Despite recent advances, the majority of patients with multiple myeloma (MM) relapse or progress. For a subset of patients, salvage high dose chemotherapy and autologous hematopoietic stem cell transplantation (auto-HCT) can yield a meaningful duration of remission1–3 and may improve time to progression in comparison to traditional salvage chemotherapy.4 Recent data has suggested that combination of lenalidomide with traditional cytotoxic chemotherapy is safe and may result in synergistic anti-myeloma effects, even in patients previously treated with lenalidomide.5, 6 We therefore hypothesized that lenalidomide could be safely combined with high dose melphalan in the salvage auto-HCT setting. We further postulated that increasing the dose of lenalidomide would not add unacceptable toxicity, as cytopenias would be ameliorated by the planned autologous rescue. Thus this setting would provide us the opportunity to study higher doses of lenalidomide which, to date, have not been examined in MM.
We conducted a phase I/II study to determine the safety and efficacy of lenalidomide in combination with high dose melphalan (NCT01079936). Patients between the ages of 18–80 who had relapsed or progressive MM7 were eligible. The study was approved by the Institutional Review Board. Patients received 7 days of lenalidomide (between 25–100 mg orally daily for the 7 days) on days (−8) – (−2). High dose melphalan (total of 200/m2) was administered as 100 mg/m2 IV on days (−3) and (−2). Rescue autologous stem cell graft was on day 0. Standard supportive care measures were provided per departmental protocol.
There were 4 doses of lenalidomide in the dose escalation phase: 25 mg, 50 mg, 75 mg and 100 mg. The first 12 patients were treated at these dose levels (3 patients per level) and safety was assessed at each level. The dose for each subsequent patient was chosen in a sequentially adaptive fashion in order to optimize the trade-off between toxicity and efficacy.8 Dose limiting toxicity (DLT) was defined as regimen-related death, graft failure, grade 3 or 4 atrial fibrillation, grade 4 deep venous thrombosis, or pulmonary embolism before day 30 after auto-HCT. Efficacy was defined as being alive in complete response (CR)7 at day 90 (+/−30 days) after auto-HCT. Multiparameter flow cytometry (MFC), (CD19, CD20, CD38, CD45, CD56, CD81, CD117, kappa or lambda light chains and CD138) further identified patients in CR with no evidence of minimal residual disease (MFC-CR).
Overall survival (OS) and progression-free survival (PFS) were estimated by the method of Kaplan and Meier. The log rank test was used to assess differences in OS or PFS between subgroups. Exact tests for association between categorical variables were carried out using the Fisher Exact and Fisher-Freeman-Halton test.9, 10 Bayesian binary regression11 was used to assess possible multivariate relationships between the probability of Day 90 CR, and patient covariates. Bayesian survival time regression12 was used to assess the relationship between OS, PFS, and patient covariates. The four lenalidomide dose levels were standardized by dividing by the mean dose, represented as 0.40, 0.80, 1.20, and 1.60. To interpret the fitted Bayesian regression models, a posterior probability Prob(β > 0 | Data) for the coefficient β of a covariate in the model of either > .95 or <.05 was considered “significant,” and a value either > .99 or < .01 was considered “highly significant.” Values in the ranges .90–.95 or .05–.10 were considered “moderately significant.”
From March 2010 to April 2013 57 patients were enrolled. Patient characteristics are summarized in Table 1. Twenty-two (39%) patients were lenalidomide-refractory, 30 (53%) bortezomib-refractory and 13 (23%) refractory to both. Patient characteristics did not differ significantly between the lenalidomide dose levels, with the exception that patients in dose level 1 were more likely to have responsive disease going into the auto-HCT.
Table 1.
Patient characteristics overall and by lenalidomide dose level (25, 50, 75, or 100 mg).
