Summary
Objective
Examine therapeutic potential of a selective serotonin reuptake inhibitor (SSRI) and a norepinephrine reuptake inhibitor (NERI) in an animal model of comorbidity between epilepsy, depression-like, and impulsive-like impairments.
Methods
Epilepsy was induced in male Wistar rats by LiCl and pilocarpine. An SSRI fluoxetine (FLX), and a NERI reboxetine (RBX) were administered either alone or as a combination over one week. Depressive-like and impulsive-like behaviors were examined using forced swim test. Fast scan cyclic voltammetry was used to analyze serotonergic transmission in the raphe nucleus (RN)-prefrontal cortex (PFC) pathway, and noradrenergic transmission in locus coeruleus (LC)-PFC, and LC-RN projections. Monoamine levels in PFC were measured using high performance liquid chromatography. Functional capacities of 5-HT1A receptors and α2A adrenoreceptors in PFC were analyzed by autoradiography.
Results
Epileptic rats showed behavioral signs of depression and hyper-impulsivity, suppressed serotonergic and noradrenergic tones, decreased levels of serotonin (5-HT) and norepinephrine (NE); 5-HT1A receptor and α2A adrenoreceptors functions remained intact. FLX failed to improve behavioral deficits, but effectively raised 5-HT level and marginally improved RN-PFC serotonergic transmission. RBX reversed impulsive-like behavior, normalized content of NE and noradrenergic tone in LC-PFC and LC-RN. FLX-RBX combination fully reversed depressive-like behavior, and normalized RN-PFC serotonergic transmission. None of the treatment modified the function of 5-HT and NE receptors.
Significance
Depressive- and impulsive-like behaviors in the pilocarpine model of epilepsy stem respectively from dysfunctions of serotonergic and noradrenergic ascending pathways. At the same time, epilepsy-associated depression is SSRI-resistant. The finding that an SSRI-NERI combination exerts antidepressant effect, along with RBX-induced improvement of LC-RN noradrenergic transmission point towards the involvement of LC-RN noradrenergic input in enabling therapeutic potential of FLX. Medications that improve serotonergic and noradrenergic transmission, such as serotonin-norepinephrine reuptake inhibitors may be effective in treating epilepsy-associated SSRI-resistant depression, as well as concurrent depression and ADHD.
Keywords: Epilepsy, depression, attention deficit/hyperactivity disorder, selective serotonin reuptake inhibitor, norepinephrine reuptake inhibitor
Introduction
Depression and attention deficit/hyperactivity disorder (ADHD) are recognized as common comorbidities of epilepsy; their prevalence among epilepsy patients is up to five times higher than in people without epilepsy 1–3. Furthermore, comorbidity between depression and ADHD is a well-known mental health problem, whereby between one and five out of ten patients with depression fit the ADHD diagnosis 4; 5. Although the issue has not been explicitly addressed in clinical studies, the latter epidemiological observation infers concurrent presence of depression and ADHD in some epilepsy patients. Such triple-morbidity (i.e. epilepsy-depression-ADHD) would have obvious diagnostic and therapeutic implications.
In rodent models of epilepsy, behavioral, neurotransmitter and neuroendocrine impairments consistent with depression have been well established 6–9. Several studies have reported on ADHD-like abnormalities in epileptic animals 10; 11. A possibility of triple morbidity has been suggested in our earlier report: in the LiCl-pilocarpine model, rats show either depressive- or ADHD-like behaviors, or both 11. The latter cohort may be especially useful for examining mechanisms of epilepsy-depression-ADHD connections, and for experimental therapy trials.
While multiple mechanisms have been implicated in depression, serotonergic hypothesis prevails. Consequently, selective serotonin reuptake inhibitors (SSRI) represent major tool for managing depressive disorders 12. In case of ADHD dopaminergic dysfunction serves as a premise for a wide use of psychostimulants 13. At the same time, established perturbations in noradrenergic transmission in both depression and ADHD justify the use of noradrenergic drugs. Indeed, a norepinephrine reuptake inhibitor (NERI) reboxetine (RBX) has been used for the treatment for depression (albeit with controversial results) 14, and another NERI atomoxetine has been approved for the treatment of ADHD 15.
In our animal studies, we traced depressive-like impairments to the dysfunction of raphe nucleus (RN)- prefrontal cortex (PFC) serotonergic pathway, and ADHD-like impairments- to the dysfunction in the locus coeruleus (LC)-PFC noradrenergic projection 11. These findings suggest that SSRI and NERI may be indeed effective in improving depression-like and ADHD-like impairments respectively, and further that SSRI-NERI combination (or serotonin-norepinephrine reuptake inhibitors, SNRI, such as venlafaxine 16; 17) may be a rational therapy choice when both behavioral disorders are present.
Here, we examined effects an SSRI fluoxetine (FLX) and of a NERI RBX administered either as monotherapies or as a combination on depressive-like and impulsive-like behaviors in rats with chronic epilepsy vis-à-vis effects of the drugs on impairments in serotonergic and noradrenergic transmissions in respective ascending pathways.
Methods
Animals
The experiments were performed in male Wistar rats (Charles River, Wilmington, MA), fifty days old at the beginning of the study, in accordance with the policies of the National Institutes of Health and of the UCLA Office of Protection of Research Subjects.
Induction of chronic epilepsy
Status epilepticus (SE) was induced by LiCl (128 mg/kg, i.p., Sigma, St. Louis, MO) and pilocarpine (40 mg/kg, s.c., Sigma) injected 24 hours apart. In order to alleviate the severity of SE and subsequent chronic epilepsy, the animals received i.p. injections of diazepam (10 mg/kg) and phenytoin (50 mg/kg) one and four hours after the first seizure 7; 11; 18. Control animals were administered with saline in lieu of pilocarpine.
Beginning from four weeks after SE and until the end of the experiments animals were continuously video-monitored for documenting spontaneous seizures.
Forced swim test (FST)
FST was used to examine depressive-like, and impulsive-like behaviors. First FST was performed 4 weeks after SE. The test consisted of a single 300-second swimming session in a tank filled with water at 22°–25°C. Behavior was video-recorded and analyzed off-line in a single-blind fashion. Cumulative durations of three distinct behavioral patterns were calculated: active adaptive behavior (i.e. swimming along the walls, climbing attempts, diving); immobility (i.e. movements are limited to maintaining head above water with no escape attempts); and non-adaptive struggle (i.e. treading water away from the walls with no attempts to escape) 7; 11. The first two behaviors are typical for both normal animals and those with depressive-like impairments. However, in depression models immobility is increased, which is thought to reflect the state of despair/hopelessness 19. Non- adaptive struggle is negligible in normal rats, but is observable in 25–50% of animals with epilepsy in different experimental series. In an earlier study we found that only those animals which display non-adaptive struggle during FST, display impulsivity in the Lateralized Reaction Time Task (LRTT) 11, which is specific for ADHD 20; 21. The downside of LRTT and similar tasks is that they require weeks to complete10; 20 and are thus associated with substantial challenges when used in chronic epilepsy10; 11. Based on the congruency between ADHD-specific behaviors during LRTT and non-adaptive struggle during FST, we proposed that the latter can be used as a simple surrogate indicator of hyper-impulsivity 11.
The second FST was performed 24 hours after the end of drug treatment.
Subject selection for drug treatment and data analysis
Two criteria were used for advancing the animals to experimental therapy and/or for including the data into the analysis.
