Abstract
Purpose
Recent evidence suggests that the heart may be injured by ionizing radiation at lower doses than was previously thought. This raises concerns about the cardiovascular risks from exposure to radiation during space travel. Since space travel is associated with exposure to both protons from solar particle events and heavy ions from galactic cosmic rays, we here examined the effects of a “priming” dose of protons on the cardiac cellular and molecular response to a “challenge” dose of 56Fe in a mouse model.
Methods
Male C57BL/6 mice at 10 weeks of age were exposed to sham-irradiation, 0.1 Gy of protons (150 MeV), 0.5 Gy of 56Fe (600 MeV/n), or 0.1 Gy of protons 24 hours prior to 0.5 Gy of 56Fe. Hearts were obtained at 7 days post-irradiation and western-blots were used to determine protein markers of cardiac remodeling, inflammatory infiltration, and cell death.
Results
Exposure to 56Fe caused an increase in expression of α-smooth muscle cell actin, collagen type III, the inflammatory cell markers mast cell tryptase, CD2 and CD68, the endothelial glycoprotein thrombomodulin, and cleaved caspase 3. Of all proteins investigated, protons at a dose of 0.1 Gy induced a small increase only in cleaved caspase 3 levels. On the other hand, exposure to protons 24 hours before 56Fe prevented all of the responses to 56Fe.
Conclusions
This study shows that a low dose of protons may prime the heart to respond differently to a subsequent challenge dose of heavy ions. Further investigation is required to identify responses at additional time points, consequences for cardiac function, threshold dose levels, and mechanisms by which a proton priming dose may alter the response to heavy ions.
Keywords: High-LET Radiation, Space Radiation, Heart, Inflammatory Infiltration, SPE, GCR
1. Introduction
Recent reports on the long-term follow-up of atomic bomb survivors have shown an increased incidence of cardiovascular disease, including ischemic heart disease and stroke, in people exposed to doses of radiation as low as 2 Gy [1-3]. Moreover, epidemiological studies in occupational exposure and low-dose exposure due to medical treatments indicate that cardiovascular disease may occur after lower doses of ionizing radiation than was previously thought [4-10]. These findings have raised concern for potential cardiovascular effects of current low-dose ionizing radiation exposures, including the radiation encountered during space travel, especially beyond Low Earth Orbit [11, 12]. However, care should be taken when the results of terrestrial radiation exposures such as those from atomic bombs are used to support the potential for a cardiovascular disease risk from space radiation, since certain conditions such as dose rate are different.
Recent studies have started to elucidate the effects of space-like radiation on cardiac function and structure in animal models. Exposure to protons (0.5 Gy, 1 GeV) and 56Fe (0.15 Gy, 1 GeV/n) in a mouse model induced cardiac infiltration of CD68-positive cells, increased DNA oxidation, myocardial fibrosis, and modified cardiac function, both at baseline and in response to myocardial infarction, in a radiation-type specific manner [13-15]. Moreover, exposure to 28Si (0.1-0.5 Gy, 300 MeV/n) caused prolonged apoptosis and increased expression of the common pro-inflammatory cytokines interleukin (IL)-1β, IL-6, or tumor necrosis factor (TNF)-α in a mouse model [16]. Deep space travel is associated with exposure to various types of high-LET radiation. It is currently unknown how the exposure to one form of radiation may alter the cardiac response to a subsequent challenge with the same or a different form of radiation.
This study used a mouse model to characterize cellular and molecular changes in the heart in response to protons, 56Fe, or consecutive irradiation. The goal was to determine whether a low dose of protons may prime the heart to respond differently to a subsequent challenge dose of heavy ions. Protons were administered at a dose of 0.1 Gy (150 MeV), a likely occurring dose during a solar particle event (SPE). The energy of 150 MeV is not only commonly used in therapeutic settings but also represents an energy near the maximum abundance of protons expected in most SPEs [11]. 56Fe was selected as one of the higher dose-equivalent heavy particles that make up galactic cosmic rays in free space and was administered at 0.5 Gy (600 MeV/n) - the minimum dose that has shown to cause cell loss in other tissues [17]. For consecutive irradiation, a priming dose of 0.1 Gy of proton was followed by a challenge dose of 0.5 Gy of 56Fe 24 hours later. While the choice of 24 hours between the two exposures was in part made out of scientific interest, it is also relevant for the radiation environment in deep space, in which astronauts will be exposed to heavy ions after having encountered an SPE.
