Abstract
Studies of sex differences in the brain range from reductionistic cell and molecular analyses in animal models to functional imaging in awake human subjects, with many other levels in between. Interpretations and conclusions about the importance of particular differences often vary with differing levels of analyses and can lead to discord and dissent. In the past two decades, the range of neurobiological, psychological and psychiatric endpoints found to differ between males and females has expanded beyond reproduction into every aspect of the healthy and diseased brain, and thereby demands our attention. A greater understanding of all aspects of neural functioning will only be achieved by incorporating sex as a biological variable. The goal of this review is to highlight the current state of the art of the discipline of sex differences research with an emphasis on the brain and to contextualize the articles appearing in the accompanying special issue.
Keywords: steroid hormones, sex chromosomes, hypothalamus
1. Introduction
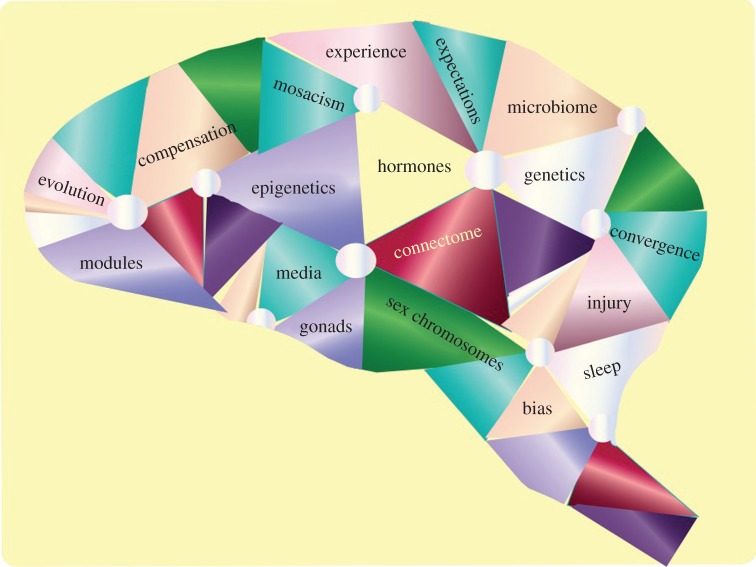
This special issue of Philosophical Transactions B of the Royal Society titled Multifaceted Origins of Sex Differences in the Brain is a combination of original research reports, expert reviews and opinion pieces. The goal is to provide in one place as many different perspectives on the question of brain sex differences as can be reasonably achieved, so that the interested reader can reach their own conclusions. This issue cannot possibly hope to create a consensus view as there are many and varied approaches at both the experimental and theoretical level (figure 1). For some, the lens through which sex differences are viewed is at the cellular level in animal models, whereas for others, the lens focuses on human behaviour. Nonetheless, it is possible to more clearly define the fault lines along which discord lies and identify future steps towards a greater understanding.
Figure 1.
Multifaceted origins of sex differences in the brain. The number of known variables impacting how sex differences in the brain are established and maintained are numerous. They vary from the purely biological, such as hormones and genetics, to those impacted by experience and environment, such as epigenetics. Cultural and societal expectations may also exert biological influences on the brain but determining these is a challenge. Media reports exaggerating the significance of sex differences confound efforts to have reasoned data-based discussions by the diverse community of scientists addressing this topic. (Online version in colour.)
It may seem odd to suggest that sex differences in the brain constitute a ‘hot topic’ given that the first reports of these date back over 40 years. Why such interest now? A perfect storm of advances in the biology, changes in policy at major granting institutions, hyperbolic exaggeration by the media and strong pushback by select scientists have generated a maelstrom of dissenting voices. Some scientists argue that sex differences in the brain are robust and widespread [1], whereas others argue that much of the science is flawed and that inherent long-standing bias has stepped in to replace objectivity [2,3]. Everyone has an opinion, sometimes personal, sometimes data-driven. Within the realm of psychological and biomedical research, the subject of sex differences in the brain is singular for both its broad importance and its impact, which ranges from societal and government policies, to approval criteria for new therapeutics and diagnostic guidelines, to fundamental biological principles at the cellular and molecular level. So it is a topic worthy of attention.
2. Sex versus gender
The majority of species in the animal kingdom are divided into two sexes based on reproductive criteria such as large and limited gametes (females—oocytes) versus small and plentiful ones (males—sperm). In species where sex is determined genetically, sex chromosomes are by definition the ones that differ between males and females, i.e. XX versus XY for female and male mammals, respectively, but ZW versus ZZ for female and male birds. In humans, however, the study of sex differences is confounded by gender, a composite term for both self and societal perception of one's sex. It is the collision of gender and biology that generates the most heat in the debate about the brain. Science often advances in fits and starts, and discord is certainly not unusual. But, in the case of sex differences in the brain, the stakes are unusually high on both sides of the argument. Misrepresentation and outright fabrication of differences between learning in boys and girls may have contributed to a nationwide movement towards same-sex education in the USA, a phase that appears to be reversing but may have done genuine harm while intending to do good (reviewed in [4]). Conversely, a wilful exclusion of female subjects in preclinical research (i.e. animal studies) has severely hampered the applicability and reproducibility of basic neuroscience research over the past several decades. Moreover, the withdrawal of 8 out of 10 drugs from the USA market between 1997 and 2000 was, in part, owing to more severe adverse effects in women (reviewed in [5]). Thus, as has been proposed by many others, it is essential to the shared mission of high-quality and impactful science that research on sex differences be expertly conducted, accurately interpreted and appropriately conveyed to the lay public and media.
Towards that end, it is beneficial to restate logical truisms that we all agree on but which are often left out of discussions on sex differences in the brain. First and probably most important is the inherently obvious fact that human brain and behaviour are far more complex and more profoundly influenced by environment and experience than commonly used animal models at every level, meaning fish, reptiles, birds, rodents and non-human primates. Humans are a singular species for the use of sophisticated numerical calculations and language, the two topics that are most likely to be misrepresented in the media. In this issue, Maney [6] reminds us of how easily work in animal models can be distorted and mis-represented and she rightly places the onus on scientists to take steps to assure their findings are appropriately conveyed to non-scientists.
Second is the pervasive assumption that a sex difference in neuroanatomy or neurophysiology is synonymous with a sex difference in behaviour. Rather than an assumption, the connection between anatomy and behaviour should be a hypothesis subject to empirical testing. In the case of human imaging studies, there is concern of pervasive reverse inference in which sex differences in fMRI signal are interpreted as empirical evidence of pre-existing stereotypes, rather than actually tested [2]. In this issue, Verma and co-workers [7] tackle this question by direct comparison of performance on a computerized battery of cognitive tasks with diffusion MRI to map the connectome of boys and girls and young men and women. They establish subnetworks based on functional and behavioural domains and find that they differ between males and females and correlate with performance on neural cognitive tasks. Future studies using additional parallel approaches of imaging and performance will be essential to reliably establishing such relationships.
Third is the notion of the brain as a unitary organ that is either ‘male’ or ‘female’. Because the majority of sex differences in the brain are established early by gonadal steroids that differ in males and females, and because the brain resides in a body that is either male or female, there is an implicit, even inherent bias, that brains are male versus female. In this issue, Joel and Fausto-Sterling [8] challenge this view and cite empirical evidence based on MRI that humans are a single heterogeneous population when considering the brain [9].