-
(i)
Spontaneous seizures. Animals which displayed no seizures during the first four weeks of observation (i.e. the presence of epilepsy could not be verified) were excluded from further procedures. At any time, rats were also excluded if they presented with more than 5 seizures per week, as such high seizure frequency renders animals unamenable to behavioral testing 11; 22.
-
(ii)
Behavior. Our earlier studies established that based on behavior in the FST, epileptic rats fall into one of four categories: those with depressive-like impairments only; with ADHD-like impairments only; with concurrent depressive-like and ADHD-like impairments; and with no behavioral deficits 11. Such diversity complicates effective design of experimental therapy trials, as each sub-population should be analyzed separately, and this would require hardly manageable sample sizes. Therefore, in order to standardize experimental conditions, we chose only those animals which presented with both depressive-like (i.e. increased immobility) and impulsive-like (i.e. increased non-adaptive struggle) behaviors. Control animals which showed ≥100 s of immobility during the first FST, were also excluded.
Drugs and treatments
One week after the first FST, the selected epileptic and control animals were randomly assigned to one of four treatment groups: saline; FLX hydrochloride (20 mg/kg, USP, Spectrum Chemical MFG Corp, Gardena, CA); RBX mesylate (40 mg/kg, USP, Ontario Chemicals Inc., Guelph, ON); FLX (20 mg/kg)+RBX (40 mg/kg). The drugs were injected i.p. once a day for seven days. Four-six animals were processed at a time, with all treatments present in each group. The procedures were repeated until the final animal numbers were reached.
One day after the last injection, the animals underwent the second FST (therefore the first and the second tests were separated by two weeks, which were proven sufficient to avoid animals’ behavior during the second test being affected by the first swimming session 7; 22). Afterwards, the rats were randomly assigned to one of the three procedures: fast scan cyclic voltammetry (FSCV), high performance liquid chromatography (HPLC), or autoradiography of 5-HT1A receptors and α2A adrenoreceptors; for each assay, the rats were processed within 48 hours after the end of the treatments.
FSCV of serotonin (5-HT) and norepinephrine (NE)
FSCV was performed in order to measure the strength of serotonergic and noradrenergic tones in the RN-PFC and the LC-PFC pathways respectively. The procedure was validated and extensively used by our group 7; 9; 11; 18; 22.
Under urethane anesthesia (1.5 mg/kg s.c.), the animals were placed in the stereotaxic apparatus. Carbon fiber electrode (32 µm, sensitivity ≥20 nA/µm, Invilog, Kuopio, Finland) was placed in the infralimbic cortex (from Bregma: anterior 2.7 mm, left 0.5 mm, ventral 5.0 mm 23). Dry reference Ag/AgCl electrode (diameter 2.5 mm, Invilog) was placed on the nasal bone. Coaxial stimulating electrode (diameter 200 µM) was placed in the RN for 5-HT detection (from Bregma: posterior −8.0 mm, midline, ventral 6.0 mm 23) and afterwards moved to LC for NE detection (from Bregma: posterior −9.7 mm, left 1.2 mm, ventral 7.2 mm 23). Separately, noradrenergic transmission was analyzed in the RN-LC pathway, whereby stimulating electrode was placed in LC and carbon fiber electrode- in RN.
FSCV was performed using In Vivo Voltammetry system (Invilog), consisting of the two-electrode head stage, connecting to carbon fiber and reference electrodes on the one end, and to the Voltammeter/Electrical stimulator unit on the other end. The stimulating electrode was connected to the stimulator output of the unit. The unit was connected to a Windows PC via the Multifunctional Data Acquisition Board (National Instruments, Austin, TX). Data acquisition, collection, analysis and electrical stimulations were performed using the proprietary Acquisition and Analysis software (Invilog).
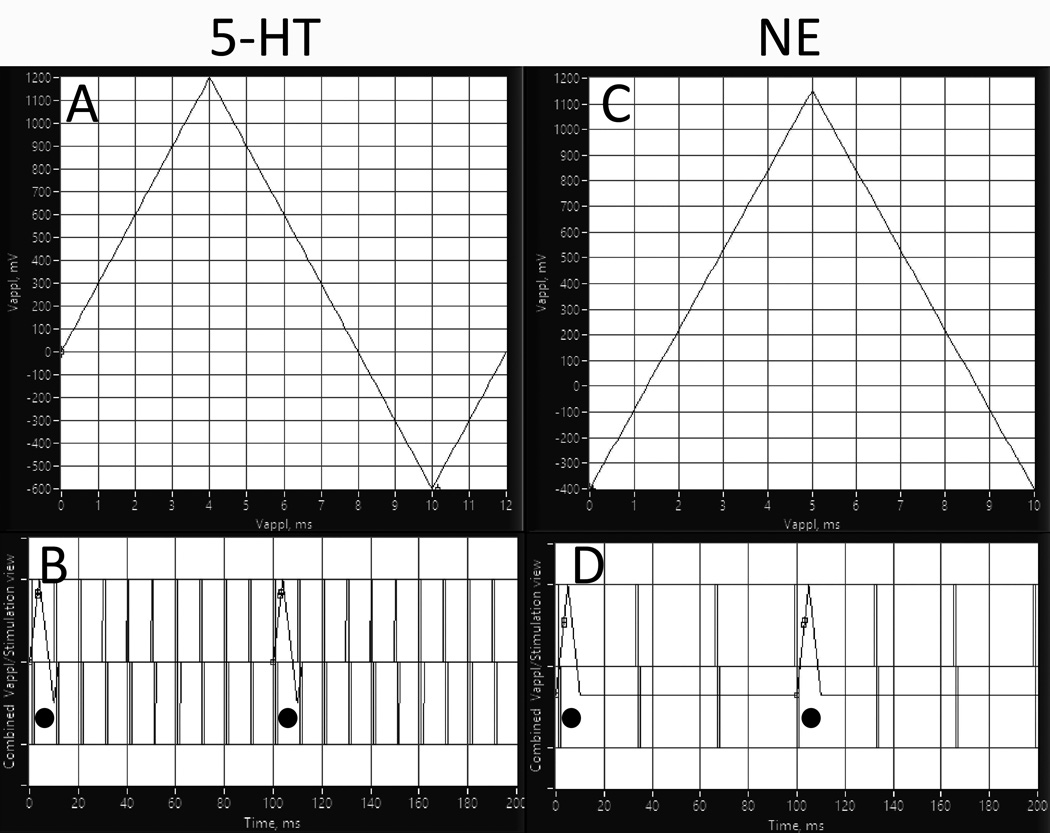
For the 5-HT detection, voltammetric waveform applied to the carbon fiber electrode consisted of a resting potential 0 V scanned to 1.2 V, then to −0.6 V and then back to 0 V at 300 V/s (Fig. 1A) 11; 24. First, to detect the baseline, the waveform was applied 5 times with 100-ms intervals without electrical stimulation. The procedure was then repeated together with electrical stimuli applied to the RN stimulating electrode: bipolar 1 ms square-wave pulses, 100 Hz, 0.35 mA (Fig. 1B) 11. The procedure was repeated 5 times, with 10 min intervals.
Fig. 1. Fast scan cyclic voltammetry (FSCV) setup.
