Abstract
Leptin is a peptide hormone produced by adipose tissue and acts in brain centers to control critical physiological functions. Leptin receptors are especially abundant in the hypothalamus and trigger specific neuronal subpopulations, and activate several intracellular signaling events, including the JAK/STAT, MAPK, PI3K, and mTOR pathway. Although most studies focus on its role in energy intake and expenditure, leptin also plays a critical role in many central nervous system diseases.
Keywords: Alzheimer’s disease, central nervous system diseases, depression, leptin, Parkinson’s disease, stroke
Leptin
The endocrine hormone leptin is an adipose-derived protein, consisting of 167 amino acid residues, and is encoded by the ob gene on chromosome 6, the murine homolog of the human leptin gene Lep. Leptin enters the brain through saturable, passive transport across the blood–brain barrier 1–3 and then influences a multitude of biological processes, including controlling food intake 4, glucose homeostasis 5, and energy expenditure 6.
Although the adipose tissue is the main source of leptin, it is also produced by other peripheral tissues, such as the stomach 7, mammary gland 8, placenta 9, skeletal muscle, heart 10,11, kidney 12, and the brain 4,13. Therefore, this hormone has a wide range of pleiotropic effects, affecting the cardiovascular, nervous, immune, and reproductive systems 14,15, all of which are dysregulated when the leptin signaling pathways are compromised. In the brain, leptin mRNA expression and immunoreactivity have been observed in the hypothalamus, cortex, dentate gyrus (DG), and the hippocampus of the rat 16.
Indeed, a lack of leptin in mice and humans leads to neuroendocrine dysfunction, including neurodegenerative disease, stroke, and cognitive impairment 17. Recent studies have reported that higher circulating leptin levels are associated with a lower risk of Alzheimer’s disease (AD), and lower circulating levels of leptin have been reported in patients with AD 18–20. Several studies have suggested that Parkinson’s disease (PD) patients have lower BMI and serum leptin levels than controls among the elderly 21,22. It is also known that patients with depression experience weight loss and a decrease in circulating leptin levels 23. Other works have suggested that higher circulating leptin levels increase the risk of vascular disease, such as stroke 24,25.
Leptin receptor
Leptin receptors (ObRs) belong to the class of the I cytokine receptor superfamily. Alternative splicings of the ObRs gene are classified as six leptin receptor forms (ObRa–ObRf), which have an identical N-terminal 26,27. In mice and humans, only five (ObRa–ObRe) and four (ObRa–ObRd) alternative spliced isoforms have been described, respectively 28,29. They all share the same complex extracellular domain, consisting of two cytokine receptor homology (CRH) domains separated by an immunoglobulin-like domain, followed by two membrane proximal fibronectin type III (FN III) domains. The membrane proximal CRH2 domain is necessary and sufficient for leptin binding with an affinity in the nanomolar range 30. The two FN III domains have no affinity for the ligand, but are nevertheless essential for receptor activation as mutation of two conserved cysteines on positions 672 and 751 completely blocks leptin signaling 31. The long isoform ObRb is essential for mediating leptin’s intracellular signal transduction 32. The ObRb is present in several neural tissues, but is mainly expressed in multiple hypothalamic regions including the arcuate nucleus (ARC), the ventromedial hypothalamus, the paraventricular nucleus, the dorsomedial hypothalamus, the lateral hypothalamic area, and the ventral premammillary nucleus 33,34. Leptin’s action on two distinct populations of ARC neurons is well described, one of which is the orexigenic neuropeptides neuropeptide Y (NPY) and agouti-related peptide (AgRP) 35, whereas the other is anorexigenic neuropeptides cocaine and amphetamine-related transcript and pro-opiomelanocortin 36,37. In contrast to ObRb, the short-form leptin receptor ObRa have short C-terminal domains and are considered to be mainly involved in endocytosis and transport of leptin across the blood–brain barrier 38. Whereas ObRe is the only soluble isoform, accumulated evidence showed that it is probably binding circulating leptin and affecting its stability and availability 39.
Leptin signaling pathways
ObRb, which is lacking in db/db mice 40, is expressed in several brain nuclei, with higher expression in the hypothalamic ARC 5. On binding to the ObRb, leptin leads to the activation of several intracellular signaling pathways 41,42, including signal transducer and activator of transcription 3 (STAT3) 43, and activates mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) 29. In addition, the mammalian target of rapamycin (mTOR) has emerged as a key downstream pathway in ObRb signaling and in mediating leptin’s effects 29,44. Whether these short isoforms are still able to bind Janus kinase 2 (JAK2) and signal in vivo is still doubtful as none of the short isoforms mediates activation of JAK2 at physiologic levels of JAK2 45,46.
