Abstract
FGF21 is a stress-induced hormone with potent anti-obesity, insulin-sensitizing, and hepatoprotective properties. Although proteolytic cleavage of recombinant human FGF21 in preclinical species has been observed previously, the regulation of endogenously produced FGF21 is not well understood. Here we identify fibroblast activation protein (FAP) as the enzyme that cleaves and inactivates human FGF21. A selective chemical inhibitor, immunodepletion, or genetic deletion of Fap stabilized recombinant human FGF21 in serum. In addition, administration of a selective FAP inhibitor acutely increased circulating intact FGF21 levels in cynomolgus monkeys. On the basis of our findings, we propose selective FAP inhibition as a potential therapeutic approach to increase endogenous FGF21 activity for the treatment of obesity, type 2 diabetes, non-alcoholic steatohepatitis, and related metabolic disorders.
Keywords: FGF, metabolic disease, protease inhibitor, proteolytic enzyme, serine protease, FGF21, dipeptidyl peptidase IV (DPPIV), fibroblast activation protein (FAP)
Introduction
Obesity is linked to various metabolic derangements such as insulin resistance, hyperinsulinemia, dyslipidemia, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis (NASH).2 These metabolic problems can often lead to severe illnesses such as type 2 diabetes, liver cirrhosis, stroke, and heart disease (1). Indeed, the global pandemic of obesity has made it the leading cause of disability around the world (2). Reflecting this rapidly growing medical issue, the list of anti-obesity and anti-diabetic agents has been increasing in recent years. However, a need for novel therapeutic agents to address obesity and related health issues clearly persists.
FGF21 is an endocrine member of the FGF superfamily that acts as a hormone circulating within the blood stream (3, 4). Supraphysiological exposure to recombinant FGF21 or its engineered analogs ameliorates obesity, insulin resistance, dyslipidemia, fatty liver, and hyperglycemia in rodents (5, 6). In mice, the metabolic activity of FGF21 is attributed to the stimulation of thermogenesis in brown adipose tissues (5, 6), white adipose tissue browning (7), hepatic fatty acid oxidation (8), amelioration of hepatic endoplasmic reticulum stress (9), and induction of the adipokine adiponectin (10, 11). Mice lacking FGF21 are refractory to diet-induced energy expenditure (12) and prone to developing NASH (8, 13, 14). The metabolic action of FGF21 appears to be conserved in obese diabetic humans, at least to a certain extent. Small clinical trials have demonstrated that FGF21 analogs are efficacious in inducing weight loss and correcting hyperinsulinemia, dyslipidemia, and hypoadiponectinemia in obese individuals with type 2 diabetes (15, 16).
Non-obese humans circulate variable levels of FGF21 protein (0.02–5 ng/ml), which is stable with time within each individual (17). In individuals with obesity or type 2 diabetes, circulating FGF21 levels are increased (18–20). In addition, a correlation between hepatic fat content and serum FGF21 in humans has been reported (21). Almost all circulating FGF21 is derived from the liver in mice and, presumably, also in other mammalian species (22). Mechanistically, the increased production of FGF21 has been attributed to an induction of hepatic FGF21 mRNA expression by fatty acids, the unfolded protein response, and glucocorticoids (9, 17, 23). In addition to the endocrine effects, FGF21 has autocrine or paracrine roles at the site of expression (3). On the basis of the beneficial activity of recombinant FGF21 and the metabolic phenotypes of FGF21-deficient mice, the observed increase in FGF21 expression in obese individuals is thought to be protective. However, the induction of endogenous FGF21 expression alone is apparently not sufficient to prevent obesity-associated metabolic derangements.
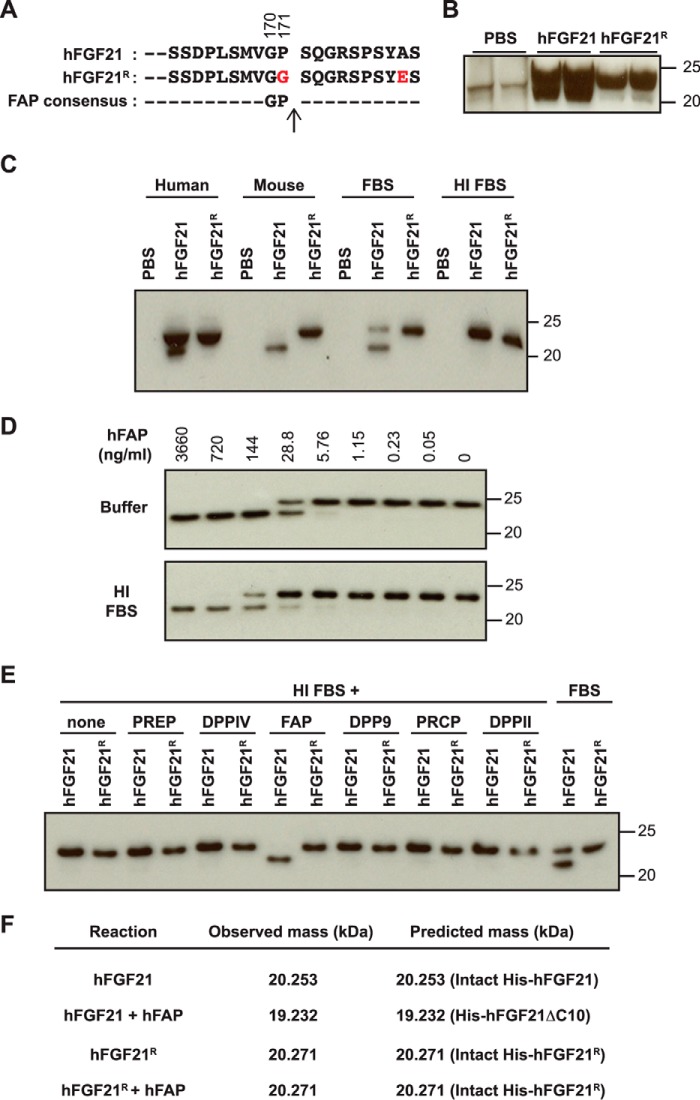
Human FGF21 (hFGF21) can be cleaved proteolytically at both the N terminus and the C terminus. At the N terminus, the first four amino acid residues, His-Pro-Ile-Pro- (Tyr-Pro-Ile-Pro- in mouse FGF21 (mFGF21)), can be removed because of an undefined dipeptidyl peptidase activity (24). FGF21 lacking these four amino acid residues is fully active (25, 26). At the C terminus, 10 amino acid residues are cleaved off rapidly upon hFGF21 injection into mice or cynomolgus monkeys (27). hFGF21 protein lacking the last 10 amino acid residues is not able to bind to the obligatory co-receptor βKlotho (KLB) and is ∼380-fold less potent in cell-based signaling assays (25, 26). Therefore, this site-specific cleavage essentially generates an inactive form of the hFGF21 protein. Using site-directed mutagenesis, two amino acid residues, Gly at position 2 (P2) and Pro at P1, have been shown to be essential for this site-specific cleavage (amino acid residues 170 and 171, respectively, Fig. 1A) (27). A mutation in one of these residues generates a proteolysis-resistant hFGF21 variant that is more efficacious than its cleavable counterpart in vivo (27).
FIGURE 1.
FGF21 cleavage in serum and by recombinant FAP. A, the C-terminal sequence of hFGF21 and the cleavage-resistant mutant (hFGF21R) used for the study. Residues in hFGF21R that differ from wild-type hFGF21 are indicated in red. Gly170 and Pro171 are required for site-specific proteolysis. B, 6-week-old C57BL/6 male mice (n = 2 mice/group) were injected with vehicle (PBS), hFGF21 (10 mg/kg), or hFGF21R (10 mg/kg). 1 h after injection, serum was prepared and subjected to immunoblot analysis to detect full-length FGF21 (top band) or cleaved FGF21 (bottom band). C, cleavage of recombinant hFGF21 and hFGF21R in serum was assessed by anti-hFGF21 immunoblot analysis after incubation in human or mouse serum, FBS, or heat-inactivated FBS overnight at 37 °C. D, hFGF21 was incubated with the indicated concentrations of recombinant hFAP in buffer or HI FBS and subjected to immunoblot analysis to detect hFGF21. E, hFGF21 or hFGF21R was incubated with the indicated human recombinant proteases in HI FBS. FGF21 cleavage was assessed by immunoblot analysis. Active FBS was used as a positive control. PRCP, lysosomal Pro-X carboxypeptidase. F, hFGF21 or hFGF21R was incubated with or without recombinant hFAP in buffer, and the mass of FGF21 the cleavage products was determined by LC/MS. The observed mass was compared with the predicted mass of the indicated full-length His-tagged hFGF21 or His-hFGF21ΔC10, which lacks 10 amino acid residues at the C terminus. C–F, hFGF21 was incubated overnight at 37 °C.
