Abstract
Candida parapsilosis is an important, emerging opportunistic fungal pathogen. Highly mannosylated fungal cell wall proteins are initial contact points with host immune systems. In Candida albicans, Och1 is a Golgi α1,6-mannosyltransferase that plays a key role in the elaboration of the N-linked mannan outer chain. Here, we disrupted C. parapsilosis OCH1 to gain insights into the contribution of N-linked mannosylation to cell fitness and to interactions with immune cells. Loss of Och1 in C. parapsilosis resulted in cellular aggregation, failure of morphogenesis, enhanced susceptibility to cell wall perturbing agents and defects in wall composition. We removed the cell wall O-linked mannans by β-elimination, and assessed the relevance of mannans during interaction with human monocytes. Results indicated that O-linked mannans are important for IL-1β stimulation in a dectin-1 and TLR4-dependent pathway; whereas both, N- and O-linked mannans are equally important ligands for TNFα and IL-6 stimulation, but neither is involved in IL-10 production. Furthermore, mice infected with C. parapsilosis och1Δ null mutant cells had significantly lower fungal burdens compared to wild-type (WT)-challenged counterparts. Therefore, our data are the first to demonstrate that C. parapsilosis N- and O-linked mannans have different roles in host interactions than those reported for C. albicans.
Keywords: cell wall, mannosylation pathway, Candida parapsilosis, host-fungus interplay, virulence, mannosyltransferase
Introduction
Candida parapsilosis is an opportunistic fungal pathogen largely associated with nosocomial infections in newborns and immunocompromised patients (Nosek et al., 2009). Over the past two decades, the incidence of C. parapsilosis has dramatically increased, such that this organism is the second most commonly isolated Candida species from blood cultures; though it is considered less virulent than C. albicans (Trofa et al., 2008). Moreover, increased resistance to some antifungal drugs, such as echinocandins, occurs in this pathogen (Trofa et al., 2008). In order to develop new and more effective drugs, important efforts are currently underway to achieve a better understanding of how fungal invasion occurs, including the pathogenesis of C. parapsilosis, as well as to identify new antifungal targets.
The cell wall is the first site of interaction between most fungal pathogens and host cells, so this structure profoundly influences the recognition of fungal cells and can trigger a protective immune defense (Netea et al., 2008; Díaz-Jiménez et al., 2012). At present, little is known about the organization and composition of the C. parapsilosis cell wall; nevertheless, as this fungus is closely related to C. albicans, it has been assumed that this organelle is similar in both organisms. In C. albicans, the cell wall is composed of an inner layer of chitin and β1,3- and β1,6-glucans that is covered with an outer layer of highly glycosylated proteins (Díaz-Jiménez et al., 2012). These proteins are covalently modified with mannose-rich oligosaccharides attached to either asparagine (N-linked mannans) or serine/threonine (O-linked mannans) residues (Klis et al., 2001). Mannans play important roles in cell wall integrity, adhesion to host tissues, virulence and in the establishment of a protective host immune response (Bates et al., 2005, 2006, 2013; Mora-Montes et al., 2007, 2009, 2010; Díaz-Jiménez et al., 2012; Hall et al., 2013; West et al., 2013). The interaction of C. albicans with immune cells has been thoroughly studied to establish the mechanisms underlying the generation of an effective anti-Candida immune response. Although, less well studied in C. parapsilosis, certain key differences in the phagocytic process and induction of the T-cell response have already been identified (Toth et al., 2013, 2014).
Thus, far, it is well established that in C. albicans the β1,3-glucans, N- and O-linked mannans are the main pathogen-associated molecular patterns (PAMPs) recognized by the immune system (Brown and Gordon, 2001; Netea et al., 2006). The mannose receptor (MR), expressed at the surface of dendritic cells, monocytes, and macrophages, recognizes α-mannose residues within the N-linked mannans (Netea et al., 2006; McKenzie et al., 2010). Other immune receptors for N-linked mannans include DC-SIGN (Cambi et al., 2008), Mincle (Wells et al., 2008), dectin-2 (Saijo et al., 2010), and dectin-3 (Zhu et al., 2013). In addition, the phosphomannan moiety contained in both N- and O-linked mannans is a key cell wall component for the effect of antimicrobial peptides, and for proper phagocytosis by murine macrophages (Harris et al., 2009; McKenzie et al., 2010). The O-linked mannans are recognized by the toll-like receptor 4 (TLR4), while β1,3-glucan is sensed through dectin-1 and TLR2 (Brown and Gordon, 2001; Netea et al., 2006).
Mannan relevance for C. albicans cell wall integrity, virulence, and sensing by innate immune cells has been mainly assessed using mutant cells lacking specific enzymes with key roles during the assembly of either N- or O-linked mannans (Bates et al., 2005, 2006, 2013; Munro et al., 2005; Prill et al., 2005; Mora-Montes et al., 2007, 2010; Hall et al., 2013). Among them, disruption of the OCH1 gene has been a valuable molecular tool to understand those cellular processes (Bates et al., 2006). This gene encodes for a Golgi-resident α1,6-mannosyltransferase that initiates the elaboration of the N-linked mannan outer chain. Null mutants lacking this gene in C. albicans exhibit reduced virulence, increased sensitivity to cell-wall perturbing agents, and reduced ability to stimulate cytokine production by human mononuclear cells (PMBCs) and dendritic cells (Bates et al., 2006; Netea et al., 2006; Cambi et al., 2008). Moreover, cell treatments with endoglycosidase H (endo H; an enzyme that trims the N-linked glycans from glycoproteins, Kobata 1979) or β-elimination, which specifically removes O-linked mannans, have further contributed to the understanding of the physiological role of these cell wall components in the biology of C. albicans (Hamada et al., 1981; Hazen and Glee, 1994; Mormeneo et al., 1994; Goins and Cutler, 2000; Spreghini et al., 2003).
In contrast, the role of C. parapsilosis mannans in cell fitness, virulence and immune sensing is unknown. Here, we disrupted C. parapsilosis OCH1 and found that loss of proper N-linked mannosylation affected cell morphology, filamentation, wall composition, susceptibility to wall perturbing agents, and fungal interaction with human PBMCs. Furthermore, we demonstrated that O-linked mannans are not required for phagocytosis by human monocyte-derived macrophages, but they play a central role in the stimulation of IL-1β by human PBMCs. Notably, the production of this cytokine required the co-stimulation of dectin-1 and TLR4. In a murine systemic infection model, but not in the alternative invertebrate model Galleria mellonella, the C. parapsilosis och1Δ null mutant showed significant defects in virulence.
Results
Deletion of CpOCH1
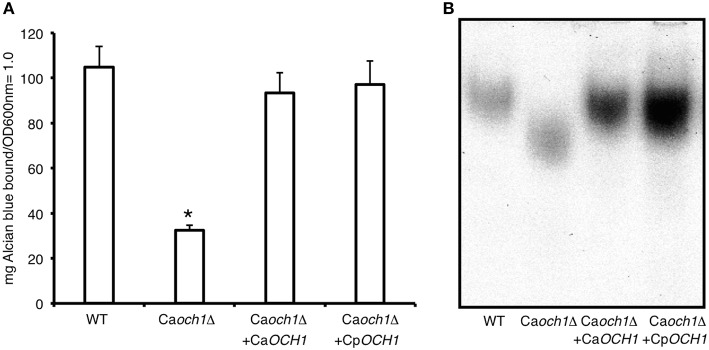
CpOCH1 was identified in the Candida genome database (http://www.candidagenome.org/) by homology to the C. albicans ortholog (Systematic name orf19.7391). The CpOCH1 open reading frame of 1089 bp (Systematic name CPAR2_404930) is predicted to encode a type-II transmembrane protein of 362 amino acids of the glycosyl transferase family 32, which shows 67 and 78% of identity and similarity to C. albicans Och1, respectively. This open reading frame is unlikely to encode the closely related α1,6-mannosyltransferase Hoc1, as it shows 39 and 57% of identity and similarity to C. albicans Hoc1 (Systematic name orf19.3445). The CpOCH1 alleles were deleted by sequential gene replacement in the CPL2H1 strain (Holland et al., 2014) as described for C. albicans, using disruption cassettes complementing leucine and histidine auxotrophies (Figure 1SA; Noble et al., 2010). Southern blotting assays were performed to discard ectopic integrations within the C. parapsilosis genome, using specific probes for CmLEU2, CdHIS1 and CpOCH1. Results showed loss of CpOCH1 gene and a single copy of each replacing cassette within the mutant strain genome (Figure 1SB), demonstrating the production of a C. parapsilosis och1Δ null mutant. As a control, the CpOCH1 open reading frame, under the control of the C. albicans TDH3 promoter was re-integrated into the C. parapsilosis och1Δ null mutant, generating a re-integrant control strain. In order to demonstrate that CpOCH1 is the functional ortholog of the CaOCH1, we complemented a C. albicans och1Δ null mutant (Bates et al., 2006) with CpOCH1. Results indicated that the C. parapsilosis gene was able to restore the levels of phosphomannosylation (Figure 1A) and the electrophoretic mobility of Hex1 (Figure 1B), a secreted protein previously used to assess the status of the N-linked glycosylation pathway (Bates et al., 2005, 2006; Mora-Montes et al., 2007; Lopes-Bezerra et al., 2015). Therefore, C. parapsilosis OCH1 is the functional ortholog of C. albicans OCH1.
Figure 1.
CpOCH1 is the functional ortholog of CaOCH1. The CpOCh1 open reading frame was expressed in the Caoch1Δ null mutant, as described in materials and methods, and the ability of cells to bind Alcian blue, an indirect measurement of the cell wall phosphomannan content, and therefore of mannan length was analyzed (A). In addition, cells were grown in minimal medium with GlcNAc added to induce the N-acetylhexosaminidase activity, broken to obtain a cell homogenate and protein samples were used to perform electrophoretic mobility shift assays of Hex1, a highly N-linked glycosylated N-acetylhexosaminidase (B). *P < 0.05. Strains used are: NGY152 (WT), Caoch1Δ (NGY357); Caoch1Δ + CaOCH1 (NGY328), Caoch1Δ + CpOCH1 (HMY163).
