Abstract
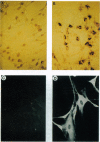
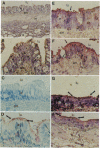
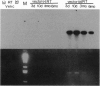
Adeno-associated virus (AAV) vectors expressing the normal cystic fibrosis transmembrane conductance regulator (CFTR) cDNA complement the cystic fibrosis (CF) defect in vitro. Unlike other DNA virus vectors, AAV is a stably integrating virus, which could make possible long-term in vivo complementation of the CF defect in the airway epithelium. We report AAV-CFTR gene transfer and expression after infection of primary CF nasal polyp cells and after in vivo delivery of AAV-CFTR vector to one lobe of the rabbit lung through a fiberoptic bronchoscope. In the rabbit, vector DNA could be detected in the infected lobe up to 6 months after administration. A 26-amino acid polypeptide sequence unique to the recombinant AAV-CFTR protein was used to generate both oligonucleotide probes and a polyclonal antibody which allowed the unambiguous identification of vector RNA and CFTR protein expression. With these reagents, CFTR RNA and protein were detected in the airway epithelium of the infected lobe for up to 6 months after vector administration. AAV vectors do, therefore, efficiently promote in vivo gene transfer to the airway epithelium which is stable over several months. These findings indicate that AAV-CFTR vectors could potentially be very useful for gene therapy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blacklow N. R., Hoggan M. D., Kapikian A. Z., Austin J. B., Rowe W. P. Epidemiology of adenovirus-associated virus infection in a nursery population. Am J Epidemiol. 1968 Nov;88(3):368–378. doi: 10.1093/oxfordjournals.aje.a120897. [DOI] [PubMed] [Google Scholar]
- Carter B. J. Adeno-associated virus vectors. Curr Opin Biotechnol. 1992 Oct;3(5):533–539. doi: 10.1016/0958-1669(92)90082-t. [DOI] [PubMed] [Google Scholar]
- Egan M., Flotte T., Afione S., Solow R., Zeitlin P. L., Carter B. J., Guggino W. B. Defective regulation of outwardly rectifying Cl- channels by protein kinase A corrected by insertion of CFTR. Nature. 1992 Aug 13;358(6387):581–584. doi: 10.1038/358581a0. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Allen E. D., Wilson J. M. Reconstitution of tracheal grafts with a genetically modified epithelium. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11192–11196. doi: 10.1073/pnas.88.24.11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte T. R., Afione S. A., Solow R., Drumm M. L., Markakis D., Guggino W. B., Zeitlin P. L., Carter B. J. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem. 1993 Feb 15;268(5):3781–3790. [PubMed] [Google Scholar]
- Flotte T. R., Solow R., Owens R. A., Afione S., Zeitlin P. L., Carter B. J. Gene expression from adeno-associated virus vectors in airway epithelial cells. Am J Respir Cell Mol Biol. 1992 Sep;7(3):349–356. doi: 10.1165/ajrcmb/7.3.349. [DOI] [PubMed] [Google Scholar]
- Hyde S. C., Gill D. R., Higgins C. F., Trezise A. E., MacVinish L. J., Cuthbert A. W., Ratcliff R., Evans M. J., Colledge W. H. Correction of the ion transport defect in cystic fibrosis transgenic mice by gene therapy. Nature. 1993 Mar 18;362(6417):250–255. doi: 10.1038/362250a0. [DOI] [PubMed] [Google Scholar]
- Kotin R. M., Linden R. M., Berns K. I. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992 Dec;11(13):5071–5078. doi: 10.1002/j.1460-2075.1992.tb05614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotin R. M., Menninger J. C., Ward D. C., Berns K. I. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics. 1991 Jul;10(3):831–834. doi: 10.1016/0888-7543(91)90470-y. [DOI] [PubMed] [Google Scholar]
- Kotin R. M., Siniscalco M., Samulski R. J., Zhu X. D., Hunter L., Laughlin C. A., McLaughlin S., Muzyczka N., Rocchi M., Berns K. I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath S. A., Basu A., Zeitlin P. L. Cystic fibrosis gene and protein expression during fetal lung development. Am J Respir Cell Mol Biol. 1993 Feb;8(2):201–208. doi: 10.1165/ajrcmb/8.2.201. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., Gallery F., MacConnell P., Becker J., Bloch W. An improved technique for the in situ detection of DNA after polymerase chain reaction amplification. Am J Pathol. 1991 Dec;139(6):1239–1244. [PMC free article] [PubMed] [Google Scholar]
- Nuovo G. J., MacConnell P., Forde A., Delvenne P. Detection of human papillomavirus DNA in formalin-fixed tissues by in situ hybridization after amplification by polymerase chain reaction. Am J Pathol. 1991 Oct;139(4):847–854. [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Samulski R. J., Chang L. S., Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989 Sep;63(9):3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R. J., Zhu X., Xiao X., Brook J. D., Housman D. E., Epstein N., Hunter L. A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991 Dec;10(12):3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., West M. H., Sandbank T., Carter B. J. A human parvovirus, adeno-associated virus, as a eucaryotic vector: transient expression and encapsidation of the procaryotic gene for chloramphenicol acetyltransferase. Mol Cell Biol. 1984 Oct;4(10):2072–2081. doi: 10.1128/mcb.4.10.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin P. L., Lu L., Rhim J., Cutting G., Stetten G., Kieffer K. A., Craig R., Guggino W. B. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am J Respir Cell Mol Biol. 1991 Apr;4(4):313–319. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]