These studies have proposed that mice and cynomolgus monkeys produce an endopeptidase that cleaves and inactivates not only recombinant FGF21 but also endogenous FGF21. Indeed, it has been reported recently that up to 35% of the circulating FGF21 may be subjected to a C-terminal truncation in healthy individuals (28), and the number may be higher in disease conditions. If this is true, we might be able to increase the activity of endogenous FGF21 by inhibiting this proteolytic activity. An impetus for this idea comes from the inactivation of glucagon-like peptide 1 (GLP-1) by dipeptidyl peptidase IV (DPPIV) (29). DPPIV-resistant GLP-1 analogs (exenatide, liraglutide, etc.) are used to increase insulin secretion in type 2 diabetics. In addition, selective DPPIV inhibitors (sitagliptin, alogliptin, linagliptin, etc.) also increase insulin secretion by augmenting the level of active GLP-1 by preventing DPPIV-mediated cleavage. Both DPPIV inhibitors and stabilized GLP-1 analogs are currently used in the clinic for the treatment of type 2 diabetes.
Here we identify fibroblast activation protein (FAP, also known as FAP-α, seprase, or circulating antiplasmin-cleaving enzyme, EC 3.4.21.B28) as a site-specific endopeptidase for hFGF21. FAP is a type II transmembrane serine protease that is also found in human serum as a soluble form lacking the transmembrane domain (30–32). In adult animals, FAP activity is found in a variety of tissues, including the pancreas, skin, lymph nodes, skeletal muscle, colon, and adipose tissues (33). FAP expression is also induced at sites of wound healing, tissue remodeling, fibrosis, inflammation, and tumorigenesis (32, 34). FAP is the closest structural relative to DPPIV (also described as CD26 or FAP-β). FAP, together with DPPIV, DPP8 and DPP9, forms a family of post-proline dipeptidyl aminopeptidases known simply as the DPPIV family of proteins (35). Both FAP and DPPIV exhibit dipeptidase activity, but only FAP exhibits endopeptidase activity as well (31, 32). Our discovery suggests FAP inhibition as a potential approach to increase the beneficial activity of endogenous FGF21 protein for the treatment of obesity-related metabolic disorders.
Experimental Procedures
Research Ethics
All animal studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (publication 8523, revised 1985). The Institutional Animal Care and Use Committee at Genentech reviewed and approved all animal protocols.
Recombinant Proteins
N-terminally His5-tagged hFGF21 (catalog no. 2539-FG/CF), untagged-mFGF21 (catalog no. 8409-FG/CF), human prolyl oligopeptidase (PREP, catalog no. 4308-SE), DPPIV (catalog no. 1180-SE), FAP (catalog no. 3715-SE), DPP9 (catalog no. 5419-SE), lysosomal Pro-X carboxypeptidase (catalog no. 7164-SE), and DPPII/DPP7 (catalog no. 3438-SE) proteins were from R&D Systems. N-terminally His5-tagged cleavage-resistant hFGF21 mutant (designated as hFGF21R, R for resistant) containing a triple L98R/P171G/A180E mutation and the N-terminally His5-tagged or human IgG1 Fc-tagged cleavage-susceptible counterpart containing the L98R variant (designated hFGF21 and Fc-hFGF21, respectively) were produced in Escherichia coli, purified, and refolded using standard methods as described previously (27). The L98R change has been reported to reduce protein aggregation and facilitate protein production without a significant effect on activity (27). The A180E change was introduced in hFGF21R to prevent a secondary cleavage event that becomes apparent only when the primary cleavage event is blocked by the P171G change (Fig. 1A) (36). N-terminal His8-tagged human, cynomolgus monkey, and murine FAPs containing the extracellular domain (residues Leu26-Asp760, Leu26-Asp760, and Leu26-Asp761, respectively) were purified from transiently transfected CHO cell supernatant by immobilized metal affinity chromatography, followed by size exclusion chromatography. FAP-cleaved hFGF21 (hFGF21ΔC10) was generated by incubating N-terminally His5-tagged hFGF21 (wild-type (R&D Systems) and the L98R version) in PBS at a 10:1 mass ratio with human FAP (hFAP) overnight at 37 °C. Complete cleavage of hFGF21 was confirmed by LC/MS. Cleaved hFGF21 was then isolated by size exclusion chromatography using an S75 10/300 gel filtration column (GE Healthcare). Coomassie staining of SDS-PAGE gels was performed using SimplyBlue SafeStain (Thermo Fisher Scientific), and BSA (Pierce) was used as a standard.
Serum Isolation and Immunodepletion
6-week-old male C57BL/6 mice (The Jackson Laboratory) or 6- 8-week-old male Fap-deficient mice and littermate controls were used to isolate serum by incubating blood for >30 min at room temperature, followed by centrifugation at 1000 rpm for 10 min. One ShotTM FBS was purchased from Life Technologies and heat-inactivated at 56 °C for a minimum of 30 min. Human serum was obtained from healthy donors. For immunodepletion, 0.5 ml of serum was mixed with 10 μg of either a biotinylated sheep anti-hFAP polyclonal antibody (R&D Systems, catalog no. BAF3716) or biotinylated sheep IgG (R&D Systems, catalog no. BAF020) for 2 h at room temperature on a rotator. Protein-antibody complexes were separated using 0.2 ml of streptavidin magnetic M-280 Dynabeads (Thermo Fisher Scientific) for 30 min for a total of three rounds.
Immunoassays
For immunoblot analysis, proteins were separated on SDS-PAGE gels and transferred to nitrocellulose membranes. Antibodies used for immunodetection were anti-FGF21 (BioVendor, catalog no. RD181108100), an in-house generated anti-hFAP mouse monoclonal antibody (15A11), a mouse anti-FAP antibody (28H1) (34), anti-human DPPIV (R&D Systems, catalog no. MAB1180), and an anti-GAPDPH antibody (Cell Signaling Technology, catalog no. 3683). Secondary antibodies were ECL HRP-linked antibodies from GE Healthcare Life Sciences (anti-rabbit, catalog no. NA934; anti-mouse, catalog no. NA931; anti-human, catalog no. NA933; and anti-rat, catalog no. NA935). Proteins were detected using the ECL Prime Western blotting detection reagent and visualized on Amersham Biosciences Hyperfilm ECL. Total (R&D Systems, catalog no. DY2539 (vendor A) or BioVendor, catalog no. RD191108200R (vendor B)) or intact (Alpco, catalog no. 43-FGFHU-E01) hFGF21 ELISAs were performed according to the instructions of the manufacturers.
FGF21 Cleavage Assay
A final concentration of 400 ng/ml recombinant FGF21 was incubated with serum at 37 °C and analyzed following overnight incubation. FAP cleavage of FGF21 was conducted either in heat-inactivated FBS or in 50 mm Tris buffer (pH 7.5) containing 1 m NaCl and 1 mg/ml BSA. PREP, DPPIV, DPP9, lysosomal Pro-X carboxypeptidase, and DPPII were used at 43 nm. Alternatively, in vitro FAP cleavage of recombinant FGF21 was conducted overnight at 37 °C in 50 mm HEPES (pH 7.2), 150 mm NaCl, 0.1 mg/ml BSA, and the resultant FGF21 masses were analyzed by standard LC/MS methods.
Assessment of FGF21 Activity
The signaling activity of FGF21 was assessed in a GAL-ELK1 luciferase assay in HEK293T cells as reported previously (37). Briefly, HEK293T cells were transfected with plasmids encoding human KLB, human fibroblast growth factor 1c (FGFR1c), Renilla luciferase (pRL-SV40, Promega), a transcriptional activator (pFA2-ELK1, Stratagene), and a firefly luciferase reporter driven by GAL4 (pFR-luc, Stratagene), using FuGENE® (Roche) according to the protocol of the manufacturer. 10 μg/ml hFGF21 proteins were incubated with or without equimolar amounts of human FAP or DPPIV in 50 mm HEPES (pH 7.2), 150 mm NaCl, and 0.1 mg/ml BSA at 37 °C. Following overnight incubation, the reaction was diluted in serum-free medium and applied to transfected cells for 6.5 h. Cellular luciferase activity was determined using the Dual-Glo® luciferase assay system (Promega) and quantified on an EnVision® multilabel reader (PerkinElmer Life Sciences). Relative light units were calculated by normalizing the firefly luciferase value by the Renilla luciferase value.
FRET-quench Assay
FRET-quench peptides of the region flanking the FGF21 cleavage site (e.g. VGPSQG) were synthesized (Anaspec), containing an amine-terminal donor (HyLiteTM Fluor 488) and dark quencher (QXLTM 520) conjugated to a supplemental C-terminal lysine. Assays were conducted at 37 °C in 50 mm HEPES (pH 7.2), 150 mm NaCl, 1 mm EDTA, and 0.1 mg/ml BSA. The excitation/emission wavelengths of cleaved peptide were 490/520 nm, and fluorescence was monitored on a Tecan Safire2 plate reader in kinetic mode. Measurements of serum or plasma FAP activity were performed at the peptide substrate Km, 3 μm (data not shown). For IC50 measurements, the FAP concentration was 0.25 nm. Data represent the mean ± S.E. of three independent experiments.
FAP Inhibitor
The FAP-specific inhibitor (S)-N-(2-(2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)-quinoline-4-carboxamide (compound 60) was synthetized according to an experimental procedure reported previously (38).
