Abstract
Mounting evidence from epidemiology studies suggests that whole grain intake may reduce pancreatic cancer risk, but convincing evidence is scarce. We conducted a meta-analysis to assess the association between whole grain intake and pancreatic cancer risk.
Relevant observational studies were identified by searching PubMed, Embase, Scopus, and Cochrane library databases for the period from January 1980 to July 2015, with no restrictions. We calculated the summary odds ratios (ORs) for pancreatic cancer using random-effects model meta-analysis. Between-study heterogeneity was analyzed using the I2 statistic.
A total of 8 studies regarding whole grain intake were included in the meta-analysis. The pooled OR of pancreatic cancer for those with high versus low whole grain intake was 0.76 (95% confidence interval [CI], 0.64–0.91; P = 0.002). There was no significant heterogeneity across these studies (I2 = 11.7%; Pheterogeneity = 0.339). In the subgroup analysis by geographic area, the summary ORs of developing pancreatic cancer were 0.64 (95% CI, 0.53–0.79; P < 0.001; I2 = 0%; Pheterogeneity = 0.482) in the United States (n = 4) and 0.95 (95% CI, 0.63–1.43; P = 0.803; I2 = 45.6%; Pheterogeneity = 0.175) in Europe (n = 2). In the subgroup analysis by type of whole grain, the summary ORs were 0.72 (95% CI, 0.60–0.87; P = .001; I2 = 0; Pheterogeneity = 0.876) for grains (n = 4) and 0.74 (95% CI, 0.27–2.02; P = 0.554; I2 = 86.3%; Pheterogeneity = 0.007) for wheat (n = 2).
A high intake of whole grains was associated with a reduced risk of pancreatic cancer. Because of the absent of more cohort studies, further prospective studies need to be conducted to ensure conclusions that are more robust.
INTRODUCTION
Pancreatic cancer (PC) is the seventh most common cause of cancer mortality, with 330,000 deaths per year worldwide, accounting for about 4.0% of all cases of cancer.1 Evidence from epidemiologic data has suggested that uneven dietary intake is a major important etiological factor of PC, and high intakes of red and processed meats and low consumptions of vegetables, fruits, and dietary fiber are considered to be dietary risk factors for this disease.2–4
In the 1990s, Howe initially proposed the hypothesis that dietary fiber should be a biologically independent protective factor of PC, based on the findings of low PC cancer rates among Canadians who have high dietary fiber intakes. Some plausible explanations for this hypothesis may be that dietary fiber intakes alter cytokine production and modulate inflammation,5 counter the carcinogenic effects of N-nitroso compounds,6 and affect the intestinal immune system.7 Whole grain, which include the bran, germ, and endosperm, is a primary source of dietary fiber and appears to be associated with a reduced risk of various types of cancer. In addition, the anticarcinogenic properties, which are based on the content of dietary fiber, folate, and various antioxidants, are purported to play a protective role in pancreatic carcinogenesis.8,9 In a systematic review and meta-analysis of 40 case-control studies of 20 cancers and colon polyps conducted in 1998 provided earlier evidence that a whole grain intake protects against multifarious cancer.10 A summary odds ratio (OR) of 0.7 for reducing PC risk in those with a high versus low intake of whole grains was reported among 4 studies in this review. The updated series of case-control studies in Italy could be used to confirm the beneficial outcomes of whole grain foods on the incidence of the most human cancers, and diets high in whole grains might have a favorable role in PC.11 In contrast, the relationship between diet and PC risk were investigated in 3 case-control studies by Gold et al12 Soler et al13 and Chatenoud et al,14 which unanimously indicated that high intakes of whole grain foods could not reduce the risk of PC. Similar controversial results were also observed in one prospective cohort studies, which showed no consistent pattern for the protective effect between wheat products and PC risk.15
Although a previous meta-analysis was conducted to examine whole grains and PC risk in 1998, the evidence from epidemiologic surveys has still been controversial in recent years. Additionally, increasing results regarding whole grain intake and PC risk have been published during the past decade; however, these need to be updated to ensure conclusions that are more robust. Therefore, we tested the hypothesis that whole grain intake is associated with PC risk by carrying out a meta-analysis of case-control and cohort studies.
MATERIALS AND METHODS
Search Strategy and Eligibility Criteria
We performed a literature search using PubMed, Embase, Scopus, and Cochrane library databases from January 1980 to July 2015 for all observational studies in which the relationship between whole grain consumption and PC risk was assessed. The studies were identified using the following Keywords: whole grain; whole wheat; grain; wheat; brown rice; cereals; barley; rye; oat; maize; corn; sorghum; PC; pancreatic neoplasms; and pancreatic tumor. In addition; we searched and systematically examined the list of references from relevant articles. Studies were included in our meta-analysis if they met the following criteria: published in English language; case-control or cohort study; the exposure was whole grain or whole wheat foods; the outcome was PC; and relative risk (RR), OR, or hazard ratio (HR) estimates with 95% confidence intervals (CIs) were reported or could be estimated based on data provided in the original articles or other relevant study. If duplicate data were published or study populations were the same; we included only the study with the largest number of sample size. Because our study was based on previous published data; therefore, we did not need to obtain ethical approval or informed consent. Additionally, the included studies in our review cited did in fact got patient consent and that each study was approved by an ethics committee.