Leptin and the JAK/STAT3 pathway
The JAK/STATS pathway is one of the best illustrated pathways in leptin signaling 4,47. The binding of leptin to ObRb results in the activation of JAK2. Activated phosphorylated-JAK2 subsequently phosphorylates conserved tyrosine residues of ObRb 48 at Tyr985, Tyr1077, and Tyr1138 49. The ObRb phosphorylated at Tyr1077 and Tyr1138 serves as a docking site and recruits Srchomology 2 (SH2) and Srchomology 3 (SH3) domain comprising proteins such as STAT3 50. The activation of STAT3 induces its dimerization and translocation into the nucleus, where it mediates changes in the expression of several genes, including suppressor of cytokine signaling 3 (SOCS3), an inhibitor of ObRb signaling 51, and coordinates the regulation of food intake and energy homeostasis by altering the expression of NPY, AgRP, and pro-opiomelanocortin 52.
In addition to STAT3, leptin also induces phosphorylation of STAT1, STAT5, and STAT6 in cultured cells 53,54, but only leptin-induced STAT5 phosphorylation in the hypothalamic ARC of mice 55 and STAT5 nuclear translocation in rat hypothalamic nuclei 56 were detected.
Leptin and the MAPK pathway
Leptin modulates the phosphorylation of ObRb tyrosine residues that activate MAPK. Then, MAPK activates cAMP response element-binding (CREB) protein, which has an antiapoptotic effect on the cell. Recent studies suggest that leptin could mediate neuroprotective effects on dopaminergic cells through the MAPK/CREB pathway in the central nervous system (CNS) 28. Leptin induces phosphorylation of Tyr985 in the ObRb, thereby creating a binding site for the carboxyterminal SH2 domain of SH2-containing protein tyrosine phosphatase 2 (SHP2) 57. Phosphorylated SHP2 then recruits the adaptor protein to induce extracellular signal-regulated kinase (ERK), a member of the MAPK family 58. Stimulation of ERK by leptin can also be achieved by direct interaction with JAK2 4. ERK-dependent upregulation of the immediate early genes egr-1 and c-fos has been shown in cell culture and in vivo in the hypothalamus 57,59. The physiological importance of the MAPK pathway is underscored by the observations that pharmacological inhibition of ERK1/2 in the hypothalamus reverses the anorectic and weight-reducing effects of leptin 60.
Other members of the MAPK family such as p38 and JNK have also been reported to be activated by leptin in several cell types 61, but the associated pathways have not been well characterized.
Leptin and the PI3K/Akt pathway
The PI3K/Akt pathway was found to be the critical pathway for the mediation of leptin-induced neuroprotection 62. ObRb activation induces phosphorylation of several members of the insulin receptor substrate (IRS) family and then IRS in turn recruits the regulatory p85 subunit and activates PI3K, and leads to sequential activation of Akt. The hypothalamic PI3K pathway of leptin signaling is impaired during the development of diet-induced obesity 63,64 and pharmacological inhibition of PI3K activity blocks the anorectic effect of leptin 5. Leptin and insulin may act in coordination to control energy homeostasis 65 as their intracellular signalings converge at the PI3K pathway. Although the relative contributions of leptin in functional hypothalamic signaling are difficult to assess, the importance of the PI3K pathway is clear.
Leptin and the mTOR pathway
The mTOR protein is a highly conserved serine–threonine kinase that regulates cell-cycle progression and growth by sensing changes in energy status. The mTOR signaling is critically involved in the regulation of several cellular functions and plays a key role in the CNS regulation of energy balance and peripheral metabolism 66. Leptin increases hypothalamic mTOR activity and inhibition of mTOR signaling by rapamycin blunts leptin’s anorectic effect 67. Systemic deletion of the ribosomal p70S6 kinase, a major physiological downstream effector of mTOR, alleviatives leptin’s acute anorexigenic action 68.
Leptin in central nervous system disease
All the above evidence suggests that leptin-binding ObRb initiates the main intracellular signaling passways to play a protective role in CNS (Fig. 1). To what extent can leptin salvage neurons affected by the pathophysiological processes of diseases involving not only neurodegeneration but also acute cerebral ischemia–reperfusion (I/R) injury? If it can be shown to be leptin-induced neuroprotection, what is the functional outcome of leptin treatment on diseases such as AD, PD, depression, and acute cerebral I/R injury?
Fig. 1.
