Abstract
A newly identified fungal pathogen, Batrachochytrium salamandrivorans(Bsal), is responsible for mass mortality events and severe population declines in European salamanders. The eastern USA has the highest diversity of salamanders in the world and the introduction of this pathogen is likely to be devastating. Although data are inevitably limited for new pathogens, disease-risk assessments use best available data to inform management decisions. Using characteristics of Bsalecology, spatial data on imports and pet trade establishments, and salamander species diversity, we identify high-risk areas with both a high likelihood of introduction and severe consequences for local salamanders. We predict that the Pacific coast, southern Appalachian Mountains and mid-Atlantic regions will have the highest relative risk from Bsal. Management of invasive pathogens becomes difficult once they are established in wildlife populations; therefore, import restrictions to limit pathogen introduction and early detection through surveillance of high-risk areas are priorities for preventing the next crisis for North American salamanders.
Keywords: fungal pathogen, amphibians, urodeles, Caudata, invasive species, disease
1. Background
Batrachochytrium salamandrivorans (Bsal), a recently described chytrid pathogen, causes morbidity and mortality in salamanders [1,2] and is expected to pose a substantial risk to global amphibian biodiversity. Bsal was first described from declining fire salamander (Salamandra salamandra) populations in Belgium and The Netherlands [1–3] and is suspected to have been introduced into Europe via the importation of Asian salamanders (Cynopsspp. and Paramesotriton spp. [2]) for the pet trade. Indeed, Bsal was subsequently identified on commercially traded salamanders in the UK and Germany [4,5]. Concern for the spread of Bsal is compounded by the continued spread of a related chytrid pathogen, B. dendrobatidis (Bd), which has contributed to global population declines (more than 200 species affected [6]) and the classification of amphibians as the most threatened vertebrate taxa worldwide [7]. Similar to the generalist nature and high pathogenicity of the pandemic strain of Bd[6], Bsal caused substantial mortality in over half of salamander species tested in laboratory infection studies (USA: 2/7 species tested; globally: 12/23 species tested [2]). Although preliminary testing of archived salamander specimens and native salamander populations has failed to detect Bsal in the USA, only a fraction of North American species have been tested (185 individuals from 7 of 191 species [8,9]). Based on the phylogenetic relationship with Bd, wide host range, and high pathogenicity [2], Bsal introduction into the USA could threaten North American salamander biodiversity.
Predicting the potential distribution of a pathogen in novel habitats is an integral part of a formal disease-risk analysis that evaluates the consequences of an invasive disease. Two modelling approaches are commonly used to predict shifts in species distributions: (i) correlative approaches that use statistical relationships between environmental characteristics and observed occurrences and (ii) mechanistic approaches that use relationships between environmental characteristics and organismal performance that are independent of current distributions [10]. Recently, a correlative approach was used to estimate the invasive range of Bsal for North America [11]. This species distribution model characterized the realized niche of potential reservoir species in their native range in Asia (identified from archived wild caught specimens [2,11]), and identified similar environmental conditions in the USA. This approach identified only climatic conditions amenable to the known host species and did not consider the ecology of the pathogen itself. Correlative species distribution models that are based on the realized niche of invasive species in their native range can underestimate the resulting invasive range [12]. Mechanistic species distribution models, on the other hand, rely on the underlying mechanisms contributing to a pathogen’s range [1,13] and may provide better predictions of range shifts during invasions [10]. More importantly, presenting multiple approaches to estimate potential distribution of pathogen invasion will increase overall confidence in predicted areas of agreement and highlight areas of disagreement, where the effect of underlying assumptions on risk predictions can be tested should introduction occur.
Emerging infectious diseases necessitate that decision-makers make decisions in the face of uncertainty. This is particularly true for wildlife diseases because pathogens established in wild populations are extremely difficult to eradicate [14]. Providing decision-makers with tools, such as multiple risk assessments based on varying underlying assumptions (about the host or pathogen for example), allows them to weigh the strength of particular predictions when making decisions about management or surveillance for a pathogen, and to update their confidence in underlying assumptions as more information becomes available. Here, we present an alternative risk assessment that uses a mechanistic approach to predict suitable habitat for Bsal, and combine this with a formal assessment of the risk of Bsal introduction to US salamander populations. Specifically, we followed the World Organisation for Animal Health disease-risk assessment framework [15], where the total risk to native amphibian populations from Bsalin the USA is the combination of two events: (i) where introductions are likely to occur (i.e. introduction assessment) and (ii) if introduced, where suitable habitat and high species diversity overlap (i.e. consequences assessment). We identify areas of the USA projected to be at the highest relative risk for Bsal introduction, and areas where consequences are expected to be most severe. We also discuss the current uncertainties and research priorities needed to better understand and manage the threat to US amphibians from Bsal.