Data Extraction and Quality Assessment
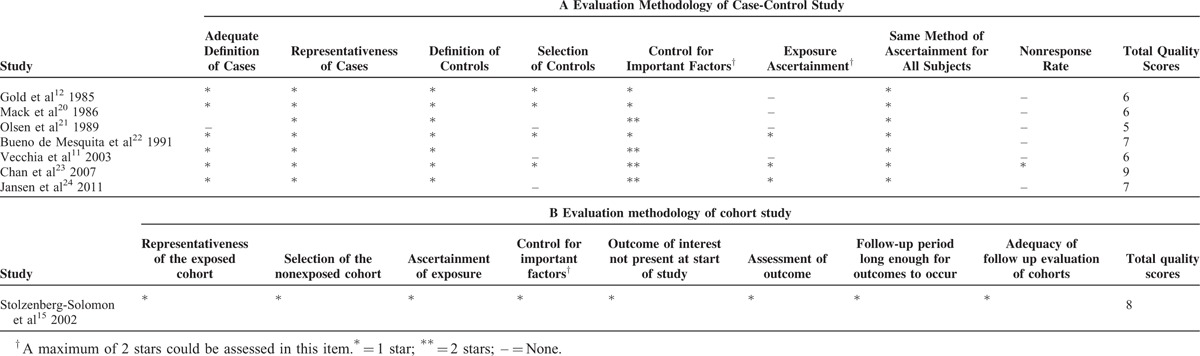
Data extraction was performed independently by 6 authors (QL, HZ, JB, FT, MX, and TJ) and quality assessment was conducted by 3 authors (QL, HZ, and JB); any disagreements were resolved by consensus and discussion among research team members. The following data were extracted from each included study: the first author's last name; publication year; type of study design; country where the study was conducted; study period; the age and sex of participants; sample size (number of cases and controls); type of whole grains; measurement of exposure; ORs, RRs, or HRs with 95% CIs; and variables matched or adjusted for the analysis. We also contacted the authors through e-mail when additional unpublished information about their studies was required for analysis. If the information was still unavailable due to nonresponse from the corresponding author or data loss, only the available results in the article were reported. Quality assessment of each study was conducted using the Newcastle–Ottawa Scale (NOS),16 which involves 2 different tools for case-control and cohort studies, and 3 aspects of study characteristics were evaluated (ie, selection, comparability, and outcome/exposure assessment). The full score was 9 stars, and high and low quality studies were defined as studies with ≥7 stars and ≤6 stars, respectively.
Statistical Analysis
The data analysis was performed using Stata software, version 12.0 (Stata, College Station, TX). The relationship between whole grain intake and PC risk was evaluated using the summary ORs. Based on Greenland's study17 and due to the low prevalence of PC, the RRs, and HRs were considered equivalent to the ORs. The summary ORs with the 95% CIs were combined using the random-effects model. Heterogeneity among studies was assessed using the I2 statistics, and a P value below 0.10 was considered significant.18 The subgroup analyses were performed according to the study design, geographic area, type of whole grains, gender, control type, study quality, and adjustments factors. Sensitivity analyses, omitting 1 study at a time, were performed to evaluate whether the pooled result was credible and stable.19 All other P values were 2-sided and P values below 0.05 were considered statistically significant.
RESULTS
Literature Search and Study Characteristics
The detailed steps of the literature search are shown in Figure 1. In total, 112 studies were originally identified from the above-mentioned databases; 106 articles were excluded based on the inclusion criteria and the remaining 6 studies were used for further review. Four studies were additionally identified form the references of one study.10 Afterward, the full text of 10 studies were systematically reviewed. There were no reported 95% CIs in 2 articles,12,22 these data of those two studies12,22 would be estimated from the previous meta-analysis.10 Additionally, 2 studies were excluded because the same study population was reported.13,14 Ultimately, 8 articles11,12,15,20–24 involving 43,629 participants and 2548 patients with PC were included in our meta-analysis. Among these 8 studies, 7 were case-control studies11,12,20–24 and 1 was a cohort study.15 The earliest and latest studies were published in 1985 and 2011, respectively. All studies were conducted in the USA except for the 3 studies conducted in Europe.11,15,22 Sample sizes ranged from 402 to 27,111. The study population comprised men and women in 6 studies11,12,20,22–24 and only men in 2 studies.15,21 All studies adjusted for age, sex, and smoking, most studies adjusted for alcohol consumption (n = 5), and only 3 studies adjusted for energy intake.15,22,24 The summary of study characteristics are shown in Table 1. According to the scoring criteria of NOS, the scores ranged from 5 to 9; there were 4 high-15,22–24 and 4 low-quality studies.11,12,20,21 In most case-control studies, there were exposure and selection biases and the nonresponse rates were not reported. Only one cohort study of high quality was included in our meta-analysis. The results of the study quality assessment are presented in Table 2.
FIGURE 1.
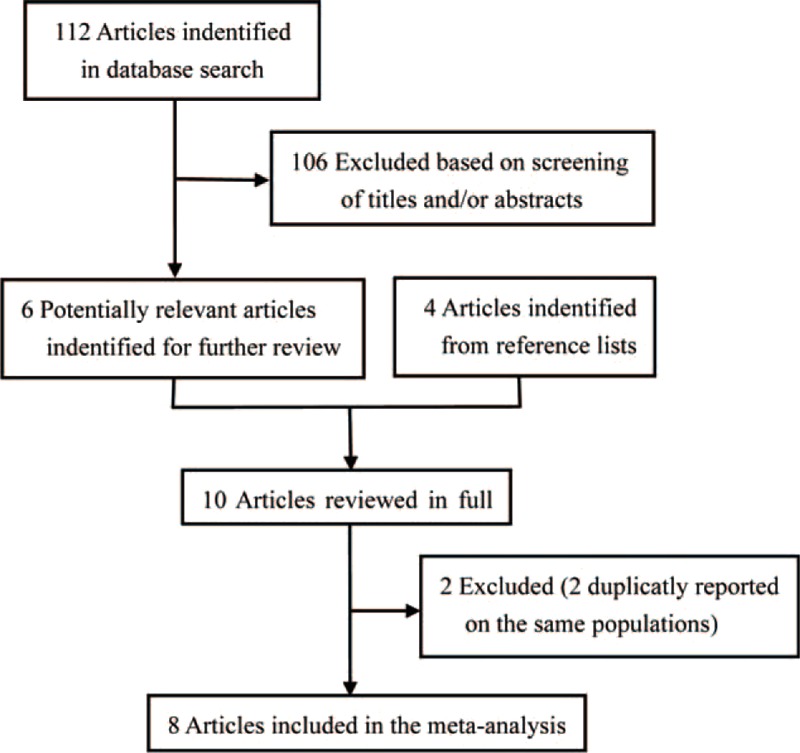
Flow-chart of study selection in the meta-analysis.