2. Materials and methods
2.1. Animals and irradiation
Male C57BL/6J mice, at 10 weeks of age (Jackson Laboratory, Bar Harbor, ME) were shipped to Brookhaven National Laboratories (BNL) in Upton, NY. After a one-week acclimation period, the mice were randomly assigned to experimental groups and were exposed to sham-irradiation (n=4), 0.1 Gy protons (150 MeV) (n=5), 0.5 Gy 56Fe (600 MeV/n) (n=5), or 0.1 Gy protons (150 MeV) at 24 h followed by 0.5 Gy 56Fe (600 MeV/n) (n=5). Dosimetry was performed by the Physics Dosimetry Group at BNL. For each exposure, animals were individually placed into clear Lucite cubes (3 in × 1½ in × 1½ in) with breathing holes. Sham-irradiated mice served as controls and were placed into the same enclosures. During the entire experiment, sham-irradiated mice were not housed together with irradiated mice. After irradiation, the mice were shipped to Loma Linda University (LLU) under climate-controlled conditions and were housed at LLU under a constant 12 h light:dark cycle. Regular chow and water were provided ad libitum. All procedures were approved by the Institutional Animal Care and Use Committees of LLU and BNL.
2.2. Tissue harvesting
Animals were sacrificed at 7 days after irradiation, and the heart was obtained and snap-frozen in liquid nitrogen. Frozen tissues were shipped on dry ice to the University of Arkansas for Medical Sciences for analysis of cellular markers with western-blots and gene expression with real-time PCR.
2.3. Analysis of cardiac protein levels with western-blots
Western-blots were performed as described previously [18] and in the Supplementary Material. Supplementary Table 1 lists all primary antibodies used for western-blotting.
2.4. RNA isolation and real-time PCR
Total cardiac RNA was isolated, cDNA synthesized, and steady-state mRNA levels were measured with real-time quantitative PCR (TaqMan™) as described before [19] and in the Supplementary Material, using the pre-designed TaqMan Gene Expression Assays™ listed in Supplementary Table 2.
2.5. Statistical analysis
All data were analyzed with the software NCSS 2007 (NCSS, Kaysville, UT) using two-way analysis of variance (ANOVA), followed by Newman-Keuls individual comparisons. The criterion for significance was a p<0.05. Data reported as average ± standard error of the mean (SEM) with n=4-5.
3. Results
3.1. A priming dose of protons altered the levels of cardiac remodeling and inflammatory infiltration markers after exposure to 56Fe
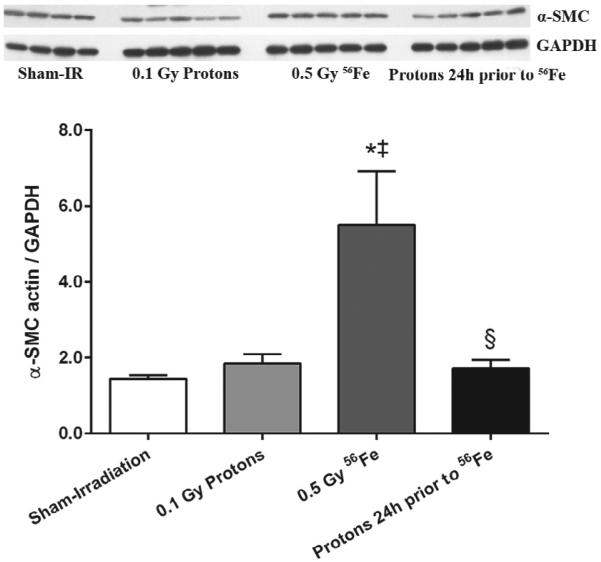
Increased collagen deposition, collagen turnover, and increased numbers of myofibroblasts that express α-smooth muscle cell (SMC) actin are common manifestations of cardiac remodeling. Cardiac levels of α-SMC actin were measured with western-blots. While cardiac levels of α-SMC actin were increased after exposure to 56Fe, they remained steady in the hearts of mice that had been primed with low dose protons (Fig. 1).
Figure 1.
Increased expression of α-SMC actin, a marker of myofibroblasts, was observed at 7 days after exposure to 56Fe. A priming dose of protons prevented the increase in α-SMC actin in response to 56Fe exposure. Western-blot image indicates 4-5 biological repeats in each radiation group. Graphs indicate average ± SEM, n=4-5. Quantification of α-SMC divided by GAPDH for sham-irradiation: 1.43 ± 0.11; for 0.1 Gy protons: 1.86 ± 0.24; for 0.5 Gy 56Fe: 5.51 ± 1.42; for the consecutive exposure group: 1.72 ± 0.23. *p<0.05 compared to sham-irradiated control, ‡p<0.05 compared to proton exposure, §p<0.05 compared to 56Fe exposure.
