Abstract
A crucial and enigmatic step in the complex biosynthesis of aflatoxin B1 is the oxidative rearrangement of versicolorin A to demethylsterigmatocystin. This step is thought to proceed by an oxidation–reduction–oxidation sequence, in which the NADPH-dependent oxidoreductase AflM catalyzes the enclosed reduction step. AflM from Aspergillus parasiticus, after heterologous production in E. coli and puriflcation, however, catalyzed the reduction of the hydroquinoid form of the starting compound versicolorin A (25% conversion) to a so far unknown product of aflatoxin biosynthesis. The asymmetric reduction of emodin hydroquinone to (R)-3,8,9,10-tetrahydroxy-6-methyl-3,4-dihydroanthracen-1(2H)-one (up to 82% for AflM) has also been observed in previous studies using MdpC from Aspergillus nidulans (mono-dictyphenone biosynthetic gene cluster). The first (non-enzymatic) reduction of emodin to emodin hydroquinone, for example with sodium dithionite, is obligatory for the enzymatic reduction by AflM or MdpC. These results imply an unprecedented role of AflM in the complex enzymatic network of aflatoxin biosynthesis.
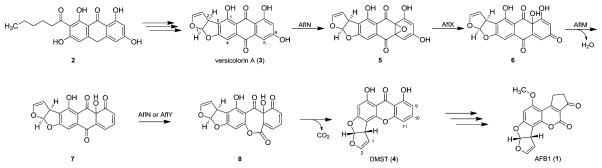
Aflatoxin B1 (AFB1, 1), a naturally occurring mycotoxin, produced by Aspergillus flavus, A. parasiticus, and A. nomius, is one of the most potent carcinogens known.1 The biosynthesis of 1 consists of a complex sequence starting with the formation of the polyketide synthase (PKS) product norsolorinic acid anthrone (2), which is subsequently converted in several enzymatic reactions into 1.2,3 A central reaction sequence in this pathway is the conversion of versicolorin A (3) into demethylsterigmatocystin (DMST, 4) (Scheme 1).4 This sequence includes loss of the C6 hydroxyl group of 3 and Baeyer-Villiger-like oxidative cleavage resulting in the loss of C10 and formation of the xanthone derivative 4. Biosynthesis studies have identified up to four genes, which encode for the enzymes AflN (also denoted as VerA), AflX, AflM, and AflY that could be involved in this process.5–9 Individual gene disruption experiments have shown an accumulation of 3 and a diminished production of 1.5–9 According to Henry and Townsend, the initial reaction step has been speculated to be an epoxidation of 3 to 5 catalyzed by AflN, a putative cytochrome P450 enzyme.4 AflX has some sequence similarity with epoxide hydrolases and thus is seemingly involved in the epoxide ring opening of 5, leading to the hydroxydienone 6.7 In the next reaction steps, a reduction supposedly catalyzed by the NADPH-dependent oxidoreductase AflM and an elimination of water, leading to 7, has been assumed.4,8 AflY, a putative Baeyer–Villiger oxidase, is assumed to catalyze the formation of the lactone 8, which is probably then nonenzymatically converted into xanthone 4.9 This reaction sequence of oxidation–reduction–oxidation (Scheme 1) under preclusion of an initial reduction step was postulated due to the failure of incorporation of other possible intermediates, such as 6-deoxy-3, and o-carboxybenzophenone derivatives of 3.4,10,11
Scheme 1.