TABLE 1.
Characteristic of Studies Regarding Whole Grain Intake and Pancreatic Cancer Risk
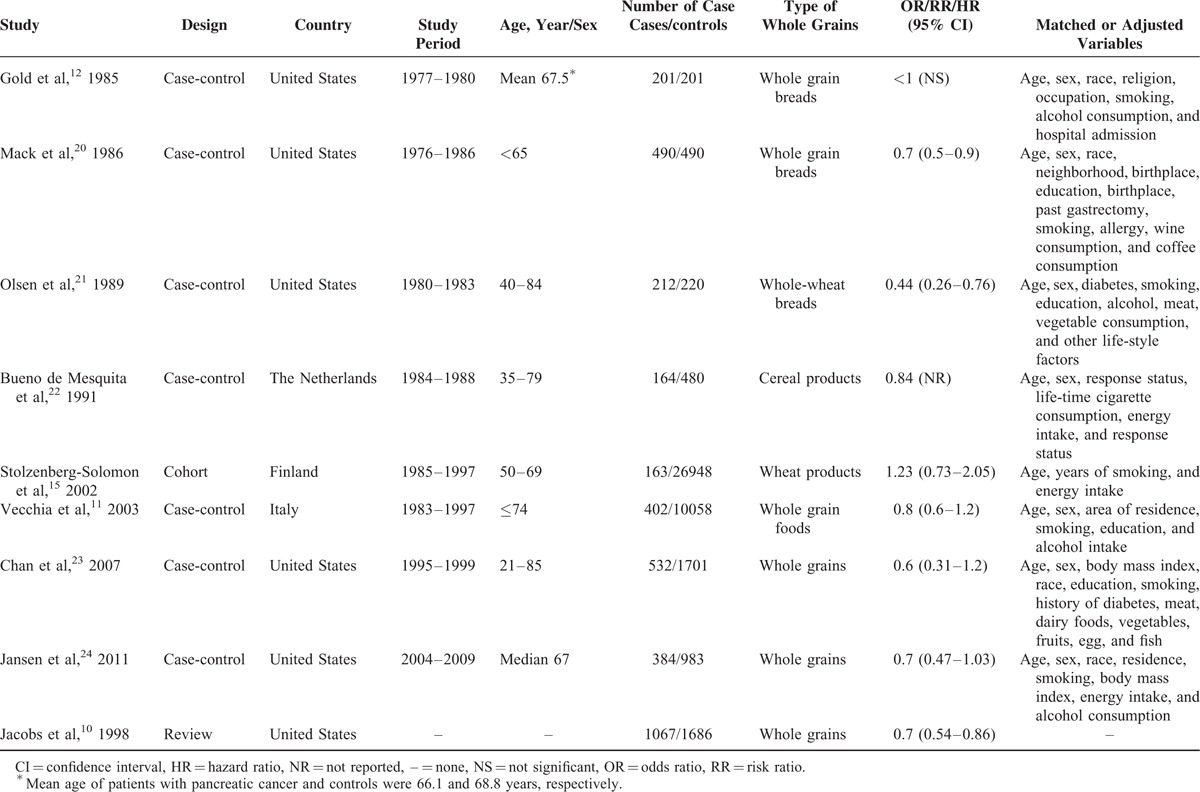
TABLE 2.
Methodologic Quality of Case-Control and Cohort Studies in Our Meta-Analysis
Effects of Whole Grains on Pancreatic Cancer
The relationship between whole grains and PC risk was evaluated in 7 case-control studies11,12,20–24 and 1 cohort study.15 There were no reported 95% CIs or ORs in 2 of the included studies12,22; therefore, we used the pooled data from 4 observational studies12,20–22 in a previous meta-analysis.10 Ultimately, a total of 5 studies10,11,15,23,24 were used to analyze the association between whole grains and PC risk. No significant heterogeneity was observed among case-control studies (I2 = 0%, Pheterogeneity = 0.872), and we found a statistically significant association between whole grain intake and PC risk with a summary OR of 0.72 (P < 0.001; 95% CI, 0.60–0.85; Table 3). However, there was no relationship between whole grain intake and PC risk in the cohort study (OR, 1.23; P = 0.54; 95% CI, 0.73–2.05); however, only 1 study was included in this assessment. Pooling the data from case-control and cohort studies yielded a summary OR of 0.76 (95% CI, 0.64–0.91) for high versus low intake of whole grains, which also indicated that a diet high in whole grains was associated with a statistically significant reduction in PC risk (P = 0.002). No significant heterogeneity was present among these studies (I2 = 11.7%; Pheterogeneity = 0.339; Figure 2).
TABLE 3.