Filamentation, colony and cell morphology of the C. parapsilosis och1Δ null mutant
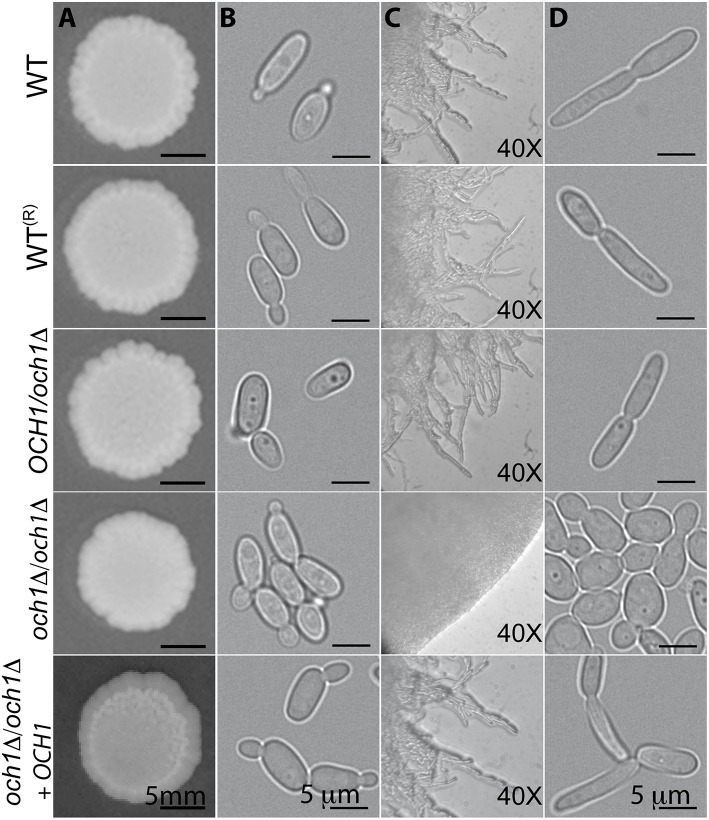
The growth rate of the Cpoch1Δ null mutant was significantly reduced, with doubling times of 2.79 ± 0.22 h for the null mutant vs. 1.54 ± 0.11 and 1.57 ± 0.15 h for the wild-type (WT) and reintegrant control strains, respectively (P < 0.05). Experiments conducted in presence of 2 units/mL chitinase to disrupt cell aggregates (Bates et al., 2006) showed similar results (data not shown). The Cpoch1Δ null cells displayed a clumpy phenotype, i.e., they tended to form cell aggregates when cultured in liquid media (Figure 2B). Colony morphology was not significantly affected when cells were grown on Sabouraud plates at 20 and 28°C, or under pH conditions ranging from 4 to 8 (Figure 2A and data not shown). However, when strains were grown at 37°C, the null mutant failed to develop pseudohyphae, even when cultured in filamentation-inducing media, such as RPMI supplemented with 10% (v/v) fetal bovine serum, Lee or Spider media (Figure 2D, and data not shown). Under such growth conditions, the Cpoch1Δ null mutant displayed well-defined colony edges on the plates and no filamentous structures were visible by light microscopy (Figure 2C). The reintegrant control strain did not show the clumpy phenotype and effectively generated pseudohyphae, confirming that the phenotypes observed in the null mutant strain are indeed related to OCH1 disruption. Therefore, loss of OCH1 affects C. parapsilosis morphogenesis.
Figure 2.
Loss of OCH1 affects C. parapsilosis morphological transition. Cells from the Cpoch1Δ null mutant grown on dextrose-Sabouraud plates at 28°C did not show differences in colonial morphology (A), but tended to form aggregates in dextrose-Sabouraud broth (B). Upon growth in RPMI 1640 plates supplemented with 10% fetal bovine serum at 37°C, the null mutant displayed rounded and well-defined colony edges, whereas the control strains generated filamentous projections outside the colony (C). When filamentation was induced using liquid medium (RPMI 1640 added with 10% fetal bovine serum and 37°C), control strains underwent morphological transition, but Cpoch1Δ null mutant was unable to form pseudohyphae (D). The strains used are: CLIB-214 (WT), CPRI (WT(R)), AP (OCH1/och1Δ), AP-1 (och1Δ/och1Δ), and AP-2 (och1Δ/och1Δ + OCH1).
Loss of CpOch1 affects cell wall composition and susceptibility to cell wall perturbing agents
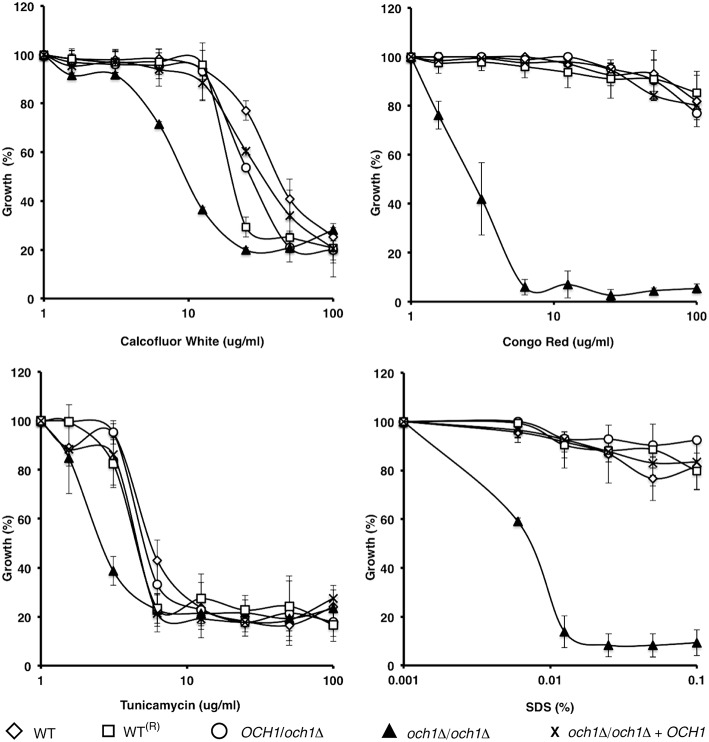
To assess the effect of CpOCH1 mutation on cell wall integrity, we tested the susceptibility of the null mutant to a range of cell wall perturbing agents and compounds associated with glycosylation defects. The Cpoch1Δ null mutant exhibited enhanced susceptibility to Calcofluor White and Congo Red (P = 0.04055 and 0.00124, respectively), which interact with cell wall chitin and β-glucans, respectively (Figure 3). Furthermore, the null mutant had an increase in susceptibility to Tunicamycin (P = 0.0278), an inhibitor of the first steps during N-linked mannan biosynthesis; and it was highly susceptible to SDS (P = 0.0074), a detergent that affects the plasma membrane (Bates et al., 2006; Mora-Montes et al., 2007); whereas the WT and control strains were largely resistant (Figure 3). Hygromycin B, vanadate, and osmotic stressors such as NaCl and KCl were also tested, but no significant differences were observed (data not shown). Similar results were generated when the experiments were performed in presence of chitinase to disaggregate cells (not shown).
Figure 3.
C. parapsilosis och1Δ null mutant displays enhanced susceptibility to specific cell wall perturbing agents. The wild type (open diamonds), wild-type plus CmLEU2 and CdHIS1 (open squares), OCH1/och1Δ (open circles), och1Δ/och1Δ (closed triangles), and och1Δ + OCH1 (X symbol) cells were incubated, using a micro-dilution method, with different concentrations of either Calcofluor White, Congo Red, Tunicamycin, or SDS, and growth was determined after incubation for 16 h at 30°C. Growth data were normalized as percentage of those generated with the same strains without treatment. The Cpoch1Δ null mutant (closed triangles) exhibited a higher susceptibility to all the perturbing agents tested. Data are means ± SD of three independent experiments performed in duplicates.
We next assessed the cell wall composition of the Cpoch1Δ null mutant. Cell walls were purified, acid hydrolyzed, washed and analyzed by High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection (HPAEC-PAD). Results indicated similar carbohydrate content among the control strains, but the null mutant displayed a reduction of about 67% in the mannan content, along with a significant increment in β-glucan and chitin levels, when compared to the control strains (Table 1). No significant differences in the cell wall protein level or the phosphomannan content were observed (Table 1). The relative cell wall porosity to polycations, an indirect reporter of the glycan arrangement (De Nobel et al., 1990; Cheng et al., 2011), was greater in the Cpoch1Δ null mutant than in the heterozygous or control strains (Table 1). Furthermore, we assessed whether rearrangements in the organization of cell wall components occurred upon disruption of CpOCH1. For this, we quantified the ability of the fluorescein isothiocyanate-wheat germ agglutinin conjugate (WGA-FITC) and IgG Fc-Dectin-1 chimera to bind chitin and β1,3-glucan, respectively (Graham et al., 2006; Mora-Montes et al., 2011; Marakalala et al., 2013). Results shown in Figure 4 indicate that both lectins displayed a weak ability to bind either chitin or β1,3-glucan at the cell wall of live WT, reconstituted WT, heterozygous mutant and re-integrant control cells. However, the live och1Δ null mutant showed an increased ability to bind both WGA-FITC and the IgG Fc-Dectin-1 chimera (Figure 4). C. albicans inactivation by heat exposes inner cell wall components at the cell surface, such as β1,3-glucan and chitin (Gow et al., 2007; Mora-Montes et al., 2011). Thus, as expected, enhanced binding by both lectins occurred upon inactivation of yeast cells by heating (Figure 4). Altogether, these results indicate that the Cpoch1Δ null mutant has significant defects in cell wall composition and fitness.
Table 1.
Cell wall analysis of Cpoch1Δ null mutant and control strains.
| Strain | Cell wall abundance | Phosphomannan content (μg)a | Porosity (%)b | Protein (μg)c | ||
|---|---|---|---|---|---|---|
| Chitin (%) | Mannan (%) | Glucan (%) | ||||
| CLIB-214 (WT) | 2.27 ± 0.7 | 27.40 ± 0.7 | 70.32 ± 1.3 | 74.25 ± 3.3 | 65.32 ± 11.1 | 164.64 ± 20.6 |
| CPRI (WTR) | 2.15 ± 0.3 | 27.07 ± 4.6 | 70.76 ± 4.9 | 66.97 ± 8.3 | 71.52 ± 8.0 | 137.5 ± 46.3 |
| AP (OCH1/och1Δ) | 2.05 ± 0.5 | 26.13 ± 4.8 | 71.80 ± 4.7 | 66.95 ± 10.7 | 65.46 ± 8.9 | 148.17 ± 35.7 |
| AP-1 (och1Δ/och1Δ) | 4.94 ± 0.8* | 9.26 ± 0.8* | 85.78 ± 1.0* | 68.03 ± 4.5 | 90.09 ± 3.8* | 135.02 ± 17.4 |
| AP-2 (och1Δ+ OCH1) | 2.12 ± 0.5 | 26.97 ± 1.8 | 71.22 ± 1.8 | 70.77 ± 5.2 | 63.34 ± 7.9 | 155.40 ± 36.2 |
| CLIB-214d (WT) | 2.27 ± 0.6 | 14.20 ± 1.3* | 86.53 ± 1.8* | ND | ND | ND |
| AP-1d (och1Δ/och1Δ) | 4.24 ± 0.5* | Traces* | 95.76 ± 0.5* | ND | ND | ND |
| AP-2d (och1Δ + OCH1) | 2.18 ± 0.4 | 14.64 ± 2.6 | 85.23 ± 1.1* | ND | ND | ND |
μg of Alcian Blue bound/OD600 = 1.
