Abstract
Circadian rhythms are endogenous cycles of physiology and behavior that align with the daily rotation of the planet and resulting light-dark cycle. The circadian system ensures homeostatic balance and regulates many aspects of physiology, including the stress response and susceptibility to and/or severity of stress-related sequelae. Both acute and chronic stressors amplify neuroinflammatory responses to a subsequent immune challenge, however it is not known whether circadian timing of the stressor regulates the priming response. Here, we test whether stress-induced neuroinflammatory priming is regulated by the circadian system. As has been previously shown, exposure to 100 inescapable tails shocks (IS) increased hippocampal cytokines following a subsequent inflammatory challenge. However, this effect was limited to animals that experienced the stressor during the light phase. Rats exposed to stress during the dark phase did not alter inflammatory potential following lipopolysaccharide (LPS) challenge. To determine whether microglia might be involved in diurnal differences in neuroinflammatory priming, microglia were isolated 24 h after stress that occurred either during the middle of the light or dark phase. Only microglia isolated from animals stressed during the light phase demonstrated an exaggerated inflammatory response when treated ex vivo with LPS. To determine possible circadian dependency of microglia responsiveness to glucocorticoids – the likely proximal mediator for stress associated neuroinflammatory priming – microglia were isolated during the middle of the light or dark phase and treated ex vivo with corticosterone. Glucocorticoids treatment downregulated CX3CR1 and CD200R, two genes involved in microglial inflammatory “off” signaling; however, there was no effect of time of day on expression of either gene. Importantly, while absolute concentrations of corticosterone were comparable following IS during the light and dark phase, the magnitude of change in corticosterone was greater during the light phase. This work highlights the importance of studying circadian rhythms to elucidate biological mechanisms of stress.
Keywords: Circadian, Microglia, Neuroinflammation, Stress
1. Background
Exaggerated innate immune/inflammatory responses in the brain are implicated in the etiology of numerous psychiatric disorders including depression, PTSD, autism, and schizophrenia (Dantzer et al., 2008). Stress is a major predisposing factor in the development of these psychiatric disorders and a potential source of elevated inflammation in the brain. In this regard, both acute and chronic stressors amplify peripheral and central inflammatory responses to a subsequent immune challenge (Johnson et al., 2002, Johnson et al., 2003, Frank et al., 2010, Wohleb et al., 2012). Glucocorticoids are implicated in mediating the effects of stress on inflammatory priming (Frank et al., 2010, Busillo et al., 2011, Frank et al., 2012, Frank et al., 2014). Although glucocorticoids are traditionally regarded as anti-inflammatory, a considerable number of studies demonstrate that glucocorticoids can simultaneously suppress ongoing inflammation while potentiating inflammatory responses to a later immune challenge (reviewed in (Frank et al., 2015). The neuroinflammatory “priming” produced by stress is regulated in part by microglia, the predominant innate immune cell of the central nervous system (Frank et al., 2007, Frank et al., 2014). While glucocorticoids are recognized as a critical mediator through which stress sensitize microglia (Frank et al., 2010, Frank et al., 2012, Frank et al., 2014, Weber et al., 2015), the exact mechanisms involved in microglia priming are not fully understood.
Circadian rhythms are endogenous cycles of physiology and behavior that have periods of about 24 hours (h) and are aligned to the timing of the daily rotation of the planet. The presence of a functional circadian system is evolutionarily advantageous as organisms that are entrained to their environment have improved survival odds (Davidson et al., 2006). Circadian rhythms allow organisms (and individual cells) to predict consistent changes in environment and temporally compartmentalize incompatible processes (Karatsoreos and McEwen, 2014). The circadian system is critical for ensuring homeostatic balance and regulates many aspects of physiology including the immune system. For example, there are circadian differences in immune system activation (Halberg et al., 1960, Marpegan et al., 2009, Spengler et al., 2012) and disruption of the circadian system can lead to heightened peripheral and central inflammatory responses (Castanon-Cervantes et al., 2010, Fonken and Nelson, 2013, Phillips et al., 2015, Wright et al., 2015). Moreover, microglia express intrinsic circadian clock mechanisms and display altered immune potential over the course of the day (Fonken et al., 2015). The time-of-day at which an inflammatory challenge occurs affects stress-induced neuroinflammatory priming (Johnson et al., 2003). However, whether diurnal timing of the stressor affects neuroinflammatory priming has not been established. Responses to stressors and susceptibility to and/or severity of stress-related comorbidities are regulated by the circadian system (Cohen et al., 2015). This suggests that microglia may also be differentially sensitive to the effects of stressors throughout the day.
Here we tested whether stress-induced inflammatory priming is time of day dependent. Furthermore, we examined the effects of stressor exposure and ex vivo corticosterone treatment on microglia inflammatory priming. Determining whether the hippocampus is more or less sensitive to the effects of stress at different times of day provides a novel platform through which it is possible to identify factors involved in stress associated neuroinflammatory priming. Better understanding of the mechanisms involved in imparting resilience or contributing to vulnerability to stress may help devise interventions to mitigate risk factors.
2. General methods
2.1. Animals
Male Sprague-Dawley rats (~3 mos. old; Harlan Sprague-Dawley, Inc, Indianapolis, IN, USA) were pair-housed with food and water available ad libitum at an ambient temperature of 22±2°C. Rats were given one month to acclimate to colony conditions before experimentation began. All rats were maintained on a 12:12 light cycle with lights on either at 0700 or 2100 h [lights on is Zeitgeber time (ZT) 0]. All experimental procedures were conducted in accordance with the University of Colorado Institutional Animal Care and Use Committee.
2.2. Experimental design
2.2.1. Experiment 1: Does stress at different times of day of differentially modulate neuroinflammatory priming to a later peripheral immune challenge?
