Abstract
The biogenic amine serotonin [5-hydroxytryptamine (5-HT)] has received considerable attention for its role in behavioral phenomena throughout a broad range of invertebrate and vertebrate taxa. Acute 5-HT infusion decreases the likelihood of crayfish to retreat from dominant opponents. The present study reports the biochemical and behavioral effects resulting from chronic treatment with 5-HT-modifying compounds delivered for up to 5 weeks via silastic tube implants. High performance liquid chromatography with electrochemical detection (HPLC-ED) confirmed that 5,7-dihydroxytryptamine (5,7-DHT) effectively reduced 5-HT in all central nervous system (CNS) areas, except brain, while a concurrent accumulation of the compound was observed in all tissues analyzed. Unexpectedly, two different rates of chronic 5-HT treatment did not increase levels of the amine in the CNS. Behaviorally, 5,7-DHT treated crayfish exhibited no significant differences in measures of aggression. Although treatment with 5-HT did not elevate 5-HT content in the CNS, infusion at a slow rate caused animals to escalate more quickly while 5-HT treatment at a faster rate resulted in slower escalation. 5,7-DHT is commonly used in behavioral pharmacology and the present findings suggest its biochemical properties should be more thoroughly examined. Moreover, the apparent presence of powerful compensatory mechanisms indicates our need to adopt an increasingly dynamic view of the serotonergic bases of behavior like crayfish aggression.
Keywords: serotonin (5-HT); 5, 7-dihydroxytryptamine (5,7-DHT); compensatory mechanisms; agonistic behavior; crustacea
INTRODUCTION
Aggression is a key element in the natural behavioral repertoire of most decapod crustacean species (Berrill, 1978; Berrill and Arsenault, 1984; Karnofsky et al., 1989). Even in the absence of an obvious resource, initial contact between two conspecifics is followed by a series of agonistic bouts that vary in length until one participant withdraws (Bovbjerg, 1953; Scrivener, 1971; Rubenstein and Hazlett, 1973; Bruski and Dunham, 1987). Encounters progress towards more intense stages of fighting in a step-wise fashion where decisions to escalate the encounter or retreat govern the temporal and probabilistic nature of subsequent behavior patterns (Huber and Kravitz, 1995; Huber et al., 2001a). In a majority of instances, physical superiority constitutes the primary determinant of fight length and outcome (Bovbjerg, 1956; Rubenstein and Hazlett, 1973; Pavey and Fielder, 1996; Barki et al., 1997; Vye et al., 1997). However, there are important caveats to viewing this behavioral framework too simplistically. For instance, previous experience, in the form of social conditioning, diminishes the initial bias of morphological asymmetries in claw and body size (Daws et al., 2001). Moreover, as crayfish hierarchies differentiate, the aggressiveness and overall success of participants converge on distinct, linear ranks due to a process of self-assembly (Goessmann et al., 2000). Nevertheless, once social status becomes established, subsequent fighting dwindles and defeated animals avoid further contact with winners (Issa et al., 1999; Goessmann et al., 2000). The fundamental characteristics of this behavioral scheme closely match predictions of game theory (Maynard-Smith and Price, 1973; Parker and Rubenstein, 1981; Enquist and Leimar, 1983; Austad, 1989) and are thus ideal for quantitative behavioral analysis (Huber and Kravitz, 1995; Huber et al., 2001a).
Decapod crustaceans have proven to be useful models for exploring proximate mechanisms in aggression (Antonsen and Paul, 1997; Krasne et al., 1997; Yeh et al., 1997; Huber and Delago, 1998; Sneddon et al., 2000; Tierney, 2000; Doernberg et al., 2001). Initial studies illustrated that direct injection of substantial amounts of 5-HT into the hemolymph (i.e., blood) of lobsters and crayfish resulted in a posture resembling “meral spread”—a common display of dominant animals (Livingstone et al., 1980). Subsequently, a small population of 5-HT-containing neurons was identified (Beltz and Kravitz, 1983; Real and Czternasty, 1990) and has since been extensively characterized (Beltz and Kravitz, 1987; Ma et al., 1992; Ma and Weiger, 1993; Heinrich et al., 1999). A subset of these neurons has both central and peripheral effects on muscle flexion (Kravitz et al., 1984; Harris-Warrick and Kravitz, 1984), may enhance the occurrence of “dominant-like” postures (Ma et al., 1992; Kravitz, 2000), and is activated by command circuits that control tail flip—a common escape behavior (Hörner et al., 1997). Mediated through lateral giant neurons, the latter mechanism exhibits 5-HT-induced activity that varies inversely as a function of social status (Glanzman and Krasne, 1983; Yeh et al., 1996, 1997). Infusion of somewhat smaller amounts of 5-HT into freely moving lobsters and crayfish produces a renewed willingness to fight their larger, dominant opponents, and 5-HT reuptake is an important underlying mechanism for such behavioral reversals (Huber et al., 1997a,b; Huber and Delago, 1998). In crayfish and lobsters, chronic treatment with fluoxetine is accompanied by an expected increase in aggressive behavior, but the behavioral effects are less pronounced compared to those produced by acute 5-HT infusion, and they do not persist across the duration of treatment (Delago et al., in review).
Consistent with evidence in the crustacean species mentioned above, 5-HT is implicated in the neurochemical control of aggression throughout a broad range of taxa (Maas, 1962; Kostowski et al., 1975; Maler and Ellis, 1987; Blanchard et al., 1991; Raleigh et al., 1991; Reisner et al., 1996; Dyakonova and Schürmann, 1999; Lesch and Merschdorf, 2000; Larson and Summers, 2001). Attempts to explain such relationships have centered predominantly on 5-HT’s presence or absence, and thereby its putative function in aggression and social dominance. Although often assumed to extend from evidence derived in acute experimental situations, the neurochemical mechanisms underlying long-term changes in aggressive motivation (Van De Poll et al., 1982; Jackson, 1991; Chase et al., 1994; Hollis et al., 1995; Whitehouse, 1997; Hsu and Wolf, 1999; Issa et al., 1999; Goessmann et al., 2000) have received comparatively little attention. Of the studies that have addressed such issues directly (e.g., Winberg and Nilsson, 1993; Kudryavtseva and Avgustinovich, 1998; Stribley and Carter, 1999), general agreement converges on the dynamic neurochemical processes that parallel long-term changes in aggressive state. Moreover, studies ranging from learning (Montarolo et al., 1986; Dale et al., 1987) and aggression (Yeh et al., 1996, 1997; Flugge et al., 1997; Kudryavtseva and Avgustinovich, 1998) to drug use (Robinson and Berridge, 1993; Bradbury, 2000; Koob and Le Moal, 2001) and depression (Hjorth and Auerbach, 1996; Blier and de Montigny, 1999) indicate an inherent, temporal complexity for studying the role of monoamines in behavioral plasticity.
In crayfish, the time course and potential reversal of 5-HT’s aggression enhancing effects have not yet been thoroughly examined. Recent experiments with chronic fluoxetine treatments have underscored the need to carefully explore the time frame of serotonergic plasticity concomitant to changes in decapod aggressive motivation (Delago et al., in review). In the present article, we report experiments that examine the neurochemical basis of crustacean fighting behavior in the crayfish, Orconectes rusticus. Dyadic agonistic encounters were analyzed following different lengths of chronic exposure to silastic tube implants containing 5-HT, 5,7-DHT, or α-methyltryptophan (AMTP). The effects of 5-HT release were additionally explored by varying the rate of delivery. Following behavioral trials, changes in nervous system levels of 5-HT were measured with high performance liquid chromatography with electrochemical detection (HPLC-ED).
MATERIALS AND METHODS
Crayfish
Crayfish (O. rusticus) from the Portage River near Bowling Green State University were socially isolated in individual containers (Ø 0.17 m, 0.1 m depth) for at least 7 days prior to behavioral trials. Only intermolt males (5.0–26.6 g) with intact appendages were used. Individual containers were maintained in fiberglass trays (2.0 × 0.7 × 0.1 m) supplied continuously with freshly filtered and aerated water (20°C) from a main holding reservoir. All animals were fed twice a week and maintained under a 16:8 h light/dark cycle.
Pharmacological Treatments
Sections of silastic tubing (Ø 0.635 mm; Konigsberg Instruments, Inc.) were cut to a length of 15 mm and loaded with crystals of 5,7-DHT creatinine sulfate (FW: 403.4; Sigma D-0136 or Fluka 37970), AMTP (FW: 218.3; Sigma M-8377), 5-HT creatinine sulfate (FW: 387.4; Sigma H-7752), or left empty in the control group. All tubes were occluded at one end with 734 Flowable Sealant (Dow Corning). A fifth group (5-HT “slow”) received tubes also loaded with 5-HT, but which had both ends of the tube sealed (compared to the 5-HT “fast” treatment that had only one end sealed). Differences in the rate of release between 5-HT treatments were confirmed by placing loaded tubes into 30 mL of 125 mM saline. Samples were taken over 1 week (ca. every 36 h) and assayed for 5-HT with HPLC-ED (see below). 5-HT remained intact within the tubes and was released continuously at stable rates in both 5-HT slow [F(1, 4) = 11.01; p < .05] and 5-HT fast [F(1, 4) = 13.39; p < .05] conditions. Rates of infusion were measured as 0.6 μg/h and 7.8 μg/h for 5-HT slow and 5-HT fast, respectively. Such treatments were expected to serve as a major challenge to aminergic systems. For instance, the total amount of serotonin infused (400 μg) over 4 weeks in the 5-HT slow condition exceeded natural levels (9.4 ± 0.85 ng) by 40,000 times.