Screen captures of the acquisition software. A. For 5-HT, voltammetric waveform consisted of a resting potential 0 V scanned to 1.2 V, then to −0.6 V and then back to 0 V at 300 V/s. Right: B. Combined scan and stimulation view for 5-HT detection. Electrical stimuli applied to RN (100 Hz) appear as vertical lines superimposed on ramp currents applied to PFC (indicated by circles). Shown are first 200 ms out of 500 ms of total stimulation. C. For NE, voltammetric waveform consisted of a rest potential −0.4 V, scanned to 1.15 V and back to −400 V at 300 V/s. D. Combined scan and stimulation view for NE detection. Electrical stimuli applied to LC (30 Hz) appear as vertical lines superimposed on ramp currents applied to PFC (indicated by circles). Shown are first 200 ms out of 500 ms of total stimulation.
NE detection was performed similarly to 5-HT scans, with two differences: voltammetric waveform consisted of a rest potential −0.4 V, scanned to 1.15 V and back to −400 V at 300 V/s (Fig. 1C); stimulation was set at 30 Hz, 0.5 mA 25 (Fig. 1D).
The amount of transmitter released was expressed as faradaic current calculated by subtracting average baseline values from average stimulation-evoked values.
Upon completing FSCV, animals were euthanized, perfused with 4% paraformaldehyde, and brains processed for the verification of proper placements of stimulating and carbon fiber electrodes.
HPLC of 5-HT and NE
HPLC was performed to measure content of 5-HT and NE in frontal/prefrontal cortex as described earlier 7; 26. Under Pentobarbital anesthesia, rats were decapitated, brains removed and placed in ice-cold saline. Frontal-prefrontal cortex was dissected upon placing the brain in the ice-cold brain matrix. Bilateral coronal 3-mm thick section was taken between coronal planes of approximately 4.6 and 1.6 mm anterior to Bregma 23. Samples were weighed, sonicated in solution containing 0.09 mol/L perchloric acid, 0.04 mmol/L EDTA and 5 mmol/L sodium bisulfite (1 ml per 100 mg of wet tissue), and supernatant was collected after centrifugation. HPLC was performed using the L-ECD-6A electrochemical detector (Shimadzu, Kyoto, Japan) on the HR-80 column (4.6×8×3 µM, ESA Biosciences, Chelmsford, MA). The separation was done in isocratic elution mode using CAT-A-Phase II mobile phase (ESA). The measurements were done at an electrode potential of +0.7 V. The concentration of 5-HT and NA in wet tissue was normalized against respective standards (Sigma). Data were analyzed using Shimadzu EZ StartTM software.
Autoradiography of 5-HT1A receptors and of α2A adrenoreceptors in PFC
Functional capacities of 5-HT1A receptors and of α2A adrenoreceptors to activate G-proteins were examined using quantitative autoradiography of a respective agonist-stimulated [35S]GTPγS binding 9; 27. Under pentobarbital anesthesia, the animals were decapitated, brains removed and frozen on dry ice. (±)-8-hydroxy-2-dipropylaminotetralin (8-OH-DPAT, Tocris, Bristol, UK) 9; 22 and dexmedetomidine (DMED, Tocris) 27 were used as 5-HT1A receptor and α2A adrenoreceptor agonists respectively. Twenty micron-thick coronal sections containing infralimbic cortex (3.2–2.7 mm anterior from Bregma 23) were rehydrated, pre-incubated in an assay buffer, and incubated for 2 hours in the assay buffer containing 40 pmol/L [35S]GTPγS (Perkin Elmer, Waltham, MA). Receptor-stimulated [35S]GTPγS binding was determined in the presence of 8-OH-DPAT (1 µM), or DMED (1 µM). Basal [35S]GTPγS binding was determined in the absence of 8-OH-DPAT and DMED. Nonspecific [35S]GTPγS binding was defined in the absence of 8-OH-DPAT/DMED and in the presence of 10 µmol/L GTPγS (Sigma). Sections were exposed to Kodak Biomax MR film (Amersham, Piscataway, New Jersey) for 48 hours. Digitized autoradiograms were analyzed using NIH Image software (ImageJ 1.42q). Autoradiograms of 8-OH-DPAT- and DMED- stimulated- [35S]GTPγS binding were quantified by the use of simultaneously exposed [14C] standards (American Radiochemicals). Nonspecific binding of [35S]GTPγS was subtracted from basal binding and from binding in the presence of 8-OH-DPAT/DMED.
Data analysis
Data were analyzed by means of Prism 6 software (GraphPad, San Diego, CA). All groups passed D’Agostino-Pearson omnibus normality test. One-way or two-way ANOVA followed by Tukey post hoc test were used where appropriate. Sample sizes and tests are indicated in respective figure legends.
Results
Effects of monoamine reuptake inhibitors on behavior
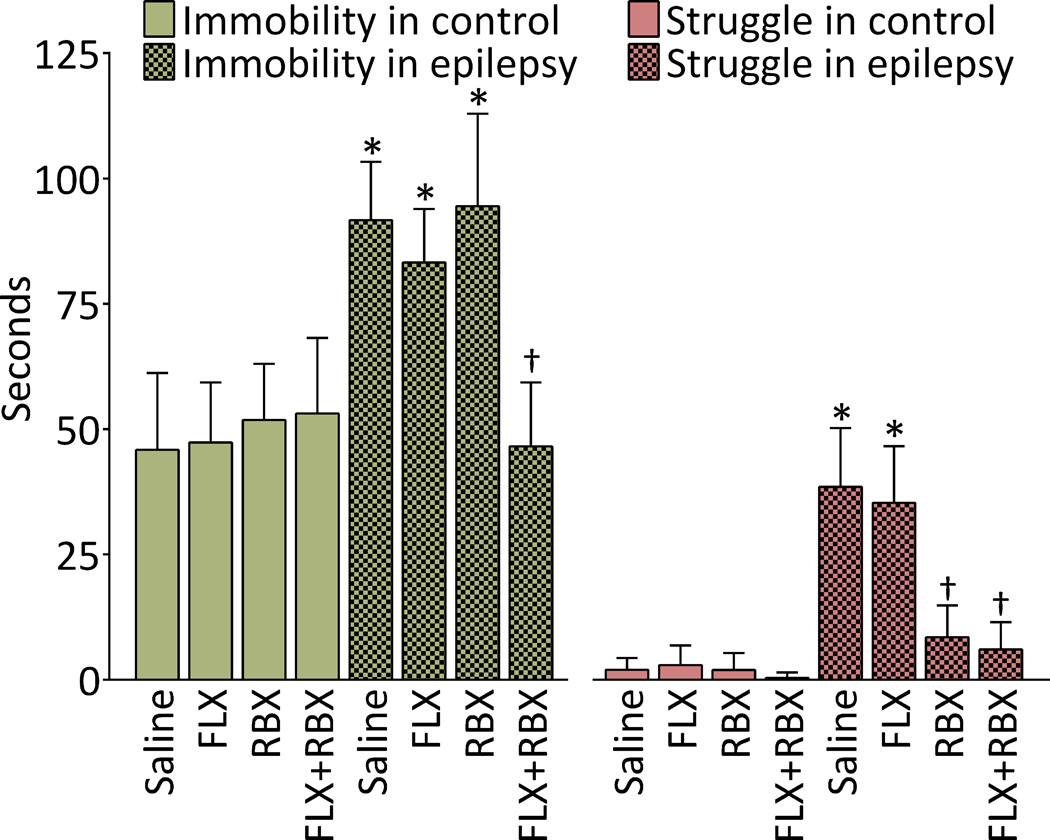
During the second FST, animals with epilepsy showed an approximately two-fold increase in the immobility time, as compared to non-epileptic controls (Fig. 2, left). In both control and epileptic rats, neither FLX, nor RBX monotherapy modified the immobility time. However, the FLX+RBX combination, while remaining inconsequential in controls, significantly reduced the immobility time in epileptic rats, and brought its value into the range typical for control subjects (Fig. 2, left, compare the last and the first columns).
