Abstract
Background
Dermatitis associated with ileostomy is an important problem that affects many people, especially children. The aim of this study was to investigate the therapeutic effects of ozone on dermatitis due to ileostomy, and to develop an alternative treatment option.
Material/Methods
A total of 28 rats were divided into 4 groups: control, ileostomy, ozone, and zinc oxide. Ileostomy was performed in all rats except the control group. After a 1-week waiting time, the ozone group was administered ozone therapy and the zinc oxide group was administered zinc oxide cream locally once a day for a total of 7 days. All rats were sacrificed at the end of this period. The efficacy of treatment was examined by biochemical, histopathological, and immunohistochemical parameters. The levels of malondialdehyde (MDA), total glutathione (tGSH), total antioxidant capacity (TAC), and total oxidant status (TOS) were measured from tissue. Vascular endothelial growth factor (VEGF) and proliferating cell nuclear antigen (PCNA) were examined immunohistochemically.
Results
Dermatitis occurred pathologically in all rats that underwent ileostomy surgery. The lowest dermatitis score was in the ozone treatment group (p<0.05). Ileostomy dermatitis caused increased levels of MDA and TOS. Ozone treatment resulted in reduced MDA and TOS levels, while the levels of tGSH and TAC were increased (p<0.05). Both VEGF and PCNA immunostaining were augmented in the ozone treatment group (p<0.05).
Conclusions
Local ozone application may be a good alternative compared to the conventional treatment methods for the prevention of skin lesions that develop after ileostomy.
MeSH Keywords: Dermatitis, Ileostomy, Immunohistochemistry, Ozone
Background
An ostomy is a surgical opening of internal structures to the outside of the body. Ileostomies, colostomies, and urostomies are the most common types. An ileostomy is a surgical procedure in which the small intestine is attached to the abdominal wall in order to bypass the large intestine. Digestive waste can then exit the body through the stoma, which is an artificial opening through which watery stool passes and collect in the ileostomy bag. The common stoma complications are peristomal dermatitis, infections, abscesses, retraction, fistula, vascular compromise, parastomal herniation, bowel obstruction, and electrolyte imbalance [1,2]. Peristomal skin problems are common and affect up to 73.0% of ostomates. Peristomal irritant dermatitis is the most frequent skin complication and can be both a cause and a consequence of poor adhesion of stoma appliances; it is associated with leakage of stoma contents. This problem is common, affecting up to 62.0% of ostomates, and can be associated with considerable social, psychological, and physical problems [3]. Irritation of the skin surrounding the stoma is more frequently observed with ileostomies than with colostomies due to greater quantities of liquid and the caustic nature of the bilious small intestinal contents [2].
Irritant contact dermatitis is the most common peristomal skin complication [4]. Chemical injury occurs by intestinal contents contacting the skin. The skin becomes red, painful, and slippery and therefore it becomes difficult to affix the ostomy bag to the skin. Preventing the progression is critical because the dermatitis can lead to worsened leakage, further irritation and pain, and ongoing complications. Medical or surgical methods have been used to prevent and treat peristomal skin complications, while topical wound care products have been used to treat the damaged skin. Ileal peristomal leakage has been shown to be controlled temporarily using a mushroom-type (de Pezzer) catheter in addition to medical treatment [5]. Eroded peristomal skin can be treated with a hydrocolloid powder before placing the stoma appliance. Topical and aerosol corticosteroids or antihistamines have been useful in speeding peristomal skin recovery [1,6], while zinc oxide has been used topically in irritant dermatitis treatment and is known for its treatment efficacy [7–12].
Ozone is a gaseous molecule consisting of 3 oxygen atoms. The oxygen molecule (O2) is stable, but ozone (O3) is an unstable structure. The use of an ozone-oxygen gas mixture for treatment is known as ozone therapy [13]. Hydrogen peroxide, a powerful oxidizing agent, is responsible for the biological and therapeutic effects of ozone. It leads to reductions in antioxidant levels and shocks in the body due to the stimulating effect of hydrogen peroxide. As a result of this effect, the defense system is stimulated and thus resistance to the oxidative process is increased, including antioxidant enzyme expression [14]. Ozone therapy can increase acute cutaneous wound healing and regulate other processes, such as inflammatory responses [15]. Many studies have shown the advantages of ozone use in infected wounds, gangrene starts, burns, circulatory disorder diseases, chronic skin wounds, and abdominal extensive infections [16–18].
