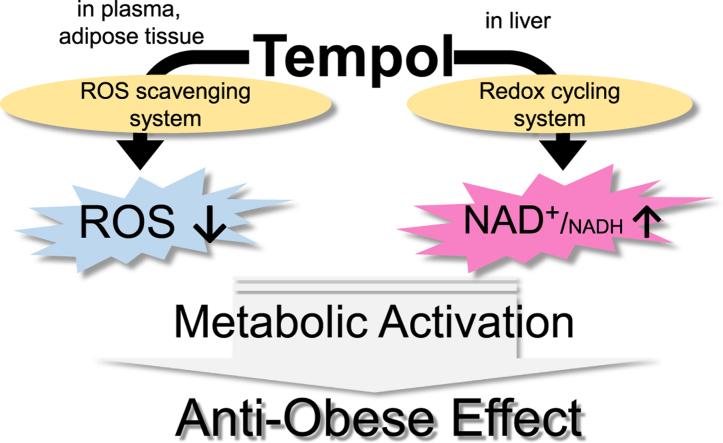
Abstract
Continuous energy conversion is controlled by reduction–oxidation (redox) processes. NAD+ and NADH represent an important redox couple in energy metabolism. 4-Hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL) is a redox-cycling nitroxide that promotes the scavenging of several reactive oxygen species (ROS) and is reduced to hydroxylamine by NADH. TEMPOL is also involved in NAD+ production in the ascorbic acid–glutathione redox cycle. We utilized the chemical properties of TEMPOL to investigate the effects of antioxidants and NAD+/NADH modulators on the metabolic imbalance in obese mice. Increases in the NAD+/NADH ratio by TEMPOL ameliorated the metabolic imbalance when combined with a dietary intervention, changing from a high-fat diet to a normal diet. Plasma levels of the superoxide marker dihydroethidium were higher in mice receiving the dietary intervention compared with a control diet, but were normalized with TEMPOL consumption. These findings provide novel insights into redox regulation in obesity.
Keywords: Obesity, Oxidative stress, Redox status, NAD+, Nitroxide
Graphical abstract
Highlights
-
•
Redox imbalance: ROS generation and NADH accumulation, occurs in obesity.
-
•
TEMPOL can normalize redox imbalance in various tissues.
-
•
Whole body metabolism was activated by TEMPOL and dietary intervention.
-
•
Redox regulation might be useful approaches for treating obesity.
1. Introduction
The worldwide prevalence of obesity is increasing each year. Obesity is associated with the risk of death from all cancers [1], shortened lifespan and decrease of healthy years [2]. However, effective therapeutic strategies for obesity are not known.
Obesity is a condition of increased adipose tissue mass, causing functional abnormalities in energy metabolism. Continuous energy conversion is tightly controlled by reduction–oxidation (redox) processes. NAD+ and NADH represent an important redox couple for energy metabolism [3]. Over the last decade, the role of NAD+ has become increasingly understood with discovery of proteins, such as sirtuins, that consume NAD+ and function as metabolic regulators [4]. In recent reports, regulation of the NAD+/NADH ratio led to increased energy metabolism [3]. Strategies for such regulation include: activation of related enzymes such as AMP-activated protein kinase [5] and nicotinamide phosphoribosyl transferase [6]; providing precursors for NAD+ de novo and salvage pathways [7], [8]; modulation of enzymes that consume NAD+ such as poly(ADP-ribose)polymerase and CD38 [9], [10]; and providing substrates of NADH:quinone oxidoreductase [11]. These studies showed that increasing the NAD+/NADH ratio, including by promoting NADH oxidation, is critical to accelerating energy metabolism. In obese mice, the NAD+/NADH ratio was lower than in lean mice [12], further suggesting that increasing the NAD+/NADH ratio would be necessary for changing metabolic state to maintain homeostasis.
In contrast, in adipose tissue and plasma, oxidative stress and generation of reactive oxygen species (ROS) is greater in obese mice than in lean mice [13]. To decrease oxidative stress and its resulting tissue damage, administration of antioxidants has been considered [14].
Both approaches have been shown to attenuate disease symptoms, yet they are completely contradictory. While one involves increasing oxidation from NADH to NAD+ the other is a process involving reduction, for example, to decrease ROS levels. We hypothesized that obesity leads to a growing imbalance between reduction–oxidation (redox) status in the body and that correction of this imbalance would be beneficial.