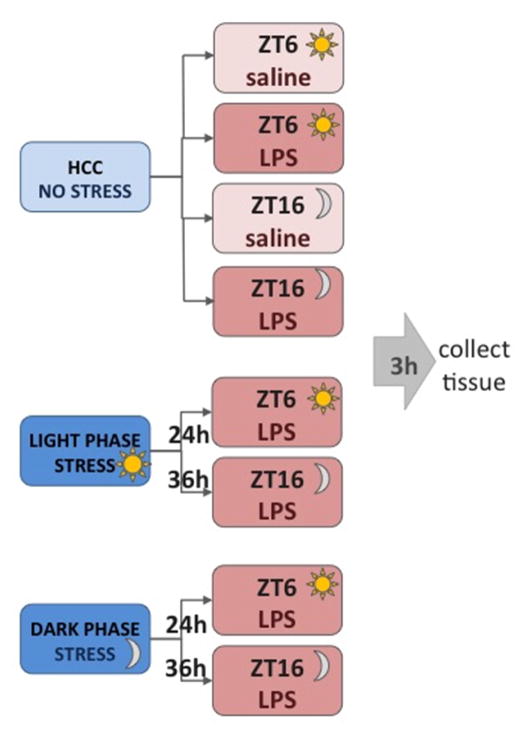
Rats received 100 trials of inescapable tail shock (IS) over 2 h during either the middle of the light phase (Zeitgeber time 5 to 7; ZT5 – ZT7) or dark phase (ZT15 – ZT17). Half of the rats were maintained on a reverse light/dark cycle (that was staggered 2 h) in order for the stress and tissue collection to occur together for the separate time points. 24 h later rats were injected intraperitoneally (i.p) with 10 μg/kg of lipopolysaccharide (LPS; E. coli serotype 0111:B4; Sigma) or saline (vehicle control). This dose of LPS was selected because it results in a sub-threshold hippocampal pro-inflammatory response (Johnson et al., 2002). Because LPS elicits a greater response during the light phase it is important to equate times of day for LPS injection. Thus, a 36 h time point was also included in which IS occurred 12 h prior to IS in the 24 h time point groups. Because the reverse light/dark cycle room was not exactly 12 h out of phase with the standard light/dark cycle room (due to animal care requirements), middle of the light phase stress occurred from ZT3 – ZT5 and dark phase stress occurred from ZT17 – ZT19. All of the groups (light and dark phase and 24 and 36 h time points) received LPS injections together which corresponded to ZT6 in standard light/dark cycle and ZT16 in the reverse light/dark room. Three hours following LPS injection hippocampal tissue was collected and cytokine protein and gene expression evaluated (see Fig. 1 for schematic of experimental design). Gene and protein expression were evaluated in the hippocampus because IS specifically potentiates hippocampal pro-inflammatory processes to peripheral LPS (Johnson et al., 2002). This experiment had 8 groups (see Figure 1): control with saline during the light phase, control with saline during the dark phase, control with LPS during the light phase, control with LPS during the dark phase, stress during the light phase with LPS during the light phase (24 h later), stress during the light phase with LPS during the dark phase (36 h later), stress during the dark phase with LPS during the light phase (36 h later), and stress at during the dark phase with LPS during the dark phase (24 h later). In order to minimize animal use and because our laboratory has previously demonstrated that 24 h post-IS rats do not exhibit elevated levels of pro-inflammatory cytokines (Johnson et al., 2003), we did not include IS/saline groups at ZT6 and ZT16.
Figure 1.
Experimental design schematic.
2.2.2. Experiment 2: Are microglia differentially sensitized by stress exposure during the light versus dark phase?
Rats received 100 trials of IS either during the middle of the light phase (ZT5 – ZT7) or dark phase (ZT15 – ZT17). 24 h later microglia were isolated from IS and HCC animals and treated ex vivo with LPS for 3 h. Cytokine mRNA expression was measured in microglia using qPCR to evaluate microglia sensitization.
2.2.3. Experiment 3: Are microglia isolated at ZT6 versus ZT16 differentially altered by ex vivo corticosterone treatment?
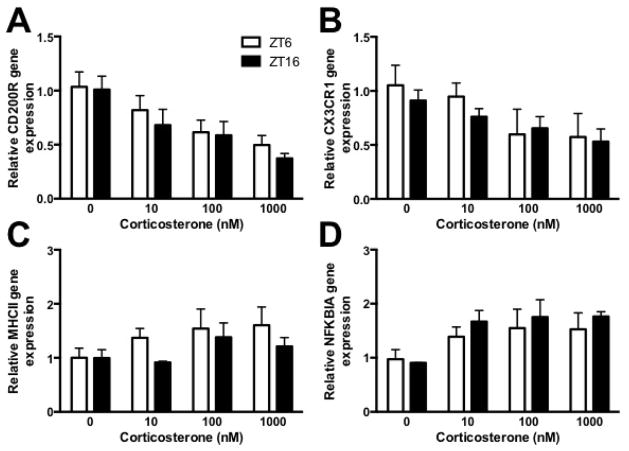
Microglia from unmanipulated rats were isolated either during the middle of the light (ZT6) or dark phase (ZT16) and cells were plated with corticosterone for 2 h. Relative mRNA expression of markers of microglia activation state such as MHCII, NFKBIA, CD200R, and CX3CR1 were measured in microglia using qPCR.
2.2.4. Experiment 4: Are there diurnal changes in inescapable stress-induced corticosterone release?
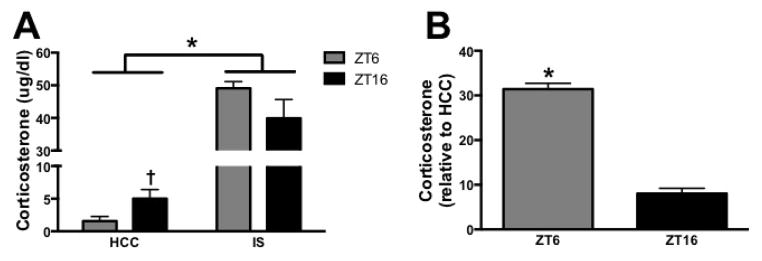
Rats received 100 trials of IS either during the middle of the light phase (ZT6) or dark phase (ZT16). Upon termination of the stress session cardiac blood was immediately collected from IS and HCC rats to assess plasma corticosterone concentrations (as described below).
2.3. General methods
2.3.1. Inescapable tail shock
The present stressor protocol has been extensively used in this laboratory and reliably potentiates hippocampal pro-inflammatory cytokine responses to a peripheral immune challenge (Johnson et al, 2002). In brief, animals were placed in Plexiglas tubes (23.4 cm in length x 7 cm in diameter) and exposed to 100 1.6 mA, 5 sec tail shocks with a variable inter-trial interval ranging from 30–90 sec (average of 60 sec). IS rats were immediately returned to their home cages upon termination of shock. During the IS session HCC rats remained undisturbed.