VEGF facilitates angiogenesis in wound healing, creating endothelial cell proliferation and migration, as well as new capillary formation. PCNA is known as a proliferation marker. In studies related to ozone therapy in wound healing, PCNA and VEGF expression has been used as an immunohistochemical marker of ozone effectiveness [15,19].
The aim of this study was to investigate ozone therapy, which has been widely used due to its antioxidative effect in medical treatment of ileostomy dermatitis. For this purpose, the relationship between antioxidative enzymes and immunohistochemical markers were analyzed in an experimental study. Development of ozone therapy as an alternative treatment option in clinical applications will also be discussed.
Material and Methods
Study groups
This study was approved by the Ethics Committee for Animal Experimentation of Atatürk University, Erzurum, Turkey (06.06.2014–91).
Twenty-eight Sprague-Dawley rats weighing 250–300 g were randomly divided into 4 distinct groups of 7 animals each. The rats used in the experiments were obtained from Atatürk University Medical Practice and Research Center and were housed in aerated plastic breeding cages at a constant temperature of 21±1°C, with a 12-h light/dark cycle. Rats were fed ad libitum using a standard pellet chow and tap water. In Group 1 (control group) and Group 2 (ileostomy group), an ileostomy was performed but no treatment was given. In Group 3 (ozone group) and Group 4 (zinc oxide group), ileostomy was performed and ozone or zinc oxide treatment was administered after surgery.
Surgery
Each rat was anesthetized using ketamine hydrochloride (80 mg/kg) (Ketalar 50 mg/ml Flakon, Pfizer®) and xylazine hydrochloride (10 mg/kg) (Rompun 100 mg/ml, Bayer®) intraperitoneally. After shaving the right abdominal region of rats, laparotomy was performed with a 1-cm transverse skin incision from the right midabdominal region. Loop ileostomy was performed from 5 cm proximal to the ileocecal junction. The ileum ans was fixed to the fascia with a 5/0 Vicryl, and the ileum tip was fixed to the skin with a 5/0 Vicryl. After the surgical procedure, rats were placed into individual cages to prevent any damage to each other. The drainage of the intestinal contents was observed from ileostomy in the postanesthetic period.
Ozone and zinc oxide application
Ozone (O3) was created by an ozone generator (OZONO-SAN Photonik 1014, Hansler GmbH; Nordring & Iffezheim, Germany). The ozone flow rate was kept constant at 60 mg/ml concentration, 97.0% oxygen +3.0% ozone gas mixture at 3 L/min. The ozone/oxygen mix was applied to 2 cm of skin around the ileostomy of rats in Group 3 using a sterile gauze once a day for 7 days. Zinc oxide (Oksizinc baby 40 g, Oxyde de Zinc 20.0%, D-Panthenol 5.0%, Dermotek Medicine, Turkey) was applied to 2 cm of skin around the ileostomy of rats in Group 4 once a day for 7 days.
Sample collection
All animals were anesthetized 7 days after the surgical procedure. The skin (2 cm2) was excised from the ileostomy around and a portion of it was reserved for histopathologic and biochemical examination. The portion reserved for histopathologic analysis was placed into 10.0% buffered formaldehyde solution. Samples reserved for MDA measurement were stored at −80°C. A 5-ml blood sample was taken from the abdominal aorta and centrifuged to separate the serum. The blood serum samples were stored at −80°C until assayed for biochemical examination (tGSH, TAC, TOS).
Biochemical evaluation
tGSH analysis
A double sandwich ELISA kit (Shanghai Sunred Biological Technology Co., Ltd; Catalog No: 201-11-5134) was used to analyze rat reduced glutathione (GSH) level in samples.
MDA analysis
An ELISA kit (Shanghai Sunred Biological Technology Co., Ltd.; Catalog No: 201-11-0157) was used to analyze rat MDA levels in the samples.
TAC detection
Serum total antioxidant capacity detection was determined by an automatic measurement method developed by Erel [21].
TOS detection
Serum total oxidant status detection was determined by an automatic measurement method developed by Erel [22].