Relative to DEAE-Dextran.
μg of protein/mg of cell wall.
Upon β-elimination.
ND Not determined.
P < 0.05.
Figure 4.
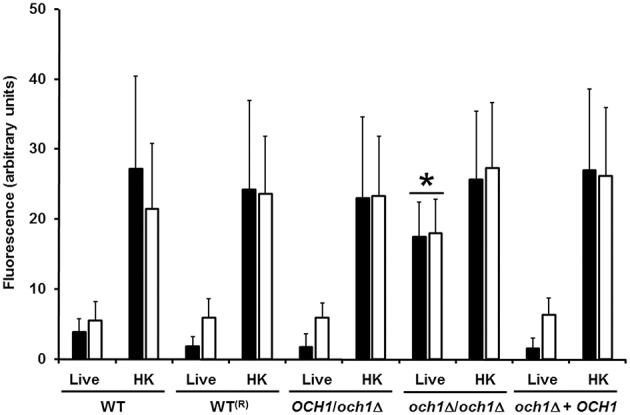
Chitin and β1,3-glucan are significantly exposed at the cell surface of the C. parapsilosis och1Δ null mutant. Live or heat-killed (HK) yeast cells were incubated with either fluorescein isothiocyanate-wheat germ agglutinin conjugate (closed bars) or IgG Fc-Dectin-1 chimera (open bars) as described in Materials and Methods, inspected under fluorescence microscopy, and the fluorescence associated to 50 individual cells recorded. The strains used are: CLIB-214 (WT), CPRI (WT(R)), AP (OCH1/och1Δ), AP-1 (och1Δ/och1Δ), and AP-2 (och1Δ + OCH1). *P < 0.05, when compared with live cells from CLIB-214, CPR1, AP, and AP-2 strains.
Loss of C. parapsilosis OCH1 affects cytokine production by human PBMCs
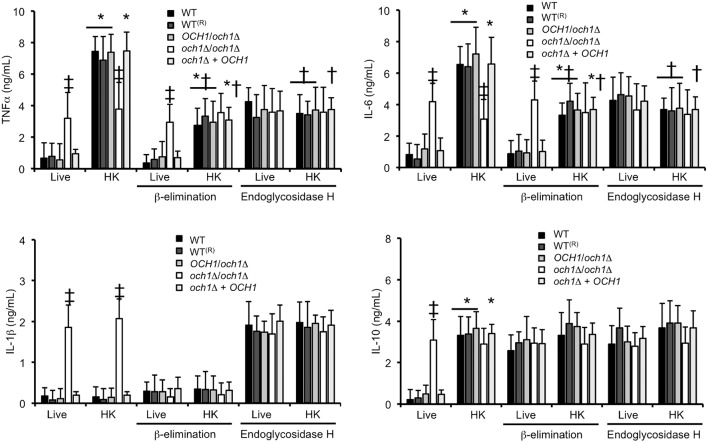
We next assessed the relevance of proper cell wall N-linked mannosylation during C. parapsilosis interaction with human PBMCs, quantifying the levels of pro- and anti-inflammatory cytokines as a read out of this interaction. Live C. parapsilosis yeast cells from WT control strains and the heterozygous mutant stimulated low and similar TNFα, IL-1β, IL-6, and IL-10 levels (Figure 5). As mentioned, C. albicans inactivation by heat exposes β1,3-glucan at the cell surface, and heat-treated C. albicans cells are therefore more immunostimulatory compared to untreated cells (Gow et al., 2007). Here, upon heat inactivation of WT C. parapsilosis cells, the interaction with PBMCs resulted in stimulation of TNFα, IL-6, and IL-10, but not IL-1β (Figure 5). Live C. parapsilosis och1Δ null mutant stimulated significantly higher levels of both pro- and anti-inflammatory cytokines compared to WT and control cells (Figure 5), and, accordingly, WT and control cells treated with endo-H, i.e., with N-linked mannan enzymatically removed from the cell wall (Kobata, 1979), induced cytokine levels similar to that with the och1Δ null mutant cells (Figure 5). The heat-killed (HK) och1Δ null mutant and the endo-H-treated WT cells stimulated significantly less TNFα and IL-6 production than the heterozygous mutant, the reintegrant control and the WT control cells (Figure 5), suggesting that recognition of both N-linked mannans and inner cell wall components is required for maximal cytokine stimulation. Production of IL-1β and IL-10 was insensitive to heat killing in both the och1Δ null mutant and the endo-H-treated WT cells. Therefore, proper N-linked mannosylation is required for stimulation of cytokines by human PBMCs.
Figure 5.
Loss of proper cell wall mannosylation affects the ability of C. parapsilosis to stimulate cytokine production by human PBMCs. Yeast cells where co-incubated with human PBMCs, the supernatant saved and used to quantify pro- and anti-inflammatory cytokines. Results (means ± SD) where obtained using samples from six donors, each assayed in duplicate wells. The strains used are: CLIB-214 (WT), CPRI (WT(R)), AP (OCH1/och1Δ), AP-1 (och1Δ/och1Δ), and AP-2 (och1Δ + OCH1). *P < 0.05, when compared with live cells; †P < 0.05, when compared with untreated cells; ‡P < 0.05, when compared with cells subjected to the same treatment.
Loss of O-linked mannans affects the ability of C. parapsilosis to stimulate cytokine production by human PBMCs
Next, to obtain insights into the role of O-linked mannans as PAMPs during C. parapsilosis sensing, we trimmed this oligosaccharide from the cell wall by β-elimination (Diaz-Jimenez et al., 2012), and used these cells to stimulate cytokine production by human PBMCs. After the removal of O-linked mannans, cell viability was not significantly altered (not shown), about 50% of mannan content was removed from the cell wall of WT and control cells, and no mannose was recorded in the cell wall preparations from och1Δ null mutant cells (Table 1). Furthermore, C. albicans β-eliminated cells stimulated similar cytokine levels than those stimulated by the C. albicans mnt1-mnt2Δ null mutant (Munro et al., 2005) that lacks long O-linked mannans from the cell wall (Figure 2S). Live WT control, heterozygous, re-integrant strain and och1Δ null mutant cells stimulated similar TNFα and IL-6 levels in comparison to the β-eliminated-treated cells (Figure 5). HK WT controls, re-integrant, and OCH1/och1Δ heterozygous cells, but not och1Δ null mutant cells, induced lower TNFα and IL-6 levels, suggesting that O-linked mannans significantly contribute to C. parapsilosis sensing by human PBMCs. Upon O-linked mannan trimming, HK och1Δ cells lost the ability to stimulate high IL-1β levels, suggesting a key role for this cell wall component in IL-1β stimulation. As in the case of N-linked mannans, loss of O-linked mannans did not affect production of IL-10 (Figure 5). Overall, these data indicate key roles of both N- and O-linked mannans during the interaction of C. parapsilosis with human PBMCs.
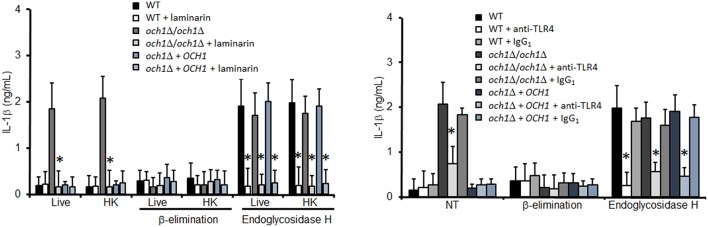
Dectin-1 and TLR4 are required for IL-1β production
Next, we analyzed the pathogen recognition receptors involved in the stimulation of IL-1β. The production of this cytokine was dependent on proper engagement of either dectin-1 or TLR4, as fungal cells lacking N-linked mannans stimulated reduced levels of IL-1β production upon blocking with laminarin and antibodies to TLR4, respectively (Figure 6). No effect was observed in the IL-1β levels when TLR2 was blocked with an antibody to TLR2, indicating this receptor does not affect stimulation of this cytokine (Figure 3S). As a control assay for this blocking agent, we found that this antibody did not affect the levels of IL-10 (Figure 4S), but significantly reduced TNFα production by WT cells harboring N-linked mannans at the cell wall (Figure 3S). Control experiments using isotype-matched, irrelevant antibodies did not show significant variations in cytokine stimulation by C. parapsilosis cells (Figure 6, Figure 3S). Overall, these data indicate that N-linked mannans are able to inhibit IL-1β production whereas stimulation of this cytokine requires presence of O-linked mannans, dectin-1 and TLR4.
Figure 6.
Blocking of dectin-1 and TLR4 affects IL-1β stimulation by C. parapsilosis cells. Human PBMCs were pre-incubated with either laminarin, antibody to TLR4, or irrelevant IgG1 for 1 h at 37°C, before incubation with yeast cells. After 24 h incubation at 37°C the supernatant were saved and used to quantify IL-1β. Results (means ± SD) where obtained using samples from six donors, each assayed in duplicate wells. The strains used are: CLIB-214 (WT), AP-1 (och1Δ/och1Δ), and AP-2 (och1Δ + OCH1). *P < 0.05, when compared to same cell type without treatment. NT, non-treated cells.
N-linked mannans are not required for the uptake of C. parapsilosis by macrophages
To assess the relevance of C. parapsilosis cell wall mannans during phagocytosis, primary human PBMC-derived macrophages were co-cultured with fluorescently labeled WT or C. parapsilosis och1Δ for 1.5 h and the ratio of phagocytosing macrophages was determined by flow cytometry. Interestingly, we found that C. parapsilosis och1Δ null mutant cells were as efficiently phagocytosed by the PBMC-derived macrophages as WT cells, indicating that N-linked mannans are not essential cell wall components for C. parapsilosis phagocytosis (Figure 5S). Furthermore, we similarly did not find a difference in the engulfment of C. parapsilosis WT or och1Δ null mutant cells by J774 mouse macrophages (data not shown).