In Vivo Studies
Fap-deficient mice were generated by conventional homologous recombination methods in embryonic stem cells to delete the first two exons of the Fap gene. Following homologous recombination, a PGK-neo-selectable marker was deleted by Cre-mediated recombination, and deletion was confirmed by Southern blotting analysis. Plasma samples were prepared in K3-EDTA MiniCollect tubes (Greiner Bio-One) and stored at −80 °C prior to ELISA analysis.
The cynomolgus monkey study was conducted at Covance Laboratories (Madison, WI). Compound 60 was formulated in 0.5% methylcellulose/0.2% Tween 80 and administered via oral gavage intubation. Blood samples were collected pre-and post-dose from the femoral vein. The plasma concentration of compound 60 was quantified using a qualified LC-MS/MS method (lower limit of quantification, 5 nm). FAP activity was measured using a six-residue FRET-quench substrate corresponding to the residues flanking the hFGF21 C-terminal cleavage site (i.e. VGP SQG). Intact FGF21 concentrations were measured by intact FGF21 ELISA (Alpco, catalog no. 43-FGFHU-E01).
Results
Recombinant Human FGF21 Undergoes Proteolytic Cleavage in Vivo and ex Vivo
A previous study has shown that recombinant hFGF21 undergoes inactivating cleavage of the 10 amino acid residues at the C terminus when administered to mice and cynomolgus monkeys (27). For this cleavage event, the Gly residue at P2 and the Pro residue at P1 are necessary (27). To confirm these findings, we generated N-terminally His-tagged recombinant hFGF21 proteins with wild-type (hFGF21) or cleavage-resistant (hFGF21R) C termini (Fig. 1A). We injected 10 mg/kg of these recombinant proteins intravenously into C57BL/6 mice, isolated serum 1 h after injection, and performed immunoblot analysis using an anti-FGF21 antibody. As shown in Fig. 1B, a mobility shift was detected with hFGF21 but not hFGF21R, consistent with the in vivo cleavage of FGF21 proposed previously.
To determine whether isolated serum contains the activity responsible for the observed hFGF21 proteolysis, we incubated recombinant hFGF21 or hFGF21R in isolated human, mouse, or fetal bovine serum at 37 °C overnight and then subjected the reactions to immunoblot analysis. Again, wild-type hFGF21, but not hFGF21R, exhibited a similar mobility shift, suggesting that a site-specific proteolytic activity for the C terminus exists in serum. Furthermore, the proteolytic activity was ablated by preincubation of FBS at 65 °C, suggesting that a heat-labile component is responsible for the observed hFGF21 proteolysis (Fig. 1C).
FAP Cleaves and Inactivates hFGF21
The MEROPS database lists 58 human proteases with known substrates that are cleaved after a Pro residue at P1 (39). Of these, 14 have known substrates that are cleaved after the Gly residue at P2 and the Pro residue at P1, and only one, FAP, that showed a clear preference for a Gly residue at P2 and a Pro residue at P1 (40) (Fig. 1A). To test the ability of FAP to cleave recombinant hFGF21 at a specific site, the extracellular domain of recombinant hFAP was incubated with hFGF21 in a serum-free buffer or heat-inactivated (HI) FBS. hFGF21 cleavage was then assessed by immunoblot analysis. As shown in Fig. 1D, recombinant hFAP cleaved hFGF21 in a dose-dependent manner in either serum-free buffer (top panel) or when mixed with heat-inactivated serum (bottom panel). This activity was not observed when hFGF21R was used as a substrate (Fig. 1E). Other related S9 family serine peptidases, PREP, DPPIV, DPP9, lysosomal Pro-X carboxypeptidase, or DPPII, did not cleave hFGF21 under these conditions (Fig. 1E). LC/MS analysis confirmed that recombinant hFAP cleaves hFGF21 in vitro specifically at the reported in vivo cleavage site, between Pro171 and Ser172, while leaving hFGF21R intact (Fig. 1F).
Previous studies using hFGF21 deletion mutants have demonstrated that the C-terminal 10 amino acid residues of hFGF21 are absolutely essential for KLB binding and signaling activity (25, 26). To confirm this notion, we incubated hFGF21 or hFGF21R with or without recombinant hFAP. The cleavage of hFGF21 but not that of hFGF21R by hFAP was confirmed by immunoblot analysis (Fig. 2A). We then assessed the signaling activity of these predigested proteins in a GAL-ELK1-based luciferase assay using HEK293T cells expressing FGFR1c alone or both FGFR1c and KLB. As expected, hFGF21 increased luciferase reporter activity in a KLB-dependent manner (Fig. 2B). This activity was ablated by preincubating hFGF21 with recombinant hFAP. In contrast, hFGF21R remained active even after preincubation with hFAP (Fig. 2B). Therefore, FAP not only cleaves hFGF21 but also inactivates its signaling capability, likely by abolishing its ability to bind to the co-receptor KLB that requires the C-terminal 10 amino acid residues of hFGF21.
FIGURE 2.
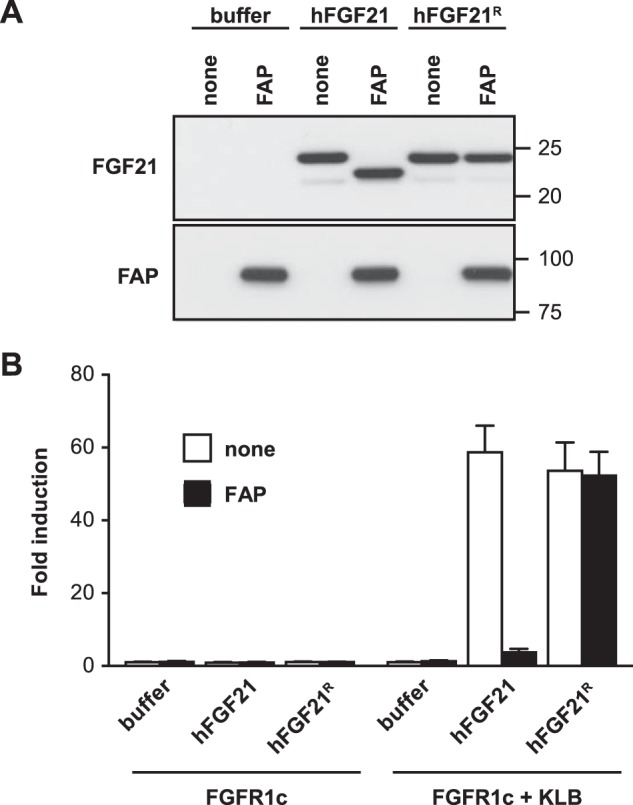
Loss of signaling activity following FAP cleavage of hFGF21. A, hFGF21 was incubated with or without recombinant hFAP in buffer and then analyzed by immunoblotting to detect FGF21 (top) or FAP (bottom). B, hFGF21 proteins preincubated with (black columns) or without hFAP (white columns) were tested using a GAL-ELK1 luciferase assay at 40 ng/ml in HEK293T cells expressing FGFR1c alone or FGFR1c and KLB (see “Experimental Procedures” for details). The experiment was performed in triplicate, and the results are shown as mean fold induction ± S.E.
FAP Is Essential for hFGF21 Cleavage
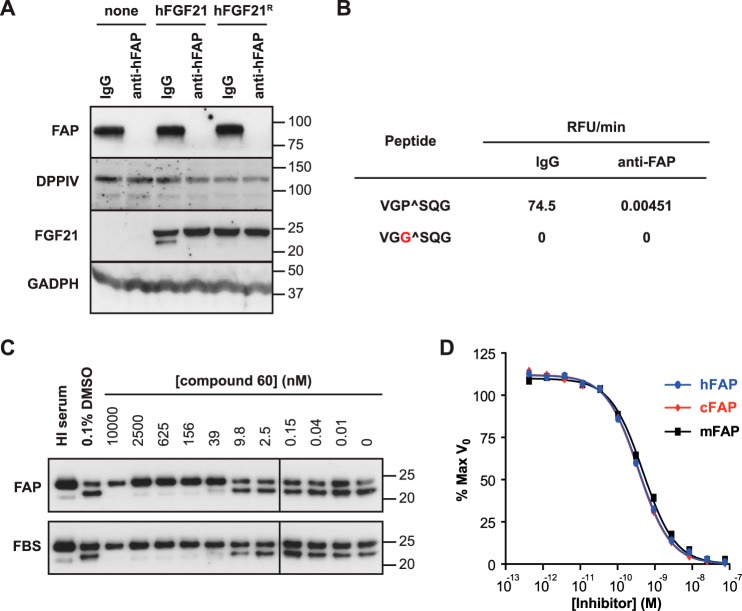
To determine whether FAP is the only enzyme in human serum responsible for the observed cleavage of hFGF21 C-terminal tail, we incubated human serum with a biotin-labeled anti-hFAP polyclonal antibody (anti-hFAP) or a control biotinylated antibody (IgG) and then removed protein-antibody complexes using immunoprecipitation with streptavidin magnetic beads. This procedure successfully depleted FAP from human serum on the basis of immunoblot analysis (Fig. 3A, first panel). The levels of DPPIV, the closest relative to FAP (51% identity), were unchanged following FAP immunodepletion (Fig. 3A, second panel). When the resulting samples were tested for hFGF21 cleavage, we found that the cleavage activity was lost specifically in the FAP-immunodepleted sample (Fig. 3A, third panel). In addition, a FRET-quench-based peptide cleavage assay using a peptide bearing the six-residue sequence that flanks the C-terminal FAP cleavage site of hFGF21 confirmed that FAP immunodepletion completely ablated FAP activity (Fig. 3B).