Before implanting each tube, crayfish were anesthetized in ice for 10 min. An 18.5 gauge needle was used to make a small incision in the abdominal cuticle directly behind the most posterior ambulatory leg. With a pair of forceps, the silastic tube was inserted into the opening and guided into the thoracic body cavity. Subsequently, animals were placed in an observation aquarium and allowed to recover from the treatment. Within minutes, all animals exhibited normal activity and exploratory behavior, and no deaths resulted from such treatment. Animals were returned to their containers and maintained as previously described.
Profiles of Pharmacological Compounds
5,7-DHT is widely used to deplete 5-HT. It acts as a classical neurotoxin in vertebrates by destroying 5-HT-containing cells, presumably through a build up of free radicals produced by its intracellular actions with monoamine oxidase (MAO; Klemm et al., 1979). However, neither MAO (Boadle and Blaschko, 1968; Dewhurst et al., 1972; Sloley and Goldberg, 1991; Sparks and Geng, 1992) nor 5-hydroxyindolacetic acid, the primary metabolite of 5-HT oxidation (Kennedy, 1978; Dubbels and Elofsson, 1989), appear to be present in many invertebrates, including crustaceans. Moreover, there is strong evidence indicating that invertebrate 5-HT neurons retain all functional membrane properties after treatment with 5,7-DHT (Lent and Dickinson, 1984; Cook and Orchard, 1993; Heinrich et al., 1999). Nevertheless, numerous studies of invertebrates have demonstrated that treatment with 5,7-DHT results in robust depletions of 5-HT (Livingstone et al., 1981; Lent, 1984; Lent and Dickinson, 1984; Glanzman and Krasne, 1986; Cook and Orchard, 1993; Sahley, 1994; Benton et al., 1997; Doernberg et al., 2001).
Likewise, AMTP has been used to evaluate the behavioral effects produced by depletion of 5-HT in the invertebrate CNS (Novak and Rowley, 1994; Dyakonova and Schürmann, 1999; Stevenson et al., 2000). AMTP leads to a general decrease in 5-HT synthesis by way of α-methylated amino acid substitutions (Sloley and Orikasa, 1988).
Quantitative Neurochemical Analysis
To verify the effects of pharmacological treatments we obtained neurochemical profiles for individuals with HPLC-ED (see Table 1). After the end of each behavioral trial (see below), the experimental animal was immediately anesthetized in ice for 30 min. The animal was then sacrificed and its entire ventral nerve cord (i.e., CNS) was removed in four sections: (A1–A6) all abdominal ganglia, (T1–T5) all thoracic ganglia, (SEG/CEG) subesophageal/circumesophageal ganglia, and (SUP) supraesophageal ganglion (i.e., brain). Tissues were placed into a 1.5 mL microfuge tube containing 200 μL 0.1 N perchloric acid, mechanically disrupted (Kontes motor driven pelleting tool equipped with a Teflon™ pestle), and cellular debris and denatured proteins were pelleted with a tabletop centrifuge (Capsulefug, 6200 RPM, 15 min). Twenty-microliter aliquots (diluted 10–100-fold with mobile phase) were separated on a C18 column (Spherisorb ODS2, 3 μm, 100 × 4.6 mm), with further details reviewed elsewhere (Panksepp et al., 2002).
Table 1.
Descriptive Statistics for Crayfish Used in Behavioral Trials and HPLC-ED Analysis
| 5,7-DHT | AMTP | Control | 5-HT “Slow” | 5-HT “Fast” | |
|---|---|---|---|---|---|
| Treatment | |||||
| Weight | 13.6 ± 1.69 g | 13.2 ± 1.64 g | 8.7 ± 0.45 g | 11.1 ± 2.20 g | 13.1 ± 1.29 g |
| Duration | 5–16 days | 12–26 days | 21–30 days | 21–30 days | 4–23 days |
| Behavioral trials | |||||
| n | 8 | 6 | 5 | 4 | 5 |
| # Interactions | 100 | 65 | 63 | 26 | 65 |
| HPLC-ED analysis | |||||
| n | 8 | 8 | 35* | 7 | 5 |
Numbers reported for weight are means ± standard error. In a few cases, crayfish were used for HPLC-ED analysis, but were not used in behavioral experiments. This accounts for the discrepancy in sample sizes (n) for treatment groups between behavioral and HPLC-ED analyses.
Thirty additional animals that were not experimentally manipulated were included in the control group for a more accurate measure of standard crayfish CNS 5-HT content. There were no statistically significant differences between untreated and control animals, or between body weight and amine content.
The mobile phase contained 50 mM sodium phosphate (6.9 g/L monobasic anhydrous; FW: 120.0; Sigma S-0751), 5 mM heptanesulfonic acid (1 g/L sodium salt; FW: 202.2; Sigma H-2766), and v/v 16% MeOH and 4% acetonitrile as organic modifiers. The final solvent buffer was adjusted to pH 3.25 with concentrated phosphoric acid (ACS reagent; FW: 98.0; Sigma P-6560), filtered through a 0.22 μm filter, and operated at ambient temperature with a flow rate of 1.5 mL/min. Eluted compounds were detected electrochemically on a BAS LC-4C single-cell, amperometric detection system. The detector potential was set to 500 mV. Chromatograms were recorded with analog/digital converter (MacLab) and strip-chart software (MacLab Chart v3.3.3) on an Apple Macintosh computer. The size of eluting peaks was analyzed with chromatography software (MacLab Peaks v1.3.1). Identification of 5-HT was based on comparison with external standards by retention time under several different mobile phase conditions. Known standards were dissolved to a final concentration (5 pg amine/μL) in mobile phase from a stock solution each day. The detection limit was 3–5 pg 5-HT injected on column. Recovery rates were close to 100% and no further correction was applied. 5,7-DHT standards were also prepared as described above and included for the analysis of crayfish treated with this compound. Standards were injected every five samples, and at the beginning and end of each day.
Quantitative Behavioral Analysis
Following different lengths of drug exposure, treated crayfish were size-matched (i.e., < 5% difference in body weight) against randomly selected opponents (see Table 1). The behavioral analysis followed standard procedures used in our lab as detailed below (reviewed in Huber et al., 2001a). Both animals were placed in a Plexiglas™ observation tank (0.45 × 0.31 × 0.15 m) that received continuous flow of freshly filtered and aerated water through evenly spaced pairs of in/out holes. Initially, an opaque Plexiglas™ divider separated opponents for a 10 min acclimation period. The divider was then raised and each trial videotaped for 30 min (Sony DCR-TR7000 digital video camera). Design and size of this arena were guided by observations of agonistic interactions in the field. O. rusticus reach densities in excess of 50 individuals/m2 at our collecting sites near Bowling Green, OH (unpublished observation) and are routinely observed fighting in shallow, shelterless pools with similar sizes and features to those used in the present study.
Behavioral measures were scored for all interactions that took place during 30 min trials. For each interaction the identities of the initiating and retreating animals, fight duration, maximum intensity reached, and the frequency of the highest intensity level (i.e., level 4) were scored. An interaction was defined as the point when the approaching animal came to within one body length of the opponent and its presence was overtly reacted to. Fighting intensity was coded on an ordinal scale: (0) no contest—one animal retreats without challenge; (1) threat displays or “meral spread”—both animals contest the interaction without physically touching the opponent; (2) restrained use of claws—both animals contest and at least one individual is antenna whipping and/or pushing its opponent without grabbing; (3) clawlock—both animals contest and at least one individual uses claws to grab and wrestle with opponent; and (4) unrestrained combat or “strike and rip”—brief periods characterized by the attempt of one animal to tear an appendage from its opponent. When one animal retreated from its opponent by at least one body length and remained separate for at least 5 s, it was considered the end of the interaction.
Statistical Evaluation of Neurochemistry and Behavior
Analysis of variance was used to test for differences in all continuous variables (e.g., fight duration, frequency of intensity level 4, and 5-HT content of all CNS tissues). To reduce heteroscedasticity (i.e., inequality of variances among treatment groups) a logarithmic transformation was applied to 5-HT content. Posthoc comparisons were explored with Tukey-Kramer HSD tests.
Following the establishment of social relationships, winning crayfish become more likely to initiate while losers become more likely to retreat (Issa et al., 1999; Goessmann et al., 2000), and thus such measures will lack independence. This dominance-associated behavioral polarity is apparent within three interactions and remains stable during fighting periods (Goessmann et al., 2000). To eliminate such lack of independence in our analyses, we restricted our analyses to those interactions that occurred prior to “established dominance”—operationally defined as the first interaction after which the identity of both initiating and retreating animals remained unchanged in subsequent interactions. Differences in initiation, retreat, and number of fights reaching a particular maximum intensity were compared with G-tests, and overall significant effects were explored with Freeman-Tukey deviates (FTD).
Understanding how different behaviors covary as interrelated behavioral dimensions has proven useful in our previous studies of fighting behavior in decapod crustaceans (Huber and Kravitz, 1995; Huber et al., 2001a; Stocker and Huber, 2001; Schroeder and Huber, 2002). For example, when agonistic encounters between decapods last longer they inevitably escalate to higher intensities (Huber et al., 1997b; Huber and Delago, 1998; Goessmann et al., 2000). In this set of experiments, we used whole-model regression analysis to measure the effects of duration, treatment, and their interaction on maximum intensity. The resulting slope (i.e., the regression coefficient) provides an estimate for the average rate of escalation during a fight. Differences in the rate of escalating were evaluated by the interaction term (duration × treatment) and 95% confidence intervals of each regression coefficient were used to identify differences between experimental groups.