The C. parapsilosis och1Δ null mutant is attenuated in virulence in the mouse model of systemic candidiasis
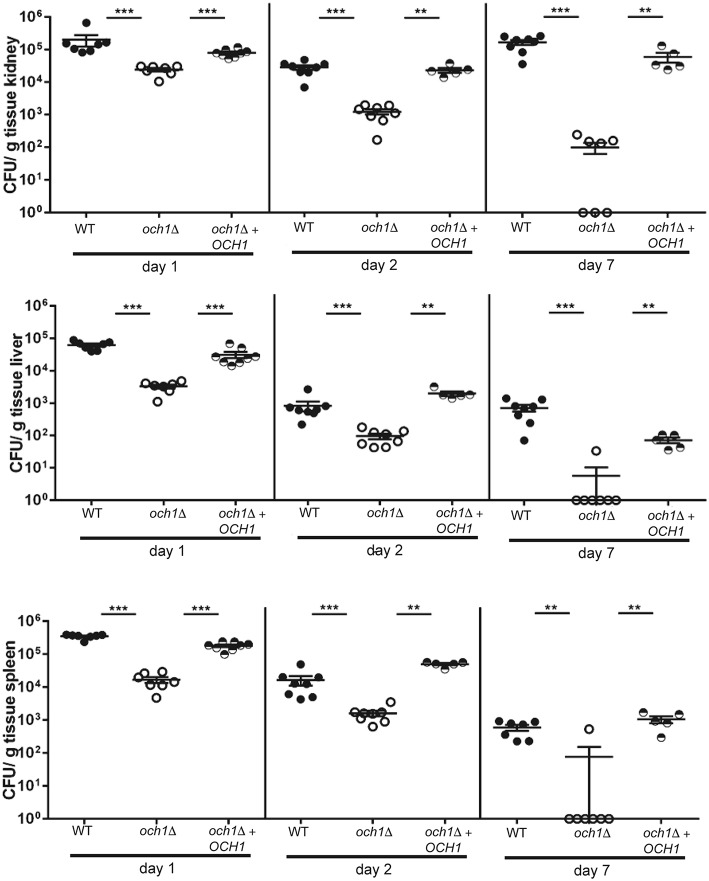
To determine how the disruption of the protein N-linked mannosylation affects the virulence of C. parapsilosis, we compared the susceptibility of BALB/c mice to C. parapsilosis WT and the och1Δ null mutant in a non-lethal experimental model of disseminated candidiasis, as previously reported (Ifrim et al., 2014). We found that mice infected with the och1Δ null mutant had significantly decreased fungal burdens in the spleen, kidneys and liver at 1, 2, and 7 days post-infection, when compared to those mice infected with the WT strain (Figure 7). Animals infected with the reintegrant control had fungal burdens similar to mice challenged with WT yeast cells (Figure 7). Therefore, these results demonstrate that the loss of OCH1 significantly affects the virulence of C. parapsilosis in vivo.
Figure 7.
The C. parapsilosis och1Δ null mutant has decreased virulence in the mouse model of systemic candidiasis. Wild-type BALB/c mice were infected i.v. with 2 × 107 cells from either C. parapsilosis wild type (CLIB-214), och1Δ (AP-1) or och1Δ + OCH1 (AP-2), and fungal burdens in kidneys, liver, and spleen were determined at 1, 2, or 7 days post-infection and expressed as CFU/g tissue (mean ± SEM). Results are pooled data from 2 separate experiments with a total of 5–8 mice per group. **P < 0.01, ***P < 0.001.
We also assessed the virulence of the mutant strains generated in this work in the Galleria mellonella model of disseminated candidiasis (Gago et al., 2014; Jacobsen, 2014). Interestingly, larvae inoculated with either the WT control cells or the Cpoch1Δ null mutant displayed similar mortality rates, with most of the larvae dying after 10 days post-inoculation (Figure 5S). As a control, we included the C. albicans och1Δ null mutant that was attenuated in a mouse model of systemic candidiasis (Bates et al., 2006), and this strain also displayed virulence attenuation in the G. mellonella infection system (Figure 6S). Therefore, these results indicate that the virulence of the C. parapsilosis och1Δ null mutant significantly depends on the host milieu.
Discussion
Thus, far, there are a limited number of disrupted genes in C. parapsilosis and most of them are involved in either lipid metabolism or biofilm formation (Ding and Butler, 2007; Gácser et al., 2007; Ding et al., 2011; Nguyen et al., 2011a,b; Connolly et al., 2013; Holland et al., 2014). Here, we report the first disruption of a gene involved in C. parapsilosis N-linked mannosylation, and assess the contribution of this metabolic pathway in the fitness of this organism and its role in the interaction with the host. In addition, we also provide for first time a comparative analysis between enzymatic removal and gene disruption to evaluate the participation of N-linked mannans during the human PBMCs-fungus interplay.
Our studies indicate that the heterozygous strain at the OCH1 locus, and the reintegrant control strain are functionally similar to the WT strain, indicating haplosufficiency, as reported in C. albicans (Bates et al., 2006). The C. parapsilosis och1Δ null mutant displayed typical phenotypes associated with defects in the N-linked mannosylation pathway, including slower growth rates, a clumpy cell phenotype, abnormal morphogenesis, and defects in the cell wall composition, porosity and fitness (Bates et al., 2005, 2006, 2013; Mora-Montes et al., 2007, 2010; Hall et al., 2013). Together with the complementation of the C. albicans och1Δ null mutant, these data strongly suggest CpOCH1 is the functional ortholog of C. albicans OCH1. Interestingly, the C. parapsilosis null mutant cells were not susceptible to hygromycin B, which strongly affects Saccharomyces cerevisiae and C. albicans cells with defects in the glycosylation pathways (Dean, 1995; Bates et al., 2005, 2006; Mora-Montes et al., 2007), suggesting that dependence on N-linked mannosylation for cell fitness is less important in C. parapsilosis compared to C. albicans. It has been reported that phosphomannosylation in C. albicans occurs in both, the N-linked mannan core and the outer chain, and the OCH1 disruption significantly affected the cell ability to bind Alcian blue (Bates et al., 2006). Since the C. parapsilosis WT and och1Δ cells have a similar ability to bind Alcian blue, it is possible to suggest that phosphomannan is mainly attached to the glycan core or to the O-linked mannans, which contrast with the current knowledge about C. albicans mannan structure (Bates et al., 2006; Mora-Montes et al., 2007).
It is well established that cell wall mannans and β1,3-glucan play a pivotal role during the C. albicans-innate immune system interaction (Netea et al., 2008; Mora-Montes et al., 2009), and truncated N-linked mannans lead to a reduced ability to stimulate cytokine production by human PBMCs (Netea et al., 2006; Mora-Montes et al., 2007, 2010). Here, we found a similar result when HK C. parapsilosis och1Δ null mutant or endo-H-treated cells were used to stimulate TNFα and IL-6 production, indicating C. parapsilosis N-linked mannans do play a significant role during stimulation of these cytokines. However, in contrast to what has been reported in C. albicans, truncated N-linked mannans did not affect the stimulation of IL-10 production during HK C. parapsilosis-human PBMCs interaction, but the cytokine levels were diminished upon dectin-1 blocking with laminarin (data not shown). IL-10 stimulation can be triggered by engagement of dectin-1 with its ligand, and simultaneous stimulation of co-receptors, such as TLR2, amplifies the cytokine production (Reid et al., 2009). Thus, it is feasible to conclude that dectin-1 engagement by β1,3-glucan triggers the signaling pathway involved in IL-10 stimulation by C. parapsilosis cells. Results obtained here with live C. parapsilosis och1Δ cells, where IL-6, IL-10, and TNFα production was higher than in the WT control cells, could be explained by the reduction in the mannan levels and the increased β-glucan content at the cell wall surface of the null mutant. Therefore, it is likely that mannans mask the β1,3-glucan layer, and thus block the triggering of cytokine production via dectin-1, as reported (Gow et al., 2007; Wheeler and Fink, 2006).
The C. albicans O-linked mannans represent a minor component of the cell wall (Munro et al., 2005), and are a non-essential wall component for cytokine stimulation by human PBMCs, as loss of these oligosaccharides has little impact in the ability of C. albicans to stimulate production of both pro-and anti-inflammatory cytokines (Netea et al., 2006). Here, we found that in C. parapsilosis the O-linked mannans play a more significant role than that reported for C. albicans. Accordingly, we showed here that O-linked mannans represent about the half of the total mannan content of the C. parapsilosis wall, which might contribute to the differential role for this component in C. albicans and C. parapsilosis. The C. parapsilosis cells lacking both, proper N- and O-linked mannans displayed a reduction of about 50% in the stimulation of TNFα and IL-6, which contrast with the poor ability of C. albicans pmr1Δ null mutant (a strain with no mannans at the wall surface) to stimulate these cytokines (30% and 20% of TNFα and IL-6, respectively; Netea et al., 2006). Therefore, our results indicate that relevance of mannans during C. parapsilosis immune sensing is different from that described in C. albicans, and other C. parapsilosis wall components might play roles that are more important during interactions with immune cells.
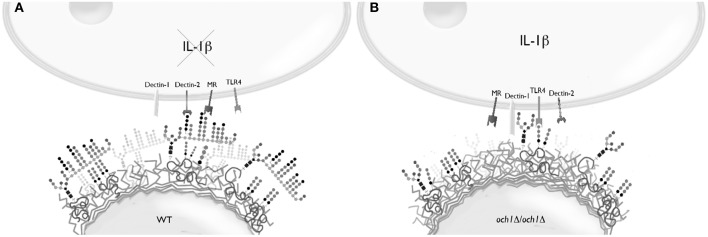
Loss of proper cell wall N-linked mannosylation in C. parapsilosis led to an increased ability to stimulate IL-1β production and secretion, a product of inflammasome activation (van de Veerdonk et al., 2009), and this response was abrogated when O-linked mannans were removed from the cell wall, suggesting a key role for these oligosaccharides in IL-1β stimulation. Our data indicate the IL-1β production depends on engagement of β1,3-glucan with dectin-1 and TLR4 with its ligand, most likely O-linked mannans (Netea et al., 2006). Thus, our model to explain this results implies that N-linked mannans mask not only β1,3-glucans as in C. albicans, but also O-linked mannans (Figure 8). Hence, removal or disruption of the N-linked mannan layer results in the recognition of β1,3-glucan and O-linked mannans by dectin-1 and TLR4, respectively, leading to the stimulation IL-1β production. This model contrasts with the one reported for C. albicans, where N-linked mannans, dectin-1 and TLR2, but not O-linked mannans nor TLR4, are the key components in the signaling pathway for this cytokine stimulation (van de Veerdonk et al., 2009). These models therefore suggest differences in the structure of both N- and O-linked mannans in these fungal organisms. Accordingly, the N-linked mannans from C. parapsilosis are shorter and less complex than those found in C. albicans (Shibata et al., 1995). Notably, a cooperation between dectin-1 and TLR4 for IL-1β production has not been previously described.