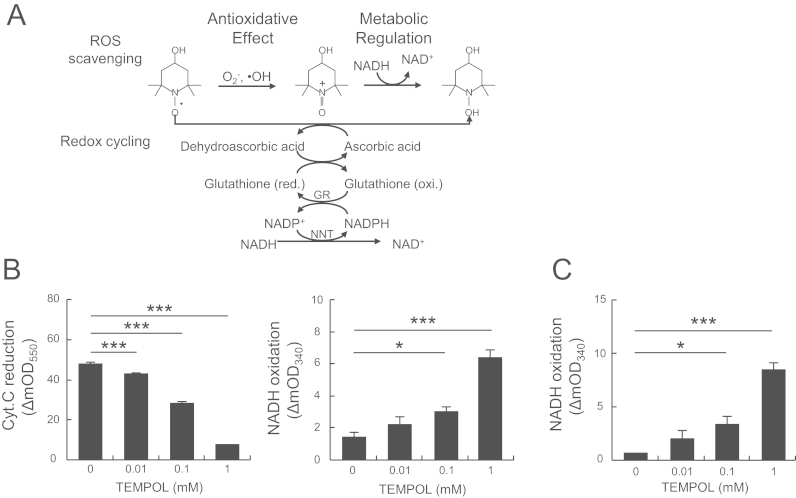
Here, we proposed a strategy to increase the NAD+/NADH ratio and decrease ROS production. To regulate redox status in the body, we focused on a small redox-cycling nitroxide antioxidant, the 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL). This molecule has been reported to have two redox potential [15]. In the oxidation process, an oxoammonium cation was generated by the diffusion-controlled reaction of superoxide or hydroxyl radical with the aminoxyl group of TEMPOL, and then was reduced by NADH to the hydroxylamine [15], [16]. During these reactions, TEMPOL can both reduce ROS and oxidize NADH to NAD+. In the reduction process, TEMPOL was directly reduced to the corresponding hydroxylamine by ascorbic acid, resulting in formation of the oxidation product dehydroascorbic acid (DHA) [17]. In this case, DHA can increase the NAD+ concentration via a redox cycling system comprised of glutathione (GSH), NADPH and NADH [18]. We therefore assumed that TEMPOL could indirectly generate NAD+ by an NADH oxidation process via two different pathways, involving either ROS scavenging or the AsA–GSH redox cycling system (Fig. 1A).
Fig. 1.
TEMPOL increased the NAD+/NADH ratio via ROS scavenging and the redox cycling system in vitro. (A) Schematic illustration of two pathways of NAD+ generation by TEMPOL. GR, glutathione reductase; NNT, nicotinamide nucleotide transhydrogenase. (B) Superoxide scavenging and simultaneous NAD+ production in the hypoxanthine–xanthine oxidase system. The reaction with superoxide was measured by the rate of reduction of ferricytochrome c at 550 nm. Reaction mixtures contained 120 µM hypoxanthine, 0.005 units xanthine oxidase, 24 μM cytochrome c and 1 mM NADH in 10 mM phosphate buffer (pH 7.4) with TEMPOL (0–1 mM, as indicated in the figure). (C) NAD+ production in the AsA–GSH redox cycle. NADH oxidation was measured at 340 nm. The reaction mixture contained 1 mM ascorbic acid (AsA), 1 mM glutathione (GSH), 1 unit of GR and 0.5 mM NADH in 10 mM phosphate buffer (pH 7.4) with TEMPOL (0–1 mM). Values are means±SD (n=4). *p<0.05 and ***p<0.005 compared with 0 mM.
In our study, we employed TEMPOL to investigate modulation of the NAD+/NADH ratio to address metabolic imbalances in obese mice. We examined effects of TEMPOL in mice under dietary intervention following a high-fat diet (HFD), to assess dietary impacts on its effectiveness.
2. Materials and methods
2.1. Materials
4-Hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL) was from Sigma-Aldrich Japan (Tokyo, Japan). Xanthine oxidase, glutathione reductase (GR), NAD+, NADH, alcohol dehydrogenase from yeast and malate dehydrogenase from yeast were from Oriental Yeast Co., Ltd (Tokyo, Japan). The following were from Wako Pure Chemical Industries (Osaka, Japan): ferricytochrome c (from horse heart), hypoxanthine, cholesterol assay kit (Cholesterol E test), triglyceride assay kit (Triglyceride E test), Tiron, ascorbic acid, and glutathione. Dihydroethidium (DHE) was purchased from Life Technologies Japan Ltd. (Tokyo, Japan).
2.2. In vitro chemical reaction of TEMPOL
Superoxide was generated by the aerobic reaction of hypoxanthine and xanthine oxidase in the presence of ferricytochrome c and NADH. To estimate its reaction with superoxide, the rate of reduction of ferricytochrome c was measured at 550 nm using a grating-based spectrometer (SH-1000 Lab; Corona Electric Co., Ltd., Tokyo, Japan). This machine was equipped with a thermostat-controlled cell for the reaction mixture, which contained 120 μM hypoxanthine, 0.005 units xanthine oxidase, 24 μM cytochrome c and 1 mM NADH in 10 mM phosphate buffer (pH 7.4) with TEMPOL (0–1 mM, as indicated).
NADH oxidation was measured at 340 nm to assess effects of TEMPOL in a redox cycling system. The reaction mixture contained 1 mM ascorbic acid (AsA), 1 mM glutathione (GSH), 1 unit GR and 0.5 mM NADH in 10 mM phosphate buffer (pH 7.4) with TEMPOL (0–1 mM).
