Abstract
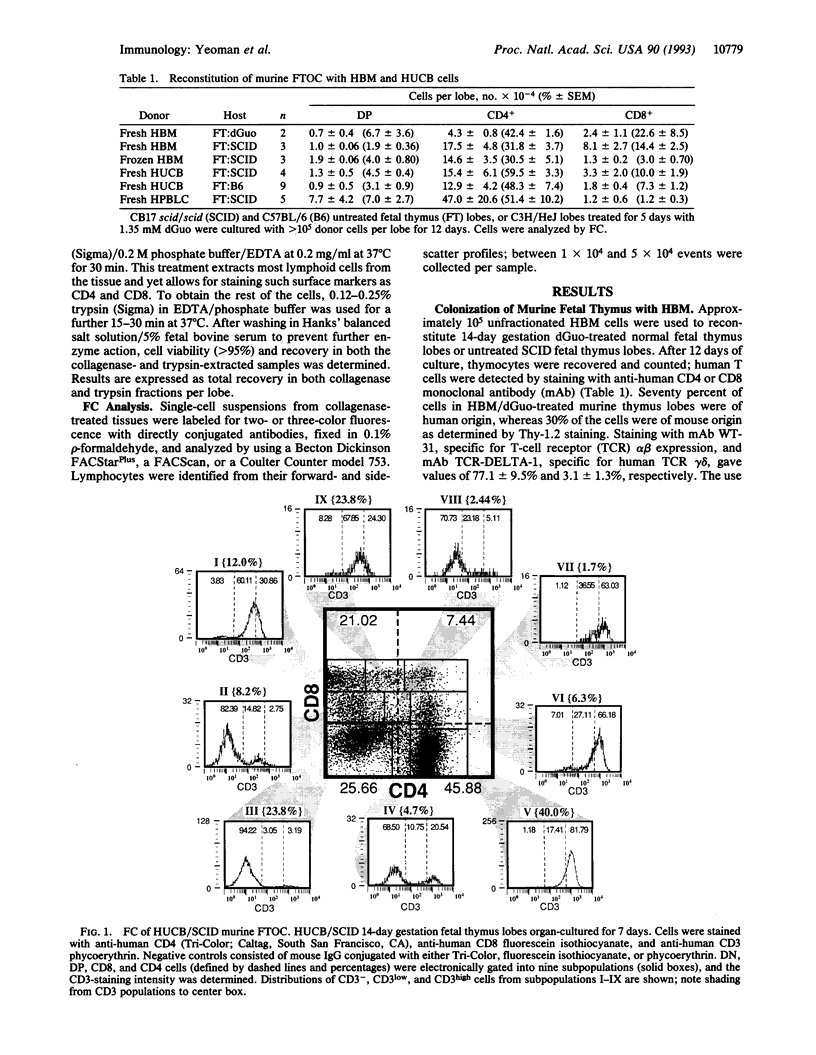
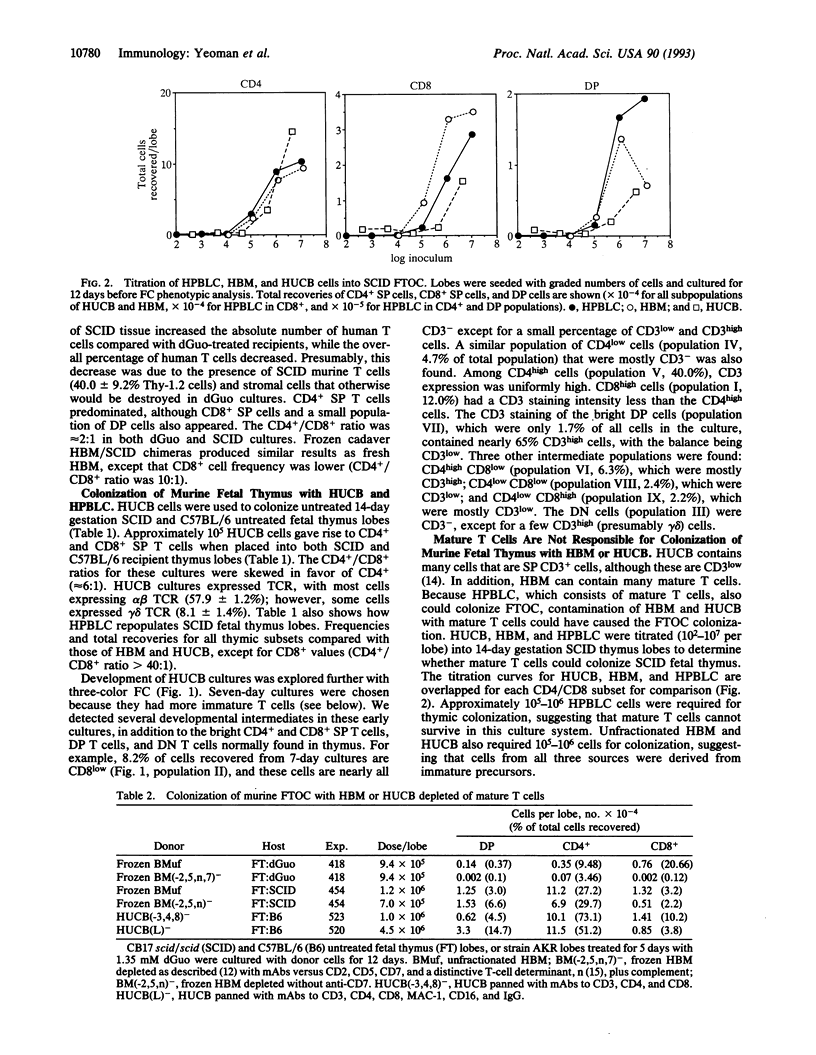
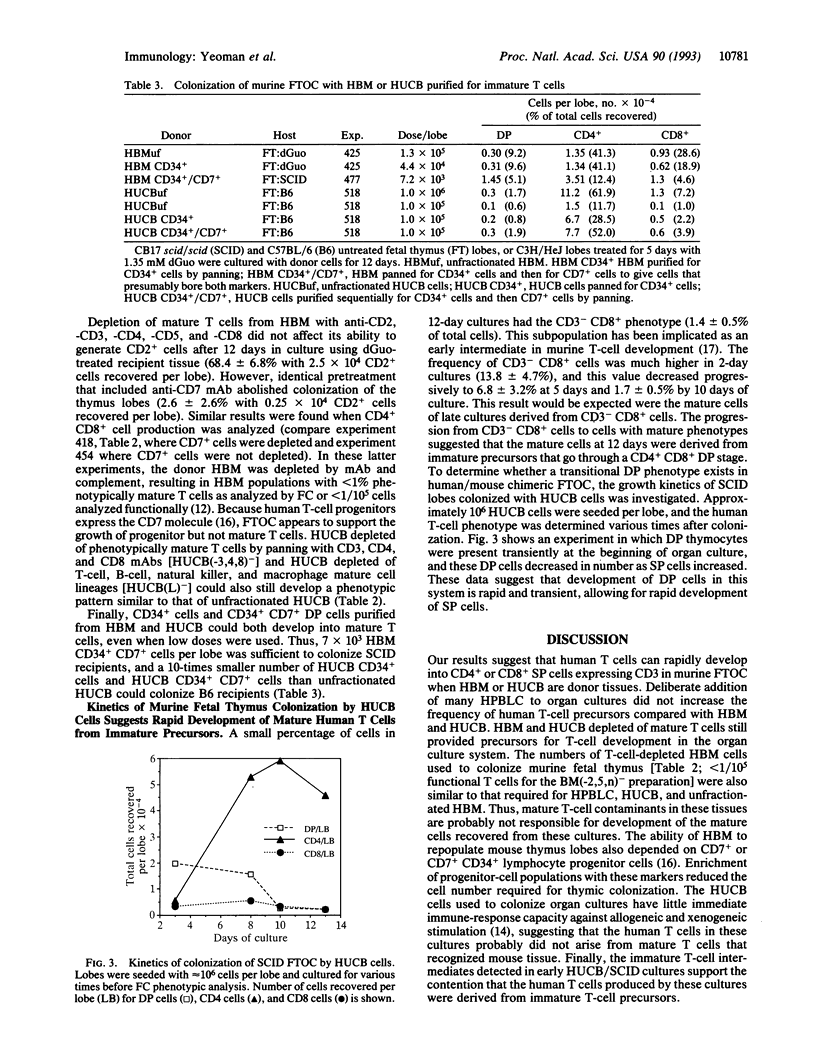
Murine fetal thymus lobes isolated from both normal and scid/scid mice can be colonized by donor cells from either human bone marrow or human umbilical cord blood in vitro. Subsequent organ culture results in a transient production of a few CD4+ CD8+ (double-positive) cells and then the accumulation of CD4+ or CD8+ (single-positive) T cells. A significant number of immature T-cell intermediates (e.g., CD8low, CD3-/low cells) were present in early organ cultures, suggesting that these were progenitors of the mature CD3+/high single-positive T cells that dominated late cultures. Depletion of mature T cells from the donor-cell populations did not affect their ability to colonize thymus lobes. However, colonization depended on the presence of CD7+ progenitor T cells. Limiting dilution experiments using mature T-cell populations (human peripheral blood leukocytes, human bone marrow cells, and human umbilical cord blood cells) suggested that thymic organ culture supports the growth of progenitor T cells but does not support the growth of mature human T cells. Each of these donor populations produced single-positive populations with different CD4/CD8 ratios, suggesting that precursor cells from different sources differ qualitatively in their capacity to differentiate into T cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B. Developmental regulation of the intrathymic T cell precursor population. J Immunol. 1991 Mar 1;146(5):1387–1393. [PubMed] [Google Scholar]
- Andrews R. G., Singer J. W., Bernstein I. D. Precursors of colony-forming cells in humans can be distinguished from colony-forming cells by expression of the CD33 and CD34 antigens and light scatter properties. J Exp Med. 1989 May 1;169(5):1721–1731. doi: 10.1084/jem.169.