2. Methods
We used a hierarchical multi-criteria evaluation approach [16], similar to those used by the International Union for Conservation of Nature’s red list and NatureServe to estimate risk of species declines when there is large uncertainty [17,18], and recently applied to predicting the risk of the fungus Phytophthora ramorum (the causative agent of sudden oak death) invasion in Oregon [19]. We combined factors with weighted linear combination (WLC), because it allows for measures of uncertainty (i.e. partial fuzzy set memberships [16]) and trade-offs among risk factors. We chose this combination method because, currently, how factors interact and affect overall risk from Bsal is unknown (i.e. the rank-importance and factor weights are equal for factors in each hierarchical level). Non-normally distributed factors were log transformed and all factors were linearly scaled from 1 to 4 (the lowest to the highest relative contribution to total risk; similar to [17]; electronic supplementary material, table S1). One of our objectives was to describe spatial variation in relative risk across the USA; therefore, the value of each contributing factor was calculated for each US county in the contiguous 48 states using ArcGIS v. 10.1 and Python v. 2.7 (raw and scaled values in the electronic supplementary material, table S1).
2.1. Introduction assessment
International trade has been implicated as the primary factor in the global spread of Bsal [2,4,5]. We assumed a linear relationship between proximity to areas with a high volume of amphibian trade (both international shipments through imports and domestic activity through pet stores) and the potential for Bsal introduction. While this is a simplification of the invasion process, it is the only step of the process that currently has available data. Thus, we estimated the relative potential of a Bsal introduction event from two derived factors, imports and the pet trade, representing the relative quantity of trade in live amphibians combined with initial spread from potential introduction locations. The import factor was based on records of all live salamander imports from 1 January 2010 to 31 December 2014 through a Freedom of Information Act request from the US Fish and Wildlife Service Law Enforcement Management Information System (LEMIS), which compiles data on international trade passing through all ports of entry. The LEMIS import information does not include the location of final sales of live salamanders; therefore, to estimate the potential introductions from the pet trade we used the US Census Bureau’s County Business Patterns [20] number of establishments reported as pet or pet supply stores (NAICS 45391) as a proxy for commercial trade of salamanders within the USA. The number of establishments and gross sales from the US Census Survey of Businesses [21] were highly correlated (R2=0.84, p<0.001) but sales for approximately half of all counties were not available due to business privacy rules.
Even with highly rigorous surveillance efforts, the probability of detecting a single introduction event of a pathogen is low (e.g. [22]), so some spread from the initial importation location is expected before detection occurs. In other words, a particular county’s likelihood of introduction is a combination of both the number of pet stores and imports within that county and the number of pet stores and imports in neighbouring counties. To account for this combined risk, we first geolocated ports by their physical address and assigned each port the respective number of salamander imports from 2010 to 2014. Similarly, we assigned the number of pet establishments by county to the respective county centroid. We created three buffers of the port and pet establishment point layers to represent the potential spread of Bsal (based on the estimated spread for Bd, 26 km yr−1 [23]) 1, 5 and 10 years post introduction (26, 130 and 260 km), assigning each buffer the value of the buffered point. For each spread scenario, we summed the value of buffers that overlapped each county (‘intersect’, ArcGIS v. 10.1), creating six layers representing the three spread scenarios for ports and pet establishments. The three spread scenarios were then averaged to create an imports layer and a pet trade layer, where the potential for introduction declines as a function of distance from each type of introduction (figure 1; electronic supplementary material, table S1 and figure S1). The relative potential for introduction of Bsal was then determined by averaging the imports and pet trade layers.
Figure 1.
