Abstract
Objective
In this work we evaluated the association of human immunodeficiency virus (HIV) infection and methamphetamine (METH) use with mitochondrial injury in the brain and its implication on neurocognitive impairment.
Design
Mitochondria carry their genome (mtDNA) and play a critical role in cellular processes in the central nervous system. METH is commonly used in HIV-infected populations. HIV infection and METH use can cause damage to mtDNA and lead to neurocognitive morbidity. We evaluated HIV infection and METH use with mitochondrial injury in the brain.
Methods
We obtained white and gray matter from Brodmann’s areas (BA) BA7, BA8, BA9, and BA46 of a) HIV-infected individuals with history of past METH use (HIV+METH+, n=16), b) HIV-infected individuals with no history of past METH use (HIV+METH−, n=11), and c) HIV-negative controls (HIV−METH−, n=30). We used the “common deletion,” a 4,977 bp mutation, as a measurement of mitochondrial injury, and quantified levels of mtDNA and “common deletion” by droplet digital PCR, and evaluated in relation to neurocognitive functioning (Global Deficit Score [GDS]).
Results
Levels of mtDNA and mitochondrial injury were highest in white matter of BA46. A higher relative proportion of mtDNA carrying the “common deletion” was associated with lower GDS (p<0.01) in HIV+METH+ but higher GDS (p<0.01) in HIV+METH−.
Conclusions
Increased mitochondrial injury was associated with worse neurocognitive function in HIV+METH− individuals. Among HIV+METH+ individuals, an opposite effect was seen.
Keywords: HIV, mitochondrial DNA, mitochondrial injury, common deletion, neurocognitive impairment, methamphetamine, GDS, droplet digital PCR
Introduction
Mitochondria are the main organelles responsible for synthesizing energy in the form of adenosine triphosphate (ATP) for metabolic and cellular processes throughout the body and in the central nervous system (CNS) [1]. In eukaryotic cellular respiration, post-glycolytic reactions occur in mitochondria, making mitochondria at risk to damage by reactive oxygen species (ROS). Oxidative damage caused by ROS can lead to mitochondrial DNA (mtDNA) mutations and deletions. Previous studies have explored the relationships between mtDNA deletions, neurodegenerative diseases, and mental illnesses [2–4]. However, little is known about mitochondrial and neurocognitive dysfunction in the setting of human immunodeficiency virus (HIV) type 1 infection.
The most frequently identified mtDNA deletion—accounting for about 40% of all mtDNA deletions—is a 4,977 bp deletion that affects RNA transfer and respiratory chain genes known as the “common deletion” [5]. The “common deletion” results in defective mitochondrial function and is often found in higher amounts in the brain tissue of individuals with neurodegenerative diseases, substance abuse, and older age [6–8]. Although each mitochondrion carries multiple copies of its genome in response to the risk incurred by ROS, somatic changes in mtDNA can proliferate within the mtDNA population over time. Accumulation of mtDNA carrying deletions within a cell can lead to reduced energy synthesis and overall mitochondrial network dysfunction.
Neurocognitive dysfunction is a common complication of HIV disease. HIV may enter the CNS and establish a latent infection. In some individuals, productive infection occurs in the CNS leading to inflammation and eventually to HIV-related brain injury, affecting pathways involved in learning, memory, attention/working memory, and executive functioning [9]. Previous studies have also demonstrated that HIV-1-infected macrophages produce factors that suppress axonal growth and induce mitochondrial membrane depolarization. This might lead to reduced ATP production, causing additional damage to neurons [10].
Methamphetamine (METH) use is associated with neurocognitive disorders; the neuropathogenesis of HIV-1 infection may also be exacerbated by METH use [11]. Some of the effects of METH use are apoptosis, oxidative stress, and neuroinflammation [12, 13]. METH is a prominent drug of choice among HIV-infected individuals engaging in high-risk behaviors [14–17]. METH use during HIV infection is associated with increased viral loads in cerebral spinal fluid (CSF), plasma, or both; enhanced HIV-1 pathogenesis [18]; and greater mtDNA damage [19]. In addition, METH has been shown to increase HIV-1 replication in dendritic cells [20] and monocyte-derived macrophages [21].
In this study, we compared the levels of mtDNA and the relative proportion of the “common deletion” in brain tissue of HIV-infected individuals with or without reported history of past METH use. We also investigated the magnitude of mitochondrial genetic defects in relation to neurocognition. Given our previous work demonstrating the correlation between cell-free CSF mtDNA levels and neurocognitive performance [22], we also investigated cell-free CSF mtDNA in these groups. Elucidation of the relationship between METH use and HIV infection on mtDNA may help improve our understanding of the role of mitochondrial injury in HIV-associated neurocognitive disorders.