Histopathologic evaluation
For histological analysis, the formalin-fixed skin samples were prepared for paraffin sections. Then, 4-μm sections were made and placed on slides. These slides were stained with hematoxylin and eosin (H&E). Histopathological examination of the rat tissue damage was done for each parameter: epidermal atrophy, hair-follicle atrophy, collagen loss, and edema, based on a scoring system where none=0, mild=1 +, moderate=2 +, and severe=3 +. Total scores were calculated according to these parameters. A score of 4 or higher was accepted as presence of dermatitis and scores of less than 4 was accepted as no dermatitis [20].
Immunohistochemical evaluation
The 4-μm sections were made and placed on positively charged slides for immunohistochemical (IHC) staining. Then, these slides were stained with the VEGF (VG-1, Code; SC-53462, Santa Cruz, dilution rate 1:50) and PCNA (PCNA, Code: SC-56, Santa Cruz, dilution rate 1:50) antibodies. A fully automated IHC device (Leica Bond-Max, Melbourne, Australia) was used for immunostaining. Histopathological and immunohistochemical evaluations were done using a light microscope (Olympus BX53, Tokyo, Japan). For VEGF antibody staining, pneumocytes and macrophages of human lung tissue were used as a positive control. For PCNA antibody, the staining of hepatocytes in human liver tumor was used as a positive control. Negative controls were tested by omission of the primary antibody (secondary only). The slides were then covered with coverslips and treated with alcohol and xylene. VEGF and PCNA immunostaining ratios were defined in the range of 0 to 3: Score 0=no staining, Score 1=weak staining (less than 10.0% focal involvement), Score 2=moderate staining (11.0–50.0% regional involvement), and Score 3=strong staining (greater than 50.0% diffuse involvement) [20].
Statistical analysis
Descriptive statistics are represented as mean ± standard deviation. The variables were assessed after controlling preconditions of sphericity and homogeneity of variances (Shapiro-Wilk and Levene test). One-way analysis of variance (ANOVA) was used in data analysis. Some variables were determined not to meet the requirements after testing prerequisites of parametric test, in which case Box-Cox data transformation was performed. A nonparametric Kruskal-Wallis test was used when preconditions were not still met after data transformation. The adjusted LSD test was used to evaluate the variables with significant differences. Data were evaluated using SPSS (Version 17, Chicago IL, USA) software. Statistical significance was accepted with a p-value of less than 0.05.
Results
A total of 3 animals died during the study period (1 each from Group 2, Group 3, and Group 4). New animals were included in the study and the project was completed with groups including 7 animals each. In the macroscopic examination of the skin around the ileostomy before sacrifice, dermatitis was found to be milder in the ozone group as compared to the ileostomy and zinc oxide groups (Figure 1).
Figure 1.
Peristomal macroscopic view of of different groups before sacrifation. (A) Severe peristomal dermatitis in a rat Group 2; (B) Minimal deramtis after ozone therapy; (C) Moderate dermatitis after zinc oxide treatment.
The biochemical analysis of MDA, tGSH, TAC, and TOS is summarized in Table 1. The MDA values were highest in Group 2 (10.75 nmol/g), while Group 3 MDA values were significantly lower than in Group 2 and Group 4 (P<0.05). tGSH (291.42 μmol/g) and TAC (2.21 μmol/g) values in Group 3 were higher than in Groups 2 and 4. The values in Group 4 were significantly higher than the values in Group 2 (P<0.05). The TOS value in Group 3 (38.21 μmol/g) was lower than in Groups 2 and 4, while the value in Group 4 was lower than in Group 2 (P<0.05) (Figure 2).
Table 1.
Statistical analysis of biochemical data
| N | Mean | Std. deviation | p | ||
|---|---|---|---|---|---|
| MDA | Group 1 | 7 | 8.32 | 2.51 | 0.015* |
| Group 2 | 7 | 10.75a | 3.71 | ||
| Group 3 | 7 | 8.48a,b | 0.44 | ||
| Group 4 | 7 | 8.57a,b,c | 0.18 | ||
| Total | 28 | 9.45 | 3.64 | ||
| tGSH | Group 1 | 7 | 243.46 | 64.68 | 0.049* |
| Group 2 | 7 | 186.81a,c,d | 54.48 | ||
| Group 3 | 7 | 291.42a,d | 17.55 | ||
| Group 4 | 7 | 286.71a | 16.73 | ||
| Total | 28 | 245.92 | 93.91 | ||
| TAC | Group 1 | 7 | 2.46 | 1.49 | 0.034* |
| Group 2 | 7 | 0.99a,c,d | 0.53 | ||
| Group 3 | 7 | 2.21a,d | 0.48 | ||
| Group 4 | 7 | 1.90a | 0.61 | ||
| Total | 28 | 1.79 | 0.99 | ||
| TOS | Group 1 | 7 | 32.50 | 20.41 | 0.045* |
| Group 2 | 7 | 49.29a,c,d | 21.69 | ||
| Group 3 | 7 | 38.21 | 28.05 | ||
| Group 4 | 7 | 42.14a,c | 25.80 | ||
| Total | 28 | 40.54 | 32.43 |
p<0.05.
