Abstract
Grip relaxation is a voluntary action that requires an increase in short-interval intracortical inhibition (SICI) in healthy young adults, rather than a simple termination of excitatory drive. The way aging affects this voluntary inhibitory action and timing of grip relaxation is currently unknown. The objective of this study was to examine aging-related delays in grip relaxation and SICI modulation for the flexor digitorum superficialis (FDS) muscle during grip relaxation. The main finding was that young adults increased SICI to relax their grips, whereas older adults did not increase SICI with a prolonged grip relaxation time (p<0.05 for both SICI modulation and grip relaxation time). A secondary experiment showed that both young and older adults did not change H reflex excitability during grip relaxation. Our data suggest that grip relaxation is mediated by increased cortical inhibitory output in young adults, and aging-related impairment in increasing cortical inhibitory output may hamper timely cessation of muscle activity. Our data also suggest a lesser role of the spinal circuits in grip muscle relaxation. This knowledge may contribute to understanding of aging-related movement deterioration and development of interventions for improving modulation of SICI to improve muscle relaxation and movement coordination.
Keywords: grip relaxation, aging, hand, TMS, SICI, H reflex
1. Introduction
Movements become slow with aging. The ability to rapidly start and swiftly execute a movement decreases with age (Fozard et al., 1994, Der and Deary, 2006, Langan et al., 2010, van de Laar et al., 2012, Wolkorte et al., 2014). Not only movement initiation, but also prompt termination of a hand movement is important for activities of daily living. For example, delay in terminating finger flexor activity while releasing a spoon may require greater antagonist activation to open the hand to release the spoon. Unwanted muscle activity and delay in terminating such activity in a timely manner may also impair the quality of dynamic movement such as reaching and walking, and hamper movement efficiency and energy expenditure. Despite the functional significance of grip muscle relaxation, whether grip relaxation is delayed with aging is unknown. Additionally, while aging-related changes in skeletal muscles have been shown (Lexell, 1995, Ohlendieck, 2011), potential cortical neural correlates of prolonged grip relaxation with aging have not been examined.
Muscle relaxation is accompanied by activation of the dorsolateral prefrontal cortex, primary, supplementary and pre-supplementary motor areas in healthy young adults (Toma et al., 1999, Spraker et al., 2009). This activity in the motor cortex is inhibitory in nature, as evidenced by increased intracortical inhibition in M1 during muscle relaxation (Buccolieri et al., 2004a, Motawar et al., 2012). This increased intracortical inhibition may be responsible for decreased spinal motor excitability during muscle relaxation in the soleus muscle in young adults (Schieppati and Crenna, 1984, Schieppati et al., 1985, 1986).
Such increase in intracortical inhibition needed for timely muscle relaxation may decline with aging. Older people have a decreased level of intracortical inhibition at rest (Oliviero et al., 2006, Marneweck et al., 2011, Heise et al., 2013). More importantly, older adults cannot modulate intracortical inhibition as much, as evidenced by the reduced ability to decrease intracortical inhibition to initiate and maintain muscle contractions (Peinemann et al., 2001, Fujiyama et al., 2012, Heise et al., 2013, Papegaaij et al., 2014). In addition to intracortical inhibition, older adults were shown to have reduced modulation of spinal excitability, as seen in soleus H reflex during walking in older adults (Raffalt et al., 2014) and during muscle relaxation in patients with upper motor neuron lesion (Schieppati et al., 1985). These changes in neurophysiology may ultimately affect their limb function. While reduced modulation of intracortical inhibition with aging was noted during movement preparation and execution, aging-related changes in modulation of intracortical inhibition during grip relaxation are unknown.
This study examined timely grip muscle relaxation in young vs. older adults and its relationship to modulation of short-interval intracortical inhibition (SICI). We hypothesized that grip muscle relaxation is delayed in older adults and that delayed grip relaxation in older adults is associated with lesser increase of intracortical inhibition during relaxation. As a secondary analysis, we examined if delayed grip relaxation was accompanied by altered modulation of spinal motoneuron excitability assessed by the H reflex during grip relaxation in older adults. In examination of SICI and H reflex, both dominant and nondominant hands’s data were collected, since they differ in regards to the motor unit firing behavior (Adam et al., 1998) and structural and functional differences at the spinal and supraspinal level (Yakovlev and Rakic, 1966, Tan, 1989, Toga and Thompson, 2003) and may have different neural mechanisms for grip relaxation.
2. Methods
2.1 Subjects
A total of 40 young (mean and standard deviation of 25±5 years old, ranging from 18 to 37 years old, 19 females) and 21 older (57±6 years old, ranging from 46 to 68 years old, 12 females) adults were tested in the study. All subjects were right-handed, as determined by the Edinburgh Inventory (Oldfield, 1971). All subjects were healthy and did not have any known neurological and orthopedic disorders affecting the upper limb. All subjects also verbally confirmed that they could clearly hear the computer-generated sounds used during the experiment. Subjects were also screened for contraindications to TMS and electrical nerve stimulation (Rossi et al., 2011). All subjects signed an informed consent form approved by the Institutional Review Board.
While relaxation time was recorded for all participants, SICI and H reflex data were not obtained from both hands of all participants due to difficulty in the retention of the subjects or difficulty in obtaining H reflex from the flexor digitorum superficialis (FDS) muscle. All subjects with MEP were included for SICI testing. Specifically, SICI data were obtained from 20 young subjects (25±5 years old, 8 females) and 20 older subjects (57±6 years old, 11 females). All subjects with H reflex for the FDS muscle at rest were included for H reflex testing. H reflex data were obtained from 25 young (26±6 years old, 14 females) and 9 older (59±5 years old, 4 females) subjects, although we screened a total of 20 older adults. The number of subjects tested for the dominant and the nondominant hand for each test is indicated in Table 1.
Table 1.
The number of subjects tested for the right dominant and left nondominant hands for SICI and H reflex. Grip relaxation time was recorded for all subjects who participated in either the SICI or H reflex testing.
| Young (n=40 total) | Older (n=21 total) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dominant only |
Nondominant only |
Both hands |
Dominant total |
Nondominant total |
Dominant only |
Nondominant only |
Both hands |
Dominant total |
Nondominant total |
|
| Grip relaxation time (n=40 young, 21 older) |
17 | 10 | 13 | 30 | 23 | 8 | 5 | 8 | 16 | 13 |
| SICI (n=20 young, 20 older) |
10 | 9 | 1 | 11 | 10 | 8 | 4 | 8 | 16 | 12 |
| H reflex (n=25 young, 9 older) |
9 | 5 | 11 | 20 | 16 | 3 | 3 | 3 | 6 | 6 |
2.2 Procedure
The effect of aging on grip relaxation time and modulations of SICI (Kujirai et al., 1993) and H reflex (Palmieri et al., 2004, Knikou, 2008) were examined in both hands of young and older adults. The SICI and H reflex tests were conducted on separate days; two hands were examined on separate days as well. Grip relaxation time was measured at the beginning of each testing day.