Subgroup Analysis of Whole Grain Intake and Pancreatic Cancer Risk
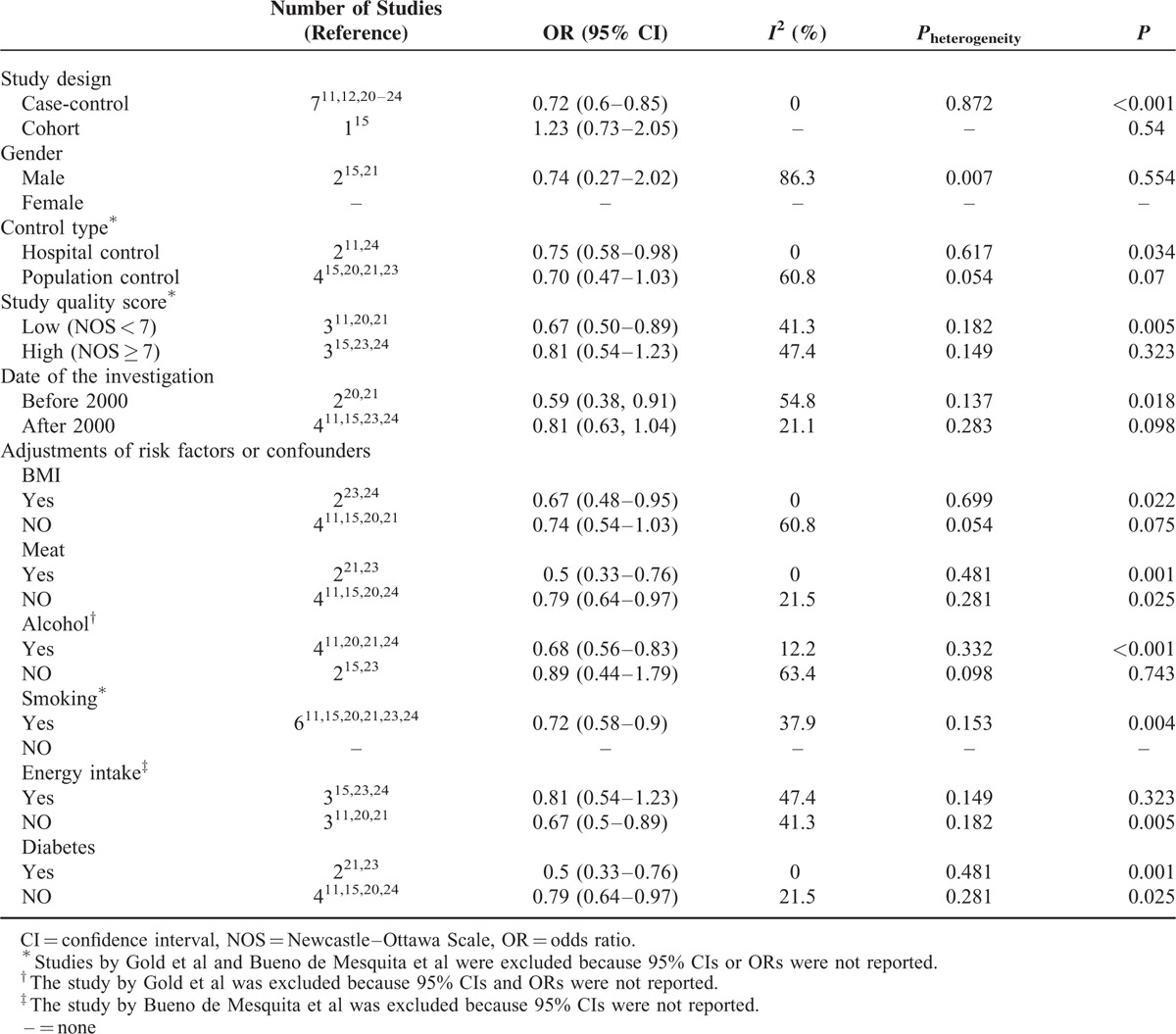
FIGURE 2.
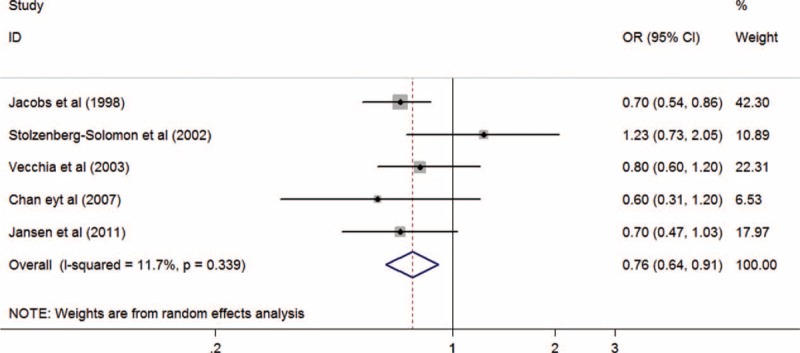
Summary odds ratios of pancreatic cancer for the highest versus lowest of whole grains intake. OR = odds ratio, CI = confidence interval.
SUBGROUP ANALYSIS
Subgroup Analysis by Geographic Area
Due to the lack of relevant data, subgroup analysis was not performed in 2 studies.12,22 OR estimates for whole grain intake and PC risk were reported in 4 studies20,21,23,24 conducted in the USA and 2 studies11,15 in Europe. When we preformed the subgroup analysis by geographic area, a statistical significant association between whole grain intake and PC risk was observed in the USA (OR, 0.64; 95% CI; 0.53–0.79; P < 0.001), but not in Europe (OR, 0.95; 95% CI, 0.63–1.43; P = 0.803). There were no significant heterogeneity between the findings from the United States (I2 = 0%; Pheterogeneity = 0.482) and Europe studies (I2 = 45.6%; Pheterogeneity = 0.175; Figure 3).
FIGURE 3.
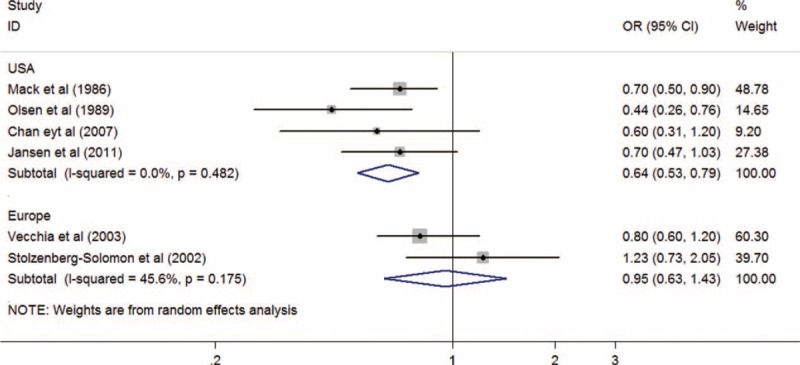
Summary odds ratios of pancreatic cancer for the highest versus lowest of whole grains intake using subgroup analysis by geographic area. OR = odds ratio, CI = confidence interval.
