Abstract
Preeclampsia is a pregnancy-specific disorder of new-onset hypertension. Unfortunately, the most effective treatment is early delivery of the fetus and placenta. Placental ischemia appears central to the pathogenesis of preeclampsia as placental ischemia/hypoxia induced in animals by reduced uterine perfusion pressure (RUPP) or in humans stimulates release of hypertensive placental factors into the maternal circulation. The anti-angiogenic factor soluble fms-like tyrosine kinase-1 (sFlt-1), which antagonizes and reduces bioavailable vascular endothelial growth factor (VEGF) and placental growth factor (PlGF), is elevated in RUPP rats and preeclampsia. Although PlGF and VEGF are both natural ligands for sFlt-1, VEGF also has high affinity to VEGFR2 (Flk-1) causing side effects like edema. PlGF is specific for sFlt-1. We tested the hypothesis that PlGF treatment reduces placental ischemia-induced hypertension by antagonizing sFlt-1 without adverse consequences to the mother or fetus. On gestational day 14, rats were randomized to four groups: normal pregnant (NP) or RUPP ± infusion of rhPlGF (180 μg/kg/day; AG31, a purified, recombinant human form of PlGF) for 5 days via intraperitoneal osmotic minipumps. On day 19, mean arterial blood pressure and plasma sFlt-1 were higher and glomerular filtration rate lower in RUPP than NP rats. Infusion of rhPlGF abolished these changes seen with RUPP along with reducing oxidative stress. These data indicate that the increased sFlt-1 and reduced PlGF resulting from placental ischemia contribute to maternal hypertension. Our novel finding that rhPlGF abolishes placental ischemia-induced hypertension, without major adverse consequences, suggests a strong therapeutic potential for this growth factor in preeclampsia.
Keywords: blood pressure, preeclampsia, pregnancy, rat, RUPP
INTRODUCTION
Preeclampsia is a dangerous disorder of pregnancy characterized by new-set hypertension on or after the twentieth week of gestation accompanied by dysfunction of cardiovascular, cerebral, visual or renal systems. Preeclampsia is a leading cause of maternal and fetal morbidity and mortality. This hypertensive disorder of pregnancy is the second leading cause of pregnancy-induced maternal death globally behind bleeding complications1. Unfortunately, the most effective treatment currently for preeclampsia is early delivery of the fetus and the ischemic placenta. Therefore, identifying novel therapeutic targets and agents against this maternal disorder is the subject of intensive experimental investigation.
Placental ischemia is strongly linked to the development of hypertension during pregnancy. The clearest evidence for this comes from experiments in non-human primates, rats and mice showing that experimentally-induced placental ischemia elicits maternal hypertension and proteinuria2–4. Reducing uterine blood flow by ~40% in the reduced uterine perfusion pressure (RUPP) rat model produces pronounced hypertension and reductions in glomerular filtration rate (GFR)3, 5. We and others have shown that placental ischemia-driven angiogenic imbalance is causative in this hypertensive response. RUPP rats and preeclamptic women have increased placental and circulating soluble fms-like tyrosine kinase-1 (sFlt-1), which quenches circulating free vascular endothelial growth factor (VEGF) levels and placental growth factor (PlGF) 6–8. Supplementing RUPP rats with recombinant VEGF abolished their hypertension9.
Patient safety is a critical factor in developing a therapy, particularly for the high-risk preeclamptic population. Although placental growth factor (PlGF) and VEGF are both natural ligands for sFlt-1, VEGF also binds with high-affinity to fetal liver kinase 1 receptor (Flk1, VEGFR2), and therefore may lead to side-effects related to excess Flk1 signaling. PlGF is uniquely specific for sFlt-1. Another advantage of PlGF is that, in contrast to small molecule therapies, PlGF circulates as a dimer (molecular weight of 45kDa) and is significantly above the threshold size for proteins that can cross the placental barrier (<5kDa). PlGF concentrations are significantly lower in patients with preeclampsia10. Therefore, we tested the hypothesis that administration of PlGF would prevent the effects of placental ischemia on blood pressure and renal hemodynamics in RUPP rats and reduce circulating sFlt-1 and oxidative stress found in this model of preeclampsia.
METHODS
Studies in Experimental Animals
All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals with all animal-use protocols approved by The University of Mississippi Medical Center’s Institutional Animal Care and Use Committee. Timed-pregnant Sprague Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN, USA; barrier # 202A). Gestational day 0 was defined as the presence of vaginal plug. All measurements were collected at day 19.
Reduced uterine perfusion pressure (RUPP) and PlGF infusion
On gestational day 14, the RUPP procedure was performed as previously detailed or rats remained in the normal pregnant (NP) group3. In a subset of NP or RUPP rats, Alzet osmotic minipumps were loaded with 180 μg/kg/day rhPlGF and placed in the intraperitoneal cavity on GD 14 and infused for 5 days until day 19. Dams that had total reabsorptions were excluded from the study.
Mean arterial blood pressure
On gestational day 19, mean arterial blood pressure and heart rates were collected as described11–14. We performed blood pressure measurements in a separate set of 6 normal pregnant rats infused with DPBS vehicle. Blood pressure was similar between NP+vehicle pump and NP without pump (data not shown), the latter which was included in the comparisons for hemodynamic and biochemical measurements. RUPP untreated rats were not infused with vehicle, but they did have a similar incision site as to those RUPP rats treated with PlGF; this is because the intraperitoneal osmotic minipumps were placed in the abdominal cavity via the incision already made for the RUPP surgery.