Different from Group 1;
different from Group 2;
different from Group 3;
Different from Group 4.
Figure 2.
(A) MDA; (B) tGSH; (C) TAC; (D) TOS levels.
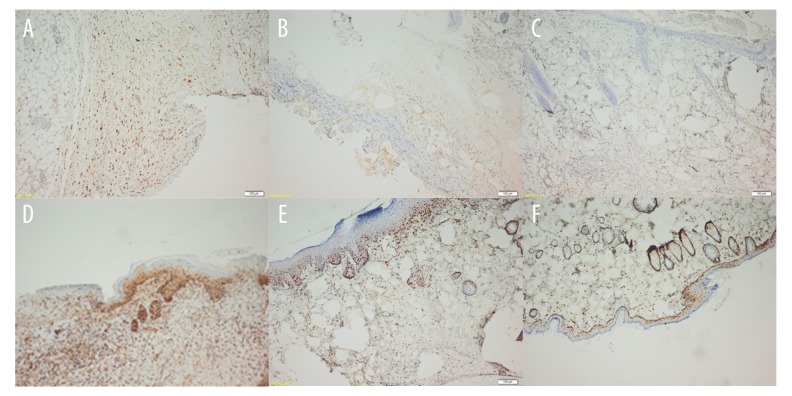
Histopathological dermatitis was not observed in the control group (Figure 3A). Group 2 had the most dermatitis (Figure 3B) and Group 3 had the least (except when compared to the control group) (Figure 3C) (P<0.05). Group 3 had the most VEGF immunostaining (Figure 4A), and it was lower in Group 4 (Figure 4B) and marginal in Group 2 (Figure 4C). The strongest PCNA immunostaining was observed in Group 3 (Figure 4D), and it was higher in Group 4 (Figure 4E) than in Group 2 (Figure 4F) (P<0.05) (Table 2).
Figure 3.
(A) Normal histopathological skin view of control group (HE ×100). (B) Histopathological examination view of a rat with severe dermatitis in Group 2 (HE ×100). Significant epidermal atrophy, hair-follicle atrophy, collagen loss, and edema are observed. (C, D) View of decreased dermatitis after ozone and zinc oxide therapy, similar to normal skin tissue (HE ×100).
Figure 4.
Immunohistochemical view of groups. (A) Group 3; Increased VEGF immunostaining with ozone therapy. (B) Group 4; Moderately increased VEGF immunostaining with zinc oxide treatment. (C) Group 2; Minimally increased VEGF immunostaining. (D) Group 3; Strong PCNA immunostaining presenting cellular proliferation increased with ozone therapy. (E) Group 4; Moderately increased PCNA immunostaining after zinc oxide treatment. (F) Group 2; Mild PCNA immunostaining presenting a small amount of cell proliferation in an untreated rat after ileostomy.
Table 2.
Statistical analysis of dermatitis development and immunohistochemical staining.
| N | Mean | Std. deviation | p | ||
|---|---|---|---|---|---|
| Dermatitis | Group 1b | 7 | .00 | .000 | 0.02* |
| Group 2a,c,d | 7 | 1.00 | .000 | ||
| Group 3a,b,d | 7 | .29 | .488 | ||
| Group 4a,b | 7 | .57 | .535 | ||
| Total | 28 | .46 | .508 | ||
| VEGF | Group 1b | 7 | .29 | .488 | 0.00* |
| Group 2a,c,d | 7 | 1.43 | .535 | ||
| Group 3a,b,d | 7 | 2.71 | .488 | ||
| Group 4a,b | 7 | 2.00 | .577 | ||
| Total | 28 | 1.61 | 1.031 | ||
| PCNA | Group 1b | 7 | .29 | .488 | 0.00* |
| Group 2a,c,d | 7 | 1.43 | .535 | ||
| Group 3a,b,d | 7 | 2.71 | .488 | ||
| Group 4a,b | 7 | 2.43 | .535 | ||
| Total | 28 | 1.71 | 1.084 |
p<0.05.