2.2.1 Measurement of grip relaxation time
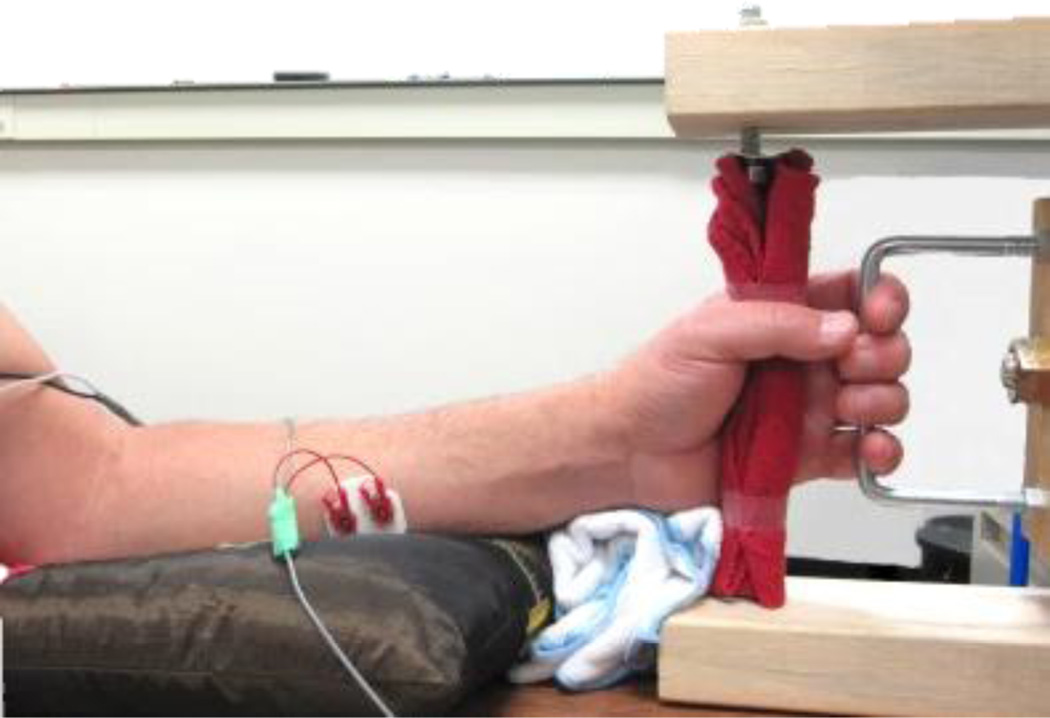
Grip relaxation time was measured during grip-and-relax trials. Subjects were seated in a chair with the upper limb relaxed, the forearm supported in the midprone position, the wrist in neutral posture, and the fingers comfortably placed around the handle in a grasping posture at rest (Figure 1). The middle phalanges pressed against the handle during grip. Subjects performed isometric grip followed by isometric grip relaxation upon cues. Specifically, subjects were instructed to grip a handle as hard as possible (maximum voluntary contraction, MVC) upon the start of a computer-generated sound and relax as quickly as possible upon the termination of the sound (Figure 1, Figure 2A). Grip relaxation time was determined as the time interval between when the sound ended and when the FDS muscle electromyogram (EMG) activity decreased to its precontraction baseline level (Figure 2A). Specifically, grip relaxation was determined to have completed when the FDS root mean square (RMS) EMG was less than the mean + 3 standard deviations (SD) of the precontraction baseline EMG level, according to the literature (Seo et al., 2009b, Motawar et al., 2012). In addition to the FDS EMG, the EMG from the extensor digitorum communis (EDC) muscle was recorded to monitor antagonist muscle activity.
Figure 1.
During grip-and-relax trials, subjects isometrically gripped a handle and relaxed upon an audio cue while the EMG was recorded from the FDS muscle. During sustained grip trials, subjects isometrically gripped the handle to match the EMG level.
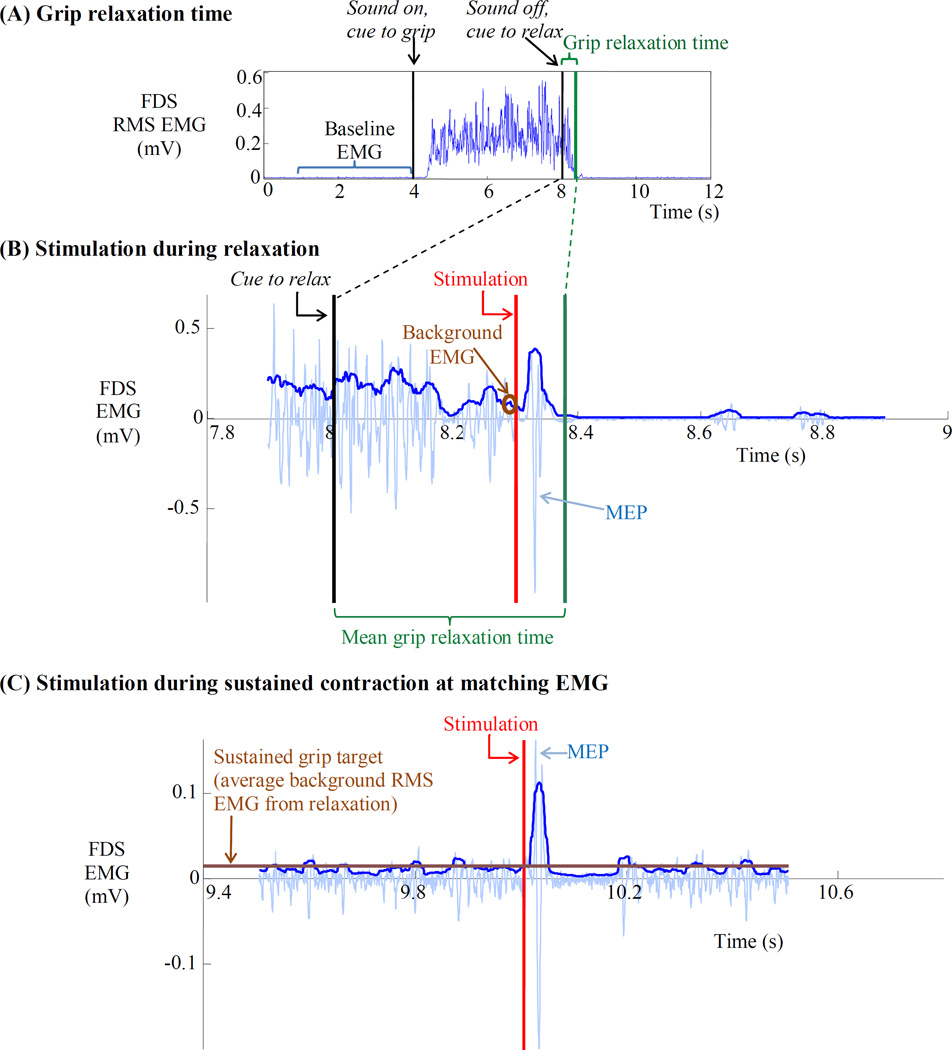
Figure 2.
(A) To measure grip relaxation time, the subject maximally and isometrically gripped the handle upon the start of a computer generated sound and relaxed the grip upon the termination of the sound. Grip relaxation time was quantified as the time in which the postcontraction FDS RMS EMG fell below mean + 3SD of the precontraction baseline FDS RMS EMG. (B) To measure SICI during relaxation, stimulation was applied at 70%, 80%, or 90% into the subject’s mean grip relaxation time during the grip-and-relax trial. The peak-to-peak MEP was used toward computation of SICI. The background RMS EMG during 20 ms immediately before stimulation was obtained during the grip-and-relax trial. The average background RMS EMG was used as a target in the subsequent measurement of SICI during sustained grip. (C) To measure SICI during sustained grip, stimulation was applied while the subject maintained a sustained grip at the target muscle activity level using visual feedback. Example trials from a single subject are shown in this figure. In (B) and (C), lighter EMG traces show raw EMG while thicker traces show RMS EMG. The same protocol was used to obtain H reflex during grip relaxation and sustained grip at a matching EMG level.
The EMGs for the FDS and EDC muscles were recorded through Ag-AgCl bipolar surface electrodes (Bortec Biomedical Ltd., Calgary, Alberta, Canada) placed according to the literature (Basmajian, 1989). The EMG data were bandwidth-filtered at 10 to 1000 Hz, amplified with a 1k gain, and recorded at 2 kHz throughout the study, using a custom-written LabVIEW program (National Instruments Corp., Austin, Texas, USA). During the grip-and-relax trials, the contralateral hand and forearm rested on a pillow on the subject’s lap for comfort. Subjects were also instructed to not open their fingers during grip relaxation and to stay relaxed until instructed otherwise. The subjects were instructed not to contract muscles in other limbs, including the contralateral hand. Practice was provided until subjects were familiarized with the grip-and-relax task. After practice, the mean grip relaxation time from 5 grip-and-relax trials determined the subject’s grip relaxation time.