FIGURE 3.
C-terminal hFGF21 cleavage in serum requires FAP. A, human serum immunodepleted by anti-hFAP or control IgG antibody was incubated with hFGF21 or hFGF21R overnight at 37 °C. Immunoblot analysis was used to assess immunodepletion of FAP and FGF21 cleavage. DPPIV and GADPH served as loading controls. B, FAP activity in the immunodepleted serum samples used in A was determined by FRET-quench using wild-type (top) or cleavage-resistant peptides. C, hFGF21 was incubated with recombinant hFAP at a molar ratio of 4:1 (top) or with FBS (bottom) and the indicated concentrations of compound 60. FGF21 cleavage was visualized by immunoblot analysis with anti-hFGF21. As a control, 0.1% dimethyl sulfoxide (DMSO, vehicle) was tested. In addition, hFGF21 incubated with HI serum was loaded also as a control. D, FRET-quench was used to determine the IC50 value for compound 60 for recombinant human (0.37 ± 0.02 nm), cynomolgus monkey (0.35 ± 0.02 nm), and mouse FAP (0.47 ± 0.03 nm).
The importance of FAP in hFGF21 cleavage was tested further by using a selective and potent FAP inhibitor, compound 60 (38). We chose to test this molecule because it is not only potent (with a reported IC50 of 3.2 nm for dipeptidyl peptidase activity) but also highly specific toward FAP compared with PREP and other S9 proteases (38). When we compared the ability of compound 60 to inhibit hFGF21 cleavage by recombinant FAP (Fig. 3C, top panel) or active FBS (Fig. 3C, bottom panel), we found that it inhibited both activities with similar concentrations, consistent with the hypothesis that FAP is indeed the enzyme responsible for FGF21 cleavage in FBS. Using an hFGF21 FRET-quench peptide substrate, compound 60 was found to inhibit endopeptidase activity of recombinant human, cynomolgus monkey, and mouse FAP proteins with similar subnanomolar IC50 values, consistent with a previous report (Fig. 3D).
Validation of Human FGF21 ELISA
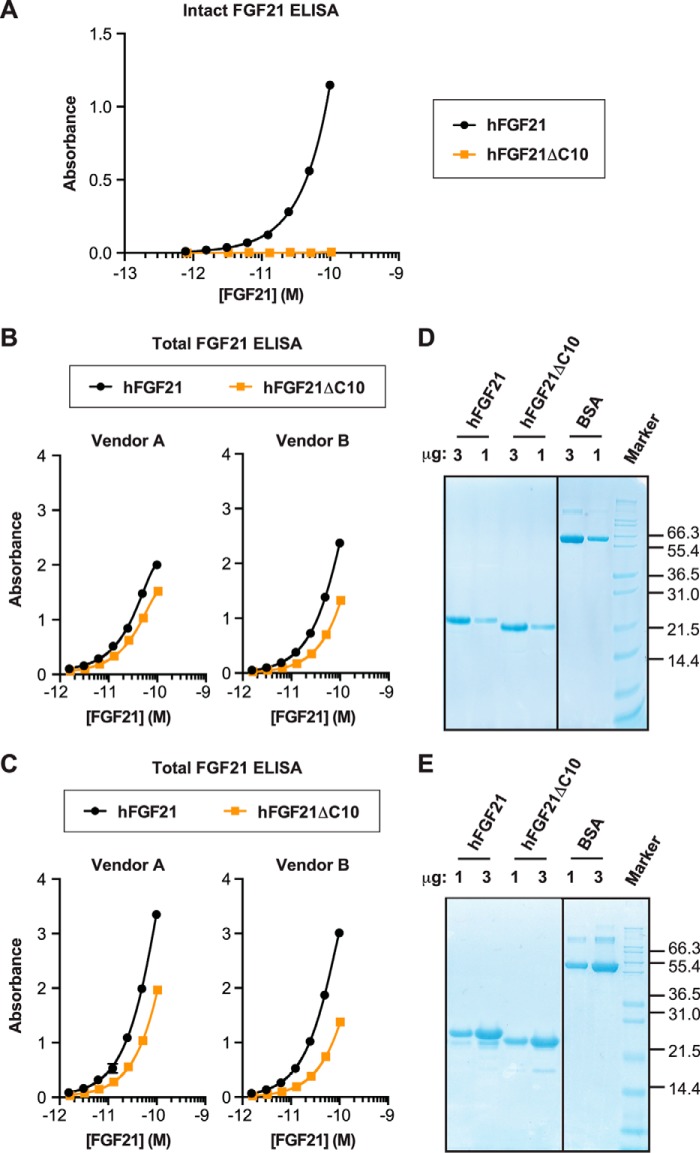
To establish appropriate assays to evaluate the effect of FAP-mediated hFGF21 cleavage in vivo, we decided to first evaluate various available ELISA kits for detection of intact and C-terminally truncated hFGF21. Assuming that the truncated hFGF21 protein in vivo is produced by FAP-mediated cleavage, we prepared truncated hFGF21 protein by digesting full-length hFGF21 protein with confirmed activity using recombinant hFAP protein rather than by expressing truncated hFGF21 from a mutated cDNA.
The intact hFGF21 ELISA kit used for this study consists of one antibody that specifically binds to the N-terminal seven amino acid residues of hFGF21 and another antibody specific for the C-terminal six amino acid residues. As expected, this assay detected only intact hFGF21 but not the purified C-terminally truncated hFGF21 (hFGF21ΔC10) (Fig. 4A). We then compared the intact and truncated hFGF21 using two ELISA kits commonly used to quantify total hFGF21 in clinical samples (e.g. Refs. 18, 20, 41, 42) (Fig. 4B). To our surprise, both ELISA kits detected intact FGF21 with ∼2-fold better efficiency than the truncated version, suggesting that these assays could actually underestimate the concentration of truncated FGF21 in plasma or serum ∼2-fold. Similar observations were made when we used another hFGF21 protein preparation and its FAP-digested derivative (Fig. 4C). SDS-PAGE analysis confirmed that the proteins tested by ELISA had the expected concentrations (Fig. 4, D and E). So far, all of our efforts to develop an appropriate ELSA-based assay that does not discriminate between intact versus truncated FGF21 have been unsuccessful. In addition, we have not been able to reliably determine the relative level of endogenous FGF21 in its intact or truncated form by mass spectrometry or various immunoassays. Therefore, we decided to focus on the determination of intact FGF21 levels for the in vivo analysis described below.
FIGURE 4.
Evaluation of FGF21 ELISA. A and B, wild-type hFGF21 and the FAP-digested derivative (hFGF21ΔC10) were tested by using intact hFGF21 ELISA (A) or total hFGF21 ELISA from two separate sources (B). C, hFGF21 (L98R) and the FAP-digested derivative (hFGF21ΔC10) were tested by using the total hFGF21 ELISA used in B. A–C, each protein was tested in duplicate at each concentration, and the results are plotted as mean ± S.E. D and E, Coomassie staining of SDS-PAGE gels. The proteins used for the ELISAs in B and C were analyzed in D and E, respectively, with BSA as a control.
Targeted Deletion of the Fap Gene Abolishes hFGF21 Cleavage ex Vivo and in Vivo
Mice bearing a homozygous deletion in the Fap gene are viable and exhibit no overt phenotype (43). To test the role of FAP in hFGF21 cleavage in mouse serum ex vivo, we isolated serum from Fap-deficient (−/−) mice as well as from wild-type (+/+) and heterozygous (+/−) littermate controls. As shown in Fig. 5A, top panel, serum isolated from heterozygous Fap mice exhibited decreased serum FAP levels, whereas serum from Fap-deficient mice lacked FAP completely. We then incubated sera from these mice with recombinant hFGF21 in vitro. We found that hFGF21 cleavage was reduced when this protein was incubated with serum from heterozygous mice and ablated completely in Fap-deficient serum (Fig. 5A, center panel). In contrast, the cleavage-resistant hFGF21R protein was unaffected by incubation with sera from either genotype.
FIGURE 5.