Materials and Methods
Study cohorts
Brain tissue samples containing white and gray matter from Brodmann’s areas (BA) BA7, BA8, BA9, and BA46 were obtained from the National NeuroAIDS Tissue Consortium (NNTC). The frontal lobe regions (BA8, BA9, BA46) were requested based upon previous work demonstrating the role of oxidative stress in neurodegeneration, methamphetamine effects on the frontal lobe [23–25]. All samples were from unique subjects, except for some paired BA7 and BA8 tissue samples which came from the same participants. Samples were obtained from subjects classified into three groups: a) HIV-infected individuals with reported history of past METH use (HIV+METH+, n=16), b) HIV-infected individuals with no reported history of past METH use (HIV+METH−, n=11), and c) HIV-negative controls (HIV−METH−, n=30). We also obtained CSF samples when available from these subjects. Participants were classified into the same three groups as above: a) HIV+METH+ (n=15), b) HIV+METH− (n=26), and c) HIV−METH− (n=17). Participants with known Alzheimer’s disease (HIV+METH− (n=1), HIV+METH+ (n=3), HIV−METH− (n=4)) were excluded from analysis, since Alzheimer’s disease is associated with higher levels of mitochondrial DNA deletions [26, 27]. Clinical assessments, socio-demographical variables and neuropsychological performance evaluations were obtained from assessments performed during the pre-mortem period.
Neuropsychological assessments
Participants underwent neuropsychological testing using neuropsychological test battery of seven ability areas summarized by the Global Deficit Score (GDS), a continuous score ranging from 0 (no impairment) to 5 (severest impairment) [28, 29]. An individual with a GDS score of greater than 0.5 was deemed to have neurocognitive impairment [22]. In this study, we analyzed GDS as a continuous variable to measure neuropsychological performance.
Brain removal and storage
All brain specimens were obtained with a postmortem interval of under 24 hours (median = 13.4 hours). The entire brain was removed, weighed, bisected longitudinally, snap frozen, and then sectioned according to NNTC brain processing protocols. The frozen tissue samples were transported on dry ice and stored at −80°C until the samples were processed. Frozen CSF samples were treated in a similar manner.
DNA extraction
Separate DNA extractions were performed from 25 mg of white matter and gray matter from each sample using the QIAamp DNA Mini Kit (Qiagen, Venlo, Holland) from BA7, BA8, BA9, and BA46. The manufacturer’s protocol was followed except that the DNA was eluted twice using 200 μl elution buffer each time to maximize DNA recovery. The first elution included a 5-minute incubation before the final spin. Quality of the extraction, both quantification and purity of the nucleic acids, was assessed using the Nanodrop 1000 Spectrophotometer (Thermo Scientific, Waltham, MA) software version 3.7.1 per manufacturer’s protocol.
Quantification of mtDNA and mitochondrial injury
DNA quantification was performed using the highly sensitive droplet digital PCR platform [30]. In lieu of a digestion step, we used QIAshredder (Qiagen) cell-lysate homogenizer on 10 ng/μL of extracted DNA in a total volume of 25 μL per manufacturer’s protocol. We quantified levels of mtDNA per cell by measuring the copy numbers of the mitochondrial NADH dehydrogenase 2 (MT-ND2) (Applied Biosystems, Waltham, MA) using a standard Applied Biosystems assay (Cat. # 4331182), and the cellular control gene ribonuclease P protein subunit p30 (RPP30) (Integrated DNA Technologies, Coralville, IA) which is present in two copies per cell as described in our previous study [22]. The names and sequences of these primer-probe sets are as follows: RPP30-F (5′-GATTTGGACCTGCGAGCG-3′), RPP30-R (5′-GCGGCTGTCTCCACAAGT-3′), and RPP30-P (5′-HEX/CTGACCTGA/ZEN/AGGCTCT/IBFQ-3′). Additionally, we quantified levels of HIV in the brain tissue samples by measuring the copy numbers of conserved regions in HIV-1, such as the long terminal repeat (LTR) and the gag gene (Integrated DNA Technologies, Coralville, IA), to look for any associations between HIV DNA levels in the brain and mitochondrial injury or mtDNA. The names and sequences of these primer-probe sets are as follows: 2LTR-F (5′-TGCCAATCAGGGAAGWAGCCTTG-3′), 2LTR-R (5′-GAACCCACTGCTTAAGCCTCAAT-3′), and 2LTR-P (5′-FAM/CTGACCTGA/ZEN/AGGCTCT/IBFQ-3′) for the LTR gene, and GAG-F (5′-AGTTGGAGGACATCAAGCAGCCATGCAAAT-3′), GAG-R (5′-TGCTATGTCAGTTCCCCTTGGTTCTCT-3′), and GAG-P (5′-HEX/CTGACCTGA/ZEN/AG-GCTCT/IBFQ/-3′) for the gag gene.
In the brain tissue samples, we measured the relative proportion of mtDNA carrying the “common deletion” and used it as our measurement of mitochondrial injury. We designed a primer-probe combination that targets the bridge region on the mitochondrial chromosome before and after the 4,977 bp “common deletion” (CD) (Integrated DNA Technologies, Coralville, IA) (Fig. 1). The names and sequences of these primer-probe sets are as follows: CD-F (5′-GGCTCAGGCGTTTGTGTATGAT-3′), CD-R (5′-TATTAAACACAAACTACCACCTACC-3′), and CD-P (5′-FAM/ACCATTGGC/ZEN/AGCCTAG/IBFQ-3′). As a result, amplification will only occur in the presence of the deletion. For quantification, we used 50 ng of DNA when measuring the “common deletion,” and 50 pg of DNA when measuring MT-ND2. In the extracted DNA from CSF samples, we only measured MT-ND2 and RPP30, given the low levels of DNA present.
Figure 1. Human mitochondrial DNA (mtDNA).