3. Animal experiments
C57BL/6N and 6J male mice were obtained from Kyudo Co. Ltd. (Saga, Japan) and housed in a room with a 12 h light/dark cycle and controlled temperature and humidity and were given free access to food and water. After 1 week acclimation, mice were commercially fed either a normal, or control, diet (CD; CE-2, Clea Japan, Inc., Tokyo, Japan) or a high fat diet (HFD; HFD32, Clea Japan, Inc.). The CD was composed of crude protein (25.1%), crude fat (4.8%), crude fiber (4.2%) and crude ash (6.7%), providing 343.1 kcal of energy per 100 g. The HFD was composed of crude protein (25.5%), crude fat (32.0%), crude fiber (2.9%) and crude ash (4.0%), providing 507.6 kcal energy per 100 g.
Individual body weights were determined every 1 or 2 week from 8–20 weeks of age. Obesity was established by feeding the mice a HFD for 8 week. In the dietary intervention (DI) groups mice were switched from HFD to CD. TEMPOL (30 mM) was added to the drinking water using previously described dosing methods [19].
At the end of the treatment schedule, mice were anesthetized using a mixed solution of hydrochloric acid medetomidine (Kyoritsu Seiyaku Corporation, Tokyo, Japan), midazolam (Sandoz K.K., Tokyo, Japan) and butorphanol (Meiji Seika Pharma Co., Ltd, Tokyo, Japan) under fasting conditions. Blood was collected from the inferior vena cava with a heparinized syringe. For experiments measuring biological activities, livers were removed and stored at −80 °C until analysis. Fat pads (inguinal, gonadal, and retroperitoneal) were dissected and weighed together. The ratio of fat pad weight divided by body weight was calculated and expressed as a percentage. All procedures and animal care were approved by the Animal Care and Use Committee, Kyushu University (Fukuoka, Japan), and performed in accordance with the Guidelines for Animal Experiments, Kyushu University.
3.1. Measurement of NAD+ and NADH in liver tissue of mice
NAD+ and NADH nucleotides were directly measured as previously described [20]. Briefly, livers dissected from mice were homogenized in 200 μL acid extraction buffer to measure NAD+ or 200 μL alkali extraction buffer to measure NADH. Homogenates were neutralized and the concentration of nucleotides was measured fluorometrically (excitation wavelength: 365 nm and emission wavelength: 460 nm) after an enzymatic cycling reaction using 5 μL of each sample. Values for both nucleotides were detected within the linear range of standard curves.
3.2. Glucose tolerance test and measurement of plasma cholesterol levels
An intraperitoneal glucose tolerance test was performed as previously described [21]. Mice were fasted overnight and then injected with glucose (2 g/kg body weight, i.p.). Blood was collected from the tail vein at 0, 15, 30, 60 and 120 min following the injection. Blood glucose levels were measured using a Glucose Pilot Monitoring System (Aventir Biotech, LLC, Carlsbad, CA, USA).
Plasma was separated by centrifugation at 800×g for 15 min at 4 °C and stored at −80 °C until analysis. Plasma cholesterol levels were determined using a commercially available assay kit.
3.3. Measurement of hepatic triglyceride levels
Liver tissue was homogenized (1:8, w-v) in a mixture of chloroform and methanol (2:1). Lipid extracts were dried and dissolved in 5% Triton X-100 in isopropanol. Hepatic triglyceride levels were determined using a commercially available assay kit.
3.4. DHE staining
To estimate in vivo oxidative stress, DHE staining was performed as previously described with minor modifications [22]. Briefly, mice were intravenously administered 0.3 mL DHE (2.5 mg/mL in PBS) under isoflurane anesthesia. Two hours later, mice were anesthetized with pentobarbital (50 mg/kg body weight, i.p.). Blood was collected from the inferior vena cava and plasma fluorescence was measured in a fluorescence reader (MTP-810Lab, Corona Electric Co., Ltd.) at excitation and emission wavelengths of 518 nm and 605 nm, respectively. Livers were removed, frozen immediately in an OCT compound (Tissue-Tech II; Sakura Fine Chemical, Tokyo, Japan) and sectioned at a thickness of 10 μm on a cryostat (Sakura Finetek Japan Co., Ltd., Tokyo, Japan). Nuclear staining was performed with DAPI (Invitrogen, Carlsbad, CA, USA) in a dark chamber. A TE2000-U Fluorescence Microscope (Nikon Corporation, Tokyo, Japan) was used for obtaining fluorescence images of liver sections. The relative intensities of tissue sections were quantified using Adobe Photoshop (Adobe Systems, San Jose, CA, USA). The mean histogram value on the red channel was recorded as the DHE fluorescence level.
3.5. Oil red O staining
To visualize lipid droplets, Oil Red O staining was performed as previously described [23]. The livers were fixed and embedded in an OCT compound and stored at −80 °C. The frozen tissues were cut into 10 μm sections and placed on glass slides. The tissues sections were stained with Oil Red O and counterstained with hematoxylin to visualize lipid droplets.
