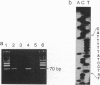
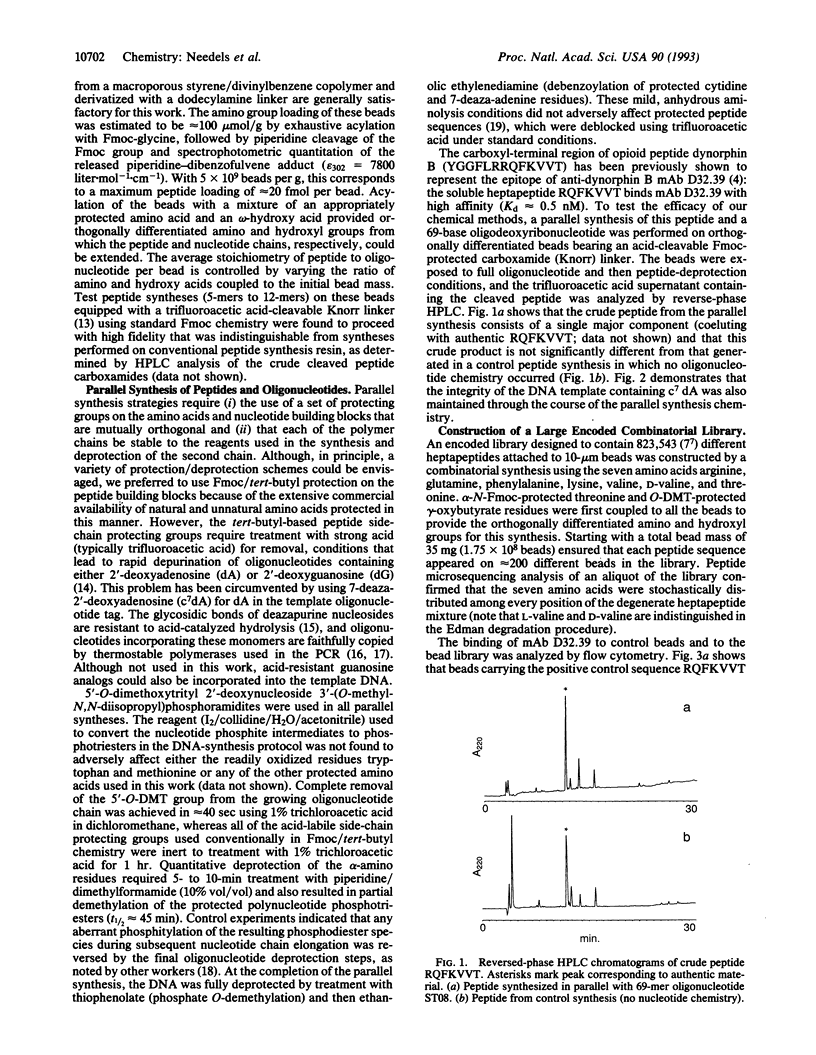
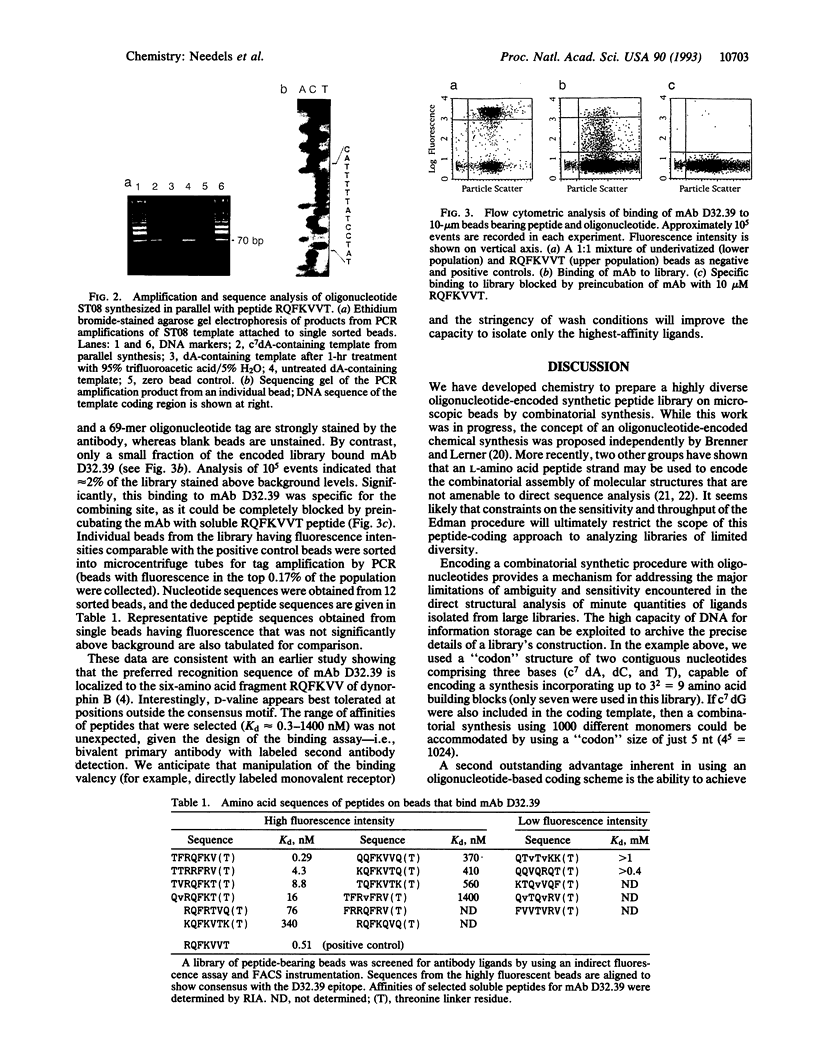
Abstract
We have prepared a library of approximately 10(6) different peptide sequences on small, spherical (10-microns diameter) beads by the combinatorial chemical coupling of both L- and D-amino acid building blocks. To each bead is covalently attached many copies of a single peptide sequence and, additionally, copies of a unique single-stranded oligonucleotide that codes for that peptide sequence. The oligonucleotide tags are synthesized through a parallel combinatorial procedure that effectively records the process by which the encoded peptide sequence is assembled. The collection of beads was screened for binding to a fluorescently labeled anti-peptide antibody using a fluorescence-activated cell sorting instrument. Those beads to which the antibody bound tightly were isolated by fluorescence-activated sorting, and the oligonucleotide identifiers attached to individual sorted beads were amplified by the PCR. Sequences of the amplified DNAs were determined to reveal the identity of peptide sequences that bound to the antibody with high affinity. By combining the capacity for information storage in an oligonucleotide code with the tremendous level of amplification possible through the PCR, we have devised a means for specifying the identity of each member of a vast library of molecules synthesized from both natural and unnatural chemical building blocks. In addition, we have shown that the use of flow cytometry instrumentation permits facile isolation of individual beads that bear high-affinity ligands for biological receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake J., Litzi-Davis L. Evaluation of peptide libraries: an iterative strategy to analyze the reactivity of peptide mixtures with antibodies. Bioconjug Chem. 1992 Nov-Dec;3(6):510–513. doi: 10.1021/bc00018a008. [DOI] [PubMed] [Google Scholar]
- Cho C. Y., Moran E. J., Cherry S. R., Stephans J. C., Fodor S. P., Adams C. L., Sundaram A., Jacobs J. W., Schultz P. G. An unnatural biopolymer. Science. 1993 Sep 3;261(5126):1303–1305. doi: 10.1126/science.7689747. [DOI] [PubMed] [Google Scholar]
- Cull M. G., Miller J. F., Schatz P. J. Screening for receptor ligands using large libraries of peptides linked to the C terminus of the lac repressor. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1865–1869. doi: 10.1073/pnas.89.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwirla S. E., Peters E. A., Barrett R. W., Dower W. J. Peptides on phage: a vast library of peptides for identifying ligands. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin J. J., Panganiban L. C., Devlin P. E. Random peptide libraries: a source of specific protein binding molecules. Science. 1990 Jul 27;249(4967):404–406. doi: 10.1126/science.2143033. [DOI] [PubMed] [Google Scholar]
- Fodor S. P., Read J. L., Pirrung M. C., Stryer L., Lu A. T., Solas D. Light-directed, spatially addressable parallel chemical synthesis. Science. 1991 Feb 15;251(4995):767–773. doi: 10.1126/science.1990438. [DOI] [PubMed] [Google Scholar]