2.2.2 SICI modulation
Modulation of SICI was assessed as SICI during grip relaxation compared to during sustained grip at matching FDS EMG activity, using our previously published protocol (Motawar et al., 2012). The reason it is important to match the muscle activity between two tasks (grip relaxation vs. sustained grip) is that SICI magnitudes are dependent on the background muscle activity level (Ortu et al., 2008). The SICI data during grip relaxation were collected first, followed by data collection for SICI during sustained grip, so that muscle activity was matched between the two tasks for individual subjects. Specifically, to record SICI during relaxation, subjects performed the grip-and relax trials while TMS was applied at 70%, 80%, and 90% of the subject’s mean grip relaxation time using computer-generated triggers. These three time points within the relaxation period were examined following the previous study (Motawar et al., 2012). To record SICI during sustained grip, subjects maintained isometric grip while matching the FDS EMG at the target level which was the background FDS EMG immediately before TMS stimulation from the grip relaxation trials collected earlier.
2.2.2.1 SICI during grip relaxation
To measure SICI during grip relaxation, TMS was delivered at 70%, 80% and 90% of the mean grip relaxation time determined for each subject immediately prior to the TMS testing. TMS was triggered by a custom-written LabVIEW program at these specific times during relaxation (Figure 2B). SICI was determined as % suppression of the conditioned MEP compared to the nonconditioned MEP observed in the FDS muscle, using paired pulse technique (Kujirai et al., 1993, Berardelli et al., 2008, Rossini et al., 2015). A higher SICI indicates greater suppression of MEP and thus greater intracortical inhibition as shown in Eq. 1 (Coxon et al., 2006, Motawar et al., 2012).
| Equation 1 |
To evoke the nonconditioned MEP, the test stimulus intensity was set at the % maximum stimulator output (MSO) that evoked peak-to-peak MEP amplitude of 1 mV in the resting FDS muscle. To evoke a conditioned MEP, the test stimulus was preceded by a conditioning stimulus with a 2 ms interstimulus interval. The conditioning stimulus intensity was set at 90% of the active motor threshold (AMT), and delivered at 70%, 80%, and 90% into grip relaxation. The AMT was determined as the %MSO that evoked peak-to-peak MEP amplitude of 100 µV, at least 5 out of 10 times during contraction of the FDS muscle at 10% MVC (Rossini et al., 1994). The MVC was determined as an average of the peak FDS RMS EMG over the 5 grip-and-relax trials collected earlier. These stimulation intensities and interstimulus interval were chosen to minimize contamination of intracortical inhibition by intracortical facilitatory pathways (Peurala et al., 2008, Rossini et al., 2015). Minimum 10 conditioned and 10 nonconditioned MEPs for each timing were obtained in a random order for each subject. Only one set of TMS stimulation (either single or paired pulse) was delivered during one grip-and-relax trial, with a minimum of 12 s interval between consecutive sets of TMS stimulation. As in the previous study (Motawar et al., 2012), trials with the background EMG (mean FDS RMS EMG for a 20 ms period immediately before the stimulus) outside the mean ± SD of the all trials were discarded, resulting in the mean trial numbers of 8.6 and 8.1 (SD = 0.7 and 0.7) for the conditioned and nonconditioned MEPs, respectively. The mean values of the conditioned MEPs and of the nonconditioned MEPs were used to compute SICI.
SICI was examined using two TMS stimulators connected through a BiStim module (BiStim2 and BiStim2002, The Magstim Company Ltd, Wales, UK). A 70-mm figure of eight coil was placed over the ‘hotspot’ of the contralateral cortex representation of the FDS muscle (approximately 6 cm anterolateral to vertex of the skull). The handle of the coil was placed postero-lateral at an approximately 45° angle to the midsagittal plane.
2.2.2.2 SICI during sustained grip
To facilitate quantification of SICI modulation for grip relaxation by controlling for the EMG level dependent changes in SICI, SICI during sustained grip at matching FDS EMG level was recorded. To evaluate SICI during sustained grip, subjects were instructed to maintain a grip exertion that matches the target FDS RMS EMG level using online visual feedback on the computer screen (Figure 2C). The target EMG level was the mean FDS RMS EMG for a 20 ms period immediately before the stimulus from the SICI during relaxation trials earlier (Figure 2B). The target was determined for the three stimulation timings individually (70%, 80% and 90% into relaxation). The same paired pulse TMS protocol described in the relaxation trials was used. The mean trial numbers used for analysis were 10 and 10 for the conditioned and nonconditioned MEPs, respectively. The mean values of the conditioned MEPs and of the nonconditioned MEPs were used to compute SICI. Five sets of TMS stimulation (either single or paired pulse), separated by 5 s each, were delivered during one recording of sustained contraction. The order of single and paired stimulation and the target levels was randomized across recordings for each subject.
2.2.3 H reflex modulation
Modulation of spinal motoneuron excitability was determined as FDS H reflex during grip relaxation compared to that during sustained grip with matching FDS EMG activity. The same approach as in SICI modulation was used since H reflex also depends on the background EMG level (Stein et al., 2007): In the same manner with the SICI modulation testing, each subject’s mean grip relaxation time was determined first, and then, H reflex at 80% into grip relaxation was determined, followed by H reflex during sustained grip at the matching muscle activity. As in the SICI testing, a computer-generated trigger evoked H reflex at 80% of the mean grip relaxation time determined at the beginning of the testing. During sustained grip, subjects matched the FDS EMG level (that was observed in the relaxation trials, immediately before the stimulation) using visual feedback. Mean H reflex values from 10 relaxation and 10 sustained grip trials were used to compute H reflex modulation.
H reflex was elicited by stimulating the median nerve in the cubital fossa using a constant current electrical stimulator (DS7A, Digitimer Ltd, Hertfordshire, UK). A single pulse electrical stimulation (square pulse width of 1 ms) was delivered through a bipolar surface electrode (3 cm inter-electrode distance with cathode proximal) (Zehr, 2002). The stimulation of the median nerve was confirmed by subjects reporting paraesthesia in the thumb, index finger, middle finger, and radial half of the ring finger. The stimulation intensity that resulted in the H reflex peak-to-peak amplitude that was approximately 10–15% M max peak-to-peak amplitude at rest was used. This stimulation intensity was suggested as the optimum intensity to examine H reflex inhibition or facilitation (Palmieri et al., 2004). The interstmulus interval was 5 seconds or longer to prevent post-activity depression of H reflex (Zehr, 2002). The peak-to-peak H reflex amplitude obtained during grip relaxation and sustained grip was normalized to the peak-to-peak M max at that background FDS EMG level to facilitate between-subject and within-subject comparisons (Zehr, 2002, Duclay and Martin, 2005).
2.3 Statistical analysis
The effects of aging and hand on grip relaxation time were examined using two-way ANOVA. For SICI, repeated measures ANOVA was used to examine if the level of SICI was affected by the within-subject variables of task (during relaxation vs. sustained contraction at matching EMG level) and time (70%, 80%, 90% into relaxation), between-subject variables of aging (young vs. older) and hand (dominant vs. nondominant), and their interactions. The factor of interest was interaction between task and aging, as it indicates whether task-specific modulation of SICI (in this case, for relaxation) is different between the two groups of different ages. Upon confirmation for the significant task × aging interaction effect, Tukey-Kramer pairwise comparison was performed to examine statistical difference between the two tasks (relaxation vs. sustained contraction) for each group. For H reflex, repeated measures ANOVA was used to examine if H reflex was affected by task (during relaxation vs. sustained contraction at matching EMG level), aging (young vs. older), hand (dominant vs. nondominant), and their interactions.