RESULTS
Neurochemistry
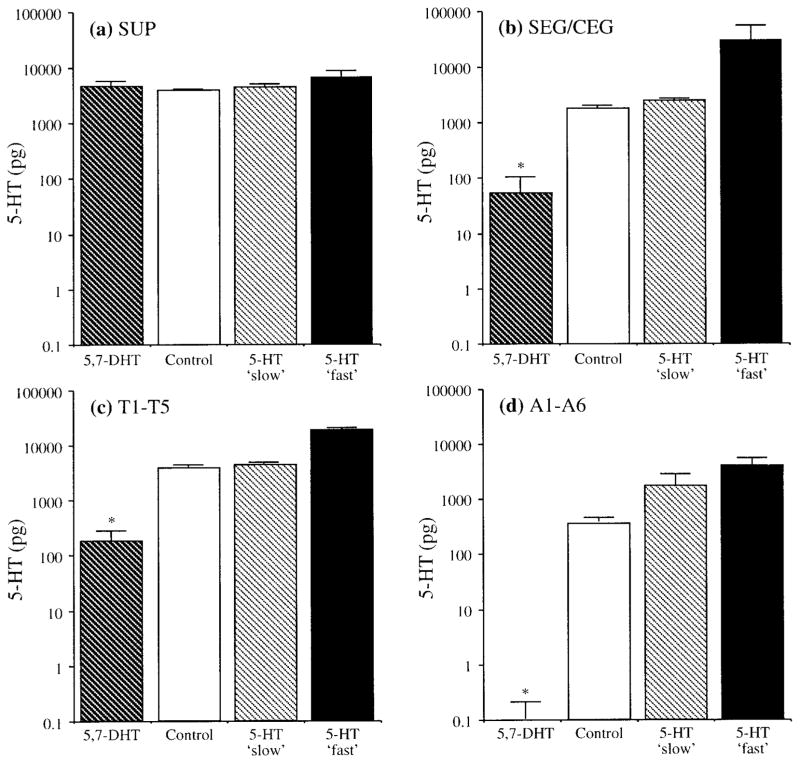
Significant differences in 5-HT (Fig. 1) were detected for A1–A6 [F(4, 53) = 10.46; p < .001], T1–T5 [F(4, 51) = 34.95; p < .001], and SEG/CEG [F(4, 52) = 69.94; p < .001]. 5,7-DHT treated animals had significantly lower levels of 5-HT in A1–A6, T1–T5, and SEG/CEG compared to controls (p < .05 for all posthoc tests). 5-HT content in SUP was not altered by 5,7-DHT [F(4, 52) = 1.54; p = .2]. Results of AMTP treatment are not reported as it neither lowered 5-HT in CNS tissues (except abdominal ganglia) nor produced behavioral effects.
Figure 1.
Graph of 5-HT content (means ± standard error) for different experimental groups plotted on log-scale: (a) SUP, (b) SEG/CEG, (c) T1–T5, and (d) A1–A6. Significant Tukey-Kramer HSD tests are indicated with asterisks. In the 5,7-DHT treated group, all tissues except SUP were significantly lower than controls.
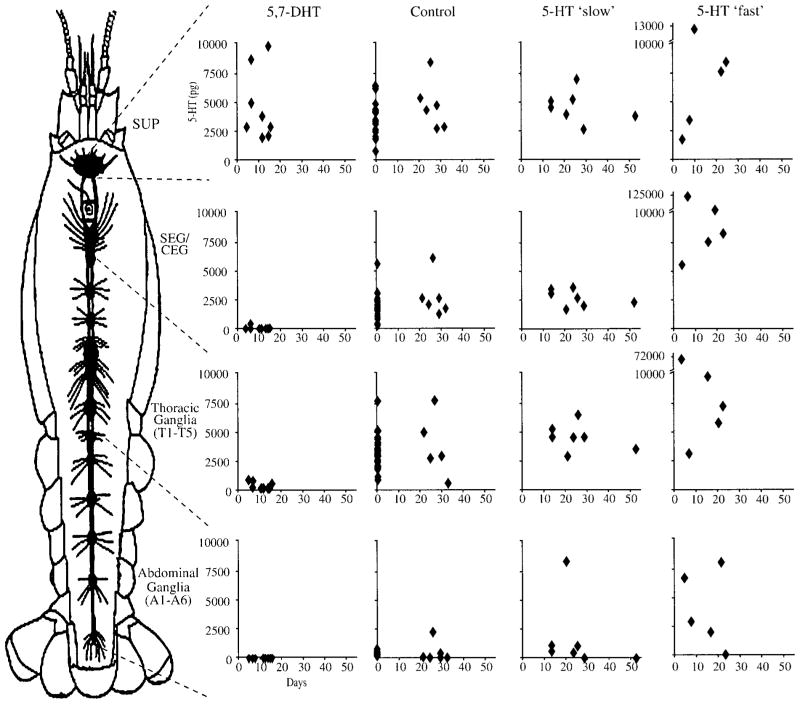
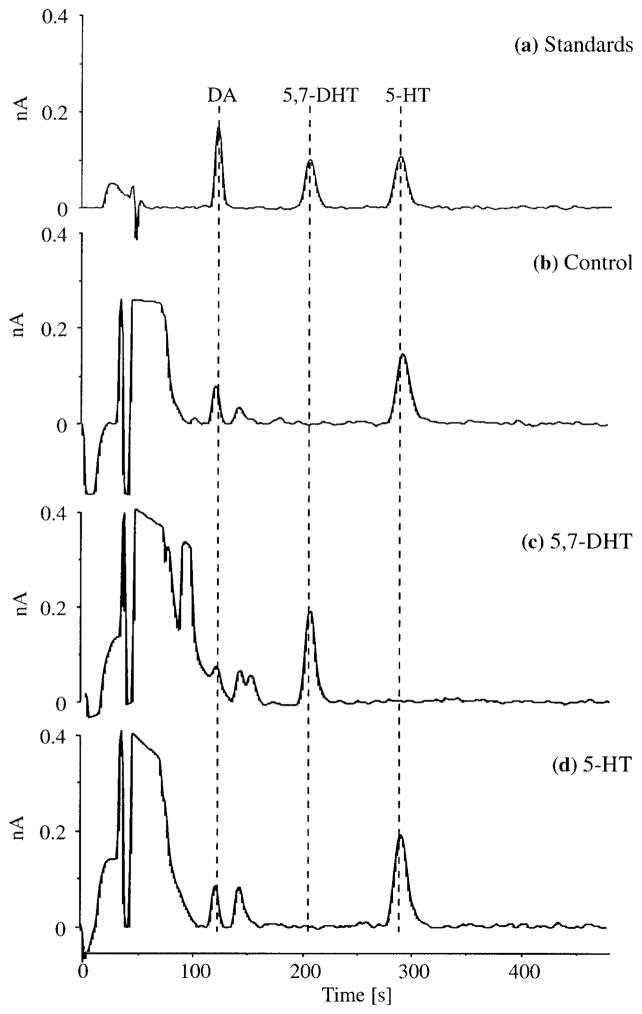
Depletion of 5-HT was evident in 5,7-DHT treated animals after 5 days of treatment and persisted for over 16 days (Fig. 2). No obvious qualitative pattern arose from different lengths of exposure to 5,7-DHT as affected tissues exhibited similar amounts of 5-HT depletion across the entire range of exposure periods. All 5,7-DHT treated crayfish contained considerable amounts of 5,7-DHT in every individual CNS segment (mean ± standard error): (A1–A6) 4.6 ± 1.24 ng, (T1–T5) 9.9 ± 3.89 ng, (SEG/CEG) 4.0 ± 1.02 ng, and (SUP) 2.0 ± 0.33 ng (Fig. 3). As 5,7-DHT may serve as a 5-HT receptor ligand (see Discussion), the CNS of 5,7-DHT treated crayfish may have thus contained elevated amounts of “neuroactive” molecules [measures of 5-HT and 5,7-DHT combined F(4, 51) 13.43; p < .001]. No significant differences in 5-HT content were found in CNS tissues that were exposed to 5-HT at different rates (see Fig. 1). 5-HT content was generally elevated after short periods of exposure to 5-HT fast tubes, but tended to return to control levels following longer treatment (see Fig. 2).
Figure 2.
Scatter plots depict the amount of 5-HT (pg) as a function of treatment duration (days). Data points represent individual tissues with treatment groups arranged in columns and tissues arranged in rows.
Figure 3.
Sample chromatograms for (a) an external standard, T1–T5 tissue from (b) a control animal, and individuals treated with (c) 5,7-DHT and (d) 5-HT “slow.” Substances are identified based on elution times across a range of mobile phase conditions: (DA) 116–120 s, (5,7-DHT) 198–202 s, and (5-HT) 290–294 s. Note the presence of a conspicuous peak (a likely 5-HT metabolite) that appears soon after the elution of DA.