At gestational day 19, blood was collected for isolation of plasma and the sum of total and average fetal and placental weights were calculated per rat then averaged per group. Total viable or reabsorbed fetuses were noted. Percent fetal absorption = (number of absorbed fetuses/total number of numbers)*100. Maternal body weight = mother weight - (fetal and placental weight).
Glomerular filtration rate
A subset of the rats used for the blood pressure measurements were included for the GFR measurements. We did not include the normal pregnant + rhPlGF group in the GFR measurements as there was no effect of rhPlGF on blood pressure in this group. We utilized a non-invasive-clearance measurement for transcutaneously assessing glomerular filtration rate (GFR), as developed by Mannheim Pharma & Diagnostics GmbH (Mannheim, Germany)15, 16. On gestational day 18, a jugular catheter was implanted. On day 19, rats were briefly anesthetized with 3–5% isoflurane to: remove hair from the nap of the neck with depilatory cream; place the USB device and attached battery on the skin using doubled-sided adhesive tape that was immobilized and protected using an infusion jacket (Kent Scientific, Torrington, CT, USA); and the jugular catheter extended. The rats were allowed to recover from anesthesia for 15–20 minutes followed by a bolus dose of 3 mg/100 g body weight FITC-Sinistrin in 0.2 mL sterile irrigation saline (Baxter Healthcare Corporation, Deerfield, IL, USA) via jugular catheter while rat moving freely in cage. Data was collected, in units of mL/min/100 g body weight, from the USB device per manufacturer’s instructions. This value for each rat was multiplied by its absolute body weight to present data as absolute GFR as mL/min.
Drug
The recombinant human (rh) PlGF protein that was used in our infusion studies was generated by Aggamin Biologics (New York, NY, USA). Briefly, cDNA encoding human PlGF131 (GenBank Accession number P49763-2) was cloned into an expression vector and electroporated into Chinese Hamster Ovary (CHO) cells. The rhPlGF-1 protein was purified to >90% purity via column chromatography and formulated in DPBS pH 7.4.
Quantification of circulating rhPlGF and sFlt-1 levels
Circulating levels of rhPlGF and sFlt-1 in plasma samples were quantitated using an enzyme-linked immunosorbent assay detecting human PlGF or mouse Flt-1, respectively (R&D Systems, Minneapolis, MN, USA) as described11. Mouse Flt1 kit measures free sFlt-1 as described elsewhere17
Marker of oxidative stress
Circulating levels of 8-iso-prostanes were quantified in plasma using ELISA kit (Cayman Chemicals, Ann Arbor, MI, USA).
Statistics
Data were graphed and statistics performed using GraphPad Prism version 6.0. Data are graph as mean±standard error of the mean (SEM). For all data, a one-way analysis of variance (ANOVA) was conducted followed by a Holm-Sidak multiple comparisons test. The exception was data in Figure 1 where a nonparametric one-way ANOVA with an experimental design not assuming Gaussian distribution was conducted followed by a Dunn’s posthoc test. Statistical significant differences were defined as P<0.05.
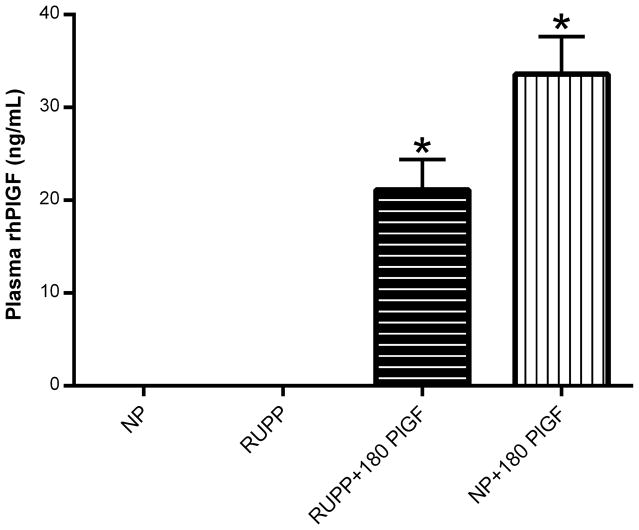
Figure 1.
Detection of rhPlGF levels in normal pregnant (NP; N=13), reduced uterine perfusion pressure (RUPP; N=18), RUPP + 180 μg/kg/day rhPlGF (N=13), and NP + 180 μg/kg/day rhPlGF rats (N=6). *P<0.05 versus corresponding untreated control pregnant rats.
RESULTS
The in vivo study demonstrated that, at gestational day 19, circulating levels of rhPlGF were readily detectable but not significantly different following five days of infusion at the dose of 180 μg/kg/day in NP and RUPP rats (Figure 1). This recombinant human protein was not detected in the non-infused NP and RUPP controls.