A diagram of the 4,977 bp “common deletion” and mitochondrial genes in human mtDNA.
Quantification was performed in triplicate using a reaction consisting of 10 μL of 2x Bio-Rad Supermix for probes, either 1 μL of 20x Primer/FAM MT-ND2 mix or 20x Primer/FAM CD mix in combination with 20x Primer/HEX RPP30 mix, 3μL of molecular grade water, and 5 μL of shredded DNA. When we measured levels of HIV, we used a reaction consisting of 10 μL of 2x Bio-Rad Supermix for probes, either 1 μL of 20x Primer/FAM 2LTR mix with 20x Primer/HEX GAG, or 20x Primer/HEX RPP30 mix with 1 μL of molecular grade water, and 8 μL of shredded DNA. The PCR thermal cycling conditions were: (1) initial activation at 95°C for 10 minutes, (2) 55 cycles at 94°C for 30 seconds and 60°C for 1 min (ramp speed 2°C per second), (3) enzyme inactivation at 98°C for 10 minutes, and a 4°C hold. Droplets were read and analyzed using the Bio-Rad QX100 droplet reader and QuantaSoft software version 1.6.6. We calculated the average amount of mtDNA per cell using our cellular control. The amount of cells tested per well were calculated by dividing the amount of RPP30 copies by 2 to adjust for two copies of RPP30 present in one cell. The relative proportion of “common deletion” was calculated by dividing the number of mitochondria carrying the “common deletion” per cell by the total number of mtDNA copies (ND2) per cell. Separate from the brain tissue samples, levels of mtDNA in CSF were expressed in log10 copies/mL of CSF.
Soluble and inflammatory markers in CSF
We measured levels of soluble CD14 (sCD14) and soluble CD163 (sCD163) in our CSF samples using Quantikine ELISA Human sCD14 and sCD163 (R&D Systems, Minneapolis, MN) following manufacturer’s protocols. Additionally, we measured neurofilament light (NF-L) and neopterin using the NF-Light (NF-L, Uman Diagnostics, Umea, Sweden) and BRAHMS (GmbH, Hennigsdorf, Germany) kits, respectively. All of these markers have been previously associated with inflammation and neurocognitive impairments in HIV infections [31–33].
Statistical analysis
All statistical analysis was performed using R statistical software Version 3.1.1. Normality of variables was assessed using the Shapiro test. Variables were either log- or square root-transformed to approximate a normal distribution. Differences in the levels of mtDNA levels per cell or relative abundance of common deletion was assessed with either a two-tailed t-test (pairwise comparisons) or by Analysis of Variance (ANOVA) with a Tukey post hoc adjustment for three-class comparisons. Univariate and multivariate association between variables were determined with either fixed-effects or mixed-effects regression analyses adjusting for repeated measures as needed.
Results
Cohort characteristics
The median age at death was 49 years. All participants were male. All HIV-infected patients both in the HIV+METH− group and HIV+METH+ group were on antiretroviral therapy (ART) when clinical and neuropsychological assessments were administered and reported until death. Neuropsychological assessments were performed on all HIV-infected participants prior to the last pre-mortem visit. CSF, blood, lymphocyte profiles, and demographic characteristics were also collected pre-mortem. There were no significant differences between the three study groups in relation to these variables. A summary of cohort characteristics is provided in Table 1. Participants with known Alzheimer’s disease were excluded from analysis.
Table 1.
Characteristics of the study participants
| HIV+METH+ (n=16) | HIV+METH− (n=11) | HIV−METH− (n=30) | p-value* | |
|---|---|---|---|---|
| Ethnicity (Hisp:Not Hisp) | 2:14 | 2:9 | 8:22 | 0.57 |
| Age | 50.2±8.1 | 46.1±7.5 | 51.4±12.1 | 0.32 |
| CSF HIV RNA Levels (log10 copies/mL) | 1.73±0.86 | 2.21±1.34 | - | 0.17 |
| Proportion of undetectability HIV RNA in CSF (<50 copies) | 3/13 | 3/8 | - | 0.66 |
| Plasma HIV RNA Levels (log10 copies/mL) | 3.36±1.52 | 3.27±2.30 | - | 0.64 |
| Proportion of undetectability HIV RNA in plasma (<50 copies) | 2/14 | 2/9 | - | 1 |
| CD4 Absolute Counts | 117±140 | 153±231 | - | 0.40 |
| GDS | 1.03±1.07 | 0.97±0.73 | - | 0.60 |
Mean values are shown for each study group.
Represent the p-value of a double-tailed Mann-Whitney or a Fisher test. See “Methods” section for the definitions of HIV+METH+, HIV+METH−, HIV−METH−.
Abbreviations: Hisp, Hispanic; CSF, cerebral spinal fluid; GDS, global deficit score.