Other secondary analyses were performed as follows. To confirm that SICI or H reflex modulation did not result from different FDS background EMG levels between the two tasks (grip relaxation vs. sustained contraction), repeated measures ANOVAs were used to test if background FDS EMG was different with task, aging, hand, and time during SICI testing and with task, aging, and hand during H reflex testing. Also, since nonconditioned MEP amplitudes may affect SICI (Sanger et al., 2001), another repeated measures ANOVA was used to examine if the nonconditioned MEP amplitude was different with task, aging, hand, time, and their interactions.
As complements to the main ANOVAs involving two aging groups, additional ANOVAs were performed to examine the effect of age as a continuous variable on SICI and H reflex. The ANOVA for SICI had age and nonconditioned MEP amplitude as covariates, task, hand, time, and their interactions. The ANOVA for H reflex had age as a covariate, task, hand, and their interactions. Lastly, upon finding aging- and task-dependent change in SICI but not H reflex, regression analysis was performed between the grip relaxation time and task-dependent SICI modulations (difference in SICI between the two task conditions) at three time points. Statistical analyses were performed with the SAS version 9.4 (SAS Institute Inc., Cary NC).
3. Results
3.1 Slowed grip relaxation in older adults
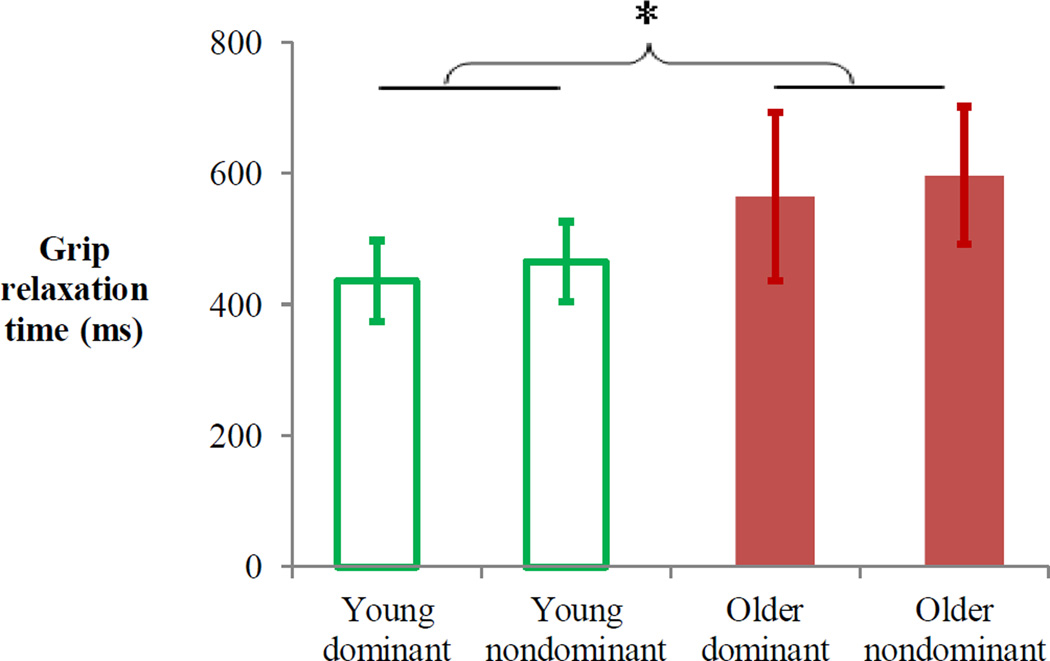
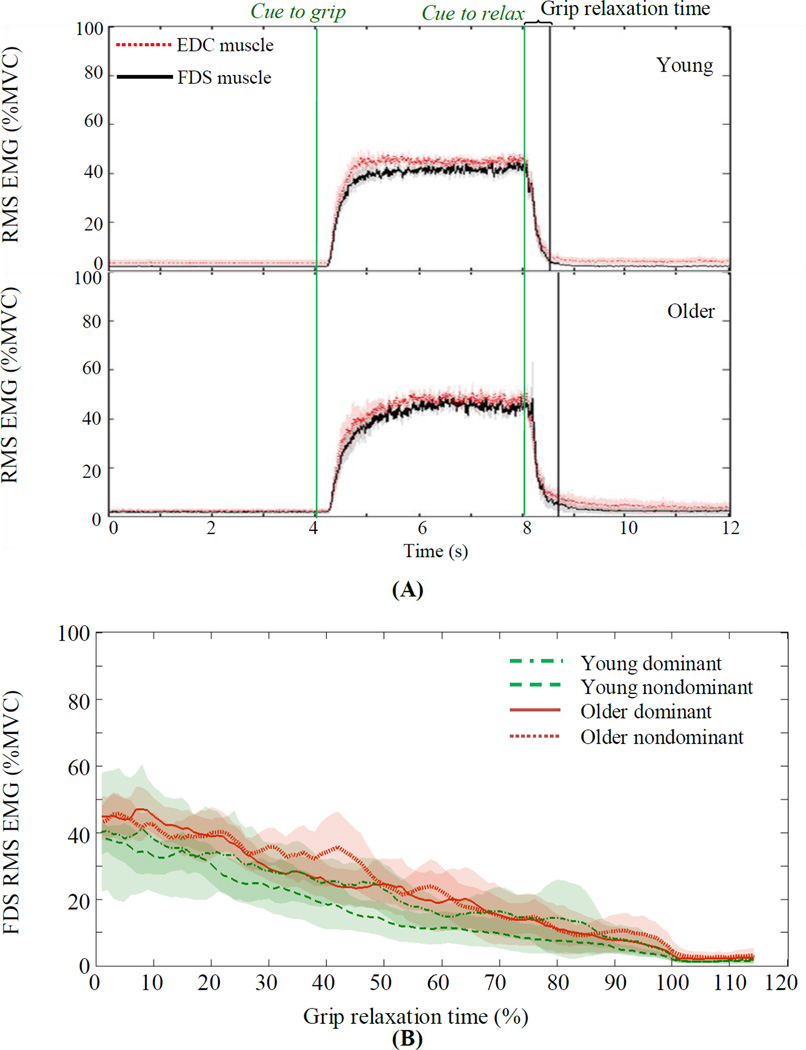
Grip relaxation time was significantly longer for older adults than young adults (F(1, 78)=9.93, p=0.002, Figure 3). There was no significant main effect of hand (dominant vs. nondominant, F(1, 78)=0.54, p=0.464) or interaction between aging and hand (F(1, 78)=0.001, p=0.977). Subjects did not activate antagonist muscles during grip relaxation, as seen by both FDS and EDC muscle activities decreasing in a consistent manner for both young and older adults (Figure 4A, after “Cue to relax”). When the FDS EMG relaxations for young and older adults are overlaid over 0–100% of individuals’ relaxation times, similar rates of reduction in the FDS RMS EMG were observed in the two aging groups (Figure 4B).
Figure 3.
Mean grip relaxation time (ms) for the dominant and nondominant hands of young and older adults. * indicates the main effect of age, p<0.05. Error bars indicate 95% confidence intervals.
Figure 4.
(A) During the grip-and-relax trial, both FDS and EDC muscles were active during grip and decreased during relaxation, similarly for young and older adults. EMGs are expressed as %MVC when MVC was the maximum RMS EMG observed during all grip-and-relax trials. (B) During the grip relaxation time (expressed as 0–100% of individuals’ grip relaxation time), FDS EMG decreased similarly for both hands of young and older adults. Shades indicate 95% confidence intervals.
