SUMMARY
The cholinergic hypothesis of schizophrenia emerged over 50 years ago based on clinical observations with both anticholinergics and pan-muscarinic agonists. Not until the 1990s did the cholinergic hypothesis of schizophrenia receive renewed enthusiasm based on clinical data with xanomeline, a muscarinic acetylcholine receptor M1/M4-preferring orthosteric agonist. In a clinical trial with Alzheimer’s patients, xanomeline not only improved cognitive performance, but also reduced psychotic behaviors. This encouraging data spurred a second clinical trial in schizophrenic patients, wherein xanomeline significantly improved the positive, negative and cognitive symptom clusters. However, the question remained: Was the antipsychotic efficacy due to activation of M1, M4 or both M1/M4? Classical orthosteric ligands lacked the muscarinic receptor subtype selectivity required to address this key question. More recently, functional assays have allowed for the discovery of ligands that bind at allosteric sites, binding sites distinct from the orthosteric (acetylcholine) site, which are structurally less conserved and thereby afford high levels of receptor subtype selectivity. Recently, allosteric ligands, with unprecedented selectivity for either M1 or M4, have been discovered and have demonstrated comparable efficacy to xanomeline in preclinical antipsychotic and cognition models. These data suggest that selective allosteric activation of either M1 or M4 has antipsychotic potential through distinct, yet complimentary mechanisms.
Muscarinic allosteric modulation will clearly be a theme driving antipsychotic drug discovery efforts for decades to come.
With an onset in late adolescence, schizophrenia, a complex psychiatric disorder characterized by a combination of negative (social withdrawal, blunting of emotional responses, anhedonia) and positive (hallucinations, delusions, paranoia, disorganized behavior) symptoms along with significant cognitive dysfunction, is a debilitating disease that affects 1% of the world population regardless of gender, race or socioeconomic status (1–6). Inclusion of schizoaffective disorder increases the incidence rate to ~6% of the world population (7, 8). Significantly, schizophrenia and related disorders require lifelong, daily maintenance therapy at a cost to society of more than USD 65 billion a year (1–6). The etiology of schizophrenia is believed to be based upon dysregulation of dopamine and glutamate neurotransmission in mesolimbic and mesocortical brain regions, and the prevailing dogma by which schizophrenia has been managed for decades states that excessive dopaminergic transmission in the forebrain underlies the disorder—the so called “dopamine hypothesis” or “dopamine hyperfunction hypothesis” (1–6, 9–12). Support for this hypothesis stems in part from neuroimaging studies using positron emission tomography to examine alterations in central dopamine levels in schizophrenic patients, which include the finding that patients given amphetamine exhibit greater increases in dopamine release than nonschizophrenic controls (13). Furthermore, rationale for this hypothesis is based on the fact that all clinically relevant antipsychotic agents, both typical (haloperidol) and atypical (clozapine, olanzapine), possess significant antagonist activity at the dopamine D2 receptor; unfortunately, these agents have a slow onset of action and mainly treat the positive symptoms of schizophrenia, with limited to no effect on the negative and cognitive symptoms, thereby representing a substantial unmet medical need (14–17). Thus, the dopamine hypothesis fails to account for all dimensions of this complex disorder, and other theories to account for the pathology of schizophrenia have been advanced.
The NMDA receptor antagonist phencyclidine has been shown to induce the positive, negative and cognitive symptoms of schizophrenia in healthy patients and elicit a resurgence of symptoms in stable schizophrenics (18, 19). In the clinic, the observation that administration of the NMDA receptor co-agonist glycine provides a modest improvement in schizophrenic patients suggests that increasing NMDA receptor activation may provide a therapeutic benefit. These observations led to the NMDA receptor hypofunction hypothesis as an alternative theory for the underlying cause of schizophrenia (18–22). According to this hypothesis, any agent that can potentiate NMDA receptor currents, either directly by action on modulatory sites on the NMDA receptor (i.e., the glycine co-agonist binding site) or indirectly by activation of G protein-coupled receptors (GPCRs) known to potentiate NMDA receptor function (i.e., muscarinic acetylcholine M1 receptor and metabotropic glutamate receptor mGlu5) has the potential to ameliorate the symptoms of schizophrenia (5). Preclinically, agents acting at three targets that can increase NMDA receptor function can have antipsychotic-like effects. These include mGlu5 positive allosteric modulators (23, 24), glycine transporter GlyT1 inhibitors (25, 26) and an M1 allosteric agonist (27). Additionally, encouraging clinical data with GlyT1 inhibitors are beginning to surface (28). While these two hypotheses have clearly dominated the development of therapeutic agents for the treatment of schizophrenia, this review will focus on the antipsychotic potential of the selective activation of muscarinic acetylcholine receptors (mAChRs) by allosteric modulation.
MUSCARINIC RECEPTORS AND THE CHOLINERGIC HYPOTHESIS
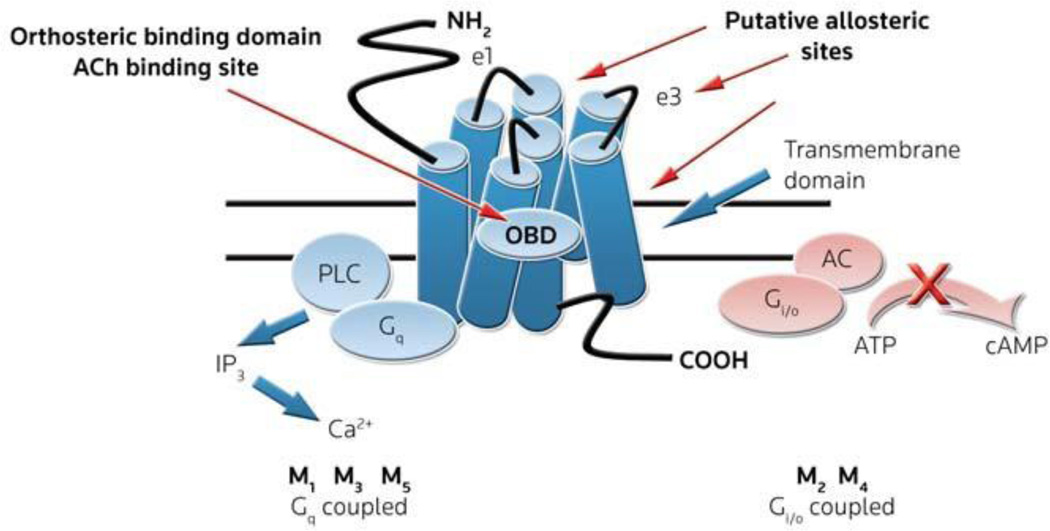
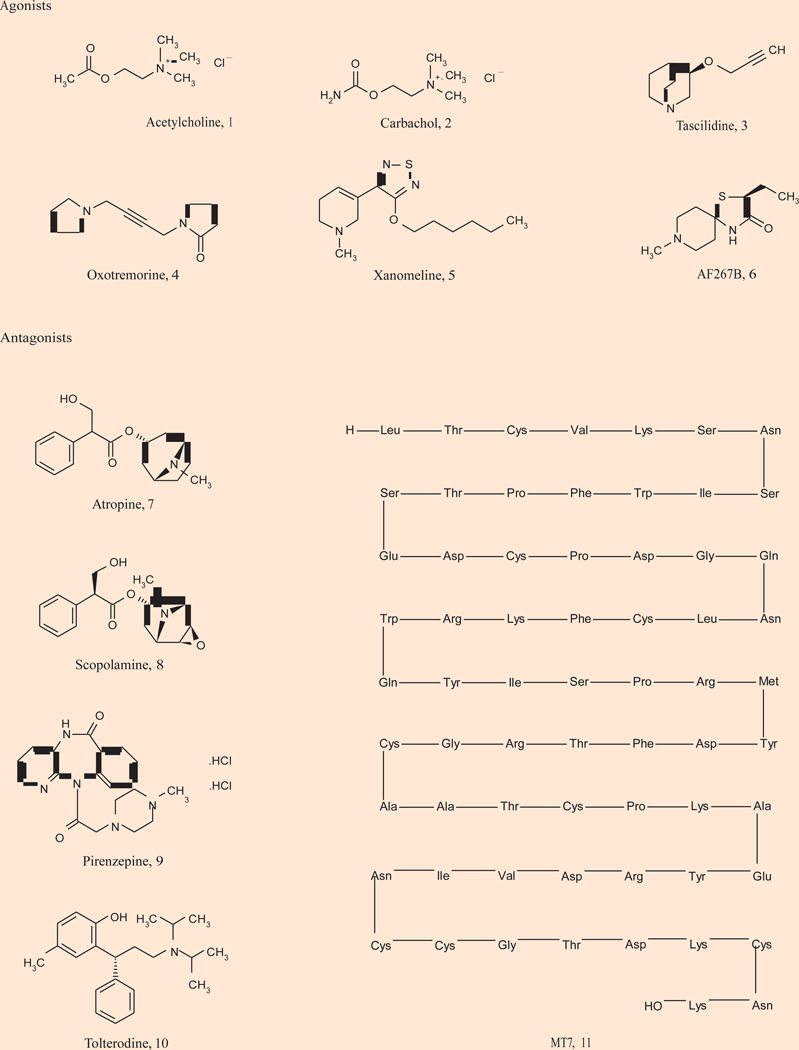
Acetylcholine (ACh) is a critical neurotransmitter in both the CNS and peripheral nervous system acting through muscarinic acetylcholine and nicotinic acetylcholine receptors. Evidence suggests that cholinergic neurotransmission in the forebrain regions and cholinergic involvement in learning and memory are mediated primarily by mAChRs (29–31). The mAChRs are members of the GPCR family A that mediate the metabotropic actions of the neurotransmitter ACh. To date, five distinct subtypes of mAChRs, M1–M5, have been cloned and sequenced. M1, M3 and M5 activate phospholipase C and calcium mobilization through Gq, whereas M2 and M4 block the action of adenylyl cyclase through Gi/o (Fig. 1) (29–31). mAChR-regulated cholinergic signaling plays a critical role in a wide variety of CNS and peripheral functions including memory and attention mechanisms, motor control, nociception, regulation of sleep-wake cycles, cardiovascular function, renal and gastrointestinal function and many others. As a result, agents that can selectively modulate the activity of specific mAChRs should have therapeutic potential in multiple pathological states (32–41). However, due to high sequence conservation within the orthosteric binding site for all five mAChR subtypes (M1–M5), it has been historically difficult to develop mAChR subtype-selective ligands that bind at the orthosteric (ACh) site (42). As a result, all historical clinical outcomes observed with orthosteric mAChR agonists 1–6 or antagonists 7–11 (Fig. 2) have proven difficult to attribute to a single receptor subtype, as these ligands activate or inhibit all five mAChRs to varying degrees.