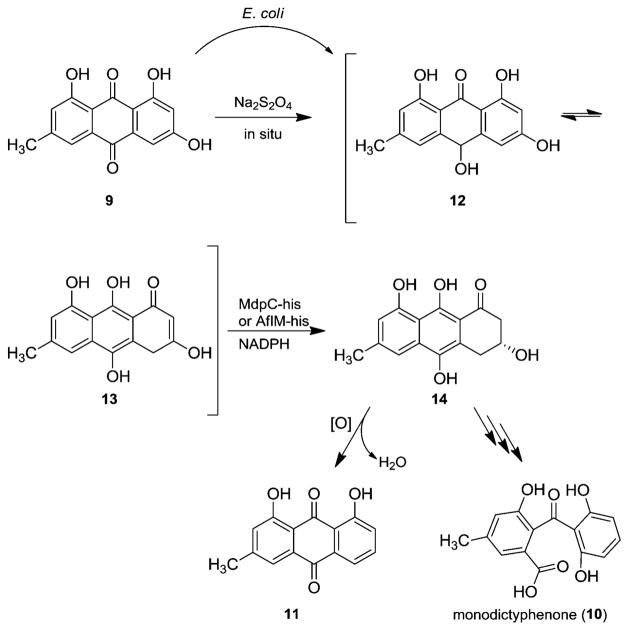
Chiang et al. have proposed a similar pathway for the biotransformation of emodin (9) to monodictyphenone (10) based on the high gene sequence identities of the monodictyphenone gene cluster of Aspergillus nidulans with the aflatoxin gene cluster; however, they could not identify a homologue of AflN.12 Nevertheless, other studies have indicated a different biosynthetic pathway via chrysophanol (11) and without the need of an epoxidation (Scheme 2).13–15
Scheme 2.
Formation of the Two Tautomers of Emodin Hydroquinone (12/13) and Enzyme-Catalyzed Reduction with MdpC-his or AflM-his13–15
To clarify the biosynthetic pathway of 1 in detail, the catalytic function of the involved enzymes, previously identified through gene disruption studies, needs to be investigated.14 Concerning the monodictyphenone gene cluster, the function of MdpC, a sequence homologue of AflM, has been elucidated in chemoenzymatic assays.15 Two tautomeric forms of emodin hydroquinone (12/13) were observed in solution after incubation of emodin (9) with sodium dithionite. Subsequent enzymatic reduction by the NADPH-dependent oxidoreductase MdpC gave (R)-3,8,9,10-tetrahydroxy-6-methyl-3,4-dihydroan-thracen-1(2H)-one (14) in 58% yield (Scheme 2).15 Due to the strong sequence similarity between MdpC and AflM from A. parasiticus (67% amino acid identity), we concluded that AflM might be involved in an analogous transformation.15
In order to confirm the activity of AflM in the conversion of anthrahydroquinones in general, the codon-optimized N-terminally His-tagged aflM was cloned into pET19b, overex-pressed in E. coli BL21, and the obtained protein purified by Ni-NTA affinity chromatography (see Supporting Information). To check for the supposed AflM-catalyzed reduction of 12/13, the anthraquinone 9 was incubated with sodium dithionite and purified AflM-his, using glucose dehydrogenase/D-glucose as an NADPH regeneration system. The reaction mixture was stirred for 24 h at room temperature under a nitrogen atmosphere to avoid prompt back-oxidation to 9. The conversion of 9 via 12/13 into 14 (up to 82%) was established by 1H NMR analysis. Purification by automated flash column chromatography yielded 20% (37 μmol) of pure 14 as an orange solid. The absolute configuration was determined as (R) by comparing the VCD spectrum of 14 with a calculated VCD spectrum and the CD spectrum of 14 with those of similar compounds (see Supporting Information).16 Moreover, during workup we observed the formation of chrysophanol (11, 8%), most probably by a nonenzymatic elimination of water and oxidation with atmospheric oxygen. Incubation of 9 with AflM-his in the absence of sodium dithionite resulted in no conversion. Thus, these results emphasize the role of hydroquinone tautomers in biosynthesis as previously described for the enzymatic reduction with MdpC.15,17 Interestingly, conversion (up to 34%) of 9 into 14 occurred when AflM-his cell-free extract in the absence of sodium dithionite was used. This result implies that E. coli possesses enzymes that are able to catalyze the first reduction step toward the two tautomers of emodin hydroquinone, 12 and 13 (Scheme 2).