Fig. 2. Effects of fluoxetine (FLX), reboxetine (RBX) and their combination on behavior in the forced swim test in control and epileptic rats.
Left: Immobility time. In untreated epileptic rats, immobility time was significantly increased as compared with untreated control non-epileptic animals. In control rats, neither of treatments modified this behavior. In animals with epilepsy, FLX and RBX monotherapy exerted no effects; however combined FLX and RBX administration decreased immobility time to the level observed in controls. Right: Non-adaptive struggle. Non-adaptive struggle was nearly absent in control animals, but was observed in epileptic rats. RBX monotherapy, as well as RBX+FLX administration decreased the time of non-cued struggle to the levels statistically similar to those in controls. Data are shown as Mean ± SD. *-p<0.05 vs. Saline control; †- p<0.05 vs. Saline epilepsy. Sample sizes: Naïve saline and RBX n=21; Naïve FLX and FLX+RBX=17; epileptic saline n=22, FLX n=16; RBX n=19; FLX+RBX n=17. Treatment-behavior interaction F (7, 282) = 13.33; effects of treatment F (7, 282) = 89.17; effects of type of behavior F (1, 282) = 1585, all p<0.0001.
In contrast to the animals of control group, epileptic rats displayed an observable non-adaptive struggling behavior (Fig. 2, right). FLX monotherapy produced no improvements in the struggling behavior. At the same time, RBX, even when administered alone significantly reduced non-adaptive struggling, duration of which was in the control range. After FLX+RBX combination, the parameter was statistically similar to the one recorded for the RBX monotherapy (Fig. 2, right).
Effects of monoamine reuptake inhibitors on neurotransmission in ascending pathways
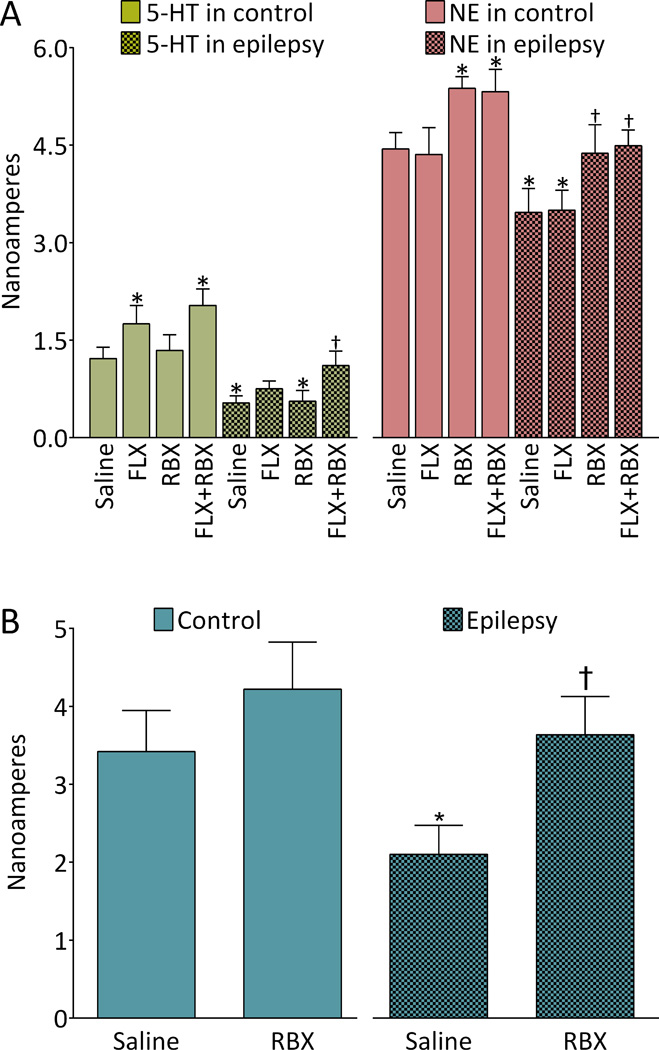
In animals of control groups, FLX significantly increased serotonergic tone in RN-PFC, and RBX increased noradrenergic transmission in LC-PFC. Combined administration of FLX and RBX had no additional effects on serotonergic and noradrenergic transmission in comparison with the effects of the drugs administered alone (Fig. 3A).
Fig. 3. Effects of fluoxetine (FLX), reboxetine (RBX) and their combination on monoamine transmission in in control and epileptic rats. A. Serotonergic and noradrenergic transmission in ascending pathways.
Left: serotonergic transmission in raphe-prefrontal cortex (RN-PFC) pathway. Suppression of serotonergic tone in RN-PFC pathway was observed in epileptic rats. In control animals, both FLX and FLX+RBX combination facilitated the neurotransmission at the same extent. In animals with epilepsy, FLX monotherapy produced a trend towards improving serotonergic transmission (P0.05 vs. untreated controls, p>0.05 vs. untreated epileptic rats). FLX+RBX combination in epileptic subjects produced significant strengthening of serotonergic transmission as compared with untreated epileptic rats. Right: noradrenergic transmission in locus coeruleus-PFC (LC-PFC) pathway. Noradrenergic responses were significantly suppressed in animals with epilepsy as compared to the rats of control untreated group. RBX and RBX+FLX treatments significantly increased noradrenergic transmission both in control and epileptic subjects. In the latter, the parameters were within statistical range observed in untreated control. Data are shown as Mean±SD. *-p<0.05 vs. Saline control; †- p<0.05 vs. Saline epilepsy. Each group included 6 animals. Two-way ANOVA + Tukey’s test for multiple comparisons. Treatment-monoamine interaction F(7,80)=9.81; effects of treatment F (7,80)=53.83; effects of type of monoamine F (1,80)=3390, all p<0.0001. B. Noradrenergic transmission in the LC-RN pathway. Diminished noradrenergic tone was observed in epileptic rats; this impairment was reversed by the RBX treatment. -p<0.05 vs. Saline control; †- p<0.05 vs. Saline epilepsy. Four animals per group. One-way ANOVA + Tukey’s multiple comparison test, F(3,12)=12.52, p<0.05.
In untreated epileptic rats, both serotonergic transmission in RN-PFC and noradrenergic transmission in LC-PFC were significantly suppressed versus controls (Fig. 3A). FLX marginally improved serotonergic transmission: while the parameter remained in statistical range found in untreated epileptic rats (p>0.05), at the same time it became statistically similar to the one observed in untreated control subjects (p>0.05). RBX alone had no effect on serotonergic tone; however, combined treatment with FLX and RBX fully normalized serotonergic transmission, which was now significantly stronger than in untreated epileptic rats (p<0.05) and similar to the one in untreated controls (p>0.05; Fig. 3A, left; compare the last and the first columns).
When administered to epileptic animals, RBX alone was sufficient to strengthen noradrenergic tone, by bringing it to normal range (i.e. the one observed in untreated controls); FLX had no effect on noradrenergic transmission. The effect of RBX+FLX combination was similar to the one seen after the RBX monotherapy (Fig. 3A, right).