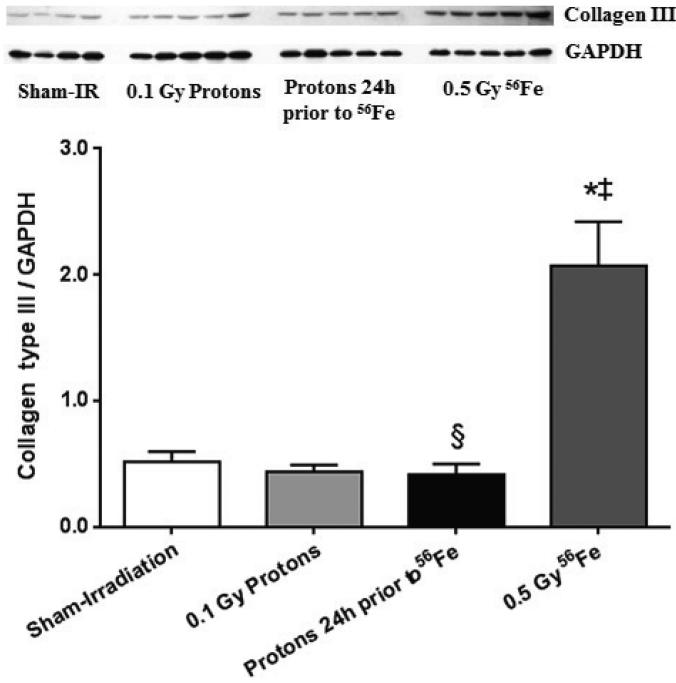
Western-blots against collagen type III, one of the main collagen types in the heart, identified two protein bands that were diminished when the anti-collagen type III antibody was pre-incubated with blocking peptide, indicating that both protein bands were fragments of collagen type III (Supplementary Fig. 1). These protein fragments are likely the result of matrix metalloprotease-induced cleavage of collagen type III, an indication of ongoing tissue remodeling. Increased levels of collagen type III fragments were observed after exposure to 56Fe, suggesting that remodeling occurred at this 7 day time point. However, when primed with low dose protons, the levels did not significantly change when compared to the hearts of sham-irradiated controls (Fig. 2).
Figure 2.
Increased cardiac levels of collagen type III (quantification of the 75 kDa fragment as indicated in Supplementary Fig. 1) was observed at 7 days after exposure to 56Fe. A priming dose of protons prevented the increase in collagen type III in response to 56Fe exposure. Western-blot image indicates 4-5 biological repeats in each radiation group. Graphs indicate average ± SEM, n=4-5. Quantification of collagen type III divided by GAPDH for sham-irradiation: 0.51 ± 0.08; for 0.1 Gy protons: 0.43 ± 0.06; for 0.5 Gy 56Fe: 2.07 ± 0.34; for the consecutive exposure group: 0.41 ± 0.09. *p<0.05 compared to sham-irradiated control, ‡p<0.05 compared to proton exposure, §p<0.05 compared to 56Fe exposure.