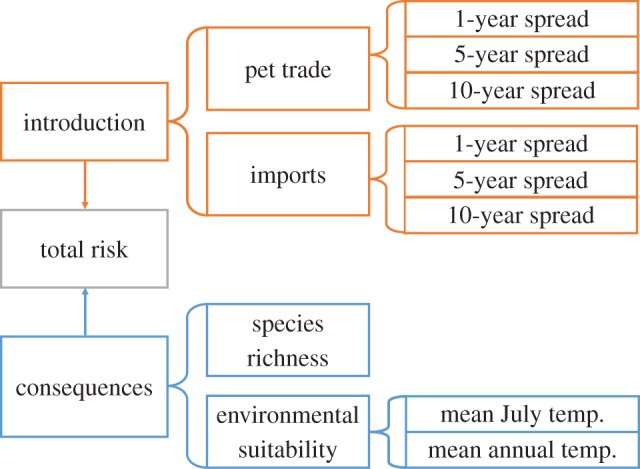
Conceptual diagram showing how each variable contributed to the estimated by-county introduction and consequences assessment for Bsal. Component variables were averaged and scaled for each hierarchical factor.
2.2. Consequences assessment
The consequences of Bsalintroduction were determined by two factors: environmental suitability and species richness (figure 1). Although many environmental requirements for Bsal survival and growth are unknown, physiological constraints (i.e. temperature of optimal growth and thermal maximum) for Bsal have been experimentally determined [1,13]. Mean annual air temperature and mean air temperature of the warmest month (July) were extracted by county from PRISM climate normals [24] using zonal statistics (spatial analyst toolbox in ArcGIS 10.1), which resulted in an average temperature value for all raster cells within each county. We scaled mean annual temperatures with the thermal optimum (15°C) as the highest score (4), representing ideal conditions for Bsal growth, and we scaled mean temperatures of the warmest month with greater than or equal to the thermal maximum of Bsal (25°C) as the lowest score (1), representing areas of the country that may have thermal refugia from Bsal infection. We then averaged these two temperature factors to derive environmental suitability (figure 1; electronic supplementary material, table S1).
Greater species richness may increase the number of available hosts, add highly susceptible species or add reservoir species, all of which may amplify disease transmission [25] (as seen for Bd in the USA [26]); therefore, species richness may be an important predictor of the consequences of Bsal introduction. To estimate species richness, we summarized the county-level occurrence of salamander species from a combination of NatureServe [27] and the USGS National Amphibian Atlas range maps [28]. We excluded two species from this list, Plethodon ainsworthi and the unisexual Jefferson salamander complex (Ambystoma sp.), because uncertainty exists in their classification as species [27]. We scaled species richness giving the county with the highest species richness the highest score (4); counties without salamander species were excluded from the risk assessment (N=116/3007, 4%; electronic supplementary material, tables S1 and S2). We calculated the potential for Bsal-caused declines (consequences assessment) by averaging environmental suitability and species richness. We averaged the introduction and consequences factors to estimate the total risk of Bsal by US county.
3. Results
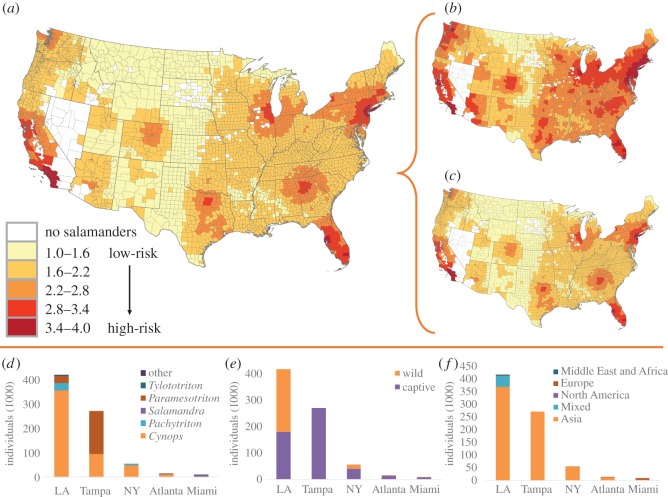
The USA imported at least 776 990 live salamanders from 1 January 2010 to 31 December 2014, through 14 ports of entry (155 398 yr−1±29 658 s.d.; electronic supplementary material, table S3). Seven ports contributed greater than 99% of all imports and had over 1000 total individuals imported: Los Angeles, CA (54%, 419 869), Tampa, FL (35%, 272 338), New York, NY (7%, 55 441), Atlanta, GA (2%, 13 310), Miami, FL (1%, 9370), San Francisco, CA (0.4%, 3243) and Chicago, IL (0.3%, 2178). Salamanders imported into the USA were predominantly reported to be captive bred (67%) and for commercial purposes (more than 99%). Cynops sp. (67%) and Paramesotriton sp. (27%), both suspected carriers ofBsal, were the two most common groups imported, followed by Pachytriton sp. (5%), Salamandra salamandra (1%; which accounted for the 1% of imports from Europe) and Tylototriton sp. (0.5%). Most shipments originated from Asia (93%), of which Hong Kong (70%) and China (27%) were the most commonly reported Asian origins (figure 2; electronic supplementary material, table S1 and figure S1).