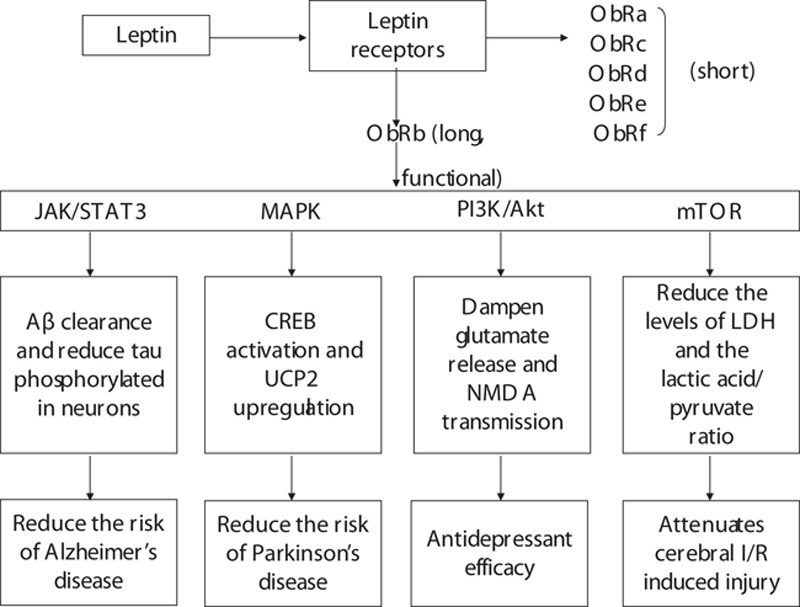
Summary of intracellular molecular signaling pathways of leptin and their possible effects for central nervous system diseases. See text for more information. CREB, cAMP response element-binding; I/R, ischemia–reperfusion; LDH, lactate dehydrogenase; MAPK, mitogen-activated protein kinase, mTOR, mammalian target of rapamycin; NMDA, N-methyl-d-aspartate; ObR, leptin receptor, UCP2, uncoupling protein-2.
Leptin in Alzheimer’s disease
AD is a progressive neurodegenerative disorder resulting in neurological deficits including memory loss and diminished cognitive function, making it the most common neurological condition in the USA 69. The brains of patients with AD, in addition to showing nerve and synapse loss, are histopathologically characterized by two hallmark lesions, amyloid-β (Aβ) 70 and neurofibrillary tangles, which are composed of hyperphosphorylated forms of the tau protein 71,72. Normally an abundant soluble protein in axons, the tau is a microtubule-associated protein that promotes assembly and stability of microtubules and vesicle transport 73. In light of the common belief that the abnormal deposition of both Aβ and neurofibrillary tangles is critical for the pathobiology of AD, it has been shown that leptin plays a role in reversing both pathological hallmarks of AD and results in better neurological outcomes for the disease state 74.
Leptin receptors are particularly vulnerable in AD 22. Leptin treatment of neuronal cells reduces the amount of Aβ secreted into the medium in a time-dependent and dose-dependent manner 75,76. Furthermore, leptin promotes ApoE-driven uptake of Aβ into neurons 77. Modulation of the tau protein phosphorylation by leptin represents a significant pathway for protection against AD pathology. Moreover, growing evidence indicates that leptin prevents the toxic accumulation of Aβ and phosphorylated tau in neurons and it has the ability to improve performance in various memory tasks in murine AD models 78.
Recent clinical research has shown that individuals with higher serum leptin levels have a much lower risk of developing AD in line with rodent models and cellular studies 20,79. Moreover, leptin levels are also significantly reduced in rodent models of AD 70. Direct injection of leptin into the hippocampus of rodents can improve memory processing and modulate long-term potentiation and synaptic plasticity 80. Recent studies have shown the potential beneficial effects of leptin as an AD therapeutic 81. Taken together, our preclinical data, showing that leptin ameliorates both Aβ-related and tau-related pathologies, along with its pharmacological profile, support its use as a novel therapeutic for AD 49.
Leptin in Parkinson’s disease
PD, following AD, is the second most common neurodegenerative disease. Epidemiological studies using 2010 US census estimates have estimated that ∼630 000 PD were diagnosed in the USA in 2010 82. PD is characterized clinically by a classic tetrad of motor symptoms: low-frequency resting tremor, rigidity of the skeletal muscles of the face and hands, reduced motor activity (bradykinesia), and in later stages of the disorder, postural instability 83. As reported earlier, leptin has been found to promote the survival of neuroblastoma and neural dopaminergic cells against 1-methyl-4-pyridinium (MPP+) toxicity (dopamine cell-specific neurotoxins commonly used in experimental Parkinsonian models) by maintaining ATP levels and mitochondrial membrane potential 84. Leptin was shown to protect the neuroblastoma cells through a PI3K/Akt-dependent pathway 85, altered Akt, and its downstream target glycogen synthase kinase-3β (GSK-3β) in depression. Meanwhile, a MEK/ERK1/2-induced increase in CREB activation preserved dopaminergic cell survival in proapoptotic conditions 85,86. In-vivo experimentation showed that up to 2 months after neurotoxin exposure, motor behavior is salvaged in leptin-treated animals compared with controls in degeneration of dopaminergic neurons’ environments in part through preservation of nigrostriatal functionality. Furthermore, leptin treatment increased the expression in neuroblastoma cells of mitochondrial uncoupling protein-2 (UCP2) and uncoupling protein-4 (UCP4), both vital to the reduction of oxidative stress in the mitochondria 84. UCP2 knockdown cells were shown to lose the protective effects of leptin when challenged with MPP+. The present study showed that leptin rescued dopaminergic neurons, reversed behavioral asymmetry, and restored striatal catecholamine levels in the unilateral 6-hydroxydopamine (6-OHDA) mouse model of dopaminergic cell death 85,87.