Last is that the level of analyses matters. Studies relying on global imaging techniques such as MRI in humans versus gene expression profiles or biochemistry in animal models are profoundly different in both technical and experimental fidelity. In the first instance, a neuroscientist may be attempting to understand language processing, whereas the latter is exploring a protein that resides at the synapse. Each has their own strengths and weaknesses, but neither the strengths nor the weaknesses are transferable. Moreover, results based on one level of analyses do not allow for sweeping conclusions. For instance, the claim that the ‘human hippocampus is not sexually dimorphic’ is unfounded because it is based entirely on volumetric analyses using MRI [10]. There are many ways for the brain to differ in males and females that do not involve size.
3. A brief history of the study of brain sex differences
When invoking the ‘history’ of sex differences, one can go back to Aristotle but for the purposes of this discussion, we will begin with the more modern era of scientific inquiry. Prior to any consideration of the brain as a driver of sex differences in behaviour, it was essential to establish how gonadal hormones determine the body phenotypes of males and females, which in most species are visibly distinct. This question was relatively easily dispatched in the late 1940s by classic experiments of Alfred Jost, establishing that gonadal secretions are the drivers of reproductive tract differentiation and establishment of secondary sex characteristics (reviewed in [11]). The dramatic nature and undeniable importance of these sex-specific features likely contributed to Frank Beach's assertions in the 1950s that behavioural differences between males and females were a function of those physical characteristics. Put simply, males behave like males because they are bigger and have a penis, full stop (reviewed in [12]). With hindsight, this may seem a silly notion, but it is not necessarily obvious that sex differences in behaviour are intrinsic to the brain. Equally plausible explanations are short-term modulatory effects of hormones or societal and cultural expectations that sculpt gender norms of behaviour with no original underlying biological basis. Even the master neuron for reproduction, the GnRH (gonadotropin releasing hormone) neuron, is decidedly not different in males and females despite markedly different physiologies of gonadotropin secretion. Thus, there are sound reasons to argue that external influences, be they of the body outside the nervous system or the world outside the body, drive sex differences in physiology and behaviour and that the brain has little to do with it. However, the notion that all sex differences are secondary to the body was largely put to rest in 1959 by a now iconic paper by Phoenix et al. [13] reporting that treatment of pregnant guinea pigs with male hormones, i.e. androgens, produced female offspring that behaved like males in a mating test. These females had masculinized genitalia, so the results were ambiguous regarding brain versus body. But, when the androgen dose was sufficiently lowered that the fetal females genitalia were not masculinized, they were still behaviourally defeminized, meaning that as adults they were no longer responsive when supplied with oestradiol and progesterone to induce female sexual receptivity. In a closing sentence reminiscent of Watson and Crick's prophetic ‘it has not escaped our notice…’, Phoenix and co-workers [13] state, ‘We are assuming that testosterone or some metabolite acts on those central nervous tissues in which patterns of sexual behavior are organized’ (p. 381). This statement preceded by a decade the discipline of neuroscience, which was not firmly established as an independent field until the 1970s.
In the time since then, extensive research on animal models irrefutably established that gonadal hormone secretions from the male testis early in development act on the brain to masculinize it (reviewed in [14–17]). In rodents, the androgens produced by the testis are aromatized to oestrogens, an essential step in the masculinization process, whereas, in primates, androgens act directly (reviewed in [18]). The development of the female brain is considered the default, meaning it is the path taken in the absence of high levels of androgens or oestrogens. These early organizational events then provide a sexually differentiated neural substrate for activation by gonadal hormones in adulthood. Steroid levels in the adult are distinguished in males versus females by patterns and levels of release (both males and females have circulating levels of androgens, oestrogens and progestins, just not in the same patterns or levels). From an evolutionary perspective, this strategy for brain sexual differentiation assures there is a match between gonadal phenotype and brain phenotype, an essential ingredient for successful reproduction.
4. Moving brain sex differences beyond reproduction
Despite the importance of the Phoenix et al. [13] paper, there was no strong feeling that the brains of males and females would be obviously different. Instead, the exact opposite view prevailed, that any sex differences that did exist would be small, subtle and limited in scope. This prejudice held true for early reports of sex differences in the mammalian brain that required electron microscopy to detect and were focused on understanding the release of gonadotropin secretion from the pituitary, not behaviour [19]. The findings were nonetheless considered of sufficient importance at the time to warrant publication in Science.
The view of small and restricted sex differences was subsequently challenged by another high-profile publication reporting on dramatic visible-to-the-unaided-eye sex differences in the brains of songbirds [20]. These consisted of brain regions or nuclei that were dramatically larger in males and gratifyingly were also the regions known to control the production of song, a decidedly male-biased behavioural trait in species such as canaries and zebra finches. A well-known piece of scientific lore, at least for this small field, tells the tale that the research in birds prompted Roger Gorski to look again at rat brains and led him to discover the celebrated sexually dimorphic nucleus of the preoptic area, or SDN for short [21]. This small collection of Nissle dense cells does not truly warrant the moniker of nucleus as it is a subdivision of a subdivision of the medial preoptic nucleus, itself a relatively small brain region. Nonetheless, the SDN is three to five times larger in males than females, and many years later, we know this is because the majority of neurons in this small region die in females very early in life because they are deprived of the oestradiol that the male generates locally in the brain by aromatizing his testicularly derived androgens [22]. There are probably more publications about the SDN than neurons actually in the SDN, yet its role in any functional difference between males and females remains elusive, a point further elaborated on below.
5. Sex differences in the human brain: fact or fiction?
The SDN did serve the important purpose of kickstarting the search for sex differences in the mammalian brain. A proliferation of ‘volumetric sex differences' followed, in which either an area, nucleus, cell layer or fibre track was found to be bigger in one sex. Early reports of sex differences in the human brain were restricted to postmortem histological analyses and focused solely on the volume of a brain region or fibre tract (reviewed in [23]). By definition, these experiments are confounded in the numerous ways in which parity within subjects of one sex cannot be achieved, much less matched to the opposite sex when dealing with postmortem tissue. This is perhaps most problematic in younger subjects in which death is, thankfully, rare, but also never owing to ‘natural causes’. Any study involving postmortem human brains can be assumed to include very few healthy controls. This approach of quantifying the size of brain regions in both humans and animals dominated for some time. However, the conversation has changed with the recent advent of readily available imaging techniques in living subjects that allow for longitudinal sampling of healthy individuals across development. There are reported sex differences in growth trajectories of various brain regions of boys and girls, with some developing faster in boys and others in girls [24–26]. Brain development involves more than just growth. Pruning of cell number and synapses is equally important, and the timing and magnitude of these critical events also appear to differ in children as a function of sex. fMRI has allowed for inferences about how men and women respond to varying stimuli [27] and more recently the use of diffusion tensor imaging (DTI) has been used to make inferences about connectivity. With these new approaches, a large number of brain sex differences have been reported, some of them quite spectacular [28], and this, in turn, has generated strong challenges and rebuttals [29]. Time and further study will sort out the answer, but important discussions about the role of brain size, movement in the scanner, implicit bias and reverse inference improve the rigour for all imaging studies.
The increasing use of imaging techniques that measure brain activity (i.e. fMRI) or connectivity (DTI) also changes the conversation about sex differences in the human brain by relying on an analytical approach that is not intuitive or accessible, as opposed to histological staining of tissue sections that everyone can readily see and comprehend. Most of us do not know how to interpret or properly ‘read’ an MRI, and the statistical approaches and algorithms used in DTI studies to assess connectivity are equally foreign. Thus, most of us cannot see the sex difference, so instead we rely on the investigators running the study to do it for us. This level of disconnect can sow suspicion.