Behavior
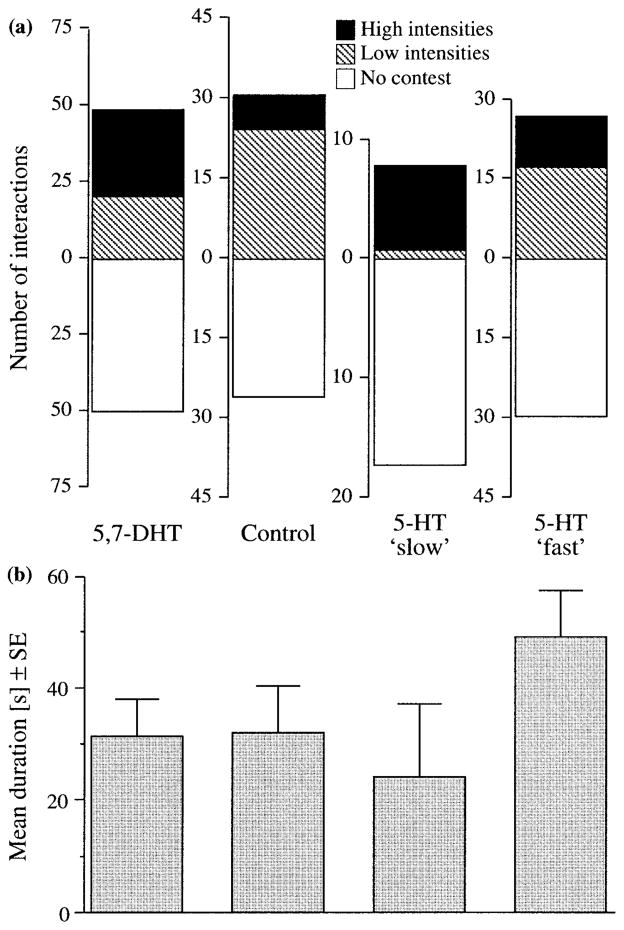
Table 2 summarizes statistical tests performed on all behavioral measures. Significant effects were found for the identity of the initiating [G(4) = 13.42; p < .01] but not the retreating [G(4) = 4.47; p = .4] animal. Decreased probability (FTDcrit = ±1.23) to initiate fights was found in opponents of 5-HT slow treated crayfish (FTD = −1.54). Moreover, the number of interactions reaching a particular fight intensity depended on experimental condition [G(8) = 27.96; p < .001]. Low fight intensities were observed in fewer instances than expected (FTDcrit ± 1.43) in trials involving 5-HT slow treated crayfish (FTD = −3.14). No significant differences (Fig. 4) were found between treatment groups for fight duration [F(4, 314) = 1.06; p = .4] or for the frequency of unrestrained combat [intensity 4; F(4, 314) = 0.44; p = .8]. Moreover, to account for the high degree of interrelatedness between fight duration and intensity (Spearman’s r = 0.59 and see below) MANOVA was performed. No significant differences in fighting behavior were apparent [Wilks’ Lambda = 0.99, F(4, 314) = 1.07; p = .4].
Table 2.
Results from Statistical Tests Performed on Behavioral Variables
| Variable | Source | df | SS or -LLH | F or G | p |
|---|---|---|---|---|---|
| Initiate | -LLH | 4 | 6.71 | 13.42 | <.01* |
| error | 28 | 16.02 | |||
| Retreat | -LLH | 4 | 2.23 | 4.47 | .346 |
| error | 38 | 27.47 | |||
| Duration | ANOVA | 4 | 19000.80 | 1.06 | .374 |
| error | 314 | 1401742.90 | |||
| Max. intensity | -LLH | 8 | 13.98 | 27.96 | <.001* |
| error | 308 | 317.08 | |||
| # Intensity 4 | ANOVA | 4 | 0.53 | 0.44 | .777 |
| error | 314 | 92.92 | |||
| Whole model regression | duration | 1 | 184.82 | 152.74 | <.001* |
| treatment | 4 | 16.28 | 3.36 | .01* | |
| dur. × treat. | 4 | 39.25 | 8.11 | <.001* |
An α of <.05 was considered significant.
Figure 4.
Measures of aggression were largely unchanged by experimental treatments. (a) Total number of interactions observed is plotted on the ordinate: (5,7-DHT) n = 100 in eight trials, (control) n = 63 in five trials, (5-HT “slow”) n = 26 in four trials, and (5-HT “fast”) n = 65 in five trials. The number of fights reaching a particular intensity is represented with different shades. Intensities were pooled to reduce empty cells in statistical analyses: (No contest) intensity 0, (low intensities) intensity 1 or 2, and (high intensities) intensity 3 or 4. (b) Average duration of interactions for experimental groups.
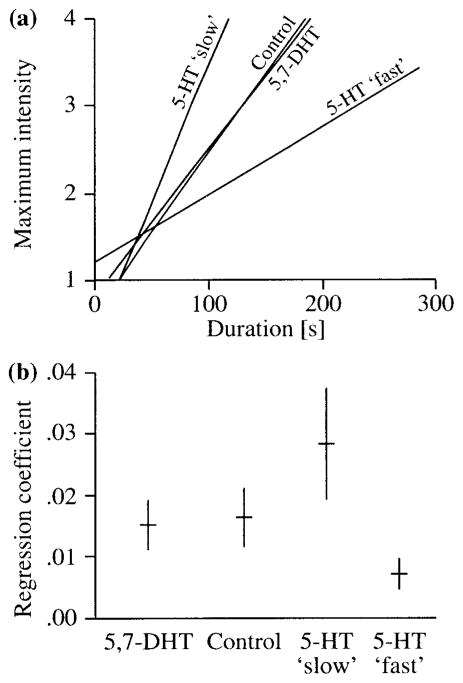
Duration predicted the maximum intensity of interactions [F(4, 309) = 152.74; p < .001] and also accounted for a large amount of the total variation in fight intensity (r2 = 0.35). In addition, significant treatment [F(4, 309) = 3.36; p = .01] and interaction [duration × treatment; F(4, 309) = 8.11; p < .001] effects were detected. The slope of each regression line, which measures the average rate of escalation, varied considerably across treatment groups. Compared to all other treatment groups, 5-HT fast escalated more slowly while 5-HT slow treated crayfish escalated more rapidly (Fig. 5).
Figure 5.
The slope (i.e., regression coefficient) of each regression line represents the average rate of escalation and provides an estimate for the maximum intensity of an agonistic encounter given a certain duration. (a) The linear regression functions for each experimental group were: (5,7-DHT) y = 0.843 + 0.017 x, (control) y = 0.668 + 0.018x, (5-HT “slow”) y = 0.334 + 0.031x, and (5-HT “fast”) y = 0.224 + 0.008x. (b) Graph of regression coefficients of the linear function for experimental groups and their respective 95% confidence intervals.
DISCUSSION
The biochemical and behavioral effects reported in this article did not result in a single, clear pattern, and thus interpretation becomes complex when such levels of analysis are considered simultaneously. The present findings suggest changes in nervous system function that occurred at levels of organization outside the focus of our experimental design. Three potential, albeit not mutually exclusive, explanations for the results of the present study are discussed.
Anatomical Location of Serotonin Neurons Involved in Decapod Aggression
Previous work implicates neurohormonal modulation as one mode of action for 5-HT in crustacean aggression (Beltz and Kravitz, 1987; Kravitz, 2000). 5-HT is released into the circulation from neurosecretory terminals originating in the first abdominal (A1) ganglion (Beltz and Kravitz, 1987), allowing it to potentially affect many areas concurrently (Pasztor and Bush, 1989; Glusman and Kravitz, 1982; Listerman et al., 2000; Santos et al., 2001). Mediated through an apparent change in activity of neurons involved in aggressive motivation, raising concentrations of 5-HT in the hemolymph fosters a decreased willingness to retreat in subordinate crayfish and lobsters (Huber et al., 1997a,b, 2001b; Huber and Delago, 1998).
In the current study, the collective lack of behavioral effects measured in individuals depleted of 5-HT in the A1 ganglion demonstrates that, at this site, decreasing levels of 5-HT does not by itself prevent the normal expression or utility of agonistic behaviors. Large reductions in 5-HT, however, were not found in all CNS tissues. For instance, 5-HT content in the supraesophageal ganglion was particularly resistant to chronic treatment as it was unaffected in all treatment conditions. HPLC/ED detected the presence of 5,7-DHT in every tissue, including the supraesophageal ganglion, suggesting that, relative to other CNS tissues, crayfish brain is resistant to such pharmacological intervention. It also indicates that failure to alter 5-HT content in crayfish brain is not simply due to the existence of a functional blood brain barrier. Treatment may, however, have potentially induced changes at a variety of other levels including synaptic availability (Doernberg et al., 2001) or the recruitment of non-5-HT neurons (Beltz et al., 1998; Musolf and Edwards, 2000).
5-HT neurons in decapod crustacean brain have been identified (Beltz and Kravitz, 1983; Sandeman et al., 1988) and studied (Sandeman and Sandeman, 1994; Benton et al., 1997), although their individual roles during agonistic encounters have not yet been explored. In our experiments, failure to alter 5-HT in the brain could account for the lack of behavioral effects found in 5,7-DHT treated crayfish. Key sites in the brain, such as the dorsal giant neurons (Benton et al., 1997; Benton and Beltz, 2001), are likely to be important during such situations—keeping in mind that complex behaviors like aggression presumably cannot be reduced to a single anatomical site or neurochemical substrate. Such “higher” brain areas may exert descending influence on postural systems or neurosecretory sites. Future studies are therefore needed to identify specific behavioral effects of 5-HT associated with the supraesophageal ganglion.
Does 5,7-DHT Have “Neuroactive” Properties in the Crustacean Nervous System?