Figure 1.
Schematic Illustration of the structures and effector systems of the muscarinic acetylcholine (ACh) receptor subtypes M1–M5. The orthosteric binding domain (OBD) is highlighted within the transmembrane domain and putative allosteric binding sites are denoted. PLC, phospholipase C; AC, adenylyl cyclase.
Figure 2.
Representative orthosteric agonists and antagonists of muscarinic acetylcholine receptors.
A third hypothesis for the etiology of schizophrenia, based on mAChRs, surfaced from clinical observations that both compliments, and yet remains distinct, from the aforementioned dominating hypotheses (5). Over 50 years ago, anticholinergic agents, such as scopolamine, were shown to induce a psychotic state similar to schizophrenia and exacerbate symptoms in schizophrenic patients. Around that same time, clinical trials provided evidence that pan-muscarinic receptor agonists were moderately effective as neuroleptic agents (43–46). These findings led to a cholinergic hypothesis of schizophrenia, decades before the dopamine hyperfunction hypothesis or the NMDA hypofunction hypothesis were proposed. In lieu of subtype-selective small-molecule ligands to probe the cholinergic hypothesis, knockout studies and other clinical, genetic, post mortem and imaging approaches have been employed to further link mAChR expression and function in the pathology of schizophrenia (8, 47).
The M1, M2 , M3 and M4 subtypes are located in the CNS, distinct regions throughout while M5 is present at low levels primarily in the midbrain, suggesting these subtypes have potential roles in the pathology of schizophrenia (48–50). From studies in knockout mice, the M1-muscarinic receptor subtype has been viewed as the most likely candidate for mediating the effects on cognition, attention mechanisms and sensory processing. Interestingly, the M1-muscarinic receptor also potentiates NMDA receptor firing (51); however, addressing NMDA hypofunction is not the only mechanism by which a cholinergic hypothesis of schizophrenia has merit. The M4 receptor is localized in dopamine-rich brain regions (the mesolimbic and nigrostriatal dopaminergic pathways), and studies in knockout mice suggest M4 regulates dopaminergic neurons involved in motor control, cognition and psychosis (52–54). Thus, activation of M1 may lead to potentiation of NMDA receptor currents and provide antipsychotic efficacy via the NMDA receptor hypofunction hypothesis, while activation of M4 regulates dopamine levels and falls under the dopamine hyperfunction hypothesis. A 2009 study found that the attenuation of amphetamine-induced activity by xanomeline was absent in M4 knockout mice and attenuated in M1 knockout mice (55). The authors conclude that the efficacy of xanomeline in amphetamine-induced hyperlocomotion is predominantly driven by M4 activation with a contribution from M1.
Data from studies using M5 knockout mice suggest that M5 is the sole mediator of ACh-induced vasodilation in the cerebral vasculature and thereby may have therapeutic relevance for cerebrovascular diseases. However, other work suggests that M5 may be relevant to schizophrenia as it is believed to regulate dopamine release in the striatum and nucleus accumbens via its midbrain localization (56). The role of M3 in the CNS is unclear from the knockout mouse with the major observable phenotype being that of hypophagy and lean (47). Again, selective small-molecule tools are required to elucidate the potential role of M3 as an antipsychotic.
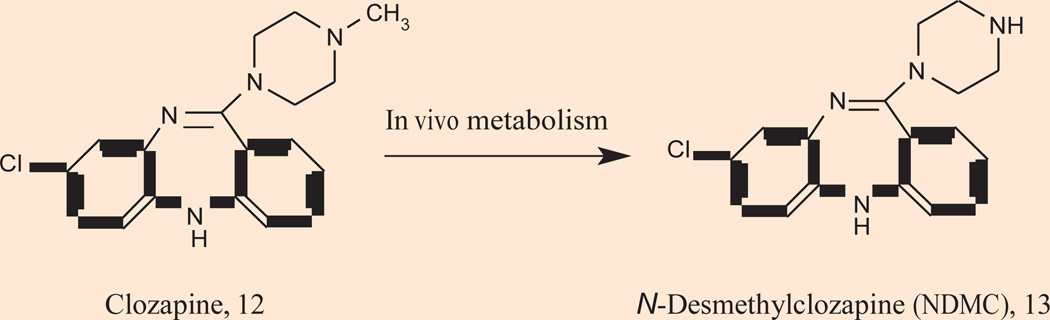
The importance of the cholinergic system in schizophrenia has been further validated clinically by clozapine 12 (Fig. 3), one of the most clinically useful atypical antipsychotics (57–59). Numerous studies suggest the unique efficacy of clozapine is due to the concentration of its major circulating metabolite, N-desmethylclozapine (NDMC) 13, an M1 receptor allosteric agonist (60–65). By no means is the efficacy purely driven by allosteric agonism of M1, but the combination of dopamine D2 receptor inhibition, coupled with selective M1 receptor agonism, may be important for the unique efficacy profile of clozapine in the treatment of recalcitrant schizophrenic patients (64, 65). mAChRs are also genetically linked to schizophrenia. De Luca and coworkers recently reported on a linkage of the M5 muscarinic gene (CHRM5) and 15q13, a gene associated with schizophrenia (66). In 2003, Liao reported on the association of M1 genetic polymorphisms with psychiatric symptoms and cognitive function in schizophrenia (67).
Figure 3.
Chemical structure of clozapine 12 and its major metabolite N-desmethylclozapine (NDMC) 13.
A role of mAChRs in schizophrenia is also suggested from post mortem CNS tissue studies. Neuropathological studies by Dean and Crook have demonstrated that levels of both M1 and M4 are significantly reduced in the prefrontal cortex, hippocampus, caudate and putamen in post mortem schizophrenic patients (68–73). Moreover, the reduction in M1 and M4 receptor expression may be specific to schizophrenia, as similar decreases were not observed in similar studies with patients suffering from bipolar disorder and major depression (74). In addition, an in vivo imaging study employing a SPECT agent, [123I]-IQNB (a pan-mAChR ligand), was performed in 12 unmedicated schizophrenic patients and findings compared to a control group of healthy subjects. mAChR receptor occupancy was diminished 20–35% in the schizophrenic patients relative to the control group (74). While clearly indicating that mAChRs are decreased in schizophrenics, the [123I]-IQNB study does not provide information on which mAChR subtype(s) are decreased. Still, this is consistent with the post mortem studies in schizophrenics.
PRECLINICAL AND CLINICAL FINDINGS WITH XANOMELINE
In numerous phase II and III clinical trials, pan-mAChR agonists were shown to improve cognitive performance in Alzheimer’s disease (AD) patients, but the gastrointestinal and/or cardiovascular side effects resulting from activation of peripheral mAChRs were deemed intolerable and the trials were discontinued (38). Importantly, several orthosteric pan-mAChR agonists demonstrated decline of Aβ42 in the cerebral spinal fluid of AD patients, suggesting that mAChR activation has the potential to be disease modifying as well as providing palliative cognitive therapy (41, 75–77). More recent studies in 3xTg-AD mice further support a disease-modifying role for mAChR activation (78). Interestingly, the M1/M4-preferring agonist xanomeline 5 (Fig. 2), in addition to improving cognitive performance, had robust therapeutic effects on the psychotic symptoms and behavioral disturbances associated with AD including hallucinations, delusions and vocal outbursts (77, 79, 80). Based on the ability of xanomeline to reduce the antipsychotic-like behaviors in AD patients, scientists at Lilly initiated an effort to evaluate xanomeline as an antipsychotic agent.
Typical and atypical antipsychotics increase dopamine release in the prefrontal cortex (PFC) and induce c-Fos expression in the PFC and nucleus accumbens. Xanomeline was found to mirror the standard antipsychotic agents clozapine and olanzapine by increasing extracellular levels of dopamine in the PFC by inducing c-Fos expression in regions in a manner comparable to clozapine (81, 82). These data suggested that xanomeline, by virtue of its M1 and M4 activation, may be a novel antipsychotic agent and led to further studies. Electrophysiologically, xanomeline, after either acute or chronic dosing, was found to inhibit A10 but not A9 dopamine cells; moreover, this effect was blocked by the muscarinic antagonist scopolamine (82). Behavioral studies (decrease in apomorphine-induced climbing, decrease in contralateral rotations in 6-OHDA-lesioned rats, inhibition of conditioned avoidance responding, reversal of apomorphine-induced deficits in prepulse inhibition, reversal of amphetamine-induced hyperlocomotion) in mice and rats followed in which xanomeline displayed comparable efficacy to clozapine without causing catalepsy contrary to the typical antipsychotic haloperidol (82, 83). In 2003, similar studies were extended to nonhuman primates (Cebus paella monkeys) with similar positive results (84). Then in 2008, the results of a phase II clinical trial in schizophrenic patients were released (85). The study was a 4-week, double-blind, placebo-controlled outcome trial in subjects with schizophrenia (N = 20) measuring the Positive and Negative Syndrome Scale (PANSS) for schizophrenia, the Brief Psychiatric Rating Scale (BPRS) and the Clinical Global Impression (CGI) scale. Impressively, subjects receiving xanomeline performed significantly better than the placebo group on both BPRS and PANSS scores (85). Cognition was also improved, with the xanomeline group displaying robust improvements in measures of vocal learning and short-term memory function. Moreover, efficacy was observed within 1 week, as opposed to the long onset of action with dopamine D2 antagonists (85). However, some adverse events were noted due to activation of peripheral mAChRs. Importantly, xanomeline afforded improvement in all three symptom clusters of schizophrenia (positive, negative and cognitive symptom clusters) with a rapid onset of action (85). One key question remained: Is the efficacy of xanomeline mediated by activation of M1, M4 or a synergy of M1 and M4 activation?