Figure 2.
Heat maps of the USA showing the Bsal introduction assessment. The introduction assessment (a) is a combination of areas with high numbers of pet trade establishments (b) and high levels of imports (c). Salamander imports from the five most active ports (more than 99% of all imports) in 2010–2014 including by genera (d), by source (e) and by region of origin (f).
There are 1235 US counties (40%) with pet or pet supply establishments according to the US Census Bureau’s County Business Patterns (2012). The average number of pet-related establishments per county was 7, with seven counties having at least 100 establishments: Los Angeles County, CA (283), Maricopa County, AZ (138), San Diego County, CA (134), Cook County, IL (133), Orange County, CA (106), King County, WA (104) and New York, NY (100). The resulting potential of Bsal introduction was highest near the major ports that also had numerous pet stores, which included central and southern Florida, southern California and near New York City, NY (figure 2; electronic supplementary material, table S1 and figure S1).
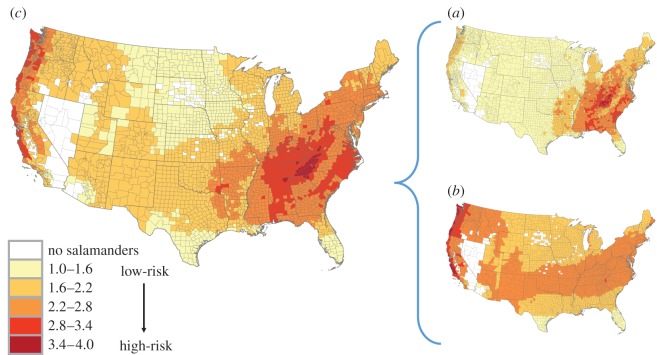
We identified the Pacific coast and Appalachian Mountains as the most likely—given an introduction—to have declines from Bsal based on environmental suitability and species richness. Environmental suitability indicated the southeast, southwest and Pacific regions of the USA as having mild climates well suited to Bsal growth (which are also suitable climates for salamanders, hence the concentration of species diversity). Species richness (range = 0–30) was the highest in the Appalachian Mountains and southeast USA (figure 3; electronic supplementary material, figure S2).
Figure 3.
Heat maps of the USA showing the consequences of Bsal introduction. The consequence assessment (c) is a combination of species richness (a) and environmental suitability (b).
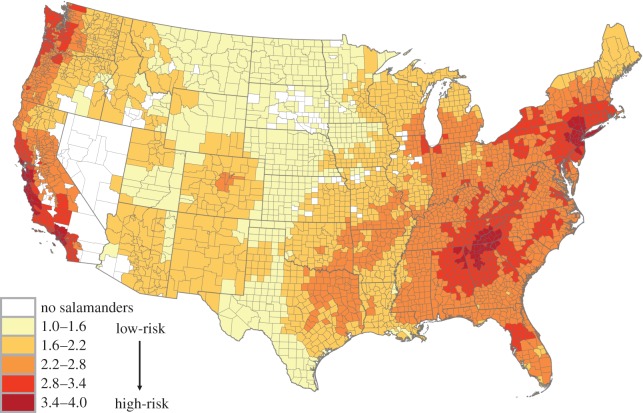
The total risk of Bsal to US salamanders, based on the introduction and consequences assessment, is elevated throughout the eastern USA. Risk is expected to be the highest for the Pacific coast, southern Appalachian Mountains and mid-Atlantic regions (figure 4).