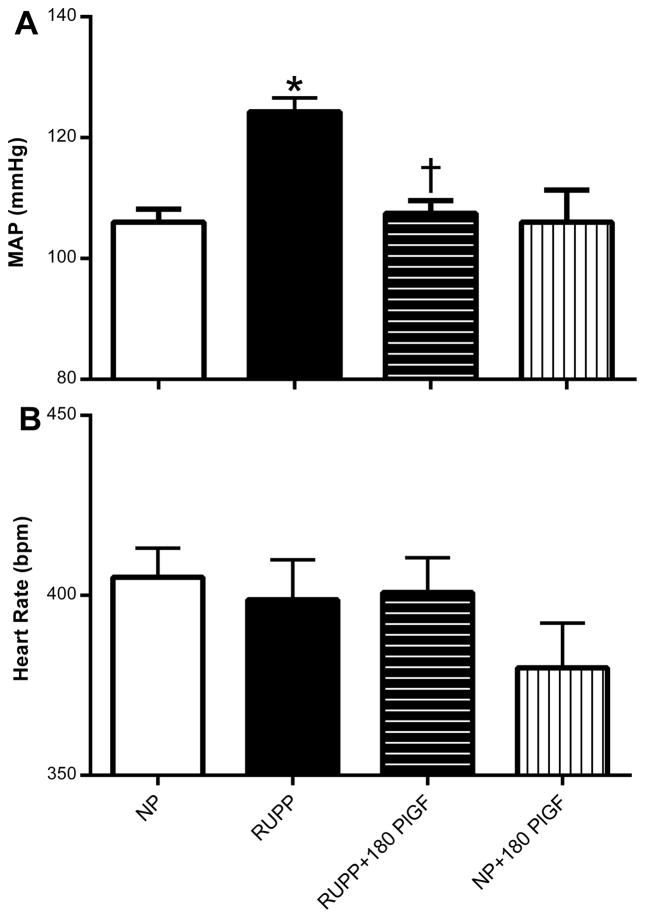
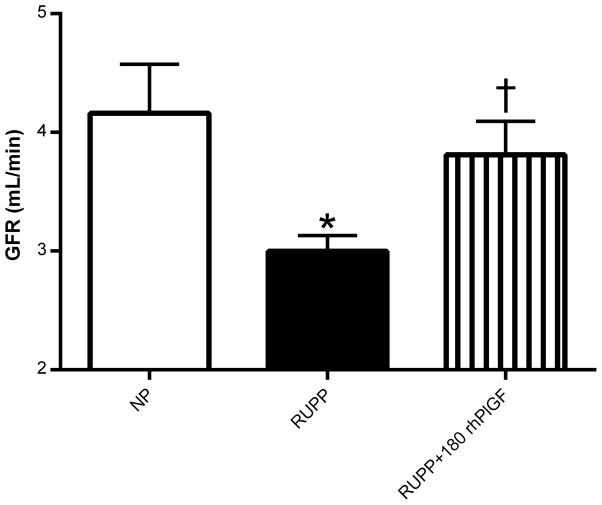
Mean arterial blood pressure was increased in response to RUPP compared to NP controls by gestational day 19 (Figure 2A). Administration of 180 μg/kg/day rhPlGF abolished the development of this placental ischemia-induced hypertension. However, this dose of rhPlGF had no effect on blood pressure levels in the NP group. Studies were also conducted to examine the effects of 90 μg/kg/day rhPlGF on blood pressure regulation in RUPP rats but did not alter the hypertensive response to RUPP (data not shown). Heart rates were similar between the NP, RUPP, RUPP + 180 μg/kg/day rhPlGF and NP + 180 μg/kg/day rhPlGF groups (Figure 2B). Figure 3 illustrates that the reduced GFR induced during placental ischemia in the RUPP rats was significantly abrogated by rhPlGF treatment.
Figure 2.
A: Mean arterial blood pressure (MAP) and B: heart rate in beats per minute (bpm) in normal pregnant (NP; N=14), reduced uterine perfusion pressure (RUPP; N=18), RUPP + 180 μg/kg/day rhPlGF (N=11), and NP + 180 μg/kg/day rhPlGF rats (N=6). *P<0.05 versus corresponding untreated control pregnant rats. *P<0.05 versus untreated NP rats; †P<0.05 versus untreated RUPP rats.
Figure 3.
Glomerular filtration rate (GFR) in normal pregnant (NP; N=9), reduced uterine perfusion pressure (RUPP; N=10), and RUPP + 180 μg/kg/day rhPlGF rats (N=6). *P<0.05 vs. NP rats; †P<0.05 versus untreated RUPP rats.