ALLOSTERIC MODULATION OF MUSCARINIC RECEPTORS
Previous attempts to develop agonists that are highly selective for individual mAChR subtypes have failed because of the high conservation of the ACh binding site, which increases the difficulty in developing compounds that are truly subtype-specific (41). To circumvent problems associated with targeting the highly conserved orthosteric ACh site, an alternative approach has focused on developing compounds that act at less highly conserved allosteric (Greek, “other site”) binding sites on the mAChRs that are spatially and often functionally distinct from the orthosteric (ACh) site. In recent years, this approach is proving to be highly successful in developing subtype-selective ligands for multiple GPCRs (e.g., mGlu, mAChR) (41, 75, 86, 87). Allosteric ligands can possess multiple modes of pharmacology. An allosteric agonist is a ligand that is capable of receptor activation in the absence of the orthosteric ligand (i.e., ACh) at a site distinct from the orthosteric (i.e., ACh) site. An allosteric modulator is a ligand that increases (positive, PAM) or decreases (negative, NAM) the action of an orthosteric agonist (i.e., ACh) by binding at an allosteric site that leads to a change in receptor conformation; however, such modulators lack intrinsic pharmacological activity at the receptor in the absence of an orthosteric ligand. A PAM may enhance the affinity of the orthosteric ligand and/or facilitate coupling to G proteins while exerting no effects alone. As opposed to a classical agonist, PAMs have three major advantages: 1) they mimic physiological signaling conditions, 2) they have greater subtype and receptor selectivity and 3) they have less risk of target-mediated toxicity due to a “ceiling effect” whereby progressively increasing doses of a PAM beyond a certain point will fail to elicit a further pharmacological response due to the limiting effect of the endogenous orthosteric agonist concentration (41, 75, 86, 87). Also, it is possible for a single molecule to have both allosteric potentiator and allosteric agonist activity (usually at high concentrations), and these ligands are referred to as ago-potentiators. Discovery of allosteric modulators typically proceeds from functional high-throughput screening using cell-based assays, which involve preincubation of the cells with test compound followed by addition of a submaximal concentration of orthosteric agonist to identify compounds that enhance the agonist response.
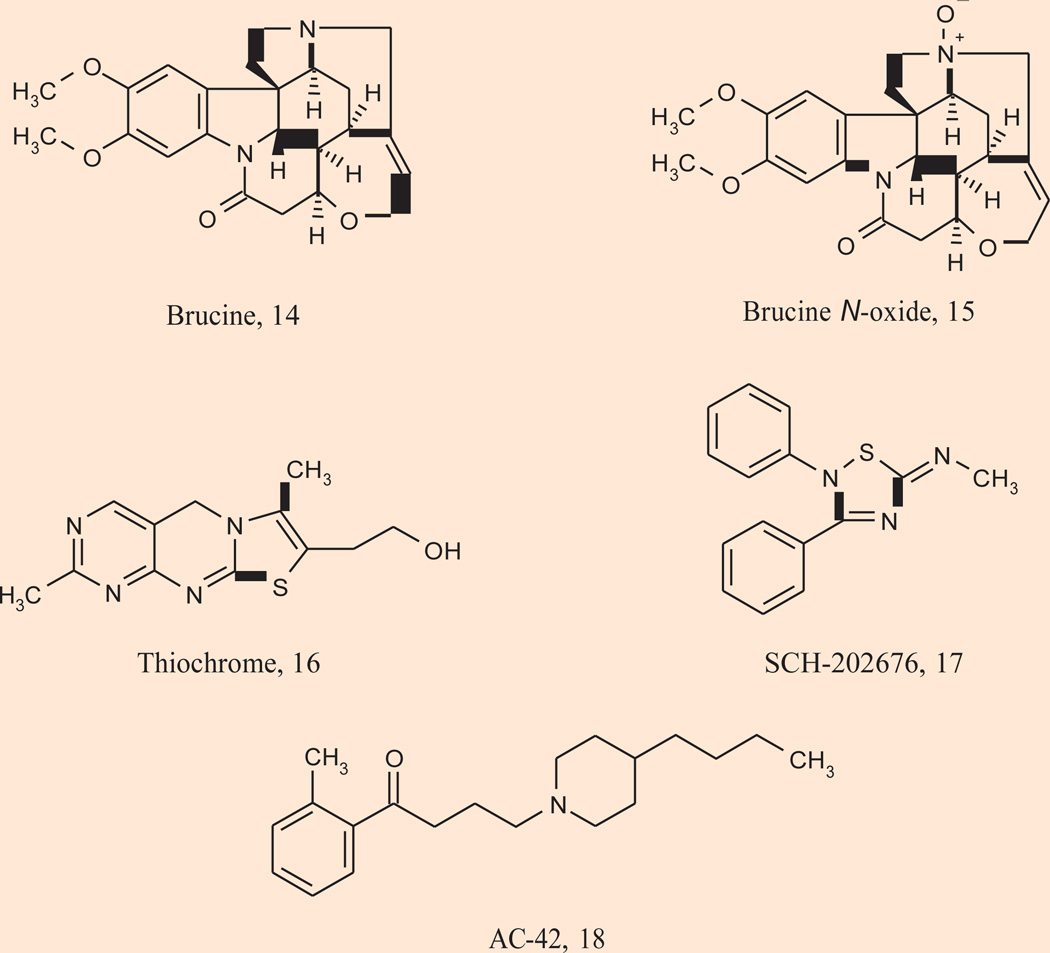
Early proof-of-concept efforts by several laboratories were successful in identifying ligands that possess PAM activity at either M1 or M4; however, these first-generation mAChR PAMs lacked efficacy and physiochemical properties to advance into in vivo studies to dissect the contribution of selective M1 and M4 activation for the efficacy of xanomeline (Fig. 4) (88–92). Brucine 14 was the first reported mAChR PAM, and it was a selective, but weak M1 PAM increasing ACh affinity only 2-fold. Surprisingly, the N-oxide of brucine 15 increased ACh affinity ~1.5-fold for M4 (90). Thiochrome 16 followed as the second reported M4 PAM and SCH-202676 17 was identified as a PAM of multiple mAChR subtypes (90, 92). For the M1 subtype, pioneering work also led to the discovery of selective allosteric agonists; however, they too suffered from ancillary pharmacology and poor physiochemical properties. AC-42 18 was the first in a novel class of M1 agonists that act by binding to an allosteric site rather than the orthosteric ACh site. AC-42 fully activates M1 and is highly selective for M1 relative to other mAChR subtypes (93). As with PAMs, this selectivity is accomplished by targeting a site distinct from the ACh binding site. Thus, mutations that render M1 insensitive to ACh do not alter activity of AC-42; however, the activity of AC-42 can be eliminated by mutations in transmembrane domains 1 and 7 that do not alter activation by ACh. NDMC 13 is another M1 agonist that demonstrates a mode of interaction with the M1 receptor that differs from that of classic orthosteric ligands and may be mechanistically similar to AC-42 (63–64). As mentioned earlier, the unique clinical efficacy of clozapine is believed to be directly related to the concentration of the major circulating metabolite NDMC (60–65).
Figure 4.
Structures of the first-generation allosteric ligands (early positive allosteric modulators and allosteric agonists) of muscarinic receptor subtypes M1 and M4.
ANTIPSYCHOTIC POTENTIAL OF M1 ALLOSTERIC MODULATION
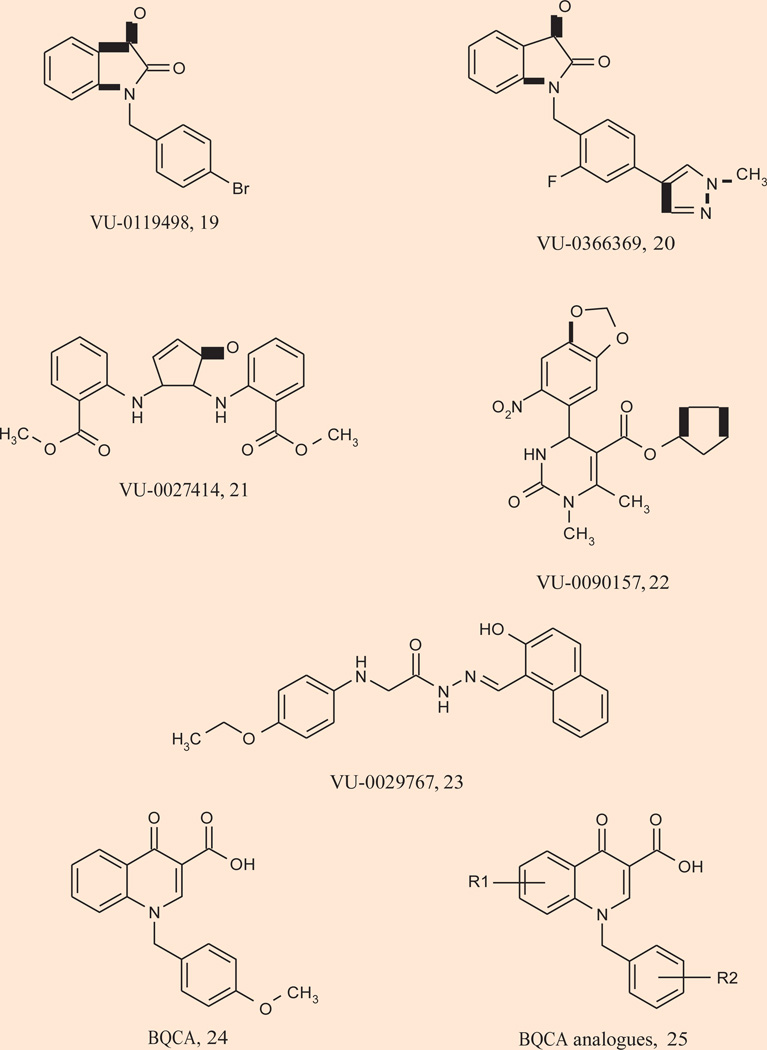
Significant advances have been made in the last 2 years in the discovery and development of both M1 PAMs and M1 allosteric agonists that possess the profile required to perform in vivo proof-of-concept studies and dissect the contribution of selective M1 activation in the preclinical and clinical efficacy of the M1/M4-preferring xanomeline 5. M1 PAMs representing multiple chemotypes 19–22 have recently been identified in a high-throughput functional screen conducted at Vanderbilt (Fig. 5) (94). None of these ligands display agonist activity, but function as pure PAMs inducing parallel leftward shifts of the ACh concentration-response curves (CRCs). PAMs 19–23 possess varying degrees of M1 selectivity (relative to the other mAChRs) with some showing complete selectivity. As anticipated, none of these M1 PAMs bind at the orthosteric site, and were shown to increase ACh affinity at M1. Interestingly, these PAMs displayed differential regulation of coupling of the M1 receptor to different signaling pathways (94). While not in vivo tools, these ligands represented a marked improvement, and demonstrated that all allosteric M1 activation is not equivalent.