Subgroup Analysis by Type of Whole Grains
Results regarding the relationship between PC risk and the high versus low intake of grains and wheats were reported in 411,20,23,24 and 2 studies,15,21 respectively. When we preformed the subgroup analysis by the type of whole grains, a statistical significant association between PC risk and grain intake was observed (OR, 0.72; 95% CI, 0.60–0.87; P = 0.001), but not for wheat intake (OR, 0.74; 95% CI, 0.27–2.02; P = 0.554). There was significant heterogeneity between the 2 studies about the intake of wheats (I2 = 86.3%; Pheterogeneity = 0.007), but not among the 4 studies about the intake of grains (I2 = 0%; Pheterogeneity = 0.876; Figure 4).
FIGURE 4.
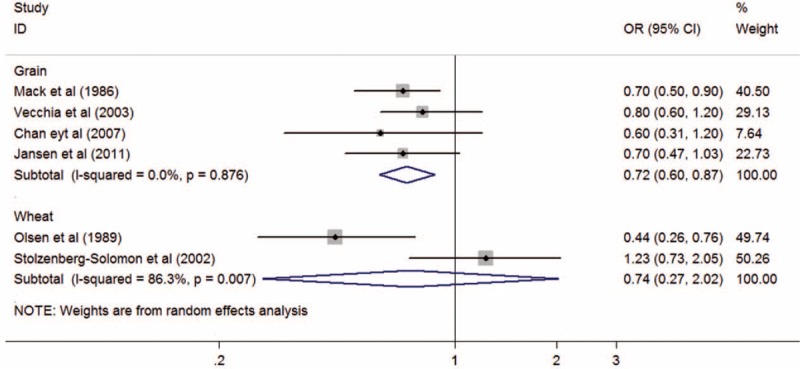
Summary odds ratios of pancreatic cancer for the highest versus lowest of whole grains intake using subgroup analysis by type of whole grains. OR = odds ratio, CI = confidence interval.
Subgroup Analysis by Other Factors
In order to minimize heterogeneity among studies, several subgroup analyses were conducted according to study design, gender, control type, study quality score, and major adjustments for potential confounders. The results of the subgroup analysis by study design are shown in Table 3. In the 2 studies that included only men,15,21 the pooled summary OR of the subgroup analyses was 0.74 (95% CI, 0.27–2.02, P = 0.554). When subgroup analyses were performed by control type, statistically significant associations between whole grain intake and PC risk were observed in the hospital-based control study (OR, 0.75; 95% CI, 0.58–0.98; P = 0.034) but not in the population-based control study (OR, 0.7; 95% CI, 0.47–1.03; P = 0.07). In further subgroup analysis by study quality score, a significant decreased risk of PC was found only for low quality studies (OR, 0.67; 95% CI, 0.50–1.09; P = 0.005); however, a significant association was not observed in the high quality studies (OR, 0.81; 95% CI, 0.54–1.23; P = 0.323). When we preformed the subgroup analysis by date of the investigation, a statistical significant decreased risk of PC was observed (OR, 0.59; 95% CI, 0.38–0.91; P = 0.018) for the study that performed prior to year 2000, but not for the study that performed after 2000 (OR, 0.81; 95% CI, 0.63–1.04; P = 0.098). We also conducted subgroup analyses by some potential confounders, such as body mass index (BMI), meat, alcohol, smoking, energy intake, and diabetes; the results were consistent with the overall data (ie, significant reduction of PC risk). The detail results are summarized in Table 3.
Sensitivity Analysis
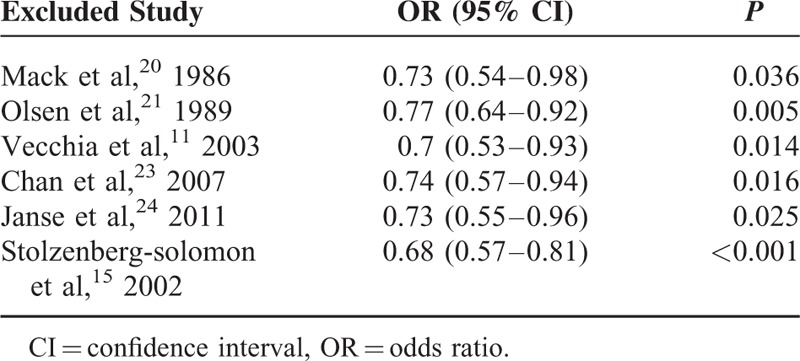
A sensitivity analysis was conducted to determine whether the results would change when one study was omitted at a time. According to our sensitivity analysis, the results regarding whole grain intake and the reduction of PC risk were robust (Figure 5). When continuously excluding one study, the results were unchanged, with a range of summary ORs from 0.68 to 0.77 and all P values were below 0.05 (Table 4).
FIGURE 5.