Figure 4.
Heat map of the USA showing the total relative risk of Bsalto native US salamanders based on the introduction and consequences assessment.
4. Discussion
Given the large number of suspected Bsal carriers imported into the USA each year (Cynops spp. and Paramesotriton spp., more than 100 000 yr−1), Bsal is likely to be introduced if no additional risk mitigation steps are taken. Though precise estimates for the invasion process (proportion of imported individuals infected, frequency of release of captive individuals, and contact of released animals with native amphibians) do not exist for Bsal, the establishment of invasive amphibians common in US amphibian trade (e.g. American bullfrogs, African clawed frogs, Asian newts [29–31]) and the patterns of global Bd spread [32] suggest these processes are also likely for Bsal. Currently,Bsal infection is known to be lethal to two US salamander species (of seven tested thus far [2]). While this number is small, these species are common and wide ranging (electronic supplementary material, figure S4). We also expect that as additional testing is completed, a larger fraction of US species may be found to be susceptible to Bsal. Thus, given the high likelihood that Bsal will be introduced and the potentially severe consequences, we categorize the risk of Bsal to US salamanders as high.
Our risk assessment predicted a higher total risk of Bsal for the northeastern and midwestern USA compared with Yap et al. [11], predicting a much larger area of the USA could be at risk for Bsal establishment. This is partially explained by the methodological differences used to estimate the potential range of Bsal in the USA between the two assessments. Our assessment uses the experimentally determined thermal range of Bsal rather than the native Asian host distribution (which may or may not be susceptible to infection across the entire range) used by Yap et al. [11]. Indeed, correlative species distribution models are suspected to under predict invasive species ranges [12], and mechanistic species distribution models often predict larger range shifts [10]. We also employed a more inclusive combinatorial method (WLC) compared with Yap et al. [11] (product); thus, our risk assessment is less restrictive and allows for trade-offs between factors. For example, an area with a high introduction score (1) but low consequences score (0) would have a medium risk score in our assessment ((1+0)/2=1/2) but a low-risk score in Yap et al.’s [11] assessment (1×0=0). Finally, we included an introduction assessment, which was not included in Yap et al. [11].
We assumed that Bsal has not been previously established in the USA; thus we estimated the potential for introduction as a function of volume of trade through ports and number of pet stores. However, the amount of salamander sales most probably varies among pet establishments due to local variation in the popularity of amphibians as pets and specialization of pet stores. Similarly, a proportion of salamander sales are through Internet breeders or hobbyist fairs, and the final location of these sales will be underrepresented in our analyses (as it is based on the physical location of the seller as reported on their tax forms, not the buyer). Additional introduction pathways are possible, such as from agricultural stowaways or infection in an already existing captive population. These represent a smaller and more difficult risk to assess, but are an important caveat—reducing commercial imports will not completely eliminate the risk of Bsal. If Bsal is established in the USA, additional information on interstate trade in salamanders, such as among-county movement of salamanders sold as fish bait, through Internet sales or hobbyists, would be useful to mitigate the impacts of human-mediated pathogen spread. The rate of spread of Bsal in wild populations is also uncertain and is expected to affect the estimate of spread from potential introduction locations. The reported rates of Bd spread have high variation (range from 1.1 to 282 km yr−1 [22]). In this assessment, we chose the estimate of Bd spread (26 km yr−1) that was associated with the most complete surveillance effort (the average rate of spread through Central America [22]). However, we acknowledge that the complexity of the terrain, typical movement of local amphibian communities and other factors may affect the realized invasion dynamics of Bsal.
When considering the impacts of an emerging wildlife disease, knowing which species may be susceptible, and thus experience population level declines, is critical. While laboratory exposure trials have shown that at least two US species, Taricha torosa and Notophthalmus viridescens, experience lethal responses to Bsal [2] (electronic supplementary material, figure S4), the responses of the other 189 US species to Bsal exposure is unknown. Similarly, we based the environmental suitability factor on the experimentally derived thermal range of Bsal, yet much is unknown about environmental characteristics in the field that will affect Bsal infection and transmission, such as amphibian behavioural thermoregulation and microhabitat use, and the persistence of the pathogen in various environmental conditions. For example, Bd was capable of infecting amphibians along a larger temperature profile than originally predicted using correlative environmental suitability approaches [32,33]. However, some of Bd’s wide range may be due to differential temperature profiles of diverse Bd strains [34,35]; an unexplored possibility for Bsal.