In PD patients who experience unintentional weight loss, circulating leptin levels have been found to be lower than in weight-stable PD patients, with lowered leptin levels consistent with reduced body fat 73. The weight-loss associated with PD would then result in less stored adipose, and thus lowered serum leptin levels 83. This scenario is an example of the association between low leptin levels in the brain and pathogenesis of neurodegenerative disease. Basic science findings suggest that leptin can reduce or prevent neuronal apoptosis induced by a variety of pathological conditions and could result in better functional outcomes for neurodegenerative disease states, especially those associated with obesity and metabolic disorders 22.
Leptin in depression disease
Depression is a chronic and debilitating mental illness with a 17% lifetime prevalence and is a major cause of morbidity, disability, and mortality 88. Currently available pharmacologic treatments for depression primarily target monoamine systems 72. Systemic administration of leptin exerts antidepressant-like behavioral effects in male rats and mice. Several lines of evidence suggest that leptin exerts its antidepressant-like effects by activating ObRb in the hippocampus. First, direct infusion of leptin into the DG of the hippocampus induces an antidepressant-like effect 89. Second, deletion of ObRb from the hippocampus causes depression-like behaviors and attenuates leptin’s antidepressant-like effects 89,90. Third, blockade of leptin signaling in the DG reverses the antidepressant-like effects of leptin. These findings support an important role of leptin actions on mood-related behavior.
Compelling evidence supports the important role of the glutamatergic system in the pathophysiology of major depression and also as a target for rapid-acting antidepressants 91. Blockade of N-methyl-d-aspartate (NMDA) receptors by intra-CA3 infusion of MK-801 (a noncompetitive NMDA receptor antagonist) reversed behavioral despair 78. A subpopulation of granule neurons that innervated the CA3 region expressed leptin receptors and these cells were not activated by stress. Leptin treatment dampened tail suspension-evoked glutamate release in CA3 92. However, intra-CA3 of the hippocampus infusion of NMDA blocked the antidepressant-like effect of leptin in reversing behavioral despair in both the tail-suspension tests and forced-swim tests, which involved activation of Akt signaling in DG 89. Results suggest that the DG–CA3 glutamatergic pathway is critical for mediating behavioral despair and antidepressant-like responses to leptin in the hippocampus 92. Elevating leptin signaling in brain represents a novel approach for the treatment of depressive disorders.
Leptin in stroke
A stroke occurs when blood flow to the brain is interrupted, either by a blockage or by a burst vessel. Stroke is responsible for roughly one-tenth of deaths, making it the second most common cause after heart disease worldwide 93. Acute ischemic stroke injuries to brain tissue are among the leading causes of death and long-term disability in humans. The pathophysiologic mechanisms of cerebral I/R injury are primarily related to the energy deficiency of neurons, cell excitatory responses, inflammation, and the start of the apoptosis cascade 62. Recent research suggested that leptin decreases tissue lactate dehydrogenase levels and thereby decreases the lactic acid/pyruvate ratio, resulting in a mitigation of acidosis because of anaerobic metabolism within the brain. This effect is reversed by LY294002, indicating that the PI3K/Akt signaling pathway plays a critical role in leptin-mediated neuroprotection 94. Research shows that the neuroprotection exerted by leptin in a rat model of permanent focal cerebral ischemia is associated with modulation of STAT3 phosphorylation in different cellular populations of the injured brain 95. The impressive positive effects of leptin administration in rodent models of ischemic stroke are promising and of potential therapeutic value to humans 94. More robust clinical and scientific studies are necessary before leptin can be used in clinical practice.