Effects of RBX on noradrenergic transmission in the LC-RN pathway
In epileptic animals, noradrenergic tone in the LC-RN projection was diminished in comparison to controls. RBX treatment did not alter significantly noradrenergic transmission in controls rats (although a trend towards the increase was observed). In rats with epilepsy, RBX reversed deficit in noradrenergic transmission, by bringing it to the level found in untreated control subjects (Fig. 3B).
Effects of monoamine reuptake inhibitors on cortical monoamine levels
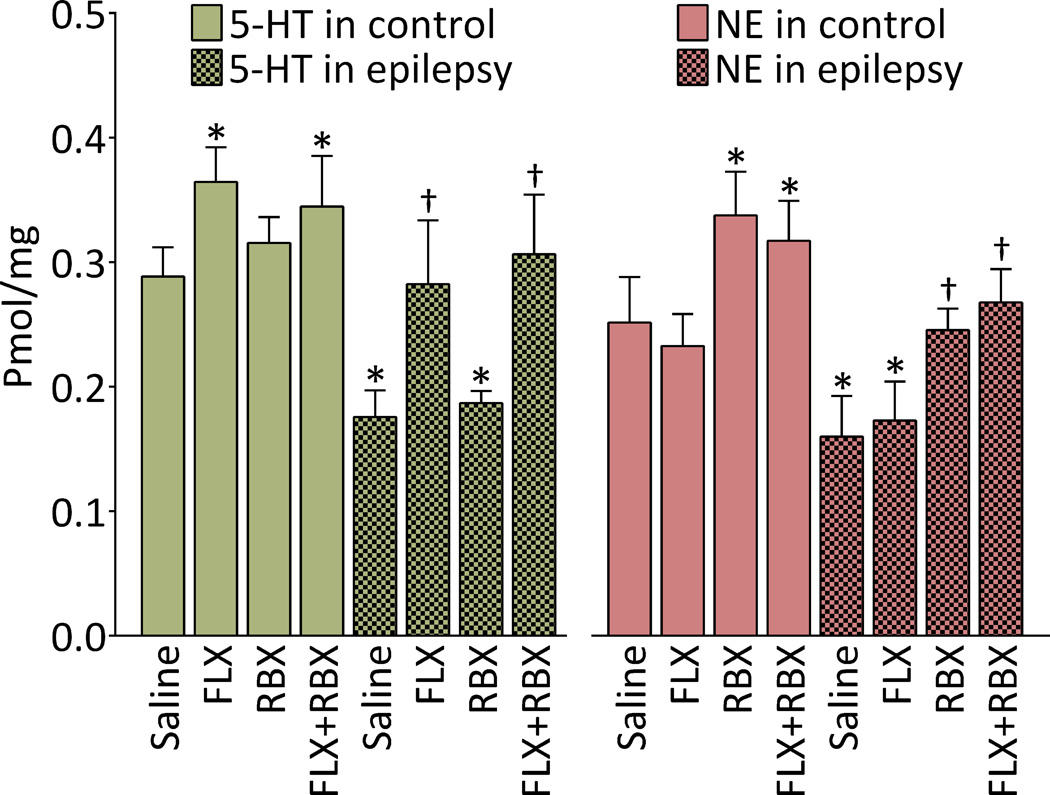
In control rats, FLX and RBX monotherapies significantly increased 5-HT and NE levels respectively, without affecting concentrations of alternate transmitters. Combined administration of FLX and RBX had no additional effects on the neurotransmitter levels, as compared to monotherapies (Fig. 4).
Fig. 4. Effects of fluoxetine (FLX), reboxetine (RBX) and their combination on monoamine content in frontal lobes of control and epileptic rats.
Left: 5-HT concentrations. In untreated epileptic rats, concentration of 5-HT was significantly lower than in untreated animals of control group. In control rats, levels of 5-HT were significantly elevated after FLX monotherapy and FLX+RBX combination, with no differences between the two treatments. In rats with epilepsy, FLX alone and FLX+RBX combination raised 5-HT concentration to levels comparable with those in untreated control subjects. Right: NE concentrations. NE levels were significantly lower in untreated epileptic rats than in their control counterparts. Administration of RBX alone and RBX+FLX combination raised NE levels both in controls and in epileptic animals; in the latter, NE concentration was brought to levels observed in untreated animals of control group. Data are shown as Mean±SD. *-p<0.05 vs. Saline control; †- p<0.05 vs. Saline epilepsy. Sample sizes: Untreated epileptic rats 8; FLX-treated epileptic rats 6, RBX-treated epileptic rats 5; each of the remaining groups 7. Two-way ANOVA + Tukey’s test for multiple comparisons. Treatment-transmitter interaction F(7,92)=11.9; effects of treatment F (7,92)=45.77; effects of transmitter F (1,92)=31.64, all p<0.0001.
In untreated epileptic animals, cortical levels of both 5-HT and NE were significantly lower than in controls (Fig. 4). FLX and RBX raised 5-HT and NE levels respectively, without affecting concentrations of alternate monoamines. Concentrations of 5-HT after FLX treatment and of NE after RBX administration were in ranges observed in the untreated control subjects. No interaction between the two drugs was found upon their combined administration (Fig. 4).
Lack of effects of monoamine reuptake inhibitors on function of 5-HT1A receptors and α2A adrenoreceptors in prefrontal cortex
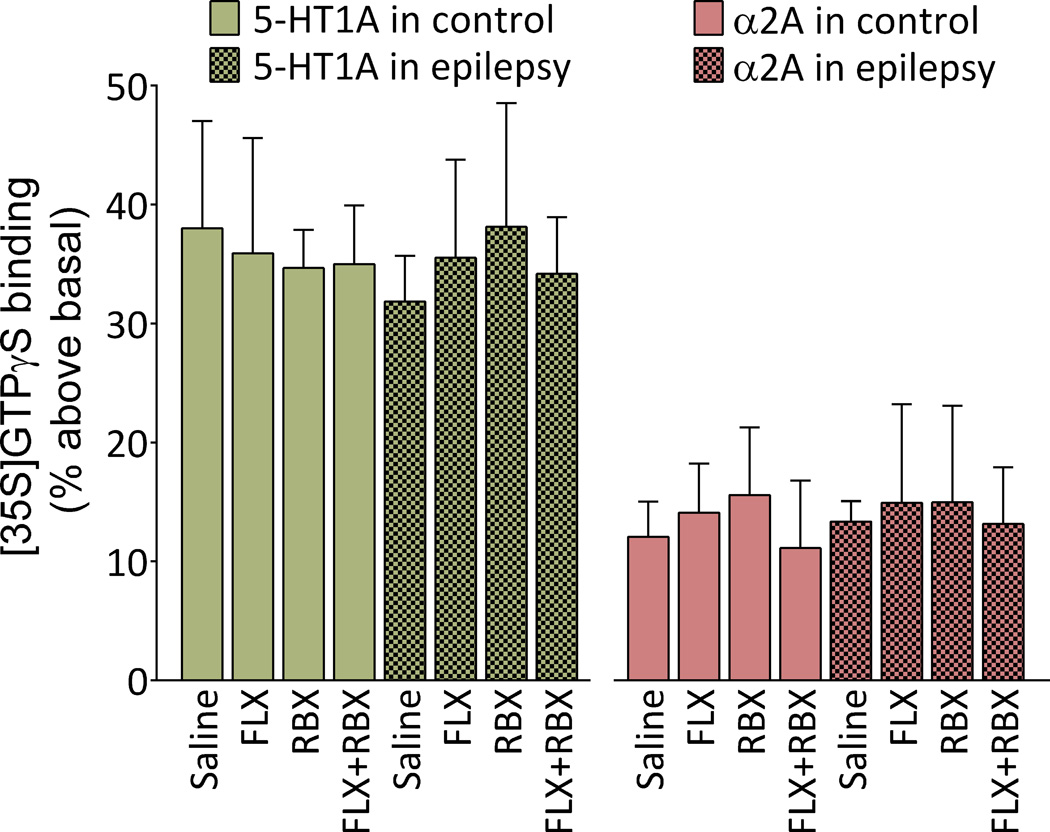
No differences were observed between control and epileptic rats for either 8-OH-DPAT or DMED-stimulated [35S]GTPγS binding. None of the treatments affected the examined parameters in either control rats or those with epilepsy (Fig. 5).