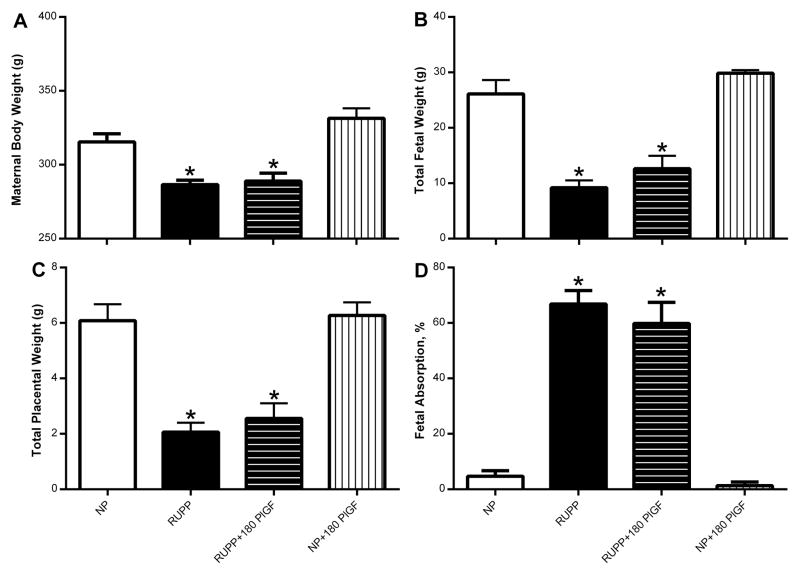
Maternal body weights were significantly lower in RUPP versus NP rats and these weights were not altered by rhPlGF administration (Figure 4A). This reduced maternal body weight in RUPP was largely due to the reductions in total fetal (Figure 4B) and placental weight (Figure 4C), and rhPlGF had no effect on these weights either. Average pup weights were reduced (P<0.05) in RUPP (2.08±0.06 g) vs. NP (2.37±0.06 g) but was not affected by rhPlGF treatment in either group (RUPP+180 rhPlGF: 2.12±0.05 g or NP+180 rhPlGF: 2.25±0.05 g). Average placental weight was similar between all groups: NP: 0.51±0.02 g; RUPP: 0.47±0.02 g; RUPP+180 rhPlGF: 0.52±0.10 g; and NP+180 rhPlGF: 0.48±0.02 g. The number of viable fetuses was reduced (P<0.05) in RUPP (4±0.6) vs. NP (13±0.7) with no influence of rhPlGF on these numbers (RUPP+180 rhPlGF: 6±0.9 or NP+180 rhPlGF: 14±0.4). Likewise, the number of reabsorptions was greater (P<0.05) in RUPP (9.3±0.9) vs. NP (1.3±0.6) and was not alter by rhPlGF (RUPP+180 rhPlGF: 8.2±1.1 or NP+180 rhPlGF: 0.5±0.3). The number of pregnancies used for these fetal biometrics were: N=10 (NP), N=16 (RUPP), N=14 (RUPP+180 rhPlGF), and N=6 (NP+180 rhPlGF). Thus, percent fetal absorption rate was significantly greater in RUPP versus NP, and rhPlGF did not influence this measure in the two pregnant groups (Figure 4D).
Figure 4.
Maternal body weight (A); total fetal weight (B); total placental weight (C) and fetal absorption as a percent in normal pregnant (NP; N=11), reduced uterine perfusion pressure (RUPP; N=15), RUPP + 180 μg/kg/day rhPlGF (N=12), and NP + 180 μg/kg/day rhPlGF rats (N=5). *P<0.05 versus untreated NP rats.
To assess the anti-angiogenic (Figure 5A) and reactive oxygen species (Figure 5B) profiles in the NP and RUPP following rhPlGF, we examined plasma levels of sFlt-1 and 8-iso-prostanes, respectively. Circulating levels of this anti-angiogenic factor was significantly greater in response to placental ischemia in untreated RUPP rats. Administration of rhPlGF dramatically reduced these levels back to NP values. The levels of sFlt-1 were similar between NP and NP + 180 μg/kg/day rhPlGF. Similarly, 8-iso-prostanes was significantly increased in RUPP over NP with rhPlGF treatment bringing these levels in RUPP qualitatively closer to NP values. Thus, there was not a significant difference in these values between the NP and RUPP + 180 μg/kg/day rhPlGF groups. The rhPlGF did not have any effect on these levels in NP rats.
Figure 5.
Circulating sFlt-1 (A) and 8-iso-prostane (B) levels in plasma from normal pregnant (NP; N=11), reduced uterine perfusion pressure (RUPP; N=15), RUPP + 180 μg/kg/day rhPlGF (N=12), and NP + 180 μg/kg/day rhPlGF rats (N=5). *P<0.05 versus untreated NP rats; †P<0.05 versus untreated RUPP rats.
DISCUSSION
Here we provide evidence that pharmacologically increasing circulating levels of PlGF has potential as an effective therapeutic strategy to reduce blood pressure during placental ischemia-induced hypertension. Administration of purified recombinant human PlGF abolished hypertension and the reductions in GFR induced in RUPP pregnant rats. There was no effect on maternal weights or fetal biometrics, as assessed by fetal/placental weights and fetal absorption rates in response to RUPP. We also show that PlGF administration in RUPP pregnant rats prevented the development of hypertension and dramatically reduced the placental ischemia-driven rise in bioavailable levels of free sFlt-1, postulated to be a major pathogenic mediator of anti-angiogenic state in preeclampsia.
The motivation for this project comes from the unmet need for interventions in preeclampsia. The need is urgent to develop safe, effective and targeted therapies for preeclampsia to reduce maternal and fetal morbidity and mortality. This is unquestionably a global public health priority. A wealth of studies in humans indicate that anti-angiogenic mechanisms are involved in the pathogenesis of preeclampsia. Elevated sFlt-1 levels precedes the onset of preeclampsia and correlates with disease severity7, 18. Moreover, well over 100 reports implicate increased sFlt-1 in reducing bioavailable VEGF and PlGF, both in humans and experimental models of preeclampsia19. Notably, the study performed by Maynard and colleagues demonstrated a dose-response effect of increasing sFlt-1 levels and reductions in VEGF and PlGF corresponding to the degree of hypertension found in their sample of preeclamptic women7. These findings suggest that targeting this sFlt1-mediated anti-angiogenic pathway may be a promising strategy to treat preeclampsia.