| Dose Level | 25 mg N=3 |
50 mg N=5 |
75 mg N=24 |
100 mg N=25 |
Total | P-value | ||
|---|---|---|---|---|---|---|---|---|
| Median age at auto-HCT (range) | 59 (50–63) | 66 (53–70) | 55 (34–72) | 60 (52–71) | 60 (34–72) | 0.07 | ||
| Prior auto-HCT | No | 1(33.3%) | 4(80%) | 16(66.7%) | 18(72%) | 39 (68%) | 0.60 | |
| Yes | 2(66.7%) | 1(20%) | 8(33.3%) | 7(28%) | 18 (32%) | |||
|
Time from diagnosis to auto-HCT (months) Median (range) |
89 (30–214) | 27 (7–68) | 25 (6–98) | 48 (5–179) | 33 (5–214) | 0.22 | ||
| Cytogenetics at auto-HCT | High-risk* | 2(66.7%) | 2(40%) | 7(29.2%) | 5(20%) | 16(28.1%) | 0.28 | |
| Standard-risk | 1(33.3%) | 3(60%) | 17(70.8%) | 20(80%) | 41(71.9%) | |||
| ISS Stage | I | 0(0%) | 2(40%) | 5(20.8%) | 7(28%) | 14(24.6%) | 0.78 | |
| II | 0(0%) | 2(40%) | 9(37.5%) | 7(28%) | 18(31.6%) | |||
| III | 1(33.3%) | 0(0%) | 3(12.5%) | 5(20%) | 9(15.8%) | |||
| NA | 2(66.7%) | 1(20%) | 7(29.2%) | 6(24%) | 16(28.1%) | |||
| Prior Lines of Rx | <3 | 0(0%) | 1(20%) | 5(21%) | 12(48%) | 18(32%) | 1.00 | |
| ≥3 | 3(100%) | 4(80%) | 19(79%) | 13(52%) | 39(68%) | |||
|
LDH at auto-HCT Median (range) |
529 (514–808) | 608 (415–775) | 469 (295–714) | 506 (365–1349) | 498(295–1349) | 0.23 | ||
|
Bone Marrow Plasma cell % at auto-HCT Median (range) |
0 (0–1) | 7 (0–14) | 5 (0–80) | 5 (0–50) | 5 (0–80) | 0.14 | ||
| Disease response prior to auto-HCT |
CR VGPR PR SD PD |
0 1 2 0 0 |
0 0 1 1 3 |
2 2 9 9 2 |
1 3 5 5 11 |
3 6 17 15 16 |
0.0013 | |
High risk =(deletion 13q, t(4:14) or del 17p) by conventional cytogenetics or (t(4:14), t(14:16) or del17p)by fluorescent in-situ hybridization.
DLTs were not seen at any of the 4 dose levels. After safely escalating to 100 mg, patients were adaptively randomized among the 4 lenalidomide dose levels (Table 1). Grade 3–4 non-hematologic toxicity was seen in 40 (70%) patients, with no significant differences between the 4 dose levels (Supplemental Table 1). Two patients died of nonrelapse causes (viral infection 1, cardiac failure 1) for a treatment-related mortality (TRM) of 3%. Median time to both neutrophil and platelet engraftment was 11 days. Thus far only 1 patient has developed a second primary malignancy (SPM) (squamous cell cancer of the skin).
By day 90 after auto-HCT, 8 (14%) patients had achieved a CR (including the 3 patients in CR before auto-HCT), 25 (44%) a CR or very good partial response (VGPR), and 42 (74%) a CR, VGPR or partial response (PR), with no significant differences in response rates among the 4 lenalidomide dose levels (Supplemental Table 1). By day 180, 12 patients (21%) had achieved a CR. Of these 12 patients, 11 had samples available for MFC; all 11 patients demonstrated a MFC-CR, indicating MRD negative disease. Of note only 1 of these patients had high risk cytogenetics at presentation.
With a median follow up of 12.3 months (range 0.5–41), estimated median PFS was 23.7 months (95% CI: 12 – [not evaluable (NE)] months) and median OS had not yet been reached (Figures 1A and 1B). By log-rank test, there were no significant differences in either PFS or OS between the doses of lenalidomide (p = 0.27 and p= 0.15 respectively, Figures 1C and 1D). Two-year PFS and OS probabilities were 48% (95% CI: 32–62%) and 72% (95% CI: 56–84%), respectively. There was no significant difference in median PFS (13 months) or OS (not yet reached) for patients undergoing a 2nd auto-HCT in comparison with patients undergoing first auto-HCT as a salvage treatment after progression (p=0.47 and 0.85, respectively). For the 38 patients with previous exposure to lenalidomide there was an improvement in PFS (but not OS) for the 26 patients who were sensitive to lenalidomide versus the 12 lenalidomide-refractory patients (23.2 versus 5.3 months respectively, P= 0.01). Of note, 36 (63%) patients went on to receive post auto-HCT maintenance therapy (lenalidomide: 32, bortezomib: 2, pomalidomide: 2).
Figure 1.