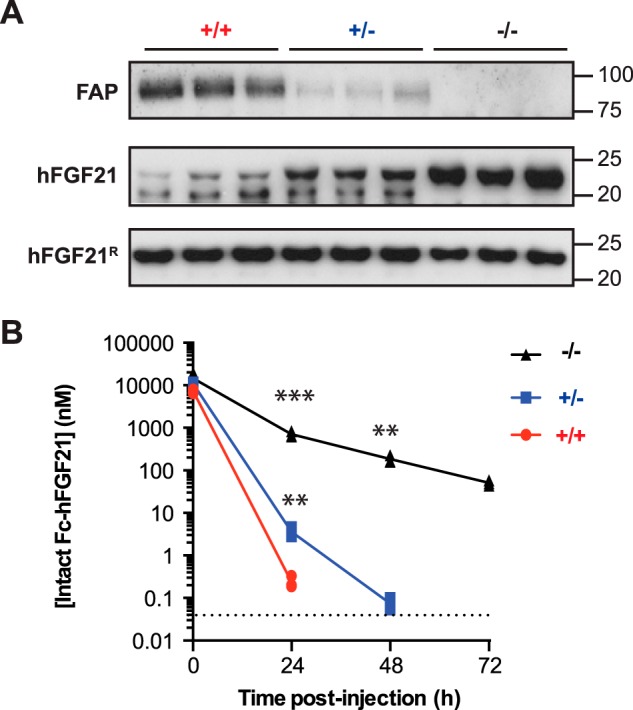
hFGF21 cleavage activity in Fap knockout mice in vitro and in vivo. A, immunoblot analysis to detect FAP in serum samples from Fap−/− mice and littermate controls (+/− and +/+) (n = 3 mice/group) (top). The same serum samples were mixed with recombinant hFGF21 (center) or hFGF21R (bottom) overnight and evaluated by immunoblot analysis to detect hFGF21. B, recombinant Fc-hFGF21 was injected intravenously into three +/+ (red lines), +/− (blue lines), or −/− (black lines) 6- to 8-week-old male mice at 20 mg/kg. Plasma was retrieved at the indicated time points and analyzed by intact FGF21 ELISA. Each point represents the intact hFGF21 concentration for one animal. **, p < 0.05; ***, p < 0.005 versus the wild-type control by two-tailed Student's t test (n = 3 mice/group).
To find out whether FAP is also responsible for hFGF21 cleavage in vivo in mice, we administered 20 mg/kg of hFGF21 fused to an Fc fragment of human IgG1 (Fc-hFGF21) intravenously to wild-type, Fap-heterozygous, or Fap-deficient mice. The serum concentration of intact Fc-hFGF21 was assessed 5 min, 24 h, 48 h, and 72 h after intravenous administration by using the specific ELISA to detect intact hFGF21 described above. As shown in Fig. 5B, the intact Fc-hFGF21 becomes undetectable in wild-type mice after 24 h, whereas the half-life of this protein is extended significantly in heterozygous mice and, to an even greater degree, in Fap-deficient mice. Therefore, decreasing the amount of FAP clearly extends the half-life of recombinant hFGF21 in its intact form.
The C-Terminus of Murine FGF21 Is Resistant to FAP Cleavage
The Gly-Pro FAP consensus residues at position 170–171 in human FGF21 (Fig. 1A) is conserved in most (70 of 79) mammalian species with available FGF21 sequences (including predicted sequences) (Supplemental Table S1). Although we cannot exclude the possibility that some of these FGF21 sequences contain sequencing errors at the putative FAP cleavage site, two closely related Muridae species, rat and mouse, clearly possess Glu-Pro instead of the conserved Gly-Pro at the putative FAP cleavage site. 12 other Rodentia species of 15 examined have the conserved Gly-Pro residues at this site, suggesting that the Gly-to-Glu change at P2 arose late during the evolution of mouse and rat. Other non-conserved species are rabbit, cat, kangaroo rat, tarsier (dry-nosed primate), and three bat species (Supplemental Table S1).
To evaluate the importance of Gly-Pro at P2-P1 and the surrounding residues for FAP cleavage, we tested the activity of hFAP with six-residue peptides corresponding to the C terminus of FGF21 from several mammalian species as well as a control substrate in which Pro at P1 is mutated to Gly (Fig. 6A). As expected from the primary protein sequence, hFAP hydrolyzed only peptides that had both Gly at P2 and Pro at P1. Therefore, hFAP failed to cleave the peptide containing the murine sequence (Fig. 6A). The corresponding peptide from guinea pig and Syrian hamster FGF21 with Leu at the P1′ position exhibited significant hydrolysis by hFAP, although at a reduced rate compared with the corresponding peptide encoding FGF21 from human, dog, cynomolgus monkey, porcine, and tree shrew (Fig. 6A). A similar sequence requirement by hFAP has been reported previously using six-residue peptides derived from the α(2)-antiplasmin sequence (40).
FIGURE 6.
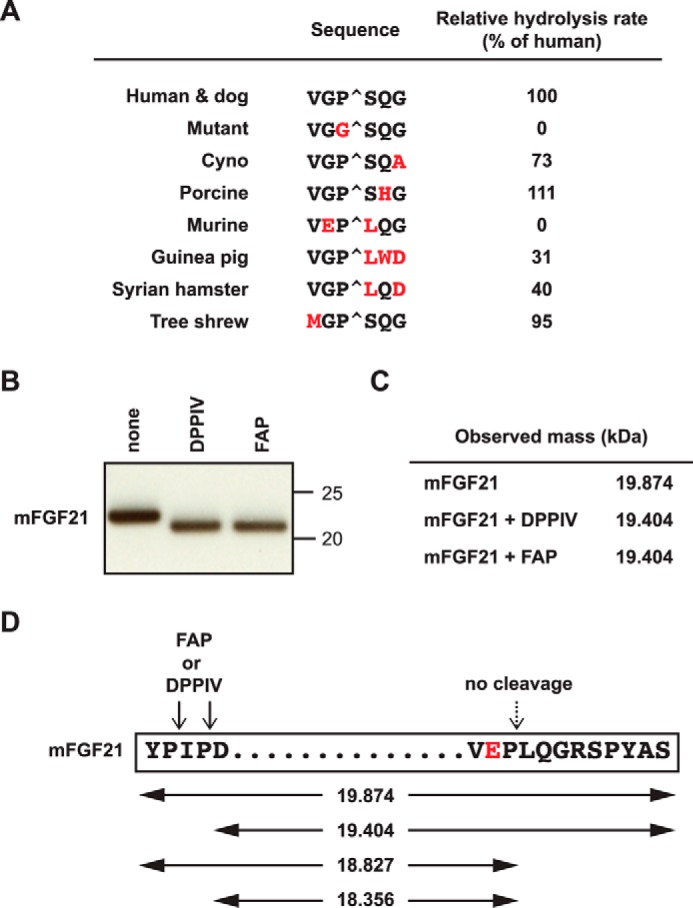
C-terminal FGF21 cleavage in different species. A, FRET-quench peptides containing the amino acid residues flanking the C terminus FAP cleavage site of hFGF21 and the corresponding mutant or orthologous sequences were used to measure hFAP cleavage efficiency relative to hFGF21 peptide. B and C, full-length recombinant mFGF21 was incubated in buffer in the presence or absence of FAP or DPPIV at 37 °C overnight and analyzed by immunoblotting (B) or LC/MS (C) to determine the molecular mass. D, schematic of mFGF21. The N-terminal and C-terminal protein sequence, putative FAP and DPPIV cleavage sites (arrows), and theoretical masses of various putative cleavage products (in kilodalton) are shown.
To confirm the resistance of the mFGF21 C terminus against FAP, full-length recombinant mFGF21 was incubated with FAP or DPPIV and then subjected to immunoblot analysis (Fig. 6B) and mass spectrometry (Fig. 6C). We observed a similar mass change that is predicted from a deletion of the N-terminal four amino acid residues, Tyr-Pro-Ile-Pro-, most likely by the dipeptidyl peptidase activity of DPPIV or FAP (Fig. 6, B–D). In contrast, we observed no evidence of FAP-specific C-terminal cleavage, consistent with the absence of the FAP consensus Gly-Pro sequence (Fig. 6D). As mentioned above, we were not able to reliably determine the level of endogenous FGF21 in its intact or truncated forms by mass spectrometry or other methods. However, an analysis of Fap-deficient and control wild-type or heterozygote littermate plasma by total mFGF21 ELISA showed that endogenous mFGF21 levels are not regulated significantly by FAP (data not shown).
FAP Inhibition in Cynomolgus Monkeys Increased Intact FGF21 Levels
To assess the effect of inhibiting FAP pharmacologically on the cleavage of endogenously produced FGF21, we took advantage of the fact that, unlike Muridae species, FAP in cynomolgus monkeys was expected to cleave endogenous FGF21 (Fig. 6A). We administered the specific FAP inhibitor compound 60 (38) to cynomolgus monkeys at a relatively low dose (5 mg/kg) per os. Plasma samples were collected before administration of the inhibitor and afterward. Compared with baseline levels, FAP activity diminished immediately after compound 60 became detectable in plasma (Fig. 7A). We then analyzed the levels of intact (i.e. uncleaved) endogenous FGF21 in these animals using an ELISA assay specific for FGF21 that has intact N- and C termini. Although the baseline levels of intact FGF21 differed between animals, this analysis revealed that the levels of intact FGF21 increased more than 3-fold in each animal treated with compound 60 (Fig. 7A, bottom row). Importantly, the time course of intact FGF21 increase correlated closely with the concentration of compound 60 and the activity of endogenous FAP in plasma. Therefore, inhibition of FAP in vivo results in an increase of intact endogenous serum FGF21, suggesting protection from cleavage by FAP.