2.3.2. Tissue collection
Animals received a lethal i.p. injection of sodium pentobarbital. After rats were completely unresponsive (as assessed by paw pinch), they were transcardially perfused with ice-cold saline (0.9%) for 3 min to remove peripheral immune cells from the CNS vasculature. Brains were then rapidly extracted and the hippocampus dissected out on ice. For in vivo experiments hippocampus was flash frozen in liquid nitrogen and stored at −80 °C. For ex vivo experiments microglia were immediately isolated as described below.
2.3.3. Microglia isolations and ex vivo treatments
Hippocampal microglia were isolated using a Percoll density gradient as described in (Frank et al., 2006). Our laboratory has previously demonstrated that this isolation procedure yields highly pure microglia (Iba-1+/MHCII+/CD163-/GFAP-) and we confirmed immunophenotype and purity of microglia with qPCR in this experiment. Microglia isolations occurred as follows: rats were saline perfused for 3 min, brains were rapidly extracted, and the hippocampus was dissected out on ice. The hippocampus was homogenized in 3 mL of 0.2% glucose in 1X DPBS (Gibco, Life Technologies) in a sterilized glass homogenizer and the homogenate was strained through a 40 μm filter (Falcon) that was rinsed with an additional 2 mL or DPBS. The homogenate was transferred to a sterile 5 mL tube and pelleted at 1000 g for 10 min at 22 C. Supernatant was aspirated off and a percoll gradient was created by resuspending the pellet in 2 mL of 70% isotonic percoll (GE Healthcare; isotonic percoll is 10:1 percoll with 10X PBS; 100% isotonic percoll is then diluted with 1X DPBS), followed by a layer of 2 mL 50% percoll and topped with 1 mL DPBS. The gradient was spun at 1200 g for 45 min at 22 C with no acceleration or break. Myelin debris was removed and then microglia were extracted from the 50/70% interface. Microglia were then washed in DPBS and pelleted at 1000 g for 10 min at 22 C. Following isolations, microglia were resuspended in media (sterile high glucose DMEM (Gibco, 11960-044) +10%FBS [Atlanta biological, S11050] that was passed through a 0.2 μm filter Millipore filter) and microglia concentration was determined by trypan blue exclusion. Microglia concentration was adjusted to a density of 8,000 cells/100 uL and cells were plated in a 96-well v-bottom plate. To assess microglia cytokine responsiveness, cells were challenged ex vivo with 10 uL of LPS (LPS; E. coli serotype 0111:B4; Sigma) at a concentration of 10 or 100 ng/mL or media alone (concentrations based on previously work (Frank et al., 2007)) for 3 h at 37 °C, 5% CO2. To determine the influence of corticosterone on microglia at different times of day, microglia were treated with 10uL of corticosterone (Sigma-C2505) at a concentration of 0, 10, 100, or 1000 nM for 2 h (this range includes physiologically relevant concentrations and is based on previous work (Jacobsson et al., 2006, Busillo et al., 2011, Chatterjee and Sikdar, 2014)). Corticosterone was first diluted to a concentration of 10mM in EtOH followed by a serial dilution in media (0 nM control contains EtOH equivalent to 1000 nM EtOH concentration). Following ex vivo manipulations, plates were centrifuged at 1000 g for 10 min at 4 °C to pellet cells and the supernatant was aspirated off. Cells were then washed with 0.1M ice cold PBS and centrifuged at 1000 g for 10 min at 4 °C. Cell lysis and cDNA synthesis was performed using SuperScript III CellsDirect cDNA Synthesis System (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol.
2.3.4. ELISA
Cardiac blood was centrifuged (14,000xg for 10 min at 4°C) and plasma collected. An enzyme immunoassay for corticosterone (Assay Designs, Inc., Ann Arbor, MI) was run in duplicate according to the manufacturer’s instructions. For the assay, plasma samples were treated with a steroid displacement reagent and then diluted 1:40 with assay buffer. The high and low limits of detectability were 80 μg/dL to 0.128 μg/dL (taking into account the dilution factor of 40). All samples fell within the range of detectability and the intra-assay coefficient of variation was 1.3 %.
Hippocampal samples were sonicated on ice using a tissue extraction reagent (Invitrogen) supplemented with protease inhibitor cocktail (Sigma). Homogenates were centrifuged (14,000xg for 10 min at 4°C) and supernatant collected and stored at −20°C. A rat IL1β ELISA (R&D systems) was run in duplicate according to the manufacturer’s instructions. All samples fell within the range of detectability (2000 pg/ml and 7.8 pg/ml respectively) with an intra-assay coefficient of variation of 5.9 %. The concentration of IL1β protein is expressed relative to total protein concentrations established in a Bradford assay (pg of IL1β/100μg of total protein).
2.3.5. Quantitative real-time PCR (qPCR)
Primers were previously designed using Genbank at the National Center for Biotechnology Information (NCBI), the Operon Oligo Analysis Tool, and the Basic Local Alignment Search Tool at NCBI and obtained from Invitrogen. Primers were designed to span exon/exon boundaries and thus exclude amplification of genomic DNA. Primer specificity was verified by melt curve analysis. Primers included β-actin (F: TTCCTTCCTGGGTATGGAAT and R: GAGGAGCAATGATCTTGATC), CD200R (F: TAGAGGGGGTGACCAATT and R: TACATTTTCTGCAGCCACT), CX3CR1 (F: TCAGGACCTCACCATGCCT and R: CGAACGTGAAGACAAGGG), IL-1β (F: CCTTGTGCAAGTGTCTGAAG and R: GGGCTTGGAAGCAATCCTTA), IL-6 (F: AGAAAAGAGTTGTGCAATGGCA and R: GGCAAATTTCCTGGTTATATCC), MHCII (F: AGCACTGGGAGTTTGAAGAG and R: AAGCCATCACCTCCTGGTAT), NFKBIA (F: CACCAACTACAACGGCCACA and R: GCTCCTGAGCGTTGACATCA), and TNFα (F: CAAGGAGGAGAAGTTCCCA and R: TTGGTGGTTTGCTACGACG). RNA was extracted from hippocampal homogenates using TRIZOL reagent and 2 μg of RNA was reversed transcribed to cDNA using Superscript II (Life Technologies) according to the manufacturer’s instructions. RNA was isolated from microglia and reversed transcribed to cDNA using SuperScript III CellsDirect cDNA Synthesis System (Life Technologies). PCR amplification of cDNA was performed using the Quantitect SYBR Green PCR Kit (Qiagen, Valencia, CA) with a MyiQ Single-Color Real-Time PCR Detection System (BioRad, Hercules, CA). Gene expression was determined in duplicate and expressed relative to β-actin.