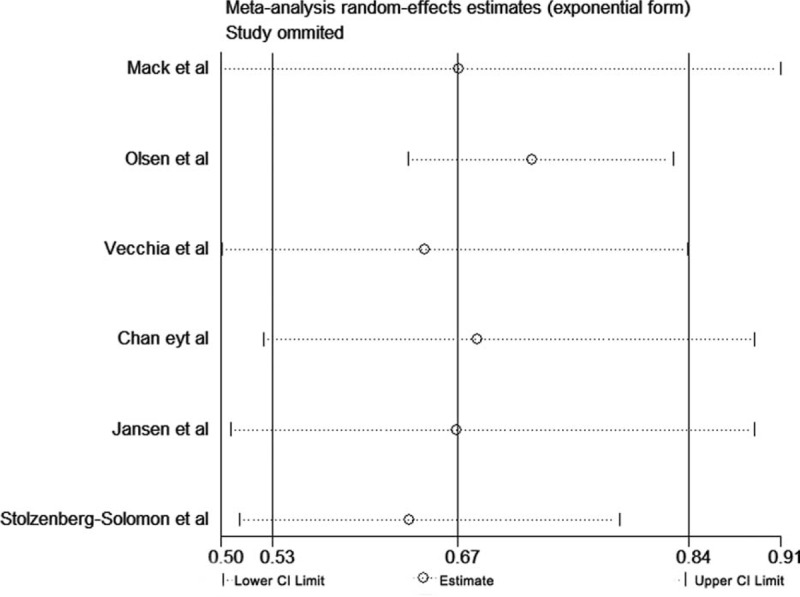
Sensitivity analysis for whole grains.
TABLE 4.
The Sensitivity Analysis Results for Whole Grains
DISCUSSION
To the best of our knowledge, this is the largest pooled analysis of the relationship between whole grain intake and PC risk based on 8 published observational studies with >2500 cases. In the present meta-analysis, we identified a significant association between whole grain intake and PC risk. Higher intake of whole grains was associated with a reduction of PC risk. Similar results were observed in the subgroup analyses.
Whole grains have various micronutrients and rich non-nutrients that are lost in the refining process25,26 and may have a benefit in preventing cancer.27 In 1986, Mack et al20 conducted a case-control study in Los Angeles, and they were the first to show that the intake of whole grain bread was inversely associated with having PC. Furthermore, Jacobs et al10 proposed and proved the hypothesis that whole grain consumption protects against many kinds of cancer, including PC. Some potential mechanisms to explain this hypothesis have been suggested. Firstly, whole grains are a rich source of dietary fiber. High fiber foods are known to have potential anticarcinogenic properties and cancer-preventive effects, such as reducing N-nitroso compounds6 and enhancing immunity,7 and particularly produce various antiinflammatory cytokines, which may be involved in the initiation and progression of PC.28 A significant inverse association between dietary fiber intake and PC risk was observed in a recent meta-analysis of epidemiological studies.29 Secondly, whole grains are concentrated sources of vitamin B, such as pantothenic acid, thiamin, niacin, riboflavin, and folate.30 Folate are of particular importance because a protective role of folate consumption on PC risk has been suggested by the World Cancer Research Fund31 and a meta-analysis (pooling of 4 case-control and 6 cohort studies).8 Thirdly, whole grains are rich in multifarious antioxidants, including vitamins (vitamin C and E and β-carotene)32 and trace minerals (selenium, zinc, copper, and manganese).32,33 Those trace elements are components of enzymes that conduct antioxidant functions, and several researchers have determined that β-carotene, vitamin E, and selenium intakes are inversely associated with PC risk.34–36 Fourthly, whole grains are significant sources of some key non-nutrients, such as phenolic acids, phytoestrogens, and lignans, which protect against pancreatic carcinogenesis by modulating hormonal pathways.33,37 Finally, a high intake of whole grains has been reported to lower the levels of C-peptide, insulin, homocysteine, leptin, total cholesterol, and low-density lipoprotein cholesterol,38,39 which may directly or indirectly guard against the development of PC.23 Although various plausible mechanisms have been proposed to explain the protective effects of consuming whole grains on developing PC, it is difficult to determine the protective bioactive components of whole grains from the epidemiologic studies. Further experimental studies are needed to confirm the underlying protective mechanisms of whole grains or their different bioactive components in PC.
In a previous expanded review and meta-analysis, based on 40 case-control studies of 20 types of cancers and colon polyps, it was demonstrated that whole grain intake would reduce the risk of various cancers, including oral, gastric, colorectal, endometrial, pancreatic, and other digestive cancers.10 In this previous meta-analysis with 4 case-control studies that involving assessed the risk of PC, the summary OR for the risk of PC in those with the highest whole grain intake, compared to the lowest intake, was 0.7 (95% CI, 0.54–0.86). Since then, some observed studies have increased yearly and inconsistent results have been published. In 2007, Chan et al23 suggested that consuming more whole grains might reduce the risk of developing PC based on a large population-based case-control study with 532 cases and 1701 controls. After 4 years, Jansen et al24 further provided evidence that lower intake of whole grains was related to developing PC. However, the hypothesis that a high intake of wheat products may increase the risk of PC was supported in a prospective study with 27,111 male smokers.15 Our meta-analysis, based on 7 case-control studies and 1 cohort study involving 2548 PC cases, should provide a better understanding about whole grain intake and the risk of PC. Interestingly, the OR for PC in those with the highest versus lowest whole grain intake in our study was similar to the previous meta-analysis. However, in order to reduce reporting bias, the estimated risk of PC was pooled using only data from published studies in which the 95% CIs or ORs were reported in the original articles (excluded the studies by Gold et al and Bueno de Mesquita et al). In the pooled analysis, we found a significant inverse association between whole grain intake and PC risk (pooled OR, 0.72; 95% CI, 0.58–0.90; P = 0.002; data not shown). Therefore, excluding 2 studies12,22 also failed to alter the estimated risk of PC; however, this result would further strengthen the association between whole grain intake and PC risk.