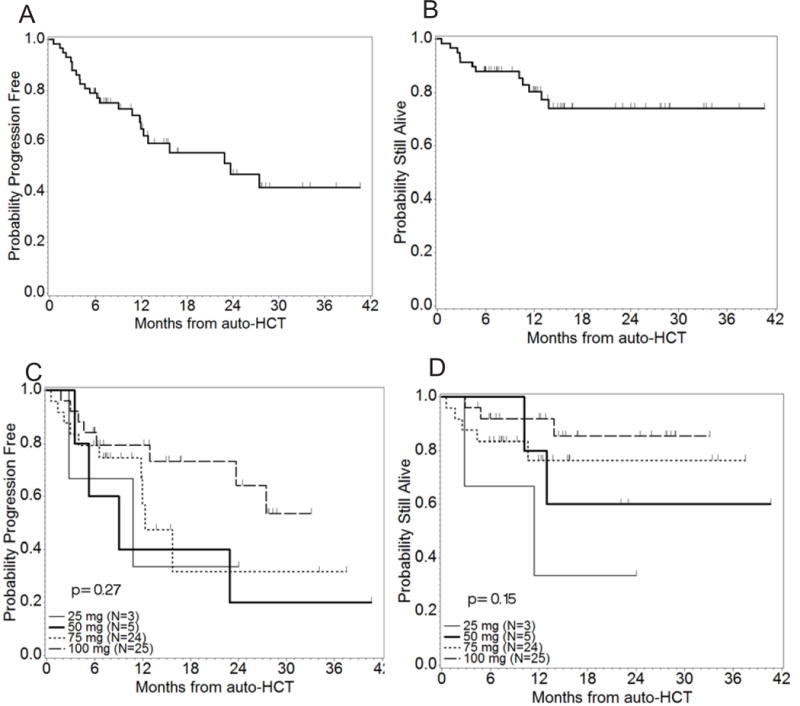
Survival curves. 1A: PFS from time of auto-HCT. The median PFS time was 23.7 months (95% CI: 12 – NE months). The upper CI could not be estimated as the largest observation was censored. 1B: OS from time of auto-HCT. At time of analysis, the median OS time had not been reached. 1C: PFS by lenalidomide dose level (N=57, Events=24). 1D: OS by lenalidomide dose level (N=57, Deaths=12).
On multivariate analysis high-risk cytogenetics, percent plasma cell infiltration of bone marrow (%PCs) and number of prior lines of treatment were each significantly associated with a lower probability of reaching CR by day 90 (Prob(β > 0) of 0.0114, 0.0430 and 0.0110 respectively). For PFS, higher lenalidomide dose level and longer time from diagnosis were significantly beneficial while higher LDH was moderately significantly harmful, even after accounting for high-risk cytogenetics and %PCs (Prob(β > 0) of 0.9770, 0.9909, 0.0936 respectively). Factors that independently affected OS included higher dose level (Prob(β > 0) = 0.9466), and longer time from initial diagnosis to auto-HCT (Prob(β > 0) = 0.9568), both of which were beneficial. In contrast, high-risk cytogenetics and higher LDH both adversely affected OS ((Prob(β > 0) = 0.0507).
Previous studies have suggested that a salvage auto-HCT, as a first or second transplant after relapse or progression is relatively well-tolerated.2, 13 In the current trial, we studied escalating doses of lenalidomide, ranging from 25 mg – 100 mg for 7 days, and determined that higher doses of lenalidomide could be safely combined with myeloablative chemotherapy with only 3% TRM. This was a high risk population with >50% refractory to lenalidomide or bortezomib and a median of 3 lines of prior therapy. Importantly, there were no significant delays in engraftment or increase in thromboembolic events, and only 1 patient developed a SPM. This suggests that the platform of autologous stem cell rescue may in fact be the ideal venue for delivering high doses of immunomodulatory agents.
The CR rate (14% at day 90) and overall response rate (74% at day 90) are comparable to those seen with other salvage regimens.14 The median PFS of 23.7 represents an improvement from our prior data in this population2 and is comparable to other salvage studies15 and a randomized study of auto-HCT versus salvage chemotherapy for relapsed MM patients.4 However, we acknowledge that this patient population was heterogeneous and thus further study is warranted in a more defined patient population. Though the small patient number places some limitation on interpretation, there is a suggestion that this treatment could still be considered for patients who may have not previously been referred for auto-HCT but who now have relapsed disease.
Our adaptive study design ultimately determined that the highest doses of lenalidomide (75 mg and 100 mg) were equivalent to achieve a balance between tolerability and efficacy. Due to relatively small patient numbers, a longer follow-up time may further differentiate the true clinical effect of the dose escalation and the impact of post-auto-HCT maintenance therapy, which 63% of patients received. Unfortunately (though not surprisingly), poor-risk cytogenetics independently predicted for poorer OS, reminding us of the continued need for innovative treatment options for these patients.