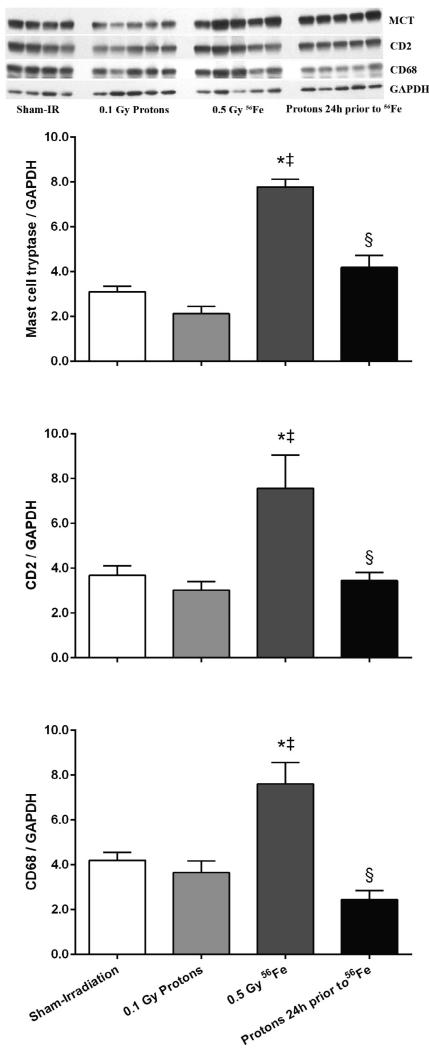
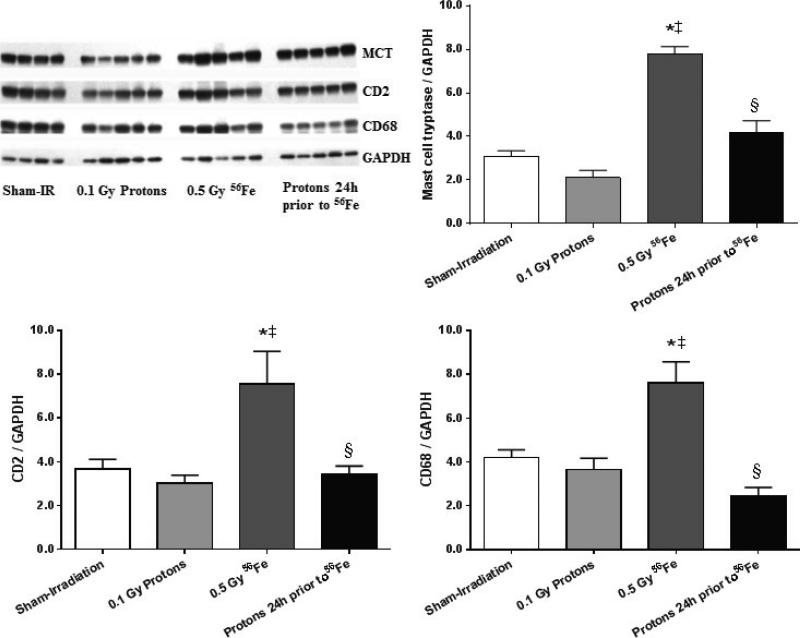
The proton exposure also altered the inflammatory infiltration response seen with 56Fe. Alone, exposure to 56Fe caused increased levels of the mast cell enzyme tryptase, the T-lymphocyte marker CD2, and the monocyte/macrophage marker CD68. When 56Fe were preceded by low dose protons, no significant changes were seen in these markers (Fig. 3). Although western-blots for mast cell tryptase, CD2, and CD68 indicate that cardiac inflammatory infiltration had occurred after exposure to 56Fe, no significant changes were seen in cardiac mRNA levels of the pro-inflammatory cytokines IL-1β, IL-6, or TNF-α (data not shown).
Figure 3.
Increased expression of markers of cardiac inflammatory cells indicated that 56Fe exposure led to increased numbers of mast cells (mast cell tryptase, MCT), T-lymphocytes (CD2), and monocytes/macrophages (CD68). No alterations in these markers were observed in the consecutive exposure group. Western-blot image indicates 4-5 biological repeats in each radiation group. Graphs indicate average ± SEM, n=4-5. Quantification of MCT/CD2/CD68 divided by GAPDH for sham-irradiation: 3.10 ± 0.24/3.68 ± 0.43/4.21 ± 0.35; for 0.1 Gy protons: 2.12 ± 0.33/3.02 ± 0.38/3.65 ± 0.51; for 0.5 Gy 56Fe: 7.78 ± 0.35/7.56 ± 1.48/7.61 ± 0.96; for the consecutive exposure group: 4.19 ± 0.53/3.44 ± 0.37/2.45 ± 0.39. *p<0.05 compared to sham-irradiated control, ‡p<0.05 compared to proton exposure, §p<0.05 compared to 56Fe exposure.
3.2. A priming dose of protons altered the induction of thrombomodulin in response to 56Fe
Thrombomodulin (TM) is a transmembrane glycoprotein located on the luminal surface of endothelial cells that plays an important role in the regulation of coagulation and fibrinolysis. Reduced TM expression on the endothelial cell surface is an indication of endothelial dysfunction [20-23]. While 56Fe caused an increase in cardiac levels of TM, its levels were not modified after proton or consecutive exposure (Fig. 4).
Figure 4.