Selection of β-actin as the housekeeping gene warrants discussion as previous reports have noted circadian differences in β-actin (Taishi et al., 1997). First, there were no circadian differences in the amplification of β-actin in this experiment (see supplemental figure 1). Second, a recent study evaluating circadian differences in 10 potential housekeeping genes found that β-actin, GAPDH, and Atp5b are the most stable for normalization of circadian related gene expression in the suprachiasmatic nucleus (master circadian pacemaker in mammals) (Cleal et al., 2014). Finally, GAPDH was also evaluated but there were statistical differences in GAPDH expression in microglia isolated in the light versus the dark phase. Thus, β-actin was used as the housekeeping gene for all of the analyses.
3.4. Statistical analyses
All data are presented as mean ± standard error of the mean (SEM). Data were analyzed with Prism 5 (GraphPad Software) using analysis of variance (ANOVA). F values are reported for each ANOVA and serve as the criteria for post hoc analysis (Tukey’s multiple comparisons test). Threshold for statistical significance was set at p < 0.05.
3. Results
3.1. Experiment 1: Stress-induced inflammatory priming was influenced by time of day
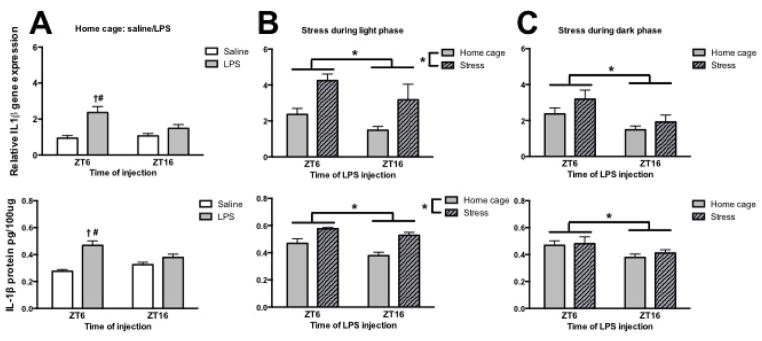
The first goal of this study was to determine whether stress at different times of day would elicit a similar priming response following peripheral immune challenge (see Fig 1 for experimental outline). As we have previously demonstrated (Fonken et al., 2015), HCC rats injected with LPS during the middle of the light as compared to the dark phase had elevated induction of hippocampal IL-1β gene expression (interaction of time x LPS: F1,22 = 5.0 and post hoc, p < 0.05) and protein (interaction of time x LPS: F1,26 = 8.8 and post hoc, p < 0.05) (Fig. 2A). Furthermore, stress significantly potentiated the LPS induced IL-1β response, but only when the stressor occurred during the light phase. Rats that were exposed to 100 trials of IS during the light phase and challenged with LPS at ZT6 or ZT16 (24 or 36 h later) showed significantly enhanced LPS-induced hippocampal IL-1β gene (main effect of time: F1,23 = 5.2, main effect of stress: F1,23 = 19.0; p < 0.05) and protein (main effect of time: F1,23 = 7.0, main effect of stress: F1,23 = 24.5; p < 0.05) expression (Fig. 2B). In contrast, IS that occurred during the dark phase did not significantly elevate IL-1β gene (main effect of time only: F1,22 = 9.1, p < 0.05) and protein (main effect of time only: F1,23 = 6.0, p < 0.05) expression after LPS (Fig. 2C). Importantly, time of day did not affect amplification of the housekeeping gene β-actin or protein concentrations as determined by a Bradford assay (see supplemental figure 1).
Figure 2. Inflammatory priming of interleukin-1β did not occur in rats that underwent inescapable stress during the dark phase.
(A) Hippocampal IL-1β gene and protein expression in rats that were injected with saline or lipopolysaccharide either during the middle of the light (ZT6) or dark (ZT16) phase. (B) Hippocampal IL-1β gene and protein expression in rats that received stress during the middle of the light phase and were injected 24 or 36 h later with LPS. (C) Hippocampal IL-1β gene and protein expression in rats that received stress during the middle of the dark phase and were injected 24 or 36 h later with LPS. Gene expression data are presented relative to β actin. All data are presented as mean ± SEM. *main effect, †simple effect of LPS, #simple effect of time, in all cases p < 0.05.
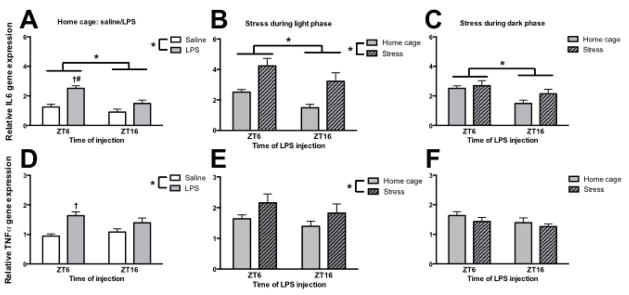
A similar pattern was observed with IL-6 and TNF-α gene expression. In HCC rats, LPS increased hippocampal IL-6 gene expression (main effect of LPS: F1,23 = 20.8, p < 0.05). Furthermore, IL-6 gene expression was elevated in rats injected with LPS during the light as compared to the dark phase (main effect of time: F1,23 = 11.3, p < 0.05; Fig. 3A). Post hoc analysis revealed that LPS during the light as compared to the dark phase significantly increased IL-6 gene expression (p < 0.05). TNFα gene expression was also elevated in HCC rats injected with LPS (main effect of LPS: F1,23 = 14.3, p < 0.05; Fig. 3D). There was no main effect of time (p > 0.05); however, post hoc comparison revealed LPS only significantly elevated TNFα expression at ZT6 (p < 0.05).
Figure 3. Tumor necrosis factor-α and interleukin-6 were not elevated by inescapable stress during the dark phase.