3.6. TBARS assay
TBARS levels in the liver were used to estimate the accumulation of lipid peroxidation products. Liver tissue was homogenized (3:7, w-v) in 1.15% KCl and 5 mM 2,6-di-t-butyl-4-methylphenol and 0.1 ml of this homogenate was mixed with reagents, to a final concentration of 2 mM EDTA, 7.5% acetic acid and 0.4% SDS. This mixture was reacted with 0.3% thiobarbituric acid (Wako Pure Chemical Industries, Osaka, Japan) in a boiling water bath for 45 min. After cooling, the chromogen was extracted in n-butanol:pyridine (15:1, v-v). TBARS levels were calculated from the absorbances of the butanol-extracted supernatants at 532 nm.
3.7. Measurement of AsA
To measure AsA in the liver and plasma, the AsA-specific fluorophore-nitroxide probe Naph-DiPy nitroxide was used [24]. Liver tissue was homogenized in 5% metaphosphoric acid and centrifuged at 14,000×g for 15 min. The supernatant was passed through a 0.45 mm filter (Minisart RC4; Sartorius Stedim Biotec GmbH, Goettingen, Germany) and analyzed for AsA content. Each 5 μL tissue or plasma sample was added to an assay solution containing 85 μL distilled water and 10 μL probe stock solution (500 μM in DMSO). Fluorescence was then measured at 310 and 430 nm excitation and emission wavelengths, respectively (Corona grading microplate reader: SH-9000Lab, Corona Electric Co., Ltd., Ibaraki, Japan).
3.8. Statistics
All results are presented as means±SEM. Statistical data were analyzed using the two-tailed t-test or the Tukey–Kramer test; p<0.05 was considered statistically significant.
4. Results and discussion
To demonstrate that TEMPOL induced NADH oxidation, increasing the NAD+/NADH ratio via two pathways, we utilized two experimental models. The first, an ROS scavenging system with TEMPOL as a reducing agent, should result in reduction of ROS and oxidation of NADH (Fig. 1A). Superoxide scavenging by TEMPOL, with superoxide generated by the hypoxanthine–xanthine oxidase system, was monitored in an assay based on ferricytochrome c reduction. Coincident with superoxide scavenging, TEMPOL, in a dose-dependent fashion, generated NAD+ (Fig. 1B). These results indicated that TEMPOL reduced superoxide levels and oxidized NADH, findings consistent with previous reports [15]. In the second system, we tested TEMPOL as an oxidizing agent, because it is readily reduced to hydroxylamine by ascorbic acid (AsA) [17]. The resulting oxidized form of AsA, dehydroascorbic acid (DHA), was reportedly reduced to AsA in the presence of GSH, glutathione reductase (GR) and NADH [18]. We found that with these substrates, TEMPOL also produced NAD+ in a dose-dependent manner (Fig. 1C). In the absence of AsA, or GSH, GR did not oxidize NADH to NAD+ in the presence of TEMPOL (Supplemental Fig. 1). These results suggested that TEMPOL can act via two pathways to produce NAD+, that is, an ROS scavenging and NADH oxidation system (ROS scavenging system) and an AsA–GSH redox cycle (redox cycling system) (Fig. 1A).
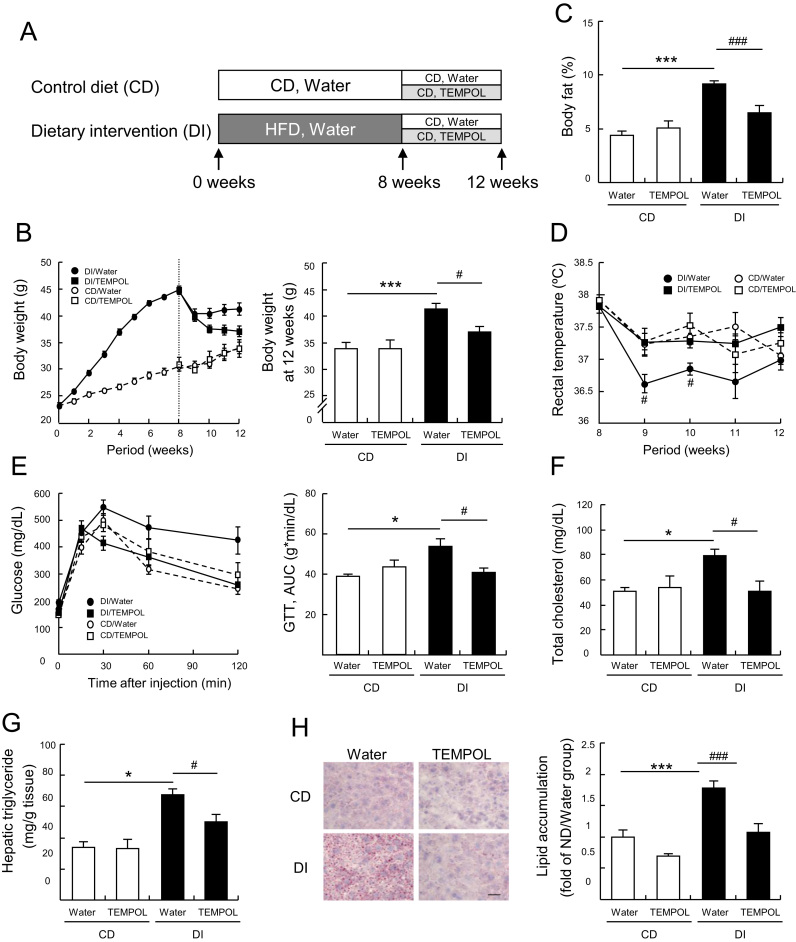