FIGURE 7.
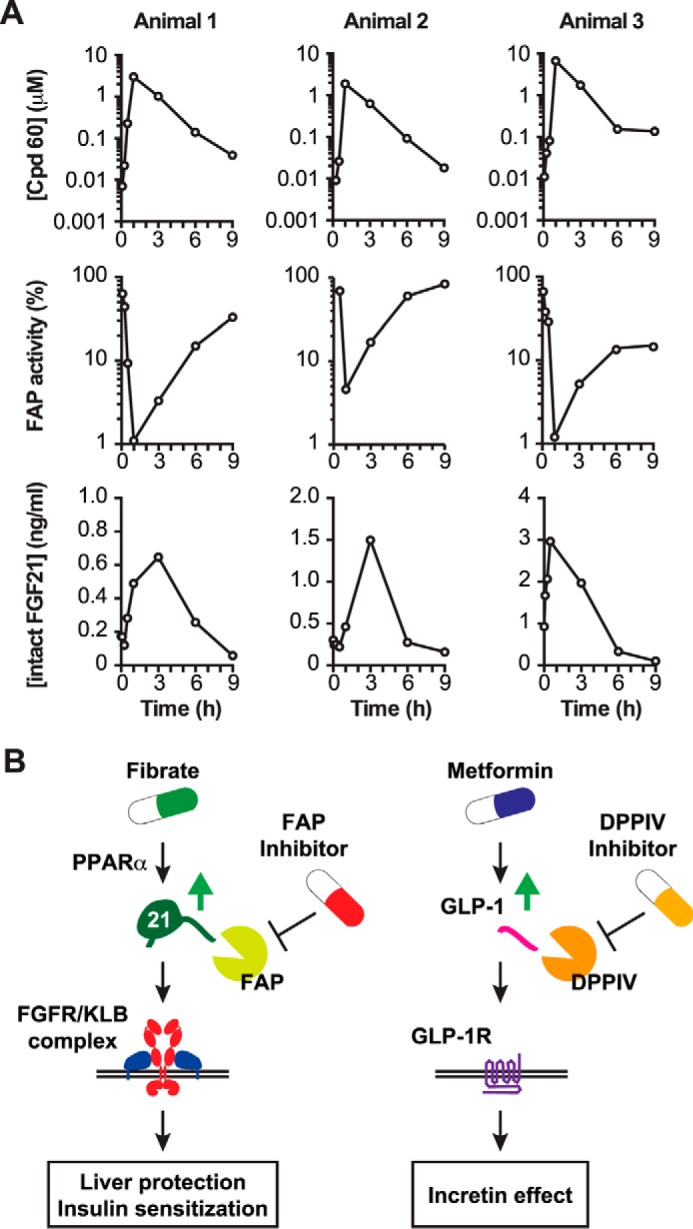
The effect of FAP inhibition on endogenous intact FGF21 levels in non-human primates. A, the FAP inhibitor compound 60 was administered at 5 mg/kg per os to three male cynomolgus monkeys (animals 1–3). The plasma concentration of compound 60 was quantified by LC-MS/MS, whereas relative FAP activity was assessed by FRET-quench using a cynomolgus-specific peptide, shown in Fig. 6A. An intact ELISA was used to measure plasma intact FGF21 concentrations. B, models outlining a therapeutic strategy to use fibrate and FAP inhibitors to augment the levels of active FGF21 for the treatment of metabolic diseases (left panel) compared with the use of metformin and DPPIV inhibitors that increase active incretin levels for the treatment of type 2 diabetes (right panel).
Discussion
Although the biology of FGF21 has been studied extensively during the last decade, not much is known about the nature of posttranslational regulation of this important metabolic hormone. This work identifies a novel evolutionarily conserved, posttranslational regulation of FGF21 by proteolytic inactivation by the serine protease FAP. The consensus Gly-Pro residues at the cleavage site are conserved in most FGF21 found in all four major branches of living placental mammals (afrotheres, xenarthrans, laurasiatheres, and euarchontoglires) (Supplemental Table 1). While this manuscript was in preparation, Zhen et al. (44) published a report that identifies FGF21 as a novel substrate for FAP, supporting our claim. Our results further extend the data from Zhen et al. (44) by demonstrating the significance of FAP-mediated FGF21 cleavage in vivo, most importantly in the regulation of endogenously produced FGF21 in cynomolgus monkeys. These discoveries immediately suggest that pharmacological FAP inhibition may confer beneficial metabolic effects via stabilization of endogenously produced FGF21. FAP exhibits 51% amino acid identity to DPPIV, the target for the gliptin class of anti-diabetic drugs that function by stabilizing the endogenously produced incretin hormone GLP-1 (29). By analogy to the relationship between DPPIV and GLP-1, we propose that selective inhibitors for the endopeptidase activity of hFAP could increase the level of active hFGF21 at the sites of production and in circulation to improve hepatic lipid metabolism and whole body insulin sensitivity (Fig. 7B). Because FGF21 expression can be augmented by fenofibrate in humans (17, 20, 45), we predict that FAP inhibition would synergize with fenofibrate or other agonists for peroxisome proliferator-activated receptor α, much like the way DPPIV inhibitors synergize with metformin, which increases the level of total GLP-1 (Fig. 7B) (46).
Among the various metabolic conditions recombinant FGF21 has been proposed to ameliorate, advanced NASH may represent the most attractive indication for the concept of FGF21 stabilization via FAP inhibition. Elevated circulating total FGF21 has been associated with fatty liver conditions (21). The liver is the most significant site of FGF21 production (3), and genetic studies in mice have demonstrated that circulating FGF21 is mostly derived from hepatocytes (22). It is believed that FGF21 in these conditions has a protective role via modulation of hepatic lipid metabolism and amelioration of endoplasmic reticulum stress (8, 13, 14). At the same time, elevated hepatic FAP expression has been observed in activated stellate cells in livers with cirrhosis (33, 47). Plasma FAP is also increased in human subjects with cirrhosis in the liver, but to a significantly lesser extent, suggesting that an increase in circulating FAP is mostly due to increased hepatic expression (33). Therefore, in livers with advanced NASH with fibrosis, FAP may negatively regulate the hepatoprotective activity of FGF21. Pharmacological FAP inhibition under these conditions may therefore prolong the activity of endogenously produced FGF21 to protect the liver via modulation of hepatic lipid handling and correction of endoplasmic reticulum stress.
One potential concern of therapeutic FAP inhibition is theoretical toxicity from FAP inhibition and an accumulation of unknown substrates. However, the evidence so far provides no indication for negative effects of FAP inhibition. Fap-deficient mice do not show an overt phenotype (43), and they exhibit less severe cartilage degradation in a chronic inflammatory arthritis model (48) and are protected from tumorigenesis (49). Recently, human individuals without detectable FAP activity in serum because of a rare inactivating variant have been identified (50). Although the detailed phenotypic analysis of these individuals has not been available, this report supports the idea that pharmacological FAP inhibition is not likely to be overtly toxic in humans (50). Besides FGF21, the existence of other endogenous FAP endopeptidase substrates remains elusive. One proposed substrate of soluble FAP is α(2)-antiplasmin, which is involved in blood clotting (30, 40). However, α(2)-antiplasmin can also be cleaved by PREP at the same site (51). Therefore, selective inhibition of FAP may have minimum effects on blood clotting. In addition, α(2)-antiplasmin may not be cleaved by FAP in vivo because of posttranslational modification (52). Another class of substrate is gelatin, after initial denaturation of collagen by matrix metalloproteinases. Again, the physiological importance of the gelatinase activity of FAP has not been demonstrated (52).
One limitation of our claim is that we do not yet have an appropriate assay to determine the level of total FGF21 or the ratio of intact/total FGF21 protein. Therefore, we cannot rule out the possibility that an acute increase in intact FGF21 levels in FAP inhibitor-treated cynomolgus monkeys may also accompany changes in total hFGF21 levels. In addition, we have not been able to determine the ratio of intact/total FGF21 protein in human subjects with various disease conditions, although we predict that individuals with elevated FAP expression would exhibit decreased intact/total FGF21 ratios. An effort is underway to develop a sensitive and reliable assay for the quantification of total FGF21 protein in human serum.
Another limitation of this study is that, because of the nature of an acute experiment with healthy cynomolgus monkeys and the lack of endogenous FGF21 cleavage in mice, it has not been addressed whether FAP inhibition could increase FGF21 to a sufficient level in vivo to confer metabolic benefits. Further experiments with higher or repeated doses of FAP inhibitors in obese and insulin-resistant non-human primates may address this question. In addition, a transgenic mouse model in which hFGF21 is produced under the regulatory control of the natural Fgf21 locus may provide proof of this therapeutic hypothesis.
Despite these limitations, our results clearly demonstrate the importance of FAP in modulating endogenous FGF21 levels in their intact form. Further study is warranted to determine whether selective inhibition of FAP activity confers metabolic benefits in individuals with obesity-related metabolic defects and liver disease via FGF21 stabilization.