Conclusion
The discovery of leptin 16 years ago was a major breakthrough. Beyond its role in glucose homeostasis and energy balance, leptin has been found to be an important protective factor contributing toward reproductive function, bone metabolism, and neuroplasticity. A large and growing basic research supports the hypothesis that leptin plays a critical role in neuroprotection. Although recent literature describes the mode of action of leptin, it remains to be seen as to how the disease-modifying effects of the hormone in preclinical trials will translate into a potential therapeutic for patients with neuroendocrine dysfunction.
Acknowledgements
This work was supported by Shandong Provincial Natural Science Foundation, China (2014ZRB14157, 2014ZRB14362) and National Natural Science Foundation of China (81500930).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372:425–432. [DOI] [PubMed] [Google Scholar]
- 2.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1995; 1:1155–1161. [DOI] [PubMed] [Google Scholar]
- 3.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides 1996; 17:305–311. [DOI] [PubMed] [Google Scholar]
- 4.Shan X, Yeo GS. Central leptin and ghrelin signalling: comparing and contrasting their mechanisms of action in the brain. Rev Endocr Metab Disord 2011; 12:197–209. [DOI] [PubMed] [Google Scholar]
- 5.Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev 2011; 91:389–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jéquier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci 2002; 967:379–388. [DOI] [PubMed] [Google Scholar]
- 7.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is a source of leptin. Nature 1998; 394:790–793. [DOI] [PubMed] [Google Scholar]
- 8.Smith-Kirwin SM, O’Connor DM, De Johnston J, Lancey ED, Hassink SG, Funanage VL. Leptin expression in human mammary epithelial cells and breast milk. J Clin Endocrinol Metab 1998; 83:1810–1813. [DOI] [PubMed] [Google Scholar]
- 9.Tessier DR, Ferraro ZM, Gruslin A. Role of leptin in pregnancy: consequences of maternal obesity. Placenta 2013; 34:205–211. [DOI] [PubMed] [Google Scholar]
- 10.Feijoo-Bandin S, Portoles M, Rosello-Lleti E, Rivera M, Gonzalez-Juanatey JR, Lago F. 20 years of leptin: role of leptin in cardiomyocyte physiology and physiopathology. Life Sci 2015; 140:10–18. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Luo W, Eitzman DT. Leptin in thrombosis and atherosclerosis. Curr Pharm Des 2014; 20:641–645. [DOI] [PubMed] [Google Scholar]
- 12.Lin TC, Lee TC, Hsu SL, Yang CS. The molecular mechanism of leptin secretion and expression induced by aristolochic acid in kidney fibroblast. PLoS One 2011; 6:e16654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson M, Morash B, Ur E. The brain is a source of leptin. Front Horm Res 2000; 26:106–125. [DOI] [PubMed] [Google Scholar]
- 14.Procaccini C, Jirillo E, Matarese G. Leptin as an immunomodulator. Mol Aspects Med 2012; 33:35–45. [DOI] [PubMed] [Google Scholar]
- 15.Donato J, Jr, Cravo RM, Frazão R, Elias CF. Hypothalamic sites of leptin action linking metabolism and reproduction. Neuroendocrinology 2011; 93:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li HY, Wang LL, Yeh RS. Leptin immunoreactivity in the central nervous system in normal and diabetic rats. Neuroreport 1999; 10:437–442. [DOI] [PubMed] [Google Scholar]
- 17.Power DA, Noel J, Collins R, O’Neill D. Circulating leptin levels and weight loss in Alzheimer’s disease patients. Dement Geriatr Cogn Disord 2001; 12:167–170. [DOI] [PubMed] [Google Scholar]
- 18.Ge JF, Qi CC, Zhou JN. Imbalance of leptin pathway and hypothalamus synaptic plasticity markers are associated with stress-induced depression in rats. Behav Brain Res 2013; 249:38–43. [DOI] [PubMed] [Google Scholar]
- 19.Johnston JM, Greco SJ, Hamzelou A, Ashford JW, Tezapsidis N. Repositioning leptin as a therapy for Alzheimer’s disease. Therapy 2011; 8:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly: findings from the health ABC study. Neurobiol Aging 2009; 30:1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evidente VG, Caviness JN, Adler CH, Gwinn-Hardy KA, Pratley RE. Serum leptin concentrations and satiety in Parkinson's disease patients with and without weight loss. Mov Disord 2001; 16:924–927. [DOI] [PubMed] [Google Scholar]
- 22.Fiszer U, Michałowska M, Baranowska B, Wolińska-Witort E, Jeske W, Jethon M, et al. Leptin and ghrelin concentrations and weight loss in Parkinson’s disease. Acta Neurol Scand 2010; 121:230–236. [DOI] [PubMed] [Google Scholar]