(A&D) Hippocampal IL-6 and TNF-α gene expression in rats that were injected with saline or lipopolysaccharide either during the middle of the light (ZT6) or dark (ZT16) phase. (B&E) Hippocampal IL-6 and TNF-α gene expression in rats that underwent stress during the middle of the light phase and were injected 24 or 36 h later with LPS. (C&F) Hippocampal IL-6 and TNF-α gene expression in rats that underwent stress during the middle of the dark phase and were injected 24 or 36 h later with LPS. Gene expression data are presented relative to β actin. All data are presented as mean ± SEM. *main effect, †simple effect of LPS, #simple effect of time, in all cases p < 0.05.
IS during the light phase significantly potentiated IL-6 (main effect of time and stress: F1,23 = 6.5 and 19.0 respectively, p < 0.05, Fig. 3B) and TNF-α (main effect of stress: F1,23 = 4.5, p < 0.05; Fig. 3E) hippocampal gene expression following LPS. However, there was no effect of stress during the dark phase on responses to LPS (main effect of time only for IL-6: F1,23 = 8.3; Fig. 3C&F).
3.2. Experiment 2: Microglia were differentially sensitized by stress exposure during the light versus dark phase
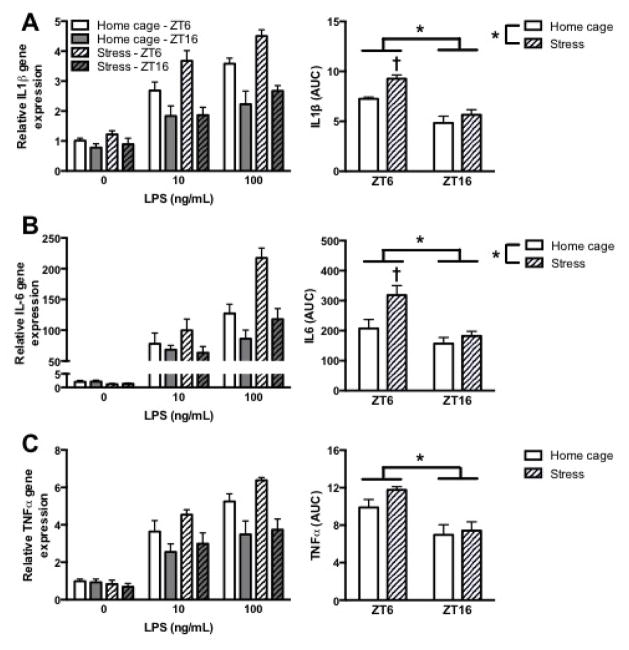
Neuroinflammatory priming in response to a stressor is typically attributed in part to microglia, the primary innate immune cell of the central nervous system (Frank et al., 2007, Frank et al., 2014). Indeed, prior exposure to acute and chronic stressors potentiates the microglia proinflammatory response to an ex vivo immune challenge (Frank et al., 2007, Frank et al., 2014). Thus, we next examined whether microglia are differentially sensitized by stress exposure in the light versus dark phase. To test this, rats received IS or remained in their home cage at either ZT6 or ZT16 and microglia were rapidly isolated 24 h later. After the isolation procedure, microglia were plated for 3 h with media alone, 10 ng/mL LPS, or 100 ng/mL LPS. To determine stress-induced sensitization of the microglia proinflammatory response, area under the curve (AUC) for LPS concentrations was calculated for each animal to reflect the cumulative proinflammatory response. Stress significantly elevated IL-1β gene expression in microglia (main effect of time and stress: F1,17 = 45.9 and 10.1, p < 0.05; Fig. 4A). Post hoc analysis demonstrated that stress only significantly potentiated IL-1β gene expression to LPS if the stressor occurred during the light phase (p < 0.05). A similar result was observed for IL-6; stress elevated microglial IL-6 gene expression to LPS if IS occurred during the light but not during the dark phase (main effect of time and stress: F1,17 = 12.7 and 6.9, and post hoc analysis, p < 0.05; Fig. 4B). Additionally, TNF- α gene expression to LPS was significantly elevated in microglia isolated in the light as compared to the dark phase (main effect of time: F1,17 = 20.0, p < 0.05, Fig. 4C). However, there was not a significant effect of stress. Overall, IL-1β and IL-6 results indicate that microglia from rats stressed during the light, but not dark phase are primed to inflammatory challenge.
Figure 4. Microglial were susceptible to priming in rats that underwent inescapable stress during the light but not the dark phase.
(A) IL-1β, (B) IL6, and (C) TNF-α mRNA expression from hippocampal microglia that were isolated from animals that underwent inescapable stress during the middle of the light (ZT 6) or dark phase (ZT 16). Microglia were stimulated ex vivo with 0, 10, or 100 ng/mL of LPS. Gene expression data are presented relative to β actin. All data are presented as mean ± SEM. *main effect, †differs from all other groups, in all cases p < 0.05.
3.3. Experiment 3: Microglia isolated at ZT6 versus ZT16 did not show different phenotypic changes induced by ex vivo corticosterone treatment
Glucocorticoids are believed to be the proximal signal through which stress primes microglia (Frank et al., 2012, Frank et al., 2014). Thus, here we evaluated whether microglia isolated from rats during the light or the dark phase exhibited differential responses to ex vivo corticosterone treatment. More specifically, we examined expression of several inflammatory pathway genes including CD200R, CX3CR1, MHCII, and NFKBIA in response to increasing concentrations of corticosterone. Corticosterone significantly decreased expression of CD200R (main effect of corticosterone: F3,24 = 8.4, p < 0.05; Fig 5A) and CX3CR1 (main effect of corticosterone: F3,24 = 3.3, p < 0.05; Fig 5B), receptors involved in the anti-inflammatory regulation of microglia. Furthermore, NFKBIA, a negative regulator of NFκB, was upregulated by corticosterone treatment (main effect of corticosterone: F3,24 = 3.2, p < 0.05; Fig 5C). There was no effect of corticosterone treatment on MHCII mRNA expression. Furthermore, there were no time-of-day dependent changes in gene regulation. That is, microglia isolated during the light and dark phase exhibited similar responses to corticosterone treatment.
Figure 5. Microglia isolated during the middle of the light or dark phase an stimulated ex vivo with corticosterone comparably regulated gene expression.
Hippocampal microglia were isolated during the middle of the light (ZT6) or dark (ZT16) phase and treated ex vivo with 0, 10, 100, or 1000 nM corticosterone for 2h. RNA was then isolated to evaluate (A) CD200R, (B) CX3CR1, (C) MHCII and (D) NFKBIA mRNA expression. Genes are expressed relative to β actin and presented as mean ± SEM.