Different from Group 1;
different from Group 2;
different from Group 3;
Different from Group 4.
Discussion
The topical effects of ozone and zinc oxide in ileostomy dermatitis treatment were compared in this experimental ileostomy model. Various topical agents other than zinc oxide have been used in the treatment of dermatitis, which is a significant complication of ileostomy. Sometimes it is not possible to cope with the location of the ileostomy; therefore, it becomes necessary to change the region of the ileostomy [7,23,24]. There have been multiple reports presenting the positive effects of ozone therapy in wound healing [15,17,25]. However, no studies have shown the relationship between ozone and ileostomy dermatitis. A week after ileostomy, noticeable dermatitis occurred on peristomal skin regions of rats. We observed that ozone therapy applied after ileostomy dermatitis provided a better healing macroscopically than zinc oxide treatment. Significant epidermal atrophy, hair-follicle atrophy, collagen loss, and edema were observed in the untreated ileostomy group based on the histopathological examination. These findings were found to decrease less in all groups when compared with ozone therapy followed by zinc oxide treatment. The most severe dermatitis was observed in the untreated ileostomy group, whereas the least severe dermatitis was observed in the ozone therapy group. In accordance with the macroscopic appearance, the dermatitis score after ozone therapy lower than after zinc oxide therapy (Table 2).
Wound healing is an active harmonic process consisting of multiple phases; it occurs as a result of inflammation, angiogenesis, granulation tissue formation, and repair of epithelium tissue around the damaged region. If the factor causing skin dermatitis continues, wound healing is delayed and the resistance to treatment increases. Biochemical parameters, such as MDA, GSH, TAC, and TOS, have been explored in investigations of oxidative damage in wound healing [25,26]. In case of tissue damage, free oxygen radicals emerge and oxidative stress occurs. In case of oxidative stress, lipid and protein oxidation products increase, while antioxidant enzymes decrease [27,28]. Ozone, an antioxidant agent, increases tGSH level and decreases MDA value, which is the final product of lipid peroxidation [29–32]. The increase in TOS and MDA values in the skin tissue of rats after ileostomy dermatitis demonstrated the presence of oxidative damage. While MDA and TOS values were found to decrease significantly after ozone therapy, tGSH and TAC values were found to increase. There was a statistically significant difference between the ozone group and the zinc oxide group in terms of these parameters. This shows that topical application of ozone having antioxidant properties provides improvement in oxidative damage and is more effective in wound healing compared to zinc oxide.
VEGF provides arteriogenesis, lumen expansion, and collateral vessel formation [33]. Angiogenesis and functional vessels play a critical role in wound healing and granulation formation. VEGF is essential for early vessel formation and angiogenesis [34]. VEGF level, which is a marker of angiogenesis, is significantly higher and its involvement in tissue increases [35–38]. VEGF expression has been observed to increase in healing of diabetic ulcers and acute cutaneus wound healing from topical ozone therapy [15,39]. In the immunohistochemical examination conducted after 7-day treatment in our study, VEGF immunostaining in the skin of the dermatitis region was more significant in the ozone therapy group compared to the zinc oxide group. This suggests that ozone therapy increases angiogenesis and thus presents more effectiveness in wound healing. VEGF expression has been reported to decrease in wounds that were almost closed (40). In our study, the increased VEGF immunostaining after ozone therapy can be explained by irritant intestinal content due to ileostomy.
In addition to the biochemical parameters, wound healing can be evaluated by monitoring cell proliferation immunohistochemically with PCNA staining [41–43]. PCNA density has been shown to increase at the 1st day, reached the peak value at the 5th day, and then decreased gradually in a study conducted in a burn model [44]. PCNA-positive cells can be identified until 21 days after injury [45]. In this study, animals were sacrificed at the 14th day after ileostomy and then skin samples were collected for analysis. Peristomal skin tissue was stained with anti-PCNA immunohistochemically and examined using a light microscope to show proliferative cells. The wound healing process still continued even at the 14th day, due to the continuation of the irritant effect of ileostomy. Therefore, we observed a more intensive immunohistochemical cellular staining. When the wound healing accelerated, cellular proliferation increased as well. These proliferative cells are stained more intensively by anti-PCNA than are other cells [46–49]. PCNA immunostaining was much lower in the untreated ileostomy group than in the treatment groups. The reason for this might be the deterioration of skin integrity caused by continuous intestinal irritant material and the insufficiency of the wound healing process. PCNA immunostaining in the ozone therapy group was observed to be the highest, and was significantly higher compared to the zinc oxide group. This suggests that ozone therapy increases cell proliferation more than zinc treatment, and ozone therapy would be more effective in the wound healing process.