Figure 5.
Structures of M1 positive allosteric modulators.
A major breakthrough resulted with the discovery of the M1 PAM coined BQCA (benzylquinolone carboxylic acid) 24 (Fig. 5) by researchers at Merck. BQCA is a potent (human M1 EC50 = 845 nM, 129-fold leftward shift of the ACh CRC), highly M1-selective PAM (no agonism, potentiation or antagonism of M2–5 observed up to 100 µM) with exceptional pharmacokinetics and CNS exposure (95). BQCA does not bind at the orthosteric ACh site, and site-directed mutagenesis experiments identified an allosteric binding site for BQCA involving residues Y179 and W400. Similar to the pre-clinical profile of xanomeline, BQCA increased c-Fos expression in critical brain regions, dose-dependently reverses amphetamine-induced hyperlocomotion in mice and reverses scopolamine-induced memory deficits in contextual fear conditioning. The procognitive phenotype of BQCA was further noted in rat sleep studies, where it modified sleep architecture (increased wakefulness with concomitant decrease in delta sleep). Quite unexpectedly, BQCA also increased blood flow to the cerebral cortex, a process formerly associated with M5 from studies in knockout mice, and another example of the critical need for selective tools to elucidate receptor function (95). Further work with BQCA by the Vanderbilt group demonstrated that activation of the M1 receptor by BQCA induces a robust inward current and an increase in spontaneous excitatory postsynaptic currents in medial prefrontal cortex (mPFC) pyramidal cells, effects which are absent in acute slices from M1 receptor knockout mice (96). To evaluate the effect of BQCA on intact and functioning brain circuits, multiple single-unit recordings were obtained from the mPFC of rats that showed BQCA increases firing of mPFC pyramidal cells in vivo. BQCA also restored discrimination reversal learning in a transgenic mouse model of Alzheimer’s disease and was found to regulate nonamyloidogenic amyloid precursor protein processing in vitro, suggesting that M1 receptor PAMs have the potential to provide both symptomatic and disease-modifying effects in Alzheimer’s disease patients. This latter study with BQCA provides compelling evidence that M1 activation induces a dramatic excitation of PFC neurons and suggests that selectively activating the M1 mAChR subtype may ameliorate impairments in cognitive function. A library of BQCA analogues 25 was also prepared, and a robust structure–activity relationship was observed, with multiple analogues displaying comparable potency, efficacy and fold-shift to BQCA (96).
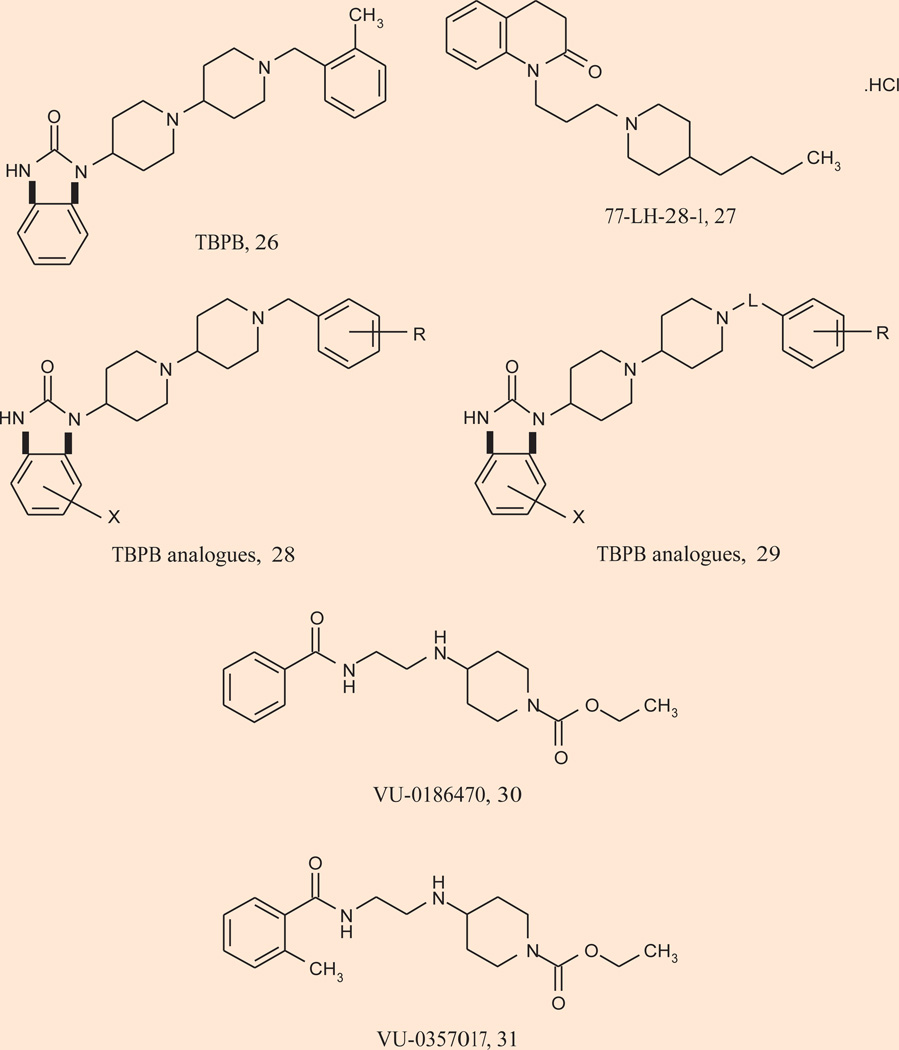
In parallel, the second-generation M1 allosteric agonists have generated equally exciting and impressive support for an antipsychotic and precognitive role of selective M1 activation (Fig. 6). TBPB 26 is a potent (EC50 = 280 nM), highly selective (> 30 µM vs. M2–5) and centrally penetrant M1 allosteric agonist (27) (Table I). A second systemically active M1 allosteric agonist, 77-LH-28-1 27, was identified from a series of AC-42 analogues (97). 77-LH-28-1 is also highly M1 selective (though weak agonism of M3 is noted), but may more accurately be referred to as a bitopic ligand. Of these, TBPB has activity in multiple electrophysiology, expression and animal models used to predict antipsychotic efficacy for the positive symptoms of schizophrenia. Activation of M1 by TBPB was shown to potentiate NMDA receptor currents, induce c-Fos expression in key brain regions, increase dopamine turnover, reverse amphetamine-induced hyperlocomotion and reverse prepulse inhibition to a degree comparable to xanomeline and other atypical antipsychotics (27). In addition, TBPB showed efficacy in reversing scopolamine-induced memory deficits in contextual fear conditioning at low doses. Similar to BQCA, TBPB was also shown to regulate nonamyloidogenic APP processing in vitro, suggesting that M1 allosteric agonists have the potential to provide both symptomatic and disease-modifying effects in Alzheimer’s disease patients (27, 97). This report was followed by accounts of TBPB analogues 28 and 29, wherein both full and partial M1 allosteric agonists were identified (98–100). Surprisingly, molecular switches (halogen substitutions) were discovered that “dialed in” dopamine D2 inhibition; thus, like NDMC, ligands could be designed to possess both D2 inhibition and M1 allosteric agonism, an attractive profile for an antipsychotic agent (98, 99).
Figure 6.
Structures of M1 allosteric agonists.
Table I.