3.4. Experiment 4: Corticosterone concentrations are comparable in rats that underwent stress during the light and dark phase
It is possible that the corticosterone signal produced by IS was different in rats that received the stressor during the light and dark phase. This could result in a different signal to microglia and explain why microglia isolated at the two different times of day show comparable responses to corticosterone (Fig. 5) but exhibit diurnal differences in responses to LPS when isolated after stress (Fig. 4). Rats received IS or HCC treatment at either ZT6 or ZT16 and then blood was immediately collected. As expected there was a significant effect of stress on corticosterone concentrations (F1,20 = 206.4, p < 0.05) and an interaction between stress and time (F1,20 = 4.9, p < 0.05; Fig. 6A). Corticosterone concentrations did not differ between the two time points following stress (post hoc). However, the percent increase in corticosterone was greater during the light as compared to the dark phase (t10 = 13.0, p < 0.05; Fig. 6B).
Figure 6. Plasma corticosterone concentrations in rats that underwent IS during the middle of the light or dark phase.
(A) Blood samples were taken directly following a 2 h IS session or from home cage control animals. (B) Percent increase in corticosterone concentrations relative to home cage control. Data are presented as mean ± SEM. *main effect, †simple effect of time, in all cases p < 0.05.
4. Discussion
Here, we provide the first evidence that the time at which rats are exposed to a stressor influences neuroinflammatory priming. Rats exposed to IS during the light (inactive) phase showed the expected enhanced neuroinflammatory response to a subsequent LPS challenge. In contrast, rats receiving IS during the dark phase did not show neuroinflammatory priming. These striking in vivo findings were mirrored by ex vivo microglial inflammatory state as microglia isolated from rats stressed during the light (but not dark) phase showed enhanced LPS-elicited activation. Our data identify time-of-day as a novel regulator of stress-induced neuroinflammatory state.
As has been previously shown, exposure to IS increased hippocampal cytokines following a subsequent inflammatory challenge. Indeed, animals that received 100 inescapable tailshocks during the light phase increased gene expression of the pro-inflammatory cytokines IL-1β, TNF-α, and IL-6 following an inflammatory challenge either 24 (ZT6) or 36 h (ZT16) later. However, this effect was limited to animals that experienced the stressor during the light (inactive) phase. Pro-inflammatory cytokine induction was not significantly amplified in rats stressed during the dark (active) phase. Previous work in our laboratory investigated whether modulating timing of the inflammatory challenge, but not stress, influences neuroinflammatory priming. This prior study demonstrated that IS during the light phase (ZT1) significantly potentiated fever, corticosterone, and ACTH following LPS during the light (ZT1) or dark phase (ZT14). However, a significant potentiation in the cytokine response was not observed in animals that received IS during the light phase and immune challenge during the dark phase. This result differed from our current findings and may be due to the timing of the stressor relative to the LPS challenge: in the study by Johnson et al. the immune challenge during the dark phase occurred 61 h following the stressors (compared to 24 and 36 h as in our experiment) (Johnson et al., 2003).
Here, both 24 and 36 h time points after LPS exposure were included following stress because inflammatory responses are elevated in the light as compared to the dark phase. A preliminary study demonstrated that rats exposed to stress at ZT16 did not show inflammatory priming 24 h later, however, it could not be ruled out that inflammatory priming was not apparent because LPS has a diminished effect at ZT16. Thus, a time point was included so that stress and LPS occurred at opposite phases of the circadian cycle (36 h later). Importantly, rats that received stress during the light phase (~ZT4) that were challenged with LPS during the dark phase (36 h later; ZT 16) still demonstrated inflammatory priming, but the converse experiment (stress during the dark at ~ZT18 and LPS at ZT6) did not produce priming.
To determine whether microglia might contribute to diurnal differences in neuroinflammatory priming we isolated microglia 24 h after stress delivered either during the middle of the light or dark phase. Only microglia isolated from rats that received IS during the light phase demonstrated an exaggerated inflammatory response when treated ex vivo with LPS. Of course, the extrapolation of results from an ex vivo model to an intact organism is difficult. However, these results do complement the in vivo findings and suggest that microglia may partially mediate circadian differences in neuroinflammatory priming. Taken together, results from both the in vivo and ex vivo studies indicate that the time at which stress occurs is critical for priming neuroinflammatory responses.
Substantial evidence indicates that glucocorticoids are the proximal signal through which acute and chronic stress primes microglia and neuroinflammatory responses (Frank et al., 2010, Frank et al., 2012, Frank et al., 2014, Chijiwa et al., 2015). Indeed, blocking the glucocorticoid response to stress (pharmacologically or surgically) prevents neuroinflammatory priming (Frank et al., 2012, Chijiwa et al., 2015). Moreover, acute and chronic glucocorticoid administration at doses that mimic the rises produced by stress primes microglia to pro-inflammatory stimuli (Frank et al., 2010, Frank et al., 2014). Thus here we tested whether microglia isolated during the light as compared to the dark phase are differentially responsive to glucocorticoids. More specifically, we examined the effects of ex vivo corticosterone on CX3CR1 (fractalkine receptor) and CD200R expression in microglia. CD200 and CX3CL1 are both considered neuroinflammatory “off” signals for microglia (Biber et al., 2007, Paolicelli et al., 2014). In support, downregulation or ablation of CX3CR1 and CD200 are associated with heightened neuroinflammatory responses (Cardona et al., 2006, Denieffe et al., 2013). Furthermore, reduced CX3CR1 may drive neuroinflammatory priming of microglia in aged mice (Wynne et al., 2010). Here, we show that in isolated microglia corticosterone decreased CX3CR1 and CD200R mRNA expression in a concentration dependent manner. These data further support the idea that glucocorticoids can have a pro-inflammatory influence and suggest a novel mechanism, the dis-inhibition of microglial pro-inflammatory pathways, for this action. However, there was no time of day effect of corticosterone on mRNA expression of either receptor. There are several limitations to the ex vivo approach that may explain the null effect of time of day on microglia responses including: (1) the isolation procedure for microglia may change responsiveness of the cells, (2) exposure to tonic corticosterone concentrations in vitro may not properly mimic the dynamic pulsatile release of glucocorticoids in vivo, and (3) the response of microglia to corticosterone in vivo may involve interactions with other cells types. Furthermore it is possible that diurnal differences in microglia priming may be due to different glucocorticoid responses to inescapable stress during the light as compared to the dark phase.