- 23.Akter S, Pham NM, Nanri A, Kurotani K, Kuwahara K, Jacka FN, et al. Association of serum leptin and ghrelin with depressive symptoms in a Japanese working population: a cross-sectional study. BMC Psychiatry 2014; 14:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Butler KR, Buxbaum SG, Sung JH, Campbell BW, Taylor HA. Leptinemia and its association with stroke and coronary heart disease in the Jackson heart study. Clin Endocrinol (Oxf) 2010; 72:32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim BJ, Lee SH, Ryu WS, Kim CK, Yoon BW. Adipocytokines and ischemic stroke: differential associations between stroke subtypes. J Neurol Sci 2012; 312 (1–2):117–122. [DOI] [PubMed] [Google Scholar]
- 26.Tartaglia LA. The leptin receptor. J Biol Chem 1997; 272:6093–6096. [DOI] [PubMed] [Google Scholar]
- 27.Tanida M, Yamamoto N, Morgan DA, Kurata Y, Shibamoto T, Rahmouni K. Leptin receptor signaling in the hypothalamus regulates hepatic autonomic nerve activity via phosphatidylinositol 3-kinase and AMP-activated protein kinase. J Neurosci 2015; 35 (1–2):474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cioffi JA, Shafer AW, Zupancic TJ, Smith-Gbur J, Mikhail A, Platika D, Snodgrass HR. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat Med 1996; 2:585–589. [DOI] [PubMed] [Google Scholar]
- 29.Wauman J, Tavernier J. Leptin receptor signaling: pathways to leptin resistance. Front Biosci (Landmark Ed) 2011; 16:2771–2793. [DOI] [PubMed] [Google Scholar]
- 30.Fong TM, Huang RR, Tota MR, Mao C, Smith T, Varnerin J, et al. Localization of leptin binding domain in the leptin receptor. Mol Pharmacol 1998; 53:234–240. [DOI] [PubMed] [Google Scholar]
- 31.Prokop JW, Duff RJ, Ball HC, Copeland DL, Londraville RL. Leptin and leptin receptor: analysis of a structure to function relationship in interaction and evolution from humans to fish. Peptides 2012; 38:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guadalupe-Grau A, Larsen S, Guerra B, Calbet JA, Dela F, Helge JW. Influence of age on leptin induced skeletal muscle signalling. Acta Physiol (Oxf) 2014; 211:214–228. [DOI] [PubMed] [Google Scholar]
- 33.Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, et al. Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci 2010; 30:5713–5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab 2011; 14:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tavares E, Maldonado R, Miñano FJ. Aminoprocalcitonin-mediated suppression of feeding involves the hypothalamic melanocortin system. Am J Physiol Endocrinol Metab 2013; 304:E1251–E1262. [DOI] [PubMed] [Google Scholar]
- 36.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1998; 1:271–272. [DOI] [PubMed] [Google Scholar]
- 37.Lemus MB, Bayliss JA, Lockie SH, Santos VV, Reichenbach A, Stark R, Andrews ZB. A stereological analysis of NPY, POMC, Orexin, GFAP astrocyte, and Iba1 microglia cell number and volume in diet-induced obese male mice. Endocrinology 2015; 156:1701–1713. [DOI] [PubMed] [Google Scholar]
- 38.Murakami T, Yamashita T, Iida M, Kuwajima M, Shima K. A short form of leptin receptor performs signal transduction. Biochem Biophys Res Commun 1997; 231:26–29. [DOI] [PubMed] [Google Scholar]
- 39.Basharat S, Parker JA, Murphy KG, Bloom SR, Buckingham JC, John CD. Leptin fails to blunt the lipopolysaccharide-induced activation of the hypothalamic–pituitary–adrenal axis in rats. J Endocrinol 2014; 221:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996; 84:491–495. [DOI] [PubMed] [Google Scholar]
- 41.Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 2008; 70:537–556. [DOI] [PubMed] [Google Scholar]
- 42.Coppari R, Bjørbæk C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov 2012; 11:692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Wu R, Chen HZ, Xiao Q, Wang WJ, He JP, et al. Enhancement of hypothalamic STAT3 acetylation by nuclear receptor Nur77 dictates leptin sensitivity. Diabetes 2015; 64:2069–2081. [DOI] [PubMed] [Google Scholar]
- 44.Fazolini NP, Cruz AL, Werneck MB, Viola JP, Maya-Monteiro CM, Bozza PT. Leptin activation of mTOR pathway in intestinal epithelial cell triggers lipid droplet formation, cytokine production and increased cell proliferation. Cell Cycle 2015; 14:2667–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bahrenberg G, Behrmann I, Barthel A, Hekerman P, Heinrich PC, Joost HG, Becker W. Identification of the critical sequence elements in the cytoplasmic domain of leptin receptor isoforms required for Janus kinase/signal transducer and activator of transcription activation by receptor heterodimers. Mol Endocrinol 2002; 16:859–872. [DOI] [PubMed] [Google Scholar]