A priming dose of protons altered the induction of TM in response to 56Fe. Western-blot image indicates 4-5 biological repeats in each radiation group. Graphs indicate average ± SEM, n=4-5. Quantification of TM divided by GAPDH for sham-irradiation: 0.43 ± 0.08; for 0.1 Gy protons: 0.60 ± 0.09; for 0.5 Gy 56Fe: 0.75 ± 0.08; for the consecutive exposure group: 0.53 ± 0.04. *p<0.05 compared to sham-irradiated control.
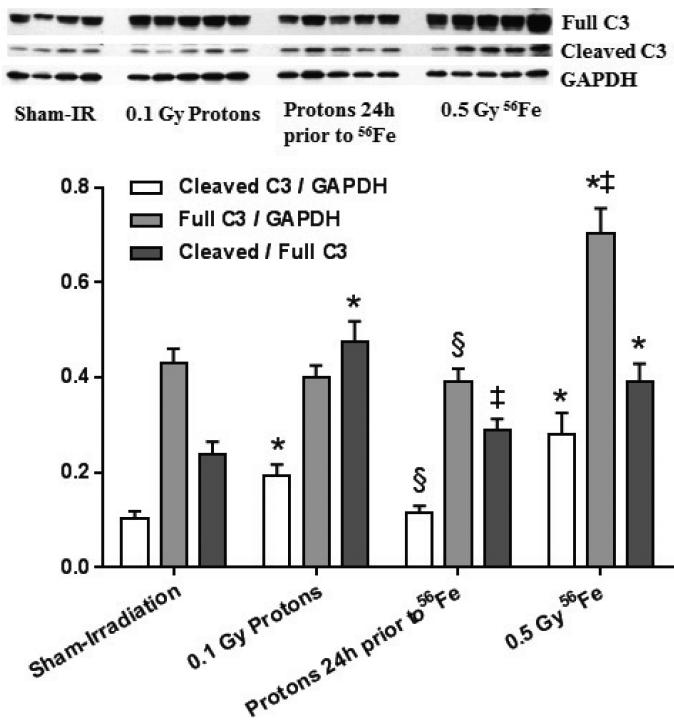
3.3. A priming dose of protons altered the levels of caspase 3
An increase in the ratio of cleaved caspase 3 to the full caspase 3 protein suggested that some apoptosis occurred after 56Fe exposure. While protons alone caused a small increase in cardiac levels of cleaved caspase 3, no significant increase was observed in cleaved caspase 3, full caspase 3 protein, or their ratio in the consecutive exposure group compared to sham-irradiated controls (Fig. 5).
Figure 5.
Effects of charged particle exposure on cardiac caspase 3 (C3). Both protons and 56Fe induced increased ratios of cleaved caspase 3 to the full caspase 3 protein, while no significant differences were observed between the consecutive exposure group and sham-irradiated controls. Western-blot image indicates 4-5 biological repeats in each radiation group. Graphs indicate average ± SEM, n=4-5. Quantification of cleaved caspase 3/full caspase 3 divided by GAPDH for sham-irradiation: 0.10 ± 0.02/0.43 ± 0.03; for 0.1 Gy protons: 0.19 ± 0.02/0.40 ± 0.02; for 0.5 Gy 56Fe: 0.28 ± 0.04/0.70 ± 0.05; for the consecutive exposure group: 0.11 ± 0.02/0.39 ± 0.03. Average ± SEM, n=4-5. *p<0.05 compared to sham-irradiated control, ‡p<0.05 compared to proton exposure, §p<0.05 compared to 56Fe exposure.
4. Discussion
The aim of this study was to determine whether a low dose of protons could alter the response of the heart to a subsequent challenge with a heavy ion. A limited number of studies have examined the effects of whole-body exposure to low doses of high-LET radiation on cardiac function and structure in animal models. Additionally, to our knowledge there are few published studies that have examined the effects of combined or consecutive exposures. While the current choice of a 24 hour interval between proton and heavy ion exposures may be relevant for certain situations in deep space, astronauts will also be exposed to different forms of radiation at the same time or within time intervals less than 24 hours. The cardiovascular effects of these radiation exposures need to be studied in the future. Also, the current experiment included only male mice. However, there are known sex differences in cardiovascular physiology and disease [24], and future studies therefore need to include female animals.
In the current study, we examined cardiac levels of protein markers of remodeling, inflammatory infiltration, apoptosis, and the endothelial glycoprotein TM at an early post-radiation time point. Due to experimental set-up and limited availability of tissues, we did not have the opportunity to include other time points or assess cardiac function or histology. However, previous research in a similar mouse model of exposure to protons (0.5 Gy, 1 GeV) and 56Fe (0.15 Gy, 1 GeV/n) showed long-term alterations in cardiac function and structure in response to these charged particles [13-15].