TEMPOL was reported to prevent obesity in mice [19], [25], with one proposed mechanism being changes to the gut microbiome [25]. Our findings suggest another potential mechanism for the anti-obesity effects of TEMPOL, involving two different redox cycling systems. We selected the diet intervention (DI) model for obesity, in which C57BL/6N male mice were switched from a high fat diet (HFD) to a normal control diet (CD), because weight loss after switching to a CD was reported to be inconsistent [26], [27]. This has led to considerations of a therapeutic strategy combining diet counseling and drug treatment, with agents such as lorcaserin [28]. Fig. 2A shows the experimental schedule. In the DI group, mice were fed a HFD for 8 week then switched to a CD. Each feeding group was further divided into two groups receiving water alone or TEMPOL (30 mM) in the drinking water. HFD fed mice developed obvious obesity (Fig. 2B). Though, body weight was reduced in the group receiving DI and water alone, it was significantly lower in the group consuming TEMPOL (Fig. 2B). The percentage of total body fat (inguinal, gonadal, and retroperitoneal) was higher in the DI than in the CD group. TEMPOL consumption decreased the percentage of total body fat in the mice under DI (Fig. 2C). To further characterize physiological changes in mice in the DI/TEMPOL group, we measured the rectal temperature at rest. The rectal temperatures of the DI/water group were significantly lower than those of mice on the CD. However, mice in the DI/TEMPOL group had a normal temperature (Fig. 2D). Glucose intolerance, higher total plasma cholesterol and triglyceride levels and the intensity of Oil Red O staining in the mice under DI were ameliorated significantly by TEMPOL consumption (Fig. 2E–H). Food and water intake and plasma concentrations of TEMPOL were virtually identical in the DI/water and DI/TEMPOL groups (Supplemental Fig. 2).
Fig. 2.
Effects of TEMPOL on the metabolic state of obese mice under a dietary intervention (DI). (A) Schematic overview of the experimental schedule. Eight weeks after feeding a high-fat diet (HFD), diets were switched from HFD to a control diet (CD) in the DI group. Each group was further divided into two groups, consuming water alone or TEMPOL (30 mM) in the drinking water. (B) Body weights in the CD and DI groups. After 8 week on a HFD, the diet was switched to CD in the DI groups and TEMPOL consumption was started, where indicated. (C) Percentage of total body fat (inguinal, gonadal, and retroperitoneal), (D) rectal temperature, (E) glucose tolerance test, (F) plasma total cholesterol (G) hepatic triglycerides, (H) hepatic lipid accumulation stained by Oil Red O (red). Scale bar in (H) represents 100 μm. Values are means±SEM (n=7 for CD and 8 for DI groups except (H) was n=6). *p<0.05 and ***p<0.005 compared with CD group. #p<0.05 and ###p<0.005 compared with the DI/water group. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To test whether the TEMPOL associated weight loss and recovery to normal conditions were caused by decreased ROS levels, we used 1,2-dihydroxy-3,5-benzenedisulfonic acid disodium salt monohydrate (Tiron), a superoxide scavenger, as a comparison treatment. In a previous report, Tiron significantly attenuated ischemia-reperfusion induced liver injury in rats [29]. Tiron scavenged free radicals but did not oxidize NADH (Supplementary Fig. 3A and B). In mice under DI, with Tiron consumption, we observed no improvements in body weight, body fat, glucose intolerance or total plasma cholesterol levels over those in mice given only water (Supplementary Fig. 3C–F). These results suggested that antioxidant properties alone are not sufficient to ameliorate metabolic imbalances in obese C57BL/6N mice under DI.