A direct role for sFlt-1 in causing hypertension during pregnancy was demonstrated by infusion or adenoviral overexpression of this factor, which resulted in the development of hypertension by the end of pregnancy in both mice and rats. The levels of sFlt-1 are increased in RUPP rodents with placental ischemia-induced hypertension compared to their NP counterparts6, 12. Our laboratory previously showed that there are significant reductions in free VEGF and PlGF levels in RUPP rats6. Supplementing these RUPP rats with recombinant VEGF121 for the duration of the placental ischemia abolished their hypertension and restored GFR to NP levels9. The rationale for administering the rhPlGF via intraperitoneal minipump was based on these previous studies, which demonstrated that infusion of VEGF via intraperitoneal infusion significantly increased circulating VEGF levels. Until now, it was not known whether PlGF can also attenuate the heightened blood pressure levels occurring in response to placental ischemia, without the risk of off-target effects associated with VEGF. Moreover, as the reductions in circulating PlGF are a better predictor than VEGF levels for the onset of preeclampsia10, we set out to examine whether PlGF has a beneficial effect on the RUPP animal model of preeclampsia.
In this study, we showed that rhPlGF abolished the development of hypertension and prevented the reductions in GFR resulting from placental ischemia. These findings implicate aberrations in circulating PlGF with subsequent reductions in renal hemodynamics in the pathogenesis of hypertension preeclampsia. Administration of rhPlGF significantly reduces RUPP-induced increases in circulating sFlt-1. We believe that a major source of sFlt-1 is the ischemic placenta. Indeed, we have demonstrated that RUPP rats have significantly increased levels of circulating and placental sFlt-16. We have also shown in ex vivo studies using isolated placental villi from normal pregnant rats that acute hypoxia stimulates the release of sFlt-112. However, this does not discount the contribution of circulating platelet-monocyte aggregates to the circulating pool of sFlt-1. Indeed, because exogenous PlGF did not alter levels of placental ischemia due to the mechanical constriction of the uterine blood vessels, it is possible that PlGF reduces sFlt-1 production from extra-placental sources as well. We have also shown previously that oxidative stress is an important mechanism that leads to endothelial dysfunction and hypertension in the RUPP model20. PlGF infusion brings the elevated plasma 8-iso-prostane levels, which a surrogate measure of oxidative stress, in RUPP closer to NP levels. However, we cannot rule out whether other pathways such as endothelin-1 mediates the blood pressure lowering and GFR raising effects of rhPlGF in RUPP rats 21.
We validated a new method for measuring GFR showing similar reduction in GFR with RUPP (delta fall = 1.2 mL/min) compared to our previous studies using urinary clearance of radioactive inulin (delta fall = 1.6 mL/min). However, we did find that the absolute values for GFR were greater in both of these groups with using the transcutaneous technique. With this technique, a continuous disappearance curve is generated therefore the number of measurement points is much higher and the 95% confidence intervals narrower compared to blood or urinary sample measurements. This results in more robust curve fitting and thus seems more sensitive than other clearance techniques for assessing GFR15.
Alternative splicing of PlGF results in 4 different isoforms in human22. PlGF-1 and PlGF-3 are diffusible because they lack heparin-binding sites. Aggamin Biologics expressed and purified a recombinant form of PlGF. We hypothesized that administration of rhPlGF for the duration of the RUPP protocol would reduce the hypertensive response to placental ischemia in rats. The dose of 180 μg/kg/day of rhPlGF is same as the dose of VEGF used in the supplementation study referenced above9. In preliminary dose response experiments using doses of 90, 180, 300, 420 μg/kg/day that maximal blood pressure lowering at 180 was not any different than the two higher doses (data not shown). We believe that the reason rhPlGF administration at the dose of 90 μg/kg/day did not reduce the RUPP hypertensive blood pressure levels is because it did not result in a significant rise in circulating PlGF levels (6±3 ng/mL) whereas those that were infused with double the dose had 21±3 ng/mL plasma levels of rhPlGF. This small rise in rhPlGF in the lower dose group did not change RUPP blood pressure levels at all compared to RUPP untreated, whereas the higher dose abolished the RUPP hypertension. This is similar to the findings in our previous VEGF supplementation paper9. Furthermore, because we used the same infusion dose of VEGF that we previously showed brought the hypertensive blood pressure levels in RUPP back down to normal pregnant levels, controlling proper PlGF availability seems just as important as VEGF in blood pressure regulation during healthy pregnancies. However, in favor of PlGF as the angiogenic therapy of choice for preeclampsia, we propose that PlGF would have fewer side effects because it does not bind VEGF receptor 2 (or Flk-1) which is thought to mediate vascular leak. In contrast, VEGF binds both Flt-1 and Flk-1, the latter of which promotes edema when pathophysiological increases in VEGF occur. Thus, we propose that PlGF administration exerts its effects by binding to Flt1 and displacing endogenous VEGF to act on Flk-1 without causing potential side effects that may arise with exogenously increasing VEGF levels. Therefore, based on our dose response data, we propose 180 μg/kg/day is the optimal dose for biological efficacy.