But there is another window into the human brain and that is through the minds of boys and girls. Hines has discovered a robust sex difference in toy preference between boys and girls and has convincingly demonstrated over many studies that girls prenatally exposed to androgen owing to a genetic anomaly (congenital adrenal hyperplasia (CAH) girls) have a boy-like toy preference [30,31]. In this issue, Hines [32] makes another major leap forward in illuminating how androgens impact the developing human brain with evidence that CAH girls are less sensitive than unaffected girls to extraneous socialization cues about gender-appropriate toy-choices. Thus, rather than concluding that there is some undiscovered ‘prefers-dolls-nucleus' in the brain, her recent work demonstrates how children are differentially sensitive to socializing cues, so that girls become even more girl-like by modelling the behaviour of other females. In this way, the nature versus nurture conundrum is broken down with the realization that nature determines the response to nurture. Whether the converse is true for boys is not yet known.
6. Animal studies inform humans about humans
The most polarized views on sex differences in humans are understandably centred around cognitive aptitude and abilities [4]. This is appropriate as we should never easily accept a scientific conclusion that could be used to justify discrimination or limit opportunities for one sex. No matter how often we repeat that different does not mean better, there is always a tendency to conclude that certain skill sets are superior over others. A good exercise to gauge how divisive a finding of sex differences associated with cognition can be is to substitute the word ‘race’ for ‘sex’. However, honest evidence-based debates on sex differences in cognitive regions of the human brain should be limited to just that, cognition, and not used to conclude there are no differences in the brains of human males and females.
In this issue, Balthazart [33] reviews the existing literature on what is arguably the most robust behavioural sex difference in humans, sexual partner preference, and he directly relates this to findings in animals involving hormonal or genetic manipulation. Evidence that partner preference is a sexually differentiated trait is found in the impact of preventing oestradiol production in the developing male rodent brain, resulting in either no preference or a reversal towards preference for mounting males [34]. A naturally occurring population of homosexual male sheep, or rams, provides further evidence for hormonally mediated sexual differentiation of partner preference in mammals [35]. The size of the SDN correlates with partner preference in rodents, sheep and humans, in which it is called INAH-3, although there is considerable controversy regarding the latter [36]. In animals, we can manipulate the size of the SDN with hormones and also change sexual orientation, thus by strong inference it is parsimonious to assume the same is occurring in humans, yet the diversity of variables impacting human brain development can never be modelled in a rodent. Moreover, indirect measures of prenatal hormone exposure correlate with partner preference in both men and women, but again are not predictive (reviewed in [37]). On balance, the preponderance of evidence is consistent with a hormonal contribution to partner preference in humans and therefore also consistent with the conclusion that humans too, like every other mammalian species, are subject to sexual differentiation of the brain.
7. The biological basis of sex differences: hormones, epigenetics and genetics
Limitations inherent to human studies preclude the ability to understand how sex differences are established or maintained, and this is where animal studies provide unique contributions. Steroid hormones are a dominant and pervasive source of sex differences by mediating developmental processes that are enduring and establish adult physiological and behavioural responses relevant to the reproductive constraints of each sex. However, as Clarkson and Herbison [38] note in this issue, the mechanism by which fetal testis androgen production is initiated is largely unknown. They report on a male-specific and transient population of kisspeptin neurons residing in the preoptic area that stimulate the GnRH neurons to release LH and FSH, thereby stimulating testicular steroidogenesis. This is related to but distinct from the role of kisspeptin neurons in puberty and ovulation by adult females (reviewed in [39,40]). Moreover, it beautifully demonstrates how a lack of sex differences in the GnRH neurons per se is irrelevant, because the drivers of those neurons appear to be highly sexually specific. Variations in the number or activity of the transient population of kisspeptin neurons could have an unappreciated impact on male brain development by changing the timing or amount of testicular androgen produced.
Epigenetic underpinnings of the enduring impact of steroids also shed new light on how the brain develops and the potential for plasticity in adulthood by reversing the ‘above the genome’ changes established by hormones earlier in life. In this issue, Forger [41] provides an overview of the current state-of-the-art and highlights future directions and unanswered questions. The study of epigenetics of sex differences in the brain is in its infancy and so much remains to be learned, but some principles are beginning to emerge. First is the observation that regions of the developing female brain have DNA that is more heavily methylated than it is in males [42]. DNA methylation is associated with repression of gene expression in most, but not all instances (reviewed in [43]). Second is that gene expression in the adult male brain is heavily influenced by hormonal status, whereas in the female, it is not (reviewed in Shah [44] and [17]). A component of the masculinization process is removal of DNA methylation early in development, at least in the preoptic area (POA) and possibly elsewhere, thus the adult pattern of gene expression in males is consistent with the developmental loss of DNA methylation. This is also consistent with a fourth independent observation of delayed changes in the epigenetic profile following exposure to gonadal hormones early in life [45,46], a sort of ‘epigenetic echo’. Challenges ahead include determining the mechanisms by which such a delay occurs, the genes that are being influenced and ultimately, the function of this type of epigenetic regulation.
Animal models have also allowed us to move beyond hormones and to an appreciation of the important role of sex chromosomes. In this issue, Arnold and co-workers [47] report on the use of novel mouse models in which the number of X chromosomes is emancipated from either gonadal sex or the presence of a Y chromosome. The four-core-genotype model allows for comparison of XX individuals with ovaries and testis and XY individuals with ovaries and testes. Study of these mice has revealed important roles for chromosome complement on body weight and feeding, aggression and habit formation, to name a few (reviewed in [48]). More recent mouse models bring new insights by varying the number of X chromosomes, so that an XXY individual can be compared with XY, thereby revealing the impact of the number of X chromosomes. Advances in determining the genes on the X that exert a modulatory influence include identification of epigenetic regulatory genes, permitting wide-ranging effects on gene expression by autosomes. In this issue, Wade [49] also explores the importance of sex chromosome complement only in the context of non-mammalian species. In birds, the homogametic sex is males (ZZ), as opposed to females (WZ), and she reviews the intersection between hormones and chromosomes to achieve full masculinization with the intriguing speculation that the homogametic sex is always the more neutral, or default sex, and thereby more responsive to manipulations that shift development towards the other sex. Wade [49] also exploits the variety of sex-determining mechanisms in lizards, which may be parthenogenic or not and which may have sex chromosomes or not. She speculates that the absence of sex chromosomes and sex determination by exogenous factors, such as temperature, further frees the nervous system from constraint by sex-differentiating hormones and allows for greater plasticity.
8. Neuroanatomical versus behavioural sex differences
Some argue it is pure folly to study neuroanatomical sex differences with the hope of understanding sex differences in behaviour as the connection between anatomy and behaviour is often weak or even non-existent [50]. Indeed, the most celebrated sex difference in the brain, the SDN, has not been clearly tied to any specific behavioural or physiological endpoint, although see above discussion regarding sexual orientation. While it is true that anatomical or physiological differences in the brain often do not map onto clear differences in behaviour, does this mean they are not important or even, not real? Many studies of sex differences in brain anatomy or neural functioning are perfectly sound, meaning they are fully powered and technically executed using well vetted and accepted approaches, yet a strong correlation between variance in anatomy and variance in behaviour is about the best one can hope for in establishing a causal relationship.
A common refrain is that behaviour is highly variable both within and between individuals. Thus, what is true on one day under one set of circumstances might not be true on another. Cognitive performance in particular can be influenced by the degree of stress, the testing conditions, prior experience, etc. Some would say this is why we will never be able to connect brain and behaviour, but this serves only to squash further inquiry, not to answer questions. Instead, perhaps we should ask when is there a disconnect between brain and behaviour and why does it occur?