To test this hypothesis, plasma corticosterone concentrations were assessed directly following IS during the middle of the light or dark phase. Corticosterone concentrations after the stressor did not differ between rats stressed at the two different time points suggesting that differential regulation of corticosterone might not mediate time of day differences in neuroinflammatory priming. However, the percent and absolute change in corticosterone concentrations differed between the light and dark phase, with a greater magnitude of change during the light phase.
Previous work indicates that the type of stressor is important for eliciting diurnal differences in glucocorticoid response (Retana-Marquez et al., 2003). For example, circadian differences in the HPA stress response do occur in some cases, with peak corticosterone induction during the light as compared to the dark phase (Dunn et al., 1972, Kant et al., 1986). However, the stressors used in these studies were milder than that used here. A recent study using inescapable predator scent also found that the glucocorticoid concentrations following a stressor were similar at different circadian phases (Cohen et al., 2015). Again, it is important to note that since basal corticosterone concentrations were lower in the light phase than in the dark phase, the increase from baseline produced by IS was greater in the light phase. It is possible that it is the increase in corticosterone produced by the stressor rather than the absolute post-stressor concentration of corticosterone that is critical. In addition, glucocorticoid receptor number and affinity might be different at different times of day (Nader et al., 2009), and so it is possible that glucocorticoid signaling in response to the stressor could still be different in the light and dark cycles.
It is also possible that the down-regulation of CD200R and CXC3CR1 in microglia treated with corticosterone is not sufficient to induce microglia priming and that other changes occur in vivo. For example, upregulation of MHCII occurs in the hippocampus following stress and has previously been implicated in stress-induced neuroinflammatory priming (Frank et al., 2010, Weber et al., 2015). However, there was no effect of corticosterone treatment on MHCII gene expression in microglia isolated during the light or the dark phase. This may indicate that interactions with other cells types (such as neurons and astrocytes) occur in the brain following stress and contribute to glucocorticoid priming of microglia. For example, neurons may respond differentially to the effects of elevated glucocorticoids at different times of day, providing an enhanced priming signal – such as HMGB1 release (Qiu et al., 2008, Weber et al., 2015) or glutamatergic signaling (Mayhew et al., 2015) – to microglia during the light but not dark phase.
Interestingly, nocturnal rodents are more susceptible to the effects of stress during the light (inactive) phase as compared to the dark phase (Cohen et al., 2015). Rats exposed to a stressor at the onset of their inactive phase displayed increased anxiety-like behaviors in the elevated plus maze and acoustic startle response tests, compared to rats exposed to the same stressor during their active phase (Cohen et al., 2015). These behavioral differences are associated with differential regulation of NPY and Yr1 in the hypothalamus. Taken together, these results may indicate that rats are buffered against the effects of glucocorticoids during the dark (active) phase when endogenous concentrations of corticosterone are higher basally. Animals are more likely to be exposed to a variety of stimuli during their active phase, and thus diminished inflammatory priming at this time may prevent potentially harmful overreactions to such stimuli. Furthermore, it may be maladaptive to respond with an energetically expensive heightened neuroinflammatory response during the active phase, when energy resources are needed for other processes (e.g. foraging, mating, fighting).
The present work highlights the importance of studying circadian rhythms to understand the biological mechanisms that mediate stress effects. The natural fluctuations in vulnerability and resilience to stress that occur throughout the day may help elucidate the biological mechanisms that underlie stressor susceptibility. In particular, these results indicate that there are diurnal differences in heightened neuroinflammatory processes, which have been implicated in the etiology of mood disorders (Dantzer et al., 2008). Overall, this work may have important implications for shift workers. Shift workers are more likely to be exposed to stress when they are working during their inactive phase, which could lead to heightened neuroinflammatory responses. Importantly, shift work is a known risk factor for the development of mood disorders (Bedrosian and Nelson, 2013).
5. Conclusions
These experiments resulted in several important findings: (1) the time at which rats are exposed to stress influences neuroinflammatory priming, (2) microglia inflammatory responses are also differentially regulated by stress at different time of day, (3) glucocorticoids directly down regulate genes involved in microglia inhibition, and (4) the magnitude of the corticosterone response but not absolute concentrations of corticosterone are enhanced following IS during the light as compared to the dark phase. While rats exposed to IS during the light phase showed increase hippocampal cytokines following a subsequent inflammatory challenge, stress during the dark phase did not significantly affect inflammatory potential. Microglia likely contribute to diurnal differences in neuroinflammatory priming as microglia also show circadian differences in stress induced inflammatory priming to an ex vivo LPS challenge. These results highlight the importance of time-of-day in regulating susceptibility to stress and inflammatory challenges, and may have key implications for improving conditions for shift workers, hospital patients, and surgeries.
Supplementary Material
Highlights.
Timing of stressor exposure influences neuroinflammatory priming
Microglia inflammatory responses are similarly regulated by time of stress exposure
The corticosterone response is enhanced following stress during the light phase
Glucocorticoids directly down-regulate genes involved in microglia inhibition
Acknowledgments
Role of the funding source
This work was supported by NIH grant R21MH096224 to S.F.M. and F32AG048672 to L.K.F. The funding sources were not involved in the design, collection, analysis or interpretation of data; the written report; or the decision to submit the article for publication.
The authors thank Andrew D. Gaudet for helpful comments and John D’Angelo and Austin Hudmon for excellent animal care. This work was supported by NIH grant R21MH096224 to S.F.M. and F32AG048672 to L.K.F.
Footnotes
Conflict of interest statement
The authors declare no competing financial interests. All authors concur with the submission of this manuscript and none of the data have been previously reported or are under consideration for publication elsewhere.