Conclusions
In conclusion, topical ozone application in the treatment of ileostomy dermatitis improves the effects of oxidative damage in tissues, increasing angiogenesis and cell proliferation, and provides more effective wound healing than zinc oxide. Clinical use of ozone would be useful as an alternative treatment option in severe dermatitis associated with ileostomy.
Acknowledgments
We would like to thank Ataturk University Experimental Research Center for their help during the experimental study.
Footnotes
Conflict of interests
The authors have declared that no conflict of interests exists.
Source of support: This research was supported by Erzincan University Unit of Scientific Projects (no. 2014-14/18), Erzincan, Turkey
References
- 1.Nicholson J, Sriskandarajah S, Moore J, et al. Aerosol steroids for the treatment of peristomal mucocutaneous breakdown due to severe eczema. Int J Surg Case Rep. 2014;5(12):1173–75. doi: 10.1016/j.ijscr.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kann BR. Early stomal complications. Clin Colon Rectal Surg. 2008;21(1):23–30. doi: 10.1055/s-2008-1055318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weidmann AK, Al-Niaimi F, Lyon CC. Correction of skin contour defects in leaking stomas by filler injection: a novel approach for a difficult clinical problem. Dermatol Ther. 2014;4(2):271–79. doi: 10.1007/s13555-014-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burch J. The management and care of people with stoma complications. Br J Nurs. 2004;13(6):307–8. doi: 10.12968/bjon.2004.13.6.12526. [DOI] [PubMed] [Google Scholar]
- 5.Banani SA, Banani SJ. Managing severe dermatitis caused by ileal peristomal leakage using a mushroom-type (de Pezzer) catheter in infants: A case series. Ostomy Wound Manage. 2013;59(12):26–31. [PubMed] [Google Scholar]
- 6.Kwiatt M, Kawata M. Avoidance and management of stomal complications. Clin Colon Rectal Surg. 2013;26(2):112–21. doi: 10.1055/s-0033-1348050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conley P, McKinsey D, Ross O, et al. Does skin care frequency affect the severity of incontinence-associated dermatitis in critically ill patients? Nursing. 2014;44(12):27–32. doi: 10.1097/01.NURSE.0000456382.63520.24. [DOI] [PubMed] [Google Scholar]
- 8.Hosseinpour M, Fazeli A, Agabeigi M. Efficacy of Acacia senegal for stoma care in children with colostomy. Eur J Pediatr Surg. 2012;22(3):234–37. doi: 10.1055/s-0032-1308706. [DOI] [PubMed] [Google Scholar]
- 9.Hoggarth A, Waring M, Alexander J, et al. A controlled, three-part trial to investigate the barrier function and skin hydration properties of six skin protectants. Ostomy Wound Manage. 2005;51(12):30–42. [PubMed] [Google Scholar]
- 10.Nielsen LF, Blume N, Romme T, et al. Skin changes induced by a zinc oxide dressing compared with a hydrocolloid dressing in healthy individuals. Skin Res Technol. 2005;11(2):140–51. doi: 10.1111/j.1600-0846.2005.00105.x. [DOI] [PubMed] [Google Scholar]
- 11.Sibbald RG, Campbell K, Coutts P, et al. Intact skin an integrity not to be lost. Ostomy Wound Manage. 2003;49(6):27–8. [PubMed] [Google Scholar]
- 12.Bhat RM, Chavda R, Ribet V. To evaluate the efficacy and safety of “RV2427B” cream in Irritant dermatitis care. Indian Dermatol Online J. 2013;4(3):180–84. doi: 10.4103/2229-5178.115511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Paolo N, Gaggiotti E, Galli F. Extracorporeal blood oxygenation and ozonation: Clinical and biological implications of ozone therapy. Redox Rep. 2005;10(3):121–30. doi: 10.1179/135100005X38888. [DOI] [PubMed] [Google Scholar]
- 14.Yıldırım AO, Eryılmaz M, Kaldırım U, et al. Effectiveness of hyperbaric oxygen and ozone applications in tissue healing in generated soft tissue trauma model in rats: an experimental study. Ulus Travma Acil Cerrahi Derg. 2014;20(3):167–75. doi: 10.5505/tjtes.2014.09465. [DOI] [PubMed] [Google Scholar]