Figure 8.
Conceptualization of the stimulation of IL-1β production by C. parapsilosis. (A) In the wild-type cells, N-linked mannans mask both β1,3-glucan and the ligand of TLR4, most likely O-linked mannans, resulting in a relatively limited production of IL-1β. (B) Once the N-linked mannan outer chain is removed, such as in the och1Δ null mutant, the ligands for dectin-1 and TLR4 are exposed enabling the induction of a strong stimulation for IL-1β production.
The role of cell wall mannans during C. albicans phagocytosis by macrophages has been previously addressed and N-linked mannans are required for proper phagocytosis, while O-linked mannans play a negative role during this interaction (McKenzie et al., 2010). Interestingly, here we found that N-linked mannans are redundant wall components during C. parapsilosis phagocytosis by human and murine macrophages, suggesting once again a differential role for these cell wall glycans during host-pathogen interactions. Despite we did not find any significant difference in the uptake of both WT and och1Δ cells by phagocytic cells, it is not possible to discard a differential ability of macrophages to kill fungal cells, as it was not investigated in this work. When we assessed the relevance of OCH1 during the host-fungus interaction, we found that C. parapsilosis och1Δ null mutant had significantly decreased virulence in a mouse model of systemic candidiasis, which is in line with the findings reported for C. albicans (Bates et al., 2006). However, it was interesting to observe that the C. parapsilosis och1Δ null mutant cells were as virulent as the control strain in the G. mellonella infection model, while the C. albicans och1Δ null mutant displayed virulence attenuation in both the insect and mouse models (Bates et al., 2006). Although, this insect model has been previously used to assess the virulence of members of the C. parapsilosis complex (Gago et al., 2014), our data suggest that this host might not be the most appropriate model to study the C. parapsilosis virulence. In addition, as phagocytic components play a very important role in the innate immune responses of G. mellonella, it is tempting to speculate that the decreased virulence of C. albicans och1Δ null mutant in this model results from its altered interaction with phagocytes.
In conclusion, we report that OCH1 regulates N-linked mannan production in C. parapsilosis and impacts biological interactions with host effector cells. Deletion of OCH1 leads to morphological alterations in C. parapsilosis, including cellular aggregation, inability to form pseudohyphae, and defects in the wall composition. Most significantly, N-linked and O-linked mannans differentially stimulate cytokine production during the interactions of C. parapsilosis with innate immune cells. Finally, the results further underscore the importance of detailed molecular and biological investigation of each of the members of the Candida genus.
Experimental procedures
Strains and culture conditions
The strains utilized in the experiments are listed in Table 2. Unless otherwise indicated, cells were maintained and propagated at 30°C in Sabouraud medium [1% (w/v) mycological peptone, 4% (w/v) glucose]. Two % (w/v) agar was included when solid medium was required. In order to prepare cells for cytokine assays and cell wall analyses, 500 μL from an overnight culture were transferred to 500 mL flasks containing 100 mL of fresh medium and incubated at 30°C with constant shaking at 200 rpm, until mid-log growth phase was reached. Filamentation was induced by growing 5 × 106 cells/mL in RPMI 1640 medium (Sigma) supplemented with 10% fetal bovine serum (Sigma) or Lee's medium (all reagents from Sigma; Lee et al., 1975), at 37°C for 4 h. Cells were heat inactivated by incubating at 56°C for 60 min (Mora-Montes et al., 2007). Loss of cells viability was confirmed by absence of fungal growth in Sabouraud broth at 30°C for 72 h. β-Elimination was achieved by incubating cells overnight with 100 mM NaOH at room temperature and mild shaking (Diaz-Jimenez et al., 2012). Cells maintained viability upon β-elimination, since they showed no significant differences in CFU/mL before and after this treatment (average loss of cell viability upon β-elimination was 5.0 ± 2.0%). Trimming of N-linked glycans was accomplished by treating cells with endo-H (New England BioLabs) as described (Mora-Montes et al., 2012). The release of cell wall mannans was assessed by quantifying the free mannan content upon either β-elimination or treatment with endo-H, using HPAEC-PAD, in an ion chromatograph Dionex ICS-3000 (USA) with a guard-column CarboPac PA-100 (4 × 50 mm) and analytical CarboPac-PA100 (4 × 250 mm) column as reported (Mora-Montes et al., 2012). In some experiments, the medium was added with 2 units/mL chitinase (Sigma) to disperse cell aggregates as reported (Bates et al., 2006).
Table 2.
Fungal strains used in this work.
| Strain | Organism | Origin | Genotype | References |
|---|---|---|---|---|
| CLIB-214 | C. parapsilosis | Clinical isolate | Type strain | Laffey and Butler, 2005 |
| CPL2H1 | C. parapsilosis | Derived from CLIB-214 | leu2Δ::FRT/leu2Δ::FRT, his1Δ::FRT/his1Δ::FRT | Holland et al., 2014 |
| CPRI | C. parapsilosis | Derived from CPL2H1 | leu2Δ::FRT/leu2Δ::FRT, his1Δ::FRT/his1Δ::FRT, FRT::CmLEU2/FRT::CdHIS1 | Holland et al., 2014 |
| AP | C. parapsilosis | Derived from CPL2H1 | leu2Δ::FRT/leu2Δ::FRT, his1Δ::FRT/his1Δ::FRT, och1Δ::CmLEU2/OCH1 | This work |
| AP-1 | C. parapsilosis | Derived from AP | leu2Δ::FRT/leu2Δ::FRT, his1Δ::FRT/his1Δ::FRT, och1Δ::CmLEU2/ och1Δ::CdHIS1 | This work |
| AP-2 | C. parapsilosis | Derived from AP-1 | As AP-1 but RPSI/rps1Δ::pTDH3-OCH1-caSAT1 | This work |
| NGY152 | C. albicans | Derived from CAI-4 | ura3Δ-iro1Δ::imm434/ ura3Δ-iro1Δ::imm434; RPSI/rps1Δ::Clp10 | Brand et al., 2004 |
| NGY205 | C. albicans | Derived from NGY204 | ura3Δ-iro1Δ::imm434/ ura3Δ-iro1Δ::imm434; och1Δ::hisG/och1Δ::hisG | Bates et al., 2006 |
| NGY357 | C. albicans | Derived from NGY205 | As NGY205, but RPSI/rps1Δ::Clp10 | Bates et al., 2006 |
| NGY328 | C. albicans | Derived from NGY205 | As NGY205, but RPSI/rps1Δ::Clp10-CaOCH1 | Bates et al., 2006 |
| HMY163 | C. albicans | Derived from NGY205 | As NGY205, but RPSI/rps1Δ::Clp10-CpOCH1 | This work |
| NGY337 | C. albicans | Derived from CAI-4 | ura3Δ-iro1Δ::imm434/ ura3Δ-iro1Δ::imm434; mnt1-mnt2Δ::hisG/mnt1-mnt2Δ::hisG, RPS1/rps1Δ::CIp10 | Munro et al., 2005 |
| NGY335 | C. albicans | Derived from NGY337 | As NGY337 but RPS1/rps1Δ::CIp10-MNT1 | Munro et al., 2005 |
Construction of the Cpoch1Δ null mutant, heterozygous and re-integrant strain
The gene disruption strategy performed here consisted in the use of a double auxotrophy (His− Leu−) system in the CLIB-214 isolate of C. parapsilosis (CPL2H1 strain) (Holland et al., 2014), as described for C. albicans (Noble et al., 2010). Briefly, disruption cassettes containing either C. dubliniensis HIS1 or C. maltosa LEU2 genes flanked by ~500 nucleotides matching sequences upstream and downstream of the C. parapsilosis OCH1 gene were generated by fusion PCR. The markers C. dubliniensis HIS1 or C. maltosa LEU2 were amplified by PCR from pSN52 or pSN40 (Noble and Johnson, 2005), respectively, using the same primers (primer pair: 5′-CCGCTGCTAGGCGCGCCGTGACCAGTGTGATGGA TATCTGC-3′and 5′-GCAGGGATGCGGCCGCTGACT CCGCTTAAACAATCGGCAAAGCTCGGATCCACTAGTAAC G-3′, underlined sequences align with the 5′ and 3′ OCH1 fragments, respectively). The C. parapsilosis OCH1 5′ and 3′ regions of homology were amplified by PCR (primer pair: 5′-CAACATTTACATTCTTTTGC-3′ and 5′-CAC GGCGCGCCTAGCAGCGGATCAACGTATAAGCACTGC C-3′; and primer pair: 5′-GTCAGCGGCCGCATCCCTGCTGCC TGAAATGCTTACATAG-3′ and 5′-AACATCTCAAAACCGC AAGA-3′; homologous sequences to C. dubliniensis HIS1 or C. maltosa LEU2 markers are underlined, respectively). The heterozygous strain lacking one OCH1 allele was constructed by chemical transformation of C. parapsilosis CPL2H1 with a LEU2-marked disruption cassette. Transformants were selected in minimal SC medium [0.67% (w/v) yeast nitrogen base, 2% (w/v) dextrose, 2% (w/v) agar] supplemented with L-histidine (1 mg/mL), and quick DNA extraction (Looke et al., 2011) was performed to screen Leu+ transformants by PCR, looking for the presence of expected 5′ and 3′ junctions of the integrated DNA. Homozygous och1Δ null mutant was constructed by transforming the heterozygous knockout strain with a HIS1-containing disruption cassette. Transformants were selected in minimal medium and quick DNA extractions (Looke et al., 2011) were performed to screen His+Leu+ mutants by PCR. Furthermore, to confirm the PCR results, genomic DNA samples were analyzed by means of southern blotting. Briefly, samples of genomic DNA from the null mutant, and control strains were digested with RI, run onto agarose gels, and transferred to nylon membranes. DNA samples were hybridized with specific probes targeting either CmLEU2, CdHIS1, or CpOCH1 genes. These probes were amplified using Digoxigenin-marked dNTPs (Invitrogen) whose signal was revealed by enzymatic means (Roche). To reintegrate OCH1 in the null mutant, the Invitrogen Gateway® cloning strategy was used. First the OCH1 orf was amplified from the genomic DNA of C. parapsilosis CLIB 214. The 1089bp PCR product was purified using the PEG/MgCl2 method according to manufacturer's instruction from Invitrogen gateway cloning kit. Next, the BP clonase was used to integrate the OCH1 ORF into the pDONR221 vector, and then subcloned into a modified destination vector, which contained a constitutive promoter, pTDH3, and a permanent URA3 terminator sequence. Nourseothricin (NAT) was used as dominant selection marker. To achieve successful integration into the C. parapsilosis RP10 locus, the destination vector was further modified by replacing the C. albicans RP10 region to an artifical StuI restriction site containing C. parapsilosis RP10 locus. The expression vector was then digested with StuI, and the linearized plasmid was transformed in the och1Δ/och1Δ null mutant by chemical transformation. Integration of the cassette in the CpRP10 locus was checked by colony PCR and Southern blotting analysis. pDONR221 and Clp10-CaTDH3-GTW-URA3 plasmid vectors were kindly provided by Prof. Christophe d'Enfert.