(a). Sex differences in behaviour are loosely tethered to neuroanatomy
As discussed above, the discovery of large sex differences in the size of nuclei essential for song production in birds was a transformative event. In this issue, Ball [51] discusses the potential power of studies on birds owing to the enormous natural variation across the approximately 6000 existing song bird species that live in different climates and habitats and are thus subject to divergent selection. Early studies confirmed a correlative relationship between the degree of sexual dimorphism in the size of song control nuclei and sex differences in song production. However, as scientists explored more species, including those rare few in which males and females duet or even rarer, those few species where females sing on average more than males, a disconnect appeared between anatomy and behaviour (reviewed in [52]). Ball [51] notes that contrary to original ideas that song is ancestral to males and was subsequently derived in females, emerging views suggest that song evolved in both males and females but then diverged. Whether this divergence was owing to sexual selection, natural selection or both is subject to debate [53], but the critical point is that preadaptation (i.e. existing song nuclei) may constrain the neuroanatomical substrates in males and females, so that the nuclei remain larger in males, regardless of singing behaviour, yet other factors could evolve to subserve duetting or female-biased song. This scenario evokes the concepts of convergence and canalization, both of which are discussed further below.
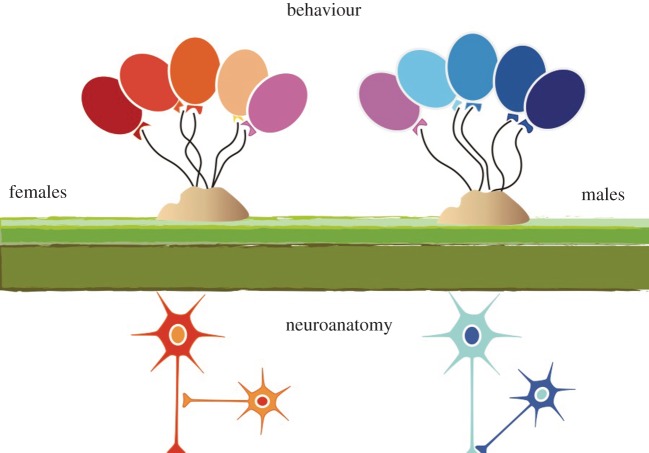
While the scenario in birds is particularly powerful, there are also numerous examples in rodent models where neuroanatomy and behaviour do not directly align. But this can be attributed more simply to the fact that behaviour is controlled by entire networks of cells, and one function of those networks is to integrate multiple sources of information. Even a behaviour as strongly regulated as mating in female rodents, which is tightly constrained by hormonal and circadian factors, is still subject to modification by fear, anxiety, hunger, etc. Thus, neuroanatomy is the ground upon which behaviour is tethered, meaning it exerts some constraining influences, but the behavioural output is subject to buffering from numerous extraneous influences (figure 2). This is true of all behaviours, regardless of whether they differ in expression in males and females. The challenge is to gain a coherent understanding of both parameters and how they relate to each other.
Figure 2.
Sex differences in the brain do not equal sex differences in behaviour. The ability to constrain variables such as genetics, age and experience in animal models has allowed for the reliable detection of robust neuroanatomical and physiological sex differences. These can be found at the level of individual neurons, their connections, the signal transduction pathways activated and neuronal physiology. But sex differences in any one of these parameters do not perfectly predict behaviour as many variables across a wide neural network must be integrated in order for a particular behaviour to be executed. There are circumstances in which the predictability is greater, such as mating behaviour where the neural substrates are well known and the motor execution is distinctly different in males and females. Others are far more challenging. These would include cognitive and affective behaviours that can diverge in males and females in response to stress or other external variables and for which the neural substrates are diffuse and often ill defined. (Online version in colour.)
(b). Modular control of behaviour via neural circuits
One of the greatest challenges to dissecting the neural control of complex social behaviours such as mating and aggression is the diversity of cell types within the relevant brain regions. Classic lesion and stimulation studies combined with tract tracing have clearly mapped the landscape and identified critical neural hubs for expression of particular behaviours, but more often than not there are multiple behaviours regulated at an individual hub. For instance, the ventromedial nucleus of the hypothalamus is essential for expression of female sexual receptivity [54,55], but also modulates male mating and aggression [56]. In this issue, Bayless and Shah [44] emphasize the power of the mouse model to allow for targeted deletion or stimulation of specific sets of neurons, such as only those expressing the aromatase enzyme or the androgen receptor, and thereby disentangling the role of those specific neurons from their immediate but distinct neighbours. This is a powerful approach for knowing that neurons of one particular phenotype in one particular region regulate aggression and mating in males but only mating in females. Ultimately, a highly refined map will guide understanding of the neurologic substrates of behaviour, but the conundrum of why behaviours vary so greatly both within and between individuals will remain.
(c). Compensation and convergence in sexually dimorphic behaviours
Compensation [57], also referred to as convergence [58], refers to the phenomenon in which the two sexes find a different way to solve the same problem. This may involve different anatomical substrates in males and females for purposes of convergence on the same behaviour. The most frequently cited example is the one that prompted the investigator who first articulated the notion, Geert De Vries. He has documented marked sex differences in vasopressin innervation of the forebrain of multiple mammalian species, with males having significantly heavier input than females, and he connected this difference to parenting behaviour in voles as a means by which male voles develop the capacity to exhibit nurturing behaviour towards their offspring (reviewed in [59]). He speculates that the males of this species of vole evolved the vasopressin innervation to solve the problem of their lack of a hormonally induced nurturing circuit that is present in females and activated by pregnancy and/or parturition, hence the term ‘compensation’. More recent are surprising reports of marked sex differences in signal transduction pathways in hippocampal circuits that also seem to represent a case of ‘convergence’ such that the same physiological problem (state) is solved differently in each sex [60]. Divergent pathways to cell death [61,62] and sexually dimorphic cellular origins of pain [63] are additional startling examples. These latter examples demonstrate convergence rather than compensation as it is not clear what one sex is lacking versus the other, but we may not have yet discovered the missing piece.
Studies of learning provide a particularly interesting perspective on how the sexes can differ in both the neurophysiological underpinnings and the expression of a behaviour. In this issue, Shors [64] takes a trip down memory lane to review her 20 year journey of deciphering how males and females learn and how that learning is impacted by stress. Using the conditioned eye-blink task, which is attractive for not being confounded by hunger, fear or motivation, she observed that males improved under stressful conditions whereas female performance deteriorated. Opposite changes in hippocampal synaptic density correlate with the divergence in behavioural responding, so there appears to be a connection between neuroanatomy and behaviour (reviewed in [65]). But as Shors [64] goes on to point out, the divergence in response to stress occurs only during a limited time in the female reproductive cycle, proestrus, a time of high circulating oestradiol, and she makes a case for the importance of considering life stage in any discussion of sex differences. This is an important caveat and its truth is borne out in studies of humans across the lifespan, which show a convergence in the size of specific nuclei in older adults compared with younger [66].
A combination of compensation and convergence is found in spatial maze learning. Under some conditions, males consistently outperform females, but in others, the reverse is true, and this appears to be largely dependent upon the learning strategy employed. Males and females attend to different cues (geographic versus local) when solving the maze, but as long as both types of cues are equally available they solve the task equally well (reviewed in [67]). How and why there would be such a dichotomy in behavioural strategies used by each sex for cognitive solutions is unknown.