In the mother, circulating levels of PlGF increase and peak at 30 weeks of gestation whereas in preeclampsia it peaks before 25 weeks and then falls23. This is similar to the timing of the onset of the hypertension in preeclampsia24. These findings and our data highlight the importance of PlGF on maternal blood pressure regulation during pregnancy and preeclampsia. It is interesting that PlGF restored blood pressure to normal pregnancy levels as we had previously observed with infusion of VEGF9. It is possible that these two angiogenic factors act cooperatively to regulate blood pressure during pregnancy. Mechanistically, PlGF only binds the Flt1 (VEGFR1) whereas VEGF binds both VEGFR1 and VEGFR2 (Flk-1)25. Therefore, it is thought that increased PlGF binding to Flt-1 shunts more VEGF to VEGFR2. The VEGFR2 receptor elicits most of the angiogenic and vasoprotective actions of VEGF. Furthermore, our data indicate that exogenously increasing circulating levels of PlGF also quenches bioavailable sFlt-1. Future studies should examine the relative importance of each of these pathways and placental production of soluble factors on the effects of rhPlGF to reduce blood pressure and increase GFR during placental ischemia. Furthermore, it is not yet clear whether rhPlGF administration had any beneficial effect on the fetus even though maternal blood pressure was reduced without changing fetal weight at gestational day 19. Future studies should examine long-term cardiovascular and metabolic outcomes in these offspring to determine if this intervention serves to benefit both mother and the baby of preeclamptic pregnancies.
As no effective treatment is available, it is urgent to develop a novel and effective drug therapy for preeclampsia. Premature delivery by induced labor or Caesarean section is the only recourse to ensure mother’s safety, but it puts the neonate at risk for multiple complications and results in poor neonatal outcomes26. Important clinical trials are directing us toward development of more rational and targeted therapies. For instance, trials with pravastatin are underway to ameliorate preeclampsia by inhibiting HMG CoA reductase, but because statins are contraindicated in pregnancy, as this class of drugs has a high risk of teratogenic potential27. Interestingly, the mechanistic rationale for pravastatin use is its potential to up-regulate VEGF and PlGF and correct the angiogenic imbalance of excess sFlt-1 in RUPP rats28. While this rationale is consistent with our therapeutic approach, administering rhPlGF is likely to be a more direct and safer strategy to treat preeclampsia. We believe PlGF therapy to be superior to the current treatment strategies as they are wrought with issues of drugs losing potency due to tolerance and their potential teratogenic effects. This latter point is especially true for ETA antagonists during pregnancy. However, based on previous studies and our results showing plasma 8-iso-prostane, a measure of reactive oxygen species, is reduced by PlGF infusion support that vascular dysfunction and oxidative stress occurs downstream of placental ischemia and increased sFlt-1 levels to elicit hypertension. Therefore, we believe that initial alterations in angiogenic balance, such as increased sFlt-1, results in these pro-hypertensive mechanisms and that rhPlGF therapy protects against this vascular dysfunction and hypertension by binding to sFlt-1 and improving oxidative stress.
PERSPECTIVES
Hypertensive disorders of pregnancy not only increases the risk for maternal/fetal morbidity and mortality, but it also predisposes the mother29 and neonate30 for a higher risk of developing hypertension later in life. Therefore, it is important to find novel therapeutic strategies to maintain healthy blood pressure regulation during preeclamptic events. As patient safety is a critical factor in developing a therapy for this high-risk population, it is important to mitigate any risk of off-target effects. Although PlGF and VEGF are both natural ligands for sFlt-1, only PlGF is specific for the Flt-1 receptor, while VEGF also binds to VEGFR2 and can promote side-effects related to excess VEGFR2 signaling such as increased vascular permeability and edema. Furthermore, PlGF-1 has the ability to form heterodimers with VEGF and this may prevent such adverse effects of increasing VEGF signaling through VEGFR2 following exogenous application of PlGF31. In models of inflammation, PlGF (but not VEGF) has been shown to protect the vasculature, which is particularly relevant in preeclampsia, a profoundly pro-inflammatory state32, 33. In conclusion, with readily-available assays to identify women most likely to benefit from PlGF therapy, our goal is to be able to guide appropriate timing of therapy initiation and discontinuation, minimizing maternal and fetal risk as clinical development progresses. We have previously shown that angiogenic imbalance with reduced PlGF and increased sFlt-1, i.e. high sFlt-1 to PlGF ratio, is a robust biomarker predicting the development preeclampsia and its related complications34. Indeed, among high-risk individuals this imbalance is present as early as 12–15 weeks of gestation, which is several months before the clinical manifestations of this disorder35.
NOVELTY AND SIGNFICANCE.
1) What is new?
The role of PlGF, an endogenous ligand for Flt-1 on the endothelium, in pregnancy-induced hypertension and preeclampsia is unclear. Our novel finding is that administration of rhPlGF abolished placental ischemia related hypertension in a pregnant rat model of preeclampsia that was accompanied by reductions in circulating free sFlt-1. Elevations of the anti-angiogenic factor sFlt-1 occurs before the onset preeclampsia in humans; is increased in RUPP rats; and is known to elicit hypertension in pregnant experimental animals on its own.
2) What is significant?