- 46.Cui MY, Hu CK, Pelletier C, Dziuba A, Slupski RH, Li C, Denver RJ. Ancient origins and evolutionary conservation of intracellular and neural signaling pathways engaged by the leptin receptor. Endocrinology 2014; 155:4202–4214. [DOI] [PubMed] [Google Scholar]
- 47.Doherty GH, Oldreive C, Harvey J. Neuroprotective actions of leptin on central and peripheral neurons in vitro. Neuroscience 2008; 154:1297–1307. [DOI] [PubMed] [Google Scholar]
- 48.Bjørbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem 1997; 272:32686–32695. [DOI] [PubMed] [Google Scholar]
- 49.Marwarha G, Ghribi O. Leptin signaling and Alzheimer’s disease. Am J Neurodegener Dis 2012; 1:245–265. [PMC free article] [PubMed] [Google Scholar]
- 50.Robertson S, Ishida-Takahashi R, Tawara I, Hu J, Patterson CM, Jones JC, et al. Insufficiency of Janus kinase 2-autonomous leptin receptor signals for most physiologic leptin actions. Diabetes 2010; 59:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bjørbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem 1999; 274:30059–30065. [DOI] [PubMed] [Google Scholar]
- 52.Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Jr, Rossetti L. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab 2006; 4:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci USA 1996; 93:6231–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang P, Yang FJ, Du H, Guan YF, Xu TY, Xu XW, et al. Involvement of leptin receptor long isoform (LepRb)-STAT3 signaling pathway in brain fat mass- and obesity-associated (FTO) downregulation during energy restriction. Mol Med 2011; 17 (5–6):523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Münzberg H, Myers MG., Jr The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem 2007; 282:31019–31027. [DOI] [PubMed] [Google Scholar]
- 56.Mütze J, Roth J, Gerstberger R, Hübschle T. Nuclear translocation of the transcription factor STAT5 in the rat brain after systemic leptin administration. Neurosci Lett 2007; 417:286–291. [DOI] [PubMed] [Google Scholar]
- 57.Bjørbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem 2001; 276:4747–4755. [DOI] [PubMed] [Google Scholar]
- 58.Sun Z, Dragon S, Becker A, Gounni AS. Leptin inhibits neutrophil apoptosis in children via ERK/NF-κB-dependent pathways. PLoS One 2013; 8:e55249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui H, Cai F, Belsham DD. Leptin signaling in neurotensin neurons involves STAT, MAP kinases ERK1/2, and p38 through c-Fos and ATF1. FASEB J 2006; 20:2654–2656. [DOI] [PubMed] [Google Scholar]
- 60.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes 2009; 58:536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ge YC, Li JN, Ni XT, Guo CM, Wang WS, Duan T, Sun K. Cross talk between cAMP and p38 MAPK pathways in the induction of leptin by hCG in human placental syncytiotrophoblasts. Reproduction 2011; 142:369–375. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Deng Z, Liao J, Song C, Liang C, Xue H, et al. Leptin attenuates cerebral ischemia injury through the promotion of energy metabolism via the PI3K/Akt pathway. J Cereb Blood Flow Metab 2013; 33:567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Metlakunta AS, Sahu M, Sahu A. Hypothalamic phosphatidylinositol 3-kinase pathway of leptin signaling is impaired during the development of diet-induced obesity in FVB/N mice. Endocrinology 2008; 149:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Myers MG., Jr Leptin keeps working, even in obesity. Cell Metab 2015; 21:791–792. [DOI] [PubMed] [Google Scholar]
- 65.Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 2015; 160 (1–2):88–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haissaguerre M, Saucisse N, Cota D. Influence of mTOR in energy and metabolic homeostasis. Mol Cell Endocrinol 2014; 397 (1–2):67–77. [DOI] [PubMed] [Google Scholar]
- 67.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science 2006; 312:927–930. [DOI] [PubMed] [Google Scholar]
- 68.Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab 2008; 8:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang MC, Lung FW. Neuroprotection of paliperidone on SH-SY5Y cells against β-amyloid peptide(25–35), N-methyl-4-phenylpyridinium ion, and hydrogen peroxide-induced cell death. Psychopharmacology (Berl) 2011; 217:397–410. [DOI] [PubMed] [Google Scholar]
- 70.Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer’s Abeta. FASEB J 2004; 18:1870–1878. [DOI] [PubMed] [Google Scholar]
