Abstract
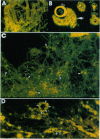
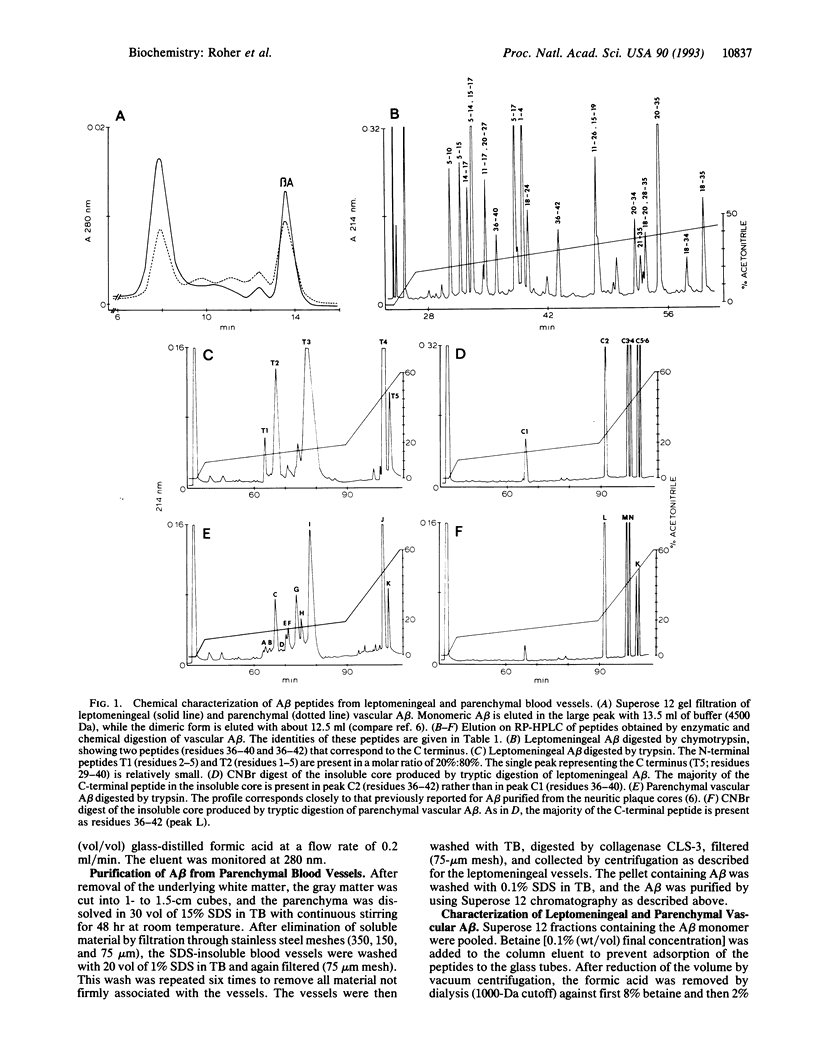
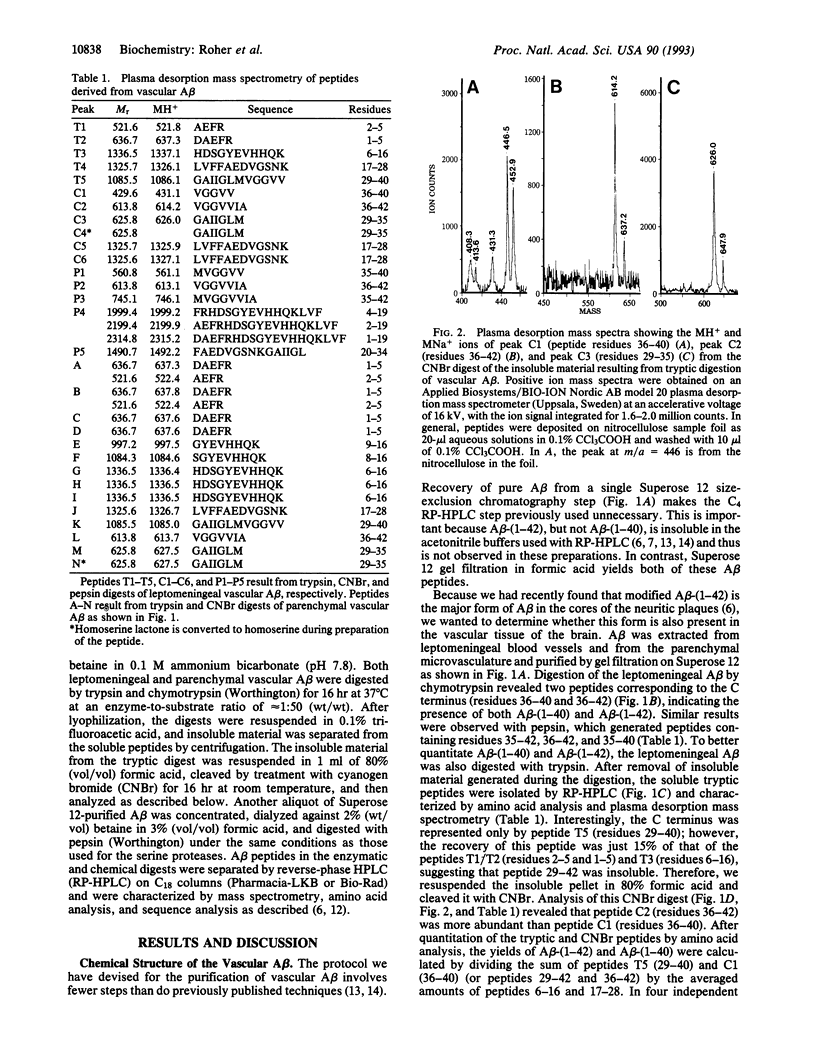
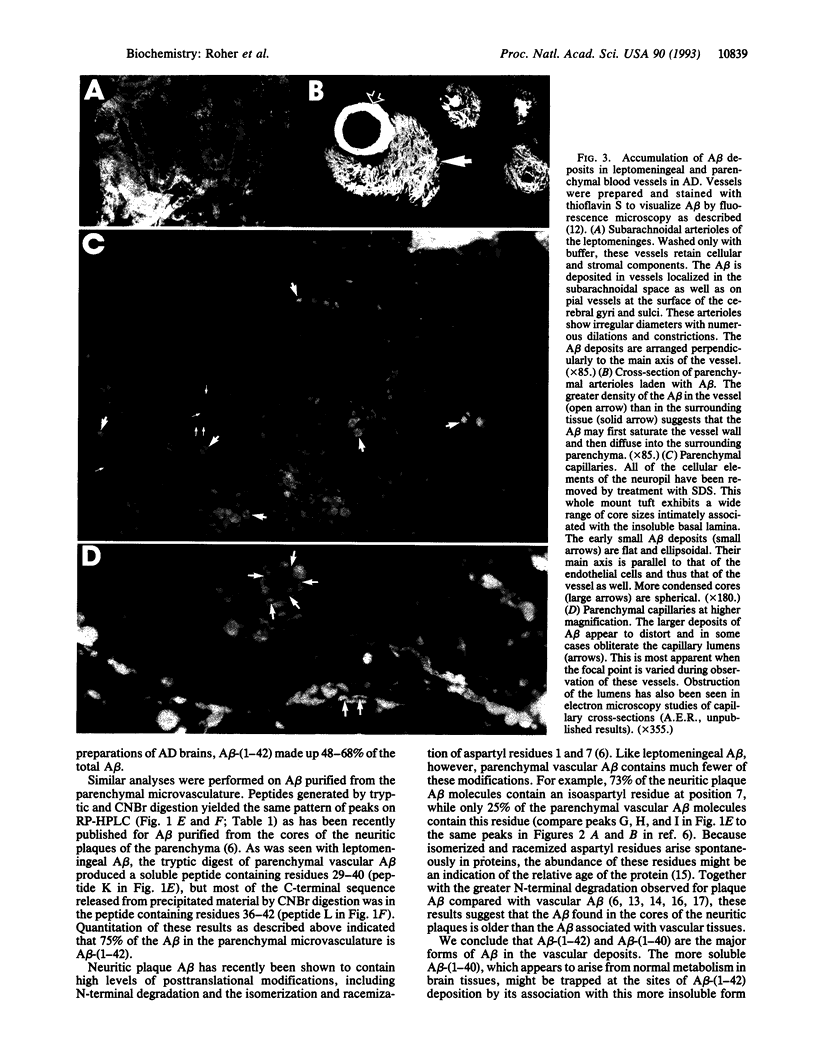
Reinvestigation of the chemical structure of beta-amyloid peptide (A beta) deposits in the vascular tissue of Alzheimer disease brains revealed that the 42-residue form A beta-(1-42), rather than the more soluble A beta-(1-40) form, is the predominant peptide. Following removal of the surrounding tissue with SDS and collagenase, A beta was solubilized in formic acid and purified by Superose 12 chromatography. Peptides generated by enzymatic and chemical digestion of the A beta were purified by HPLC and characterized by amino acid analysis, sequence analysis, and mass spectrometry. In the leptomeningeal vessels, the average ratio of A beta-(1-42)/A beta-(1-40) was 58:42, whereas in the parenchymal vessels this ratio was 75:25. Interestingly, vascular A beta contains considerably less isomerized and racemized aspartyl residues than does neuritic plaque A beta, suggesting that the vascular amyloid is "younger." The discrete nature of the bands and spherical deposits of A beta associated with arterioles and capillaries, respectively, suggests that this amyloid arises from the vascular tissue itself. Increasing A beta deposition appears to lead to the distortion and occlusion of capillaries, which may contribute significantly to the pathology of Alzheimer disease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow C. J., Yasuda A., Kenny P. T., Zagorski M. G. Solution conformations and aggregational properties of synthetic amyloid beta-peptides of Alzheimer's disease. Analysis of circular dichroism spectra. J Mol Biol. 1992 Jun 20;225(4):1075–1093. doi: 10.1016/0022-2836(92)90106-t. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Selkoe D. J. The seminal role of beta-amyloid in the pathogenesis of Alzheimer disease. Alzheimer Dis Assoc Disord. 1992 Spring;6(1):7–34. doi: 10.1097/00002093-199205000-00003. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Knauer M. F., Soreghan B., Burdick D., Kosmoski J., Glabe C. G. Intracellular accumulation and resistance to degradation of the Alzheimer amyloid A4/beta protein. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7437–7441. doi: 10.1073/pnas.89.16.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra S. S., Hart M. N., Terry R. D. Making the diagnosis of Alzheimer's disease. A primer for practicing pathologists. Arch Pathol Lab Med. 1993 Feb;117(2):132–144. [PubMed] [Google Scholar]
- Miyakawa T., Katsuragi S., Yamashita K., Ohuchi K. Morphological study of amyloid fibrils and preamyloid deposits in the brain with Alzheimer's disease. Acta Neuropathol. 1992;83(4):340–346. doi: 10.1007/BF00713523. [DOI] [PubMed] [Google Scholar]
- Mori H., Takio K., Ogawara M., Selkoe D. J. Mass spectrometry of purified amyloid beta protein in Alzheimer's disease. J Biol Chem. 1992 Aug 25;267(24):17082–17086. [PubMed] [Google Scholar]
- Pardridge W. M., Vinters H. V., Yang J., Eisenberg J., Choi T. B., Tourtellotte W. W., Huebner V., Shively J. E. Amyloid angiopathy of Alzheimer's disease: amino acid composition and partial sequence of a 4,200-dalton peptide isolated from cortical microvessels. J Neurochem. 1987 Nov;49(5):1394–1401. doi: 10.1111/j.1471-4159.1987.tb01005.x. [DOI] [PubMed] [Google Scholar]