Acute injection of 5,7-DHT into decapod crustaceans elicits a posture resembling the “dominant-like” stance characteristic of 5-HT injections (Livingstone et al., 1980; Glanzman and Krasne, 1986; Antonsen and Paul, 1997). Such results could be due to 5,7-DHT’s releasing properties (Cook and Orchard, 1993) or an agonist-effect at 5-HT binding sites. At least at some synapses, however, different physiological effects result from application of 5-HT and 5,7-DHT. 5,7-DHT produces modest depolarization of specific neurons in the A1 ganglion (Doernberg et al., 2001), while 5-HT mediates comparatively stronger depolarizing activity followed by periods of prolonged inhibition (Heinrich et al., 1999). Nevertheless, 5,7-DHT treatment reduces 5-HT content in decapod CNS (cf. Doernberg et al., 2001 and this article). With regard to the present experiments, however, care must be invoked when interpreting the general lack of behavioral effects accompanying such depletions. Namely, is reducing 5-HT levels (with 5,7-DHT treatment) equivalent to depressing 5-HT function? The present results raise the caveat that nonspecific actions of 5,7-DHT, at least to an extent, may counterbalance its depleting effect on 5-HT content.
Compensatory Responses to Pharmacological Interventions
The complex array of physiological and behavioral results illustrated in this study indicate that no simple pattern links long-term changes in 5-HT levels and fighting behavior. For instance, neither 5-HT treatment significantly altered 5-HT content in the CNS, although such treatments produced opposite effects on the rate of fight escalation. Resistance in altering 5-HT levels due to chronic pharmacological intervention may result from the system’s ability to maintain a homeostatic set point (i.e., a functional balance). The existence of compensatory mechanisms is consistent with similar reports of failure to disrupt 5-HT levels in the brain of a sea slug, Tritonia diomedea (Fickbohm et al., 2000), where 5-HT was uninfluenced by most pharmacological treatments although its precursors and metabolites were. The notion that “appropriate” levels of 5-HT are needed for a given site and time (Doernberg et al., 2001) corroborates the suggestion that crustacean 5-HT systems can compensate to maintain functional integrity. Moreover, compensatory feedback between monoamine systems and chronic biochemical intervention has been demonstrated at the level of receptor turnover (Patel et al., 1996; Woo et al., 1996), synthesis (Stachowiak et al., 1986; Sivam, 1995), metabolism (Ase et al., 2000), reuptake (Gobbi et al., 1994; Pan et al., 2001), and synaptic availability (Lent, 1984; Hall et al., 1999). As is apparent from results in other invertebrate systems (O’Gara et al., 1991), the present experiments suggest that such mechanisms were not only activated, but were ultimately manifested at the behavioral level.
The behavioral effects associated with chronic 5-HT treatment (in this study) were unlike those that accompanied acute treatment with 5-HT (Huber and Delago, 1998). Decreased retreat, the main behavioral effect of acute 5-HT infusion, closely parallels the behavior of dominant crayfish during the initial periods of hierarchy formation (Goessmann et al., 2000). In contrast, slow 5-HT treatment produced a behavioral change (i.e., increased escalation) similar to what is found in dominant crayfish after several days of behavioral reinforcement (Issa et al., 1999; Goessmann et al., 2000). Such findings have implications for the formation of social dominance in crayfish. Namely, are compensatory mechanisms, similar to those apparent in 5-HT treated animals, also activated within natural contexts? Recent work supports such a possibility. For instance, CNS levels of 5-HT do not vary as a function of social rank (Huber et al., 2001b). Moreover, established dominance facilitates dynamic, synaptic alterations that occur at multiple time frames (Yeh et al., 1996,1997; Krasne et al., 1997). Rather than the absolute amount of 5-HT in the CNS, it is likely that magnitude, duration, and temporal pattern of release, as well as synaptic neuromodulator ratios, are critical for determining the resulting behavioral phenotype (Huber and Delago, 1998; Crider and Cooper, 1999; Listerman et al., 2000; Peeke et al., 2000; Sneddon et al., 2000; Kravitz, 2000; Teshiba et al., 2001).
CONCLUSIONS
As a motivational state, the term aggression is invoked as an intervening concept because it is of inherent utility for predicting a range of behavioral acts. The explanatory power afforded by such a concept, however, is diminished when each of its proximate mechanisms are viewed as static characteristics. Previous attempts to study “aggression” have centered on implementing pharmacological compounds with broad and often unknown actions (Olivier et al., 1989; Fuller, 1996), or “knocking out” one component of a particular system for a prolonged duration (Chen et al., 1994; Saudou et al., 1994; Cases et al., 1995). Despite the information derived from such approaches, the current status of our understanding is, in fact, that we understand very little about 5-HT’s role in aggression without considering specific brain regions (Blanchard et al., 1991; Amstislavskaya and Kudryavtseva, 1997; Korzan et al., 2000), genetic history (Brunner et al., 1993; Cases et al., 1995), or social context (Ison et al., 1996; Yeh et al., 1997; Berton et al., 1999; Ferris, 2000). Moreover, the role of 5-HT in aggression may depend heavily on its interaction with other molecules (Ferris and Delville, 1994). A single neural substrate, such as amounts of a particular neuromodulator, is unlikely to explain the functional role of monoamine systems in behavior (Lederhendler and Schulkin, 2000). Similar perspectives have emerged from work on steroid hormones in developing insect (Henrich and Brown, 1995) and avian (Arnold and Schlinger, 1993; Ottinger et al., 1997) species, the effects of neuromodulators on network properties (Johnson and Harris-Warrick, 1990; Katz et al., 1994; Stevenson and Meuser, 1997), and environment-mediated alterations in neurochemical systems (Fox et al., 1997; Yeh et al., 1997; Soma et al., 2000). Such systems seem to foster adaptive behavior within the proper context (Kravitz, 1988).
Taken together, the biochemical and behavioral results of the present experiments make clear that although our goal was to measure effects of absolute 5-HT content on aggression, it is likely that we observed the behavioral effects caused by a complex cascade of neurochemical changes. Moreover, the present study underscores the importance of verifying the effectiveness and specificity of pharmacological substances in every system in which they are used. In conjunction with findings of Doernberg et al. (2001), an increasingly dynamic view of the neurochemical basis of aggression has emerged from recent work in crustacean species. A more productive framework for studying the interface between neuromodulators and behavior may be to view such molecules only as short-term modulatory components embedded within larger, dynamically organized systems that are critical for governing behavior. Chronic removal of one element will thus fail to cause a single, well-defined deficit, but rather it will reconstitute the entire neuronal environment. Accordingly, combining physiology and molecular biology with quantitative behavioral analyses is a principle goal for future research. In decapod crustaceans, such a combination offers a fruitful avenue for bringing behavioral analyses down to the level of individual neurons and thereby allowing for a more thorough understanding of causal mechanisms in aggression.
Acknowledgments
Contract grant sponsor: NSF; contract grant numbers: IBN-9874608 and DBI-0070334 (R.H.).
Contract grant sponsor: NIH; contract grant number: MH62557-01 (R.H.).
We are grateful to S. Tuttle, A. Daws, J. Panksepp, V. Bingman, P. Moore, members of the Huber Laboratory, and the Laboratory for Sensory Ecology for comments on earlier versions of this manuscript. We also thank three anonymous reviewers for their helpful comments and suggestions. The Bowling Green State University honors department supported this research as part of the requirement for completion of a senior honors thesis to JBP.