- 71.Ittner LM, Götz J. Amyloid-β and tau – a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci 2011; 12:65–72. [DOI] [PubMed] [Google Scholar]
- 72.Guo M, Lu Y, Garza JC, Li Y, Chua SC, Zhang W, et al. Forebrain glutamatergic neurons mediate leptin action on depression-like behaviors and synaptic depression. Transl Psychiatry 2012; 2:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lorefält B, Toss G, Granérus AK. Weight loss, body fat mass, and leptin in Parkinson’s disease. Mov Disord 2009; 24:885–890. [DOI] [PubMed] [Google Scholar]
- 74.Magalhães CA, Carvalho MG, Sousa LP, Caramelli P, Gomes KB. Leptin in Alzheimer’s disease. Clin Chim Acta 2015; 450:162–168. [DOI] [PubMed] [Google Scholar]
- 75.Greco SJ, Sarkar S, Johnston JM, Tezapsidis N. Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells. Biochem Biophys Res Commun 2009; 380:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, et al. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis 2010; 19:1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greco SJ, Sarkar S, Johnston JM, Zhu X, Su B, Casadesus G, et al. Leptin reduces Alzheimer's disease-related tau phosphorylation in neuronal cells. Biochem Biophys Res Commun 2008; 376:536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zarate C, Jr, Machado-Vieira R, Henter I, Ibrahim L, Diazgranados N, Salvadore G. Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry 2010; 18:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oania R, McEvoy LK. Plasma leptin levels are not predictive of dementia in patients with mild cognitive impairment. Age Ageing 2015; 44:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci USA 2006; 103:1593–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beccano-Kelly D, Harvey J. Leptin: a novel therapeutic target in Alzheimer’s disease? Int J Alzheimers Dis 2012; 2012:594137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson’s disease in the United States. Mov Disord 2013; 28:311–318. [DOI] [PubMed] [Google Scholar]
- 83.Ozdilek B, Kenangil G. Serum leptin concentrations in Turkish Parkinson’s disease population. Parkinsons Dis 2014; 2014:576020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ho PW, Liu HF, Ho JW, Zhang WY, Chu AC, Kwok KH, et al. Mitochondrial uncoupling protein-2 (UCP2) mediates leptin protection against MPP+ toxicity in neuronal cells. Neurotox Res 2010; 17:332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weng Z, Signore AP, Gao Y, Wang S, Zhang F, Hastings T, et al. Leptin protects against 6-hydroxydopamine-induced dopaminergic cell death via mitogen-activated protein kinase signaling. J Biol Chem 2007; 282:34479–34491. [DOI] [PubMed] [Google Scholar]
- 86.Morroni F, Sita G, Tarozzi A, Cantelli-Forti G, Hrelia P. Neuroprotection by 6-(methylsulfinyl)hexyl isothiocyanate in a 6-hydroxydopamine mouse model of Parkinson’s disease. Brain Res 2014; 1589:93–104. [DOI] [PubMed] [Google Scholar]
- 87.Lu X, Kim-Han JS, Harmon S, Sakiyama-Elbert SE, O’Malley KL. The Parkinsonian mimetic, 6-OHDA, impairs axonal transport in dopaminergic axons. Mol Neurodegener 2014; 9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, Meader N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol 2011; 12:160–174. [DOI] [PubMed] [Google Scholar]
- 89.Paz-Filho G, Wong ML, Licinio J. Leptin levels and Alzheimer disease. JAMA 2010; 303:1478. [DOI] [PubMed] [Google Scholar]
- 90.Guo M, Huang TY, Garza JC, Chua SC, Lu XY. Selective deletion of leptin receptors in adult hippocampus induces depression-related behaviours. Int J Neuropsychopharmacol 2013; 16:857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 2013; 73:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X, Zhang D, Lu XY. Dentate gyrus-CA3 glutamate release/NMDA transmission mediates behavioral despair and antidepressant-like responses to leptin. Mol Psychiatry 2015; 20:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brambilla R, Couch Y, Lambertsen KL. The effect of stroke on immune function. Mol Cell Neurosci 2013; 53:26–33. [DOI] [PubMed] [Google Scholar]
- 94.Savopoulos C, Michalakis K, Apostolopoulou M, Miras A, Hatzitolios A. Adipokines and stroke: a review of the literature. Maturitas 2011; 70:322–327. [DOI] [PubMed] [Google Scholar]
- 95.Amantea D, Tassorelli C, Russo R, Petrelli F, Morrone LA, Bagetta G, Corasaniti MT. Neuroprotection by leptin in a rat model of permanent cerebral ischemia: effects on STAT3 phosphorylation in discrete cells of the brain. Cell Death Dis 2011; 2:e238. [DOI] [PMC free article] [PubMed] [Google Scholar]