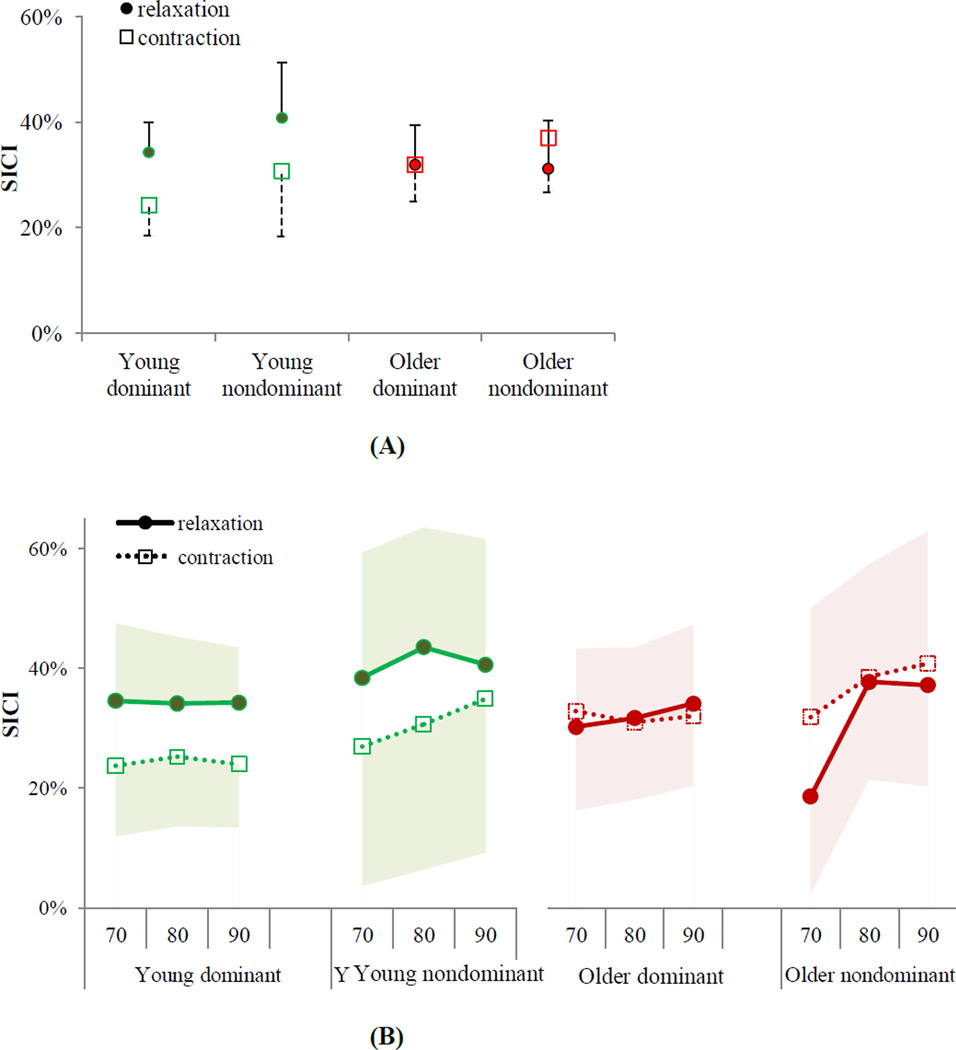
3.2 Lack of increase in SICI during grip relaxation in older adults
While young adults increased SICI during grip relaxation compared to sustained contraction at the matching EMG level by an average of 36%, older adults had an average of 7% decrease in SICI for relaxation (Figure 5). The ANOVA results showed that SICI significantly varied by task × aging interaction (F(1, 241)=8.11, p=0.005), but not by the task main effect (F(1, 241)=2.59, p=0.109), aging main effect (F(1, 241)=0.04, p=0.845), dominant vs. nondominant hand (F(1, 241)=0.02, p=0.883), time (F(2, 241)=2.00, p=0.138), and other interactions (p>0.05). Tukey-Kramer pairwise comparisons showed that young adults significantly increased intracortical inhibition during grip relaxation compared to sustained grip contraction at matching muscle activity (t(241)=−2.95, p=0.018, Figure 5). In contrast, older adults did not increase their intracortical inhibition during grip relaxation compared to sustained grip (Tukey-Kramer t(241)= 0.94, p=0.785, Figure 5).
Figure 5.
(A) Mean SICI during grip relaxation and during sustained grip contraction at matching muscle activity averaged for three time points showed that young adults, on average, increased SICI during grip relaxation compared to sustained grip in both hands, while older adults did not (p=0.005 for ANOVA task × aging interaction). (B) Mean SICI for the two tasks are shown separately for three time points (70%, 80%, 90% of the relaxation and their EMG-matching contractions). Error bars/shades show upper or lower bound 95% confidence intervals.
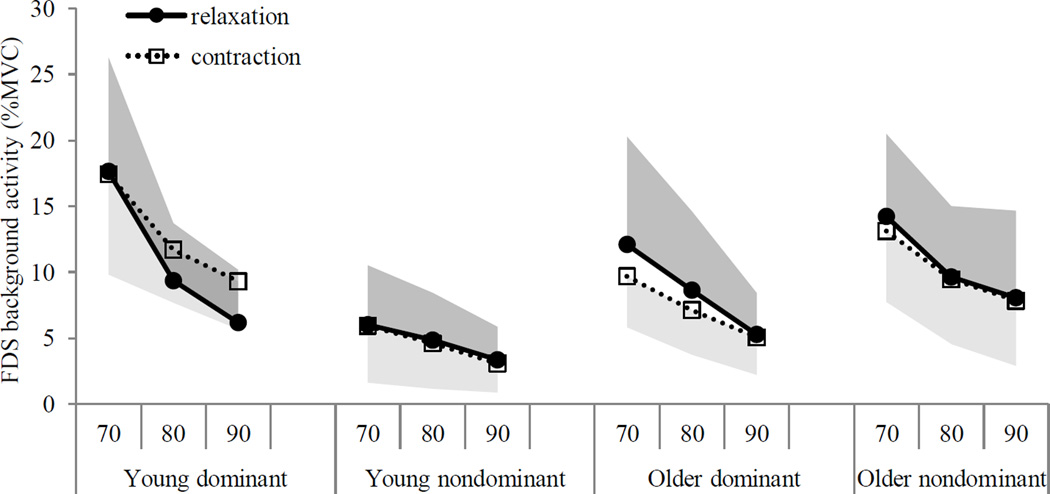
The secondary statistical analysis results are as follows. The background FDS RMS EMG did not significantly vary with task (relaxation vs. sustained contraction), aging, and second-order interactions (p>0.05), except for time (F(2, 279)=12.59, p<0.001), hand (F(1, 279)=6.24, p=0.013), and aging × hand interaction (F(1, 279)=24.53, p<0.001). As expected, the background FDS RMS EMG decreased as the time progressed (Figure 6). The background FDS RMS EMG was greater for the dominant hand than the nondominant hand in young adults (12±2% vs. 5±1%MVC), whereas it was comparable between the two hands in older adults (8±2%MVC vs. 10±2%MVC) (Figure 6).
Figure 6.
Mean background FDS EMG levels are shown for both groups and both hands. The background FDS muscle activity was not statistically different during grip relaxation vs. during sustained grip contraction within each aging group and hand. Shades show upper bound 95% confidence intervals for relaxation and lower bound 95% confidence intervals for sustained contraction.
In addition, the nonconditioned MEP peak-to-peak amplitudes did not significantly vary with task, aging, hand, and any interactions among task, aging, hand, and time (p>0.05). The nonconditioned MEP decreased with time (F(2, 241)=6.3, p=0.002), as expected with decreased background FDS EMG with time. In summary, these secondary analysis results suggest that the background FDS EMG level was well controlled between the two tasks for both aging groups (Figure 6), and that the finding of aging- and task-dependent SICI modulation was not confounded by different background EMG levels or nonconditioned MEP amplitudes.
As for the complementary analysis, the ANOVA with age as a continuous covariate showed that the task-related SICI modulation was dependent on age. SICI significantly varied with task and age × task interaction (F(1,239)=8.5, p=0.004 for task, F(1,239)=7.08, p=0.008 for age × task interaction), but not with other factors including unconditioned MEP (p>0.05). This result supports the finding of aging- and task-dependent SICI modulation. Lastly, the regression analysis showed that the grip relaxation time was significantly and negatively related to the task-dependent SICI modulation at the 70% time point (p=0.045) but not at the 80% and 90% (p>0.05). A greater SICI modulation at the 70% time point was associated with a shorter grip relaxation time.