Author Contributions
J. S. conceived the project. D. R. D. conducted a protease database search and identified FAP as a putative protease for FGF21. D. R. D., T. W. B., A. C. S., J. A. E., and J. S. designed and performed the assays. T. W. B., R. Corpuz, M. W., R. Chan, and J. A. E. contributed to the purification of recombinant proteins. D. R. D., N. M. K., and J. Z. S. performed the mouse experiments. D. P. S. directed the synthesis of compound 60. D. P. S., G. D., and J. L. designed and analyzed the non-human primate study and pharmacokinetic analysis. W. Z. generated the construct for Fap-deficient mice. J. S., D. R. D., T. W. B., A. C. S., D. P. S., J. L., and J. A. E. analyzed the data. J. S., D. R. D., and T. W. B. drafted the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Supplementary Material
Acknowledgments
We thank our Genentech colleagues in Molecular Biology, Protein Chemistry, the Bio-Molecular Engineering Group, and Laboratory Animal Resources for technical assistance. Anti-mFAP (28H1) was provided by Christian Klein and Anne Freimoser-Grundschober.
This work was funded by Genentech, Inc. All authors are present or former paid employees of Genentech with no further conflict of interest.

This article contains supplemental Table S1.
- NASH
- non-alcoholic steatohepatitis
- KLB
- βklotho
- DPPIV
- dipeptidyl peptidase IV
- FAP
- fibroblast activation protein
- PREP
- prolyl endopeptidase
- hFAP
- human fibroblast activation protein
- HI
- heat-inactivated.
References
- 1. Shulman G. I. (2014) Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 371, 1131–1141 [DOI] [PubMed] [Google Scholar]
- 2. Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., Mullany E. C., Biryukov S., Abbafati C., Abera S. F., Abraham J. P., Abu-Rmeileh N. M., Achoki T., AlBuhairan F. S., and Alemu Z. A., et al. (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Owen B. M., Mangelsdorf D. J., and Kliewer S. A. (2015) Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol. Metab. 26, 22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gimeno R. E., and Moller D. E. (2014) FGF21-based pharmacotherapy: potential utility for metabolic disorders. Trends Endocrinol. Metab. 25, 303–311 [DOI] [PubMed] [Google Scholar]
- 5. Coskun T., Bina H. A., Schneider M. A., Dunbar J. D., Hu C. C., Chen Y., Moller D. E., and Kharitonenkov A. (2008) Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149, 6018–6027 [DOI] [PubMed] [Google Scholar]
- 6. Xu J., Lloyd D. J., Hale C., Stanislaus S., Chen M., Sivits G., Vonderfecht S., Hecht R., Li Y. S., Lindberg R. A., Chen J. L., Jung D. Y., Zhang Z., Ko H. J., Kim J. K., and Véniant M. M. (2009) Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58, 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fisher F. M., Kleiner S., Douris N., Fox E. C., Mepani R. J., Verdeguer F., Wu J., Kharitonenkov A., Flier J. S., Maratos-Flier E., and Spiegelman B. M. (2012) FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fisher F. M., Chui P. C., Nasser I. A., Popov Y., Cunniff J. C., Lundasen T., Kharitonenkov A., Schuppan D., Flier J. S., and Maratos-Flier E. (2014) Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology 147, 1073–1083.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang S., Yan C., Fang Q. C., Shao M. L., Zhang Y. L., Liu Y., Deng Y. P., Shan B., Liu J. Q., Li H. T., Yang L., Zhou J., Dai Z., Liu Y., and Jia W. P. (2014) Fibroblast growth factor 21 is regulated by the IRE1α-XBP1 branch of the unfolded protein response and counteracts endoplasmic reticulum stress-induced hepatic steatosis. J. Biol. Chem. 289, 29751–29765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin Z., Tian H., Lam K. S., Lin S., Hoo R. C., Konishi M., Itoh N., Wang Y., Bornstein S. R., Xu A., and Li X. (2013) Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 17, 779–789 [DOI] [PubMed] [Google Scholar]
- 11. Holland W. L., Adams A. C., Brozinick J. T., Bui H. H., Miyauchi Y., Kusminski C. M., Bauer S. M., Wade M., Singhal E., Cheng C. C., Volk K., Kuo M. S., Gordillo R., Kharitonenkov A., and Scherer P. E. (2013) An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 17, 790–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laeger T., Henagan T. M., Albarado D. C., Redman L. M., Bray G. A., Noland R. C., Münzberg H., Hutson S. M., Gettys T. W., Schwartz M. W., and Morrison C. D. (2014) FGF21 is an endocrine signal of protein restriction. J. Clin. Invest. 124, 3913–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim S. H., Kim K. H., Kim H. K., Kim M. J., Back S. H., Konishi M., Itoh N., and Lee M. S. (2015) Fibroblast growth factor 21 participates in adaptation to endoplasmic reticulum stress and attenuates obesity-induced hepatic metabolic stress. Diabetologia 58, 809–818 [DOI] [PubMed] [Google Scholar]
- 14. Tanaka N., Takahashi S., Zhang Y., Krausz K. W., Smith P. B., Patterson A. D., and Gonzalez F. J. (2015) Role of fibroblast growth factor 21 in the early stage of NASH induced by methionine- and choline-deficient diet. Biochim. Biophys. Acta 1852, 1242–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaich G., Chien J. Y., Fu H., Glass L. C., Deeg M. A., Holland W. L., Kharitonenkov A., Bumol T., Schilske H. K., and Moller D. E. (2013) The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18, 333–340 [DOI] [PubMed] [Google Scholar]
- 16. Dong J. Q., Rossulek M., Somayaji V. R., Baltrukonis D., Liang Y., Hudson K., Hernandez-Illas M., and Calle R. A. (2015) Pharmacokinetics and pharmacodynamics of PF-05231023, a novel long-acting FGF21 mimetic, in a first-in-human study. Br. J. Clin. Pharmacol. 80, 1051–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gälman C., Lundåsen T., Kharitonenkov A., Bina H. A., Eriksson M., Hafström I., Dahlin M., Amark P., Angelin B., and Rudling M. (2008) The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARα activation in man. Cell Metab. 8, 169–174 [DOI] [PubMed] [Google Scholar]
- 18. Zhang X., Yeung D. C., Karpisek M., Stejskal D., Zhou Z.-G., Liu F., Wong R. L., Chow W.-S., Tso A. W., Lam K. S., and Xu A. (2008) Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57, 1246–1253 [DOI] [PubMed] [Google Scholar]
- 19. Chen W. W., Li L., Yang G. Y., Li K., Qi X. Y., Zhu W., Tang Y., Liu H., and Boden G. (2008) Circulating FGF-21 levels in normal subjects and in newly diagnose patients with Type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 116, 65–68 [DOI] [PubMed] [Google Scholar]
- 20. Mraz M., Bartlova M., Lacinova Z., Michalsky D., Kasalicky M., Haluzikova D., Matoulek M., Dostalova I., Humenanska V., and Haluzik M. (2009) Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin. Endocrinol. (Oxf) 71, 369–375 [DOI] [PubMed] [Google Scholar]
- 21. Yan H., Xia M., Chang X., Xu Q., Bian H., Zeng M., Rao S., Yao X., Tu Y., Jia W., and Gao X. (2011) Circulating fibroblast growth factor 21 levels are closely associated with hepatic fat content: a cross-sectional study. PLoS ONE 6, e24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Markan K. R., Naber M. C., Ameka M. K., Anderegg M. D., Mangelsdorf D. J., Kliewer S. A., Mohammadi M., and Potthoff M. J. (2014) Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 63, 4057–4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel R., Bookout A. L., Magomedova L., Owen B. M., Consiglio G. P., Shimizu M., Zhang Y., Mangelsdorf D. J., Kliewer S. A., and Cummins C. L. (2015) Glucocorticoids regulate the metabolic hormone FGF21 in a feed-forward loop. Mol. Endocrinol. 29, 213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kharitonenkov A., Beals J. M., Micanovic R., Strifler B. A., Rathnachalam R., Wroblewski V. J., Li S., Koester A., Ford A. M., Coskun T., Dunbar J. D., Cheng C. C., Frye C. C., Bumol T. F., and Moller D. E. (2013) Rational design of a fibroblast growth factor 21-based clinical candidate, LY2405319. PLoS ONE 8, e58575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yie J., Hecht R., Patel J., Stevens J., Wang W., Hawkins N., Steavenson S., Smith S., Winters D., Fisher S., Cai L., Belouski E., Chen C., Michaels M. L., Li Y.-S., Lindberg R., Wang M., Véniant M., and Xu J. (2009) FGF21 N- and C-termini play different roles in receptor interaction and activation. FEBS Lett. 583, 19–24 [DOI] [PubMed] [Google Scholar]