Abbreviated summary of muscarinic acetylcholine receptor (mAChR) ligands discovered by Vanderbilt University.
| Compound | Type/mode | Potency in cell-based Ca2+ assay |
Efficacy in rodent behavioral models |
Electrophysiology/ neurochemistry |
|---|---|---|---|---|
| TBPB (26) | M1 agonist | 158 nM at rat M1, > 30 µM vs. all other mAChRs |
Amphetamine-induced hyperlocomotion, apomorphine-induced PPI |
Potentiates NMDAR current in CA1, induces fos expression in forebrain |
| VU-0357017 (31) | M1 agonist | 198 nM at rat M1, > 30 µM vs. all other mAChRs |
Scopolamine-induced disruption of contextual fear conditioning (acquisition) |
Potentiates NMDAR current in CA1 |
| VU-0366369 (20) | M1 PAM | 830 nM at rat M1, > 30 µM vs. all other mAChRs |
Not determined/unpublished |
Not determined/unpublished |
| VU-0152100 (34) | M4 PAM | 380 nM at rat M4, > 30 µM vs. all other mAChRs |
Amphetamine-induced hyperlocomotion |
Not determined/unpublished |
| VU-0238429 (38) | M5 PAM | 1.1 µM at human M5, > 30 µM vs. all other mAChRs |
Not determined/unpublished |
Not determined/unpublished |
Recently, a third generation of exquisitely potent (EC50s 150–200 nM), selective (> 50 µM vs. M2–5 and clean ancillary pharmacology) and centrally penetrant (brain:plasma ratio of 4.0) M1 allosteric agonists, VU-0186470 30 and VU-0357017 31, were reported by the Vanderbilt group (100). Unlike all the predecessors that bind at an allosteric site in the 7 transmembrane domain, VU-0186470 and VU-0357017 act at a novel allosteric site in the third extracellular loop. Importantly, these new M1 allosteric agonists potentiate NMDA currents and were shown to provide a full reversal of scopolamine-induced memory deficits in contextual fear conditioning at a 10 mg/kg dose (100). Preclinical antipsychotic efficacy is eagerly awaited. Thus, selective activation of M1 by either a PAM or an allosteric agonist does mimic some of the effects of xanomeline in animal models that are relevant to clinical efficacy of antipsychotic agents.
ANTIPSYCHOTIC POTENTIAL OF M4 ALLOSTERIC MODULATION
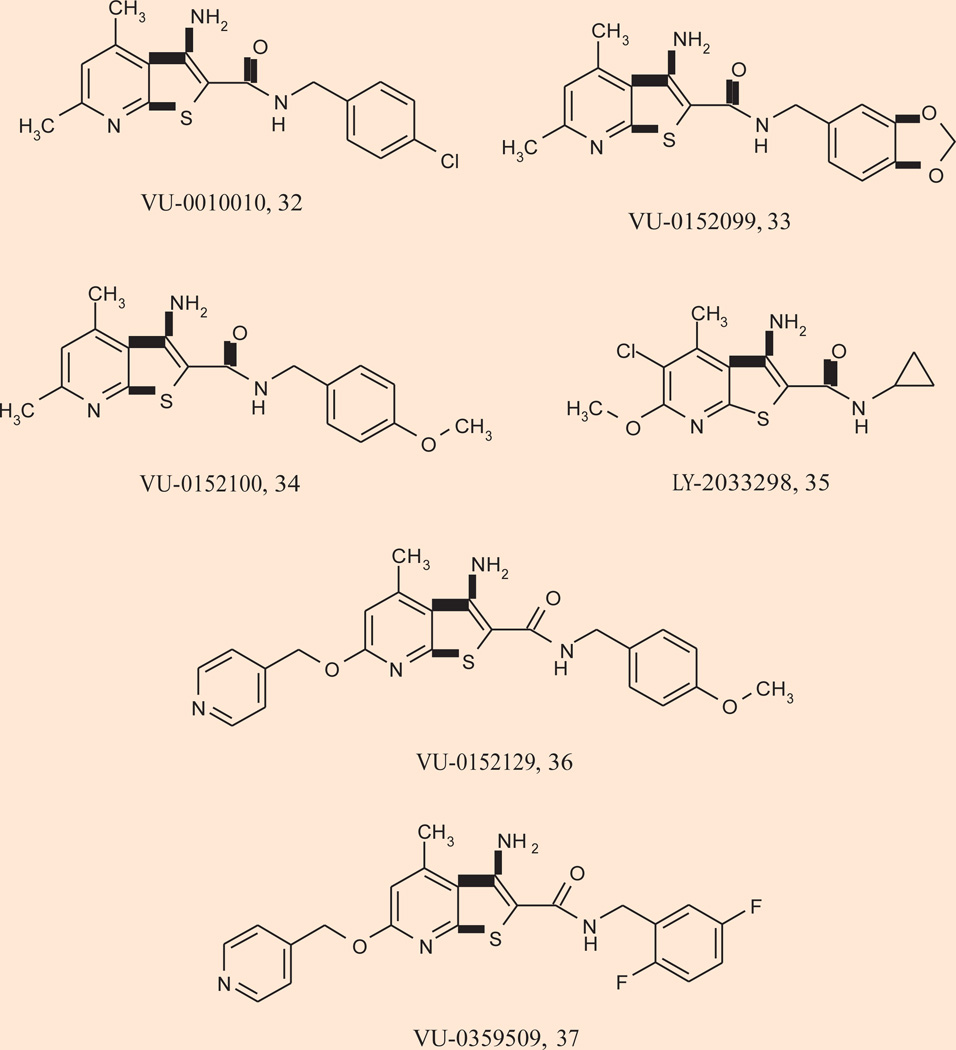
A major breakthrough for selective activation of M4 occurred with the discovery of VU-0010010 32 as a highly selective M4 PAM (> 30 µM vs. M1,2,3,5) (101). VU-0010010 is an allosteric potentiator and does not activate M4 directly but dramatically potentiates the response of the receptor to ACh. Extensive in vitro pharmacological characterization reveals that VU-0010010 binds to an allosteric site to increase both the affinity of M4 for ACh and efficiency of coupling of M4 to G proteins (102). More recently, two related compounds were reported, VU-0152099 33 and VU-0152100 34, that are also highly selective for rat M4 (rat M4 EC50s in the 350–400 nM range, > 30 µM vs. M1,2,3,5, 30-to 70-fold leftward shift of the ACh CRC), readily cross the blood–brain barrier and have pharmacokinetic properties making them highly suitable for behavioral studies (103). Interestingly, both VU-0152100 and VU-0152099 almost fully reverse amphetamine-induced hyperlocomotion in rats relying on endogenous ACh, suggesting that M4 PAMs have efficacy in at least one model used to predict antipsychotic efficacy similar to xanomeline (103).
Chan and coworkers reported the discovery of a similar, but structurally distinct M4 PA M , termed LY-2033298 35 (101). This compound exhibits highly selective and potent human M4 potentiator activity, whereas VU-0010010, VU-0152099 and VU-0152100 show equivalent potency at both human and rat M4. Interestingly, LY-2033298 has also been shown to possess in vivo efficacy in reducing conditioned avoidance response and in reversal of apomorphine-induced disruption of prepulse inhibition, two additional rodent paradigms predictive of antipsychotic drug efficacy and in which xanomeline showed similar positive effects. Importantly, due to the weaker activity of LY-2033298 at the rat (vs. human) receptor, in vivo studies in rats required co-dosing with a low dose of oxotremorine for the PAM to potentiate receptor function; in contrast, the VU compounds were efficacious in vivo relying solely on endogenous ACh (101, 103).
A second generation of M4 PAMs from Vanderbilt incorporated basic residues in the Western portion and noted a unique species difference, wherein VU-0152129 36 and VU-0359509 37 (Fig. 7) were an order of magnitude more potent on human versus rat M4 (human EC50s of 100 nM and 183 nM, respectively; rat EC50s of 2.0 µM and 3.8 µM, respectively, but with > 50-fold shifts on both cell lines) (104). Despite the weaker potency on rat M4, both compounds still provided modest reversal of amphetamine-induced hyperlocomotion activity in rats, relying on endogenous ACh for receptor potentiation.
Figure 7.
Structures of M4 positive allosteric modulators.
Collectively, the studies with M4 allosteric modulators clearly demonstrate that selective activation of M4 is a viable target for the development of novel antipsychotic agents. Moreover, the observed efficacy in three pre-clinical antipsychotic models where xanomeline affords similar positive results implicates M4 as a major contributor to the mechanism of action of xanomeline.
ANTIPSYCHOTIC POTENTIAL OF M5 ALLOSTERIC MODULATION
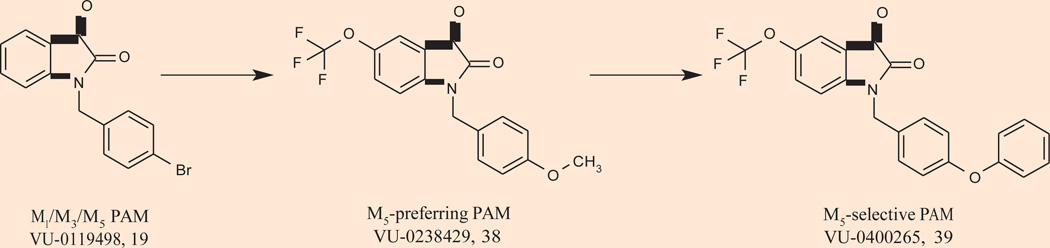
Though implicated as a potential antipsychotic target from a combination of knockout and genetic studies mentioned earlier, M5 is far less advanced than M1 and M4, due to a lack of ligands to study receptor function. In fact, prior to 2009, no selective M5 ligands existed. Interestingly, the p-bromobenzyl-substituted isatin M1 PAM screening hit VU-0119498 19 (Fig. 8) was found to exhibit allosteric potentiator activity at not only M1, but also M3 and M5 receptors with comparable potency and efficacy in Ca2+ mobilization assays, but lacked potentiator effects at M2 and M4 (94). Chemical optimization of this M1/M3/M5 PAM led to the discovery of VU-0238429 38, the first M5-preferring ligand, an M5 PAM (M5 EC50 = 1.1 µM, > 30 µM vs. M1–4, 14-fold shift) (105). Similar to other mAChR PAMs, VU-0238429 did not compete for binding at the orthosteric ACh binding site and increased affinity of M5 for ACh (105). Further optimization of VU-0238429 led to the discovery of the phenoxybenzyl analogue VU-0400265 39, which displayed similar potency (M5 EC50 = 1.9 µM) but was completely selective for M5 in functional assays with recombinant cells expressing each of the other mAChRs (106). This initial disclosure is very exciting, and in vivo data with this class of M5 PAMs, and hopefully M5 NAMs, will be essential to determine the antipsychotic potential of selective M5 modulation.