References
- Amstislavskaya TG, Kudryavtseva NN. Effect of repeated experience of victory and defeat in daily agonistic confrontations on brain tryptophan hydroxylase activity. Fed Euro Biochem Soc. 1997;406:106–108. doi: 10.1016/s0014-5793(97)00252-4. [DOI] [PubMed] [Google Scholar]
- Antonsen BL, Paul DH. Serotonin and octopamine elicit sterotypical agonistic behaviors in the squat lobster Munidia quadrispina (Anomura, Galatheidae) J Comp Physiol A. 1997;181:501–510. [Google Scholar]
- Arnold AP, Schlinger BA. Sexual-differentiation of brain and behavior: the zebra finch is not just a flying rat. Brain Behav Evol. 1993;42:231–241. doi: 10.1159/000114157. [DOI] [PubMed] [Google Scholar]
- Ase AR, Reader TA, Hen R, Riad M, Descarries L. Altered serotonin and dopamine metabolism in the CNS of serotonin 5-HT1A or 5-HT1B receptor knockout mice. J Neurochem. 2000;75:2415–2426. doi: 10.1046/j.1471-4159.2000.0752415.x. [DOI] [PubMed] [Google Scholar]
- Austad SN. Game theory and the evolution of animal contests. Trends Ecol Evol. 1989;4:2–3. [Google Scholar]
- Barki A, Harpaz S, Karplus I. Contradictory asymmetries in body and weapon size, and assessment in fighting male prawns, Macrobrachium rosenbergii. Aggressive Behav. 1997;23:81–91. [Google Scholar]
- Beltz BS, Kravitz EA. Mapping of serotonin-like immunoreactivity in the lobster nervous system. J Neurosci. 1983;3:585–602. doi: 10.1523/JNEUROSCI.03-03-00585.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz BS, Kravitz EA. Physiological identification, morphological analysis and development of identified serotonin-proctolin containing neurons in the lobster ventral nerve chord. J Neurosci. 1987;7:533–547. doi: 10.1523/JNEUROSCI.07-02-00533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz BS, Richards K, Marder E. The serotonin transporter and receptor mature prior to serotonin appearance in embryonic STG: a borrowed transmitter hypothesis. Soc Neurosci Abstr. 1998;24:107. [Google Scholar]
- Benton J, Huber R, Ruchhoeft M, Helluy S, Beltz B. Serotonin depletion by 5,7 dihydroxytryptamine alters deutocerebral development in the lobster, Homarus americanus. J Neurobiol. 1997;33:357–373. doi: 10.1002/(sici)1097-4695(199710)33:4<357::aid-neu2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Benton JL, Beltz BS. Effects of serotonin depletion on local interneurons in the developing olfactory pathway of lobsters. J Neurobiol. 2001;46:193–205. doi: 10.1002/1097-4695(20010215)46:3<193::aid-neu1002>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Berrill M. Distribution and ecology of crayfish in the Kawartha Lakes region of southern Ontario. Can J Zool. 1978;56:166–177. [Google Scholar]
- Berrill M, Arsenault M. The breeding behaviour of a northern temperate orconectid crayfish, Orconectes rusticus. Anim Behav. 1984;32:333–339. [Google Scholar]
- Berton O, Durand M, Aguerre S, Mormède P, Chaouloff F. Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the lewis rat strain. Neurosci. 1999;92:327–341. doi: 10.1016/s0306-4522(98)00742-8. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Panrapee C, Blanchard RJ, Clow DW, Hammer RP, Jr, Rowlett JK, Bardo MT. Serotonin, but not dopamine, metabolites are increased in selected brain regions of subordinate male rats in a colony environment. Brain Res. 1991;568:61–66. doi: 10.1016/0006-8993(91)91379-f. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Serotonin and drug-induced therapeutic responses in major depression, obsessive-compulsive and panic disorders. Neuropsychopharmacol. 1999;21:91–98. doi: 10.1016/S0893-133X(99)00036-6. [DOI] [PubMed] [Google Scholar]
- Boadle MC, Blaschko H. Cockroach amine oxidase: classification and substrate specificity. Comp Biochem Physiol. 1968;25:129–138. doi: 10.1016/0010-406x(68)90919-5. [DOI] [PubMed] [Google Scholar]
- Bovbjerg RV. Dominance order in the crayfish Orconectes virilis (Hagen) Physiol Zool. 1953;26:127–136. [Google Scholar]
- Bovbjerg RV. Some factors affecting aggressive behavior in crayfish. Physiol Zool. 1956;29:127–136. [Google Scholar]
- Bradbury CW. Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine use. J Neurosci. 2000;20:7109–7115. doi: 10.1523/JNEUROSCI.20-18-07109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Bruski CA, Dunham DW. The importance of vision in agonistic communication of crayfish Orconectes rusticus: an analysis of bout dynamics. Behaviour. 1987;63:83–107. [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, De Maeyer E. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase ID, Costanza B, Dugatkin LA. Aggressive interactions and inter-contest interval: how long do winners keep winning? Anim Behav. 1994;48:393–400. [Google Scholar]
- Chen C, Rainnie D, Greene RW, Tonegawa S. Abnormal fear response and aggressive behavior in mutant mice deficient for alpha-calcium-calmodulin kinase II. Science. 1994;266:291–294. doi: 10.1126/science.7939668. [DOI] [PubMed] [Google Scholar]
- Cook H, Orchard I. The short term effects of 5, 7 dihydroxytryptamine on peripheral serotonin stores in Rhodnius prolixus and their long term recovery. Insect Biochem Molec Biol. 1993;23:895–904. [Google Scholar]
- Crider ME, Cooper RL. Importance of stimulation paradigm in determining facilitation and effects of neuromodulation. Brain Res. 1999;842:324–331. doi: 10.1016/s0006-8993(99)01816-8. [DOI] [PubMed] [Google Scholar]
- Dale N, Kandel ER, Schacher S. Serotonin produces long-term in the excitability of Aplysia sensory neurons in culture that depend on new protein synthesis. J Neurosci. 1987;7:2232–2238. doi: 10.1523/JNEUROSCI.07-07-02232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws AG, Grills J, Konzen K, Moore PA. Previous experiences alter the outcome of aggressive interactions between males in the crayfish, Procambarus clarkii. J Mar Freshwater Behav Physiol. 2001 in press. [Google Scholar]
- Delago A, Kravitz EA, Huber R. Effects of chronic fluoxetine on agonistic behavior of crayfish and lobsters. in review. [Google Scholar]
- Dewhurst SA, Croker SG, Ikeda K, McCaman RE. Metabolism of biogenic amines in Drosophilla nervous tissue. Comp Biochem Physiol. 1972;43B:975–981. doi: 10.1016/0305-0491(72)90241-6. [DOI] [PubMed] [Google Scholar]
- Doernberg SB, Cromarty SI, Heinrich R, Beltz BS, Kravitz EA. Agonistic behavior in naive juvenile lobsters depleted of serotonin by 5,7-dihydroxytryptamine. J Comp Physiol A. 2001;187:91–103. doi: 10.1007/s003590000178. [DOI] [PubMed] [Google Scholar]
- Dubbels R, Elofsson R. N-acetylation of arylalkylamines (serotonin and tryptamine) in the crayfish brain. Comp Biochem Physiol. 1989;93C:307–312. [Google Scholar]
- Dyakonova VE, Schürmann FW. Effects of serotonergic and opiodergic drugs on escape behaviors and social status of male crickets. Naturwissenschaften. 1999;86:435–437. doi: 10.1007/s001140050647. [DOI] [PubMed] [Google Scholar]
- Enquist M, Leimar O. Evolution of fighting behaviour: decision rules and assessment of relative strength. J Theor Biol. 1983;102:387–410. [Google Scholar]
- Ferris CF. Adolescent stress and neural plasticity in hamsters: a vasopressin-serotonin model of inappropriate aggressive behaviour. Exp Physiol. 2000;85:85S–90S. doi: 10.1111/j.1469-445x.2000.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Delville Y. Vasopressin and serotonin interactions in the control of agonistic behavior. Psychoneuroendocrinol. 1994;19:593–601. doi: 10.1016/0306-4530(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Fickbohm DJ, Spitzer N, Katz PS. Serotonin homeostasis in the brain of Tritonia Diomedea. Soc Neurosci Abstr. 2000;26:343.5. [Google Scholar]
- Flugge G, Ahrens O, Fuchs E. Monoamine receptors in the prefrontal cortex of Tupaia belangeri during chronic psychosocial stress. Cell Tissue Res. 1997;288:1–10. doi: 10.1007/s004410050787. [DOI] [PubMed] [Google Scholar]
- Fox HE, White SA, Kao MHF, Fernald RD. Stress and dominance in a social fish. J Neurosci. 1997;17:6463–6469. doi: 10.1523/JNEUROSCI.17-16-06463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RW. The influence of fluoxetine on aggressive behavior. Neuropsychopharmacol. 1996;14:77–81. doi: 10.1016/0893-133X(95)00110-Y. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Krasne FB. Serotonin and octopamine have opposite modulatory effects on the crayfish’s lateral giant escape reaction. J Neurosci. 1983;3:2263–2269. doi: 10.1523/JNEUROSCI.03-11-02263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzman DL, Krasne FB. 5,7-dihydroxytryptamine lesions of crayfish serotonin-containing neurons: effect on the lateral giant escape reaction. J Neurosci. 1986;6:1560–1569. doi: 10.1523/JNEUROSCI.06-06-01560.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glusman S, Kravitz EA. The action of serotonin on excitatory nerve terminals in lobster nerve-muscle preparations. J Physiol (Lond) 1982;325:223–241. doi: 10.1113/jphysiol.1982.sp014147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi M, Regodi MC, Pompeiano M, Palacios JM, Mennini T. Differential effects of 5,7-dihydroxytryptamine-induced serotonergic degeneration on 5-HT1A receptors and 5-HT uptake sites in the rat brain. J Chem Neuroanat. 1994;7:65–73. doi: 10.1016/0891-0618(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Goessmann C, Hemelrijk C, Huber R. The formation and maintenance of crayfish hierarchies: behavioral and self-structuring properties. Behav Ecol Sociobiol. 2000;48:418–428. [Google Scholar]