- Prelli F., Castaño E., Glenner G. G., Frangione B. Differences between vascular and plaque core amyloid in Alzheimer's disease. J Neurochem. 1988 Aug;51(2):648–651. doi: 10.1111/j.1471-4159.1988.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Roher A. E., Lowenson J. D., Clarke S., Wolkow C., Wang R., Cotter R. J., Reardon I. M., Zürcher-Neely H. A., Heinrikson R. L., Ball M. J. Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer's disease. J Biol Chem. 1993 Feb 15;268(5):3072–3083. [PubMed] [Google Scholar]
- Roher A. E., Palmer K. C., Chau V., Ball M. J. Isolation and chemical characterization of Alzheimer's disease paired helical filament cytoskeletons: differentiation from amyloid plaque core protein. J Cell Biol. 1988 Dec;107(6 Pt 2):2703–2716. doi: 10.1083/jcb.107.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J. The molecular pathology of Alzheimer's disease. Neuron. 1991 Apr;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Covalent modification reactions are marking steps in protein turnover. Biochemistry. 1990 Jul 10;29(27):6323–6331. doi: 10.1021/bi00479a001. [DOI] [PubMed] [Google Scholar]
- Vinters H. V. Cerebral amyloid angiopathy. A critical review. Stroke. 1987 Mar-Apr;18(2):311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Yamazaki T., Lemere C. A., Frosch M. P., Selkoe D. J. Beta amyloid is focally deposited within the outer basement membrane in the amyloid angiopathy of Alzheimer's disease. An immunoelectron microscopic study. Am J Pathol. 1992 Jul;141(1):249–259. [PMC free article] [PubMed] [Google Scholar]