Figure 8.
Structure of the first M5-preferring ligand VU-0238429 38, an M5 positive allosteric modulator (PAM).
CONCLUSIONS
Historical clinical data from over 50 years ago suggested a cholinergic hypothesis for the pathophysiology of schizophrenia. Preclinical and clinical data with xanomeline, an M1/M4-preferring orthosteric agonist, suggest mAChR activation has high antipsychotic potential. Only recently has science evolved to a point where highly selective small molecules can be developed to activate individual mAChRs, by virtue of allosteric modulation. Collectively, the studies with M1 and M4 allosteric modulators, both allosteric agonists and PAMs, clearly demonstrate the potential of targeting allosteric sites for developing highly selective activators of individual mAChR subtypes and suggest that both M1 and M4 may provide viable targets for the development of novel antipsychotic agents. Moreover, these preclinical animal model studies suggest that the antipsychotic efficacy of xanomeline is due to a synergy of M1 and M4 activation, although M4 may play a dominant role. The antipsychotic potential of M3 is unclear, but with the emergence of new selective M5 PAMs, we may soon know the contribution, if any, of selective M5 activation, and potentially M5 NAMs on the etiology of schizophrenia. Based on all of these data, muscarinic allosteric modulation will clearly be a theme driving antipsychotic drug discovery efforts for decades to come.
Acknowledgments
P.J. Conn has received compensation over the past 2 years as a consultant from Merck & Co., Johnson & Johnson, Hoffman-La Roche, GlaxoSmithKline, Lundbeck Research USA, Epix Pharmaceuticals, Invitrogen Life Technologies, Evotec Inc., Addex Pharmaceuticals, Michael J. Fox Foundation, Seaside Therapeutics, Cephalon Inc., AstraZeneca USA, NeurOp Inc., Forest Research Institute, LEK Consulting, The Frankel Group, Prestwick Chemical Co., Millipore Corp., Genentech, IMS Health, Primary Insight and Otsuka. Dr. Conn has also received honoraria as a speaker from University of Toronto, American Society for Bone and Mineral Research, University of Alabama Birmingham, University of Michigan, Southern Research Inst., Harvard Medical School and the University of North Carolina. Dr. Conn receives research support that includes salary support from NIH, Michael J. Fox Foundation, Seaside Therapeutics, and Vanderbilt University. C.K. Jones is the recipient of Research Grant R01 MH086601-01 (PI, Jones) NIH/NIMH, M4 positive allosteric modulators for the treatment of schizophrenia.
Footnotes
DISCLOSURES
T.M. Bridges, E.P. LeBois, C.R. Hopkins, M.R. Wood and C.W. Lindsley state that they have no potential conflicts of interest to disclose.
Contributor Information
Thomas M. Bridges, Department of Pharmacology, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Evan P. LeBois, Department of Pharmacology, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Corey R. Hopkins, Department of Pharmacology, Vanderbilt Program in Drug Discovery and Vanderbilt Specialized Chemistry Center (MLPCN), Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Michael R. Wood, Department of Pharmacology, Vanderbilt Program in Drug Discovery and Vanderbilt Specialized Chemistry Center (MLPCN), Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Carrie K. Jones, Department of Pharmacology, Vanderbilt Program in Drug Discovery and Vanderbilt Specialized Chemistry Center (MLPCN), Vanderbilt University Medical Center, and U.S. Department of Veterans Affairs, Tennessee Valley Healthcare System (TVHS), Nashville, Tennessee, USA.
P. Jeffrey Conn, Department of Pharmacology, Vanderbilt Program in Drug Discovery and Vanderbilt Specialized Chemistry Center (MLPCN), Vanderbilt University Medical Center, Nashville, Tennessee, USA..
Craig W. Lindsley, Department of Pharmacology, Vanderbilt Program in Drug Discovery and Vanderbilt Specialized Chemistry Center (MLPCN), Vanderbilt University Medical Center, and Department of Chemistry, Vanderbilt University, Nashville, Tennessee, USA..
REFERENCES
- 1.Andreasen NC. Schizophrenia: the fundamental questions. Brain Res Rev. 2000;31(2–3):106–112. doi: 10.1016/s0165-0173(99)00027-2. [DOI] [PubMed] [Google Scholar]
- 2.Karayiorgou M. Genetic aspects of schizophrenia. Clin Neurosci Res. 2001;1(1–2):158–163. [Google Scholar]
- 3.Meador-Woodruff JH, Healy DJ. Glutamate receptor expression in schizophrenic brain. Brain Res Rev. 2000;31(2–3):288–294. doi: 10.1016/s0165-0173(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 4.Jablensky A. Subtyping schizophrenia: Implications for genetic research. Mol Psychiatry. 2006;11(9):815–836. doi: 10.1038/sj.mp.4001857. [DOI] [PubMed] [Google Scholar]
- 5.Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL, Sur C, Kinney GG. Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr Top Med Chem. 2006;6(8):771–785. doi: 10.2174/156802606777057599. [DOI] [PubMed] [Google Scholar]
- 6.Tamminga CA. The neurobiology of cognition in schizophrenia. J Clin Psychiatry. 2006;67(Suppl. 9):9–13. [PubMed] [Google Scholar]
- 7.Felder CC, Bymaster FP, Ward J, DeLapp N. Therapeutic opportunities for muscarinic receptors in the central nervous system. J Med Chem. 2000;43(23):4333–4353. doi: 10.1021/jm990607u. [DOI] [PubMed] [Google Scholar]
- 8.Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry. 2007;12(3):232–246. doi: 10.1038/sj.mp.4001924. [DOI] [PubMed] [Google Scholar]
- 9.Marino PJ, Conn PJ. Direct and indirect modulation of the N-methyl-D-aspartate receptor: potential for the development of novel antipsychotic therapies. Curr Drug Targets CNS Neurol Disord. 2002;1(1):1–16. doi: 10.2174/1568007023339544. [DOI] [PubMed] [Google Scholar]
- 10.Carlsson A, Lindqvist M. Effects of chlorpromazine or haloperidol on formation of 3-methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol. 1963;20(2):140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 11.Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261(5562):717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 12.Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188(4194):1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 13.Verhoeff N. Radiotracer imaging of dopaminergic transmission in neuropsychiatric disorders. Psychopharmacology. 1999;147(3):217–249. doi: 10.1007/s002130051163. [DOI] [PubMed] [Google Scholar]
- 14.Bertolino A, Breier A, Callicott JH, et al. The relationship between dorsolateral pre-frontal neuronal N-acetylaspartate and evoked release of striatal dopamine in schizophrenia. Neuropsychopharmacology. 2000;22(2):125–132. doi: 10.1016/S0893-133X(99)00096-2. [DOI] [PubMed] [Google Scholar]
- 15.Laruelle M, Abi-Dargham A, van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93(17):9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laruelle M, D’Souza CD, Baldwin RM, et al. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology. 1997;17(3):162–174. doi: 10.1016/S0893-133X(97)00043-2. [DOI] [PubMed] [Google Scholar]
- 17.Angrist BM. Amphetamine Its Analogs: Psychopharmacology Toxicology. In: Cho AK, Segal DS, editors. Amphetamine psychosis: clinical variations of the syndrome. Academic Press; San Diego: 1994. pp. 387–414. [Google Scholar]
- 18.Javitt DC, Zukin SR. Recent advances in the phenylcyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 19.Millan MJ. N-Methyl-D-aspartate receptor-coupled glycineB receptors in the pathogenesis and treatment of schizophrenia. Curr Drug Target CNS Neurol Disord. 2002;1(2):191–213. doi: 10.2174/1568007024606258. [DOI] [PubMed] [Google Scholar]
- 20.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52(12):998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 21.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatry Res. 1999;33(6):523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto K, Okamura N, Shimizu E, Iyo M. Glutamate hypothesis of schizophrenia and approach for possible therapeutics. Curr Med Chem: CNS Agent. 2004;4(2):147–154. [Google Scholar]
- 23.Lindsley CW, Wisnoski DD, Leister WH, et al. Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1,3-diphenyl-1H–pyrazol-5-yl)benzamides that potentiate receptor function in vivo. J Med Chem. 2004;47(24):5825–5828. doi: 10.1021/jm049400d. [DOI] [PubMed] [Google Scholar]
- 24.Kinney GG, O’Brien JA, Lemaire W, et al. A novel selective allosteric modulator of metabotropic glutamate receptor subtype 5 (mGluR5) has an antipsychotic profile in rat behavioral models. J Pharmacol Exp Ther. 2005;313(1):199–211. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- 25.Depoortere R, Daraganzanil G, Estenne-Bouhtou G, et al. Neurochemical, electrophysiological and pharmacological profiles of the selective inhibitor of the glycine transporter-1 SSR504734, a potential new antipsychotic. Neuropsychopharmacology. 2005;30(11):1963–1985. doi: 10.1038/sj.npp.1300772. [DOI] [PubMed] [Google Scholar]
- 26.Lindsley CW, Zhao Z, Leister WH, et al. Design, synthesis and in vivo efficacy of novel glycine transporter-1 (GlyT1) inhibitors derived from a series of [4-phenyl-1-(propylsulfonyl)-piperidin-4-yl] methyl benzamides. Chem Med Chem. 2006;1(8):807–813. doi: 10.1002/cmdc.200600097. [DOI] [PubMed] [Google Scholar]