- Hall FS, Devries AC, Fong GW, Huang S, Pert A. Effects of 5,7-dihydroxytryptamine depletion of tissue serotonin levels on extracellular serotonin in the striatum assessed with in vivo microdialysis: relationship to behavior. Synapse. 1999;33:16–25. doi: 10.1002/(SICI)1098-2396(199907)33:1<16::AID-SYN2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick R, Kravitz EA. Cellular mechanisms for modulation of posture by octopamine and serotonin in the lobster. J Neurosci. 1984;4:1976–1993. doi: 10.1523/JNEUROSCI.04-08-01976.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich R, Cromarty SI, Horner M, Edwards DH, Kravitz EA. Autoinhibition of serotonin cells: an intrinsic regulatory mechanism sensitive to the pattern of usage of the cells. Proc Natl Acad Sci USA. 1999;96:2473–2478. doi: 10.1073/pnas.96.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich VC, Brown NE. Insect nuclear receptors a development and comparative perspective. Insect Biochem Molec. 1995;25:881–897. doi: 10.1016/0965-1748(95)00030-y. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Auerbach SB. 5-HT 1A autoreceptors and the mode of action of SSRI’s. Behav Brain Res. 1996;73:281–283. doi: 10.1016/0166-4328(96)00113-1. [DOI] [PubMed] [Google Scholar]
- Hollis KL, Dumas MJ, Singh P, Fakelman P. Pavlovian conditioning of aggressive behavior in blue gourami fish (Trichogaster trichopterus): winners become winners and losers stay losers. J Comp Psychol. 1995;2:123–133. [Google Scholar]
- Hörner M, Weiger WA, Edwards DH, Kravitz EA. Excitation of identified serotonergic neurons by escape command neurons in lobsters. J Exp Biol. 1997;200:2017–2033. doi: 10.1242/jeb.200.14.2017. [DOI] [PubMed] [Google Scholar]
- Hsu Y, Wolf LL. The winner and loser effect: integrating multiple experiences. Anim Behav. 1999;57:903–910. doi: 10.1006/anbe.1998.1049. [DOI] [PubMed] [Google Scholar]
- Huber R, Daws A, Tuttle S, Panksepp JB. Quantitative behavioral techniques for the study of crustacean aggression. In: Wiese K, Schmidt M, editors. Physiology of the crustacean nervous system. Berlin: Springer; 2001a. [Google Scholar]
- Huber R, Delago A. Serotonin alters decisions to withdraw in fighting crayfish, Astacus astacus: the motivational concept revisited. J Comp Physiol A. 1998;182:573–583. [Google Scholar]
- Huber R, Kravitz EA. A quantitative analysis of agonistic behavior in juvenile american lobsters (Homarus americanus) Brain Behav Evol. 1995;46:72–83. doi: 10.1159/000113260. [DOI] [PubMed] [Google Scholar]
- Huber R, Orzeszyna M, Pokorny N, Kravitz EA. Biogenic amines and aggression: experimental approaches in crustaceans. Brain Behav Evol. 1997a;50(suppl 1):60–68. doi: 10.1159/000113355. [DOI] [PubMed] [Google Scholar]
- Huber R, Panksepp JB, Yue Z, Delago A, Moore P. Dynamic interactions of behavior and amine neurochemistry in acquisition and maintenance of social rank in crayfish. Brain Behav Evol. 2001b;57:271–282. doi: 10.1159/000047245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Smith K, Delago A, Isaksson K, Kravitz EA. Serotonin and aggressive motivation in crustaceans: altering the decision to retreat. Proc Natl Acad Sci USA. 1997b;94:5939–5942. doi: 10.1073/pnas.94.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison M, Fachinelli C, Rodríguez ELE. Effect of the ICV injection of 5,7-di-hydroxytryptamine on the aggressive behavior of dominant and submissive pigeons (Columba livia) Pharmacol Biochem Behav. 1996;53:951–955. doi: 10.1016/0091-3057(95)02005-5. [DOI] [PubMed] [Google Scholar]
- Issa FA, Adamson DJ, Edwards DH. Dominance hierarchy formation in juvenile crayfish Procambarus clarkii. J Exp Biol. 1999;202:3497–3506. doi: 10.1242/jeb.202.24.3497. [DOI] [PubMed] [Google Scholar]
- Jackson WM. Why do winners keep winning? Behav Ecol Sociobiol. 1991;28:271–276. [Google Scholar]
- Johnson BR, Harris-Warrick RM. Aminergic modulation of graded synaptic transmission in the lobster stomatogastric ganglion. J Neurosci. 1990;10:2066–2076. doi: 10.1523/JNEUROSCI.10-07-02066.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnofsky EB, Atema J, Elgin RH. Field observations of social behavior, shelter use, and foraging in the lobster, Homarus americanus. Biol Bull. 1989;176:239–246. doi: 10.2307/1541982. [DOI] [PubMed] [Google Scholar]
- Katz PS, Getting PA, Frost WN. Dynamic neuromodulation of synaptic strength intrinsic to a central pattern generator circuit. Nature. 1994;367:729–731. doi: 10.1038/367729a0. [DOI] [PubMed] [Google Scholar]
- Kennedy MB. Products of biogenic amine metabolism in the lobster: sulfate conjugates. J Neurochem. 1978;30:315–320. doi: 10.1111/j.1471-4159.1978.tb06532.x. [DOI] [PubMed] [Google Scholar]
- Klemm HP, Baumgarten HP, Schlossberger HG. In vitro studies on the interaction of brain monoamine oxidase with 5,6 dihydroxytryptamine and 5,7 dihydroxytryptamine. J Neurochem. 1979;32:111–119. doi: 10.1111/j.1471-4159.1979.tb04517.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacol. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Korzan WJ, Summers TR, Summers CH. Monoaminergic activities of limbic regions are elevated during aggression: influence of sympathetic social signalling. Brain Res. 2000;970:170–178. doi: 10.1016/s0006-8993(00)02420-3. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Markowska L, Markiewicz L. On the role of serotonin in aggressive behaviour of ants Genus Formica. J Pharmacol Pharmacy (Poland) 1975;27:237–239. [PubMed] [Google Scholar]
- Krasne FB, Shamsian A, Kulkarni R. Altered excitability of the crayfish lateral giant escape reflex during agonistic encounters. J Neurosci. 1997;17:709–716. doi: 10.1523/JNEUROSCI.17-02-00709.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz EA. Hormonal control of behavior: amines and the biasing of behavioral output in lobsters. Science. 1988;241:1775–1780. doi: 10.1126/science.2902685. [DOI] [PubMed] [Google Scholar]
- Kravitz EA. Serotonin and aggression: insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. J Comp Physiol A. 2000;186:221–238. doi: 10.1007/s003590050423. [DOI] [PubMed] [Google Scholar]
- Kravitz EA, Beltz B, Glusman S, Goy M, Harris-Warrick R, Johnston M, Livingstone M, Schwarz T. The well-modulated lobster: the roles of serotonin, octopamine and proctolin in the lobster nervous system. Pest Biochem Physiol. 1984;22:133–147. [Google Scholar]
- Kudryavtseva NN, Avgustinovich DF. Behavioral and physiological markers of experimental depression induced by social conflicts (DISC) Aggressive Behav. 1998;24:271–286. [Google Scholar]
- Larson ET, Summers CH. Serotonin reverses dominant social status. Behav Brain Res. 2001;121:95–102. doi: 10.1016/s0166-4328(00)00393-4. [DOI] [PubMed] [Google Scholar]
- Lederhendler I, Schulkin J. Behavioral Neuroscience: challenges for the era of molecular biology. Trends Neurosci. 2000;23:451–454. doi: 10.1016/s0166-2236(00)01636-2. [DOI] [PubMed] [Google Scholar]
- Lent CM. Quantitative effects of a neurotoxin upon serotonin levels within tissue compartments of the medicinal leech. J Neurobiol. 1984;15:309–323. doi: 10.1002/neu.480150502. [DOI] [PubMed] [Google Scholar]
- Lent CM, Dickinson MH. Retzius cells retain functional membrane properties following ‘ablation’ by the neurotoxin 5,7-DHT. Brain Res. 1984;300:167–171. doi: 10.1016/0006-8993(84)91353-2. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Merschdorf U. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behav Sci Law. 2000;18:581–604. doi: 10.1002/1099-0798(200010)18:5<581::aid-bsl411>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Listerman LR, Deskins J, Bradacs H, Cooper RL. Heart rate within male crayfish: social interactions and effects of 5-HT. Comp Biochem Physiol A. 2000;125:251–263. doi: 10.1016/s1095-6433(99)00180-4. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Harris-Warrick R, Kravitz EA. Serotonin and octopamine produce opposite postures in lobsters. Science. 1980;208:76–79. doi: 10.1126/science.208.4439.76. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Schaeffer SF, Kravitz EA. Biochemistry and ultrastructure serotonin nerve endings in the lobster: serotonin and octopamine are contained in different nerve endings. J Neurobiol. 1981;12:27–54. doi: 10.1002/neu.480120104. [DOI] [PubMed] [Google Scholar]
- Ma P, Beltz B, Kravitz EA. Serotonin-containing neurons in lobsters: their role as gain-setters in postural control mechanisms. J Neurophysiol. 1992;68:36–53. doi: 10.1152/jn.1992.68.1.36. [DOI] [PubMed] [Google Scholar]
- Ma P, Weiger W. Serotonin-containing neurons in lobsters: the actions of GABA, octopamine, serotonin and proctolin on activity of a pair of identified neurons in the first abdominal ganglia. J Neurosci. 1993;69:2015–2029. doi: 10.1152/jn.1993.69.6.2015. [DOI] [PubMed] [Google Scholar]
- Maas JW. Neurochemical differences between two strains of mice. Science. 1962;137:621–622. doi: 10.1126/science.137.3530.621. [DOI] [PubMed] [Google Scholar]
- Maler L, Ellis WG. Inter-male aggressive signals in weakly electric fish are modulated by monoamines. Behav Brain Res. 1987;25:75–81. doi: 10.1016/0166-4328(87)90046-5. [DOI] [PubMed] [Google Scholar]
- Maynard-Smith J, Price GR. The logic of animal conflict. Nature. 1973;246:15–18. [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel EA, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1253. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Musolf BE, Edwards DH. Crayfish hindgut neurons take up serotonin from different 5-HT sources in the terminal ganglion. Soc Neurosci Abstr. 2000;26:643.16. [Google Scholar]