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardavin C., Wu L., Li C. L., Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993 Apr 22;362(6422):761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Douglas G. W., Hangoc G., Cooper S., Bard J., English D., Arny M., Thomas L., Boyse E. A. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R. Differentiation potential of 14-day fetal mouse thymocytes in organ culture. Analysis of CD4/CD8-defined single-positive and double-negative cells. J Immunol. 1988 Jul 15;141(2):355–362. [PubMed] [Google Scholar]
- DeLuca D. Ia-positive nonlymphoid cells and T cell development in murine fetal thymus organ cultures: monoclonal anti-Ia antibodies inhibit the development of T cells. J Immunol. 1986 Jan;136(2):430–439. [PubMed] [Google Scholar]
- DeLuca D., Mandel T. E., Luckenbach G. A., Kennedy M. M. Tolerance induction by fusion of fetal thymus lobes in organ culture. J Immunol. 1980 Apr;124(4):1821–1829. [PubMed] [Google Scholar]
- DeLuca D., Mizel S. B. I-A-positive nonlymphoid cells and T cell development in murine fetal thymus organ cultures: interleukin 1 circumvents the block in T cell differentiation induced by monoclonal anti-I-A antibodies. J Immunol. 1986 Sep 1;137(5):1435–1441. [PubMed] [Google Scholar]
- Fisher A. G., Larsson L., Goff L. K., Restall D. E., Happerfield L., Merkenschlager M. Human thymocyte development in mouse organ cultures. Int Immunol. 1990;2(6):571–578. doi: 10.1093/intimm/2.6.571. [DOI] [PubMed] [Google Scholar]
- Fung-Leung W. P., Schilham M. W., Rahemtulla A., Kündig T. M., Vollenweider M., Potter J., van Ewijk W., Mak T. W. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991 May 3;65(3):443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- Harris D. T., Schumacher M. J., Locascio J., Besencon F. J., Olson G. B., DeLuca D., Shenker L., Bard J., Boyse E. A. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10006–10010. doi: 10.1073/pnas.89.21.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Denning S. M., Le P. T., Singer K. H. Human intrathymic T cell differentiation. Semin Immunol. 1990 Jan;2(1):67–77. [PubMed] [Google Scholar]