Levels of mtDNA and mitochondrial injury in different brain regions
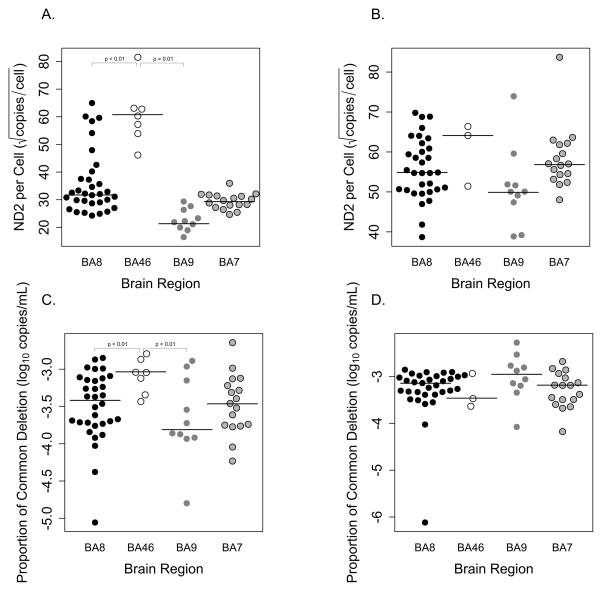
When comparing the levels of mtDNA in white matter between sampled brain regions BA8, BA9, and BA46 we found that BA46 had significantly higher levels of mtDNA when compared to BA8 (p<0.01) and BA9 (p<0.01) (Fig. 2A). For a subset of the subjects, we received brain tissues from both BA7 and BA8 from the same participants. As a result of dependent data in the repeated measurements from both BA7 and BA8 for the same participants, we used a mixed model to adjust for variance. We found that there was no statistical difference between the two brain regions. There were no statistical differences between brain regions and mtDNA levels in gray matter (Fig. 2B).
Figure 2. Levels of mitochondrial DNA (mtDNA) and mitochondrial injury in different brain regions.
(A, B) Levels of mtDNA per cell and (C, D) the abundance of the “common deletion” in different brain regions in white and gray matter. Significant differences in levels of mtDNA and the abundance of the “common deletion” was seen in white matter. In addition, BA46 showed the highest levels of mtDNA and mitochondrial injury compared to BA8 and BA9. There was no statistical difference in levels of mtDNA and mitochondrial injury in gray matter.
Abbreviations: ND2, NADH dehydrogenase 2; BA, Brodmann Area.
By quantifying the “common deletion,” we compared levels of mitochondrial injury in white matter from BA8, BA9, and BA46. We found that BA46 had the highest relative proportion of mitochondrial injury when compared to BA8 (p<0.01) and BA9 (p=0.01) (Fig. 2C). Again, for a subset of the subjects with brain tissue samples from both BA8 and BA7, we used a mixed model to adjust for variance and found that there were no statistical differences. We did not find any statistical difference between brain regions and mitochondrial injury in gray matter (Fig. 2D), even though higher levels of “common deletion” were present in gray matter rather than white matter. However, we still found that white matter had greater differences between the brain regions and the proportions of mtDNA with the “common deletion” when compared to gray matter in a mixed-model analysis.
Levels of mtDNA and mitochondrial injury in subject groups
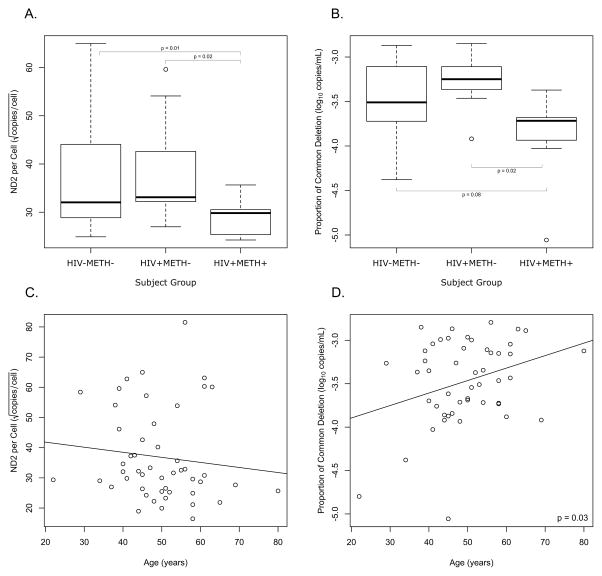
Significant differences in mtDNA copy number and mitochondrial injury were found only in white matter rather than gray matter. Additionally, we collected the largest amount of samples from BA8. Because of these reasons, we decided to focus our analysis on white matter from BA8. In this brain region, participants in the HIV+METH+ group had significantly higher levels of mtDNA per cell when compared to HIV+METH− (p=0.02) and HIV−METH− (p<0.01) (Fig. 3A). HIV+METH+ group showed significantly less mitochondrial injury when compared to the HIV+METH− group (p=0.02) and HIV−METH− group (p=0.08) (Fig. 3B). When we compared the three study groups by ANOVA followed by the Tukey adjustment, we again found significantly less mitochondrial injury in the HIV+METH+ group when compared to the HIV+METH− (p=0.01) and HIV−METH− groups (p=0.06). However, we did not find any significant differences in the levels of mtDNA with these additional tests.
Figure 3. Levels of mitochondrial DNA (mtDNA) and mitochondrial injury in different subject groups and association with age.
(A) HIV+METH− participants had significantly higher levels of mtDNA per cell compared to HIV+METH+ and HIV−METH−. (B) However, HIV+METH+ showed significantly lower levels of mitochondrial injury compared to HIV+METH− and HIV−METH−.
(C) While there was no association between the levels of mtDNA per cell and age, (D) higher abundance of the “common deletion” was associated with older age.
See “Methods” section for the definitions of HIV+METH+, HIV+METH−, HIV−METH−.