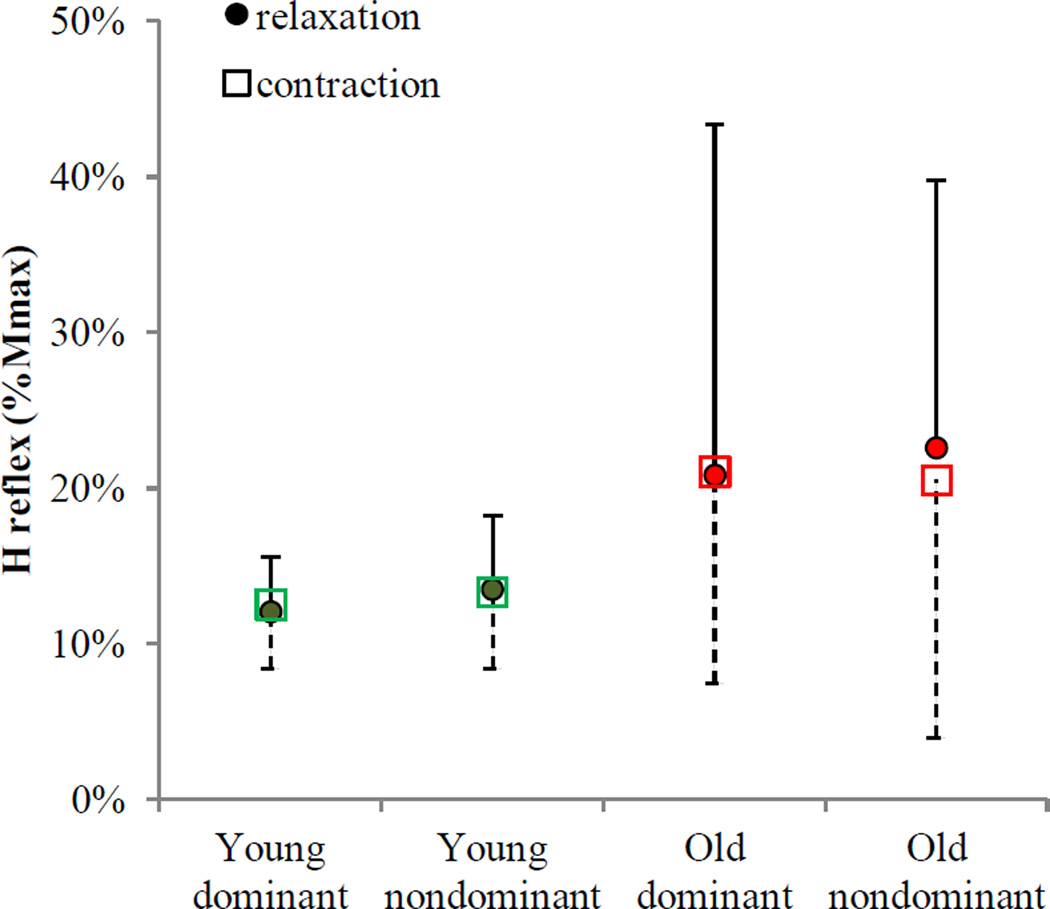
3.3 H reflex
H reflex modulation was not seen for both young and older adults (Figure 7). The ANOVA results showed that H reflex was not affected by task (F(1, 57)=0.04, p=0.848), hand (F(1, 57)=0.28, p=0.596), interaction between aging and task (F(1, 57)=0.06, p=0.808), or any other interactions (p>0.05). Older adults had overall higher H reflex amplitudes compared to young adults (21±7 vs. 13±2%Mmax, F(1, 57)=5.62, p=0.021), which was not specific to the relaxation or sustained contraction task. Both young and older adults maintained spinal motoneuron excitability during grip relaxation compared to sustained grip, as indicated by no task-specific change in H reflex for both hands in both aging groups.
Figure 7.
Mean H reflex during grip relaxation and during sustained grip contraction at matching muscle activity. No change in H reflex (normalized to Mmax) was observed between relaxation and sustained contraction at matching muscle activity for both groups and both hands. Error bars show upper bound 95% confidence intervals for relaxation and lower bound 95% confidence intervals for sustained contraction.
The secondary statistical analysis for the background FDS EMG for H reflex testing showed that the background FDS RMS EMG was not significantly different between the two tasks (18±5%MVC during grip relaxation vs.17±4 %MVC during sustained grip, F(1, 57)=0.33, p=0.566), nor with hand (F(1, 57)=0.53, p=0.470), and interactions among task, aging, and hand (p>0.05). The mean background FDS RMS EMG was greater for young adults compared to older adults (21±4 vs. 9±3%MVC, F(1, 57)=4.64, p=0.035). Yet, within the group, the background FDS EMG level was well controlled between the two tasks. This secondary analysis suggests that relaxation-specific modulation of H reflex (or lack thereof) was not confounded by different background EMG levels between two tasks.
When age was treated as a continuous covariate in the statistical model for the H reflex, the same results were obtained. H reflex was not affected by task (F(1,89)=0.1, p=0.758), hand (F(1,89)=0.01, p=0.927), interaction between age and task (F(1,89)=0.06, p=0.811), and any other interactions (p>0.05). H reflex amplitudes were greater for older adults than young adults (F(1,89)=5.95, p=0.017).
4. Discussion
The main finding was that older adults were slower in relaxing their grip compared to young adults, and this delay in grip relaxation time was associated with a lack of increase in short-interval intracortical inhibition during grip relaxation in older adults. H reflex did not change for grip relaxation compared to sustained contraction at the matching muscle activity, for both young and older adults. This finding suggests a lesser role of spinal motor neuron excitability for grip relaxation. The present study expands previous knowledge by demonstrating that older adults not only have a slowed reaction time (Fozard et al., 1994, Smith et al., 1999, Der and Deary, 2006) but also a slowed grip relaxation time (Figure 3). In addition, while previous research studied the role of intracortical inhibition alone in timely cessation of the hand muscle relaxation in healthy young adults (Buccolieri et al., 2004a, Begum et al., 2005, Motawar et al., 2012), the current study is the first to examine grip relaxation for both young and older adults at both cortical and spinal levels.
Increased short-interval intracortical inhibition may inhibit corticospinal motor neurons and thus contribute to terminating grip activity in young adults (Buccolieri et al., 2004a, Begum et al., 2005, Motawar et al., 2012). This study results suggest that older adults’ reduced ability to increase the cortical inhibitory action during their attempt to relax the muscle may be responsible for slower muscle relaxation time. Older adults’ reduced ability to decrease SICI to initiate a movement has previously been demonstrated (Marneweck et al., 2011, Heise et al., 2013, Papegaaij et al., 2014), associating the lack of SICI modulation with the declined performance of functional tasks (Heise et al., 2013, Papegaaij et al., 2014). Our study expands the previous literature by demonstrating that this lack of SICI modulation exists not only at movement initiation but also at movement termination, contributing to decline in motor performance in older adults. This aging-related change in modulation of short-interval intracortical inhibition may be related to changes in the GABA-A circuits (Ziemann et al., 1998, Di Lazzaro et al., 2005, 2006) with aging, such as changes in the structural properties of the GABA-A receptors in addition to reduced GABA content and transport in the aging brain (see Wong (2002) for review).