- Furka A., Sebestyén F., Asgedom M., Dibó G. General method for rapid synthesis of multicomponent peptide mixtures. Int J Pept Protein Res. 1991 Jun;37(6):487–493. doi: 10.1111/j.1399-3011.1991.tb00765.x. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A., Pinilla C., Blondelle S. E., Appel J. R., Dooley C. T., Cuervo J. H. Generation and use of synthetic peptide combinatorial libraries for basic research and drug discovery. Nature. 1991 Nov 7;354(6348):84–86. doi: 10.1038/354084a0. [DOI] [PubMed] [Google Scholar]
- Lam K. S., Salmon S. E., Hersh E. M., Hruby V. J., Kazmierski W. M., Knapp R. J. A new type of synthetic peptide library for identifying ligand-binding activity. Nature. 1991 Nov 7;354(6348):82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- Lehmann C., Xu Y. Z., Christodoulou C., Tan Z. K., Gait M. J. Solid-phase synthesis of oligoribonucleotides using 9-fluorenylmethoxycarbonyl (Fmoc) for 5'-hydroxyl protection. Nucleic Acids Res. 1989 Apr 11;17(7):2379–2390. doi: 10.1093/nar/17.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon P. E., Raventos H., Lynch E., Morrow J., King M. C. The gene for an inherited form of deafness maps to chromosome 5q31. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5181–5184. doi: 10.1073/pnas.89.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. L., Langer J. A., Schwartz B., Pestka S. Creation of phosphorylation sites in proteins: construction of a phosphorylatable human interferon alpha. Proc Natl Acad Sci U S A. 1989 Jan;86(2):558–562. doi: 10.1073/pnas.86.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo M. C., Berninger M. S., Hartley J. L. Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene. 1990 Sep 1;93(1):125–128. doi: 10.1016/0378-1119(90)90145-h. [DOI] [PubMed] [Google Scholar]
- McConlogue L., Brow M. A., Innis M. A. Structure-independent DNA amplification by PCR using 7-deaza-2'-deoxyguanosine. Nucleic Acids Res. 1988 Oct 25;16(20):9869–9869. doi: 10.1093/nar/16.20.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltenyi S., Müller W., Weichel W., Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11(2):231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- Nikolaiev V., Stierandová A., Krchnák V., Seligmann B., Lam K. S., Salmon S. E., Lebl M. Peptide-encoding for structure determination of nonsequenceable polymers within libraries synthesized and tested on solid-phase supports. Pept Res. 1993 May-Jun;6(3):161–170. [PubMed] [Google Scholar]
- Scott J. K., Smith G. P. Searching for peptide ligands with an epitope library. Science. 1990 Jul 27;249(4967):386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- Simon R. J., Kania R. S., Zuckermann R. N., Huebner V. D., Jewell D. A., Banville S., Ng S., Wang L., Rosenberg S., Marlowe C. K. Peptoids: a modular approach to drug discovery. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9367–9371. doi: 10.1073/pnas.89.20.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]