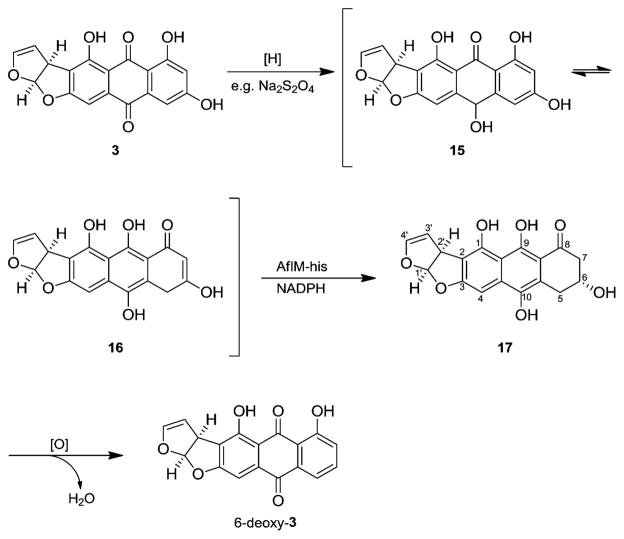
Accordingly, these results strengthen our hypothesis regarding possible reduction of the tautomers of versicolorin A hydroquinone (15/16) by AflM in the biosynthesis of aflatoxin B1 (1). Analogous to the reduction of 9, we tested 3 or rather its hydroquinone as a putative substrate of AflM (see Supporting Information). As expected, we observed a dearomatization leading to (1′R,2′S,6R)-1,6,9,10-tetrahydroxy-2′,5,6,7-tetrahydroanthra[3,2-b]furo[2′,1′-d]furan-8(1′H)-one (17, Scheme 3). The conversion (25%) into 17 was ascertained by 1H NMR analysis of the crude product. The structure of 17 was confirmed by total correlated spectroscopic experiments. The absolute configuration at C1′ and C2′ is according to the absolute configuration of substrate 3.18 C6 was predicted to have the (R) configuration, assuming a similar reaction mechanism as that for compound 14. The NMR spectrum shows only one diastereomer of 17, which implies a stereospecific enzyme reaction.
Scheme 3.
Conversion of Versicolorin A (3) into 17 with Na2S2O4 and AflM-his
In summary, we have shown that AflM from the aflatoxin B1 biosynthetic gene cluster is active in converting the tautomers of emodin hydroquinone (12/13) and versicolorin A hydroquinone (15/16) into the 3-hydroxy-3,4-dihydroanthracen-1(2H)-one derivatives 14 and 17, respectively. AflM, as well as MdpC, specifically accept the tautomers of hydroquinone as a substrate, but not the anthraquinone itself.15 Moreover, 17 is most likely the intermediate in the biosynthesis of 6-deoxy-3 in accord with similarities to the biosynthesis of chrysophanol (11, Scheme 2).
This result contrasts with the previously postulated biosynthesis of 6-deoxy-3 and 1, for which an initial reduction of 3 has been precluded (as shown in Scheme 1). The potential roles of the tautomers of versicolorin A hydroquinone (15/16) and the AflM product 17 in aflatoxin formation remain to be determined. Nevertheless, our results afford a chemically plausible alternative to account for the extensive deuterium incorporation observed at carbons 9, 10, and 11 in DMST (4) from D2O and NADPD11 and provide a basis to re-evaluate the sequence of reactions that relate the anthraquinone versicolorin A (3) to the xanthone DMST (4). These findings lead to a consistent picture of aromatic deoxygenations extending from chrysophanol (11, Scheme 2) to 6-deoxy-3 (Scheme 3).14,15,19 Although 6-deoxy-3 has failed to support aflatoxin biosynthesis, keto tautomers or oxidized derivatives of dihydroanthracenone 17 capable of Baeyer–Villiger oxidation can be visualized to undergo cleavage, reclosure, and dehydration in a conceptually efficient biogenesis of xanthone 4 that parallels related pathways to fungal metabolites as tajixanthone, shamixanthone, and ergochromes.14,20–23
Supplementary Material
Acknowledgments
Financial support of this work by the Deutsche Forschungs-gemeinschaft (IRTG 1038) is gratefully acknowledged. We thank Sascha Ferlaino for measurement of the NMR spectra and Dr. Steffen Lüdeke for help with the VCD measurements and interpretation.
Footnotes
Notes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.5b06770.