(d). Canalization of neuroanatomical sex differences
Canalization is a biological process whereby variability is constrained within a certain domain. The ‘canal’ refers to the path not taken, such that once a particular developmental programme is initiated, others are precluded, i.e. the process must continue along that canal and not others. This concept was originally elucidated by Waddington in the 1940s as part of understanding the epigenetic regulation of cell fate but is now widely used in discussions of biological processes of various sorts (reviewed in [68]). For instance, eye size in cichlid fishes is very tightly constrained, with little variability. This is achieved by a chaperone protein, HSP90, which assists in protein trafficking and proper folding so that minor challenges, such as in water salinity or pH, do not dramatically impact cellular function. If HSP90 is blocked or eliminated, then eye size variation increases dramatically, so that there are rapidly large eyes, small eyes and medium size eyes. If conditions are such that eyes are superfluous, as is the case for cave-dwelling cichlids, then selection quickly drives eye size down to nothing owing to the energetic demands of these complex sensory organs [69]. In this way, HSP90 serves as a capacitor, restraining variation under one set of conditions but freeing it under another.
Of interest to the topic of sex differences in the brain is whether there could also be cellular agents that maintain male versus female canals for some neuroanatomical endpoints. Steroid-induced masculinization has many attributes one would associate with canalization, most prominent of which are the evidence of a threshold effect and a ceiling effect. In other words, masculinization of an endpoint is achieved only if a threshold is reached, meaning there is rarely partial masculinization of a neuroanatomical endpoint, and likewise, there are few examples of super-masculinization, i.e. a ceiling. Common portrayals of hormone-mediated sexual differentiation imply that males are exposed to high levels of gonadal steroids, whereas females see none. But in reality, the level of hormones does not differ all that greatly in brain tissue, and in some regions that are sexually differentiated, the levels of steroid do not differ at all between males and females [70]. Moreover, if males are injected with a dose of steroid that would masculinize a female, there is no greater masculinization seen in those males. Thus, something acts as a governor both to prevent females from being masculinized by their own steroids (levels of which are lower than in males but still present) and to keep males from being super masculinized when steroid levels are exceedingly high. There are multiple mechanisms that can establish thresholds or ceilings. The heat shock family of proteins, of which HSP90 is one, are also essential chaperone proteins for steroid receptors such as the oestrogen and androgen receptors (reviewed in [71,72]), but they have received little attention in the context of sex differences. These proteins could easily maintain a threshold for steroid action by preventing dimerization or promoting receptor translocation to the nucleus. MicroRNAs are also agents of canalization by generating a threshold for translation of mRNA into protein [73]. MicroRNAs inhibit translation by binding to and destroying mRNA, thereby providing a break. If the amount of microRNA is saturated by excess mRNA, however, then there is a sudden uptick or jump in translation as the break has been removed, i.e. a threshold is breached and thus, a new canal is entered. Both we [17] and Morgan & Bale [74] have detected sex differences in microRNAs in the developing brain. Epigenetic modifications were the original basis for the concept of canalization and the widespread detection of modifications to both the DNA and histones of males and females suggests canalization takes place. At least in the case of the synaptic density of the POA, these epigenetic changes have been linked to a neuroanatomical sex difference [42]. Other agents may also exist, including in humans those considered cultural. Telling boys not to cry and be a man, dressing girls in clothing that restricts their movements, these could easily be agents of canalization that by reinforcing gender norms actually impact the developing brain. The data report of Hines and co-workers [32] in this issue in which girls model their behaviour after what they see other females doing is evidence that such processes can occur, although at this time there is no clear neuroanatomical substrate upon which socialization pressure would act.
9. Sex differences in diseases and disorders of the nervous system
Human health afflictions that originate in or impact the nervous system are vast in number and scope. They range from simple injury secondary to trauma or oxygen deprivation, to developmental perturbations of genetic or physiological origins that derail healthy maturation to neurodegenerative diseases associated with autoimmunity and/or aging. Almost without exception, there are gender biases either in the diagnosis, frequency, severity or timing of nervous system diseases and disorders as well as the enduring consequences of brain trauma (figure 3). For some disorders, such as multiple sclerosis—which is four times more frequent in women it has been argued that the protective effect of being male far outweighs any treatment currently available [75]. Thus, it is incumbent upon us to exploit the traction created by a sex difference to discover the biological factors that either afford protection or generate vulnerability in one sex versus the other for the ultimate benefit of both sexes.
Figure 3.
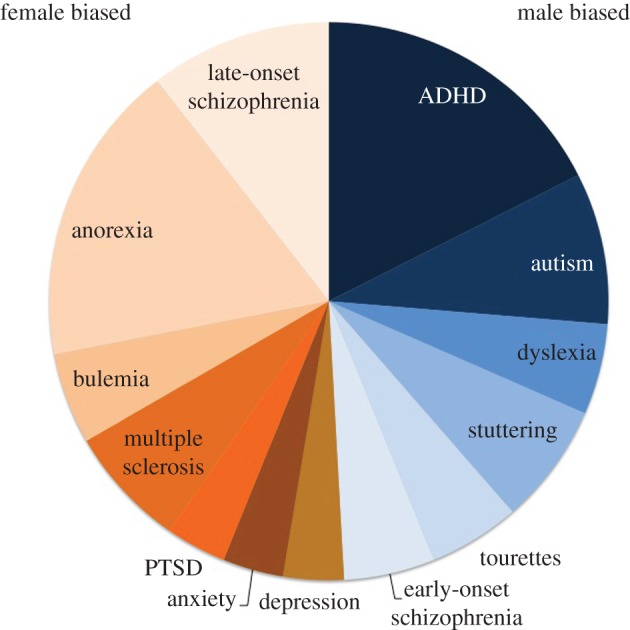
Gender bias in neurologic and neuropsychiatric disorders. A compelling reason for studying sex as a biological variable is the pervasive gender bias in the frequency of diagnosis of numerous disorders. Many factors go into the determination of a disease state, but identification of biological variables clarifies those influences that are not biological in origin (i.e. diagnosis bias, different symptomology, etc.). This diagram presents only a small number of the many diseases and disorders known to differ in frequency between boys and girls, men and women. The size of the wedge for each disorder represents the relative degree of bias in favour of females (left) versus males (right) and is loosely based on [75–87]. ADHD, attention deficit hyperactivity disorder; PTSD, post-traumatic stress disorder. (Online version in colour.)
Most neuropsychiatric disorders are heavily gender-biased. Autism spectrum disorder is diagnosed in boys four to five times more often than girls [76,77], schizophrenia manifests differently in men and women across the lifespan [78], and affective disorders such as unipolar depression and PTSD are up to twice as frequent in women and girls [79,88]. The latter may be skewed by social factors such as willingness to seek treatment. Likewise, varying rates of drug and alcohol abuse in men and women are speculated to be based in sex differences in risk-seeking and reward systems, as opposed to a neural substrate of addiction that is sexually dimorphic [89].
Insults to the brain come in a variety of forms and the impact varies by whether they occur prenatally, after birth or as a consequence of prematurity. In this issue, Terasaki et al. [90] note that fetal and infant exposure to drugs and alcohol is one of the most devastating and preventable forms of injury to the developing brain. They also note that the majority of research to-date using animal models has either focused exclusively on males or, if both sexes were included, has not analysed for sex as a variable. However, with just an initial foray into the effects of alcohol and opiate exposure on developing rodents, they find widespread sex differences that vary with the timing of the exposure and the endpoint under study. Long-term deleterious consequences associated with cognitive tasks and social behaviours may be best attenuated by sex-specific interventions and these authors convincingly argue that all future research on fetal drug and alcohol exposure should compare effects in males and females.