Author contributions
L.K.F., M.D.W., and S.F.M designed experiments, L.K.F, M.D.W, R.A.D., and M.M.K performed experiments, L.K.F and S.F.M analyzed results, L.K.F., M.G.F., L.R.W., and S.F.M. wrote the manuscript, and all authors contributed to editing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bedrosian TA, Nelson RJ. Influence of the modern light environment on mood. Mol Psychiatry. 2013;18:751–757. doi: 10.1038/mp.2013.70. [DOI] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. The Journal of biological chemistry. 2011;286:38703–38713. doi: 10.1074/jbc.M111.275370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nature neuroscience. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Sikdar SK. Corticosterone targets distinct steps of synaptic transmission via concentration specific activation of mineralocorticoid and glucocorticoid receptors. J Neurochem. 2014;128:476–490. doi: 10.1111/jnc.12478. [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Oka T, Lkhagvasuren B, Yoshihara K, Sudo N. Prior chronic stress induces persistent polyI:C-induced allodynia and depressive-like behavior in rats: Possible involvement of glucocorticoids and microglia. Physiol Behav. 2015;147:264–273. doi: 10.1016/j.physbeh.2015.04.050. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Shepherd JN, Shearer JL, Bruce KD, Cagampang FR. Sensitivity of housekeeping genes in the suprachiasmatic nucleus of the mouse brain to diet and the daily light-dark cycle. Brain Res. 2014;1575:72–77. doi: 10.1016/j.brainres.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Cohen S, Vainer E, Matar MA, Kozlovsky N, Kaplan Z, Zohar J, Mathe AA, Cohen H. Diurnal Fluctuations in HPA and Neuropeptide Y-ergic Systems Underlie Differences in Vulnerability to Traumatic Stress Responses at Different Zeitgeber Times. Neuropsychopharmacology. 2015;40:774–790. doi: 10.1038/npp.2014.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Current biology : CB. 2006;16:R914–916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denieffe S, Kelly RJ, McDonald C, Lyons A, Lynch MA. Classical activation of microglia in CD200-deficient mice is a consequence of blood brain barrier permeability and infiltration of peripheral cells. Brain Behav Immun. 2013;34:86–97. doi: 10.1016/j.bbi.2013.07.174. [DOI] [PubMed] [Google Scholar]
- Dunn J, Scheving L, Millet P. Circadian variation in stress-evoked increases in plasma corticosterone. Am J Physiol. 1972;223:402–406. doi: 10.1152/ajplegacy.1972.223.2.402. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun. 2015;45:171–179. doi: 10.1016/j.bbi.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Nelson RJ. Mice exposed to dim light at night exaggerate inflammatory responses to lipopolysaccharide. Brain Behav Immun. 2013;34:159–163. doi: 10.1016/j.bbi.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Frank MG, Hershman SA, Weber MD, Watkins LR, Maier SF. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology. 2014;40:191–200. doi: 10.1016/j.psyneuen.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun. 2012;26:337–345. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF. The permissive role of glucocorticoids in neuroinflammatory priming: mechanisms and insights. Curr Opin Endocrinol Diabetes Obes. 2015;22:300–305. doi: 10.1097/MED.0000000000000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. Journal of neuroscience methods. 2006;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Halberg F, Johnson EA, Brown BW, Bittner JJ. Susceptibility rhythm to E. coli endotoxin and bioassay. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1960;103:142–144. doi: 10.3181/00379727-103-25439. [DOI] [PubMed] [Google Scholar]
- Jacobsson J, Persson M, Hansson E, Ronnback L. Corticosterone inhibits expression of the microglial glutamate transporter GLT-1 in vitro. Neuroscience. 2006;139:475–483. doi: 10.1016/j.neuroscience.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Hansen MK, Watkins LR, Maier SF. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol. 2003;284:R422–432. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- Kant GJ, Mougey EH, Meyerhoff JL. Diurnal variation in neuroendocrine response to stress in rats: plasma ACTH, beta-endorphin, beta-LPH, corticosterone, prolactin and pituitary cyclic AMP responses. Neuroendocrinology. 1986;43:383–390. doi: 10.1159/000124553. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, McEwen BS. Timing is everything: a collection on how clocks affect resilience in biological systems. F1000Research. 2014;3:273. doi: 10.12688/f1000research.5756.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marpegan L, Leone MJ, Katz ME, Sobrero PM, Bekinstein TA, Golombek DA. Diurnal variation in endotoxin-induced mortality in mice: correlation with proinflammatory factors. Chronobiol Int. 2009;26:1430–1442. doi: 10.3109/07420520903408358. [DOI] [PubMed] [Google Scholar]
- Mayhew J, Beart PM, Walker FR. Astrocyte and microglial control of glutamatergic signalling: a primer on understanding the disruptive role of chronic stress. Journal of neuroendocrinology. 2015;27:498–506. doi: 10.1111/jne.12273. [DOI] [PubMed] [Google Scholar]
- Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J. 2009;23:1572–1583. doi: 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bisht K, Tremblay ME. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front Cell Neurosci. 2014;8:129. doi: 10.3389/fncel.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DJ, Savenkova MI, Karatsoreos IN. Environmental disruption of the circadian clock leads to altered sleep and immune responses in mouse. Brain Behav Immun. 2015;47:14–23. doi: 10.1016/j.bbi.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- Retana-Marquez S, Bonilla-Jaime H, Vazquez-Palacios G, Dominguez-Salazar E, Martinez-Garcia R, Velazquez-Moctezuma J. Body weight gain and diurnal differences of corticosterone changes in response to acute and chronic stress in rats. Psychoneuroendocrinology. 2003;28:207–227. doi: 10.1016/s0306-4530(02)00017-3. [DOI] [PubMed] [Google Scholar]
- Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, Gitlin II, Artemicheva NM, Deluca KA, Gudkov AV, Antoch MP. Core circadian protein CLOCK is a positive regulator of NF-kappaB-mediated transcription. Proc Natl Acad Sci U S A. 2012;109:E2457–2465. doi: 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taishi P, Bredow S, Guha-Thakurta N, Obal F, Jr, Krueger JM. Diurnal variations of interleukin-1 beta mRNA and beta-actin mRNA in rat brain. Journal of neuroimmunology. 1997;75:69–74. doi: 10.1016/s0165-5728(97)00002-7. [DOI] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF. Stress Induces the Danger-Associated Molecular Pattern HMGB-1 in the Hippocampus of Male Sprague Dawley Rats: A Priming Stimulus of Microglia and the NLRP3 Inflammasome. J Neurosci. 2015;35:316–324. doi: 10.1523/JNEUROSCI.3561-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–1505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr, Drake AL, Frey DJ, Fleshner M, Desouza CA, Gronfier C, Czeisler CA. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.