- 27.Jones CK, Brady AE, Davis AA, et al. Novel selective allosteric activator of the M1 muscarinic receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci. 2008;28(41):10422–10433. doi: 10.1523/JNEUROSCI.1850-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolkenberg ES. Glycine transporter 1 (GlyT1) inhibitors for the treatment of schizophrenia. 236th ACS Natl Meet; August 17–21; Philadelphia. 2008. Abst MEDI 220. [Google Scholar]
- 29.Levey AI. Immunological localization of M1-M5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993;52(5–6):441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- 30.Abrams P, Andersson KE, Buccafusco JJ, et al. Muscarinic receptors: Their distribution and function in body systems, and their implications for treating overactive bladder. Br J Pharmacol. 2006;148(5):565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volpicelli LA, Levey AI. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog Brain Res. 2004;145(1):59–66. doi: 10.1016/S0079-6123(03)45003-6. [DOI] [PubMed] [Google Scholar]
- 32.Felder CC, Porter AC, Skillman TL, et al. Elucidating the role of muscarinic receptors in psychosis. Life Sci. 2001;68(22–23):2605–2613. doi: 10.1016/s0024-3205(01)01059-1. [DOI] [PubMed] [Google Scholar]
- 33.Anagnostaras SG, Murphy GG, Hamilton SE, et al. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6(1):51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- 34.Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6(9):721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- 35.Bartus RT. On neurodegenerative diseases, models, and treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163(2):495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- 36.Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol & Ther. 2008;117(2):232–243. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Fisher A. M1 muscarinic agonists target major hallmarks of Alzheimer’s Disease — the pivotal role of brain M1 receptors. Neurodegener Dis. 2008;5(3–4):237–240. doi: 10.1159/000113712. [DOI] [PubMed] [Google Scholar]
- 38.Mirza NR, Peters D, Sparks RG. Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists. CNS Drug Rev. 2003;9(2):159–186. doi: 10.1111/j.1527-3458.2003.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psych. 2008;165(8):1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- 40.Scarr E, Sundrarn S, Keriakous D, Dean B. Altered hippocampal muscarinic M4, but not M1, receptor expression from subjects with schizophrenia. Biol Psych. 2007;61(10):1161–1170. doi: 10.1016/j.biopsych.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 41.Conn PJ, Jones CK, Lindsley CW. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci. 2009;30(3):148–155. doi: 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heinrich JN, Butera JA, Carrick T, et al. Pharmacological comparison of muscarinic ligands: Historical versus more recent muscarinic M1-preferring receptor agonists. Eur J Pharmacol. 2009;605(1–3):53–56. doi: 10.1016/j.ejphar.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 43.Biel JH, Nuhfer PA, Hoya WK, et al. Cholinergic blockade as an approach to the development of new psychotropic agents. Ann New York Acad Sci. 1962;96(1):251–262. doi: 10.1111/j.1749-6632.1962.tb50120.x. [DOI] [PubMed] [Google Scholar]
- 44.Fisher CM. Visual hallucinations on eye closure associated with atropine toxicity A neurological analysis and comparison with other visual hallucinations. Can J Neur Sci. 1991;18(1):18–27. doi: 10.1017/s0317167100031255. [DOI] [PubMed] [Google Scholar]
- 45.Mego DM, Omori JM, Hanley JF. Transdermal scopolamine as a cause of transient psychosis in two elderly patients. South Med J. 1988;81(3):394–395. doi: 10.1097/00007611-198803000-00025. [DOI] [PubMed] [Google Scholar]
- 46.Fisch RZ. Trihexyphenidyl abuse: therapeutic implications for negative symptoms of schizophrenia? Acta Psychiatr Scand. 1987;75(1):91–94. doi: 10.1111/j.1600-0447.1987.tb02757.x. [DOI] [PubMed] [Google Scholar]
- 47.Bymaster FP, McKinzie DL, Felder CC, Wess J. Use of M1-M5 muscarinic receptor knock out mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem Res. 2003;28(3–4):437–442. doi: 10.1023/a:1022844517200. [DOI] [PubMed] [Google Scholar]
- 48.Buckley NJ, Bonner TI, Bran MR. Localization of a family of muscarinic receptor RNAs in rat brain. J Neurosci. 1988;8(12):4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao CF, Themmen AP, Joho R, Barberis C, Birnbaumer M, Birnbaumer L. Molecular cloning and expression of a fifth muscarinic acetylcholine receptor. J Biol Chem. 1989;264(13):7328–7337. [PubMed] [Google Scholar]
- 50.Vilaro MT, Palacios JM, Mengod G. Localization of M5 muscarinic receptor RNA in rat brain by in situ hybridization histochemistry. Neurosci Lett. 1990;114(2):154–159. doi: 10.1016/0304-3940(90)90064-g. [DOI] [PubMed] [Google Scholar]
- 51.Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ. Activation of the genetically defined M1 muscarinic receptor potentiates N-methyl-D-asparate (NMDA) receptor currents in hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1998;95(19):11465–11470. doi: 10.1073/pnas.95.19.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yasuda RP, Ciesla W, Flores LR, Wall SJ, et al. Development of antiseraselective for M4 and M5 muscarinic cholinergic receptors: Distribution of M4 and M5 receptors in the brain. Mol Pharmacol. 1993;43(2):149–157. [PubMed] [Google Scholar]
- 53.Wirtshafter D, Osbron CV. The distribution of M4 muscarinic acetylcholine receptors in the islands of Calleja and striatum of rats and cynomolgus monkeys. J Chem Neuroanat. 2004;28(3):107–116. doi: 10.1016/j.jchemneu.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Tzavara ET, Bymaster FP, Davis RJ, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: Relevance to the pathophysiology and treatment of CNS pathologies. FASEB J. 2004;18(12):1410–1412. doi: 10.1096/fj.04-1575fje. [DOI] [PubMed] [Google Scholar]
- 55.Wool ML, Carter HJ, Gartlon JE, Watson JM, Dawson LA. Attenuation of amphetamine-induced activity by the non-selective muscarinic receptor agonist, xanomeline, is absent in muscarinic M4 receptor knockout mice and attenuated in muscarinic M1 receptor knockout mice. Eur J Pharmacol. 2009;603(1–3):147–149. doi: 10.1016/j.ejphar.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 56.Eglen RM, Nahorski SR. The muscarinic M5 receptor: a silent or emerging subtype? Br J Pharmacol. 2000;130(1):13–21. doi: 10.1038/sj.bjp.0703276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kane JM, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 58.Hagger C, Buckley P, Kenny JT, Friedman L, Ubogy D, Meltzer HY. Improvement in cognitive function and psychiatric symptoms in treatment-refractory schizophrenic patients receiving clozapine. Biol Psychiatry. 1993;34(10):702–712. doi: 10.1016/0006-3223(93)90043-d. [DOI] [PubMed] [Google Scholar]
- 59.Goldberg TE, Weinberger DR. The effects of clozapine on neurocognition: an overview. J Clin Psychiatry. 1994;55(Suppl B):88–90. [PubMed] [Google Scholar]
- 60.Weiner DM, Meltzer HY, Veinbergs I, et al. The role of M1 muscarinic receptor agonism of N-desmethylclozapine in the unique clinical efficacy of clozapine. Psychopharmacology. 2004;177(1–2):207–216. doi: 10.1007/s00213-004-1940-5. [DOI] [PubMed] [Google Scholar]
- 61.Davies MA, Compton-Toth BA, Hufeisen SJ, Meltzer HY, Roth BL. The highly efficacious actions of N-desmethylclozapine at muscarinic receptors are unique and not a common property of either typical or atypical antipsychotic drugs: is M1 agonism a prerequisite for mimicking clozapine’s actions. Psychopharmacology. 2005;178(4):451–460. doi: 10.1007/s00213-004-2017-1. [DOI] [PubMed] [Google Scholar]
- 62.Burstein ES, Ma JN, Wong S, et al. Intrinsic efficacy of antipsychotics at human D2, D3 and D4 dopamine receptors: identification of the clozapine metabolite N-desmethylclozapine as a D2/3 partial agonist. J Pharmacol Exp Ther. 2005;315(3):1272–1278. doi: 10.1124/jpet.105.092155. [DOI] [PubMed] [Google Scholar]
- 63.Li Z, Huang M, Ichikawa J, Dai J, Meltzer HY. N-Desmethylclozapine, a major metabolite of clozapine, increases cortical acetylcholine and dopamine release in vivo via stimulation of M1 muscarinic receptors. Neuropsychopharmacology. 2005;30(11):1986–1995. doi: 10.1038/sj.npp.1300768. [DOI] [PubMed] [Google Scholar]
- 64.Sur C, Mallogora PJ, Wittmann M, et al. N-Desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiate N-methyl-D-aspartate receptor activity. Proc Natl Acad Sci U S A. 2003;100(23):13674–13679. doi: 10.1073/pnas.1835612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller AD, Blaha CD. Midbrain muscarinic receptor mechanisms underlying regulation of mesoaccumbens and nigrostriatal dopaminergic neurotransmission. Eur J Neurosci. 2005;21(7):1837–1846. doi: 10.1111/j.1460-9568.2005.04017.x. [DOI] [PubMed] [Google Scholar]
- 66.De Luca V, Wang H, Squassina A, Wong GW, Yeomans J, Kennedy JL. Linkage of M5 muscarinic and alpha-7 nicotinic receptor genes on 15q13 in schizophrenia. Neuropsychobiology. 2004;50(2):124–127. doi: 10.1159/000079102. [DOI] [PubMed] [Google Scholar]
- 67.Liao D, Hong CJ, Chen HM, et al. Association of muscarinic M1 receptor genetic polymorphisms with psychiatric symptoms and cognitive function in schizophrenic patients. Neuropsychobiology. 2003;48(2):72–76. doi: 10.1159/000072880. [DOI] [PubMed] [Google Scholar]
- 68.Dean D, Crook JM, Opeskin K, Hill C, Keks N, Copolov DL. The density of muscarinic M1 receptors is decreased in the caudate-putamen of subjects with schizophrenia. Mol Psychiatry. 1996;1(1):54–58. [PubMed] [Google Scholar]