- Novak MG, Rowley WA. Serotonin depletion affects blood-feeding but not host-seeking in Aedes triseriatus. J Med Entomol. 1994;31:599–606. doi: 10.1093/jmedent/31.4.600. [DOI] [PubMed] [Google Scholar]
- O’Gara BA, Heechin C, Latham LB, Friesen O. Modification of leech behavior patterns by reserpine-induced amine depletion. J Neurosci. 1991;11:96–110. doi: 10.1523/JNEUROSCI.11-01-00096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier B, Mos J, van der Heyden J, Hartog J. Serotonergic modulation of social interactions in isolated male mice. Psychopharmacol. 1989;97:154–156. doi: 10.1007/BF00442239. [DOI] [PubMed] [Google Scholar]
- Ottinger MA, Thompson N, Viglietti-Panzica C, Panzica GC. Neuroendocrine regulation of GnRH and behavior during aging in birds. Brain Res Bull. 1997;44:471–477. doi: 10.1016/s0361-9230(97)00228-1. [DOI] [PubMed] [Google Scholar]
- Pan Y, Gembom E, Peng W, Lesch KP, Mossner R, Simantov R. Plasticity in serotonin uptake in primary neuronal cultures of serotonin transporter knockout mice. Dev Brain Res. 2001;126:125–129. doi: 10.1016/s0165-3806(00)00145-0. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Yue Z, Drerup C, Huber R. Amine neurochemistry and aggression in crayfish. Microsc Res Tech. 2002 doi: 10.1002/jemt.10274. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GA, Rubenstein DI. Role assessment, reserve strategy, and the acquisition of information in asymmetric animal conflicts. Anim Behav. 1981;29:221–240. [Google Scholar]
- Pasztor VM, Bush BMH. Primary afferent responses of a crustacean mechanoreceptor are modulated by proctolin, octopamine and serotonin. J Neurobiol. 1989;20:234–254. doi: 10.1002/neu.480200406. [DOI] [PubMed] [Google Scholar]
- Patel TD, Azmitia EC, Zhou FC. Increased 5-HT 1A receptor immunoreactivity in the rat hippocampus following 5,7-DHT lesions in the cingulum bundle and fimbria-fornix. Behav Brain Res. 1996;73:319–323. doi: 10.1016/0166-4328(96)00122-2. [DOI] [PubMed] [Google Scholar]
- Pavey CR, Fielder DR. The influence of size differential on agonistic behaviour in the freshwater crayfish, Cherax cuspidatus. J Soc Zoo Lond. 1996;238:445–457. [Google Scholar]
- Peeke HVS, Blank GS, Figler MH, Chang ES. Effects of exogenous serotonin on a motor behavior and shelter competition in juvenile lobsters (Homarus americanus) J Comp Physiol A. 2000;186:575–582. doi: 10.1007/s003590000113. [DOI] [PubMed] [Google Scholar]
- Raleigh MJ, Mcguire MT, Brammer GL, Pollack DB, Yuwiler A. Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Brain Res. 1991;559:181–190. doi: 10.1016/0006-8993(91)90001-c. [DOI] [PubMed] [Google Scholar]
- Real D, Czternasty G. Mapping of serotonin-like immunoreactivity in the ventral nerve cord of crayfish. Brain Res. 1990;521:203–212. doi: 10.1016/0006-8993(90)91544-q. [DOI] [PubMed] [Google Scholar]
- Reisner IR, Mann JJ, Stanley M, Huang Y-Y, Houpt KA. Comparison of cerebrospinal fluid monoamine metabolite levels in dominant-aggressive and non-aggressive dogs. Brain Res. 1996;714:57–64. doi: 10.1016/0006-8993(95)01464-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rubenstein DI, Hazlett B. Examination of the agonistic behaviour of the crayfish Orconectes virilis by character analysis. Behaviour. 1973;20:193–216. [Google Scholar]
- Sahley CL. Serotonin depletion impairs but does not eliminate classical conditioning in the leech Hirudo medicinalis. Behav Neurosci. 1994;108:1043–1052. doi: 10.1037//0735-7044.108.6.1043. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Sandeman RE. Electrical responses and synaptic connections of giant serotonin-immunoreactive neurons in crayfish olfactory and accessory lobes. J Comp Neurol. 1994;341:130–144. doi: 10.1002/cne.903410111. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Sandeman RE, Aitken AR. Atlas of serotonin-containing neurons in the optic lobes and brain of the crayfish, Cherax destructor. J Comp Neurol. 1988;269:465–478. doi: 10.1002/cne.902690402. [DOI] [PubMed] [Google Scholar]
- Santos EA, Keller R, Rodriguez E, Lopez L. Effects of serotonin and fluoxetine on blood glucose regulation in two decapod species. Braz J Med Biol Res. 2001;34:75–80. doi: 10.1590/s0100-879x2001000100009. [DOI] [PubMed] [Google Scholar]
- Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacing 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- Schroeder L, Huber R. Fighting strategies in small and large individuals of the crayfish, Orconectes rusticus. Behaviour. 2002 in press. [Google Scholar]
- Scrivener JCE. Agonistic behaviour of the American lobster, Homarus americanus (Milne-Edwards) Fish Res Board Can Tech Rep. 1971;235:1–126. [Google Scholar]
- Sivam SP. Dopamine, serotonin and tachykinin in self-injurious behavior. Life Sci. 1996;58:2367–2375. doi: 10.1016/0024-3205(96)00121-x. [DOI] [PubMed] [Google Scholar]
- Sloley BD, Goldberg JI. Determination of gamma-glutamyl conjugates of monoamines by means of high-performance liquid chromatography with electrochemical detection and application to gastropod tissues. J Chromatogr. 1991;567:49–56. doi: 10.1016/0378-4347(91)80308-y. [DOI] [PubMed] [Google Scholar]
- Sloley BD, Orikasa S. Selective depletion of dopamine, octopamine and 5-HT in the nervous tissue of the cockroach (Periplaneta americana) J Neurochem. 1988;51:535–541. doi: 10.1111/j.1471-4159.1988.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Sneddon LU, Taylor AC, Huntingford FA, Watson DG. Agonistic behaviour and biogenic amines in shore crabs Carcinus maenas. J Exp Biol. 2000;230:537–545. doi: 10.1242/jeb.203.3.537. [DOI] [PubMed] [Google Scholar]
- Soma KK, Sullivan KA, Tramontin AD, Saldanha CJ, Schlinger BA, Wingfield JC. Acute and chronic effects of aromatase inhibitor on territorial aggression in breeding and nonbreeding male song sparrows. J Comp Physiol A. 2000;186:759–769. doi: 10.1007/s003590000129. [DOI] [PubMed] [Google Scholar]
- Sparks TC, Geng C. Analysis of the biogenic amines in the central nervous system of the tobacco hornworm by high-performance liquid chromatography with 16-sensor electrochemical detection. Analyt Biochem. 1992;205:319–325. doi: 10.1016/0003-2697(92)90442-a. [DOI] [PubMed] [Google Scholar]
- Stachowiak MK, Stricker EM, Jacoby JH, Zigmond MJ. Increased tryptophan hydroxylase activity in serotonergic nerve terminals spared by 5,7-dihydroxytryptamine. Biochem Pharmacol. 1986;35:1241–1248. doi: 10.1016/0006-2952(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Stevenson PA, Hofmann HA, Schoch K, Schildberger K. The fight and flight responses of crickets depleted of biogenic amines. J Neurobiol. 2000;43:107–120. [PubMed] [Google Scholar]
- Stevenson PA, Meuser S. Octopaminergic innervation and modulation of a locust flight steering muscle. J Exp Biol. 1997;200:633–642. doi: 10.1242/jeb.200.3.633. [DOI] [PubMed] [Google Scholar]
- Stocker AM, Huber R. Fighting strategies in crayfish Orconectes rusticus (Decapoda: Cambaridae) differ with hunger state and the presence of food cues. Ethology. 2001;107:727–736. [Google Scholar]
- Stribley JM, Carter CS. Developmental exposure to vasopressin increases aggression in adult prairie voles. Proc Natl Acad Sci USA. 1999;96:12601–12604. doi: 10.1073/pnas.96.22.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshiba T, Shamsian A, Yashar B, Yeh SR, Edwards DH, Krasne FB. Dual and opposing modulatory effects of serotonin on crayfish lateral giant escape command neurons. J Neurosci. 2001;21:4523–4529. doi: 10.1523/JNEUROSCI.21-12-04523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AJ. Effects of serotonin receptor agonists on posture and aggressive behavior in crayfish. Soc Neurosci Abstr. 2000;26:657.11. [Google Scholar]
- Van De Poll NE, De Jonge F, Van Oyen HG, Van Pelt J. Aggressive behavior in rats: effects of winning or losing on subsequent aggressive interactions. Behav Processes. 1982;7:143–155. doi: 10.1016/0376-6357(82)90023-7. [DOI] [PubMed] [Google Scholar]
- Vye C, Cobb JS, Bradley T, Gabbay J, Genizi A, Karplus I. Predicting the winning and losing of symmetrical contests in the American lobster Homarus americanus (Milne-Edwards) J Exp Mar Biol Ecol. 1997;217:19–29. [Google Scholar]
- Whitehouse MEA. Experience influences male-male contests in the spider Argyodes antipodiana (Theridiidae: Areneae) Anim Behav. 1997;53:913–923. [Google Scholar]
- Winberg S, Nilsson GE. Time-course of changes in brain serotonergic activity and brain tryptophan levels in dominant and subordinate juvenile arctic charr. J Exp Biol. 1993;179:181–195. [Google Scholar]
- Woo CC, Wilson DA, Sullivan RM, Leon M. Early locus coruleus lesions increase the density of B-adernergic receptors in the rat main olfactory bulb of rats. Int J Dev Neurosci. 1996;14:913–919. doi: 10.1016/s0736-5748(96)00041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh S-R, Fricke RA, Edwards DH. The effect of social experience on serotonergic modulation of the escape circuit of crayfish. Science. 1996;271:366–369. doi: 10.1126/science.271.5247.366. [DOI] [PubMed] [Google Scholar]
- Yeh S-R, Musolf BE, Edwards DH. Neuronal adaptations to changes in the social dominance status of crayfish. J Neurosci. 1997;17:697–708. doi: 10.1523/JNEUROSCI.17-02-00697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]