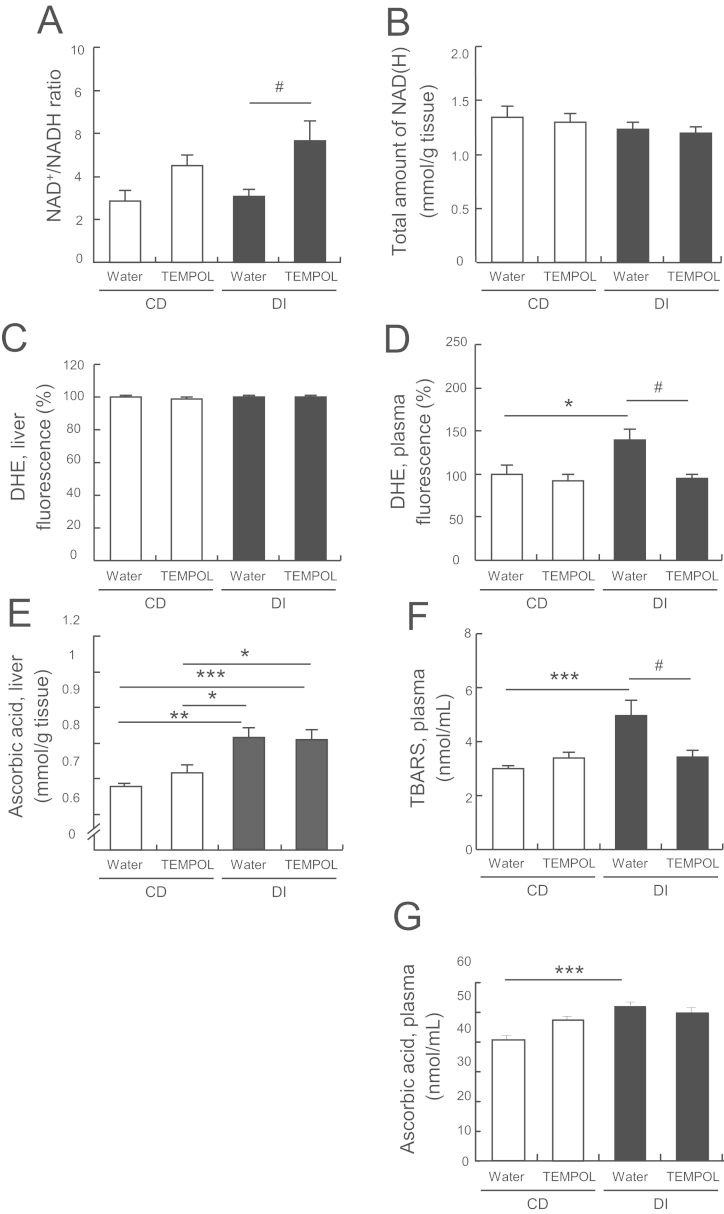
We next investigated whether TEMPOL, in addition to its antioxidant effects, altered the NAD+/NADH ratio in the livers of mice under DI. We found that TEMPOL consumption significantly increased the NAD+/NADH ratio (Fig. 3A) over that in the livers of mice given only water, though the total amount of NADH was unchanged between the groups (Fig. 3B). In the liver samples, both the intensity of oxidized dihydroethidium (DHE), a relatively specific marker of superoxide, were virtually identical in mice under DI and those on the CD (Fig. 3C). However, in plasma samples, the fluorescence intensity of oxidized DHE, and the accumulation of thiobarbituric acid reactive substances (TBARS), an index of lipid peroxidation, were much higher in the mice under DI than in those on the CD (Fig. 3D and F). These indications of oxidative stress were consistent with previous findings in obese mice [13]. With TEMPOL consumption, DHE fluorescence intensity and TBARs levels returned to control values in the mice under DI (Fig. 3D and F). These results indicated that mice under DI showed signs of oxidative stress in the plasma, though not in the liver, and TEMPOL improved this via its antioxidant effects.
Fig. 3.
Hepatic redox states in mice fed only a CD or undergoing DI. (A) Ratio of NAD+/NADH in the liver. (B) Total amount of NADH (oxidized and reduced forms of NAD) in the liver. For estimation of oxidative stress in plasma and the liver in the CD and DI groups: (C) Fluorescence intensity of oxidized DHE, a superoxide indicator, in the liver. (D) Fluorescence intensity of DHE in the plasma. (E) AsA levels in the liver. (F) TBARS levels in the plasma. (G) AsA levels in the plasma. Values are means±SEM (n=7 for CD and 8 for DI groups except (C), (E) and (H) was n=4). *p<0.05, **p<0.01, and ***p<0.005 compared with the corresponding CD group; #p<0.05 compared with the DI/water group.
To further characterize redox status in the liver, we estimated antioxidant enzyme activities and AsA concentrations. Superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities in the liver did not differ between mice fed a CD and those subjected to DI (Supplemental Fig. 4). However, hepatic AsA was higher in the mice under DI than in those on the CD though levels in plasma (Fig. 3E and G). TEMPOL was present in the liver in its hydroxylamine form (Supplementary Fig. 2D). Therefore, TEMPOL may modulate NAD+ production through the AsA–GSH redox cycle, becoming reduced to its hydroxylamine.