Control of wildlife diseases is more challenging and expensive than prevention [14] and expedient action is required to have the best opportunity to reduce the risk of Bsal invasion. There are few known viable treatment or management options for responding to the introduction of Bsal [36,37], and despite the fact that Bd has been identified and described for more than 15 years [38], there are currently no effective treatments available for wild populations. Strategies focused on prevention or reduction of introduction events remain the best control option for emerging diseases. Currently, Bd and ranaviruses are categorized as reportable diseases by the World Organisation for Animal Health and import restrictions and veterinary certifications can be required [15]. Similar import restrictions and health certificate requirements for Bsal would reduce the potential for introduction into the USA. Additional reductions in the potential for introduction are possible with the implementation of industry best-practices for treatment of waste, voluntary quarantine and treatment of imported amphibians (e.g. at least 10 days above 25°C [13]). Public awareness campaigns in partnership with private businesses and non-profit conservation organizations that highlight the potential for Bsal invasion and provide guidelines to prevent spread from captive populations may also be effective for reducing the risk of Bsal to wild populations.
5. Conclusion
Based on the information available for our assessment, the highest total risk of Bsalfor wild salamander populations in the US occurs along the Pacific coast, southern Appalachian Mountains and mid-Atlantic regions. This risk assessment provides separate and combined estimates for two different types of risk, introduction and consequences, which can be used independently or together to inform policy decisions. Similarly, this study can be used to both assist in the development of a surveillance strategy for Bsal and direct applied research to areas with high uncertainty where additional information could make the largest impact on mitigation and prevention. Critical knowledge gaps which may refine future assessments include identifying the prevalence of Bsal in imported animals through sampling shipments upon arrival at US ports, determining the susceptibility of US salamander species through exposure experiments, and further refining the environmental conditions necessary for establishment of Bsal.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Craig Stephen and the Canadian Wildlife Health Cooperative for discussions about Bsal. The manuscript was significantly improved by comments from Dan Grear, Susan Jewell, Jonathan Kolby and four anonymous reviewers. We thank the US Fish and Wildlife Service for providing LEMIS wildlife trade data records. K.L.D.R. performed this work with the US Geological Survey while employed as a postdoctoral researcher at the University of Wisconsin. The use of trade or product names does not imply endorsement by the US Government.
Data accessibility
Datasets from this article are available in the electronic supplementary material.
Authors' contributions
K.L.D.R. collected data, performed spatial analyses and drafted the manuscript. K.L.D.R., R.E.R., M.J.A., E.H.C.G. and C.L.W. conceived and designed the study and helped draft the manuscript. All authors gave final permission for publication.
Competing interests
We declare we have no competing interests.
Funding
The Bsal risk assessment was funded by the US Geological Survey’s National Wildlife Health Center and completed in cooperation with the US Geological Survey’s Amphibian Research and Monitoring Initiative (product number 530).