Heterologous complementation in C. albicans
To confirm that CpOCH1 is the functional ortholog of CaOCH1, we performed the complementation of a Caoch1Δ null mutant (Bates et al., 2006). The CpOCH1 open reading frame, along with 1 Kbp upstream and 582 bp downstream sequences, was amplified by PCR (primer pair 5′- GCGGCCGCAGGCTAATCAAGAGGTTCCTG-3′ and 5′- GCGGCCGCTTCATAGCGAGTGAGAGAC-3′, with the bases to generate a NotI site underlined), cloned into pCR®2.1-TOPO® (Invitrogen), and subcloned into the NotI site of CIp10 (Murad et al., 2000). The resulting plasmid was linearized with StuI before transformation of the Caoch1Δ null mutant.
Hex1 electrophoretic mobility shift assays
Cells in mid-log phase were collected by centrifugation, washed twice with deionized water, and mechanically broken, under a CO2 stream, in a Braun homogenizer during 5 min. Cell disruption was performed in cycles of 1 min, with 2-min resting periods on ice. Then, the homogenate was centrifuged for 10 min at 13 206 × g, 4°C and the supernatant was recovered. The samples were loaded onto a 4% PAGE gel and run for 11 h at 40 V under native conditions. The N-acetylhexosaminidase activity was determined by incubating with 0.4 mM 4-methylumbelliferyl N-acetyl-β-D-glucosamine (Sigma) in 0.1 M citrate-KOH buffer (pH 4.5) during 30 min at 37°C, and the results were observed by exposing the gel to UV light (Hernandez-Cervantes et al., 2012).
Analysis of cell wall composition
Cells were mechanically broken as above described, the homogenate was centrifuged, the pellet recovered and extensively washed with deionized water for debris elimination. Cell walls were lyophilized and acid-hydrolyzed as described (Mora-Montes et al., 2007). Acid-hydrolyzed samples were analyzed by HPAEC-PAD and the carbohydrate separation was achieved with a gradient of sodium acetate in 150 mM NaOH (flow 0.5 mL/min) as follows: 0–5 min = 45–75 mM NaOH, 5.1–15.0 min = 90 mM NaOH, 15.1–17.0 min = 105 mM NaOH + 75 mM sodium acetate, 17.1–20.0 min = 75 mM NaOH + 150 mM sodium acetate, and 20.1–25.0 min = 45 mM NaOH, at a column temperature of 25°C. Applied potentials, for detection by the amperometric pulse were: E1 (400 ms), E2 (20 ms), E3 (20 ms), and E4 (60 ms) of + 0.1, −2.0, + 0.6, and −0.1 V, respectively. For quantification of cell wall protein content, lyophilized cell walls were alkali-hydrolyzed as reported (Mora-Montes et al., 2007), and analyzed using the Bradford protein assay.
Cell wall porosity assay
Cell wall porosity was determined by relative porosity to polycations as described (De Nobel et al., 1990). Briefly, overnight-grown cells were inoculated into fresh Sabouraud broth, incubated for 4 h at 30°C and 200 rpm, and washed twice with PBS. Cell pellets containing 1 × 108 cells were suspended in either 10 mM Tris-HCl, pH 7.4 (buffer A), buffer A plus 30 μg/mL poly-L-lysine (MW 30–70 kDa, Sigma Cat. No. P-2636) or buffer A plus 30 μg/mL DEAE-dextran (MW 500 kDa, Sigma Cat. No. D-9885), and incubated for 30 min at 30°C with constant shaking at 200 rpm. Preparations were centrifuged to pellet cells, supernatants recovered and further centrifuged before absorbance at 260 nm was measured. The relative cell wall porosity to DEAE-dextran was calculated as described (De Nobel et al., 1990).
Alcian blue binding assays
Cells grown at mid-log phase were pelleted, washed twice with deionized water and adjusted at an OD600 of 0.2 in deionized water. Aliquots of 1 mL were pelleted and cells suspended in 1 mL of Alcian blue (Sigma; 30 μ g/mL, in 0.02 M HCl) and assayed as described (Hobson et al., 2004).
Susceptibility to cell wall perturbing agents
Strains were tested for susceptibility to cell wall perturbing agents using a microdilution method as described (Bates et al., 2005). Briefly, cells from overnight culture in medium containing 2 units/mL chitinase were washed with water, passaged through a syringe with a 32-gauge needle, and suspended at an OD600 = 1. These cells were then inoculated into fresh medium at an OD600 of 0.01 and 95 μL of this suspension were dispensed into 96-well plates. A 5 μL volume of the cell wall perturbing agents was added to each well by duplicate across a range of double dilutions. The final OD600 was determined after 16 h of incubation at 30°C. The maximum concentrations tested for each agent were Calcofluor White (Sigma), Congo Red (Sigma) and tunicamycin (Sigma, 100 μ g/mL, each), SDS (BioRad, 0.1%, w/v), hygromycin B (Sigma, 500 μ g/mL), NaCl2 and KCl (1M, each), caffeine (Sigma; 50 mM) and vanadate (Sigma, 80 mM). Cells were grown in medium with no stressor added to normalize data. Growth data were normalized as percentage of those generated with the same strains without treatment.
Fluorochrome staining
The assay was prepared as previously described (Mora-Montes et al., 2011). For chitin staining, cells were stained with 1 mg/mL WGA-FITC (Sigma). For β1,3-glucan staining cells were incubated with 5 μg/mL IgG Fc-Dectin-1 chimera (Graham et al., 2006) for 40 min at room temperature, followed by incubation with 1 μg/mL donkey anti Fc IgG-FITC for 40 min at room temperature (Marakalala et al., 2013). Samples were examined by fluorescence microscopy using a Zeiss Axioscope-40 microscope and an Axiocam MRc camera. From the pictures acquired, the fluorescence quantification of 50 cells was achieved using Adobe Photoshop™ CS6 with the next formula: [(total of green pixels-background green pixels) × 100]/total pixels.
Ethics statement
The Ethics Committee from Universidad de Guanajuato approved the use of human cells in this study (permission number 17082011); while University of Szeged granted permission XII./00455/2011 to work with mice. Human cells were collected from healthy adult volunteers after information about the study was provided and written informed consent was obtained.
Isolation and stimulation of human PBMCs with Candida cells
Human PBMCs were isolated by density centrifugation using Histopaque-1077 (Sigma) as described (Endres et al., 1988). For stimulation experiments, the interactions were performed in round-bottom 96-well microplates with 100 μL of cells adjusted to 5 × 105 PBMCs in RPMI 1640 Dutch modification (added with 2 mM glutamine, 0.1 mM pyruvate and 0.05 mg/mL gentamycin; all reagents from Sigma) and 100 μL with 1 × 105 fungal cells freshly harvested or treated. The interactions were incubated for 24 h at 37°C with 5% (v/v) CO2. In some experiments, PBMCs were preincubated for 1 h at 37°C with either laminarin (200 μg/mL), anti-TLR2 (10 μg/mL, eBioscience, Cat. No. 16-9922) or antibodies to TLR4 (10 μg/mL, Santa Cruz Biotechnology, Cat. No. sc-293072) prior to stimulation with Candida cells. Isotype matched, irrelevant antibodies, IgG2aκ (10 μg/mL, eBioscience, Cat. No. 14-4724-85) and IgG1(10 μg/mL, Santa Cruz Biotechnology, Cat. No. sc-52003) were used as controls for experiments assessing TLR2 and TLR4, respectively. All reagents used for the pre-incubation experiments were negative to contamination with LPS (tested with the Limulus amebocyte lysate from Sigma), and all reactions were performed in presence of 5 μ g/mL polymyxin B (Sigma) (Schwartz et al., 1972). Plates were centrifuged for 10 min at 3000 × g at 4°C, the supernatant saved and kept at −20°C until used.
Cytokine quantification
The concentration of TNFα, IL-6, and IL-10 was quantified by ELISA (Peprotech), according to the manufacturer's instructions. The IL-1β levels were measured using a commercial ELISA kit from R&D Systems.
Differentiation of human PBMC-derived macrophages
After isolation, aliquots of 1 mL containing 2 × 107 PBMCs in RPMI supplemented with 1% (v/v) penicillin-streptomycin solution (PS, Sigma-Aldrich) were placed in flat bottom 12-well plates, and incubated 1.5 h at 37°C, 5% (v/v) CO2. Non-adherent cells were removed gently with the medium and adherent cells were washed with PBS at 37°C. One mL of X-VIVO 15 serum-free medium (Lonza) supplemented with 1% (v/v) PS and 10 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF, Sigma) were added into each well and incubated for 6–7 days at 37°C, 5% (v/v) CO2, with fresh medium exchanged every 2–3 days (Netea et al., 2009).
Phagocytosis assays
Phagocytosis of C. parapsilsosis cells was performed as previously described (Nemeth et al., 2013). Briefly, C. parapsilosis cells were labeled with AlexaFlour488 Succinimidyl ester (Life Technologies) and subsequently co-incubated with human PBMC-derived macrophages at an effector:target ratio of 1:5 for 1.5 h. Non-phagocytosed yeast cells were removed by gentle washing and macrophages were detached from cell culture plates by using TrypLE™ Express solution (Gibco). To inhibit phagocytosis, macrophages were pre-incubated for 30 min with 2.5 μM cytochalasin D (R&D Systems) before infection. Samples were measured on a FACSCalibur instrument and analyzed by FlowJo vX.0.7 software.
