Abstract
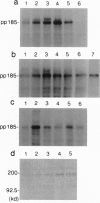
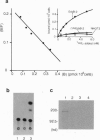
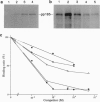
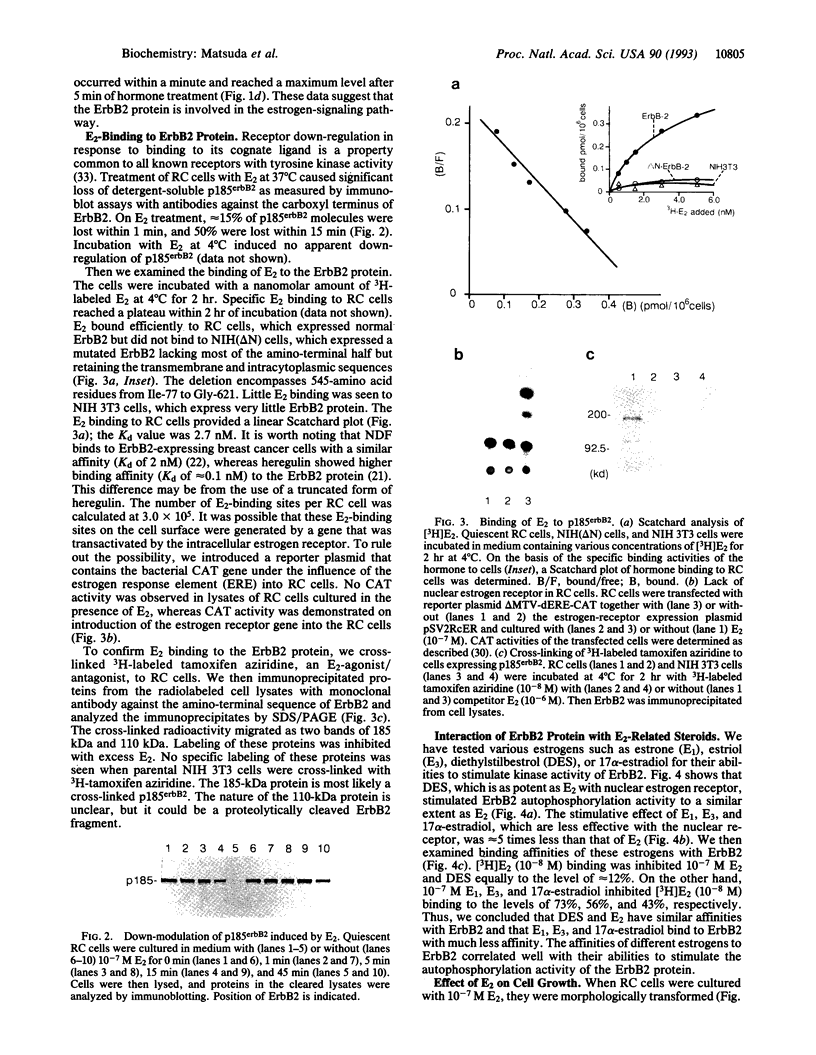
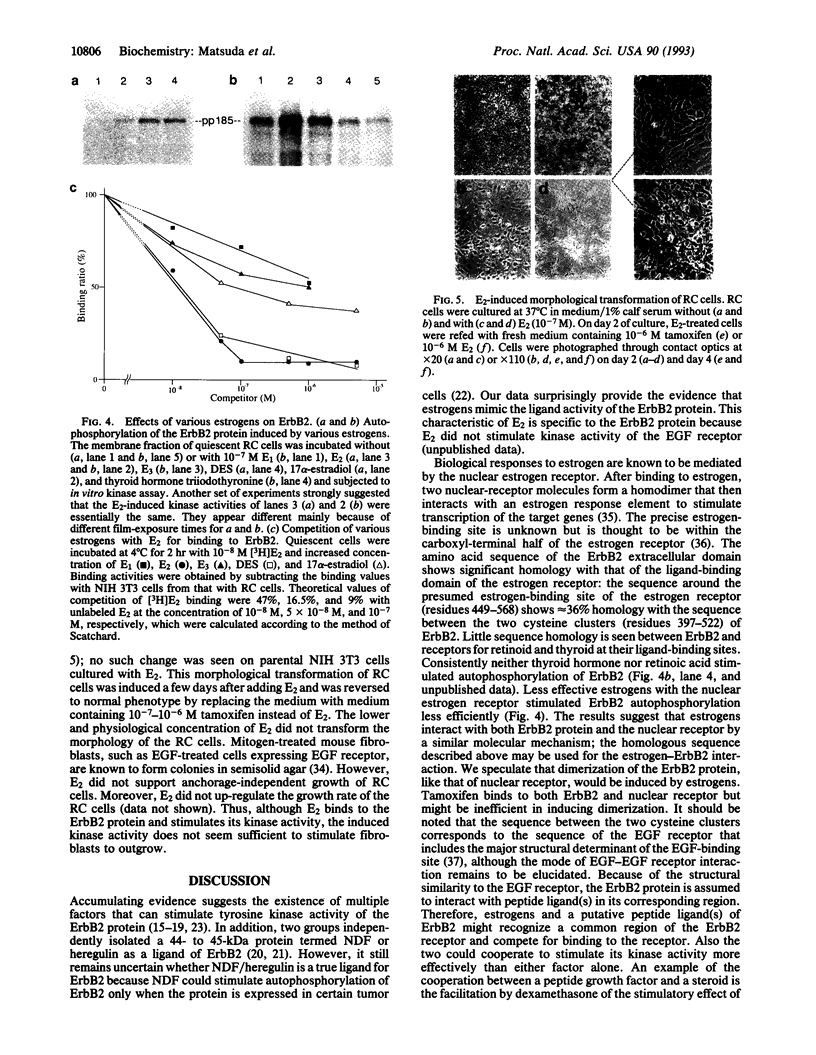
We report here the physical and functional interaction of estrogen with the ErbB2 protein p185c-erbB2. The ErbB2 protein immunoprecipitated from estrogen-treated [10(-8) to 10(-6) M 17 beta-estradiol (E2)] RC cells showed higher autophosphorylation activity than that from untreated cells. Likewise autophosphorylation activity of ErbB2 protein from untreated cells was stimulated in vitro by E2. In addition, E2 treatment induced down-regulation of ErbB2 protein from the detergent-soluble fraction of the RC cells within 15 min. E2 also induced morphological transformation of the RC cells but not of the parental NIH 3T3 cells, which express little c-erbB2 under the same experimental conditions. This morphological transformation of RC cells was reversed by tamoxifen. However, E2 treatment did not induce anchorage-independent growth of RC cells. Scatchard analysis revealed E2 binding to the ErbB2 protein on RC cells; the Kd value was 2.7 nM. E2 did not bind appreciably to the parental NIH 3T3 cells or cells expressing an ErbB2 protein lacking most of its extracellular domain. These data suggest that estrogen plays an important role in ErbB2-mediated signaling.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Matsuda S., Namba Y., Saito T., Toyoshima K., Yamamoto T. The transforming potential of the c-erbB-2 protein is regulated by its autophosphorylation at the carboxyl-terminal domain. Mol Cell Biol. 1991 Feb;11(2):833–842. doi: 10.1128/mcb.11.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T., Saito T., Ogawara H., Toyoshima K., Yamamoto T. Tumor promoter and epidermal growth factor stimulate phosphorylation of the c-erbB-2 gene product in MKN-7 human adenocarcinoma cells. Mol Cell Biol. 1988 Mar;8(3):1019–1026. doi: 10.1128/mcb.8.3.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T., Sudo C., Ogawara H., Toyoshima K., Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986 Jun 27;232(4758):1644–1646. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- An G., Hidaka K., Siminovitch L. Expression of bacterial beta-galactosidase in animal cells. Mol Cell Biol. 1982 Dec;2(12):1628–1632. doi: 10.1128/mcb.2.12.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986 Jan 16;319(6050):226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Weinberg R. A. Increased tyrosine kinase activity associated with the protein encoded by the activated neu oncogene. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5394–5398. doi: 10.1073/pnas.85.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L., Yang-Feng T. L., Liao Y. C., Chen E., Gray A., McGrath J., Seeburg P. H., Libermann T. A., Schlessinger J., Francke U. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985 Dec 6;230(4730):1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- Davis J. G., Hamuro J., Shim C. Y., Samanta A., Greene M. I., Dobashi K. Isolation and characterization of a neu protein-specific activating factor from human ATL-2 cell conditioned medium. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1536–1542. doi: 10.1016/0006-291x(91)91747-z. [DOI] [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Fleming T. P., Hazan R., Ullrich A., King C. R., Schlessinger J., Aaronson S. A. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987 Dec 24;51(6):1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- Dobashi K., Davis J. G., Mikami Y., Freeman J. K., Hamuro J., Greene M. I. Characterization of a neu/c-erbB-2 protein-specific activating factor. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8582–8586. doi: 10.1073/pnas.88.19.8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974 May 10;249(453):123–127. doi: 10.1038/249123a0. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Evans R. M. Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell. 1988 Dec 2;55(5):899–906. doi: 10.1016/0092-8674(88)90145-6. [DOI] [PubMed] [Google Scholar]
- Holmes W. E., Sliwkowski M. X., Akita R. W., Henzel W. J., Lee J., Park J. W., Yansura D., Abadi N., Raab H., Lewis G. D. Identification of heregulin, a specific activator of p185erbB2. Science. 1992 May 22;256(5060):1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- Huang S. S., Koh H. A., Konish Y., Bullock L. D., Huang J. S. Differential processing and turnover of the oncogenically activated neu/erb B2 gene product and its normal cellular counterpart. J Biol Chem. 1990 Feb 25;265(6):3340–3346. [PubMed] [Google Scholar]
- Hudziak R. M., Schlessinger J., Ullrich A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7159–7163. doi: 10.1073/pnas.84.20.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Iwase H., Itoh Y., Fukuoka H., Yamashita H., Kuzushima T., Iwata H., Masaoka A., Kimura N. Estrogen receptor, c-erbB-2 and nm23/NDP kinase expression in the intraductal and invasive components of human breast cancers. Jpn J Cancer Res. 1992 Aug;83(8):859–865. doi: 10.1111/j.1349-7006.1992.tb01991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S., Sakai M., Muramatsu M. Molecular cloning and characterization of rat estrogen receptor cDNA. Nucleic Acids Res. 1987 Mar 25;15(6):2499–2513. doi: 10.1093/nar/15.