- Jenkinson E. J., Franchi L. L., Kingston R., Owen J. J. Effect of deoxyguanosine on lymphopoiesis in the developing thymus rudiment in vitro: application in the production of chimeric thymus rudiments. Eur J Immunol. 1982 Jul;12(7):583–587. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- Lucas P. J., Quinones R. R., Moses R. D., Nakamura H., Gress R. E. Alternative donor sources in HLA-mismatched marrow transplantation: T cell depletion of surgically resected cadaveric marrow. Bone Marrow Transplant. 1988 May;3(3):211–220. [PubMed] [Google Scholar]
- Mandel T. E., Kennedy M. M. The differentiation of murine thymocytes in vivo and in vitro. Immunology. 1978 Aug;35(2):317–331. [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager M., Fisher A. G. Human postnatal thymocytes generate phenotypically immature CD3dim, CD5dim, CD1abright progeny in organ culture. J Immunol. 1992 Feb 15;148(4):1012–1015. [PubMed] [Google Scholar]
- Ogimoto M., Yoshikai Y., Matsuzaki G., Matsumoto K., Kishihara K., Nomoto K. Expression of T cell receptor V gamma 5 in the adult thymus of irradiated mice after transplantation with fetal liver cells. Eur J Immunol. 1990 Sep;20(9):1965–1970. doi: 10.1002/eji.1830200914. [DOI] [PubMed] [Google Scholar]
- Petrie H. T., Hugo P., Scollay R., Shortman K. Lineage relationships and developmental kinetics of immature thymocytes: CD3, CD4, and CD8 acquisition in vivo and in vitro. J Exp Med. 1990 Dec 1;172(6):1583–1588. doi: 10.1084/jem.172.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones R. R., Lunney J., Gress R. E. An anti-human-T-cell monoclonal antibody with specificity for a novel determinant. Transplantation. 1988 Jul;46(1):143–150. doi: 10.1097/00007890-198807000-00026. [DOI] [PubMed] [Google Scholar]
- Riggs J. E., Stowers R. S., Mosier D. E. Adoptive transfer of neonatal T lymphocytes rescues immunoglobulin production in mice with severe combined immune deficiency. J Exp Med. 1991 Jan 1;173(1):265–268. doi: 10.1084/jem.173.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]