Indeed, sex differences in vulnerability or resilience to disorders of the nervous system can be found in unlikely places, even those that would seem the least likely to be different in males and females. In this issue, Jašarević et al. [91] shine a light on the microbiome as a source of sexually dimorphic disease risk. The coevolution of gut bacteria and the metabolic demands of the brain are postulated to have created a critical alliance that must be tightly orchestrated across the lifespan as both components in this partnership mature. Surprisingly, the gut microbiome differs in males and females, and in a direct tie-in to sexual differentiation, the male microbiome appears to stimulate androgen production that protects against autoimmune disorders [92]. Bale and co-workers [91] provide a different angle in the discovery that stress during pregnancy alters the vaginal microbiota, which is a primary source seeding the newborn infant gut microbiome. The impact seems to be sex-specific as evidenced by opposite changes in amino acid profiles in the paraventricular nucleus of neonates [93]. The paraventricular nucleus is a key component of the stress axis, and so sex-specific changes in the development of this brain region could have enduring consequences for later life stress management. Bale et al. [91] speculate that changes in stress responsivity, both physiologically and behaviourally, may have origins in the microbiota and that sex differences in the latter may impact the former. Again, there is much work to be done.
Last, there is per chance to sleep. Here too, great advances are being made in fundamental understanding of how sleep is controlled in the brain as well as how sleep impacts brain function. But very few investigators consider the impact of sex on sleep. In this issue, Mong and Cusmano [94] detail sex differences in various aspects of sleep and note that sleep disorders in women may manifest differently than in men, requiring a new set of diagnostic criteria. Similar arguments have been made for the substantially higher rates of diagnosis of autism spectrum disorders in boys, meaning, because girls externally manifest the disorder differently from boys, they may be under-diagnosed and thereby under-served. Surprisingly, sex differences in sleep appear to have origins in development and to be sexually differentiated by hormones in the same manner as so many other traits [95]. Inappropriate attention to sleep disorders in women has compounding effects. Insomnia is the most common sleep complaint and is 40% more frequent in women. Sleep deficit is a risk factor for depressive disorders, and women are also about 40% more likely to be diagnosed with depression [96]. Thus, a full understanding of each and how they differ in males and females will more fully inform the other.
10. Conclusion
The continued interest in and study of sex differences in brain and behaviour are not going away anytime soon. The pervasive impact of sex on so many aspects of neural functioning in both the healthy and diseased brain demands that as neuroscientists, we incorporate sex as a biological variable in order to reach conclusions that are applicable to all. The exploratory power of comparing and contrasting males and females cannot be overstated. There are many ways to solve a biological problem and evolution often finds different ways in males and females. By studying both, we open ourselves to a richer and more complex view of the nervous system and enhance our understanding beyond that achievable by a unisex approach. However, the importance of gender, in all its guises, also cannot be over stated. Any study of humans should consider the impact of both personal and societal perceptions of gender, in both the subjects of study and the investigators conducting the research. Consideration of how gender guides subject responses and effects investigators’ interpretations will improve the reliability of the work. Only through the inclusion of both sex and gender can understanding of the full multifaceted origins of sex differences in the brain be achieved.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Cahill L. 2006. Why sex matters for neuroscience. Nat. Rev. Neurosci. 7, 477–484. ( 10.1038/nrn1909) [DOI] [PubMed] [Google Scholar]
- 2.Rippon G, Jordan-Young R, Kaiser A, Fine C. 2014. Recommendations for sex/gender neuroimaging research: key principles and implications for research design, analysis, and interpretation. Front. Hum. Neurosci. 8, 650 ( 10.3389/fnhum.2014.00650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eliot L. 2011. The trouble with sex differences. Neuron 72, 895–898. ( 10.1016/j.neuron.2011.12.001) [DOI] [PubMed] [Google Scholar]
- 4.Maney DL. 2015. Just like a circus: the public consumption of sex differences. Curr. Top. Behav. Neurosci. 19, 279–296. ( 10.1007/7854_2014_339) [DOI] [PubMed] [Google Scholar]
- 5.Klein SL, et al. 2015. Opinion: sex inclusion in basic research drives discovery. Proc. Natl Acad. Sci. USA 112, 5257–5258. ( 10.1073/pnas.1502843112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maney DL. 2016. Perils and pitfalls of reporting sex differences. Phil. Trans. R. Soc. B 371, 20150119 ( 10.1098/rstb.2015.0119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tunç B, et al. 2016. Establishing a link between sex-related differences in the structural connectome and behaviour. Phil. Trans. R. Soc. B 371, 20150111 ( 10.1098/rstb.2015.0111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joel D, Fausto-Sterling A. 2016. Beyond sex differences: new approaches for thinking about variation in brain structure and function. Phil. Trans. R. Soc. B 371, 20150451 ( 10.1098/rstb.2015.0451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joel D, et al. 2015. Sex beyond the genitalia: the human brain mosaic. Proc. Natl Acad. Sci. USA 112, 15 468–15 473. ( 10.1073/pnas.1509654112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan A, Ma W, Vira A, Marwha D, Eliot L. 2016. The human hippocampus is not sexually-dimorphic: meta-analysis of structural MRI volumes. Neuroimage 124, 350–366. ( 10.1016/j.neuroimage.2015.08.050) [DOI] [PubMed] [Google Scholar]
- 11.Josso N. 2008. Professor Alfred Jost: the builder of modern sex differentiation. Sex Dev. 2, 55–63. ( 10.1159/000129690) [DOI] [PubMed] [Google Scholar]
- 12.Nelson RJ. 1995. An introduction to behavioral endocrinology. Sunderland, MA: Sinauer Associates Inc. [Google Scholar]
- 13.Phoenix CH, Goy RW, Gerall AA, Young WC. 1959. Organizing action of prenatally administered testosterone proprionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382. ( 10.1210/endo-65-3-369) [DOI] [PubMed] [Google Scholar]
- 14.Simerly RB. 2002. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 25, 507–536. ( 10.1146/annurev.neuro.25.112701.142745) [DOI] [PubMed] [Google Scholar]
- 15.Morris JA, Jordan CL, Breedlove SM. 2004. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 7, 1034–1039. ( 10.1038/nn1325) [DOI] [PubMed] [Google Scholar]
- 16.De Vries GJ, Simerly RB. 2002. Anatomy, development and funtion of sexually dimorphic neural circuits in the mammalian brain. In Hormones, brain and behavior (eds Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RG), pp. 137–192. New York, NY: Academic Press. [Google Scholar]
- 17.McCarthy MM, Pickett LA, VanRyzin JW, Kight KE. 2015. Surprising origins of sex differences in the brain. Horm. Behav. 76, 3–10. ( 10.1016/j.yhbeh.2015.04.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallen K. 2005. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front. Neuroendocrinol. 26, 7–26. ( 10.1016/j.yfrne.2005.02.001) [DOI] [PubMed] [Google Scholar]
- 19.Raisman G, Field PM. 1971. Sexual dimorphism in the preoptic area of the rat. Science 173, 731–733. ( 10.1126/science.173.3998.731) [DOI] [PubMed] [Google Scholar]