Our finding of stable spinal motor excitability during grip relaxation was somewhat different from the previous studies concerning the soleus muscle (Schieppati and Crenna, 1984, Schieppati et al., 1985, 1986). The previous studies showed that the H reflex in the soleus muscle, an antigravity postural muscle in the leg, was decreased during and after muscle relaxation compared to the resting state in healthy young adults (Schieppati et al., 1986). The motor control of the hand and leg muscles may be distinct from each other because of their different functional demands. It is likely that the leg muscles may have greater spinal control through reflex circuits contributing to the maintenance of balance and posture as well as gait, a function not shared by hand muscles. The hand muscles are known to have greater cerebral control that likely contributes to the hand’s ability to perform fine motor tasks with great precision (Brouwer and Ashby, 1990, de Noordhout et al., 1999). Thus, it is possible that the spinal circuits may play a lesser role for hand muscle control compared to leg muscles owing to their different functional roles and neural connectivity. Likewise, relaxation from a contraction may be under greater cortical control for hand muscles than leg muscles.
This study identifies a neural mechanism of timely muscle relaxation. While this study described differences in neurophysiology and function between older and young adults, causality between neurophysiology and function was not demonstrated. Future studies may explore experimental manipulation of the brain network, such as using rTMS to impair normal function, to reveal direct causal relationships. Future research may also examine interventions to restore SICI modulation in older adults to facilitate timely muscle relaxation and improve movement quality. Such interventions may include neuromodulation by brain stimulation (Boros et al., 2008, Stagg et al., 2009, Stetkarova and Kofler, 2013), operant conditioning (Wolf and Segal, 1990, Tenteromano et al., 2012, Arduin et al., 2013), and GABA agonists (Misgeld et al., 1995, Di Lazzaro et al., 2005, Di Lazzaro et al., 2006). This study only examined the cortical inhibitory and spinal mechanisms and thus does not tease apart the relative contributions of the changes in the skeletal muscles and cortical inhibitory activity on delayed grip relaxation with aging.
5. Conclusion
This study demonstrated that grip relaxation is delayed in older adults and this delay was associated with their lack of increase in short-interval intracortical inhibition during grip relaxation. These delays in terminating muscle activity, in addition to general aging-related slowness in movement initiation and execution, may contribute to a deterioration of motor control in older adults. Interventions to increase the plasticity of GABA-Aergic inhibitory cortical circuits may be useful in improving muscle relaxation and general motor control in older adults.
Acknowledgements
The authors would like to acknowledge funding agencies, the American Heart Association, the National Institutes of Health grant P20GM109040 awarded to the Medical University of South Carolina, the Clinical and Translational Science Award program of the National Center for Research Resources, National Institute of Health through, Grant Number 1UL1RR031973, the University of Wisocnsin-Milwaukee College of Health Sciences, the American Society of Biomechanics, and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 8UL1TR000055 for providing support for this research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest: None of the authors have potential conflicts of interest to be disclosed.
References
- Adam A, Luca CJD, Erim Z. Hand Dominance and Motor Unit Firing Behavior. 1998 doi: 10.1152/jn.1998.80.3.1373. [DOI] [PubMed] [Google Scholar]
- Arduin PJ, Fregnac Y, Shulz DE, Ego-Stengel V. "Master" neurons induced by operant conditioning in rat motor cortex during a brain-machine interface task. J Neurosci. 2013;33:8308–8320. doi: 10.1523/JNEUROSCI.2744-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basmajian JV. Biofeedback: Principles and Practice for Clinicians. Baltimore: Williams & Wilkins; 1989. [Google Scholar]
- Begum T, Mima T, Oga T, Hara H, Satow T, Ikeda A, Nagamine T, Fukuyama H, Shibasaki H. Cortical mechanisms of unilateral voluntary motor inhibition in humans. Neurosci Res. 2005;53:428–435. doi: 10.1016/j.neures.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Abbruzzese G, Chen R, Orth M, Ridding MC, Stinear C, Suppa A, Trompetto C, Thompson PD. Consensus paper on short-interval intracortical inhibition and other transcranial magnetic stimulation intracortical paradigms in movement disorders. Brain Stimul. 2008;1:183–191. doi: 10.1016/j.brs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Boros K, Poreisz C, Munchau A, Paulus W, Nitsche MA. Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. Eur J Neurosci. 2008;27:1292–1300. doi: 10.1111/j.1460-9568.2008.06090.x. [DOI] [PubMed] [Google Scholar]
- Brouwer B, Ashby P. Corticospinal projections to upper and lower limb spinal motoneurons in man. Electroencephalogr Clin Neurophysiol. 1990;76:509–519. doi: 10.1016/0013-4694(90)90002-2. [DOI] [PubMed] [Google Scholar]
- Buccolieri A, Abbruzzese G, Rothwell JC. Relaxation from a voluntary contraction is preceded by increased excitability of motor cortical inhibitory circuits. J Physiol. 2004a;558:685–695. doi: 10.1113/jphysiol.2004.064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccolieri A, Avanzino L, Marinelli L, Trompetto C, Marchese R, Abbruzzese G. Muscle relaxation is impaired in dystonia: a reaction time study. Mov Disord. 2004b;19:681–687. doi: 10.1002/mds.10711. [DOI] [PubMed] [Google Scholar]
- Chae J, Yang G, Park BK, Labatia I. Delay in initiation and termination of muscle contraction, motor impairment, and physical disability in upper limb hemiparesis. Muscle Nerve. 2002;25:568–575. doi: 10.1002/mus.10061. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol. 2006;95:3371–3383. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- de Noordhout AM, Rapisarda G, Bogacz D, Gerard P, De Pasqua V, Pennisi G, Delwaide PJ. Corticomotoneuronal synaptic connections in normal man: an electrophysiological study. Brain. 1999;122(Pt 7):1327–1340. doi: 10.1093/brain/122.7.1327. [DOI] [PubMed] [Google Scholar]
- Der G, Deary IJ. Age and sex differences in reaction time in adulthood: results from the United Kingdom Health and Lifestyle Survey. Psychology and aging. 2006;21:62–73. doi: 10.1037/0882-7974.21.1.62. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Dileone M, Pilato F, Nardone R, Ranieri F, Musumeci G, Fiorilla T, Tonali P. Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J Physiol. 2005;564:661–668. doi: 10.1113/jphysiol.2004.061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Ranieri F, Ricci V, Profice P, Bria P, Tonali PA, Ziemann U. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J Physiol. 2006;575:721–726. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclay J, Martin A. Evoked H-reflex and V-wave responses during maximal isometric, concentric, and eccentric muscle contraction. J Neurophysiol. 2005;94:3555–3562. doi: 10.1152/jn.00348.2005. [DOI] [PubMed] [Google Scholar]
- Fozard JL, Vercryssen M, Reynolds SL, Hancock PA, Quilter RE. Age differences and changes in reaction time: the Baltimore Longitudinal Study of Aging. Journal of gerontology. 1994;49:P179–P189. doi: 10.1093/geronj/49.4.p179. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Hinder MR, Schmidt MW, Garry MI, Summers JJ. Age-related differences in corticospinal excitability and inhibition during coordination of upper and lower limbs. Neurobiology of aging. 2012;33:1484, e1481–e1414. doi: 10.1016/j.neurobiolaging.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Grasso M, Mazzini L, Schieppati M. Muscle relaxation in Parkinson's disease: a reaction time study. Mov Disord. 1996;11:411–420. doi: 10.1002/mds.870110410. [DOI] [PubMed] [Google Scholar]