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Lax I., Burgess W. H., Bellot F., Ullrich A., Schlessinger J., Givol D. Localization of a major receptor-binding domain for epidermal growth factor by affinity labeling. Mol Cell Biol. 1988 Apr;8(4):1831–1834. doi: 10.1128/mcb.8.4.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu R., Colomer R., Zugmaier G., Sarup J., Shepard M., Slamon D., Lippman M. E. Direct interaction of a ligand for the erbB2 oncogene product with the EGF receptor and p185erbB2. Science. 1990 Sep 28;249(4976):1552–1555. doi: 10.1126/science.2218496. [DOI] [PubMed] [Google Scholar]
- Masuko T., Sugahara K., Kozono M., Otsuki S., Akiyama T., Yamamoto T., Toyoshima K., Hashimoto Y. A murine monoclonal antibody that recognizes an extracellular domain of the human c-erbB-2 protooncogene product. Jpn J Cancer Res. 1989 Jan;80(1):10–14. doi: 10.1111/j.1349-7006.1989.tb02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Akiyama T., Yamada Y., Morishita Y., Sugawara I., Toyoshima K., Yamamoto T. C-erbB-2 gene product, a membrane protein commonly expressed on human fetal epithelial cells. Lab Invest. 1989 Jul;61(1):93–97. [PubMed] [Google Scholar]
- Nomura Y., Kobayashi S., Takatani O., Sugano H., Matsumoto K., McGuire W. L. Estrogen receptor and endocrine responsiveness in Japanese versus American breast cancer patients. Cancer Res. 1977 Jan;37(1):106–110. [PubMed] [Google Scholar]
- Peles E., Bacus S. S., Koski R. A., Lu H. S., Wen D., Ogden S. G., Levy R. B., Yarden Y. Isolation of the neu/HER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992 Apr 3;69(1):205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- Peles E., Ben-Levy R., Tzahar E., Liu N., Wen D., Yarden Y. Cell-type specific interaction of Neu differentiation factor (NDF/heregulin) with Neu/HER-2 suggests complex ligand-receptor relationships. EMBO J. 1993 Mar;12(3):961–971. doi: 10.1002/j.1460-2075.1993.tb05737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato B., Miyashita Y., Maeda Y., Noma K., Kishimoto S., Matsumoto K. Effects of estrogen and vanadate on the proliferation of newly established transformed mouse Leydig cell line in vitro. Endocrinology. 1987 Mar;120(3):1112–1120. doi: 10.1210/endo-120-3-1112. [DOI] [PubMed] [Google Scholar]