- 20.Nottebohm F, Arnold AP. 1976. Sexual dimorphism in vocal control areas of the songbird brain. Science 194, 211–213. ( 10.1126/science.959852) [DOI] [PubMed] [Google Scholar]
- 21.Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. 1980. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J. Comp. Neurol. 193, 529–539. ( 10.1002/cne.901930214) [DOI] [PubMed] [Google Scholar]
- 22.Davis EC, Popper P, Gorski RA. 1996. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 734, 10–18. ( 10.1016/0006-8993(96)00298-3) [DOI] [PubMed] [Google Scholar]
- 23.Hines M. 2002. Sexual differentiation of human brain and behavior. In Hormones, brain and behavior (ed. Pfaff D.), pp. 425–462. London, UK: Academic Press. [Google Scholar]
- 24.Raznahan A, et al. 2010. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc. Natl Acad. Sci. USA 107, 16 988–16 993. ( 10.1073/pnas.1006025107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenroot RK, et al. 2007. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36, 1065–1073. ( 10.1016/j.neuroimage.2007.03.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustacci K, Masalehdan A, Noll J, Boring AM. 2001. Sex differences in brain maturation during childhood and adolescence. Cereb. Cortex 11, 552–557. ( 10.1093/cercor/11.6.552) [DOI] [PubMed] [Google Scholar]
- 27.Gur RC, et al. 2000. An fMRI study of sex differences in regional activation to a verbal and a spatial task. Brain Lang. 74, 157–170. ( 10.1006/brln.2000.2325) [DOI] [PubMed] [Google Scholar]
- 28.Ingalhalikar M, et al. 2014. Sex differences in the structural connectome of the human brain. Proc. Natl Acad. Sci. USA 111, 823–828. ( 10.1073/pnas.1316909110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joel D, Tarrasch R. 2014. On the mis-presentation and misinterpretation of gender-related data: the case of Ingalhalikar's human connectome study. Proc. Natl Acad. Sci. USA 111, E637 ( 10.1073/pnas.1323319111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamminmaki A, Hines M, Kuiri-Hänninen T, Kilpeläinen L, Dunkel L, Sankilampi U. 2012. Testosterone measured in infancy predicts subsequent sex-typed behavior in boys and in girls. Horm. Behav. 61, 611–616. ( 10.1016/j.yhbeh.2012.02.013) [DOI] [PubMed] [Google Scholar]
- 31.Pasterski V, Geffner ME, Brain C, Hindmarsh P, Brook C, Hines M. 2005. Prenatal hormones and postnatal socialization by parents as determinants of male-typical toy play in girls with congenital adrenal hyperplasia. Child Dev. 76, 264–278. ( 10.1111/j.1467-8624.2005.00843.x) [DOI] [PubMed] [Google Scholar]
- 32.Hines M, Pasterski V, Spencer D, Neufeld S, Patalay P, Hindmarsh PC, Hughes IA, Acerini CL. 2016. Prenatal androgen exposure alters girls' responses to information indicating gender-appropriate behaviour. Phil. Trans. R. Soc. B 371, 20150125 ( 10.1098/rstb.2015.0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balthazart J. 2016. Sex differences in partner preferences in humans and animals. Phil. Trans. R. Soc. B 371, 20150118 ( 10.1098/rstb.2015.0118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakker J, Brand T, Van Ophemert J, Slob AK. 1993. Hormonal regulation of adult partner preference behavior in neonatally ATD-treated male rats. Behav. Neurosci. 107, 480–487. ( 10.1037/0735-7044.107.3.480) [DOI] [PubMed] [Google Scholar]
- 35.Roselli CE, Reddy RC, Kaufman KR. 2011. The development of male-oriented behavior in rams. Front. Neuroendocrinol. 32, 164–169. ( 10.1016/j.yfrne.2010.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byne W, Tobet S, Mattiace LA, Lasco MS, Kemether E, Edgar MA, Morgello S, Buchsbaum MS, Jones LB. 2001. The interstitial nuclei of the human anterior hypothalamus: an investigation of variation with sex, sexual orientation, and HIV status. Horm. Behav. 40, 86–92. ( 10.1006/hbeh.2001.1680) [DOI] [PubMed] [Google Scholar]
- 37.Roselli C, Balthazart J. 2011. Sexual differentiation of sexual behavior and its orientation. Front. Neuroendocrinol. 32, 109 ( 10.1016/j.yfrne.2011.03.002) [DOI] [PubMed] [Google Scholar]
- 38.Clarkson J, Herbison AE. 2016. Hypothalamic control of the male neonatal testosterone surge. Phil. Trans. R. Soc. B 371, 20150115 ( 10.1098/rstb.2015.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dungan HM, Clifton DK, Steiner RA. 2006. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology 147, 1154–1158. ( 10.1210/en.2005-1282) [DOI] [PubMed] [Google Scholar]
- 40.Kauffman AS. 2010. Coming of age in the kisspeptin era: sex differences, development and puberty. Mol. Cell Endocrinol. 324, 51–63. ( 10.1016/j.mce.2010.01.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forger NG. 2016. Epigenetic mechanisms in sexual differentiation of the brain and behaviour. Phil. Trans. R. Soc. B 371, 20150114 ( 10.1098/rstb.2015.0114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM. 2015. Brain feminization requires active repression of masculinization via DNA methylation. Nat. Neurosci. 18, 690–697. ( 10.1038/nn.3988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sweatt JD. 2013. The emerging field of neuroepigenetics. Neuron 80, 624–632. ( 10.1016/j.neuron.2013.10.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayless DW, Shah NM. 2016. Genetic dissection of neural circuits underlying sexually dimorphic social behaviours. Phil. Trans. R. Soc. B 371, 20150109 ( 10.1098/rstb.2015.0109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwarz JM, Nugent BM, McCarthy MM. 2010. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology 151, 4871–4881. ( 10.1210/en.2010-0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghahramani NM, et al. 2014. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol. Sex Differ. 5, 8 ( 10.1186/2042-6410-5-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnold AP, et al. 2016. The importance of having two X chromosomes. Phil. Trans. R. Soc. B 371, 20150113 ( 10.1098/rstb.2015.0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arnold AP, Chen X. 2009. What does the ‘four core genotypes’ mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 30, 1–9. ( 10.1016/j.yfrne.2008.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wade J. 2016. Genetic regulation of sex differences in songbirds and lizards. Phil. Trans. R. Soc. B 371, 20150112 ( 10.1098/rstb.2015.0112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Vries GJ, Södersten P. 2009. Sex differences in the brain: the relation between structure and function. Horm. Behav. 55, 589–596. ( 10.1016/j.yhbeh.2009.03.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ball GF. 2016. Species variation in the degree of sex differences in brain and behaviour related to birdsong: adaptations and constraints. Phil. Trans. R. Soc. B 371, 20150117 ( 10.1098/rstb.2015.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ball GF, MacDougall-Shackleton SA. 2001. Sex differences in songbirds 25 years later: what have we learned and where do we go? Microsc. Res. Tech. 54, 327–334. ( 10.1002/jemt.1146) [DOI] [PubMed] [Google Scholar]
- 53.Dale J, Dey CJ, Delhey K, Kempenaers B, Valcu M. 2015. The effects of life history and sexual selection on male and female plumage colouration. Nature 527, 367–370. ( 10.1038/nature15509) [DOI] [PubMed] [Google Scholar]
- 54.Pfaff DW, Sakuma Y. 1979. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J. Physiol. 288, 203–210. [PMC free article] [PubMed] [Google Scholar]
- 55.Pfaff DW, Sakuma Y. 1979. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J. Physiol. 288, 189–202. [PMC free article] [PubMed] [Google Scholar]
- 56.Kunwar PS, Zelikowsky M, Remedios R, Cai H, Yilmaz M, Meister M, Anderson DJ. 2015. Ventromedial hypothalamic neurons control a defensive emotion state. Elife 4, e06633 ( 10.7554/eLife.06633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Vries GJ. 2004. Minireview: sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145, 1063–1068. ( 10.1210/en.2003-1504) [DOI] [PubMed] [Google Scholar]