Abbreviations: ND2, NADH dehydrogenase 2.
Mitochondrial injury is associated with age
Since the relative proportion of mtDNA carrying the “common deletion” is known to be associated with increasing age and mortality [34, 35], we performed fixed-effect regression analysis to determine the association of both measurements of mtDNA copy number and mitochondrial injury with age. After correcting for the subset with measurements from both BA7 and BA8, we found that there was no association between the levels of mtDNA per cell and age (Fig. 3C); however, a higher abundance of the “common deletion” was associated with increasing age (Fig. 3D, p=0.03).
MtDNA and mitochondrial injury is associated with cognitive function
We performed a multivariate analysis to evaluate the association of levels of mtDNA and mitochondrial injury with GDS while adjusting for age and brain region. We used recursive partitioning to determine the effects of age and brain region in the model. If a variable did not contribute significantly to the model, it was removed from the model. In the HIV+METH+ group, we found that a higher abundance of “common deletion” was associated with lower GDS (p<0.01); however, in the HIV+METH− group, higher abundance of “common deletion” was associated with higher GDS (p<0.01) (Table 2).
Table 2.
P-values of the association of the relative abundance of the “common deletion” with clinical variables
| Clinical Variable | HIV+METH+ | HIV+METH− |
|---|---|---|
| GDS | 0.006 | 0.001 |
| CSF VL (log10 copies/mL) | 0.19 | 0.17 |
| Plasma VL (log10 copies/mL) | 0.25 | 0.72 |
| CD4 counts (cells/μL) | 0.89 | 0.86 |
| CD4 percentage (%) | 0.59 | 0.22 |
| CD8 counts (cells/μL) | 0.87 | 0.94 |
| CD8 percentage (%) | 0.81 | 0.55 |
| 2-LTR (copy per 1E6 cells) | 0.58 | 0.15 |
| HIV gag (copy per 1E6 cells) | 0.0003 | 0.0001 |
See “Methods” section for the definitions of HIV+METH+, HIV+METH−.
Abbreviations: GDS, global deficit score; CSF, cerebral spinal fluid; VL, viral load; LTR, long terminal repeat; 1E6, 1,000,000.
HIV DNA in brain tissue is associated with mitochondrial injury
We performed a multivariate analysis to evaluate the association between levels of HIV DNA and mitochondrial injury while adjusting for age and brain region. In both HIV+ groups, we found that higher abundance of “common deletion” was associated with higher levels of HIV copies per million cells (p<0.01) found in brain tissue (Table 2). We did not find any associations between levels of HIV DNA and mtDNA.
Levels of cell free mtDNA, soluble and inflammatory markers in CSF
There were no significant associations between cell-free mtDNA in CSF, in relation to subject groups, GDS, or other clinical and immunological variables. There were also no significant associations between soluble and inflammatory markers, such as sCD14, sCD163, NFL, and neopterin, in relation to subject groups and other clinical and immunological variables.
Discussion
This is the first study to measure and compare the levels of mtDNA and the abundance of the “common deletion” in brain tissue in HIV-infected individuals with or without reported history of past METH use. We found significant differences in levels of mtDNA per cell and the abundance of “common deletion” in white matter of brain tissue in HIV+METH+ and HIV+METH− groups. We also found a relationship between the abundance of the “common deletion” with levels of HIV in brain tissue. Previous studies have shown that high levels of mitochondrial injury and cell death are associated with increased environmental stress, increased production of ROS, and decreased respiration, Ca2+ regulation, and electron transport chain activity [36, 37]. A significant increase in ROS is associated with oxidative DNA damage and mtDNA mutations, causing a chain of events that would lead to mitochondrial injury and cell death [38]. In cultured media, toxicity is directly related to Ca2+ influx, as Ca2+ is a major determinant of glutamate receptor excitotoxicity [39]. A greater abundance of the “common deletion” could indicate a larger amount of mitochondrial injury due to oxidative stress, which may be worsened by HIV infection.
Highest levels of mtDNA and mitochondrial injury were seen in the white matter of BA46 when compared to BA7, BA8, and BA9. We found that only brain tissue from white matter demonstrated statistically significant variation in mtDNA levels and mitochondrial injury. This is supported by previous observations demonstrating that glutamate excitotoxicity and downstream free radical attack by ROS frequently occurs in glial cells like oligodendrocytes and myelinated axons present in white matter [40, 41], while gray matter primarily contains unmyelinated axons [42]. Observable differences in mtDNA levels and injury may vary more between myelinated and unmyelinated axons. In addition, we found that higher abundance of “common deletion” was associated with higher levels of HIV in brain tissue, perhaps indicating a relationship between HIV viral loads in the brain with mitochondrial injury.