- Shan R. J., Matsuda S., Ichino M., Yamamoto T. Detection of the ligand activity of the c-erbB-2 protein in calf serum. Jpn J Cancer Res. 1992 Jan;83(1):15–19. doi: 10.1111/j.1349-7006.1992.tb02345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Stancovski I., Hurwitz E., Leitner O., Ullrich A., Yarden Y., Sela M. Mechanistic aspects of the opposing effects of monoclonal antibodies to the ERBB2 receptor on tumor growth. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8691–8695. doi: 10.1073/pnas.88.19.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kamps M. P., Cao H. Oncogenic activation of p185neu stimulates tyrosine phosphorylation in vivo. Mol Cell Biol. 1988 Sep;8(9):3969–3973. doi: 10.1128/mcb.8.9.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Velu T. J., Beguinot L., Vass W. C., Willingham M. C., Merlino G. T., Pastan I., Lowy D. R. Epidermal-growth-factor-dependent transformation by a human EGF receptor proto-oncogene. Science. 1987 Dec 4;238(4832):1408–1410. doi: 10.1126/science.3500513. [DOI] [PubMed] [Google Scholar]
- Wittliff J. L. Steroid-hormone receptors in breast cancer. Cancer. 1984 Feb 1;53(3 Suppl):630–643. doi: 10.1002/1097-0142(19840201)53:3+<630::aid-cncr2820531308>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Wright C., Nicholson S., Angus B., Sainsbury J. R., Farndon J., Cairns J., Harris A. L., Horne C. H. Relationship between c-erbB-2 protein product expression and response to endocrine therapy in advanced breast cancer. Br J Cancer. 1992 Jan;65(1):118–121. doi: 10.1038/bjc.1992.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Ikawa S., Akiyama T., Semba K., Nomura N., Miyajima N., Saito T., Toyoshima K. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature. 1986 Jan 16;319(6050):230–234. doi: 10.1038/319230a0. [DOI] [PubMed] [Google Scholar]
- Yamanashi Y., Kakiuchi T., Mizuguchi J., Yamamoto T., Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science. 1991 Jan 11;251(4990):192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Weinberg R. A. Experimental approaches to hypothetical hormones: detection of a candidate ligand of the neu protooncogene. Proc Natl Acad Sci U S A. 1989 May;86(9):3179–3183. doi: 10.1073/pnas.86.9.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota J., Yamamoto T., Toyoshima K., Terada M., Sugimura T., Battifora H., Cline M. J. Amplification of c-erbB-2 oncogene in human adenocarcinomas in vivo. Lancet. 1986 Apr 5;1(8484):765–767. doi: 10.1016/s0140-6736(86)91782-4. [DOI] [PubMed] [Google Scholar]
- Zhou D., Battifora H., Yokota J., Yamamoto T., Cline M. J. Association of multiple copies of the c-erbB-2 oncogene with spread of breast cancer. Cancer Res. 1987 Nov 15;47(22):6123–6125. [PubMed] [Google Scholar]