- 58.McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. 2012. Sex differences in the brain: the not so inconvenient truth. J. Neurosci. 32, 2241–2247. ( 10.1523/JNEUROSCI.5372-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Vries GJ. 2008. Sex differences in vasopressin and oxytocin innervation of the brain. Prog. Brain Res. 170, 17–27. ( 10.1016/S0079-6123(08)00402-0) [DOI] [PubMed] [Google Scholar]
- 60.Huang GZ, Woolley CS. 2012. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron 74, 801–808. ( 10.1016/j.neuron.2012.03.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill CA, Fitch RH. 2012. Sex differences in mechanisms and outcome of neonatal hypoxia-ischemia in rodent models: implications for sex-specific neuroprotection in clinical neonatal practice. Neurol. Res Int. 2012, 867531 ( 10.1155/2012/867531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li H, Pin S, Zeng Z, Wang MM, Andreasson KA, McCullough LD. 2005. Sex differences in cell death. Ann. Neurol. 58, 317–321. ( 10.1002/ana.20538) [DOI] [PubMed] [Google Scholar]
- 63.Sorge RE, et al. 2015. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 18, 1081–1083. ( 10.1038/nn.4053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shors TJ. 2016. A trip down memory lane about sex differences in the brain. Phil. Trans. R. Soc. B 371, 20150124 ( 10.1098/rstb.2015.0124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shors TJ. 2006. Stressful experience and learning across the lifespan. Annu. Rev. Psychol. 57, 55–85. ( 10.1146/annurev.psych.57.102904.190205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung WC, De Vries GJ, Swaab DF. 2002. Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. J. Neurosci. 22, 1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCarthy MM, Konkle AT. 2005. When is a sex difference not a sex difference? Front. Neuroendocrinol. 26, 85–102. ( 10.1016/j.yfrne.2005.06.001) [DOI] [PubMed] [Google Scholar]
- 68.Gursky V, Surkova SY, Samsonova MG. 2012. Mechanisms of developmental robustness. Biosystems 109, 329–335. ( 10.1016/j.biosystems.2012.05.013) [DOI] [PubMed] [Google Scholar]
- 69.Rohner N, Jarosz DF, Kowalko JE, Yoshizawa M, Jeffery WR, Borowsky RL, Lindquist S, Tabin CJ. 2013. Cryptic variation in morphological evolution: HSP90 as a capacitor for loss of eyes in cavefish. Science 342, 1372–1375. ( 10.1126/science.1240276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Konkle AT, McCarthy MM. 2011. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology 152, 223–235. ( 10.1210/en.2010-0607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beato M. 1989. Gene regulation by steroid hormones. Cell 56, 335–344. ( 10.1016/0092-8674(89)90237-7) [DOI] [PubMed] [Google Scholar]
- 72.Beato M, Klug J. 2000. Steroid hormone receptors: an update. Hum. Reprod. Update 6, 225–236. ( 10.1093/humupd/6.3.225) [DOI] [PubMed] [Google Scholar]
- 73.Posadas DM, Carthew RW. 2014. MicroRNAs and their roles in developmental canalization. Curr. Opin. Genet. Dev. 27, 1–6. ( 10.1016/j.gde.2014.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morgan CP, Bale TL. 2012. Sex differences in microRNA regulation of gene expression: no smoke, just miRs. Biol. Sex Differ. 3, 22 ( 10.1186/2042-6410-3-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Voskuhl R. 2011. Sex differences in autoimmune diseases. Biol. Sex Differ. 2, 1 ( 10.1186/2042-6410-2-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Halladay AK, et al. 2015. Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol. Autism 6, 36 ( 10.1186/s13229-015-0019-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Werling DM, Geschwind DH. 2013. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 26, 146–153. ( 10.1097/WCO.0b013e32835ee548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abel KM, Drake R, Goldstein JM. 2010. Sex differences in schizophrenia. Int. Rev. Psychiatry 22, 417–428. ( 10.3109/09540261.2010.515205) [DOI] [PubMed] [Google Scholar]
- 79.Altemus M. 2006. Sex differences in depression and anxiety disorders: potential biological determinants. Horm. Behav. 50, 534–538. ( 10.1016/j.yhbeh.2006.06.031) [DOI] [PubMed] [Google Scholar]
- 80.Swaab DF, Hofman MA. 1995. Sexual differentiation of the human hypothalamus in relation to gender and sexual orientation. Trends Neurosci. 18, 264–270. ( 10.1016/0166-2236(95)80007-O) [DOI] [PubMed] [Google Scholar]
- 81.Bale TL, et al. 2010. Early life programming and neurodevelopmental disorders. Biol. Psychiatry 68, 314–319. ( 10.1016/j.biopsych.2010.05.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Auyeung B, Wheelwright S, Allison C, Atkinson M, Samarawickrema N, Baron-Cohen S. 2009. The children's empathy quotient and systemizing quotient: sex differences in typical development and in autism spectrum conditions. J. Autism Dev. Disord. 39, 1509–1521. ( 10.1007/s10803-009-0772-x) [DOI] [PubMed] [Google Scholar]
- 83.Bangasser DA, Valentino RJ. 2014. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 35, 303–319. ( 10.1016/j.yfrne.2014.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schaafsma SM, Pfaff DW. 2014. Etiologies underlying sex differences in autism spectrum disorders. Front. Neuroendocrinol. 35, 255–271. ( 10.1016/j.yfrne.2014.03.006) [DOI] [PubMed] [Google Scholar]
- 85.Volkmar FR, Szatmari P, Sparrow SS. 1993. Sex differences in pervasive developmental disorders. J. Autism Dev. Disord. 23, 579–591. ( 10.1007/BF01046103) [DOI] [PubMed] [Google Scholar]
- 86.Waddell J, McCarthy MM. 2010. Sexual differentiation of the brain and ADHD: what is a sex difference in prevalence telling us? Curr. Top. Behav. Neurosci. 9, 341–360. ( 10.1007/7854_2010_114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Young LJ, Pfaff DW. 2014. Sex differences in neurological and psychiatric disorders. Front. Neuroendocrinol. 35, 253–254. ( 10.1016/j.yfrne.2014.05.005) [DOI] [PubMed] [Google Scholar]
- 88.Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. 2003. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. J. Consult. Clin. Psychol. 71, 692–700. ( 10.1037/0022-006X.71.4.692) [DOI] [PubMed] [Google Scholar]
- 89.Becker JB, Perry AN, Westenbroek C. 2012. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol. Sex Differ. 3, 14 ( 10.1186/2042-6410-3-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Terasaki LS, Gomez J, Schwarz JM. 2016. An examination of sex differences in the effects of early-life opiate and alcohol exposure. Phil. Trans. R. Soc. B 371, 20150123 ( 10.1098/rstb.2015.0123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jašarević E, Morrison KE, Bale TL. 2016. Sex differences in the gut microbiome–brain axis across the lifespan. Phil. Trans. R. Soc. B 371, 20150122 ( 10.1098/rstb.2015.0122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Markle JG, et al. 2013. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088. ( 10.1126/science.1233521) [DOI] [PubMed] [Google Scholar]
- 93.Jasarevic E, Howerton CL, Howard CD, Bale TL. 2015. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology 156, 3265–3276. ( 10.1210/en.2015-1177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mong JA, Cusmano DM. 2016. Sex differences in sleep: impact of biological sex and sex steroids. Phil. Trans. R. Soc. B 371, 20150110 ( 10.1098/rstb.2015.0110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cusmano DM, Hadjimarkou MM, Mong JA. 2014. Gonadal steroid modulation of sleep and wakefulness in male and female rats is sexually differentiated and neonatally organized by steroid exposure. Endocrinology 155, 204–214. ( 10.1210/en.2013-1624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goldstein JM, Holsen L, Handa R, Tobet S. 2014. Fetal hormonal programming of sex differences in depression: linking women's mental health with sex differences in the brain across the lifespan. Front. Neurosci. 8, 247 ( 10.3389/fnins.2014.00247) [DOI] [PMC free article] [PubMed] [Google Scholar]