In the past, interventions evaluated for their ability to prolong pregnancy in patients with preeclampsia (e.g., treatment for mild hypertension, plasma-volume expansion, digibind, heparin, and corticosteroid use) have been unsuccessful or are not recommended for prevention of preeclampsia due to adverse effects to the fetus 36–41. Our data have significant relevance toward the development of the novel treatment strategies for preeclampsia.
3) Summary
PlGF is abundantly made in normal pregnancy, but due to abnormally high circulating levels of sFlt-1 in preeclampsia, levels of free PlGF are insufficient. Correcting this angiogenic imbalance with a naturally occurring protein may limit the occurrence of unwanted systemic side effects. As rhPlGF protein is identical in structure and function to human PlGF, it is unlikely to be antigenic or induce an immune reaction. Our data support the development of a treatment strategy for preeclampsia using naturally occurring human PlGF.
Acknowledgments
We would like to that Marietta Arany and Kathy Cockrell for their expert technical assistance.
SOURCES OF FUNDING
Funding includes: SBIR 1R43HD082657 (A.Y.T.), T32HL105324-01 (F.T.S), P01HL051971 (J.P.G).
Footnotes
CONFLICTS OF INTEREST
S.A.K is a co-inventor on patents related to the use of angiogenic factors for the diagnosis and treatment of preeclampsia that are held by the Beth Israel Deaconess Medical Center. S.A.K has financial interest in Aggamin LLC and reports serving as a consultant to Roche, Siemens and Thermofisher Scientific. A.Y.T, W.S.J, G.D, and P.K. are employees of Aggamin LLC. All other authors disclose no conflict.
References
- 1.Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, Gulmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: A who systematic analysis. Lancet Glob Health. 2014;2:e323–333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 2.Makris A, Yeung K, Farrell P, Heffernan S, Thompson J, Xu B, Woolcock J, Ogle R, Thadhani R, Karumanchi A, Hennessy A. Os061. Placental growth factor reduces blood pressure and proteinuria in experimental preeclampsia. Pregnancy Hypertens. 2012;2:210. doi: 10.1016/j.preghy.2012.04.062. [DOI] [PubMed] [Google Scholar]
- 3.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 4.Intapad S, Warrington JP, Spradley FT, Palei AC, Drummond HA, Ryan MJ, Granger JP, Alexander BT. Reduced uterine perfusion pressure induces hypertension in the pregnant mouse. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1353–1357. doi: 10.1152/ajpregu.00268.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol. 2007;293:H2080–2084. doi: 10.1152/ajpheart.00667.2007. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 7.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sflt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension. 2010;55:380–385. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46:1077–1085. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 11.Spradley FT, Palei AC, Granger JP. Obese melanocortin-4 receptor-deficient rats exhibit augmented angiogenic balance and vasorelaxation during pregnancy. Physiol Rep. 2013;1:e00081. doi: 10.1002/phy2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy SR, LaMarca BB, Parrish M, Cockrell K, Granger JP. Control of soluble fms-like tyrosine-1 (sflt-1) production response to placental ischemia/hypoxia: Role of tumor necrosis factor-alpha. Am J Physiol Regul Integr Comp Physiol. 2013;304:R130–135. doi: 10.1152/ajpregu.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41:457–462. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 14.Banek CT, Bauer AJ, Needham KM, Dreyer HC, Gilbert JS. Aicar administration ameliorates hypertension and angiogenic imbalance in a model of preeclampsia in the rat. Am J Physiol Heart Circ Physiol. 2013;304:H1159–1165. doi: 10.1152/ajpheart.00903.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schock-Kusch D, Xie Q, Shulhevich Y, Hesser J, Stsepankou D, Sadick M, Koenig S, Hoecklin F, Pill J, Gretz N. Transcutaneous assessment of renal function in conscious rats with a device for measuring fitc-sinistrin disappearance curves. Kidney Int. 2011;79:1254–1258. doi: 10.1038/ki.2011.31. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber A, Shulhevich Y, Geraci S, Hesser J, Stsepankou D, Neudecker S, Koenig S, Heinrich R, Hoecklin F, Pill J, Friedemann J, Schweda F, Gretz N, Schock-Kusch D. Transcutaneous measurement of renal function in conscious mice. Am J Physiol Renal Physiol. 2012;303:F783–788. doi: 10.1152/ajprenal.00279.2012. [DOI] [PubMed] [Google Scholar]
- 17.Holwerda KM, Burke SD, Faas MM, Zsengeller Z, Stillman IE, Kang PM, van Goor H, McCurley A, Jaffe IZ, Karumanchi SA, Lely AT. Hydrogen sulfide attenuates sflt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J Am Soc Nephrol. 2014;25:717–725. doi: 10.1681/ASN.2013030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bian Z, Shixia C, Duan T. First-trimester maternal serum levels of sflt1, pgf and adma predict preeclampsia. PLoS One. 2015;10:e0124684. doi: 10.1371/journal.pone.0124684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens. 2008;21:1152–1156. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37:485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 22.Dewerchin M, Carmeliet P. Plgf: A multitasking cytokine with disease-restricted activity. Cold Spring Harb Perspect Med. 2012;2:a011056. doi: 10.1101/cshperspect.a011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, Edwin S, Chaiworapongsa T, Levine RJ, Karumanchi SA. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: Two different maternal hemodynamic states in the latent phase of the disease. Hypertension. 2008;52:873–880. doi: 10.1161/HYPERTENSIONAHA.108.117358. [DOI] [PubMed] [Google Scholar]