In the subgroup analysis, we found that the risk of PC decreased with increasing whole grain intake in the USA. However, the association between whole grain intake and PC risk was not found in Europe. This might be due to the difference in the type of study conducted in Europe because only 1 cohort study was included. The study type is a factor that should not be ignored when analyzing the results. In a pooled analysis of 5 case-control studies, a stronger association was observed when the study type was used to assess the relationship between whole grain intake and PC risk, however, no significant association was found in the European cohort study. There was a statistically significant inverse association between PC risk and intake of grains, but not wheats, when we performed a subgroup analysis according to the type of whole grains. Some other reasons may be the heterogeneity of study type (I2 = 83.6%, Pheterogeneity = 0.007) and the limited number of studies, which may have been too small to significantly reduce the risk of PC. Researchers have indicated that BMI,40 alcohol consumption,41 and smoking42 might increase the risk of PC; a significant inverse association between whole grain intake and PC risk was observed in our subgroup analysis that adjusted for these potential confounders. Furthermore, meat consumption3 and diabetes history43 were risk factors for PC. We respectively adjusted for those 2 risk factors in our subgroup analysis, and the statistical significant association between whole grain intake and PC risk was confirmed; however, the relationship was also observed when we performed the analysis without any adjustments. In addition, the relationship between whole grain intake and PC risk was completely consistent and stable according to our sensitivity analysis, which was conducted by excluding one study at a time.
Our meta-analysis also has several limitations. Firstly, our analysis was based on 7 case-control studies and only 1 cohort study. Therefore, the recall bias from case-control investigations was likely to be acknowledged. Secondly, the quality of our included studies was moderate. The association between whole grain intake and PC risk was not found in high quality studies (NOS ≥ 7) when we performed a subgroup analysis based on quality score. Further investigations are necessary to determine whether the quality of study affects the significance of the association. Third, most of studies may exist introduce bias because age, sex, and smoking status were controlled for in almost all the included studies; other potential confounders (ie, BMI, meat consumption, energy intake, and diabetes history) were also adjusted for in a few studies. Additionally, although the ORs that we extracted from the included studies would contain the greatest degree of control for main potential confounders, confounding by other unmeasured factors (ie, coffee,44 citrus fruits,45 and green tea consumption46), which may threaten the validity of observed results to some extent, was not adjusted for in the majority of included studies. Fourthly, the 95% CIs or ORs were not reported in 2 of the included studies and therefore, these values were only pooled from a previous meta-analysis, which may lead to reporting bias. Fifthly, the results obtained from the subgroup analysis by specific categories (ie, geographic area and type of whole grains) and adjustments of confounders were based on a limited number of studies. Sixthly, the intake levels of whole grains were mentioned in only 2 included studies;23,24 however, we did not pool the data and conducted a dose-response meta-analysis due to the inconsistencies in the unit of measurement for whole grain intake and the evaluation methods. In addition, differences in the definitions of whole grain intake among studies might result in heterogeneity.
In conclusion, dietary intake of whole grains was appreciably and inversely related to PC risk according to our meta-analysis of all relevant case-control and cohort studies. To fully understand the relationship between whole grain intake and PC risk, larger well-designed prospective cohort studies that consider whole grain subgroups (eg, grain and wheat) are required in the future.
Acknowledgments
This study was supported by a Chinese research grant from the Special Science and Technology Research Funds for Public Welfare Projects (201502022).
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, HR = hazard ratio, NOS = Newcastle–Ottawa Scale, OR = odds ratio, PC = Pancreatic cancer, RR = relative risk.
QL, HZ, and JB contributed equally to this work.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2014; 136:359–386. [DOI] [PubMed] [Google Scholar]
- 2.Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012; 62:30–67. [DOI] [PubMed] [Google Scholar]
- 3.Larsson SC, Wolk A. Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies. Br J Cancer 2012; 106:603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bidoli E, Pelucchi C, Zucchetto A, et al. Fiber intake and pancreatic cancer risk: a case-control study. Ann Oncol 2012; 23:264–268. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, James R, Hébert, et al. Association between dietary fiber and markers of systemic inflammation in the Women's Health Initiative Observational Study. Nutrition 2008; 24:941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Møller ME, Dahl R, Bøckman OC. A possible role of the dietary fibre product, wheat bran, as a nitrite scavenger. Food Chem Toxicol 1988; 26:841–845. [DOI] [PubMed] [Google Scholar]
- 7.Volman JJ, Ramakers JD, Plat J. Dietary modulation of immune function by beta-glucans. Physiol Behav 2008; 94:276–284. [DOI] [PubMed] [Google Scholar]
- 8.Lin HL, An QZ, Wang QZ, et al. Folate intake and pancreatic cancer risk: an overall and dose-response meta-analysis. Public Health 2013; 127:607–613. [DOI] [PubMed] [Google Scholar]
- 9.Banim PJ, Robert L, Alison MT, et al. Dietary antioxidants and the etiology of pancreatic cancer: a cohort study using data from food diaries and biomarkers. Gut 2013; 62:1489–1496. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs DR, Jr, Marquart L, Slavin J, et al. Whole-grain intake and cancer: an expanded review and meta-analysis. Nutr Cancer 1998; 30:85–96. [DOI] [PubMed] [Google Scholar]
- 11.La Vecchia C, Chatenoud L, Negri E, et al. Session: whole cereal grains, fibre and human cancer wholegrain cereals and cancer in Italy. Proc Nutr Soc 2003; 62:45–49. [DOI] [PubMed] [Google Scholar]