We observed that levels of mtDNA and mitochondrial damage in white matter were highest among HIV-infected without METH individuals but lowest in HIV-infected with METH users. In addition, we observed that a higher proportion of mtDNA carrying the common deletion was associated with better neurocognitive function, (i.e., lower GDS), in HIV-infected METH users, but worse neurocognitive function in HIV-infected non-METH users. The reason for the reduced levels of mitochondrial injury in METH users remains unclear, but possible explanations include: 1) A sampling bias from where the brain tissue was sampled; 2) Induction of mitophagy by METH—thus the measureable amount of mitochondrial injury is reduced due to selective degradation of damaged mitochondrial DNA [43, 44]; 3) Neuroprotection by METH—previous work has shown that METH may mediate neuroprotection at low doses through moderate activation of dopamine receptors via the phosphoinositol-3 kinase and protein kinase B (Akt) pathways, both of which are responsible for apoptosis and cell proliferation [45], while dopamine receptor D2 activation plays an important role in protecting the brain against glutamate cytotoxicity through Akt signaling and up-regulation of Bcl-2 expression, an antiapoptotic protein [46, 47]; and 4) Reduced ART exposure—although HIV-infected participants (HIV+METH−, HIV+METH+) in our study were on ART until the last visit prior to death, previous studies have shown that HIV-infected METH users are prone to medication non-adherence [48]. ART has been known to induce oxidative stress [49, 50], and increase mitochondrial toxicity, particularly with the use of nucleoside reverse transcriptase inhibitors [51]. Therefore, reduced ART exposure could lead to lower levels of mitochondrial injury.
As with any evaluation of a clinical cohort, this study has several limitations. Primarily, all participants were enrolled into the NNTC at the time of death. Ante-mortem clinical data was only abstracted from medical records and sometimes acquired through family reports. For example, a urine toxicology test for amphetamine was not administered to everyone in the HIV+METH+ group. We cannot adequately determine the length of history of the participants’ exposure to METH, whether it is past, current, or lifetime, abuse or dependence. We were also limited to only the four BAs that were provided in our study as well as the number of available samples for each group. In addition, in our analysis of HIV viral loads and mitochondrial injury, not all the participants had undetectable HIV viral loads and were in different stages of infection during the time clinical and neuropsychological assessments were administered. The small numbers in our cohort limited our ability to correct for other potential confounders, such as disease stage, ART use, and other demographic factors.
In conclusion, our data indicate that levels of mtDNA per cell and the abundance of “common deletion” are significant in white matter of brain tissue in BA46 when compared to BA7, BA8, and BA9. Participants who were HIV+METH+ had the lowest levels of mitochondrial injury per cell in white matter when compared to the HIV+METH− and HIV−METH− groups, as well as lowest mtDNA copy number. There was also a significant association between greater mitochondrial injury with higher HIV DNA levels in brain tissue. As expected, increased mitochondrial injury was associated with worse neurocognitive function in HIV-infected individuals. However, in those individuals using METH, an opposite effect was seen. Further work is needed to clarify the relationships between the presence of mitochondrial injury and neurocognitive function in HIV-infected individuals and users of METH.
Acknowledgments
Funding
This work was supported by the Department of Veterans Affairs [SRM and DMS]; National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) [grants K23 AI093163 to SRM, K24 AI100665 to DMS, U19 AI090970, University of California Center for AIDS Research (P30 AI036214), AIDS Training Grant (T32 AI007384)], National Institute of Drug Abuse, NIH [grants Translational Methamphetamine AIDS Research Center (P30 DA026306), DP1 DA034978 to DMS], National Institute of Mental Health [grants R01 MH094159 & R01 MH105319 to CLS, Human Neurobehavioral Research Center MH062512, R01 MH096648, R01 AG043384, R01 MH097520 to DMS, Interdisciplinary Fellowship in NeuroAIDS (R25 MH081482)] and the National NeuroAIDS Tissue Consortium, which consists of the National Neurological AIDS Bank (U01-MH08021, U24 MH100929 and R24-NS38841; Singer), the Texas NeuroAIDS Research Center (U01-MH083507, U24 MH100930 and R24-NS45491; Benjamin, Gelman, principal investigator, University of Texas Medical Branch), the Manhattan HIV Brain Bank (U01-MH083501, U24 MH100931 and R24-MH59724; Susan Morgello, principal investigator, Mt. Sinai Medical Center), and the California NeuroAIDS Tissue Network (U01-MH083506, U24 MH100928 and R24-MH59745; David Moore, principal investigator, UCSD); the National Science Foundation [DMS0714991]; and the James B. Pendleton Charitable Trust.
We would like to thank Lucas Barwick of the National NeuroAIDS Tissue Consortium for his adept assistance in providing us with clinical data. The authors acknowledge the contribution of the research volunteers, Translational Methamphetamine AIDS Research Center, and the Center for AIDS Research Genomics and Translational Virology Cores, and the National NeuroAIDS Tissue Consortium, of which members included National Neurological AIDS Bank, Texas NeuroAIDS Research Center, California NeuroAIDS Tissue Network, and Manhattan HIV Brain Bank. We also would like to thank the Aging and Inflammation Scientific Working Group of the University of California San Diego Center for AIDS Resesarch.
Footnotes
Meetings
Some of these data were presented at the Conference on Retroviruses and Opportunistic Infections in Seattle, Washington from February 23–26, 2015.
Author Contributions
SRV performed DNA extraction, ddPCR quantification experiments, and measurement of soluble and inflammatory markers, performed the data analyses, performed the statistical analyses, and wrote the primary version of the manuscript. TRCD participated in measurement of soluble and inflammatory markers. AV participated in ddPCR quantification experiments, and figure design. DMS participated in data analysis and revision of the manuscript. VS obtained samples, performed brain dissections, and data interpretation. DJM participated in neuropsychological testing, data interpretation, and revision of the manuscript. JPS participated in DNA extraction and statistical analyses. CLA, SRM, and JPS designed the present study, participated in data analysis, and revision of the manuscript. All authors read and approved the final manuscript.
Conflict of interest
SRV, TRCD, AV, VS, DJM, CLA, SRM, and JPS do not have any commercial or other associations that might pose a conflict of interest. DMS has received grant support from ViiV and worked as a consultant for Hologic.
References
- 1.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Block ER, Patel JM. Down-regulation of mitochondrial cytochrome c oxidase in senescent porcine pulmonary artery endothelial cells. Mech Ageing Dev. 2002;123:1363–1374. doi: 10.1016/s0047-6374(02)00075-1. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- 4.Mamdani F, Rollins B, Morgan L, Sequeira PA, Vawter MP. The somatic common deletion in mitochondrial DNA is decreased in schizophrenia. Schizophr Res. 2014;159:370–375. doi: 10.1016/j.schres.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen B, Zhong Y, Peng W, Sun Y, Hu YJ, Yang Y, et al. Increased mitochondrial DNA damage and decreased base excision repair in the auditory cortex of D-galactose-induced aging rats. Mol Biol Rep. 2011;38:3635–3642. doi: 10.1007/s11033-010-0476-5. [DOI] [PubMed] [Google Scholar]
- 6.Trifunovic A, Larsson NG. Mitochondrial dysfunction as a cause of ageing. J Intern Med. 2008;263:167–178. doi: 10.1111/j.1365-2796.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 7.Perfeito R, Cunha-Oliveira T, Rego AC. Revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease--resemblance to the effect of amphetamine drugs of abuse. Free Radic Biol Med. 2012;53:1791–1806. doi: 10.1016/j.freeradbiomed.2012.08.569. [DOI] [PubMed] [Google Scholar]
- 8.Langford D, Grigorian A, Hurford R, Adame A, Crews L, Masliah E. The role of mitochondrial alterations in the combined toxic effects of human immunodeficiency virus Tat protein and methamphetamine on calbindin positive-neurons. J Neurovirol. 2004;10:327–337. doi: 10.1080/13550280490520961. [DOI] [PubMed] [Google Scholar]
- 9.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitayama H, Miura Y, Ando Y, Hoshino S, Ishizaka Y, Koyanagi Y. Human immunodeficiency virus type 1 Vpr inhibits axonal outgrowth through induction of mitochondrial dysfunction. J Virol. 2008;82:2528–2542. doi: 10.1128/JVI.02094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol. 2011;24:275–283. doi: 10.1097/WCO.0b013e32834695fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah A, Kumar S, Simon SD, Singh DP, Kumar A. HIV gp120- and methamphetamine-mediated oxidative stress induces astrocyte apoptosis via cytochrome P450 2E1. Cell Death Dis. 2013;4:e850. doi: 10.1038/cddis.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semple SJ, Patterson TL, Grant I. Motivations associated with methamphetamine use among HIV+ men who have sex with men. J Subst Abuse Treat. 2002;22:149–156. doi: 10.1016/s0740-5472(02)00223-4. [DOI] [PubMed] [Google Scholar]
- 15.Urbina A, Jones K. Crystal methamphetamine, its analogues, and HIV infection: medical and psychiatric aspects of a new epidemic. Clin Infect Dis. 2004;38:890–894. doi: 10.1086/381975. [DOI] [PubMed] [Google Scholar]
- 16.Halkitis PN, Fischgrund BN, Parsons JT. Explanations for methamphetamine use among gay and bisexual men in New York City. Subst Use Misuse. 2005;40:1331–1345. doi: 10.1081/JA-200066900. [DOI] [PubMed] [Google Scholar]
- 17.Shoptaw S. Methamphetamine use in urban gay and bisexual populations. Top HIV Med. 2006;14:84–87. [PubMed] [Google Scholar]
- 18.Mantri CK, Mantri JV, Pandhare J, Dash C. Methamphetamine inhibits HIV-1 replication in CD4+ T cells by modulating anti-HIV-1 miRNA expression. Am J Pathol. 2014;184:92–100. doi: 10.1016/j.ajpath.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potula R, Hawkins BJ, Cenna JM, Fan S, Dykstra H, Ramirez SH, et al. Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J Immunol. 2010;185:2867–2876. doi: 10.4049/jimmunol.0903691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair MP, Saiyed ZM. Effect of methamphetamine on expression of HIV coreceptors and CC-chemokines by dendritic cells. Life Sci. 2011;88:987–994. doi: 10.1016/j.lfs.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang H, Wang X, Chen H, Song L, Ye L, Wang SH, et al. Methamphetamine enhances HIV infection of macrophages. Am J Pathol. 2008;172:1617–1624. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Santiago J, Schrier RD, de Oliveira MF, Gianella S, Var SR, Day TR, et al. Cell-free mitochondrial DNA in CSF is associated with early viral rebound, inflammation, and severity of neurocognitive deficits in HIV infection. J Neurovirol. 2015 doi: 10.1007/s13365-015-0384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung YH, Yurgelun-Todd DA, Shi XF, Kondo DG, Lundberg KJ, McGlade EC, et al. Decreased frontal lobe phosphocreatine levels in methamphetamine users. Drug Alcohol Depend. 2013;129:102–109. doi: 10.1016/j.drugalcdep.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales AM, Lee B, Hellemann G, O’Neill J, London ED. Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend. 2012;125:230–238. doi: 10.1016/j.drugalcdep.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkateshappa C, Harish G, Mahadevan A, Srinivas Bharath MM, Shankar SK. Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: implications for neurodegeneration in Alzheimer’s disease. Neurochem Res. 2012;37:1601–1614. doi: 10.1007/s11064-012-0755-8. [DOI] [PubMed] [Google Scholar]
- 26.Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochim Biophys Acta. 2010;1802:29–44. doi: 10.1016/j.bbadis.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Stichel CC, Zhu XR, Bader V, Linnartz B, Schmidt S, Lubbert H. Mono- and double-mutant mouse models of Parkinson’s disease display severe mitochondrial damage. Hum Mol Genet. 2007;16:2377–2393. doi: 10.1093/hmg/ddm083. [DOI] [PubMed] [Google Scholar]
- 28.Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, et al. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol. 2012;26:894–908. doi: 10.1080/13854046.2012.694479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 30.Strain MC, Lada SM, Luong T, Rought SE, Gianella S, Terry VH, et al. Highly precise measurement of HIV DNA by droplet digital PCR. PLoS One. 2013;8:e55943. doi: 10.1371/journal.pone.0055943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS. 2013;27:1387–1395. doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13:976–986. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60:234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuningas M, Estrada K, Hsu YH, Nandakumar K, Uitterlinden AG, Lunetta KL, et al. Large common deletions associate with mortality at old age. Hum Mol Genet. 2011;20:4290–4296. doi: 10.1093/hmg/ddr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Y, Luo X, Zhu J, Zhang X, Zhu Y, Cheng H, et al. Mitochondrial DNA 4977 bp deletion is a common phenomenon in hair and increases with age. Bosn J Basic Med Sci. 2012;12:187–192. doi: 10.17305/bjbms.2012.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liedhegner EAS, Gao XH, Mieyal JJ. Mechanisms of Altered Redox Regulation in Neurodegenerative Diseases-Focus on S-Glutathionylation. Antioxidants & Redox Signaling. 2012;16:543–566. doi: 10.1089/ars.2011.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trachootham D, Zhang H, Zhang W, Feng L, Du M, Zhou Y, et al. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood. 2008;112:1912–1922. doi: 10.1182/blood-2008-04-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Domercq M, Sanchez-Gomez MV, Areso P, Matute C. Expression of glutamate transporters in rat optic nerve oligodendrocytes. Eur J Neurosci. 1999;11:2226–2236. doi: 10.1046/j.1460-9568.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 39.Matute C, Alberdi E, Domercq M, Sanchez-Gomez MV, Perez-Samartin A, Rodriguez-Antiguedad A, et al. Excitotoxic damage to white matter. J Anat. 2007;210:693–702. doi: 10.1111/j.1469-7580.2007.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lassmann H. Mechanisms of white matter damage in multiple sclerosis. Glia. 2014;62:1816–1830. doi: 10.1002/glia.22597. [DOI] [PubMed] [Google Scholar]
- 42.Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cadet JL, Ali SF, Rothman RB, Epstein CJ. Neurotoxicity, drugs and abuse, and the CuZn-superoxide dismutase transgenic mice. Mol Neurobiol. 1995;11:155–163. doi: 10.1007/BF02740692. [DOI] [PubMed] [Google Scholar]
- 44.Chu CT. Diversity in the regulation of autophagy and mitophagy: lessons from Parkinson’s disease. Parkinsons Dis. 2011;2011:789431. doi: 10.4061/2011/789431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rau CR, Hein K, Sattler MB, Kretzschmar B, Hillgruber C, McRae BL, et al. Anti-inflammatory effects of FTY720 do not prevent neuronal cell loss in a rat model of optic neuritis. Am J Pathol. 2011;178:1770–1781. doi: 10.1016/j.ajpath.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou S, Li L, Pei L, Vukusic B, Van Tol HH, Lee FJ, et al. Protein-protein coupling/uncoupling enables dopamine D2 receptor regulation of AMPA receptor-mediated excitotoxicity. J Neurosci. 2005;25:4385–4395. doi: 10.1523/JNEUROSCI.5099-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Kanki R, et al. Protective effect of dopamine D2 agonists in cortical neurons via the phosphatidylinositol 3 kinase cascade. J Neurosci Res. 2002;70:274–282. doi: 10.1002/jnr.10426. [DOI] [PubMed] [Google Scholar]
- 48.Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2007;11:185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma B. Oxidative stress in HIV patients receiving antiretroviral therapy. Curr HIV Res. 2014;12:13–21. doi: 10.2174/1570162x12666140402100959. [DOI] [PubMed] [Google Scholar]
- 50.Mandas A, Iorio EL, Congiu MG, Balestrieri C, Mereu A, Cau D, et al. Oxidative imbalance in HIV-1 infected patients treated with antiretroviral therapy. J Biomed Biotechnol. 2009;2009:749575. doi: 10.1155/2009/749575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schweinsburg BC, Taylor MJ, Alhassoon OM, Gonzalez R, Brown GG, Ellis RJ, et al. Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J Neurovirol. 2005;11:356–364. doi: 10.1080/13550280591002342. [DOI] [PubMed] [Google Scholar]