In conclusion, combination high dose melphalan with high dose lenalidomide appears to be a well-tolerated and safe preparative regimen in the setting of salvage auto-HCT. As novel agents, maintenance regimens and immunotherapies emerge, it is conceivable that they too may be combined with this auto-HCT platform to offer the relapsed patient yet another chance at a durable response.
Supplementary Material
Footnotes
Conflict of interest disclosures
Drs. N. Shah, Bashir, J. Shah, Hosing and Orlowski receive research funding from Celgene. Drs. N. Shah, J. Shah, and Orlowski have served on Celgene Advisory Boards.
References
- 1.Auner HW, Szydlo R, Rone A, Chaidos A, Giles C, Kanfer E, et al. Salvage autologous stem cell transplantation for multiple myeloma relapsing or progressing after up-front autologous transplantation. Leukemia & lymphoma. 2013 Oct;54(10):2200–2204. doi: 10.3109/10428194.2013.773998. [DOI] [PubMed] [Google Scholar]
- 2.Shah N, Ahmed F, Bashir Q, Qureshi S, Dinh Y, Rondon G, et al. Durable remission with salvage second autotransplants in patients with multiple myeloma. Cancer. 2012 Jul 15;118(14):3549–3555. doi: 10.1002/cncr.26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemieux E, Hulin C, Caillot D, Tardy S, Dorvaux V, Michel J, et al. Autologous stem cell transplantation: an effective salvage therapy in multiple myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013 Mar;19(3):445–449. doi: 10.1016/j.bbmt.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Cook G, Williams C, Brown JM, Cairns DA, Cavenagh J, Snowden JA, et al. High-dose chemotherapy plus autologous stem-cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem-cell transplantation (NCRI Myeloma X Relapse [Intensive trial]): a randomised, open-label, phase 3 trial. The Lancet Oncology. 2014 Jul;15(8):874–885. doi: 10.1016/S1470-2045(14)70245-1. [DOI] [PubMed] [Google Scholar]
- 5.Kumar SK, Lacy MQ, Hayman SR, Stewart K, Buadi FK, Allred J, et al. Lenalidomide, cyclophosphamide and dexamethasone (CRd) for newly diagnosed multiple myeloma: results from a phase 2 trial. American journal of hematology. 2011 Aug;86(8):640–645. doi: 10.1002/ajh.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Donk NW, Wittebol S, Minnema MC, Lokhorst HM. Lenalidomide (Revlimid) combined with continuous oral cyclophosphamide (endoxan) and prednisone (REP) is effective in lenalidomide/dexamethasone-refractory myeloma. British journal of haematology. 2010 Jan;148(2):335–337. doi: 10.1111/j.1365-2141.2009.07931.x. [DOI] [PubMed] [Google Scholar]
- 7.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006 Sep;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 8.Thall PF, Cook JD. Dose-finding based on efficacy-toxicity trade-offs. Biometrics. 2004 Sep;60(3):684–693. doi: 10.1111/j.0006-341X.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 9.Fisher R. On the interpretation of χ2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society. 1922;85(1):87–94. [Google Scholar]
- 10.Freeman GHHJ. Note on exact treatment of contingency, goodness of fit and other problems of significance. Biometrika. 1951;38:141–149. [PubMed] [Google Scholar]
- 11.Gelman ACJ, Stern HS, Rubin DB. Bayesian Data Analysis. 2nd. Chapman & Hall/CRC; New York: 2004. [Google Scholar]
- 12.Ibrahim JGCM-H, Sinha D. Bayesian Survival Analysis. Springer; New York: 2001. [Google Scholar]
- 13.Doo NW, Thompson PA, Prince HM, Seymour JF, Ritchie D, Stokes K, et al. Bortezomib with high dose melphalan conditioning for autologous transplant is safe and effective in patients with heavily pretreated and high risk multiple myeloma. Leukemia & lymphoma. 2013 Jul;54(7):1465–1472. doi: 10.3109/10428194.2012.746682. [DOI] [PubMed] [Google Scholar]
- 14.Orlowski RZ. Novel agents for multiple myeloma to overcome resistance in phase III clinical trials. Seminars in oncology. 2013 Oct;40(5):634–651. doi: 10.1053/j.seminoncol.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012 Jan;26(1):149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