Fig. 5. Lack of effects of fluoxetine (FLX), reboxetine (RBX) and their combination on the function of 5-HT1A receptors and of α2A adrenoreceptors receptors in prefrontal cortex of control and epileptic rats.
The function of 5HT-1A receptors (examined by measuring 8-OH-DPAT-stimulated [35S] GTPγS binding), and of α2A adrenoreceptors (examined by measuring DMED-stimulated [35S] GTPγS binding) were neither changed in animals with epilepsy, nor modified by any of the treatments. Data are shown as Mean±SD. Four samples per group. Two-way ANOVA + Tukey’s test for multiple comparisons. Treatment-transmitter interaction F (7, 48) = 0.07, p>0.05; effects of treatment F (7, 48) = 0.08, p>0.05; effects of transmitter F (1, 48) = 42.78, p<0.05.
Effects of other doses of FLX and RBX
In additional studies (4–6 animals per group) we examined effects of other doses of the drugs (data not shown). FLX (10 mg/kg) had no effects on behavior, monoamine concentration, or on the strength of monoaminergic transmission. At 40 mg/kg, FLX treatment led to weight loss (>10% of the pre-treatment weight), but to no further improvements in any of the assays as compared with 20 mg/kg. RBX at 20 mg/kg had no effects on behavior, monoamine concentration and release; no other doses of RBX were tested.
Discussion
In rats with epilepsy and concurrent depressive- and impulsive-like impairments, FLX and RBX selectively improved behavior and monoamine transmission (Table 1). While FLX and RBX given separately failed to improve depressive behavior and dysfunctional RN-PFC serotonergic tone, their combined administration led to full recovery from behavioral and serotonergic deficits. These effects were likely due to facilitating LC-RN noradrenergic transmission by RBX, with subsequent disinhibition of ascending serotonergic RN-PFC pathway by FLX.
Table 1.
Summary of effects of treatments with monoamine reuptake inhibitors on behavior and neurotransmission
| Groups | Control | Epilepsy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Measurements | Saline | FLX | RBX | FLX+RBX | Saline | FLX | RBX | FLX+RBX | |
| Behavior in FST |
Immobility | - | No effect | No effect | No effect | Increased | No effect | No effect | Normalized |
| Non-adaptive struggle |
Not present |
Not present |
Not present |
Not present |
Increased | No effect | Normalized | Normalized | |
| 5-HT- related |
RN-PFC tone | - | Increased | No effect | Increased (=FLX) |
Decreased | Trend towards increase |
No effect | Normalized |
| 5-HT concentration in frontal lobes |
- | Increased | No effect | Increased (=FLX) |
Decreased | Normalized | No effect | Normalized (=FLX) |
|
| 5-HT1A receptor function in PFC |
- | No effect | No effect | No effect | No change |
No effect | No effect | No effect | |
| NE- related |
LC-PFC tone | - | No effect | Increased | Increased (=RBX) |
Decreased | No effect | Normalized | Normalized (=RBX) |
| LC-RN tone | - | - | No effect | - | Decreased | - | Normalized | - | |
| NE concentration in frontal lobes |
- | No effect | Increased | Increased (=RBX) |
Decreased | No effect | Normalized | Normalized (=RBX) |
|
| α2A adrenoreceptors function in PFC |
- | No effect | No effect | No effect | No change |
No effect | No effect | No effect | |
Impairments in epileptic animals
Perturbations observed in the pilocarpine model corroborated earlier reports 7; 11; 22. Epileptic rats presented with both despair/hopelessness-like and impulsive-like behaviors. They had suppressed RN-PFC serotonergic tone, suppressed LC-PFC noradrenergic tone11, as well as newly shown LC-RN noradrenergic dysfunction and low cortical contents of 5-HT and NE. We found no alterations in the function of 5-HT1A receptors and α2A adrenoreceptors in PFC. This does not exclude a possibility of changes occurring to other receptors; however given major roles that these receptors play in depression 28; 29, and ADHD 30, it is reasonable to assume that in the pilocarpine model substrates of behavioral abnormalities are mostly confined to presynaptic components of monoamine transmission. From the therapy perspective, the preservation of postsynaptic targets for 5-HT and NE justifies the use of SSRI and NERI.
Effects of FLX
As it has been shown earlier 7; 18, neither depressive-like behavior, nor impaired serotonergic transmission in epileptic rats were improved by FLX. SSRI resistance is quite common in depression, reaching 30%–50% of patients 31. In the pilocarpine model, we attributed FLX resistance to the sustained drive exerted on RN by hyperactive inflammatory-neuroendocrine axis: seizure-induced overexpression of hippocampal interleukin-1β 32 and subsequent dysregulation of the hypothalamo-pituitary-adrenocortical axis 8; 22 leads to the upregulation of 5-HT1A autoreceptors and ultimate hyper-inhibition of 5-HT release, which cannot be compensated by the FLX-induced 5-HT1A autoreceptor desensitization 9; 18. Congruently with effects of SSRI, FLX raised cortical level of 5-HT. This rise alone however, was insufficient (although it still might be necessary) for translating into antidepressant effect. Rather, as further discussion suggests, the strength of the RN-PFC serotonergic tone may be more important for improving depression symptoms. The lack of effects of FLX therapy on 5-HT1A receptors was expected: while presynaptic 5-HT1A receptors are subject to the ligand-mediated desensitization, postsynaptic 5-HT1A receptors are not 33.
FLX had no effect on non-adaptive struggle. Neither did FLX modify any of the aspects noradrenergic transmission; while high doses of FLX can inhibit NE reuptake 34, this appeared not to be the case for the dose used in our study.
Effects of RBX
RBX had no effects on the immobility, but reversed the non-adaptive struggle in epileptic rats. The behavioral effect of RBX was accompanied by effective improvement of noradrenergic transmission: both the strength of noradrenergic tone in LC-PFC and the content of cortical NE increased (although from our experiments it cannot be deduced whether both effects were necessary for RBX to exert therapeutic effects, or one of the two was sufficient). These findings agree with previously reported correlation between impulsive behaviors and suppressed noradrenergic LC-PFC transmission in the pilocarpine model 11, although anti-ADHD effects of RBX in the pilocarpine model will have to be corroborated directly using a specific test 10; 11.
Mechanisms via which RBX improves ascending noradrenergic tone require further studies; it is conceivable that desensitization of presynaptic α2A adreno-autoreceptors is involved 35; 36 (much like desensitization of 5-HT1A autoreceptors is responsible effects of SSRI).