Experimental details and characterization data (PDF)
References
- 1.Minto RE, Townsend CA. Chem Rev. 1997;97(7):2537. doi: 10.1021/cr960032y. [DOI] [PubMed] [Google Scholar]
- 2.Mahanti N, Bhatnagar D, Cary JW, Joubran J, Linz JE. Appl Environ Microbiol. 1996;62(1):191. doi: 10.1128/aem.62.1.191-195.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Townsend CA. Nat Prod Rep. 2014;31:1260. doi: 10.1039/c4np00092g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry KM, Townsend CA. J Am Chem Soc. 2005;127(11):3724. doi: 10.1021/ja0455188. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Chang PK, Ehrlich KC, Cary JW, Bhatnagar D, Cleveland TE, Payne GA, Linz JE, Woloshuk CP, Bennett JW. Appl Environ Microbiol. 2004;70(3):1253. doi: 10.1128/AEM.70.3.1253-1262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu J, Bhatnagar D, Cleveland TE. FEBS Lett. 2004;564(1–2):126. doi: 10.1016/S0014-5793(04)00327-8. [DOI] [PubMed] [Google Scholar]
- 7.Cary JW, Ehrlich KC, Bland JM, Montalbano BG. Appl Environ Microbiol. 2006;72(2):1096. doi: 10.1128/AEM.72.2.1096-1101.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skory CD, Chang PK, Cary J, Linz JE. Appl Environ Microbiol. 1992;58(11):3527. doi: 10.1128/aem.58.11.3527-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrlich KC, Montalbano B, Boúe SM, Bhatnagar D. Appl Environ Microbiol. 2005;71(12):8963. doi: 10.1128/AEM.71.12.8963-8965.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry KM, Townsend CA. J Am Chem Soc. 2005;127(10):3300. doi: 10.1021/ja045520z. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe CMH, Townsend CA. J Am Chem Soc. 1998;120(25):6231. [Google Scholar]
- 12.Chiang YM, Szewczyk E, Davidson AD, Entwistle R, Keller NP, Wang CCC, Oakley BR. Appl Environ Microbiol. 2010;76(7):2067. doi: 10.1128/AEM.02187-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez JF, Entwistle R, Hung JH, Yaegashi J, Jain S, Chiang YM, Wang CCC, Oakley BR. J Am Chem Soc. 2011;133(11):4010. doi: 10.1021/ja1096682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson TJ. ChemBioChem. 2012;13(11):1680. doi: 10.1002/cbic.201200014. [DOI] [PubMed] [Google Scholar]
- 15.Schätzle MA, Husain SM, Ferlaino S, Müller M. J Am Chem Soc. 2012;134(36):14742. doi: 10.1021/ja307151x. [DOI] [PubMed] [Google Scholar]
- 16.Müller M. Thesis. Ludwig-Maximilians-Universität; München: 1995. Synthesis of (R)- and (S)-atrochrysone and investigations in the biosynthesis of dimeric preanthraquinones. [Google Scholar]
- 17.Husain SM, Schätzle MA, Lüdeke S, Müller M. Angew Chem, Int Ed. 2014;53(37):9806. doi: 10.1002/anie.201404560. [DOI] [PubMed] [Google Scholar]
- 18.Yabe K, Hamasaki T. Appl Environ Microbiol. 1993;59(8):2493. doi: 10.1128/aem.59.8.2493-2500.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson JA, Lin BK, Williams HJ, Scott AI. J Am Chem Soc. 1988;110:1623. [Google Scholar]
- 20.Ahmed SA, Bardshiri E, McIntyre CR, Simpson TJ. Aust J Chem. 1992;45:249. [Google Scholar]
- 21.Franck B, Backhaus H, Rolf M. Tetrahedron Lett. 1980;21(13):1185. [Google Scholar]
- 22.Wezeman T, Bräse S, Masters KS. Nat Prod Rep. 2015;32(1):6. doi: 10.1039/c4np00050a. [DOI] [PubMed] [Google Scholar]
- 23.Franck B, Bringmann G, Flohr G. Angew Chem, Int Ed Engl. 1980;19(6):460. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.