References
- 1.Martel A. et al. 2013. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc. Natl Acad. Sci. USA 110, 15 325–15 329. (doi:10.1073/pnas.1307356110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martel A. et al. 2014. Recent introduction of a chytrid fungus endangers Western palearctic salamanders. Science 346, 630–631. (doi:10.1126/science.1258268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spitzen-van der Sluijs A, Spikmans F, Bosman W, de Zeeuw M, van der Meij T, Goverse E, Kik M, Pasmans F, Martel A. 2013. Rapid enigmatic decline drives the fire salamander (Salamandra salamandra) to the edge of extinction in the Netherlands. Amphibia-Reptilia 34, 233–239. (doi:10.1163/15685381-00002891) [Google Scholar]
- 4.Cunningham AA. et al. 2015. Emerging disease in UK amphibians. Vet. Rec. 176, 468 (doi:10.1136/vr.h2264) [DOI] [PubMed] [Google Scholar]
- 5.Sabino-Pinto J. et al. 2015. First detection of the emerging fungal pathogen Batrachochytrium salamandrivorans in Germany. Amphibia-Reptilia 36, 411–416. (doi:10.1163/15685381-00003008) [Google Scholar]
- 6.Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, Hines HB, Kenyon N. 2007. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 4, 125–134. (doi:10.1007/s10393-007-0093-5) [Google Scholar]
- 7.Kilpatrick AM, Briggs CJ, Daszak P. 2010. The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends Ecol. Evol. 25, 109–118. (doi:10.1016/j.tree.2009.07.011) [DOI] [PubMed] [Google Scholar]
- 8.Muletz C, Caruso NM, Fleischer RC, McDiarmid RW, Lips KR. 2014. Unexpected rarity of the pathogen Batrachochytrium dendrobatidis in Appalachian Plethodon salamanders: 1957–2011. PLoS ONE 9, e103728 (doi:10.1371/journal.pone.0103728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bales EK, Hyman OJ, Loudon AH, Harris RN, Lipps G, Chapman E, Roblee K, Kleopfer JD, Terrell KA. 2015. Pathogenic chytrid fungus Batrachochytrium dendrobatidis, but not B. salamandrivorans, detected on eastern hellbenders. PLoS ONE 10, e0116405 (doi:10.1371/journal.pone.0116405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckley LB, Urban MC, Angilletta MJ, Crozier LG, Rissler LJ, Sears MW. 2010. Can mechanism inform species’ distribution models? Ecol. Lett. 13, 1041–1054. (doi:10.1111/j.1461-0248.2010.01479.x) [DOI] [PubMed] [Google Scholar]
- 11.Yap BTA, Koo MS, Ambrose RF, Wake DB, Vredenburg VT. 2015. Averting a North American biodiversity crisis. Science 349, 481–482. (doi:10.1126/science.aab1052) [DOI] [PubMed] [Google Scholar]
- 12.Tingley R, Vallinoto M, Sequeira F, Kearney MR. 2014. Realized niche shift during a global biological invasion. Proc. Natl Acad. Sci. USA 111, 10 233–10 238. (doi:10.1073/pnas.1405766111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blooi M, Martel A, Haesebrouck F, Vercammen F, Bonte D, Pasmans F. 2015. Treatment of urodelans based on temperature dependent infection dynamics of Batrachochytrium salamandrivorans. Sci. Rep. 5, 8037 (doi:10.1038/srep08037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karesh WB, Cook RA, Bennett EL, Newcomb J. 2005. Wildlife trade and global disease emergence. Emerg. Infect. Dis. 11, 1000–1002. (doi:10.3201/eid1107.020194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Organisation for Animal Health (OiE). 2014. Aquatic animal health code. See http://www.oie.int/international-standard-setting/aquatic-code/access-online/ (accessed 4 May 2015).
- 16.Jiang H, Eastman JR. 2000. Application of fuzzy measures in multi-criteria evaluation in GIS. Int. J. Geogr. Inf. 14, 173–184. (doi:10.1080/136588100240903) [Google Scholar]
- 17.Faber-Langendoen D. et al. 2012. NatureServe conservation status assessments: methodology for assigning ranks. NatureServe, Arlington, VA. See http://www.natureserve.org/conservation-tools/conservation-rank-calculator.