- 15.Kim HS, Noh SU, Han YW, et al. Therapeutic effects of topical application of ozone on acute cutaneous wound healing. J Korean Med Sci. 2009;24(3):368–74. doi: 10.3346/jkms.2009.24.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulmen S, Kurtoglu T, Meteoglu I, et al. Ozone therapy as an adjunct to vancomycin enhances bacterial elimination in methicillin resistant Staphylococcus aureus mediastinitis. J Surg Res. 2013;185(1):64–69. doi: 10.1016/j.jss.2013.05.085. [DOI] [PubMed] [Google Scholar]
- 17.Guven A, Gundogdu G, Sadir S, et al. The efficacy of ozone therapy in experimental caustic esophageal burn. J Pediatr Surg. 2008;43(9):1679–84. doi: 10.1016/j.jpedsurg.2008.01.064. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez ZZ, Guanche D, Alvarez RG, et al. Preconditioning with ozone/oxygen mixture induces reversion of some indicators of oxidative stress and prevents organic damage in rats with fecal peritonitis. Inflamm Res. 2009;58(7):371–75. doi: 10.1007/s00011-009-0001-2. [DOI] [PubMed] [Google Scholar]
- 19.Valacchi G, Pagnin E, Corbacho AM, et al. In vivo ozone exposure induces antioxidant/stress-related responses in murine lung and skin. Free Radic Biol Med. 2004;36(5):673–81. doi: 10.1016/j.freeradbiomed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Yirmibesoglu E, Karahacioglu E, Kilic D, et al. The protective effects of Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: An experimental study. Clin Exp Dermatol. 2012;37(4):387–94. doi: 10.1111/j.1365-2230.2011.04253.x. [DOI] [PubMed] [Google Scholar]
- 21.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37(2):112–19. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Banu T, Talukder R, Chowdhury TK, et al. Betel leaf in stoma care. J Pediatr Surg. 2007;42(7):1263–65. doi: 10.1016/j.jpedsurg.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 24.Bafford AC, Irani JL. Management and complications of stomas. Surg Clin North Am. 2013;93(1):145–66. doi: 10.1016/j.suc.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Erdemci F, Gunaydin Y, Sencimen M, et al. Histomorphometric evaluation of the effect of systemic and topical ozone on alveolar bone healing following tooth extraction in rats. Int J Oral Maxillofac Surg. 2014;43(6):777–83. doi: 10.1016/j.ijom.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Tascilar O, Cakmak G, Emre A, et al. N-acetylcycsteine attenuates the deleterious effects of radiation therapy on inci-sional wound healing in rats. Hippokratia. 2014;18(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- 27.Holroyd S, Graham K, et al. Prevention and management of incontinence-associated dermatitis using a barrier cream. Br J Community Nurs. 2014:S32–38. doi: 10.12968/bjcn.2014.19.Sup6.S32. Suppl Wound Care. [DOI] [PubMed] [Google Scholar]
- 28.Hamdy Abdelaziz M, Fouad Ghoneim D, Abdelkawi Ahmed S, et al. The effect of anterior stromal puncture using Q-switched Nd: YAG laser on corneal wound healing. J Lasers Med Sci. 2014;5(3):121–29. [PMC free article] [PubMed] [Google Scholar]
- 29.Koksal H, Kurban S. Total oxidant status, total antioxidant status, and paraoxonase and arylesterase activities during laparoscopic cholecystectomy. Clinics. 2010;65(3):285–90. doi: 10.1590/S1807-59322010000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peralta C, León OS, Xaus C, et al. Protective effect of ozone treatment on the injury associated with hepatic ischemia-reperfusion: antioxidant-prooxidant balance. Free Radic Res. 1999;31(3):191–96. doi: 10.1080/10715769900300741. [DOI] [PubMed] [Google Scholar]
- 31.Ajamieh HH, Menéndez S, Martínez-Sánchez G, et al. Effects of ozone oxidative preconditioning on nitric oxide generation and cellular redox balance in a rat model of hepatic ischaemia-reperfusion. Liver Int. 2004;24(1):55–62. doi: 10.1111/j.1478-3231.2004.00885.x. [DOI] [PubMed] [Google Scholar]