C. parapsilosis infection model and fungal burden
A non-lethal experimental model of disseminated candidiasis was used as reported previously (Ifrim et al., 2014). Briefly, groups containing 8–12-weeks-old male Balb/c WT mice (22–27 g. of weight) were injected via the lateral tail vein with 2 × 107 C. parapsilosis cells, previously passaged through a syringe with a 32-gauge needle, in 100 μL of sterile PBS. A group of control mice was injected with 100 μL of sterile PBS. Animals were mantained with sterile water and pet aliment ad libitum. After 1, 2, or 7 days post infection, animals were humanitarianly euthanatized, and liver, kidneys and spleen were aseptically removed, weighed, and homogenized in sterile PBS in a tissue grinder. The fungal burden in these tissues was determined by plating serial dilutions on three YPD agar plates per tissue. The CFU were counted after 48 h of incubation at 30°C and expressed as CFU/g tissue.
G. mellonella survival assays
Wax moth larvae killing assays were performed as described (Mylonakis et al., 2005). Briefly, 10 μL of a cell suspension prepared in PBS and containing 2 × 107 yeast cells, passaged through a syringe with a 32-gauge needle, were injected directly into the haemocele, through last left pro-leg of the larva, using a 26-gauge needle and a Hamilton syringe. Larvae were incubated at 25°C after injection and survival monitored daily. Groups of 10 larvae were used for each strain analyzed. A group of untreated larvae and a PBS-injected group were included in each experiment as controls.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 software. Results obtained upon incubation with the cell wall perturbing agents were analyzed by two-way ANOVA. Cytokine stimulation using human PMBCs was performed in duplicate with six healthy donors, whereas the rest of the experiments were performed at least thrice in duplicate. Data represent cumulative results of all experiments performed. The Mann-Whitney U test or unpaired t-test was used to establish statistical significance (see figure legends for details), with a significance level set at P < 0.05.
Author contributions
AF, AG, and HM conceived the study. LP, KC, EE, EM, TN, LL, RT, CV, AM, and AT performed experiments. LP, AF, ML, JN, AG, and HM analyzed data. LP, JN, AG, and HM drafted the manuscript. LP, KC, AF, EE, EM, TN, LL, RT, ML, CV, AM, AT, JN, AG, and HM revised and approved the manuscript. AG and HM equally contributed to this work, and are corresponding authors.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Professor Gordon Brown (University of Aberdeen) for the donation of the IgG Fc-Dectin-1 chimera. This work was supported by Consejo Nacional de Ciencia y Tecnología (ref. CB2011/166860), Universidad de Guanajuato (ref. 0025/11) and Programa de Mejoramiento de Profesorado (ref. UGTO-PTC-261). AG is supported by NKFIH NN113153 and by NF84006. AG is further supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. TN is supported by postdoctoral fellowship from the Hungarian Academy of Sciences.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00306
References
- Bates S., Hall R. A., Cheetham J., Netea M. G., MacCallum D. M., Brown A. J., et al. (2013). Role of the Candida albicans MNN1 gene family in cell wall structure and virulence. BMC Res. Notes 6:294. 10.1186/1756-0500-6-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates S., Hughes H. B., Munro C. A., Thomas W. P., MacCallum D. M., Bertram G., et al. (2006). Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J. Biol. Chem. 281, 90–98. 10.1074/jbc.M510360200 [DOI] [PubMed] [Google Scholar]
- Bates S., MacCallum D. M., Bertram G., Munro C. A., Hughes H. B., Buurman E. T., et al. (2005). Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J. Biol. Chem. 280, 23408–23415. 10.1074/jbc.M502162200 [DOI] [PubMed] [Google Scholar]
- Brand A., MacCallum D. M., Brown A. J., Gow N. A., Odds F. C. (2004). Ectopic expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Eukaryot Cell 3, 900–909. 10.1128/EC.3.4.900-909.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. D., Gordon S. (2001). Immune recognition. A new receptor for beta-glucans. Nature 413, 36–37. 10.1038/35092620 [DOI] [PubMed] [Google Scholar]
- Cambi A., Netea M. G., Mora-Montes H. M., Gow N. A., Hato S. V., Lowman D. W., et al. (2008). Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J. Biol. Chem. 283, 20590–20599. 10.1074/jbc.M709334200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. C., van de Veerdonk F. L., Lenardon M., Stoffels M., Plantinga T., Smeekens S., et al. (2011). The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J. Leukoc. Biol. 90, 357–366. 10.1189/jlb.1210702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly L. A., Riccombeni A., Grózer Z., Holland L. M., Lynch D. B., Andes D. R., et al. (2013). The APSES transcription factor Efg1 is a global regulator that controls morphogenesis and biofilm formation in Candida parapsilosis. Mol. Microbiol. 90, 36–53. 10.1111/mmi.12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean N. (1995). Yeast glycosylation mutants are sensitive to aminoglycosides. Proc. Natl. Acad. Sci. U.S.A. 92, 1287–1291. 10.1073/pnas.92.5.1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nobel J. G., Klis F. M., Munnik T., Priem J., Van Den Ende H. (1990). An assay of relative cell wall porosity in Saccharomyces cerevisiae, Kluyveromyces lactis and Schizosaccharomyces pombe. Yeast 6, 483–490. 10.1002/yea.320060605 [DOI] [PubMed] [Google Scholar]
- Diaz-Jimenez D. F., Mora-Montes H. M., Hernandez-Cervantes A., Luna-Arias J. P., Gow N. A., Flores-Carreon A. (2012). Biochemical characterization of recombinant Candida albicans mannosyltransferases Mnt1, Mnt2 and Mnt5 reveals new functions in O- and N-mannan biosynthesis. Biochem. Biophys. Res. Commun. 419, 77–82. 10.1016/j.bbrc.2012.01.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Jiménez D. F., Pérez-García L. A., Martínez-Álvarez J. A., Mora-Montes H. M. (2012). Role of the fungal cell wall in pathogenesis and antifungal resistance. Curr. Fungal. Infect. Rep. 6, 275–282. 10.1007/s12281-012-0109-7 [DOI] [Google Scholar]
- Ding C., Butler G. (2007). Development of a gene knockout system in Candida parapsilosis reveals a conserved role for BCR1 in biofilm formation. Eukaryot. Cell 6, 1310–1319. 10.1128/EC.00136-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C., Vidanes G. M., Maguire S. L., Guida A., Synnott J. M., Andes D. R., et al. (2011). Conserved and divergent roles of Bcr1 and CFEM Proteins in Candida parapsilosis and Candida albicans. PLoS ONE 6:e28151. 10.1371/journal.pone.0028151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres S., Ghorbani R., Lonnemann G., van der Meer J. W., Dinarello C. A. (1988). Measurement of immunoreactive interleukin-1 beta from human mononuclear cells: optimization of recovery, intrasubject consistency, and comparison with interleukin-1 alpha and tumor necrosis factor. Clin. Immunol. Immunopathol. 49, 424–438. 10.1016/0090-1229(88)90130-4 [DOI] [PubMed] [Google Scholar]
- Gácser A., Trofa D., Schäfer W., Nosanchuk J. D. (2007). Targeted gene deletion in Candida parapsilosis demonstrates the role of secreted lipase in virulence. J. Clin. Invest. 117, 3049–3058. 10.1172/JCI32294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago S., García-Rodas R., Cuesta I., Mellado E., Alastruey-Izquierdo A. (2014). Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis virulence in the non-conventional host Galleria mellonella. Virulence 5, 278–285. 10.4161/viru.26973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goins T. L., Cutler J. E. (2000). Relative abundance of oligosaccharides in Candida species as determined by fluorophore-assisted carbohydrate electrophoresis. J. Clin. Microbiol. 38, 2862–2869. Available online at: http://jcm.asm.org/content/38/8/2862.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow N. A. R., Netea M. G., Munro C. A., Ferwerda G., Bates S., Mora-Montes H. M., et al. (2007). Immune recognition of Candida albicans beta-glucan by dectin-1. J. Infect. Dis. 196, 1565–1571. 10.1086/523110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L. M., Tsoni S. V., Willment J. A., Williams D. L., Taylor P. R., Gordon S., et al. (2006). Soluble Dectin-1 as a tool to detect beta-glucans. J. Immunol. Methods 314, 164–169. 10.1016/j.jim.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Hall R. A., Bates S., Lenardon M. D., Maccallum D. M., Wagener J., Lowman D. W., et al. (2013). The Mnn2 mannosyltransferase family modulates mannoprotein fibril length, immune recognition and virulence of Candida albicans. PLoS Pathog 9:e1003276. 10.1371/journal.ppat.1003276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T., Nakajima T., Izaki K., Matsuda K. (1981). Comparison of the mannan structure from the cell-wall mutant Candida sp. M-7002 and its wild type. I. Characterization of the proteo-mannan from the mutant and the wild-type cells. Eur. J. Biochem. 119, 365–371. 10.1111/j.1432-1033.1981.tb05617.x [DOI] [PubMed] [Google Scholar]
- Harris M., Mora-Montes H. M., Gow N. A., Coote P. J. (2009). Loss of mannosylphosphate from Candida albicans cell wall proteins results in enhanced resistance to the inhibitory effect of a cationic antimicrobial peptide via reduced peptide binding to the cell surface. Microbiology 155 (Pt 4), 1058–1070. 10.1099/mic.0.026120-0 [DOI] [PubMed] [Google Scholar]
- Hazen K. C., Glee P. M. (1994). Hydrophobic cell wall protein glycosylation by the pathogenic fungus Candida albicans. Can. J. Microbiol. 40, 266–272. 10.1139/m94-043 [DOI] [PubMed] [Google Scholar]
- Hernandez-Cervantes A., Mora-Montes H. M., Alvarez-Vargas A., Jimenez D. F., Robledo-Ortiz C. I., Flores-Carreon A. (2012). Isolation of Sporothrix schenckii MNT1 and the biochemical and functional characterization of the encoded alpha1,2-mannosyltransferase activity. Microbiology 158 (Pt 9), 2419–2427. 10.1099/mic.0.060392-0 [DOI] [PubMed] [Google Scholar]
- Hobson R. P., Munro C. A., Bates S., MacCallum D. M., Cutler J. E., Heinsbroek S. E., et al. (2004). Loss of cell wall mannosylphosphate in Candida albicans does not influence macrophage recognition. J. Biol. Chem. 279, 39628–39635. 10.1074/jbc.M405003200 [DOI] [PubMed] [Google Scholar]
