Abstract
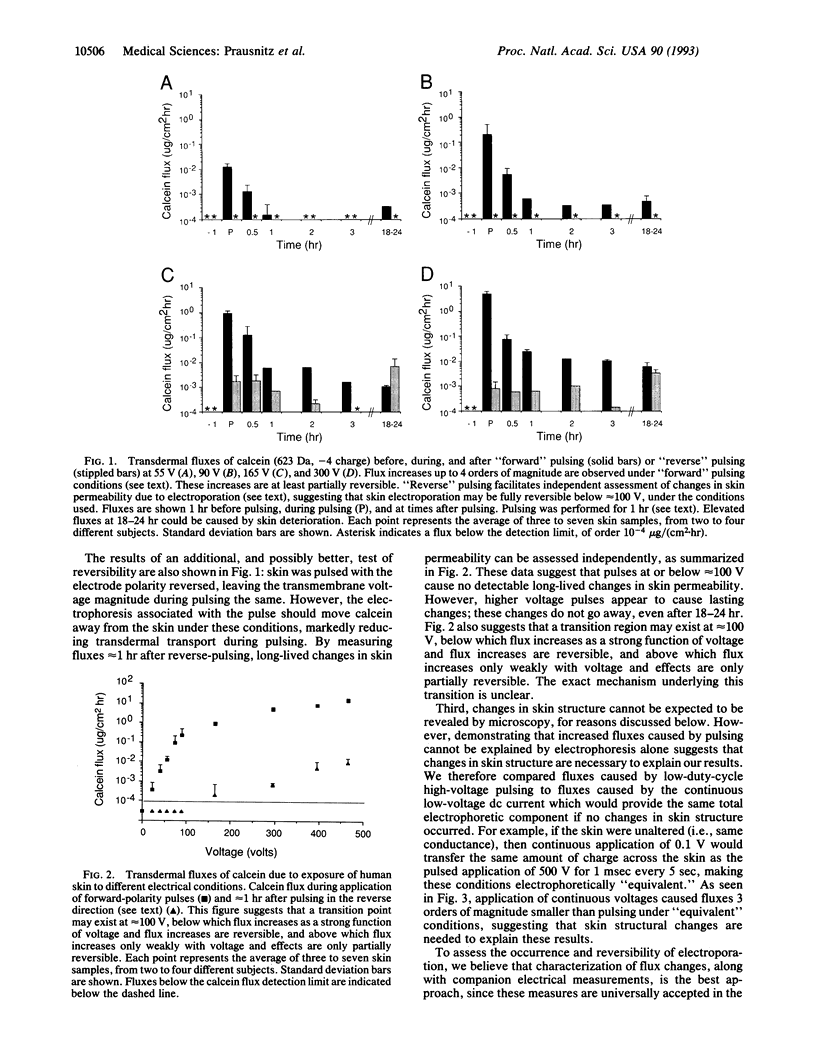
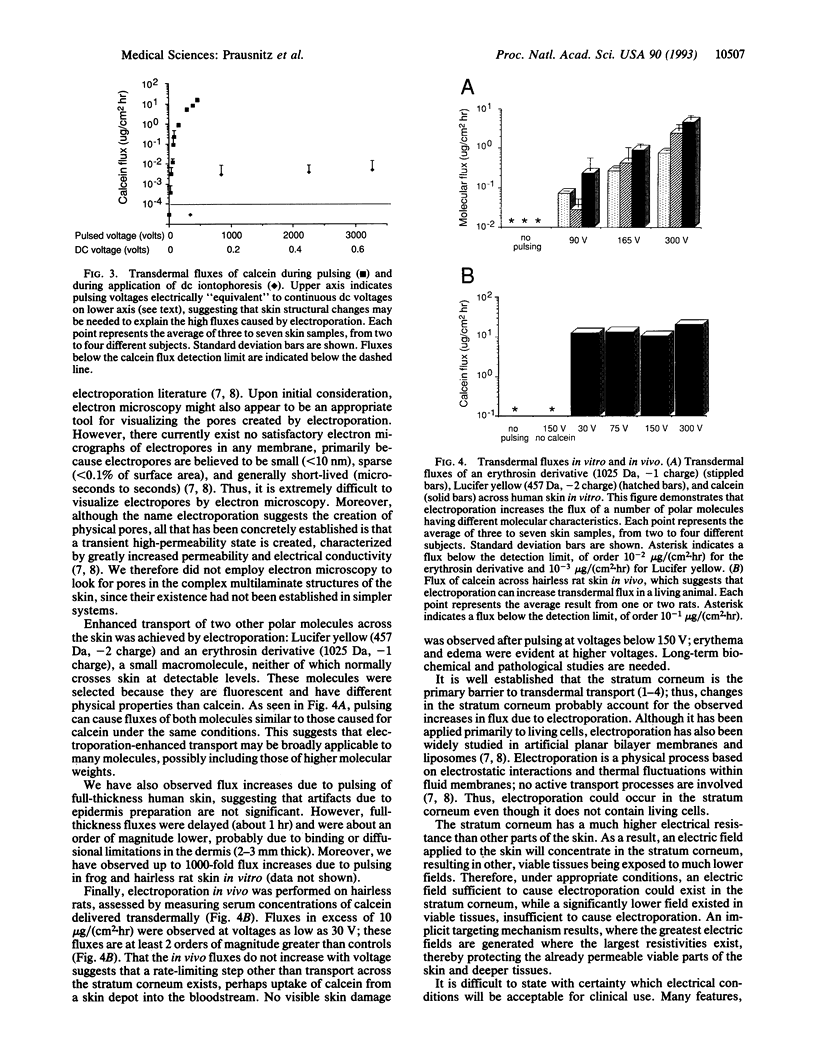
Mammalian skin owes its remarkable barrier function to its outermost and dead layer, the stratum corneum. Transdermal transport through this region occurs predominantly through intercellular lipids, organized largely in bilayers. Electroporation is the creation of aqueous pores in lipid bilayers by the application of a short (microseconds to milliseconds) electric pulse. Our measurements suggest that electroporation occurs in the intercellular lipid bilayers of the stratum corneum by a mechanism involving transient structural changes. Flux increases up to 4 orders of magnitude were observed with human skin in vitro for three polar molecules having charges between -1 and -4 and molecular weights up to slightly more than 1000. Similar flux increases were observed in vivo with animal skin. These results may have significance for drug delivery and other medical applications.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hibino M., Shigemori M., Itoh H., Nagayama K., Kinosita K., Jr Membrane conductance of an electroporated cell analyzed by submicrosecond imaging of transmembrane potential. Biophys J. 1991 Jan;59(1):209–220. doi: 10.1016/S0006-3495(91)82212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir L. M., Orlowski S., Belehradek J., Jr, Paoletti C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer. 1991;27(1):68–72. doi: 10.1016/0277-5379(91)90064-k. [DOI] [PubMed] [Google Scholar]
- Okino M., Mohri H. Effects of a high-voltage electrical impulse and an anticancer drug on in vivo growing tumors. Jpn J Cancer Res. 1987 Dec;78(12):1319–1321. [PubMed] [Google Scholar]
- Powell K. T., Morgenthaler A. W., Weaver J. C. Tissue electroporation. Observation of reversible electrical breakdown in viable frog skin. Biophys J. 1989 Dec;56(6):1163–1171. doi: 10.1016/S0006-3495(89)82763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag W. An automatic microspectrophotometric scanning method for the measurement of bone formation rates in vivo. Calcif Tissue Int. 1980;32(1):63–68. doi: 10.1007/BF02408522. [DOI] [PubMed] [Google Scholar]
- Suzuki H. K., Mathews A. Two-color fluorescent labeling of mineralizing tissues with tetracycline and 2,4-bis[N,N'-di-(carbomethyl)aminomethyl] fluorescein. Stain Technol. 1966 Jan;41(1):57–60. doi: 10.3109/10520296609116280. [DOI] [PubMed] [Google Scholar]
- Titomirov A. V., Sukharev S., Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim Biophys Acta. 1991 Jan 17;1088(1):131–134. doi: 10.1016/0167-4781(91)90162-f. [DOI] [PubMed] [Google Scholar]