- 12.Gold EB, Gordis L, Diener MD, et al. Diet and other risk factors for cancer of the pancreas. Cancer 1985; 55:460–467. [DOI] [PubMed] [Google Scholar]
- 13.Soler M, Chatenoud L, La Vecchia C, et al. Diet, alcohol, coffee and pancreatic cancer: final results from an Italian study. Eur J Cancer Prev 1998; 7:455–460. [DOI] [PubMed] [Google Scholar]
- 14.Chatenoud L, Tavani A, La Vecchia C, et al. Whole grain food intake and cancer risk. Int J Cancer 1998; 77:24–28. [DOI] [PubMed] [Google Scholar]
- 15.Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, et al. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol 2002; 155:783–792. [DOI] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute 2005; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 17.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987; 9:1–30. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 19.Xu Q, Guo W, Shi X, et al. Association between alcohol consumption and the risk of Barrett's esophagus: a meta-analysis of observational studies. Medicine (Baltimore) 2015; 94:e1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mack TM, Yu MC, Hanisch R, et al. Pancreas cancer and smoking, beverage consumption, and past medical history. J Natl Cancer Inst 1986; 76:49–60. [PubMed] [Google Scholar]
- 21.Olsen GW, Mandel JS, Gibson RW, et al. A case-control study of pancreatic cancer and cigarettes, alcohol, coffee and diet. Am J Public Health 1989; 79:1016–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bueno de Mesquita HB, Maisonneuve P, Runia S, et al. Intake of foods and nutrients and cancer of the exocrine pancreas: a population-based case-control study in The Netherlands. Int J Cancer 1991; 48:540–549. [DOI] [PubMed] [Google Scholar]
- 23.Chan JM, Wang F, Holly EA. Whole grains and risk of pancreatic cancer in a large population-based case-control study in the San Francisco Bay Area, California. Am J Epidemiol 2007; 166:1174–1185. [DOI] [PubMed] [Google Scholar]
- 24.Jansen RJ, Robinson DP, Stolzenberg-Solomon RZ, et al. Fruit and vegetable consumption is inversely associated with having pancreatic cancer. Cancer Causes Control 2011; 22:1613–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clydesdale FM. Optimizing the diet with whole grains. Crit Rev Food Sci Nutr 1994; 34:453–471. [DOI] [PubMed] [Google Scholar]
- 26.Slavin JL. Whole grains, refined grains and fortified refined grains: what's the difference? Asia Pac J Clin Nutr 2000; 9:23–27. [DOI] [PubMed] [Google Scholar]
- 27.Gil A, Ortega RM, Maldonado J. Wholegrain cereals and bread: a duet of the Mediterranean diet for the prevention of chronic diseases. Public Health Nutr 2011; 14:2316–2322. [DOI] [PubMed] [Google Scholar]
- 28.Gukovsky I, Li N, Todoric J, et al. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology 2013; 144:1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang CH, Qiao C, Wang RC, et al. Dietary fiber intake and pancreatic cancer risk: a meta-analysis of epidemiologic studies. Sci Rep 2015; 5:10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slavin J. Whole grains and human health. Nutr Res Rev 2004; 17:99–110. [DOI] [PubMed] [Google Scholar]
- 31.World Cancer Research Fund and American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington (DC): AICR; 2007. [Google Scholar]
- 32.Slavin JL. Mechanisms for the impact of whole grain foods on cancer risk. J Am Coll Nutr 2000; 19:300–307. [DOI] [PubMed] [Google Scholar]
- 33.Thompson LU. Antioxidants and hormone-mediated benefits of whole grains. Crit Rev Food Sci Nutr 1994; 34:473–497. [DOI] [PubMed] [Google Scholar]
- 34.Jeurnink SM, Ros MM, Leenders M, et al. Plasma carotenoids, vitamin C, retinol and tocopherols levels and pancreatic cancer risk within the European Prospective Investigation into cancer and nutrition: a nested case-control study: plasma micronutrients and pancreatic cancer risk. Int J Cancer 2015; 136:665–676. [DOI] [PubMed] [Google Scholar]
- 35.Stolzenberg-Solomon RZ, Sheffler-Collins S, Weinstein S, et al. Vitamin E intake, alpha-tocopherol status, and pancreatic cancer in a cohort of male smokers. Am J Clin Nutr 2009; 89:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han X, Li J, Brasky TM, et al. Antioxidant intake and pancreatic cancer risk: the Vitamins and Lifestyle (VITAL) Study. Cancer 2013; 119:1314–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyn-Cook BD, Stottman HL, Yan Y, et al. The effects of phytoestrogens on human pancreatic tumor cells in vitro. Cancer Lett 1999; 142:111–119. [DOI] [PubMed] [Google Scholar]
- 38.Jensen MK, Koh-Banerjee P, Franz M, et al. Whole grains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation 1. Am J Clin Nutr 2006; 83:275–283. [DOI] [PubMed] [Google Scholar]
- 39.Jarosz M, Sekuła W, Rychlik E. Influence of diet and tobacco smoking on pancreatic cancer incidence in Poland in 1960–2008. Gastroenterol Res Pract 2012; 2012:682156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arslan AA, Helzlsouer KJ, Kooperberg C, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium (PanScan). Arch Intern Med 2010; 170:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.La Torre G, Sferrazza A, Gualano MR, et al. Investigating the synergistic interaction of diabetes, tobacco smoking, alcohol consumption, and hypercholesterolemia on the risk of pancreatic cancer: a case-control study in Italy. Biomed Res Int 2014; 2014:481019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye X, Lu G, Huai J, et al. Impact of smoking on the risk of pancreatitis: a systematic review and meta-analysis. PLoS One 2015; 10:e0124075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valent F. Diabetes mellitus and cancer of the digestive organs: an Italian population-based cohort study. J Diabetes Complications 2015; 29:1056–1061. [DOI] [PubMed] [Google Scholar]
- 44.Dong J, Zou J, Yu XF. Coffee drinking and pancreatic cancer risk: a meta-analysis of cohort studies. World J Gastroenterol 2011; 17:1204–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bae JM, Lee EJ, Guyatt G. Citrus fruit intake and pancreatic cancer risk: a quantitative systematic review. Pancreas 2009; 38:168–174. [DOI] [PubMed] [Google Scholar]
- 46.Zeng JL, Li ZH, Wang ZC, et al. Green tea consumption and risk of pancreatic cancer: a meta-analysis. Nutrients 2014; 6:4640–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]


