C. Sleep habits and circadian preference in Italian children and adolescents. J. Sleep Res. 2007;16:163–169. doi: 10.1111/j.1365-2869.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Dahl RE, Shahar G, Rosenblat-Stein S. Sleep and the transition to adolescence: a longitudinal study. Sleep. 2009;32:1602–1609. doi: 10.1093/sleep/32.12.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellix MT, Menaker M. Circadian clocks in the ovary. Trends Endocrinol. Metab. 2010;21:628–636. doi: 10.1016/j.tem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffery JP, Lopez J, Bissette G, Roffwarg HP. Rapid eye movement sleep deprivation in post-critical period, adolescent rats alters the balance between inhibitory and excitatory mechanisms in visual cortex. Neurosci. Lett. 2006;393:131–135. doi: 10.1016/j.neulet.2005.09.051. [DOI] [PubMed] [Google Scholar]
- Sharkey KM, Carskadon MA, Figueiro MG, Zhu Y, Rea MS. Effects of an advanced sleep schedule and morning short wavelength light exposure on circadian phase in young adults with late sleep schedules. Sleep Med. 2011;12:685–692. doi: 10.1016/j.sleep.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Ramaley JA, Harper RM, Taylor AN. Sleep-wakefulness changes at the time of puberty in the female rat. Brain Res. 1976;116:346–352. doi: 10.1016/0006-8993(76)90915-x. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Ramaley JA, Harper RM, Taylor AN. Puberty-related alterations in the organization of sleep-wakefulness states: differences between spontaneous and induced pubertal conditions. Exp. Neurol. 1978;61:407–420. doi: 10.1016/0014-4886(78)90256-x. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat. Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk C, Zehr J. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA, Achermann P. Dissipation of sleep pressure is stable across adolescence. Neuroscience. 2012;216:167–177. doi: 10.1016/j.neuroscience.2012.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. J. Sleep Res. 2005;14:239–244. doi: 10.1111/j.1365-2869.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- Terman LM, Hocking A. The sleep of school age children: its distribution according to age and its relation to physical and mental efficiency. Part 1: the distribution of sleep according to age. J. Educ. Psychol. 1913a;4:138–147. [Google Scholar]
- Terman LM, Hocking A. The sleep of school age children: its distribution according to age and its relation to physical and mental efficiency. Part 3: the conditions of children's sleep. J. Educ. Psychol. 1913b;4(5):269–282. [Google Scholar]
- Thorleifsdottir B, Bjornsson JK, Benediktsdottir B, Gislason TH, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J. Psychosom. Res. 2002;53:529–537. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- Tobler I, Borbeley AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr. Clin. Neurophysiol. 1986;64:74–76. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- Tobler I, Franken P, Trachsel L, Borbeley AA. Models of sleep regulation in mammals. J. Sleep Res. 1992;1:125–127. doi: 10.1111/j.1365-2869.1992.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Turek FW, Van Cauter E. Rhythms in reproduction. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven: 1994. pp. 487–540. [Google Scholar]
- Van den Bulck J. Television viewing, computer game playing, and internet use and self-reported time to bed and time out of bed in secondary school children. Sleep. 2004;27:101–104. doi: 10.1093/sleep/27.1.101. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Uylings HBM. Postnatal volumetric development of the prefrontal cortex in the rat. J. Comp. Neurol. 1985;241:268–274. doi: 10.1002/cne.902410303. [DOI] [PubMed] [Google Scholar]
- Vollmer C, Michel U, Randler C. Outdoor light at night (LAN) is correlated with eveningness in adolescents. Chronobiol. Int. 2012;29(4):502–508. doi: 10.3109/07420528.2011.635232. [DOI] [PubMed] [Google Scholar]
- Weinert D, Kompauerova V. Light induced phase and period responses of circadian activity rhythms in laboratory mice of different age. Zoology. 1998;101:45–52. [Google Scholar]
- Weinert D, Waterhouse J. Daily activity and temperature rhythms do not change spontaneously with age in laboratory mice. Physiol. Behav. 1999;66:605–612. doi: 10.1016/s0031-9384(98)00342-4. [DOI] [PubMed] [Google Scholar]
- Weinert D, Eimert H, Erkert HG, Schneyer U. Resynchronization of the circadian corticosterone rhythm after a light/dark shift in juvenile and adult mice. Chronobiol. Int. 1994;11:222–231. doi: 10.3109/07420529409067791. [DOI] [PubMed] [Google Scholar]
- Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005;115:250–260. doi: 10.1542/peds.2004-0815G. [DOI] [PubMed] [Google Scholar]
- Yasenkov R, Deboer T. Circadian regulation of sleep and the sleep EEG under constant sleep pressure in the rat. Sleep. 2010;33(5):631–641. doi: 10.1093/sleep/33.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]