In studies on single ion exposure, 28Si (0.1-0.5 Gy, 300 MeV/n) caused prolonged apoptosis and increased expression of the common pro-inflammatory cytokines IL-1β, IL-6, or TNF-α in a mouse model [16]. Similarly, we found increased levels of cleaved caspase 3 in response to 56Fe. On the other hand, while we observed increased markers of inflammatory infiltration after exposure to 56Fe, in contrast with exposure to 28Si, no significant changes were seen in the (mRNA) expression of the pro-inflammatory cytokines IL-1β, IL-6, or TNF-α. The more energetic 56Fe nuclei may generate a unique spectrum of secondary particles in the tissue. Our results may therefore suggest that the cardiac response is radiation type-specific. However, we cannot exclude that different results are in part due to the use of different mouse strains [25].
Endothelial dysfunction, which is characterized by a proinflammatory and profibrogenic phenotype of endothelial cells, is a critical contributor to the pathophysiological manifestations of radiation injury [26-30]. A prominent feature of endothelial dysfunction associated with radiation injury is an imbalance in the TM system [20-23]. TM, a transmembrane glycoprotein located on the luminal surface of endothelial cells, has anti-inflammatory and anti-fibrogenic properties [31-33]. During endothelial dysfunction, TM is cleaved from the endothelial cell surface. Cardiac levels of TM were enhanced after exposure to 56Fe, suggesting that modifications in the TM system occurred. Our western-blots were unable to distinguish endothelial surface TM from soluble TM, and the exact role of TM in high-LET induced cardiac injury needs further investigation.
Interestingly, we here show that priming mice with a small dose of protons altered the response to a subsequent challenge with 56Fe. While proton exposure at a dose of 0.1 Gy had no effect on protein markers of cardiac tissue remodeling, inflammatory infiltration, or the endothelial glycoprotein TM, when administered 24 hours before 0.5 Gy of 56Fe proton exposure prevented the effects of 56Fe on these proteins. Moreover, while proton exposure caused a small increase in cleaved caspase 3 levels, no significant alterations were seen in caspase 3 in the consecutive exposure group compared to sham-irradiated controls. These results suggest that, even though not visible in our protein readouts, proton exposure primed the cardiac tissue by inducing certain defense mechanisms that diminished the cardiac response to a subsequent challenge. Similar to our results, recent work on tissue samples of lung [34] and brain (Antiño Allen, PhD, personal communication) of mouse models showed that protons followed at 24 hours by 56Fe resulted in molecular and functional alterations that were significantly different when compared to either source of radiation alone. Moreover, in mice that were challenged with an acute myocardial infarction, prior proton irradiation increased the expression of pro-survival genes, improved the restoration of cardiac function, and enhanced the process of cardiac remodeling when examined from hours to months after irradiation [13]. Our work confirms these earlier findings and demonstrates that the molecular and cellular response of the heart can be modified by a priming dose of protons at lower energy levels (150 MeV) than in the previous report by Yan et al. (1 GeV). Interestingly, there were no clear alterations in genome-wide gene expression arrays performed on mouse heart tissues at 7 days after exposure to protons [35]. Hence, at this time is difficult to speculate on possible mechanisms by which low-dose proton exposure may prime the heart to respond differently to a subsequent exposure to heavy ions. Previous work in cell culture models has recently shown that a low dose of protons protected against chromosomal damage induced by heavy ions administered 24 hours later, and that intercellular communication via soluble factors contributed to the cellular response [36]. We cannot exclude that similar mechanisms played a role in the cardiac response in the current study.
In conclusion, molecular alterations at 7 days after exposure to 56Fe in the mouse heart suggest that heavy ion irradiation may cause early cardiac adverse remodeling and inflammatory infiltration. Moreover, we show that a low dose of protons may prime the heart to respond differently to a subsequent challenge dose of heavy ions. Further investigation is required to identify responses at additional time points, consequences for cardiac function, threshold dose levels, and mechanisms by which a proton priming dose may alter the response to heavy ions.
Supplementary Material
Highlights.
Iron ions (0.5 Gy) induced proteins related to remodeling in the mouse heart.
Iron ions (0.5 Gy) induced markers of cardiac inflammatory infiltration.
Given 24 h before iron ions, protons (0.1 Gy) prevented effects of iron ions in the heart.