- 69.Crook JM, Dean B, Pavey G, Copolov D. The binding of [3H]AFDX384 is reduced in the caudate-putamen of subjects with schizophrenia. Life Sci. 1999;64(19):1761–1771. doi: 10.1016/s0024-3205(99)00114-9. [DOI] [PubMed] [Google Scholar]
- 70.Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol Psychiatry. 2000;48(5):381–388. doi: 10.1016/s0006-3223(00)00918-5. [DOI] [PubMed] [Google Scholar]
- 71.Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Low muscarinic receptor binding in prefrontal cortex from subjects with schizophrenia: a study of Brodmann’s areas 8, 9, 10, and 46 and the effects of neuroleptic drug treatment. Am J Psychiatry. 2001;158(6):918–925. doi: 10.1176/appi.ajp.158.6.918. [DOI] [PubMed] [Google Scholar]
- 72.Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E. Decreased muscarinic(1) receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2002;7(10):1083–1091. doi: 10.1038/sj.mp.4001199. [DOI] [PubMed] [Google Scholar]
- 73.Zavitsanou K, Katsifis A, Mattner F, Huang XF. Investigation of m1/m4 muscarinic receptors in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression disorder. Neuropsychopharmacology. 2004;29(3):619–625. doi: 10.1038/sj.npp.1300367. [DOI] [PubMed] [Google Scholar]
- 74.Raedler TJ, Knable MB, Jones DW, et al. In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am J Psychiatry. 2003;160(1):118–127. doi: 10.1176/appi.ajp.160.1.118. [DOI] [PubMed] [Google Scholar]
- 75.Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8(1):41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pakrasi S, Colloby SJ, Firbank MJ, et al. Muscarinic acetylcholine receptor status in Alzheimer’s disease assessed using (R, R) 123I–QNB SPECT. J Neurol. 2007;254(7):907–913. doi: 10.1007/s00415-006-0473-8. [DOI] [PubMed] [Google Scholar]
- 77.Shinoe T, Matsui M, Taketo MM, Manabe T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci. 2005;25(48):11194–11200. doi: 10.1523/JNEUROSCI.2338-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caccamo A, Oddo S, Billings LM, et al. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49(5):671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 79.Bodick NC, Offen WW, Shannon HE, et al. The selective muscarinic agonist xanomeline improves both cognitive deficits and behavioral symptoms of Alzheimer disease. Alz Dis & Assoc Disord. 1997;11(Suppl 4):S16–S22. [PubMed] [Google Scholar]
- 80.Bymaster FP, Whitesitt CA, Shannon HA, et al. Xanomeline: a selective muscarinic agonist for the treatment of Alzheimer’s disease. Drug Dev Res. 1997;40(2):158–170. [Google Scholar]
- 81.Perry KW, Nisenbaum LK, George CA, Shannon HE, Felder CC, Bymaster FP. The muscarinic agonist xanomeline increases monoamine release and immediate gene expression in the rat prefrontal cortex. Biol Psychiatry. 2001;49(8):716–725. doi: 10.1016/s0006-3223(00)01017-9. [DOI] [PubMed] [Google Scholar]
- 82.Shannon HE, Rasmussen K, Bymaster FP, et al. Xanomeline, an M1/M4 preferring muscarinic cholinergic receptor agonist, produces antipsychotic-like activity in rats and mice. Schiz Res. 2000;42(3):249–259. doi: 10.1016/s0920-9964(99)00138-3. [DOI] [PubMed] [Google Scholar]
- 83.Stanhope KJ, Mirza NR, Bickerdike MJ, et al. The muscarinic receptor agonist xanomeline has antipsychotic-like activity in the rat. J Pharmacol Exp Ther. 2001;299(2):782–792. [PubMed] [Google Scholar]
- 84.Andersen MB, Fink-Jensen A, Peacock L, et al. The muscarinic M1/M4 receptor agonist xanomeline exhibits antipsychotic-like activity in the Cebus paella monkey. Neuropsychopharmacology. 2003;28(6):1168–1175. doi: 10.1038/sj.npp.1300151. [DOI] [PubMed] [Google Scholar]
- 85.Lieberman JA, Javitch JA, Moore H. Cholinergic agonists as novel treatments for schizophrenia: the promise of rational drug development for psychiatry. Am J Psychiatry. 2008;165(8):931–936. doi: 10.1176/appi.ajp.2008.08050769. [DOI] [PubMed] [Google Scholar]
- 86.Lewis J, Lebois EP, Lindsley CW. Allosteric modulators of kinases and GPCRs Design principles and structural diversity. Curr Opin Chem Biol. 2008;12(3):269–280. doi: 10.1016/j.cbpa.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 87.Bridges TM, Lindsley CW. G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem Biol. 2008;3(9):530–541. doi: 10.1021/cb800116f. [DOI] [PubMed] [Google Scholar]
- 88.Jakubik J, Bacakova L, El-Fakahany EE, Tucek S. Positive cooperativity of acetylcholine and other agonists with allosteric ligands on muscarinic acetylcholine receptors. Mol Pharmacol. 1997;52(1):172–179. doi: 10.1124/mol.52.1.172. [DOI] [PubMed] [Google Scholar]
- 89.Lanzafame A, Christopoulos A. Investigation of the interaction of a putative allosteric modulator, N-(2,3-diphenyl-1,2,4-thiadiazole-5-(2H)-ylidene) methanamine hydrobromide (SCH-202676), with M1 muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2004;308(3):830–837. doi: 10.1124/jpet.103.060590. [DOI] [PubMed] [Google Scholar]
- 90.Lazareno S, Dolezal V, Popham A, Birdsall NJ. Thiochrome enhances acetylcholine affinity at muscarinic M4 receptors: receptor subtype selectivity via cooperativity rather than affinity. Mol Pharmacol. 2004;65(1):257–266. doi: 10.1124/mol.65.1.257. [DOI] [PubMed] [Google Scholar]
- 91.Birdsall NJ, Farries T, Gharagozloo P, Kobayashi S, Lazareno S, Sugimoto M. Subtype-selective positive cooperative interactions between brucine analogues and acetylcholine at muscarinic receptors: radioligand binding studies. Mol Pharmacol. 1993;55(4):778–786. [PubMed] [Google Scholar]
- 92.Fawzi AB, Macdonald D, Benbow LL, et al. SCH-202676: an allosteric modulator of both agonist and antagonist binding to G protein-coupled receptors. Mol Pharmacol. 2001;59(1):30–37. doi: 10.1124/mol.59.1.30. [DOI] [PubMed] [Google Scholar]
- 93.Spalding TA, Trotter C, Skjaerbaek N, et al. Discovery of an ectopic activation site on the M1 muscarinic receptor. Mol Pharmacol. 2002;61(6):1297–1302. doi: 10.1124/mol.61.6.1297. [DOI] [PubMed] [Google Scholar]
- 94.Marlo JE, Niswender CM, Luo Q, et al. Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol Pharm. 2009;75(3):577–589. doi: 10.1124/mol.108.052886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma L, Seager M, Wittman M, et al. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci U S A. 2009;106(37):15950–15995. doi: 10.1073/pnas.0900903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brady AE, Shirey JK, Jones PJ, et al. . A selective allosteric potentiator of the M1 Muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and can restore impairments of reversal learning J Neurosci. 2009;29(45):14271–14286. doi: 10.1523/JNEUROSCI.3930-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Langmead CJ, Austin NE, Branch CL, et al. Characterization of a CNS penetrant, selective M1 muscarinic receptor agonist, 77-LH-28-1. Br J Pharmacol. 2008;154(5):1104–1115. doi: 10.1038/bjp.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bridges TM, Brady AE, Kennedy JP, et al. Synthesis and SAR of analogues of the M1 allosteric agonist TBPB. Part I. Exploration of alternative benzyl and privileged structure moieties. Bioorg Med Chem Lett. 2008;18(2):5439–5442. doi: 10.1016/j.bmcl.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miller NR, Daniels NR, Bridges TM, et al. Synthesis and SAR of analogues of the M1 allosteric agonist TBPB. Part II Amides, sulfonamides ands ureas: the effect of capping the distal basic piperidine nitrogen. Bioorg Med Chem Lett. 2008;18(20):5443–5446. doi: 10.1016/j.bmcl.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lebois EP, Bridges TM, Dawson ES, et al. Discovery and development of novel subtype-selective M1 allosteric agonists for the investigation of M1 receptor function. ACS Chem Neurosci. doi: 10.1021/cn900003h. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chan WY, McKinzie DL, Bose S, et al. Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci U S A. 2008;105(31):10978–10983. doi: 10.1073/pnas.0800567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shirey JK, Xiang Z, Orton D, et al. An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission. Nat Chem Bio. 2008;4(1):42–50. doi: 10.1038/nchembio.2007.55. [DOI] [PubMed] [Google Scholar]
- 103.Brady A, Jones CK, Bridges TM, et al. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotion behavior in rats. J Pharm Exp Ther. 2008;327(3):941–954. doi: 10.1124/jpet.108.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kennedy JP, Bridges TM, Gentry PR, et al. Synthesis and structure-activity-relationships of positive allosteric modulators of the muscarinic acetylcholine receptor subtype 4 (mACh4 or M4) Chem Med Chem. 2009;4(10):1600–1607. doi: 10.1002/cmdc.200900231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bridges TM, Marlo JE, Niswender CM, et al. Discovery of the first highly M5-preferring muscarinic acetylcholine receptor ligand, an M5 positive allosteric modulator derived from a series of 5-trifluoromethoxy N-benzyl isatins. J Med Chem. 2009;52(11):3445–3448. doi: 10.1021/jm900286j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bridges TM, Kennedy JP, Cho HP, et al. Chemical lead optimization of a pan Gq mAChR M1, M3, M5 positive allosteric modulator (PAM) lead. Part I: Development of the first highly selective M5 PAM. Bioorg Med Chem Lett. 2010;20(2):558–562. doi: 10.1016/j.bmcl.2009.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]