- 32.Gultekin FA, Bakkal BH, Guven B, et al. Effects of ozone oxidative preconditioning on radiation-induced organ damage in rats. J Radiat Res. 2013;54(1):36–44. doi: 10.1093/jrr/rrs073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kofler NM, Simons M. Angiogenesis versus arteriogenesis: neuropilin 1 modulation of VEGF signaling. F1000Prime Rep. 2015;3(7):26. doi: 10.12703/P7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Liu J, Liu Y, et al. Negative pressure wound therapy accelerates rats diabetic wound by promoting agenesis. Int J Clin Exp Med. 2015;8(3):3506–13. [PMC free article] [PubMed] [Google Scholar]
- 35.Sayar H, Gergerlioglu N, Seringec N, et al. Comparison of efficacy of topical phenytoin with hypericin in second-degree burn wound healing: an experimental study in rats. Med Sci Monit Basic Res. 2014;20:36–46. doi: 10.12659/MSMBR.890337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li RL, Huang JJ, Shi YQ, et al. Pulsed electromagnetic field improves postnatal neovascularization in response to hindlimb ischemia. Am J Transl Res. 2015;7(3):430–44. [PMC free article] [PubMed] [Google Scholar]
- 37.Kandhare AD, Alam J, Patil MV, et al. Wound healing potential of naringin ointment formulation via regulating the expression of inflammatory, apoptotic and growth mediators in experimental rats. Pharm Biol. 2015;20:1–14. doi: 10.3109/13880209.2015.1038755. [DOI] [PubMed] [Google Scholar]
- 38.Kao HK, Hsu HH, Chuang WY, et al. Experimental study of fat grafting under negative pressure for wounds with exposed bone. Br J Surg. 2015;102(8):998–1005. doi: 10.1002/bjs.9826. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Guan M, Xie C, et al. Increased growth factors play a role in wound healing promoted by noninvasive oxygen-ozone therapy in diabetic patients with foot ulcers. Oxid Med Cell Longev. 2014;2014:273475. doi: 10.1155/2014/273475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JC, Lin BB, Hu HW, et al. NGF accelerates cutaneous wound healing by promoting the migration of dermal fibroblasts via the PI3K/Akt-Rac1-JNK and ERK pathways. Biomed Res Int. 2014;2014:547187. doi: 10.1155/2014/547187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin P. Wound healing – aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 42.Gupta A, Dai T, Hamblin MR. Effect of red and near-infrared wavelengths on low-level laser (light) therapy-induced healing of partial-thickness dermal abrasion in mice. Lasers Med Sci. 2014;29(1):257–65. doi: 10.1007/s10103-013-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu YS, Huang SL, Nan FH, et al. Over-inhibition of NADPH oxidase reduce the wound healing in liver of finfish. Fish Shellfish Immunol. 2014;40(1):174–81. doi: 10.1016/j.fsi.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Shi Y, Shu B, et al. The effect of porcine ADM to improve the burn wound healing. Int J Clin Exp Pathol. 2013;6(11):2280–91. [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JW, Lee JH, Lyoo YS, et al. The effects of topical mesenchymal stem cell transplantation in canine experimental cutaneous wounds. Vet Dermatol. 2013;24(2):242–e53. doi: 10.1111/vde.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Şimşek G, Ciftci O, Karadag N, et al. Effects of topical phenytoin on nasal wound healing after mechanical trauma: An experimental study. Laryngoscope. 2014;124(12):E449–54. doi: 10.1002/lary.24811. [DOI] [PubMed] [Google Scholar]
- 47.Zhang B, Wang M, Gong A, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33(7):2158–68. doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- 48.Shu B, Xie JL, Xu YB, et al. Effects of skin-derived precursors on wound healing of denervated skin in a nude mouse model. Int J Clin Exp Pathol. 2015;8(3):2660–69. [PMC free article] [PubMed] [Google Scholar]
- 49.Valacchi G, Zanardi I, Lim Y, et al. Ozonated oils as functional dermatological matrices: Effects on the wound healing process using SKH1 mice. Int J Pharm. 2013;458(1):65–73. doi: 10.1016/j.ijpharm.2013.09.039. [DOI] [PubMed] [Google Scholar]