- 26. Micanovic R., Raches D. W., Dunbar J. D., Driver D. A., Bina H. A., Dickinson C. D., and Kharitonenkov A. (2009) Different roles of N- and C- termini in the functional activity of FGF21. J. Cell. Physiol. 219, 227–234 [DOI] [PubMed] [Google Scholar]
- 27. Hecht R., Li Y. S., Sun J., Belouski E., Hall M., Hager T., Yie J., Wang W., Winters D., Smith S., Spahr C., Tam L. T., Shen Z., Stanislaus S., Chinookoswong N., Lau Y., Sickmier A., Michaels M. L., Boone T., Véniant M. M., and Xu J. (2012) Rationale-based engineering of a potent long-acting FGF21 analog for the treatment of type 2 diabetes. PLoS ONE 7, e49345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Umberger T. S., Sloan J. H., Chen J., Cheng C., Siegel R. W., Qian Y., Troutt J. S., and Konrad R. J. (2014) Novel sandwich immunoassays for the measurement of total and active FGF21. Bioanalysis 6, 3283–3293 [DOI] [PubMed] [Google Scholar]
- 29. Drucker D. J., and Nauck M. A. (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705 [DOI] [PubMed] [Google Scholar]
- 30. Lee K. N., Jackson K. W., Christiansen V. J., Lee C. S., Chun J. G., and McKee P. A. (2006) Antiplasmin-cleaving enzyme is a soluble form of fibroblast activation protein. Blood 107, 1397–1404 [DOI] [PubMed] [Google Scholar]
- 31. Liu R., Li H., Liu L., Yu J., and Ren X. (2012) Fibroblast activation protein: a potential therapeutic target in cancer. Cancer Biol. Ther. 13, 123–129 [DOI] [PubMed] [Google Scholar]
- 32. Hamson E. J., Keane F. M., Tholen S., Schilling O., and Gorrell M. D. (2014) Understanding fibroblast activation protein (FAP): substrates, activities, expression and targeting for cancer therapy. Proteomics Clin. Appl. 8, 454–463 [DOI] [PubMed] [Google Scholar]
- 33. Keane F. M., Yao T. W., Seelk S., Gall M. G., Chowdhury S., Poplawski S. E., Lai J. H., Li Y., Wu W., Farrell P., Vieira de Ribeiro A. J., Osborne B., Yu D. M., Seth D., Rahman K., Haber P., Topaloglu A. K., Wang C., Thomson S., Hennessy A., Prins J., Twigg S. M., McLennan S. V., McCaughan G. W., Bachovchin W. W., and Gorrell M. D. (2013) Quantitation of fibroblast activation protein (FAP)-specific protease activity in mouse, baboon and human fluids and organs. FEBS Open Bio 4, 43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laverman P., van der Geest T., Terry S. Y., Gerrits D., Walgreen B., Helsen M. M., Nayak T. K., Freimoser-Grundschober A., Waldhauer I., Hosse R. J., Moessner E., Umana P., Klein C., Oyen W. J., Koenders M. I., and Boerman O. C. (2015) Immuno-PET and immuno-SPECT of rheumatoid arthritis with radiolabeled anti-fibroblast activation protein antibody correlates with severity of arthritis. J. Nucl. Med. 56, 778–783 [DOI] [PubMed] [Google Scholar]
- 35. Yu D. M., Yao T. W., Chowdhury S., Nadvi N. A., Osborne B., Church W. B., McCaughan G. W., and Gorrell M. D. (2010) The dipeptidyl peptidase IV family in cancer and cell biology. FEBS J. 277, 1126–1144 [DOI] [PubMed] [Google Scholar]
- 36. Belouski E. J. C., Ellison M. M., Hamburger A. E., Hecht R. I., Li Y.-S, Michaels M. L., Sun J., and Xu J. (2014) FGF21 polypeptides comprising two or more mutations and uses thereof. U. S. Patent 8,835,385
- 37. Wu A. L., Kolumam G., Stawicki S., Chen Y., Li J., Zavala-Solorio J., Phamluong K., Feng B., Li L., Marsters S., Kates L., van Bruggen N., Leabman M., Wong A., West D., Stern H., Luis E., Kim H. S., Yansura D., Peterson A. S., Filvaroff E., Wu Y., and Sonoda J. (2011) Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci. Transl. Med. 3, 113ra126. [DOI] [PubMed] [Google Scholar]
- 38. Jansen K., Heirbaut L., Verkerk R., Cheng J. D., Joossens J., Cos P., Maes L., Lambeir A. M., De Meester I., Augustyns K., and Van der Veken P. (2014) Extended structure-activity relationship and pharmacokinetic investigation of (4-quinolinoyl)glycyl-2-cyanopyrrolidine inhibitors of fibroblast activation protein (FAP). J. Med. Chem. 57, 3053–3074 [DOI] [PubMed] [Google Scholar]
- 39. Rawlings N. D., Waller M., Barrett A. J., and Bateman A. (2014) MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 42, D503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Edosada C. Y., Quan C., Tran T., Pham V., Wiesmann C., Fairbrother W., and Wolf B. B. (2006) Peptide substrate profiling defines fibroblast activation protein as an endopeptidase of strict Gly2-Pro1-cleaving specificity. FEBS Lett. 580, 1581–1586 [DOI] [PubMed] [Google Scholar]
- 41. Bobbert T., Schwarz F., Fischer-Rosinsky A., Pfeiffer A. F., Möhlig M., Mai K., and Spranger J. (2013) Fibroblast growth factor 21 predicts the metabolic syndrome and type 2 diabetes in Caucasians. Diabetes Care 36, 145–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hanssen M. J., Broeders E., Samms R. J., Vosselman M. J., van der Lans A. A., Cheng C. C., Adams A. C., van Marken Lichtenbelt W. D., and Schrauwen P. (2015) Serum FGF21 levels are associated with brown adipose tissue activity in humans. Sci. Rep. 5, 10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niedermeyer J., Kriz M., Hilberg F., Garin-Chesa P., Bamberger U., Lenter M. C., Park J., Viertel B., Püschner H., Mauz M., Rettig W. J., and Schnapp A. (2000) Targeted disruption of mouse fibroblast activation protein. Mol. Cell. Biol. 20, 1089–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhen E. Y., Jin Z., Ackermann B. L., Thomas M. K., and Gutierrez J. A. (2015) Circulating FGF21 proteolytic processing mediated by fibroblast activation protein. Biochem. J. 10.1042/BJ20151085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ong K. L., Rye K. A., O'Connell R., Jenkins A. J., Brown C., Xu A., Sullivan D. R., Barter P. J., Keech A. C., and FIELD Study Investigators (2012) Long-term fenofibrate therapy increases fibroblast growth factor 21 and retinol-binding protein 4 in subjects with type 2 diabetes. J. Clin. Endocrinol. Metab. 97, 4701–4708 [DOI] [PubMed] [Google Scholar]
- 46. Migoya E. M., Bergeron R., Miller J. L., Snyder R. N., Tanen M., Hilliard D., Weiss B., Larson P., Gutierrez M., Jiang G., Liu F., Pryor K. A., Yao J., Zhu L., Holst J. J., Deacon C., Herman G., Thornberry N., Amatruda J., Williams-Herman D., Wagner J. A., and SinhaRoy R. (2010) Dipeptidyl peptidase-4 inhibitors administered in combination with metformin result in an additive increase in the plasma concentration of active GLP-1. Clin. Pharmacol. Ther. 88, 801–808 [DOI] [PubMed] [Google Scholar]
- 47. Levy M. T., McCaughan G. W., Marinos G., and Gorrell M. D. (2002) Intrahepatic expression of the hepatic stellate cell marker fibroblast activation protein correlates with the degree of fibrosis in hepatitis C virus infection. Liver 22, 93–101 [DOI] [PubMed] [Google Scholar]
- 48. Wäldele S., Koers-Wunrau C., Beckmann D., Korb-Pap A., Wehmeyer C., Pap T., and Dankbar B. (2015) Deficiency of fibroblast activation protein α ameliorates cartilage destruction in inflammatory destructive arthritis. Arthritis Res. Ther. 17, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Santos A. M., Jung J., Aziz N., Kissil J. L., and Puré E. (2009) Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J. Clin. Invest. 119, 3613–3625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Osborne B., Yao T. W., Wang X. M., Chen Y., Kotan L. D., Nadvi N. A., Herdem M., McCaughan G. W., Allen J. D., Yu D. M., Topaloglu A. K., and Gorrell M. D. (2014) A rare variant in human fibroblast activation protein associated with ER stress, loss of enzymatic function and loss of cell surface localisation. Biochim. Biophys. Acta 1844, 1248–1259 [DOI] [PubMed] [Google Scholar]
- 51. Agustí-Cobos E., and Tenorio-Laranga J. (2011) Enhancement of fibrinolysis by inhibiting enzymatic cleavage of precursor alpha2-antiplasmin: a rebuttal. J. Thromb. Haemost. 9, 1266–1267; author reply 1268–1269 [DOI] [PubMed] [Google Scholar]
- 52. Huang C. H., Suen C. S., Lin C. T., Chien C. H., Lee H. Y., Chung K. M., Tsai T. Y., Jiaang W. T., Hwang M. J., and Chen X. (2011) Cleavage-site specificity of prolyl endopeptidase FAP investigated with a full-length protein substrate. J. Biochem. 149, 685–692 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.