Our study did not address effects of changes in enzyme activity and downstream related protein expression regulated by NAD+. Therefore, to confirm our interpretations and explore the mechanism of anti-obesity effects of TEMPOL, further experiments in the appropriate cell and animal models will be required. TEMPOL did not decrease body weight in C57BL/6J mice (Supplemental Fig. 5) which do not express the nicotinamide nucleotide transhydrogenase (NNT) gene [30]. NNT transfers reducing equivalents from NADH to NADP+, resulting in increased NAD+ and NADPH (Fig. 1A) [31]. This suggests that TEMPOL activated the redox cycling system by oxidizing AsA to DHA, increasing NAD+ through NNT. Indeed, previous reports demonstrated anti-obesity effects with TEMPOL in C3H and C57BL/6N mice [19], [25], which express the NNT genes [32]. Therefore, anti-obesity activity of TEMPOL might be increasing NAD+ through the redox cycling system, in addition to its antioxidant effects.
In conclusion, TEMPOL showed anti-obesity activity through two pathways, one involving direct ROS scavenging and the other an activation of the redox cycling system, resulting in an increase of the NAD+/NADH ratio. In mice subjected to DI, additional consumption of TEMPOL, led to recovery of body weight and redox state imbalances to those of normal-fed mice within a short period of time. Thus, though the in vivo benefits of antioxidants is controversial, restoration of balance in the redox status of obese mice represents a new therapeutic concept. Appropriate compounds that, like TEMPOL, have two redox potentials enabling both reduction and oxidation, or combination therapy with both an antioxidant and oxidant acting via different routes, might be useful approaches for treating obesity.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgment
This research was supported in part by Japan Society for the Promotion of Science KAKENHI grant number 24590052 to M.Y., grant 26670017 to K.Y., by Japan Science and Technology PRESTO to K.Y., and by the Platform Project for Supporting in Drug Discovery and Life Science Research from Japan Agency for Medical Research and development (AMED), Japan. We appreciate the technical support provided by the Research Support Center, Graduate School of Medical Sciences, Kyushu University.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2016.02.007.
Contributor Information
Mayumi Yamato, Email: yamato68@redoxnavi.med.kyushu-u.ac.jp.
Ken-ichi Yamada, Email: kenyamada@phar.kyushu-u.ac.jp.
Appendix A. Supplementary material
Supplementary Figures
References
- 1.Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N. Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Grover S.A., Kaouache M., Rempel P., Joseph L., Dawes M., Lau D.C.W., Lowensteyn I. Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in overweight and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3:114–122. doi: 10.1016/S2213-8587(14)70229-3. [DOI] [PubMed] [Google Scholar]
- 3.Houtkooper R.H., Canto C., Wanders R.J., Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 5.Canto C., Gerhart-Hines Z., Feige J.N., Lagouge M., Noriega L., Milne J.C., Elliott P.J., Puigserver P., Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi S.-E., Fu T., Seok S., Kim D.-H., Yu E., Lee K.-W., Kang Y., Li X., Kemper B., Kemper J.K. Elevated microRNA-34a in obesity reduces NAD(+) levels and SIRT1 activity by directly targeting NAMPT. Aging Cell. 2013;12:1062–1072. doi: 10.1111/acel.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canto C., Houtkooper R.H., Pirinen E., Youn D.Y., Oosterveer M.H., Cen Y., Fernandez-Marcos P.J., Yamamoto H., Andreux P.A., Cettour-Rose P., Gademann K., Rinsch C., Schoonjans K., Sauve A.A., Auwerx J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshino J., Mills K.F., Yoon M.J., Imai S.-i. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai P., Canto C., Oudart H., Brunyanszki A., Cen Y., Thomas C., Yamamoto H., Huber A., Kiss B., Houtkooper R.H., Schoonjans K., Schreiber V., Sauve A.A., Menissier-de Murcia J., Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbosa M.T.P., Soares S.M., Novak C.M., Sinclair D., Levine J.A., Aksoy P., Chini E.N. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 2007;21:3629–3639. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- 11.Hwang J.H., Kim D.W., Jo E.A., Kim Y.K., Jo Y.S., Park J.H., Yoo S.K., Park M.K., Kwak T.H., Kho Y.L., Han J., Choi H.-S., Lee S.-H., Kim J.M., Lee I., Kyung T., Jang C., Chung J., Kweon G.R., Shong M. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes. 2009;58:965–974. doi: 10.2337/db08-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H.-J., Kim J.H., Noh S., Hur H.J., Sung M.J., Hwang J.-T., Park J.H., Yang H.J., Kim M.-S., Kwon D.Y., Yoon S.H. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J. Proteome Res. 2011;10:722–731. doi: 10.1021/pr100892r. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent H.K., Taylor A.G. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int. J. Obes. 2006;30:400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 15.Krishna M.C., Grahame D.A., Samuni A., Mitchell J.B., Russo A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc. Natl. Acad. Sci. USA. 1992;89:5537–5541. doi: 10.1073/pnas.89.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo W., Yamato M., Yamada K., Kinoshita Y., Shiba T., Watanabe T., Utsumi H. Formation of TEMPOL-hydroxylamine during reaction between TEMPOL and hydroxyl radical: HPLC/ECD study. Free Radic. Res. 2008;42:505–512. doi: 10.1080/10715760802112809. [DOI] [PubMed] [Google Scholar]