- Heise KF, Zimerman M, Hoppe J, Gerloff C, Wegscheider K, Hummel FC. The aging motor system as a model for plastic changes of GABA-mediated intracortical inhibition and their behavioral relevance. J Neurosci. 2013;33:9039–9049. doi: 10.1523/JNEUROSCI.4094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knikou M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods. 2008;171:1–12. doi: 10.1016/j.jneumeth.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan J, Peltier SJ, Bo J, Fling BW, Welsh RC, Seidler RD. Functional implications of age differences in motor system connectivity. Frontiers in systems neuroscience. 2010;4:17. doi: 10.3389/fnsys.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J. Human aging, muscle mass, and fiber type composition. The journals of gerontology Series A, Biological sciences and medical sciences. 1995;50 doi: 10.1093/gerona/50a.special_issue.11. Spec No: 11–16. [DOI] [PubMed] [Google Scholar]
- Marneweck M, Loftus A, Hammond G. Short-interval intracortical inhibition and manual dexterity in healthy aging. Neurosci Res. 2011;70:408–414. doi: 10.1016/j.neures.2011.04.004. [DOI] [PubMed] [Google Scholar]
- McGinnis SM, Brickhouse M, Pascual B, Dickerson BC. Age-related changes in the thickness of cortical zones in humans. Brain topography. 2011;24:279–291. doi: 10.1007/s10548-011-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Progress in neurobiology. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- Motawar B, Hur P, Stinear J, Seo NJ. Contribution of intracortical inhibition in voluntary muscle relaxation. Exp Brain Res. 2012;221:299–308. doi: 10.1007/s00221-012-3173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Akiguchi I, Kameyama M, Mizuno N. Age-related changes of pyramidal cell basal dendrites in layers III and V of human motor cortex: A quantitative Golgi study. Acta Neuropathol. 1985;65:281–284. doi: 10.1007/BF00687009. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K. Proteomic Profiling of Fast-To-Slow Muscle Transitions during Aging. Frontiers in physiology. 2011;2:105. doi: 10.3389/fphys.2011.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V. Effects of aging on motor cortex excitability. Neuroscience Research. 2006;55:74–77. doi: 10.1016/j.neures.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Ortu E, Deriu F, Suppa A, Tolu E, Rothwell JC. Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J Physiol. 2008;586:5147–5159. doi: 10.1113/jphysiol.2008.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri RM, Ingersoll CD, Hoffman MA. The hoffmann reflex: methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train. 2004;39:268–277. [PMC free article] [PubMed] [Google Scholar]
- Papegaaij S, Taube W, Baudry S, Otten E, Hortobagyi T. Aging causes a reorganization of cortical and spinal control of posture. Frontiers in aging neuroscience. 2014;6:28. doi: 10.3389/fnagi.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Conrad B, Siebner HR. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett. 2001;313:33–36. doi: 10.1016/s0304-3940(01)02239-x. [DOI] [PubMed] [Google Scholar]
- Peurala SH, Muller-Dahlhaus JF, Arai N, Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF) Clin Neurophysiol. 2008;119:2291–2297. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Raffalt PC, Alkjaer T, Simonsen EB. Changes in soleus H-reflex during walking in middle-aged healthy subjects. Muscle Nerve. 2014 doi: 10.1002/mus.24279. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Screening questionnaire before TMS: An update. Clinical Neurophysiology. 2011;122:1686. doi: 10.1016/j.clinph.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. The Journal of Physiology. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieppati M, Crenna P. From activity to rest: gating of excitatory autogenetic afferences from the relaxing muscle in man. Exp Brain Res. 1984;56:448–457. doi: 10.1007/BF00237985. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Nardone A, Musazzi M. Modulation of the Hoffmann reflex by rapid muscle contraction or release. Hum Neurobiol. 1986;5:59–66. [PubMed] [Google Scholar]
- Schieppati M, Poloni M, Nardone A. Voluntary muscle release is not accompanied by H-reflex inhibition in patients with upper moto neuron lesions. Neurosci Lett. 1985;61:177–181. doi: 10.1016/0304-3940(85)90421-5. [DOI] [PubMed] [Google Scholar]
- Seo NJ, Fischer HW, Bogey RA, Rymer WZ, Kamper DG. Effect of a serotonin antagonist on delay in grip muscle relaxation for persons with chronic hemiparetic stroke. Clin Neurophysiol. 2009a;122:796–802. doi: 10.1016/j.clinph.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Seo NJ, Rymer WZ, Kamper DG. Delays in grip initiation and termination in persons with stroke: Effects of arm support and active muscle stretch exercise. J Neurophysiol. 2009b;101:3108–3115. doi: 10.1152/jn.91108.2008. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Gordon AD, Allen JS, Phillips KA, Erwin JM, Hof PR, Hopkins WD. Aging of the cerebral cortex differs between humans and chimpanzees. Proceedings of the National Academy of Sciences. 2011;108:13029–13034. doi: 10.1073/pnas.1016709108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Umberger GH, Manning EL, Slevin JT, Wekstein DR, Schmitt FA, Markesbery WR, Zhang Z, Gerhardt GA, Kryscio RJ, Gash DM. Critical decline in fine motor hand movements in human aging. Neurology. 1999;53:1458–1461. doi: 10.1212/wnl.53.7.1458. [DOI] [PubMed] [Google Scholar]
- Spraker MB, Corcos DM, Vaillancourt DE. Cortical and subcortical mechanisms for precisely controlled force generation and force relaxation. Cereb Cortex. 2009;19:2640–2650. doi: 10.1093/cercor/bhp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, Bestmann S. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009;101:2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB, Estabrooks KL, McGie S, Roth MJ, Jones KE. Quantifying the effects of voluntary contraction and inter-stimulus interval on the human soleus H-reflex. Exp Brain Res. 2007;182:309–319. doi: 10.1007/s00221-007-0989-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetkarova I, Kofler M. Differential effect of baclofen on cortical and spinal inhibitory circuits. Clin Neurophysiol. 2013;124:339–345. doi: 10.1016/j.clinph.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience & Biobehavioral Reviews. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Tan U. The H-reflex recovery curve from the wrist flexors: lateralization of motoneuronal excitability in relation to handedness in normal subjects. Int J Neurosci. 1989;48:271–284. doi: 10.3109/00207458909002170. [DOI] [PubMed] [Google Scholar]
- Tenteromano L, Amsterdam AJ, Brangaccio J, Sniffen J, Thompson AK. Society for Neuroscience 42ndAnnual Meeting. Vol. 378.08. New Orleans, LA: 2012. Operant conditioning of the ankle dorsiflexor motor evoked potential (MEP) in people with and without CNS damage: Changes MEP size and silent period. [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Toma K, Honda M, Hanakawa T, Okada T, Fukuyama H, Ikeda A, Nishizawa S, Konishi J, Shibasaki H. Activities of the primary and supplementary motor areas increase in preparation and execution of voluntary muscle relaxation: an event-related fMRI study. J Neurosci. 1999;19:3527–3534. doi: 10.1523/JNEUROSCI.19-09-03527.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Laar MC, van den Wildenberg WP, van Boxtel GJ, Huizenga HM, van der Molen MW. Lifespan changes in motor activation and inhibition during choice reactions: a Laplacian ERP study. Biological psychology. 2012;89:323–334. doi: 10.1016/j.biopsycho.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Segal RL. Conditioning of the spinal stretch reflex: implications for rehabilitation. Phys Ther. 1990;70:652–656. doi: 10.1093/ptj/70.10.652. [DOI] [PubMed] [Google Scholar]
- Wolkorte R, Kamphuis J, Zijdewind I. Increased reaction times and reduced response preparation already starts at middle age. Frontiers in aging neuroscience. 2014;6:79. doi: 10.3389/fnagi.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. Aging of the Cerebral Cortex. McGill Journal of Medicine. 2002;6:104–113. [Google Scholar]
- Yakovlev PI, Rakic P. Patterns of decussation of bulbar pyramids and distribution of pyramidal tracts on two sides of the spinal cord. Trans Am Neurol Assoc. 1966;91:366–367. [Google Scholar]
- Zehr EP. Considerations for use of the Hoffmann reflex in exercise studies. European journal of applied physiology. 2002;86:455–468. doi: 10.1007/s00421-002-0577-5. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wischer S, Hildebrandt J, Paulus W. Pharmacological control of facilitatory I-wave interaction in the human motor cortex. A paired transcranial magnetic stimulation study. Electroencephalogr Clin Neurophysiol. 1998;109:321–330. doi: 10.1016/s0924-980x(98)00023-x. [DOI] [PubMed] [Google Scholar]