- 18.IUCN. 2001. IUCN Red List Categories and Criteria: Version 3.1. IUCN Species Survival Commission. Cambridge, UK: IUCN; (https://www.iucn.org/about/union/secretariat/offices/iucnmed/iucn_med_programme/species/red_list/) [Google Scholar]
- 19.Václavík T, Kanaskie A, Hansen EM, Ohmann JL, Meentemeyer RK. 2010. Predicting potential and actual distribution of sudden oak death in Oregon: prioritizing landscape contexts for early detection and eradication of disease outbreaks. For. Ecol. Manage. 260, 1026–1035. (doi:10.1016/j.foreco.2010.06.026) [Google Scholar]
- 20.US Census Bureau. 2012. County business statistics. Washington, DC: Industry Statistics Portal; (https://www.census.gov/econ/isp/) (accessed 10 March 2015). [Google Scholar]
- 21.US Census Bureau. 2007. Survey of businesses. Washington, DC: Industry Statistics Portal; (https://www.census.gov/econ/isp/) (accessed 10 March 2015). [Google Scholar]
- 22.Royle JA. 2006. Site occupancy models with heterogeneous detection probabilities. Biometrics 62, 97–102. (doi:10.1111/j.1541-0420.2005.00439.x) [DOI] [PubMed] [Google Scholar]
- 23.Lips KR, Diffendorfer J, Mendelson JR, Sears MW. 2008. Riding the wave: reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 6, e72 (doi:10.1371/journal.pbio.0060072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.PRISM Climate Group. 2015. 30-year normals, annual and monthly mean temperature, 800 m resolution. Corvallis, OR: Oregon State University; (http://prism.oregonstate.edu) (accessed 23 February 2015). [Google Scholar]
- 25.Keesing F, Holt RD, Ostfeld RS. 2006. Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498. (doi:10.1111/j.1461-0248.2006.00885.x) [DOI] [PubMed] [Google Scholar]
- 26.Peterson AC, McKenzie VJ. 2014. Investigating differences across host species and scales to explain the distribution of the amphibian pathogen Batrachochytrium dendrobatidis. PLoS ONE 9, e107441 (doi:10.1371/journal.pone.0107441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natureserve. 2015. NatureServe Explorer: an online encyclopedia of life. Version 7.0. Arlington, VA: NatureServe; (http://explorer.natureserve.org) (accessed 12 February 2015). [Google Scholar]
- 28.USGS National Amphibian Atlas. 2014. Salamanders (Caudata). Version Number 3.0. Laurel, MD: USGS Patuxent Wildlife Research Center; (www.pwrc.usgs.gov/naawww.pwrc.usgs.gov/naawww.pwrc.usgs.gov/naa) (accessed 1 February 2015). [Google Scholar]
- 29.Ficetola GF, Thuiller W, Miaud C. 2007. Prediction and validation of the potential global distribution of a problematic alien invasive species—the American bullfrog. Divers. Distrib. 13, 476–485. (doi:10.1111/j.1472-4642.2007.00377.x) [Google Scholar]
- 30.Measey GJ, Rödder D, Green SL, Kobayashi R, Lillo F, Lobos G, Rebelo R, Thirion J-M. 2012. Ongoing invasions of the African clawed frog, Xenopus laevis: a global review. Biol. Invasions 14, 2255–2270. (doi:10.1007/s10530-012-0227-8) [Google Scholar]
- 31.EDDMapS. 2015. Early detection & distribution mapping system. Athens, GA: The University of Georgia—Center for Invasive Species and Ecosystem Health; (http://www.eddmaps.org/) (accessed 21 May 2015). [Google Scholar]
- 32.Fisher MC, Garner TWJ, Walker SF. 2009. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu. Rev. Microbiol. 63, 291–310. (doi:10.1146/annurev.micro.091208.073435) [DOI] [PubMed] [Google Scholar]
- 33.Ron SR. 2005. Predicting the distribution of the amphibian pathogen Batrachochytrium dendrobatidis in the New World. Biotropica 37, 209–221. (doi:10.1111/j.1744-7429.2005.00028.x) [Google Scholar]
- 34.Voyles J, Johnson LR, Briggs CJ, Cashins SD, Alford RA, Berger L, Skerratt LF, Speare R, Rosenblum EB. 2012. Temperature alters reproductive life history patterns in Batrachochytrium dendrobatidis, a lethal pathogen associated with the global loss of amphibians. Ecol. Evol. 2, 2241–2249. (doi:10.1002/ece3.334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevenson LA, Alford RA, Bell SC, Roznik EA, Berger L, Pike DA. 2013. Variation in thermal performance of a widespread pathogen, the amphibian chytrid fungus Batrachochytrium dendrobatidis. PLoS ONE 8, e73830 (doi:10.1371/journal.pone.0073830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skerratt LF, Garner TWJ, Hyatt AD. 2009. Determining causality and controlling disease is based on collaborative research involving multidisciplinary approaches. Ecohealth 6, 331–334. (doi:10.1007/s10393-010-0292-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodhams DC. et al. 2014. Interacting symbionts and immunity in the amphibian skin mucosome predict disease risk and probiotic effectiveness. PLoS ONE 9, e96375 (doi:10.1371/journal.pone.0096375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longcore JE, Pessier AP, Nichols DK. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91, 219–227. (doi:10.2307/3761366) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets from this article are available in the electronic supplementary material.