Hence, a low dose of protons may prime the heart to a challenge dose of heavy ions.
ACKNOWLEDGMENTS
This work was supported by National Aeronautics and Space Administration [NNX10AD59G to GN], the National Space Biomedical Research Institute [RE03701 through NCC 9-58 to MB], the National Institutes of Health [CA148679 to MB and Clinical and Translational Science Award UL1TR000039 and KL2TR000063 to IK], and the Arkansas Biosciences Institute [to IK].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no conflicts of interest.
REFERENCES
- 1.Preston DL, Shimizu Y, Pierce DA, et al. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950-1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 2.Yamada M, Wong FL, Fujiwara S, et al. Noncancer disease incidence in atomic bomb survivors, 1958-1998. Radiat Res. 2004;161:622–32. doi: 10.1667/rr3183. [DOI] [PubMed] [Google Scholar]
- 3.Little MP, Azizova TV, Bazyka D, et al. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect. 2012;120:1503–11. doi: 10.1289/ehp.1204982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azizova TV, Muirhead CR, Druzhinina MB, et al. Cardiovascular diseases in the cohort of workers first employed at Mayak PA in 1948-1958. Radiat Res. 2010;174:155–68. doi: 10.1667/RR1789.1. [DOI] [PubMed] [Google Scholar]
- 5.Adams MJ, Grant EJ, Kodama K, et al. Radiation dose associated with renal failure mortality: a potential pathway to partially explain increased cardiovascular disease mortality observed after whole-body irradiation. Radiat Res. 2012;177:220–8. doi: 10.1667/rr2746.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Little MP, Tawn EJ, Tzoulaki I, et al. A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects, and their possible mechanisms. Radiat Res. 2008;169:99–109. doi: 10.1667/RR1070.1. [DOI] [PubMed] [Google Scholar]
- 7.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: Is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–8. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 8.Stewart AM, Kneale GW. A-bomb survivors: factors that may lead to a re-assessment of the radiation hazard. Int J Epidemiol. 2000;29:708–14. doi: 10.1093/ije/29.4.708. [DOI] [PubMed] [Google Scholar]
- 9.Carr ZA, Land CE, Kleinerman RA, et al. Coronary heart disease after radiotherapy for peptic ulcer disease. Int J Radiat Oncol Biol Phys. 2005;61:842–50. doi: 10.1016/j.ijrobp.2004.07.708. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov VK, Maksioutov MA, Chekin SY, et al. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys. 2006;90:199–207. doi: 10.1097/01.HP.0000175835.31663.ea. [DOI] [PubMed] [Google Scholar]
- 11.Townsend et al. Information Needed to Make Radiation Protection Recommendations for Space Missions Beyond Low-Earth Orbit. National Council on Radiation Protection & Measurements Report No. 153; Bethesda: 2006. [Google Scholar]
- 12.Committee on the Evaluation of Radiation Shielding for Space Exploration NRC . Managing Space Radiation Risk in the New Era of Space Exploration. The National Academies Press; Washington: 2008. [Google Scholar]
- 13.Yan X, Sasi SP, Gee H, et al. Cardiovascular risks associated with low dose ionizing particle radiation. PLoS ONE. 2014;9:e110269. doi: 10.1371/journal.pone.0110269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan X, Sasi SP, Gee H, et al. Radiation-associated cardiovascular risks for future deep-space missions. J Radiat Res. 2014;55(Suppl 1):i37–i39. [Google Scholar]
- 15.Sasi SP, Yan X, Lee J, et al. Radiation-associated degenerative cardiovascular risks during normal aging and after adverse CV event 10 months post-initial exposure. J Radiat Res. 2014;55(Suppl 1):i111–i112. [Google Scholar]
- 16.Tungjai M, Whorton EB, Rithidech KN. Persistence of apoptosis and inflammatory responses in the heart and bone marrow of mice following whole-body exposure to (2)(8)Silicon ((2)(8)Si) ions. Radiat Environ Biophys. 2013;52:339–50. doi: 10.1007/s00411-013-0479-4. [DOI] [PubMed] [Google Scholar]