- 25.Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ. Binding and neutralization of vascular endothelial growth factor (vegf) and related ligands by vegf trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sipola-Leppanen M, Vaarasmaki M, Tikanmaki M, Matinolli HM, Miettola S, Hovi P, Wehkalampi K, Ruokonen A, Sundvall J, Pouta A, Eriksson JG, Jarvelin MR, Kajantie E. Cardiometabolic risk factors in young adults who were born preterm. Am J Epidemiol. 2015;181:861–873. doi: 10.1093/aje/kwu443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brownfoot FC, Tong S, Hannan NJ, Binder NK, Walker SP, Cannon P, Hastie R, Onda K, Kaitu’u-Lino TJ. Effects of pravastatin on human placenta, endothelium, and women with severe preeclampsia. Hypertension. 2015;66:687–697. doi: 10.1161/HYPERTENSIONAHA.115.05445. [DOI] [PubMed] [Google Scholar]
- 28.Bauer AJ, Banek CT, Needham K, Gillham H, Capoccia S, Regal JF, Gilbert JS. Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia-induced hypertension. Hypertension. 2013;61:1103–1110. doi: 10.1161/HYPERTENSIONAHA.111.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heida KY, Franx A, van Rijn BB, Eijkemans MJ, Boer JM, Verschuren MW, Oudijk MA, Bots ML, van der Schouw YT. Earlier age of onset of chronic hypertension and type 2 diabetes mellitus after a hypertensive disorder of pregnancy or gestational diabetes mellitus. Hypertension. 2015;66:1116–1122. doi: 10.1161/HYPERTENSIONAHA.115.06005. [DOI] [PubMed] [Google Scholar]
- 30.Cirillo PM, Cohn BA. Pregnancy complications and cardiovascular disease death: 50-year follow-up of the child health and development studies pregnancy cohort. Circulation. 2015;132:1234–1242. doi: 10.1161/CIRCULATIONAHA.113.003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Falco S. The discovery of placenta growth factor and its biological activity. Exp Mol Med. 2012;44:1–9. doi: 10.3858/emm.2012.44.1.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yano K, Okada Y, Beldi G, Shih SC, Bodyak N, Okada H, Kang PM, Luscinskas W, Robson SC, Carmeliet P, Karumanchi SA, Aird WC. Elevated levels of placental growth factor represent an adaptive host response in sepsis. J Exp Med. 2008;205:2623–2631. doi: 10.1084/jem.20080398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armanini D. Preeclampsia: The role of aldosterone in hypertension and inflammation. Hypertension. 2012;59:1099–1100. doi: 10.1161/HYPERTENSIONAHA.112.193011. [DOI] [PubMed] [Google Scholar]
- 34.Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim KH, Wenger JB, Thadhani R, Karumanchi SA. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911–919. doi: 10.1161/CIRCULATIONAHA.111.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MY, Buyon JP, Guerra MM, Rana S, Zhang D, Laskin CA, Petri M, Lockshin MD, Sammaritano LR, Branch DW, Porter TF, Merrill JT, Stephenson MD, Gao Q, Karumanchi SA, Salmon JE. Angiogenic factor imbalance early in pregnancy predicts adverse outcomes in patients with lupus and antiphospholipid antibodies: Results of the promisse study. Am J Obstet Gynecol. 2016;214:108.e101–108 e114. doi: 10.1016/j.ajog.2015.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolte AC, van Geijn HP, Dekker GA. Management and monitoring of severe preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2001;96:8–20. doi: 10.1016/s0301-2115(00)00383-3. [DOI] [PubMed] [Google Scholar]
- 37.Clasp (collaborative low-dose aspirin study in pregnancy) collaborative group. Clasp: A randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. Lancet. 1994;343:619–629. [PubMed] [Google Scholar]
- 38.McCoy S, Baldwin K. Pharmacotherapeutic options for the treatment of preeclampsia. Am J Health Syst Pharm. 2009;66:337–344. doi: 10.2146/ajhp080104. [DOI] [PubMed] [Google Scholar]
- 39.Paternoster DM, Stella A, Simioni P, Girolami A, Plebani M. Fibronectin and antithrombin as markers of pre-eclampsia in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;70:33–39. doi: 10.1016/s0301-2115(96)02550-x. [DOI] [PubMed] [Google Scholar]
- 40.Unemori E, Sibai B, Teichman SL. Scientific rationale and design of a phase i safety study of relaxin in women with severe preeclampsia. Ann N Y Acad Sci. 2009;1160:381–384. doi: 10.1111/j.1749-6632.2009.03838.x. [DOI] [PubMed] [Google Scholar]
- 41.Adair CD, Buckalew VM, Graves SW, Lam GK, Johnson DD, Saade G, Lewis DF, Robinson C, Danoff TM, Chauhan N, Hopoate-Sitake M, Porter KB, Humphrey RG, Trofatter KF, Amon E, Ward S, Kennedy L, Mason L, Johnston JA. Digoxin immune fab treatment for severe preeclampsia. Am J Perinatol. 2010;27:655–662. doi: 10.1055/s-0030-1249762. [DOI] [PubMed] [Google Scholar]