While psychostimulants remain a major tool for ADHD therapy, noradrenergic treatments have also been used. A NERI atomoxetine is effective in managing ADHD both in experimental 21 and clinical 15 settings. Potent effects of RBX in our experiments confirm important role that noradrenergic dysfunction plays in ADHD-like abnormalities in the pilocarpine model.
The lack of effects of RBX on depressive behavior is not surprising. Although noradrenergic deficits have been documented in depression, the efficacy of noradrenergic drugs, and specifically RBX has been disputed 14. It is therefore possible that noradrenergic deficits, while occurring in depression, play secondary role in its mechanisms.
Lack of effects of RBX on serotonergic transmission is congruent with its high selectivity towards norepinephrine transporter vs. serotonin transporter 37.
Effects of FLX and RBX combination and role of the LC-RN noradrenergic pathway
With regard to cortical levels of 5-HT and NE, noradrenergic transmission in the LC-PFC, 5-HT1A and α2A receptor function, and non-adaptive struggle, we found no interaction between FLX and RBX when the drugs were administered together. Such segregation of effects of the two drugs suggests that even though FLX and RBX may cross-inhibit NE and 5-HT transporters, such inhibition did not occur in the applied doses.
At the same time, the FLX-RBX combination fully reversed the increase in the immobility, and restored serotonergic transmission in the RN-PFC pathway. LC send excitatory projection into RN, where via α2A adrenoreceptors NE activates serotonergic neurons 38. It is plausible that while FLX alone was not sufficient to normalize serotonergic transmission (although, as it has been noted, marginal improvement was observed), adding RBX stimulated 5-HT output from raphe to a level whereby serotonergic tone was effectively restored and this translated into the improvement of depressive-like behavior. This scenario was confirmed in our experiments: the diminished noradrenergic LC-RN input in epileptic rats may contribute to the dysfunction in the ascending serotonergic pathway, and RBX-induced strengthening of the LC-RN noradrenergic tone may improve 5-HT output from RN to the forebrain.
A SNRI venlafaxine has been effective in patients with SSRI-resistant depression 16, as well as in ADHD 17. Our data which show a complex interplay between noradrenergic and serotonergic transmission, may explain at least in part, the efficacy of SNRI in some cases of SSRI-resistant depression, and furthermore justify the use of such drugs in those patients (including patients with epilepsy), who present with both depression and ADHD.
FLX did not modify NE release from LC. LC does receive an input from RN; however, activation of RN serotonergic neurons reportedly had no effects on the release of NE from LC 38, and the lack of interaction between FLX and RBX on the LC level is congruent with this finding.
Although natural history of behavioral impairments in the FST has never been reported explicitly, based on our repeatedly corroborated observations, and as it can be inferred from published data 7; 22, the deficits presented by individual animals, are stable over time. Therefore, even though spontaneous recovery (as opposed to effects of treatments) cannot be excluded with absolute certainty, it is highly unlikely. This unlikelihood is further supported by specific nature of behavioral and biochemical modifications observed after respective treatments.
A note regarding spontaneous seizures
We did not examine effects of monoamine reuptake inhibitors on spontaneous seizures due to specifics of the study design. Low seizure frequency inherent to the employed protocol and short observation period precluded objective evaluation of modification of seizures by FLX and RBX. Anticonvulsant effects of both FLX 39 and RBX 40 have been reported in the pilocarpine model, although we failed to find a correlation between frequency of seizures and the severity of depressive and ADHD-like abnormalities 7; 11; 22. We can confirm that all animals continued showing spontaneous seizures during all treatments. While it was possible that both drugs were able to decrease seizure frequency, this would be an unlikely explanation for the observed therapeutic effects of FLX, RBX or FLX+RBX, which were selective towards concrete behavioral patterns and types of monoamine transmission.
In conclusion, our study suggests that some epilepsy patients with SSRI-resistant depression, as well as those who present with either ADHD alone, or with depression and ADHD concurrently, may benefit from combined treatment with SSRI and NERI, or SNRI.
Key Points.
Selected population of rats with chronic epilepsy present with concurrent depressive- and impulsive-like behaviors
Serotonergic and noradrenergic transmission in ascending pathways are dysfunctional in epileptic rats
Depression is resistant to a selective serotonin reuptake inhibitor (SSRI); impulsivity is reversed by a norepinephrine reuptake inhibitor (NERI)
Depressive impairments and serotonergic dysfunction are reversed by the SSRI-NERI combination
Epilepsy patients with SSRI resistant depression may benefit from SSRI-NERI combination; epilepsy patients with ADHD may benefit from NERI
Acknowledgements
This work was supported by research grants R01NS065783 and R21NS089396 from the National Institutes of Health to AM; and by research grant from the Today and Tomorrow Children’s Fund to AM. SM was supported by postdoctoral fellowship No 237168 from National Council of Science and Technology (CONACYT, Mexico). HMR was supported by R25 NS080684.
Footnotes
Conflict of interest
The authors report no conflicts of interest.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Seidenberg M, Pulsipher DT, Hermann B. Association of epilepsy and comorbid conditions. Future Neurol. 2009;4:663–668. doi: 10.2217/fnl.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ettinger AB, Ottman R, Lipton RB, et al. Attention-deficit/hyperactivity disorder symptoms in adults with self-reported epilepsy: Results from a national epidemiologic survey of epilepsy. Epilepsia. 2015;56:218–224. doi: 10.1111/epi.12897. [DOI] [PubMed] [Google Scholar]
- 3.Kanner AM, Schachter SC, Barry JJ, et al. Depression and epilepsy: epidemiologic and neurobiologic perspectives that may explain their high comorbid occurrence. Epilepsy Behav. 2012;24:156–168. doi: 10.1016/j.yebeh.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Daviss WB. A review of co-morbid depression in pediatric ADHD: etiology, phenomenology, and treatment. J Child Adolesc Psychopharmacol. 2008;18:565–571. doi: 10.1089/cap.2008.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntosh D, Kutcher S, Binder C, et al. Adult ADHD and comorbid depression: A consensus-derived diagnostic algorithm for ADHD. Neuropsychiatr Dis Treat. 2009;5:137–150. doi: 10.2147/ndt.s4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker C, Bouvier E, Ghestem A, et al. Predicting and treating stress-induced vulnerability to epilepsy and depression. Ann Neurol. 2015 doi: 10.1002/ana.24414. [DOI] [PubMed] [Google Scholar]