- Holland L. M., Schroder M. S., Turner S. A., Taff H., Andes D., Grozer Z., et al. (2014). Comparative phenotypic analysis of the major fungal pathogens Candida parapsilosis and Candida albicans. PLoS Pathog 10:e1004365. 10.1371/journal.ppat.1004365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifrim D. C., Bain J. M., Reid D. M., Oosting M., Verschueren I., Gow N. A. R., et al. (2014). Role of dectin-2 for host defense against systemic infection with Candida glabrata. Infect. Immun. 82, 1064–1073. 10.1128/IAI.01189-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen I. D. (2014). Galleria mellonella as a model host to study virulence of Candida. Virulence 5, 237–239. 10.4161/viru.27434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis F. M., de Groot P., Hellingwerf K. (2001). Molecular organization of the cell wall of Candida albicans. Med. Mycol. 39, 1–8. 10.1080/744118876 [DOI] [PubMed] [Google Scholar]
- Kobata A. (1979). Use of endo- and exoglycosidases for structural studies of glycoconjugates. Anal. Biochem. 100, 1–14. 10.1016/0003-2697(79)90102-7 [DOI] [PubMed] [Google Scholar]
- Laffey S. F., Butler G. (2005). Phenotype switching affects biofilm formation by Candida parapsilosis. Microbiology 151, 1073–1081. 10.1099/mic.0.27739-0 [DOI] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. (1975). An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia 13, 148–153. 10.1080/00362177585190271 [DOI] [PubMed] [Google Scholar]
- Looke M., Kristjuhan K., Kristjuhan A. (2011). Extraction of genomic DNA from yeasts for PCR-based applications. Biotechniques 50, 325–328. 10.2144/000113672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Bezerra L. M., Lozoya-Perez N. E., Lopez-Ramirez L. A., Martinez-Alvarez J. A., Teixeira M. M., Felipe M. S., et al. (2015). Functional characterization of Sporothrix schenckii glycosidases involved in the N-linked glycosylation pathway. Med. Mycol. 53, 60–68. 10.1093/mmy/myu057 [DOI] [PubMed] [Google Scholar]
- Marakalala M. J., Vautier S., Potrykus J., Walker L. A., Shepardson K. M., Hopke A., et al. (2013). Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog 9:e1003315. 10.1371/journal.ppat.1003315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie C. G. J., Koser U., Lewis L. E., Bain J. M., Mora-Montes H. M., Barker R. N., et al. (2010). Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect. Immun. 78, 1650–1658. 10.1128/IAI.00001-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Montes H. M., Bates S., Netea M. G., Castillo L., Brand A., Buurman E. T., et al. (2010). A multifunctional mannosyltransferase family in Candida albicans determines cell wall mannan structure and host-fungus interactions. J. Biol. Chem. 285, 12087–12095. 10.1074/jbc.M109.081513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Montes H. M., Bates S., Netea M. G., Diaz-Jimenez D. F., Lopez-Romero E., Zinker S., et al. (2007). Endoplasmic reticulum alpha-glycosidases of Candida albicans are required for N glycosylation, cell wall integrity, and normal host-fungus interaction. Eukaryot. Cell 6, 2184–2193. 10.1128/EC.00350-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Montes H. M., McKenzie C., Bain J. M., Lewis L. E., Erwig L. P., Gow N. A. (2012). Interactions between macrophages and cell wall oligosaccharides of Candida albicans. Methods Mol. Biol. 845, 247–260. 10.1007/978-1-61779-539-8_16 [DOI] [PubMed] [Google Scholar]
- Mora-Montes H. M., Netea M. G., Ferwerda G., Lenardon M. D., Brown G. D., Mistry A. R., et al. (2011). Recognition and blocking of innate immunity cells by Candida albicans chitin. Infect. Immun. 79, 1961–1970. 10.1128/IAI.01282-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Montes H. M., Ponce-Noyola P., Villagómez-Castro J. C., Gow N. A. R., Flores-Carreón A., López-Romero E. (2009). Protein glycosylation in Candida. Future Microbiol. 4, 1167–1183. 10.2217/fmb.09.88 [DOI] [PubMed] [Google Scholar]
- Mormeneo S., Marcilla A., Iranzo M., Sentandreu R. (1994). Structural mannoproteins released by beta-elimination from Candida albicans cell walls. FEMS Microbiol. Lett. 123, 131–136. [DOI] [PubMed] [Google Scholar]
- Munro C. A., Bates S., Buurman E. T., Hughes H. B., Maccallum D. M., Bertram G., et al. (2005). Mnt1p and Mnt2p of Candida albicans are partially redundant alpha-1,2-mannosyltransferases that participate in O-linked mannosylation and are required for adhesion and virulence. J. Biol. Chem. 280, 1051–1060. 10.1074/jbc.M411413200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad A. M., Lee P. R., Broadbent I. D., Barelle C. J., Brown A. J. (2000). CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast 16, 325–327. [DOI] [PubMed] [Google Scholar]
- Mylonakis E., Moreno R., El Khoury J. B., Idnurm A., Heitman J., Calderwood S. B., et al. (2005). Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immun. 73, 3842–3850. 10.1128/IAI.73.7.3842-3850.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth T., Toth A., Szenzenstein J., Horvath P., Nosanchuk J. D., Grozer Z., et al. (2013). Characterization of virulence properties in the C. parapsilosis sensu lato species. PLoS ONE 8:e68704. 10.1371/journal.pone.0068704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M. G., Brown G. D., Kullberg B. J., Gow N. A. (2008). An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 6, 67–78. 10.1038/nrmicro1815 [DOI] [PubMed] [Google Scholar]
- Netea M. G., Gow N. A., Munro C. A., Bates S., Collins C., Ferwerda G., et al. (2006). Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Invest. 116, 1642–1650. 10.1172/JCI27114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M. G., Nold-Petry C. A., Nold M. F., Joosten L. A., Opitz B., van der Meer J. H., et al. (2009). Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 113, 2324–2335. 10.1182/blood-2008-03-146720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L. N., Gacser A., Nosanchuk J. D. (2011a). The stearoyl-coenzyme A desaturase 1 is essential for virulence and membrane stress in Candida parapsilosis through unsaturated fatty acid production. Infect. Immun. 79, 136–145. 10.1128/IAI.00753-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L. N., Hamari Z., Kadereit B., Trofa D., Agovino M., Martinez L. R., et al. (2011b). Candida parapsilosis fat storage-inducing transmembrane (FIT) protein 2 regulates lipid droplet formation and impacts virulence. Microbes Infect. 13, 663–672. 10.1016/j.micinf.2011.02.009 [DOI] [PubMed] [Google Scholar]
- Noble S. M., French S., Kohn L. A., Chen V., Johnson A. D. (2010). Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat. Genet. 42, 590–598. 10.1038/ng.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S. M., Johnson A. D. (2005). Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot. Cell 4, 298–309. 10.1128/EC.4.2.298-309.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosek J., Holesova Z., Kosa P., Gacser A., Tomaska L. (2009). Biology and genetics of the pathogenic yeast Candida parapsilosis. Curr. Genet. 55, 497–509. 10.1007/s00294-009-0268-4 [DOI] [PubMed] [Google Scholar]
- Prill S. K., Klinkert B., Timpel C., Gale C. A., Schroppel K., Ernst J. F. (2005). PMT family of Candida albicans: five protein mannosyltransferase isoforms affect growth, morphogenesis and antifungal resistance. Mol. Microbiol. 55, 546–560. 10.1111/j.1365-2958.2004.04401.x [DOI] [PubMed] [Google Scholar]
- Reid D. M., Gow N. A. R., Brown G. D. (2009). Pattern recognition: recent insights from Dectin-1. Curr. Opin. Immunol. 21, 30–37. 10.1016/j.coi.2009.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo S., Ikeda S., Yamabe K., Kakuta S., Ishigame H., Akitsu A., et al. (2010). Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32, 681–691. 10.1016/j.immuni.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Schwartz S. N., Medoff G., Kobayashi G. S., Kwan C. N., Schlessinger D. (1972). Antifungal properties of polymyxin B and its potentiation of tetracycline as an antifungal agent. Antimicrob. Agents Chemother. 2, 36–40. 10.1128/AAC.2.1.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N., Ikuta K., Imai T., Satoh Y., Satoh R., Suzuki A., et al. (1995). Existence of branched side chains in the cell wall mannan of pathogenic yeast, Candida albicans. Structure-antigenicity relationship between the cell wall mannans of Candida albicans and Candida parapsilosis. J. Biol. Chem. 270, 1113–1122. 10.1074/jbc.270.3.1113 [DOI] [PubMed] [Google Scholar]
- Spreghini E., Davis D. A., Subaran R., Kim M., Mitchell A. P. (2003). Roles of Candida albicans Dfg5p and Dcw1p cell surface proteins in growth and hypha formation. Eukaryot. Cell 2, 746–755. 10.1128/EC.2.4.746-755.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth A., Csonka K., Jacobs C., Vagvolgyi C., Nosanchuk J. D., Netea M. G., et al. (2013). Candida albicans and Candida parapsilosis induce different T-cell responses in human peripheral blood mononuclear cells. J. Infect. Dis. 208, 690–698. 10.1093/infdis/jit188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth R., Toth A., Papp C., Jankovics F., Vagvolgyi C., Alonso M. F., et al. (2014). Kinetic studies of Candida parapsilosis phagocytosis by macrophages and detection of intracellular survival mechanisms. Front. Microbiol. 5:633. 10.3389/fmicb.2014.00633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofa D., Gacser A., Nosanchuk J. D. (2008). Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol. Rev. 21, 606–625. 10.1128/CMR.00013-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk F. L., Joosten L. A., Devesa I., Mora-Montes H. M., Kanneganti T. D., Dinarello C. A., et al. (2009). Bypassing pathogen-induced inflammasome activation for the regulation of interleukin-1beta production by the fungal pathogen Candida albicans. J. Infect. Dis. 199, 1087–1096. 10.1086/597274 [DOI] [PubMed] [Google Scholar]
- Wells C. A., Salvage-Jones J. A., Li X., Hitchens K., Butcher S., Murray R. Z., et al. (2008). The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J. Immunol. 180, 7404–7413. 10.4049/jimmunol.180.11.7404 [DOI] [PubMed] [Google Scholar]
- West L., Lowman D. W., Mora-Montes H. M., Grubb S., Murdoch C., Thornhill M. H., et al. (2013). Differential virulence of Candida glabrata glycosylation mutants. J. Biol. Chem. 288, 22006–22018. 10.1074/jbc.M113.478743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler R. T., Fink G. R. (2006). A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog 2:e35. 10.1371/journal.ppat.0020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L. L., Zhao X. Q., Jiang C., You Y., Chen X. P., Jiang Y. Y., et al. (2013). C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 39, 324–334. 10.1016/j.immuni.2013.05.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.