- 17.Tada M., Yokoyama H., Ito O., Ohya H., Ogata T. Evaluation of the hepatic reduction of a nitroxide radical in rats receiving ascorbic acid, glutathione or ascorbic acid oxidase by in vivo electron spin resonance study. J. Gastroenterol. Hepatol. 2004;19:99–105. doi: 10.1111/j.1440-1746.2004.03201.x. [DOI] [PubMed] [Google Scholar]
- 18.Wells W.W., Xu D.P. Dehydroascorbate reduction. J. Bioenerg. Biomembr. 1994;26:369–377. doi: 10.1007/BF00762777. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell J.B., Xavier S., DeLuca A.M., Sowers A.L., Cook J.A., Krishna M.C., Hahn S.M., Russo A. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic. Biol. Med. 2003;34:93–102. doi: 10.1016/s0891-5849(02)01193-0. [DOI] [PubMed] [Google Scholar]
- 20.Lin S.S., Manchester J.K., Gordon J.I. Enhanced gluconeogenesis and increased energy storage as hallmarks of aging in Saccharomyces cerevisiae. J. Biol. Chem. 2001;276:36000–36007. doi: 10.1074/jbc.M103509200. [DOI] [PubMed] [Google Scholar]
- 21.Yamato M., Shiba T., Yoshida M., Ide T., Seri N., Kudou W., Kinugawa S., Tsutsui H. Fatty acids increase the circulating levels of oxidative stress factors in mice with diet-induced obesity via redox changes of albumin. FEBS J. 2007;274:3855–3863. doi: 10.1111/j.1742-4658.2007.05914.x. [DOI] [PubMed] [Google Scholar]
- 22.Maeda Y., Inoguchi T., Takei R., Sawada F., Sasaki S., Fujii M., Kobayashi K., Urata H., Nishiyama A., Takayanagi R. Inhibition of chymase protects against diabetes-induced oxidative stress and renal dysfunction in hamsters. Am. J Physiol.-Ren. Physiol. 2010;299:F1328–F1338. doi: 10.1152/ajprenal.00337.2010. [DOI] [PubMed] [Google Scholar]
- 23.Mark M., Teletin M., Antal C., Wendling O., Auwerx J., Heikkinen S., Khetchoumian K., Argmann C.A., Dgheem M. Histopathology in mouse metabolic investigations. In: Ausubel Frederick M., editor. Current Protocols In Molecular Biology. 2007. Chapter 29: Unit 29B.24–Unit 29B.24. [DOI] [PubMed] [Google Scholar]
- 24.Matsuoka Y., Yamato M., Yamasaki T., Mito F., Yamada K. Rapid and convenient detection of ascorbic acid using a fluorescent nitroxide switch. Free Radic. Biol. Med. 2012;53:2112–2118. doi: 10.1016/j.freeradbiomed.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 25.Li F., Jiang C., Krausz K.W., Li Y., Albert I., Hao H., Fabre K.M., Mitchell J.B., Patterson A.D., Gonzalez F.J. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 2013;4 doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P., Lu M., Nguyen M.T.A., Bae E.J., Chapman J., Feng D., Hawkins M., Pessin J.E., Sears D.D., Nguyen A.-K., Amidi A., Watkins S.M., Nguyen U., Olefsky J.M. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J. Biol. Chem. 2010;285:15333–15345. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q., Perrard X.D., Perrard J.L., Mansoori A., Raya J.L., Hoogeveen R., Smith C.W., Ballantyne C.M., Wu H. Differential effect of weight loss with low-fat diet or high-fat diet restriction on inflammation in the liver and adipose tissue of mice with diet-induced obesity. Atherosclerosis. 2011;219:100–108. doi: 10.1016/j.atherosclerosis.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith S.R., Weissman N.J., Anderson C.M., Sanchez M., Chuang E., Stubbe S., Bays H., Shanahan W.R. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 29.Bowes J., Thiemermann C. Effects of inhibitors of the activity of poly (ADP-ribose) synthetase on the liver injury caused by ischaemia-reperfusion: a comparison with radical scavengers. Br. J. Pharmacol. 1998;124:1254–1260. doi: 10.1038/sj.bjp.0701930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mekada K., Abe K., Murakami A., Nakamura S., Nakata H., Moriwaki K., Obata Y., Yoshiki A. Genetic differences among C57BL/6 substrains. Exp. Anim. 2009;58:141–149. doi: 10.1538/expanim.58.141. [DOI] [PubMed] [Google Scholar]
- 31.Olgun A. Converting NADH to NAD(+) by nicotinamide nucleotide transhydrogenase as a novel strategy against mitochondrial pathologies during aging. Biogerontology. 2009;10:531–534. doi: 10.1007/s10522-008-9190-2. [DOI] [PubMed] [Google Scholar]
- 32.Freeman H.C., Hugill A., Dear N.T., Ashcroft F.M., Cox R.D. Deletion of nicotinamide nucleotide transhydrogenase-A new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes. 2006;55:2153–2156. doi: 10.2337/db06-0358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures