Abstract
The enzyme FabH catalyzes the initial step of fatty acid biosynthesis via a type II dissociated fatty acid synthase. The pivotal role of this essential enzyme, combined with its unique structural features and ubiquitous occurrence in bacteria, has made it an attractive new target for the development of antibacterial and antiparasitic compounds. We have searched the National Cancer Institute database for compounds bearing structural similarities to thiolactomycin, a natural product which exhibits a weak activity against FabH. This search has yielded several substituted 1,2-dithiole-3-ones that are potent inhibitors of FabH from both Escherichia coli (ecFabH) and Staphylococcus aureus (saFabH). The most potent inhibitor was 4,5-dichloro-1,2-dithiole-3-one, which had 50% inhibitory concentration (IC50) values of 2 μM (ecFabH) and 0.16 μM (saFabH). The corresponding 3-thione analog exhibited comparable activities. Analogs in which the 4-chloro substituent was replaced with a phenyl group were also potent inhibitors, albeit somewhat less effectively (IC50 values of 5.7 and 0.98 μM for ecFabH and saFabH, respectively). All of the 5-chlorinated inhibitors were most effective when they were preincubated with FabH in the absence of substrates. The resulting enzyme-inhibitor complex did not readily regain activity after excess inhibitor was removed, suggesting that a slow dissociation occurs. In stark contrast, a series of inhibitors in which the 5-chloro substituent was replaced with the isosteric and isoelectronic trifluoromethyl group were poorer inhibitors (IC50 values typically ranging from 25 to >100 μM for both ecFabH and saFabH), did not require a preincubation period for maximal activity, and generated an enzyme-inhibitor complex which readily dissociated. Possible modes of binding of 5-chloro-1,2-dithiole-3-ones and 5-chloro-1,2-dithiole-3-thiones with FabH which account for the role of the 5-chloro substituent were considered.
Fatty acid biosynthesis, an essential process for all organisms, is catalyzed in plants and bacteria by a series of discrete dissociable enzymes and a central acyl carrier protein (ACP) (43). This set of enzymes is known collectively as a type II fatty acid synthase (FAS) and differs significantly from the type I FAS of metazoans, in which all of the enzymatic activities are contained on one or two polypeptides (12, 31, 67). The structural and mechanistic differences between the two FAS systems, in conjunction with the fact that the type I FAS is down regulated in well-nourished mammals (38, 39), have led to significant interest in components of the type II FAS as targets for the development of new antibacterial agents. Such agents may also have promise as novel antimalarials because protozoan parasites of the genus Plasmodium have been shown recently to contain a type II FAS in their apicoplast (53, 62, 63).
Significant efforts and progress have been made in the understanding of small-molecule inhibition of two different components of the type II FAS. FabI (InhA), the enoyl ACP reductase that catalyzes the last reductive step in the biosynthetic cycle, is inhibited by triclosan, ethionamide, and isoniazid (22, 28, 49). The β-ketoacyl ACP synthase condensing enzymes (FabB, FabF, and FabH) catalyze the cysteine-mediated Claisen condensation between malonyl ACP (MACP) and an enzyme-bound acyl group derived from an acyl thioester (usually ACP) and are responsible for the elongation step in each cycle. The natural products cerulenin and thiolactomycin (TLM) inhibit fatty acid synthesis by inhibiting one or more type II FAS condensing enzymes. Cerulenin forms a covalent adduct with and does not discriminate between condensing enzymes of the type I and type II FASs (52). TLM (Fig. 1), a unique thiolactone antibiotic, is selective for the type II FAS and mimics MACP binding in the condensing enzymes (29, 47, 52). The low toxicity of TLM and its promising antibacterial and antiparasitic activities have led to numerous synthetic approaches for the generation of novel TLM analogues with improved activities (23, 37, 45, 58).
FIG. 1.
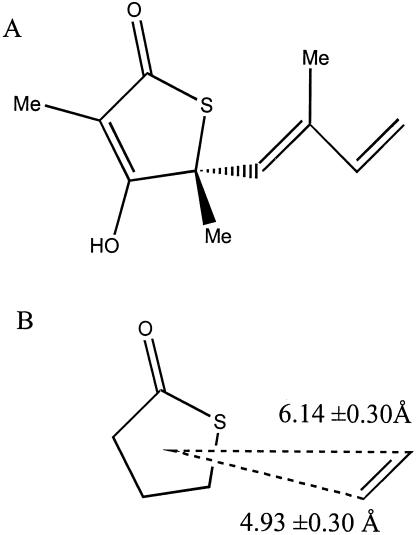
Structure of TLM (A) and the corresponding 3D Unity constraints (B) used to search for related compounds in the NCI database.
More recently, FabH, a separate condensing enzyme that initiates the fatty acid biosynthetic pathway (16, 64), has garnered both interest and acceptance as a unique target for drug discovery (66). Initial work focused on Escherichia coli FabH (ecFabH) (64) but has been extended to include FabH proteins from important human pathogens, such as Mycobacterium tuberculosis (mtFabH) (14, 59), Staphylococcus aureus (saFabH) (27), Streptococcus pneumoniae (spFabH) (34), and Plasmodium falciparum (pfFabH) (53). In all cases, FabH catalyzes a direct condensation between the acyl coenzyme A (CoA) primer used to initiate fatty acid biosynthesis and MACP (13, 14, 16, 25, 64). The enzyme is distinct in that it utilizes an acyl CoA substrate rather than the acyl ACP substrate used by other condensing enzymes. FabH also has a different catalytic triad (Cys, His, and Asn) from that observed for condensing enzymes involved in subsequent elongation steps (Cys, His, and His), and it is divergent in both primary amino acid sequence and structure (21, 54, 55, 59). The enzyme is ubiquitous in bacteria, catalyzes the key initiation step of fatty acid biosynthesis, has a regulatory role, and is reported to be essential for viability (54, 57).
TLM is a significantly less effective inhibitor of FabH than the other condensing enzymes of type II FASs, which has prompted interest in identifying potent FabH inhibitors. In one case, potent inhibitors of ecFabH and spFabH were identified through a high-throughput screening effort, and a cocrystal of a substituted indole inhibitor with ecFabH has been reported (20). The inhibitor binds in the active-site tunnel extending from the surface residues (where Arg 35 and Arg 151 form interactions with the two carboxylic acid groups of the inhibitor) to a hydrophobic region of the protein close to the active site (which is filled by a 2,6-dichlorobenzyl group in the inhibitor). The inhibitor bears no structural similarity to TLM and does not interact directly with residues of the catalytic triad (Cys 112, His 244, and Asn 279). We describe herein a separate approach in which we screened the National Cancer Institute (NCI) database for compounds that possess some of the structural characteristics of TLM which have been shown to be important for inhibition of the condensing enzymes of type II FASs. This work has led to the identification of substituted 1,2-dithiole-3-ones, which preliminary analyses have demonstrated to be potent inhibitors of ecFabH, saFabH, and pfFabH (27, 53). A series of analogs based on this basic skeleton have been prepared and tested against both ecFabH and saFabH. The results from this investigation have revealed that the presence of a C-5 chlorine substituent gives rise to the most potent FabH inhibitors, which do not readily dissociate from the enzyme. The mode of binding of the 5-chlorinated 1,2-dithiole-3-ones to FabH appears to differ significantly from both that of TLM and those of recently reported indole derivatives (20).
MATERIALS AND METHODS
Inhibitor preparation.
Small-molecule inhibitors, including compounds 1, 2, 3 (HR12), and 4, were obtained from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute. Compound 5 was obtained from Fluka. The remaining inhibitors were prepared as described below. All synthetically prepared inhibitors were purified by either preparative thin-layer chromatography or flash column chromatography. All inhibitor structures were confirmed by melting-point-determination spectral analyses (1H and 13C nuclear magnetic resonance and infrared analysis) and elemental analyses. For known compounds 6 (68), 8 (60), and 9 (1), there was consistency with previously reported properties and spectral analyses.
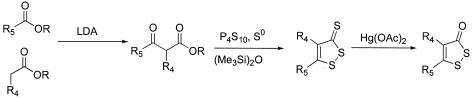
Compound 6 (4,5-dichloro-1,2-dithiole-3-thione) was prepared at a 32% yield by heating 4,5-dichloro-1,2-dithiole-3-one (compound 5) (309 mg, 1.0 eq) in P4S10 (183 mg, 0.25 eq) and hexamethyldisiloxane (1.76 ml, 5.0 eq) in 10 ml of dry toluene under reflux for 4 h (68). Compounds 7 to 14 were all prepared according to the general scheme shown in Fig. 2. The β-ketoesters were obtained by introducing the relevant phenylacetate ester (2.0 mmol) dropwise into a solution of lithium diisopropylamide (2.6 mmol, 1.3 eq) in dry tetrahydrofuran at −78°C. Ethyltrifluoroacetate (3.0 mmol, 1.5 eq) was subsequently added, providing (after workup and purification) the desired β-ketoester. This ester was then converted according to protocols in the literature, first to a 1,2-dithiole-3-thione (17-19) and subsequently, by the use of Hg(OAc)2, to the corresponding 1,2-dithiole-3-one (36). 4-Chloro-5-methyl-1,2-dithiole-3-thione (compound 7) was produced from ethyl 2-chloroacetoacetate (12% yield). 4,5-Dimethyl-1,2-dithiole-3-thione (compound 8) was prepared from ethyl 2-methylacetoacetate (82% yield). 4-Phenyl-5-ethyl-1,2-dithiole-3-thione (compound 9) was prepared from ethyl 2-phenyl-3-oxopentanoate (66% yield). Ethyl 4,4,4-trifluoro-2-phenyl-3-oxophenylbutyrate was prepared at a 31% yield and used to generate 4-phenyl-5-trifluoromethyl-1,2-dithiole-3-thione (compound 10) (51%). Compound 11 was generated from this at an 83% yield. Ethyl 2-(4-bromophenyl)-4,4,4-trifluoro-3-oxobutyrate was prepared at a 34% yield and used to generate 4-(4-bromophenyl)-5-trifluoromethyl-1,2-dithiole-3-thione (compound 12) (66% yield). Compound 13 was generated from this at a 77% yield. Finally, methyl 2-(4-methoxyphenyl)-4,4,4-trifluoro-3-oxobutyrate was prepared at a 74% yield and used to generate 4-(4-methoxyphenyl)-5-trifluoromethyl-1,2-dithiole-3-thione (compound 14) (45% yield). Compound 15 was subsequently obtained at an 80% yield.
FIG. 2.
General synthesis of 1,2-dithiole-3-thiones and 1,2-dithiole-3-ones. R4 and R5 are shown in Table 1. R was generally either a methyl or ethyl group. LDA, lithium diisopropylamide.
Unity database search.
Three-dimensional (3D) searches using ligand-based constraints were performed with Unity 4.2 in SYBYL 6.6. The NCI database integrated with SYBYL, with a total of 117,568 compounds, was screened. As supplied by Tripos, all of the compounds in the database were stored as 3D structures converted from their 2D forms by Concord (SYBYL 6.6). Although only one conformation is stored for each entry, Unity uses a conformationally flexible 3D searching algorithm for ligands such that molecules with matching conformations can be identified regardless of the conformation stored.
Molecular modeling.
Molecular modeling was carried out by using SYBYL, version 6.6 (Tripos, Inc.), running on a Silicon Graphics O2 (R10000) UNIX workstation. Flexible docking was performed by using FlexX (56), version 1.7.6, within SYBYL.
Enzyme structure.
The coordinates for the crystal structure of the ecFabH-malonyl CoA complex possessing the highest crystallographic resolution (1.46 Å) (55) were downloaded from the Protein Data Bank (entry 1hnj) (55) and used for docking studies.
Docking evaluation of hits identified by Unity search.
All candidate compounds identified from the NCI database were docked into the active site of ecFabH by the program FlexX interfaced to SYBYL to evaluate their potential interaction energies. The active site was defined as the collection of amino acids enclosed within a 6.5-Å-radius sphere centered on the bound ligand CoA (entry 1hnj). The first 100 solutions with the lowest FlexX energies were considered. Ligand conformation was first optimized by energy minimization using the Powell optimization method and the Tripos force field (15) for 1,500 steps or until an energy gradient of <0.001 kcal mol−1 Å−1 was reached.
HINT analysis.
To evaluate the important interactions of inhibitors with the residues in the enzyme active site, we employed HINT (hydropathic interactions), version 2.35S, as an add-on module within the SYBYL program (32). For each ligand, the FlexX solution with the lowest energy was subjected to HINT analysis. Each complex was first minimized to relax the ligand conformation into a neighboring local energy minimum, which in all cases retained the binding pattern of the original conformation generated by FlexX. A minimized subset was defined in which 3- and 6-Å spheres of the ligand were set as the hot and interesting regions for annealing, respectively; all portions of the structure outside of this 6-Å sphere were defined as an aggregate to maintain the structural integrity of the enzyme. Energy minimization was then carried out by using the Powell optimization method and Tripos force field for 1,500 steps or until the energy difference was <0.001 kcal mol−1 Å−1. The interactions within the complex were then analyzed with the HINT program. HINT score calculations for the ligand-enzyme interactions were conducted as follows. The ecFabH and ligand were partitioned individually by a partitioning algorithm based on the CLOGP method. The amino acid dictionary method was applied for the protein molecule, and only essential polar hydrogen atoms were considered. The resulting HINT tables were used to determine the magnitude and type of interaction between each amino acid residue and the ligand.
Materials for enzyme assays.
The following reagents were used for enzyme assays: E. coli ACP, malonyl-CoA, imidazole, and 2-mercaptoethanol (Sigma Chemical Company); [3H]acetyl-CoA (specific activity, 4.6 Ci/mmol) and [1-14C]acetyl-CoA (specific activity, 50 mCi/mmol) (Moravek Biochemicals); and Mueller-Hinton broth (Remel). TLM and the ecFabH expression plasmid were kindly provided by Pfizer Inc. The E. coli BL21-CodonPlus-RP expression strain was obtained from Stratagene. E. coli K-12 strain ATCC 10798 was purchased from the American Type Culture Collection. S. aureus RN 450 was generously provided by G. Archer and A. Rosato (Virginia Commonwealth University). All other chemicals were reagent grade or better and were obtained from VWR Scientific or Fisher Scientific.
Enzyme expression and purification.
The ecFabH and saFabH proteins were overexpressed in E. coli with an N-terminal polyhistidine tag and subsequently purified by metal chelation chromatography as described previously (26, 27).
Enzyme inhibition.
FabH activity was determined by standard methodologies that monitor the conversion of radioactive acetyl-CoA and MACP substrates to a radiolabeled 3-ketoacyl-ACP (26, 27). All of the MACP used for these assays was generated from malonyl-CoA and ACP by using FabD as previously described (25). For ecFabH assays, the MACP substrate was prepared from E. coli ACP (Sigma) and purified as described previously (25). For saFabH assays, MACP was prepared from Streptomyces glaucescens ACP (FabC). FabC was used in these studies because it was readily generated in our laboratory (42) and because a reliable commercial source for E. coli ACP was no longer available. We observed a Km for MACP (FabC) of 2.4 ± 0.5 μM in a saFabH-coupled assay, which is indistinguishable from the 1.76 ± 0.4 μM value previously reported for the assay with purified MACP made from E. coli ACP (27). The 50% inhibitory concentration (IC50) of 0.98 μM obtained for compound 3 (HR12) by this alternative assay did not differ significantly from the 1.87 μM value that was previously reported for purified MACP (E. coli AcpP) (27), indicating that either assay format was appropriate for these studies.
For inhibition studies, the test compounds were dissolved in dimethyl sulfoxide (DMSO) and then diluted in DMSO-water (1:10) to the desired concentration. The final DMSO concentration in the enzyme assays was below 1%. FabH and inhibitors were incubated together for 15 min at room temperature (23°C) prior to the addition of acetyl-CoA and MACP. For ecFabH assays, the reaction mixture contained 100 mM sodium phosphate buffer (pH 7.3), 70 nM ecFabH, 1.8 μM MACP, and 2.2 μM [3H]acetyl-CoA in a final volume of 20 μl. For saFabH assays, the following components were present in a final volume of 25 μl: 100 mM sodium phosphate buffer (pH 7.2), 70 nM saFabH, 2.4 μM MACP, and 12 μM [1-14C]acetyl-CoA. The reaction was initiated by the addition of the substrates (acetyl-CoA and MACP) and was incubated at 30°C for 10 min (ecFabH) or 23°C for 5 min (saFabH.) Substrate protection studies of saFabH were carried out by adding either the MACP or acetyl-CoA during the preincubation period. Assays conducted without a preincubation period were initiated by the addition of enzyme. In all cases, the enzyme-catalyzed reaction was terminated by the addition of 10% trichloroacetic acid and analyzed in the standard way.
Initial inhibitor screening was performed with concentrations of 75 to 100 μM. For those compounds that exhibited >50% inhibition at the initial concentration, the IC50 (concentration of inhibitor at which a 50% inhibition of FabH is achieved) was determined by duplicate or triplicate assays with a minimum of six concentrations of inhibitor. A control in which FabH was preincubated with 1% DMSO for 15 min in the absence of inhibitors was used for all of these experiments. IC50 values were calculated with Grafit 4.012 (Middlesex, United Kingdom).
Reversibility tests.
Four aliquots of ecFabH (4 μl of 18.6 μM ecFabH in 100 mM sodium phosphate [pH 7.3] and 20% glycerol [buffer A]) were preincubated with compound 3 (5 μl of 415 μM compound 3 in 10% DMSO; ratio of inhibitor to ecFabH, 28:1), compound 11 (5 μl of 2,250 μM compound 11 in 10% DMSO; ratio of inhibitor to ecFabH, 85:1), or 10% DMSO (5 μl [control]) for 15 min at room temperature. Two samples from each group were assayed under standard conditions without further treatment. The remaining two samples from each group were diluted at least 10-fold with buffer A and concentrated to the initial volume in a Micron 50 microconcentrator. This dilution concentration cycle was repeated twice more to yield an overall dilution factor for the inhibitor of at least 1,000. The final volumes were adjusted to a uniform level and the samples were assayed under standard conditions.
The use of a microconcentrator with saFabH led to significant and variable losses of the enzyme in the control (10% DMSO) experiment. For this reason, a simple dilution experiment was utilized to test the reversibility of the binding. Briefly, a solution of saFabH (3.5 μM in buffer A) was combined with the inhibitor at 10 times its IC50. The ratio of inhibitor to enzyme was at least 20:1 and the inhibitor was contained in a volume such that the total concentration of DMSO did not exceed 5%. A control solution contained enzyme and DMSO but no inhibitor. After 15 min at room temperature, the mixture was diluted 10-fold with the same buffer. An aliquot of this solution (5 μl) was introduced into assay mixtures to yield the normally used concentration of enzyme and a dilution of the inhibitor to 0.2 IC50. Assays were conducted in the usual manner in comparison to appropriate controls.
Intrinsic fluorescence titration.
Fluorescence emission spectra were created in a PC1 single-photon-counting fluorescence spectrophotometer (ISS Instruments), with excitation at 280 nm and with the emission scanned from 290 to 400 nm. Titrations were performed with a 0.2-cm-path-length quartz cuvette at 23°C by serial additions of inhibitor from a stock solution in DMSO to 0.6 ml of enzyme solution in 100 mM sodium phosphate (pH 7.3). Control titrations of FabH with DMSO alone did not change the intrinsic fluorescence over the entire volume range used. Simultaneous fluorescence measurements were performed with inhibitors alone in the same buffer for background subtraction.
Fluorescence emission spectrum measurement of enzyme inhibitor complex.
ecFabH was first incubated with a ligand (compound 3 or compound 11) or an equivalent volume of DMSO (5% of the total assay volume) alone as a control in buffer A at 23°C for 15 min. The final concentrations of enzyme and inhibitor in a volume of 126 μl were 20.5 μM ecFabH and 204.6 μM compound 3 or 406 μM compound 11. Each sample was then subjected to three cycles of dilution and concentration with buffer A in a Microcon microconcentrator 50 (Amicon) to remove unbound ligands. The ecFabH solution was brought up to a final volume of 600 μl with 100 mM sodium phosphate, pH 7.3, for fluorescence measurements. Fluorescence emission spectra were taken with an emission wavelength range of 290 to 400 nm and an excitation wavelength of 280 nm. Each set of experiments (control and with compounds 3 and 11) was conducted at least in duplicate.
MIC determination.
Susceptibility testing in Mueller-Hinton broth was performed by a standard twofold serial dilution method with an inoculum of 5 × 105 cells/ml. The data were reported as MICs, or the lowest concentrations of antibiotic inhibiting visible growth after 16 h of incubation at 37°C.
RESULTS
Identification of 1,2-dithiole-3-ones as FabH inhibitors.
We examined the TLM-FabB cocrystal structure (52) and used it in conjunction with data from analyses of TLM analogs (23, 30, 58) and our own preliminary TLM-FabH docking study to identify key structural features of TLM that are responsible for its activity against condensing enzymes of type II fatty acid synthesis. As a result, three important structural components were identified. The five-member thiolactone ring was important for mimicking MACP. The carbonyl of the same ring was found to form a critical hydrogen bond with one or more of the catalytic residues (52) that was important for placing TLM in the active site of the enzyme. Also, the alkene functionality at the distal end of the isoprenoid tail of TLM contributed a stabilizing hydrophobic interaction in the substrate binding cleft.
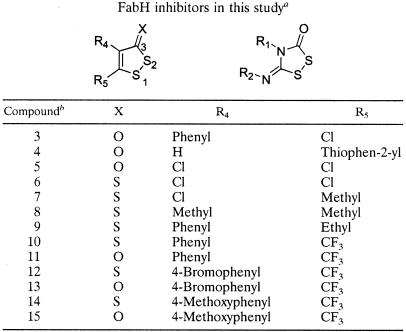
These three structural features were integrated into the 3D Unity search constraint (Fig. 1) of the 3D NCI database. This search generated 72 hits, for which 39 compounds were available for the subsequent biological assay. Initial screening for the inhibition of ecFabH used inhibitor concentrations of 75 to 100 μM. Nine compounds inhibited the enzymatic activity at least 50%. Four of these compounds were 1,2-dithiole-3-ones and related compounds (compounds 1 to 4) (Table 1). Among these was compound 1 (Table 1), which was found to be the most potent inhibitor, with >90% enzyme activity inhibition. The IC50 values of compounds 1 to 3 for ecFabH were determined to be in the 5 to 10 μM range (Table 2), and that of compound 4 was 29 μM. With these structurally similar inhibitors of high potency in hand, we performed a 2D similarity search to identify other compounds bearing the 1,2-dithiole-3-one scaffold within the NCI database. As a result, 13 analogs were identified and requested. Three of the six available compounds were shown to be active against ecFabH. Among these was compound 5, or 4,5-dichloro-1,2-dithiole-3-one, which was subsequently shown to be the most effective ecFabH inhibitor tested, with an IC50 of 2 μM (Table 2).
TABLE 1.
1,2-Dithiole-3-(thi)one compounds tested as FabH inhibitors in this studya
Compounds 1 to 4 were identified and obtained from NCI during the initial screening process. Compound 5 was identified in a second search and obtained from NCI. The remaining compounds were synthesized. Compounds 1, 3, and 5 have previously been referred to as HR19, HR12 (RWJ-3981), and HR45, respectively (4, 26, 53).
For compound 1, R1 = R2 = phenyl. For compound 2, R1 = R2 = CH2CH2Ph.
TABLE 2.
IC50 values of 1,2-dithiole-3-(thi)one inhibitors for ecFabH
| Inhibitor | IC50 (μM)a | FlexX energy scores with ecFabH (kcal/mol)b |
|---|---|---|
| 1 | 6.9 ± 2.9 | −16.0 |
| 2 | 6.0 ± 1.5 | −17.0 |
| 3 | 5.7 ± 0.4 | −10.4 |
| 4 | 29.0 ± 15.0 | −3.7 |
| 5 | 2.0 ± 0.1 | −3.6 |
| 6 | 1.0 ± 0.1 | NC |
| 7 | >100 | NC |
| 8 | 31.7 ± 8.7 | NC |
| 10 | 35.8 ± 3.2 | NC |
| 11 | >100 | NC |
Assays for IC50 values were conducted with a 15-min preincubation.
NC, not calculated.
The two most potent 1,2-dithiole-3-one inhibitors (compounds 3 and 5) of ecFabH were also shown to be active against saFabH (IC50, <1 μM) (Table 3). Compound 5 was more effective against saFabH than ecFabH by an order of magnitude (IC50, 156 nM) and has also proved to be the most effective of the 1,2-dithiole-3-one inhibitors tested to date against P. falciparum FabH (53). The dithiole-3-ones were not active against the FabD protein (malonyl-CoA-ACP transacylase) used to generate MACP for these assays. These observations indicate that the 1,2-dithiole-3-ones may be general FabH inhibitors.
TABLE 3.
IC50 values of 1,2-dithiole-3-(thi)one inhibitors for saFabH
| Inhibitor | IC50 (μM)a
|
|
|---|---|---|
| Preincubation | No preincubation | |
| 3 | 0.983 ± 0.041 | 1.92 ± 0.17 (2) |
| 5 | 0.156 ± 0.010 | 2.25 ± 0.18 (14) |
| 6 | 0.109 ± 0.028 | 3.19 ± 0.43 (30) |
| 7 | 26.0 ± 4.9 | 31.0 ± 5.1 |
| 8 | 22.4 ± 4.6 | 22.0 ± 2.8 |
| 9 | 50.5 ± 11.8 | 51.9 ± 9.3 |
| 10 | 35.2 ± 3.0 | 32.2 ± 3.9 |
| 11 | 102 ± 11 | ND |
| 12 | 15.68 ± 0.54 | 30.0 ± 4.6 (2) |
| 13 | 9.3 ± 1.5 | 29.2 ± 2.1 (3) |
| 14 | >100 | >100 |
| 15 | >100 | >100 |
Assays for IC50 values were conducted with a 15-min preincubation or without preincubation. For values obtained without preincubation, the fold differences from values obtained with preincubation are shown in parentheses. ND, not determined.
Docking studies and HINT analysis of FabH and 1,2-dithiole-3-one interaction.
Protein-ligand docking studies were performed by using FlexX, a fast docking method with an incremental construction algorithm, as an aid to determine the likely mode of binding of the 1,2-dithiole-3-ones to ecFabH. For each FlexX solution, the estimated binding energies of protein-ligand complexes were scored, and the docked sets of solutions were ranked based on the scores. In our hands, there was little correlation between the best score for docked solutions of an inhibitor and the actual IC50 value (Table 2).
We also used the HINT computational model to probe the possible intermolecular interactions between ligands and ecFabH within its active site. Two of the resulting docked complexes of compounds 3 and 5 with the best FlexX scores were examined. In the modeled compound 3-ecFabH complex, most of the predicted intermolecular stabilization was provided by a strong 1.91-Å hydrogen bond between the carbonyl oxygen of compound 3 and the active-site residue Asn274. We were unable to find a docked solution in which hydrogen bonds were made between the carbonyl oxygen of either compound 3 or TLM and both Asn274 and His244 of the FabH Cys-Asn-His catalytic triad. In contrast, the FabB-TLM crystal structure revealed two strong hydrogen bonds to His298 and His333 of the FabB Cys-His-His catalytic triad (52). Our docked solution also suggested that the chlorine substituent and the phenyl ring of compound 3 occupy a hydrophobic region of the active site and that these hydrophobic interactions make only a small contribution to the binding energy. Similar results were obtained with compound 5. However, subsequent structure-activity relationship studies (see below) indicated a major role for the chlorine substituent in binding. In addition, the overall binding scores for these docked solutions were low and thus inconsistent with the observed IC50 values. These inconsistencies may indicate either that the scoring function for the predicted interaction is insufficiently robust or that the actual binding mode is different from that predicted. It is notable in this regard that FlexX docking is limited to noncovalent interactions.
Preparation and analysis of analogs of compounds 3 and 5.
The most potent inhibitors identified from the screening of the NCI compounds were the chlorinated 1,2-dithiole-3-ones 3 and 5. We decided to prepare a series of analogs based on these in order to construct a structure-activity relationship. 1,2-Dithiole-3-thiones first appeared in the literature in the 1940s (51). Since then, a number of synthetic routes to this heterocycle have been reported. Unfortunately, most of these syntheses lack generality, proceed with a poor yield, entail multiple synthetic steps, or require tedious purification protocols (2, 50, 65). Recently, an efficient general preparative method was reported by Curphey (17, 18) (Fig. 2). Thus, β-ketoester precursors were treated with P4S10 in the presence of elemental sulfur and hexamethyldisiloxane to produce the 1,2-dithiole-3-thione skeleton in a one-pot conversion. The oxidation to the corresponding 1,2-dithiole-3-one by using Hg(OAc)2 in a mixture of acetone and glacial acetic acid (48) proceeded with a very high yield for compounds 10, 12, and 14 but was unsuccessful for thiones 7 and 8, which lacked an aromatic substituent at C-4.
The assay conditions for the initial screening of NCI compounds were used to evaluate the activities of all of the synthetic compounds against saFabH (Table 3). A representative set was also evaluated against ecFabH (Table 2). saFabH was chosen for the most detailed analysis because compounds 3 and 5 were 6- and 13-fold more potent, respectively, against saFabH than against ecFabH. Several observations can be made from the data shown in Tables 2 and 3. First, while the details of the structure-activity relationship (absolute magnitude and rank order of IC50 values) were not identical between saFabH and ecFabH, the overall trends were very similar. That is, in general, poor inhibitors of one enzyme were also poor inhibitors of the other. The converse was also true; the most potent compounds were identical for the two enzymes. Second, the ketone and thione at C-3 appeared to be interchangeable. This structural change produced, at most, a threefold difference in the IC50, the direction of which was unpredictable. For ketone-thione pairs 5-6 and 10-11, the thione was more active, while for pair 12-13 the ketone was the more effective inhibitor. Third, for inhibitors without a C-5 chlorine substituent, the presence of the aromatic moiety at C-4 also seemed to have a variable effect. For example, compounds 12 and 13 were as much as 1 order of magnitude more effective than compounds 11, 14, and 15. Thiones 7 and 8, which lack a C-4 aromatic substituent, were intermediate in potency, comparable to the 4-phenylthione compound 10. The binding site seemed to be somewhat sterically flexible, tolerating a substituent up to the size of 4-bromophenyl. Compounds 14 and 15, with a 4-methoxyphenyl substituent, were very poor inhibitors either due to the electron-releasing properties of this group or because they exceed the steric constraints of the binding site. The final and most significant observation was that the chlorine substituent at the C-5 position was a critical component of potency. Analogs bearing the isosteric and isoelectronic trifluoromethyl group were substantially less active, suggesting a role for the chlorine beyond altering the electron density of the dithiole ring. Moreover, the electron-withdrawing effect of the CF3 group seemed rather unimportant; only a twofold difference in IC50 was observed between compounds 10 and 9.
Time dependence of inhibition by 1,2-dithiole-3-(thi)ones.
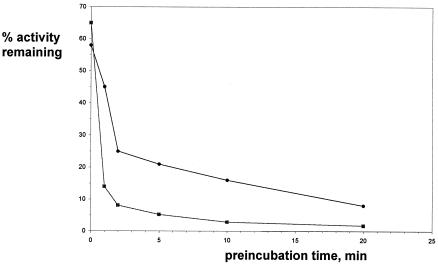
The initial assays described above were conducted under one set of preincubation conditions in order to directly compare the efficacies of the inhibitors. We examined whether this preincubation period was required for the maximal activity of any of the inhibitors against saFabH. The inhibitors that contained a chlorine substituent at C-5 were invariably found to have a time-dependent course of action (Fig. 3), with the majority of the inhibition attained within a 10- to 15-min preincubation time. For the two most potent inhibitors of saFabH, compounds 5 and 6 (bearing a C-5 chloro group), the IC50 value increased (poorer inhibitors) at least 10-fold if there was no preincubation step (Table 3). In contrast, the IC50 values for the majority of the 1,2-dithiole-3-ones that did not bear a C-5 chlorine were not statistically different if there was no preincubation period. A small increase (two- to threefold) in the IC50 values was observed with preincubation for compounds 12 and 13. Compound 3, which contains a C-5 chlorine, does not appear to fit with these observations, as preincubation led to only a twofold difference in the observed IC50 values. However, in single concentration assays in which saFabH was preincubated with compound 3, we clearly observed a time-dependent inhibition. The maximum inhibition of saFabH was accomplished within the first 2 min of the preincubation time with compound 3, which was substantially quicker than that observed for compound 5 (Fig. 4). At the higher concentrations used to determine IC50 values, the rates of inhibition will be higher, leading to the small differences observed for the effect of enzyme-inhibitor preincubation on IC50 values.
FIG. 3.
Effect of preincubation time with compound 5 (squares) or compound 3 (circles) on residual activity of saFabH.
FIG. 4.
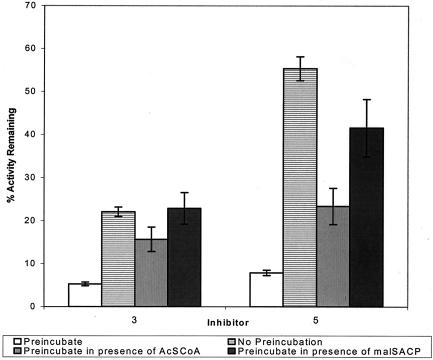
Effect of added substrate on residual activity of saFabH inhibited with compounds 3 and 5.
The presence of either acetyl-CoA or MACP during the preincubation period decreased the overall level of inhibition by both compounds 3 and 5 (Fig. 4). For compound 3, preincubation did not provide the normal increase in inhibitor potency if MACP was present. In the case of compound 5, the preincubation effect was significantly reduced by MACP. Acetyl-CoA appeared to offer somewhat less, but still significant, protection. These results suggest that compounds 3 and 5 bind slowly at sites that are occupied by these substrates.
The marked time dependence coupled with the significantly increased potency of the C-5 chlorinated inhibitors compared with the nonchlorinated inhibitors (bearing the CF3 substituent) both indicate that the modes of binding to FabH of these two classes of inhibitors are significantly different. Thus, the simple hydrophobic interaction with the chlorine atom in compounds 3 and 5 that was suggested by the FlexX analysis is unlikely to be correct. A more important role for the chlorine atom can be postulated (see below).
Reversibility of binding by 1,2-dithiole-3-ones.
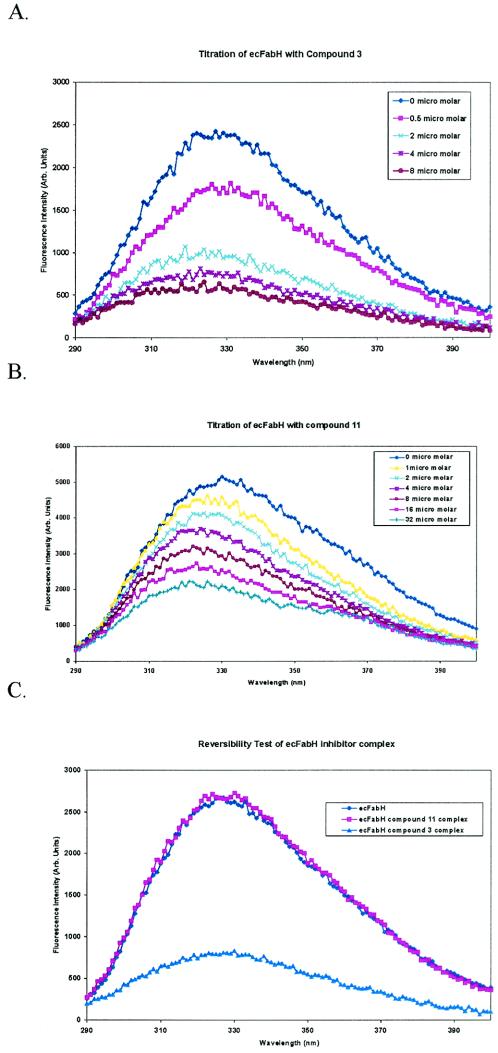
The reversibility of binding of compound 3 and the corresponding CF3 analog (compound 11) to both ecFabH and saFabH was examined by both enzyme assays and fluorescence. For this experiment, the ecFabH-inhibitor complex was first formed by preincubation of the enzyme with excess inhibitor (28-fold and 85-fold for compounds 3 and 11, respectively). Subsequent enzyme assays indicated 5% (compound 3) and 30% (compound 11) residual ecFabH activity. After multiple washing steps to remove the excess inhibitor, ecFabH treated with compound 11 regained activity to 63% that of the control. In contrast, for the enzyme treated with compound 3, washing failed to restore activity above the 5% residual level observed prior to the removal of excess inhibitor.
Differential binding of the two inhibitors was also observed by fluorescence analysis. Free ecFabH displays an intrinsic fluorescence signal when it is excited at 280 nm (33). With increasing concentrations of ligand, for both compounds 3 and 11, the fluorescence spectra of ecFabH showed a significant concentration-dependent decrease in emission intensity at the fluorescence maximum. A fivefold decrease in fluorescence intensity was observed for ecFabH in the presence of compound 3 at a concentration of 8 μM (Fig. 5A). With compound 11, significantly larger quantities of the inhibitor were required to effect the maximum change in fluorescence, consistent with its higher IC50. With 32 μM compound 11, the fluorescence emission intensity was reduced to 30% that of the original (Fig. 5B).
FIG. 5.
Decrease in intrinsic fluorescence of ecFabH with addition of compounds 3 (A) and 11 (B) and restoration of fluorescence of ecFabH after removal of unbound inhibitor (C).
For a test of the reversibility of the formation of enzyme-inhibitor complexes, these complexes were formed by incubation of the enzyme in the presence of a 10-fold excess of inhibitor. Unbound inhibitor and any inhibitor that readily dissociated from the enzyme were then removed from these samples by a 90-min successive dilution-concentration cycle (1,000-fold overall dilution). The fluorescence emission spectrum of each sample was then determined and compared to that of an ecFabH control treated in the same manner, but without an inhibitor. As shown in Fig. 5C, ecFabH treated with compound 11 regained a complete fluorescence emission intensity. In contrast, no significant restoration of fluorescence was seen for ecFabH treated with compound 3. These observations suggest that under the conditions of these experiments, compound 3 produces a complex with ecFabH that dissociates slowly, whereas compound 11 readily dissociates from the enzyme.
A comparable set of conclusions was reached when similar experiments were conducted with saFabH. The removal of excess C-5 chlorinated inhibitors by simple dilution below the IC50 (compound 3) or by successive dilution-concentration cycles (compounds 5 and 6) led, at best, to only a partial (25 to 30%) restoration of enzymatic activity, suggesting the formation of a tight complex. On the other hand, 92 to 100% activity could be recovered by the same process for compounds 7 (a C-4 chlorinated inhibitor), 8, 10, and 11.
The use of two different FabH enzymes, two different analysis methods (enzyme activity and fluorescence), and a variety of inhibitors resulted in some variability in our observations. For example, partial activity could sometimes be restored for C-5 chlorinated analogues, and a complete restoration of activity or fluorescence was not always observed for the corresponding nonchlorinated 1,2-dithiole-3-one inhibitor. The reasons for these variations are at present unclear. Even so, the pattern was absolutely reproducible: a significant restoration of both the enzymatic activity and the intrinsic fluorescence of FabH after inhibitor treatment and washing was observed only for inhibitors without the C-5 chlorine substituent.
Cell-based assays of 1,2-dithiole-3-one activity.
Cell-based assays of the inhibitory effects of representative 1,2-dithiole-3-ones involved determining their MICs for both E. coli and S. aureus (Table 4). Compound 3 was similarly active against both microorganisms. Compound 5 was the most active compound and was more active against E. coli than against S. aureus, in spite of the lower IC50 for saFabH than for ecFabH. Compound 1 was the least effective of the three inhibitors tested and was more potent against S. aureus. All three inhibitors were more effective than TLM, but not as effective as vancomycin or oxacillin under comparable conditions. As noted previously (4, 27), it is unlikely that the antibacterial activity of the 1,2-dithiole-3-ones is from a specific inhibition of FabH. Rather, the data demonstrate that these potent FabH inhibitors are able to cross the cell wall and possess significant antibacterial activities.
TABLE 4.
MIC determination for 1,2-dithiole-3-ones against E. coli K-12 (ATCC 10798) and S. aureusa
| Strain | MIC (μg/ml) of inhibitor for indicated strain
|
|||
|---|---|---|---|---|
| Compound 1 | Compound 3 | Compound 5 | TLM | |
| E. coli K-12 | 100 | 4.0 | 1 | 140 |
| S. aureus RN 450 | 35 | 8.7 | 25 | 300 |
| S. aureus N315 | 17.5 | 8.7 | 25 | 300 |
Some of these data were reported previously (29).
DISCUSSION
1,2-Dithiole-3-ones have been recognized for a considerable time; they have been used industrially as microbiocides, although their exact mechanism of action is unclear (61). At least two 1,2-dithiole-3-thiones have clinical applications, and this class of compounds has been extensively evaluated as anticarcinogens and antioxidants. Oltipraz (4-methyl-5-pyrazinyl-1,2-dithiole-3-thione) and related compounds have been used successfully to treat schistosomiasis and have been considered chemoprotective agents against a variety of cancers (33, 40, 44). Anetholedithione (5-[4-methoxyphenyl]-1,2-dithiole-3-thione) also has chemoprotective activity (46) and is used in many countries as a choleretic and to increase salivary flow (35).
1,2-Dithiole-3-ones bearing an essential C-5 halogen (including compound 3) have recently been identified as potent inhibitors of UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) through the screening of a large chemical library (4). In the present study, similar compounds were also identified as potential FabH inhibitors from an in silico screen with a parameter of structural similarity to TLM. Enzyme assays subsequently confirmed that these 1,2-dithiole-3-ones are potent inhibitors of saFabH and ecFabH. Nonetheless, further analysis suggested that their mode of binding is probably very different from that of TLM and that their identification through the database search was fortuitous.
It is currently unclear how 1,2-dithiole-3-ones inhibit either MurA or FabH. We (27, 53) and others (4) have shown that 1,2-dithiole-3-ones have antibacterial activity as well as inhibit specific bacterial enzymes. Preliminary data do not support the inhibition of either of these enzymes as the primary site of antibacterial activity (4). Thus, it is likely that these inhibitors target multiple intracellular processes and that 1,2-dithiole-3-ones will emerge as effective inhibitors of other important enzyme targets in future screening efforts. Many 1,2-dithiole-3-(thi)ones are chemically reactive, are unstable in aqueous solution, and react with nucleophiles by several mechanisms, at nearly every atom in the five-member ring (11, 51). Therefore, it is possible that they react in multiple ways with protein targets. Understanding the structural features that provide the key activity of these inhibitors against a specific target and understanding the primary mode of binding to such a target are necessary steps towards developing more selective, stable inhibitors.
In the present case, we addressed the former issue by demonstrating that the chlorine atom is important for effective inhibition. The crucial role played by chlorine as a determinant of potency suggests that one mode of binding may be a Michael-type addition in which the chlorine is displaced by an appropriate nucleophilic residue, a reaction for which chemical precedents exist (3, 5, 10). Alternative reactions are also possible; nucleophiles have been shown to react with 1,2-dithiole-3-thiones at S-2, C-3, C-4, and C-5, which are four of the five ring positions (6-9, 24, 41), but for most of these reaction pathways it is not easy to envision a chemically compelling role for the C-5 chlorine that would differ from a C-5 CF3 group. Thus, there is the possibility that these inhibitors can react in multiple ways with a single protein target and that any particular mode of interaction, once it is determined, cannot be generalized between targets. Two of the more probable binding modes suggested by the time-dependent inhibition and slow dissociation of the enzyme-inhibitor complex and supported by chemical precedents involve covalent linkage of the C-5 chlorinated 1,2-dithiole-3-ones to the enzyme, either through the aforementioned Michael addition or by a ring-opening reaction at S-2 by a Cys residue, forming an enzyme-bound disulfide. The likelihood of multiple binding modes, including covalent enzyme-inhibitor complexes, explains why the simple docking methods used to determine and evaluate binding were misleading. As further evidence, the behavior exhibited by compound 3 and several analogs with MurA shows remarkable similarity to our results with FabH, even though the two enzymes share no structural commonalities beyond an active-site Cys residue (4). For MurA, the chlorine at C-5 was important for potency and the inhibition appeared to be irreversible, even though HINT analysis showed no significant interaction between enzyme active-site residues and the chlorine atom (4).
The major mode of binding of 5-chloro-1,2-dithiole-3-ones to FabH remains to be determined and is being investigated through mutational, X-ray cocrystallization, and mass spectral studies. It is clear from the present studies that these inhibitors bind in a different manner than the recently reported indole-based FabH inhibitors (20). Therefore, there are now two different scaffolds that can be used either independently or potentially in conjunction to develop stable and selective broad-spectrum antibacterial agents that inhibit FabH proteins from many different organisms.
Acknowledgments
We are grateful to Tonie Wright (VCU) for helpful discussions. Gordon L. Archer and Adriana E. Rosato (VCU) kindly provided both S. aureus RN 450 and MRSA N315 strains for MIC determination and gave helpful suggestions. We also thank the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, for providing the chemical samples for the biological evaluations in this study.
This work was supported by NIAID grant AI44772 from the National Institutes of Health.
REFERENCES
- 1.Abazid, M., H. O. Bertrand, M. Christen, and J. L. Burgot. 1994. A general synthesis of new dithiolethione derivatives: 5-(1-hydroxyimino alkyl)-1,2-dithiole-3-thiones and 5-acyl-1,2-dithiole-3-thiones. Phosphorus Sulfur Silicon 88:195-206. [Google Scholar]
- 2.Aimar, M. L., and R. H. Rossi. 1996. One-pot synthesis of 5-alkylthio-3H-1,2-dithiole-3-thiones. Tetrahedron Lett. 37:2137-2140. [Google Scholar]
- 3.Bader, J. 1968. 5-Schwefelsubstituierte 1,2-dithiol-3-one. Helv. Chim. Acta 51:1409-1420. [Google Scholar]
- 4.Baum, E. Z., D. A. Montenegro, L. Licata, I. Turchi, G. C. Webb, B. D. Foleno, and K. Bush. 2001. Identification and characterization of new inhibitors of the Escherichia coli MurA enzyme. Antimicrob. Agents Chemother. 45:3182-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boberg, F. 1965. Über 1.2-dithia-cyclopentene. VII. 4-Chlor-5-amino-1.2-dithia-cyclopentenone-(3). Liebigs Ann. Chem. 681:169-177. [Google Scholar]
- 6.Boberg, F., and M. Ghoudikian. 1975. 1,2-Dithiacyclopentene. XXVII. 2-Chloromethylene-1,3-dithioles from 4-chloro-3H-1,2-dithiol-3-ones; preparation and mechanism. Liebigs Ann. Chem. 1975:1513-1520.
- 7.Boberg, F., M. Ghoudikian, and M. H. Khorgam. 1974. On 1,2-dithia-cyclopentenes. XXVI. Ring cleavage of 5-chloro-3H-1,2-dithiole-3-ones by Grignard compounds. Liebigs Ann. Chem. 1974:1261-1268. [Google Scholar]
- 8.Boberg, F., and J. Knoop. 1967. Über 1.2-dithia-cyclopentene. XI. 1.2-Dithia-cyclopentenone-(3) aus trithionen. Liebigs Ann. Chem. 708:148-154. [Google Scholar]
- 9.Boberg, F., H. Niemann, and J. Jovanovic. 1968. Über 1.2-dithia-cyclopentene. XIII. Befunde zur dithiacyclobuten-bzw. thiacyclopropen-zwischenstufe und synthese von 1.4-dipiperidino-2.3-dimethoxycarbonylbutadien-(1.3). Liebigs Ann. Chem. 717:154-168. [Google Scholar]
- 10.Bourzat, J. D., C. Cotrel, D. Farge, J. M. Paris, and G. Taurand. 1985. Derivatives of 1,2-dithiole-3-one and medicinal compositions containing them. French patent 2541679.
- 11.Carey, K. A., T. W. Kensler, and J. C. Fishbein. 2001. Kinetic constraints for the thiolysis of 4-methyl-5-(pyrazin-2-yl)-1,2-dithiole-3-thione (oltipraz) and related dithiole-3-thiones in aqueous solution. Chem. Res. Toxicol. 14:939-945. [DOI] [PubMed] [Google Scholar]
- 12.Chirala, S. S., M. A. Kuziora, D. M. Spector, and S. J. Wakil. 1987. Complementation of mutations and nucleotide sequence of FAS1 gene encoding beta-subunit of yeast fatty acid synthase. J. Biol. Chem. 262:4231-4240. [PubMed] [Google Scholar]
- 13.Choi, K. H., R. J. Heath, and C. O. Rock. 2000. β-Ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis. J. Bacteriol. 182:365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi, K. H., L. Kremer, G. S. Besra, and C. O. Rock. 2000. Identification and substrate specificity of beta-ketoacyl (acyl carrier protein) synthase III (mtFabH) from Mycobacterium tuberculosis. J. Biol. Chem. 275:28201-28207. [DOI] [PubMed] [Google Scholar]
- 15.Clark, M., R. D. I. Cramer, and N. Van Opdenbosch. 1989. The Tripos force field. J. Comput. Chem. 10:982-1012. [Google Scholar]
- 16.Clough, R. C., M. Matthis, S. R. Barnum, and J. G. Jaworski. 1992. Purification and characterization of 3-ketoacyl-acyl carrier protein synthase III from spinach; a condensing enzyme utilizing acetyl coenzyme A to initiate fatty acid synthesis. J. Biol. Chem. 267:20992-20998. [PubMed] [Google Scholar]
- 17.Curphey, T. J. 2000. A superior procedure for the conversion of 3-oxoesters to 3H-1,2-dithiole-3-thiones. Tetrahedron Lett. 41:9963-9966. [Google Scholar]
- 18.Curphey, T. J. 2002. Thionation of esters and lactones with the reagent combination of phosphorous pentasulfide and hexamethyldisiloxane. Tetrahedron Lett. 43:371-373. [DOI] [PubMed] [Google Scholar]
- 19.Curphey, T. J. 2002. Thionation with the reagent combination of phosphorous pentasulfide and hexamethyldisiloxane. J. Org. Chem. 67:6461-6473. [DOI] [PubMed] [Google Scholar]
- 20.Daines, R. A., I. Pendrak, K. Sham, G. S. Van Aller, A. K. Konstantinidis, J. T. Lonsdale, C. A. Janson, X. Qiu, M. Brandt, S. S. Khandekar, C. Silverman, and M. S. Head. 2003. First X-ray cocrystal structure of a bacterial FabH condensing enzyme and a small molecule inhibitor achieved using rational design and homology modeling. J. Med. Chem. 46:5-8. [DOI] [PubMed] [Google Scholar]
- 21.Davies, C., R. J. Heath, S. W. White, and C. O. Rock. 2000. The 1.8 A crystal structure and active-site architecture of beta-ketoacyl-acyl carrier protein synthase III (FabH) from Escherichia coli. Struct. Fold Des. 8:185-195. [DOI] [PubMed] [Google Scholar]
- 22.DeBarber, A. E., K. Mdluli, M. Bosman, L. G. Bekker, and C. E. Barry III. 2000. Ethionamide activation and sensitivity in multidrug-resistant Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 97:9677-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douglas, J. D., S. J. Senior, C. Morehouse, B. Phetsukiri, I. B. Campbell, G. S. Besra, and D. E. Minnikin. 2002. Analogues of thiolactomycin: potential drugs with enhanced anti-mycobacterial activity. Microbiology 148:3101-3109. [DOI] [PubMed] [Google Scholar]
- 24.Fleury, M. B., and M. Largenon. 1985. Studies on the reaction of 1,2-dithiole-3-thiones with nucleophiles. Tetrahedron 41:3705-3715. [Google Scholar]
- 25.Han, L., S. Lobo, and K. A. Reynolds. 1998. Characterization of beta-ketoacyl-acyl carrier protein synthase III from Streptomyces glaucescens and its role in initiation of fatty acid biosynthesis. J. Bacteriol. 180:4481-4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He, X., J. P. Mueller, and K. A. Reynolds. 2000. Development of a scintillation proximity assay for beta-ketoacyl-acyl carrier protein synthase III. Anal. Biochem. 282:107-114. [DOI] [PubMed] [Google Scholar]
- 27.He, X., and K. A. Reynolds. 2002. Purification, characterization, and identification of novel inhibitors of the beta-ketoacyl-acyl carrier protein synthase III (FabH) from Staphylococcus aureus. Antimicrob. Agents Chemother. 46:1310-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heath, R. J., J. Li, G. E. Roland, and C. O. Rock. 2000. Inhibition of the Staphylococcus aureus NADPH-dependent enoyl-acyl carrier protein reductase by triclosan and hexachlorophene. J. Biol. Chem. 275:4654-4659. [DOI] [PubMed] [Google Scholar]
- 29.Heath, R. J., S. W. White, and C. O. Rock. 2001. Lipid biosynthesis as a target for antibacterial agents. Prog. Lipid Res. 40:467-497. [DOI] [PubMed] [Google Scholar]
- 30.Jones, A. L., J. E. Dancer, and J. L. Harwood. 1994. The effect of thiolactomycin analogues on fatty acid synthesis in peas (Pisum sativum). Biochem. Soc. Trans. 22:285. [DOI] [PubMed] [Google Scholar]
- 31.Joshi, A. K., A. Witkowski, and S. Smith. 1997. Mapping of functional interactions between domains of the animal fatty acid synthase by mutant complementation in vitro. Biochemistry 36:2316-2322. [DOI] [PubMed] [Google Scholar]
- 32.Kellogg, G. E., S. F. Semus, and D. J. Abraham. 1991. HINT: a new method of empirical hydrophobic field calculation for CoMFA. J. Comput. Aided Mol. Des. 5:545-552. [DOI] [PubMed] [Google Scholar]
- 33.Kensler, T. W., J. D. Groopman, T. R. Sutter, T. J. Curphey, and B. D. Roebuck. 1999. Development of cancer chemopreventive agents: oltipraz as a paradigm. Chem. Res. Toxicol. 12:113-126. [DOI] [PubMed] [Google Scholar]
- 34.Khandekar, S. S., D. R. Gentry, G. S. Van Aller, P. Warren, H. Xiang, C. Silverman, M. L. Doyle, A. K. Konstantinidis, M. Brandt, R. A. Daines, and J. T. Lonsdale. 2001. Identification, substrate specificity, and inhibition of the Streptococcus pneumoniae β-ketoacyl-acyl carrier protein synthase III (FabH). J. Biol. Chem. 274:30024-30030. [DOI] [PubMed] [Google Scholar]
- 35.Khanna, S., C. K. Sen, S. Roy, M. O. Christen, and L. Packer. 1998. Protective effects of anethole dithiolethione against oxidative stress-induced cytotoxicity in human Jurkat T cells. Biochem. Pharmacol. 56:61-69. [DOI] [PubMed] [Google Scholar]
- 36.Klingsberg, E. 1972. The 1,2-dithiolium cation. XI. Polycyclic dithiole and “no-bond resonance” compounds. J. Org. Chem. 37:3226-3229. [Google Scholar]
- 37.Kremer, L., J. D. Douglas, A. R. Baulard, C. Morehouse, M. R. Guy, D. Alland, L. G. Dover, J. H. Lakey, W. R. Jacobs, Jr., P. J. Brennan, D. E. Minnikin, and G. S. Besra. 2000. Thiolactomycin and related analogues as novel anti-mycobacterial agents targeting KasA and KasB condensing enzymes in Mycobacterium tuberculosis. J. Biol. Chem. 275:16857-16864. [DOI] [PubMed] [Google Scholar]
- 38.Kuhajda, F. P. 2000. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition 16:202-208. [DOI] [PubMed] [Google Scholar]
- 39.Kuhajda, F. P., E. S. Pizer, J. N. Li, N. S. Mani, G. L. Frehywot, and C. A. Townsend. 2000. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc. Natl. Acad. Sci. USA 97:3450-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langouet, S., L. L. Furge, N. Kerriguy, K. Nakamura, A. Guillouzo, and F. P. Guengerich. 2000. Inhibition of human cytochrome P450 enzymes by 1,2-dithiole-3-thione, oltipraz and its derivatives, and sulforaphane. Chem. Res. Toxicol. 13:245-252. [DOI] [PubMed] [Google Scholar]
- 41.Largeron, M., T. Martens, and M. B. Fleury. 1987. Reactivity of substituted 1,2-dithiole-3-thiones with sodium ethanethiolate: a convenient route to a novel heterocycle. Tetrahedron Lett. 43:3421-3428. [Google Scholar]
- 42.Lobo, S., G. Florova, and K. A. Reynolds. 2001. A Streptomyces collinus thiolase with novel acetyl-CoA:acyl carrier protein transacylase activity. Biochemistry 40:11955-11964. [DOI] [PubMed] [Google Scholar]
- 43.Magnusson, K., S. Jackowski, C. O. Rock, and J. E. Cronan. 1993. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol. Rev. 57:522-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maxuitenko, Y. Y., A. H. Libby, H. H. Joyner, T. J. Curphey, D. L. MacMillan, T. W. Kensler, and B. D. Roebuck. 1998. Identification of dithiolethiones with better chemopreventive properties than oltipraz. Carcinogenesis 19:1609-1615. [DOI] [PubMed] [Google Scholar]
- 45.McFadden, J. M., G. L. Frehywot, and C. A. Townsend. 2002. A flexible route to (5R)-thiolactomycin, a naturally occurring inhibitor of fatty acid synthesis. Org. Lett. 4:3859-3862. [DOI] [PubMed] [Google Scholar]
- 46.Nagano, T., and M. Takeyama. 2001. Enhancement of salivary secretion and neuropeptide (substance P, alpha-calcitonin gene-related peptide) levels in saliva by chronic anethole trithione treatment. J. Pharm. Pharmacol. 53:1697-1702. [DOI] [PubMed] [Google Scholar]
- 47.Nishida, I., A. Kawaguchi, and M. Yamada. 1986. Effect of thiolactomycin on the individual enzymes of the fatty acid synthase system in Escherichia coli. J. Biochem. 99:1447-1454. [DOI] [PubMed] [Google Scholar]
- 48.Pagani, G., M. Pregnolato, D. Ubiali, M. Terreni, C. Piersimoni, F. Scaglione, F. Fraschini, A. R. Gascon, and J. L. P. Munoz. 2000. Synthesis and in vitro anti-mycobacterium activity of N-alkyl-1,2-dihydro-2-thioxo-3-pyridinecarbothioamides. Preliminary toxicity and pharmacokinetic evaluation. J. Med. Chem. 43:199-204. [DOI] [PubMed] [Google Scholar]
- 49.Parikh, S. L., G. Xiao, and P. J. Tonge. 2000. Inhibition of InhA, the enoyl reductase from Mycobacterium tuberculosis, by triclosan and isoniazid. Biochemistry 39:7645-7650. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen, B. S., and S.-O. Lawesson. 1979. Studies on organophosphorous compounds. XXVIII. Synthesis of 3H-1,2-dithiole-3-thiones and 4H-1,3,2-oxazaphosphorine derivatives from the dimer of methoxyphenylthionophosphine sulfide and derivatives of 3-oxocarboxylic acids. Tetrahedron 35:2433-2437. [Google Scholar]
- 51.Pedersen, C. T. 1995. 1,2-Dithiole-3-thiones and 1,2-dithiol-3-ones. Sulfur Rep. 16:173-221. [Google Scholar]
- 52.Price, A. C., K. H. Choi, R. J. Heath, Z. Li, S. W. White, and C. O. Rock. 2000. Inhibition of beta-ketoacyl-acyl carrier protein synthases by thiolactomycin and cerulenin: structure and mechanism. J. Biol. Chem. 276:6551-6559. [DOI] [PubMed] [Google Scholar]
- 53.Prigge, S. T., X. He, L. Gerena, N. C. Waters, and K. A. Reynolds. 2003. The initiating steps of a type II fatty acid synthase in Plasmodium falciparum are catalyzed by pfACP, pfMCAT, and pfKASIII. Biochemistry 42:1160-1169. [DOI] [PubMed] [Google Scholar]
- 54.Qiu, X., C. A. Janson, A. K. Konstantinidis, S. Nwagwu, C. Silverman, W. W. Smith, S. Khandekar, J. Lonsdale, and S. S. Abdel-Meguid. 1999. Crystal structure of beta-ketoacyl-acyl carrier protein synthase III. A key condensing enzyme in bacterial fatty acid biosynthesis. J. Biol. Chem. 274:36465-36471. [DOI] [PubMed] [Google Scholar]
- 55.Qiu, X., C. A. Janson, W. W. Smith, M. Head, J. Lonsdale, and A. K. Konstantinidis. 2001. Refined structures of beta-ketoacyl-acyl carrier protein synthase III. J. Mol. Biol. 307:341-356. [DOI] [PubMed] [Google Scholar]
- 56.Rarey, M., B. Kramer, T. Lengauer, and G. Klebe. 1996. A fast flexible docking method using an incremental construction algorithm. J. Mol. Biol. 261:470-489. [DOI] [PubMed] [Google Scholar]
- 57.Revill, W. P., M. J. Bibb, A. K. Scheu, H. J. Kieser, and D. A. Hopwood. 2001. β-Ketoacyl acyl carrier protein synthase III (FabH) is essential for fatty acid biosynthesis in Streptomyces coelicolor A3(2). J. Bacteriol. 183:3526-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakya, S. M., M. Suarez-Contreras, J. P. Dirlam, T. N. O'Connell, S. F. Hayashi, S. L. Santoro, B. J. Kamicker, D. M. George, and C. B. Ziegler. 2001. Synthesis and structure-activity relationships of thiotetronic acid analogues of thiolactomycin. Bioorg. Med. Chem. Lett. 11:2751-2754. [DOI] [PubMed] [Google Scholar]
- 59.Scarsdale, J. N., G. Kazanina, X. He, K. A. Reynolds, and H. T. Wright. 2001. Crystal structure of the Mycobacterium tuberculosis beta-ketoacyl-acyl carrier protein synthase III. J. Biol. Chem. 276:20516-20522. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt, U., A. Lüttringhaus, and H. Trefzger. 1960. Über trithione. XII. Trithione aus-ketoestern. Ann. Chem. 631:129-138. [Google Scholar]
- 61.Shema, B. F., R. H. Brink, Jr., and P. Swered. April1974. Synergistic combinations containing organo-bromine compounds and their use in the control of Aerobacter aerogenes. U.S. patent 3879513.
- 62.Suguna, K., A. Surolia, and N. Surolia. 2001. Structural basis for triclosan and NAD binding to enoyl-ACP reductase of Plasmodium falciparum. Biochem. Biophys. Res. Commun. 283:224-248. [DOI] [PubMed] [Google Scholar]
- 63.Surolia, N., and A. Surolia. 2001. Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat. Med. 7:167-173. [DOI] [PubMed] [Google Scholar]
- 64.Tsay, J. T., W. Oh, T. J. Larson, S. Jackowski, and C. O. Rock. 1992. Isolation and characterization of the β-keto-acyl carrier protein synthase III gene (fabH) from Escherichia coli K-12. J. Biol. Chem. 267:6807-6814. [PubMed] [Google Scholar]
- 65.Viana, M. N., C. Vaccher, P. Berthelot, J.-L. Burgot, M. Debaert, M. Luyckx, J.-C. Cazin, and S. Deblock. 1986. Recherche de propriétés antibilharziennes de dithiole-thiones, d'intermédiaires et de derives. Eur. J. Med. Chem. 21:123-130. [Google Scholar]
- 66.Volker, C., and J. R. Brown. 2002. Bioinformatics and the discovery of novel anti-microbial targets. Curr. Drug Targets Infect. Disord. 2:279-290. [DOI] [PubMed] [Google Scholar]
- 67.Wakil, S. J. 1989. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry 28:4523-4530. [DOI] [PubMed] [Google Scholar]
- 68.Wentrup, G.-J., M. Koepke, and F. Boberg. 1975. Über 1,2-dithiacyclopentene. XXIX. 3-Thioxo-3H-1,2-dithiole aus 3-chloro-1,2-dithioliumchloriden. Synthesis 1975:525-526. [Google Scholar]