- 7.Mazarati A, Siddarth P, Baldwin RA, et al. Depression after status epilepticus: behavioural and biochemical deficits and effects of fluoxetine. Brain. 2008;131:2071–2083. doi: 10.1093/brain/awn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazarati AM, Shin D, Kwon YS, et al. Elevated plasma corticosterone level and depressive behavior in experimental temporal lobe epilepsy. Neurobiol Dis. 2009;34:457–461. doi: 10.1016/j.nbd.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pineda EA, Hensler JG, Sankar R, et al. Plasticity of presynaptic and postsynaptic serotonin 1A receptors in an animal model of epilepsy-associated depression. Neuropsychopharmacology. 2011;36:1305–1316. doi: 10.1038/npp.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faure JB, Marques-Carneiro JE, Akimana G, et al. Attention and executive functions in a rat model of chronic epilepsy. Epilepsia. 2014;55:644–653. doi: 10.1111/epi.12549. [DOI] [PubMed] [Google Scholar]
- 11.Pineda E, Jentsch JD, Shin D, et al. Behavioral impairments in rats with chronic epilepsy suggest comorbidity between epilepsy and attention deficit/hyperactivity disorder. Epilepsy Behav. 2014;31:267–275. doi: 10.1016/j.yebeh.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohler S, Cierpinsky K, Kronenberg G, et al. The serotonergic system in the neurobiology of depression: Relevance for novel antidepressants. J Psychopharmacol. 2015 doi: 10.1177/0269881115609072. [DOI] [PubMed] [Google Scholar]
- 13.Genro JP, Kieling C, Rohde LA, et al. Attention-deficit/hyperactivity disorder and the dopaminergic hypotheses. Expert Rev Neurother. 2010;10:587–601. doi: 10.1586/ern.10.17. [DOI] [PubMed] [Google Scholar]
- 14.Sepede G, Corbo M, Fiori F, et al. Reboxetine in clinical practice: a review. Clin Ter. 2012;163:e255–e262. [PubMed] [Google Scholar]
- 15.Clemow DB, Bushe CJ. Atomoxetine in patients with ADHD: A clinical and pharmacological review of the onset, trajectory, duration of response and implications for patients. J Psychopharmacol. 2015 doi: 10.1177/0269881115602489. [DOI] [PubMed] [Google Scholar]
- 16.De Nayer A, Geerts S, Ruelens L, et al. Venlafaxine compared with fluoxetine in outpatients with depression and concomitant anxiety. Int J Neuropsychopharmacol. 2002;5:115–120. doi: 10.1017/S1461145702002857. [DOI] [PubMed] [Google Scholar]
- 17.Findling RL, Greenhill LL, McNamara NK, et al. Venlafaxine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17:433–445. doi: 10.1089/cap.2007.0119. [DOI] [PubMed] [Google Scholar]
- 18.Pineda EA, Hensler JG, Sankar R, et al. Interleukin-1beta causes fluoxetine resistance in an animal model of epilepsy-associated depression. Neurotherapeutics. 2012;9:477–485. doi: 10.1007/s13311-012-0110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005;182:335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- 20.Jentsch JD. Impaired visuospatial divided attention in the spontaneously hypertensive rat. Behavioural brain research. 2005;157:323–330. doi: 10.1016/j.bbr.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Jentsch JD, Aarde SM, Seu E. Effects of atomoxetine and methylphenidate on performance of a lateralized reaction time task in rats. Psychopharmacology (Berl) 2009;202:497–504. doi: 10.1007/s00213-008-1181-0. [DOI] [PubMed] [Google Scholar]
- 22.Mazarati AM, Pineda E, Shin D, et al. Comorbidity between epilepsy and depression: role of hippocampal interleukin-1beta. Neurobiol Dis. 2010;37:461–467. doi: 10.1016/j.nbd.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paxions G, Watson C. The Rat brain in stereotaxic coordinates. San Diego: Academic Press; 1986. et al. [Google Scholar]
- 24.John CE, Jones SR. Fast Scan Cyclic Voltammetry of Dopamine and Serotonin in Mouse Brain Slices. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. Boca Raton (FL): 2007. [PubMed] [Google Scholar]
- 25.Herr NR, Park J, McElligott ZA, et al. In vivo voltammetry monitoring of electrically evoked extracellular norepinephrine in subregions of the bed nucleus of the stria terminalis. Journal of neurophysiology. 2012;107:1731–1737. doi: 10.1152/jn.00620.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazarati AM, Baldwin RA, Shinmei S, et al. In vivo interaction between serotonin and galanin receptors types 1 and 2 in the dorsal raphe: implication for limbic seizures. J Neurochem. 2005;95:1495–1503. doi: 10.1111/j.1471-4159.2005.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Happe HK, Bylund DB, Murrin LC. Alpha(2)-adrenoceptor-stimulated GTP gamma S binding in rat brain: an autoradiographic study. Eur J Pharmacol. 2000;399:17–27. doi: 10.1016/s0014-2999(00)00380-0. [DOI] [PubMed] [Google Scholar]
- 28.Puig MV, Gulledge AT. Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol. 2011;44:449–464. doi: 10.1007/s12035-011-8214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albert PR, Vahid-Ansari F, Luckhart C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci. 2014;8:199. doi: 10.3389/fnbeh.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cinnamon Bidwell L, Dew RE, Kollins SH. Alpha-2 adrenergic receptors and attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2010;12:366–373. doi: 10.1007/s11920-010-0136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbui C, Hotopf M, Garattini S. Fluoxetine dose and outcome in antidepressant drug trials. Eur J Clin Pharmacol. 2002;58:379–386. doi: 10.1007/s00228-002-0497-7. [DOI] [PubMed] [Google Scholar]
- 32.Ravizza T, Gagliardi B, Noe F, et al. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Hensler JG. Differential regulation of 5-HT1A receptor-G protein interactions in brain following chronic antidepressant administration. Neuropsychopharmacology. 2002;26:565–573. doi: 10.1016/S0893-133X(01)00395-5. [DOI] [PubMed] [Google Scholar]
- 34.Koch S, Perry KW, Nelson DL, et al. R-fluoxetine increases extracellular DA, NE, as well as 5-HT in rat prefrontal cortex and hypothalamus: an in vivo microdialysis and receptor binding study. Neuropsychopharmacology. 2002;27:949–959. doi: 10.1016/S0893-133X(02)00377-9. [DOI] [PubMed] [Google Scholar]
- 35.Callado LF, Stamford JA. Alpha2A- but not alpha2B/C-adrenoceptors modulate noradrenaline release in rat locus coeruleus: voltammetric data. Eur J Pharmacol. 1999;366:35–39. doi: 10.1016/s0014-2999(98)00889-9. [DOI] [PubMed] [Google Scholar]
- 36.Lee A, Rosin DL, Van Bockstaele EJ. alpha2A–adrenergic receptors in the rat nucleus locus coeruleus: subcellular localization in catecholaminergic dendrites, astrocytes, and presynaptic axon terminals. Brain research. 1998;795:157–169. doi: 10.1016/s0006-8993(98)00266-2. [DOI] [PubMed] [Google Scholar]
- 37.Hajos M, Fleishaker JC, Filipiak-Reisner JK, et al. The selective norepinephrine reuptake inhibitor antidepressant reboxetine: pharmacological and clinical profile. CNS Drug Rev. 2004;10:23–44. doi: 10.1111/j.1527-3458.2004.tb00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pudovkina OL, Cremers TI, Westerink BH. The interaction between the locus coeruleus and dorsal raphe nucleus studied with dual-probe microdialysis. Eur J Pharmacol. 2002;445:37–42. doi: 10.1016/s0014-2999(02)01663-1. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez EJ, Williams PA, Dudek FE. Effects of fluoxetine and TFMPP on spontaneous seizures in rats with pilocarpine-induced epilepsy. Epilepsia. 2002;43:1337–1345. doi: 10.1046/j.1528-1157.2002.48701.x. [DOI] [PubMed] [Google Scholar]
- 40.Vermoesen K, Massie A, Smolders I, et al. The antidepressants citalopram and reboxetine reduce seizure frequency in rats with chronic epilepsy. Epilepsia. 2012;53:870–878. doi: 10.1111/j.1528-1167.2012.03436.x. [DOI] [PubMed] [Google Scholar]