- 17.Rola R, Fishman K, Baure J, et al. Hippocampal neurogenesis and neuroinflammation after cranial irradiation with (56)Fe particles. Radiat Res. 2008;169:626–32. doi: 10.1667/RR1263.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sridharan V, Aykin-Burns N, Tripathi P, et al. Radiation-Induced Alterations in Mitochondria of the Rat Heart. Radiat Res. 2014;181:324–34. doi: 10.1667/RR13452.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boerma M, Wang J, Sridharan V, et al. Pharmacological induction of transforming growth factor-beta1 in rat models enhances radiation injury in the intestine and the heart. PLoS One. 2013;8:e70479. doi: 10.1371/journal.pone.0070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Albertson CM, Zheng H, et al. Short-term inhibition of ADP-induced platelet aggregation by clopidogrel ameliorates radiation-induced toxicity in rat small intestine. Thromb Haemost. 2002;87:122–8. [PubMed] [Google Scholar]
- 21.Wang J, Hauer-Jensen M. Radiation toxicity and proteinase-activated receptors. Drug Dev Res. 2003;60:1–8. [Google Scholar]
- 22.Wang J, Zheng H, Ou X, et al. Hirudin ameliorates intestinal radiation toxicity in the rat: support for thrombin inhibition as strategy to minimize side-effects after radiation therapy and as countermeasure against radiation exposure. J Thromb Haemost. 2004;2:2027–35. doi: 10.1111/j.1538-7836.2004.00960.x. [DOI] [PubMed] [Google Scholar]
- 23.Geiger H, Pawar SA, Kerschen EJ, et al. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med. 2012;18:1123–9. doi: 10.1038/nm.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prabhavathi K, Selvi KT, Poornima KN, et al. Role of biological sex in normal cardiac function and it its disease outcome - a review. J Clin Diagn Res. 2014;8:BE01–4. doi: 10.7860/JCDR/2014/9635.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pecaut MJ, Gridley DS. The impact of mouse strain on iron ion radio-immune response of leukocyte populations. Int J Radiat Biol. 2010;86:409–19. doi: 10.3109/09553000903567995. [DOI] [PubMed] [Google Scholar]
- 26.Lauk S. Endothelial alkaline phosphatase activity loss as an early stage in the development of radiation-induced heart disease in rats. Radiat Res. 1987;110:118–28. [PubMed] [Google Scholar]
- 27.Schultz-Hector S, Balz K. Radiation-induced loss of endothelial alkaline phosphatase activity and development of myocardial degeneration. An ultrastructural study. Lab Invest. 1994;71:252–60. [PubMed] [Google Scholar]
- 28.Fajardo LF. The endothelial cell is a unique target of radiation: an overview. In: Rubin DB, editor. Radiation biology of the vascular endothelium. CRC Press LLC; London: 1998. pp. 1–12. [Google Scholar]
- 29.Grabham P, Sharma P, Bigelow A, et al. Two distinct types of the inhibition of vasculogenesis by different species of charged particles. Vasc Cell. 2013;5:16. doi: 10.1186/2045-824X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma P, Templin T, Grabham P. Short term effects of gamma radiation on endothelial barrier function: uncoupling of PECAM-1. Microvasc Res. 2013;86:11–20. doi: 10.1016/j.mvr.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Bizios R, Lai L, Fenton JW, et al. Thrombin-induced chemotaxis and aggregation of neutrophils. J Cell Physiol. 1986;128:485–90. doi: 10.1002/jcp.1041280318. [DOI] [PubMed] [Google Scholar]
- 32.Chambers RC, Dabbagh K, McAnulty RJ, et al. Thrombin stimulates fibroblast procollagen production via proteolytic activation of protease-activated receptor 1. Biochem J. 1998;333(Pt 1):121–7. doi: 10.1042/bj3330121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esmon CT. Regulation of blood coagulation. Biochim Biophys Acta. 2000;1477:349–60. doi: 10.1016/s0167-4838(99)00266-6. [DOI] [PubMed] [Google Scholar]
- 34.Nzabarushimana E, Prior S, Miouse IR, et al. Combined exposure to protons and 56Fe lead to overexpression of Il13 and reactivation of repetitive elements in the mouse lung. Life Sci Space Res. 2015 doi: 10.1016/j.lssr.2015.08.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coleman MA, Sasi SP, Onufrak J, et al. Low dose radiation affects cardiac physiology: gene networks and molecular signaling in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2015 doi: 10.1152/ajpheart.00050.2015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buonanno M, De Toledo SM, Howell RW, et al. Low-dose energetic protons induce adaptive and bystander effects that protect human cells against DNA damage caused by a subsequent exposure to energetic iron ions. J Radiat Res. 2015;56:502–8. doi: 10.1093/jrr/rrv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.