Abstract
Background and Aims
Dietary quality affects cardiometabolic risk, yet its pathways of influence on regional adipose tissue depots involved in metabolic and diabetes risk are not well established. We aimed to investigate the relationship between dietary quality and regional adiposity.
Methods and Results
We investigated 5,079 individuals in the Multi-Ethnic Study of Atherosclerosis (MESA) who had food-frequency questionnaires and measurement of pericardial fat and hepatic attenuation at the baseline study visit in MESA, as well as a subgroup with imaging for visceral and subcutaneous fat (N=1,390). A dietary quality score (DietQuality) was constructed to include established food group constituents of a Mediterranean-type diet. Linear models estimated associations of dietary score as well as its constituents with regional adiposity. Baseline mean age was 61 (±10) years, and approximately half of the participants (47%) were male. Those with a higher DietQuality score were generally older, female, with a lower body mass index, C-reactive protein, and markers of insulin resistance. After adjustment, a higher DietQuality score was associated with lower visceral fat (lowest vs. highest dietary score quartile: 523.6 vs. 460.5 cm2/m; P<0.01 for trend), pericardial fat (47.5 vs. 41.3 cm3/m; P<0.01 for trend), lesser hepatic steatosis (by hepatic attenuation; 58.6 vs. 60.7 Hounsfield units; P<0.01 for trend), but not subcutaneous fat (P=0.39). Greater fruits, vegetables, whole grains, seeds/nuts and yogurt intake were associated with decreased adiposity, while red/processed meats were associated with greater regional adiposity.
Conclusion
A higher quality diet pattern is associated with less regional adiposity, suggesting a potential mechanism of beneficial dietary effects on diabetes, metabolic, and cardiovascular risk.
Introduction
The role of optimal nutrition in cardiovascular health is increasingly recognized, with recent evidence linking diet and cardiovascular risk1. In response, the American Heart Association (AHA) has established major dietary goals as part of its “Life's Simple 7” guidelines, emphasizing a healthful diet pattern resembling a Mediterranean- or Dietary Approaches to Stop Hypertension (DASH)-style diet as one of the cornerstones of “ideal cardiovascular health”2. While clinical and policy interventions often target generalized obesity, it is increasingly clear that specific regional adiposity (i.e. higher levels of pericardial, hepatic, and visceral fat) may significantly contribute to insulin resistance, inflammation, arrhythmia burden3, subclinical myocardial remodeling4, and cardiometabolic events5. Indeed, both obese and lean individuals with increased fat in these regions experience higher cardiovascular and metabolic risk not explained by weight alone6-8. As such, dietary interventions that influence these regional fat depots may more effectively target the cardiovascular and metabolic consequences of obesity.
Investigations into the relationship between dietary quality and adiposity have largely relied on waist circumference8-11. Adherence to a Mediterranean-style diet appears to mitigate insulin resistance12, 13, systemic inflammation14, and oxidative stress15, features linked to alterations in metabolically relevant regional fat depots. In a seminal study of dietary associations with precise imaging-defined measures of regional adiposity in Framingham, Molenaar and colleagues demonstrated that higher dietary quality (by the 2005 Dietary Guidelines Adherence Index) was associated with lower subcutaneous and visceral fat16, though specific food groups were not explored17, 18. Nevertheless, racial differences in dietary composition and adiposity distribution suggest that racial heterogeneity in food intake may impact fat distribution and downstream cardiometabolic risk. Elucidating links between dietary quality and directly quantified regional adiposity depots in diverse individuals would provide important evidence to further substantiate the importance of diet on adiposity in different anatomic locations.
To address these gaps in knowledge, we performed a cross-sectional analysis of individuals enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA), defining a healthy diet pattern that is based on prior Mediterranean-style diet pattern established in MESA19, with specific addition of sugar-sweetened beverages as harmful, inclusion of yogurt intake as beneficial, and removal of dairy products as harmful, given their proven impact on cardiometabolic health2, 20-22; Table 1). This expanded score encompassed We hypothesized that a healthier diet would be associated with decreased regional adiposity independent of major demographics and cardiometabolic risk factors. Using specific, quantitative phenotypes of ectopic fat relevant to cardiometabolic health, our ultimate goal was to provide evidence to support the role of a healthy dietary pattern on different adiposity phenotypes.
Table 1.
Modified diet pattern score construction.
| Food Group | Constituent Foods | Weighting in Optimal Diet Score |
|---|---|---|
|
| ||
| Vegetables | All vegetables (cruciferous, dark yellow, green leafy, other vegetables; not potatoes) | Quartiles (+4 points for greatest intake; +1 point for least intake) |
|
| ||
| Whole grains | All whole grain or containing products (oatmeal, dark bread, bran muffin, brown rice, selected cereals) | Quartiles (+4 points for greatest intake; +1 point for least intake) |
|
| ||
| Seeds and nuts | Nuts (including peanuts, sunflower seeds, etc.) | Quartiles (+4 points for greatest intake; +1 point for least intake) |
|
| ||
| Legumes (including soy) | Legume and legume-containing products (e.g., pea soup, beans, burritos, enchiladas, meat chile, fried beans) and soy products (e.g., soy milk, miso soup, stirfriend tofu, tofu desserts) | Quartiles (+4 points for greatest intake; +1 point for least intake) |
|
| ||
| Yogurt | Plain and flavored yogurt | Quartiles (+4 points for greatest intake; +1 point for least intake*) |
| Fish | Fish-containing foods (e.g., boiled fish, tuna fish, shrimp, fried fish) | Quartiles (+4 points for greatest intake; +1 point for least intake) |
| Alcohol | Total alcohol intake (beer, wine, other) | +4 for 5-15 grams/day consumption, otherwise 0 |
| Unsaturated to saturated fat ratio | Mono- and polyunsaturated fat to total saturated fat ratio | Quartiles (+4 points for highest ratio; +1 point for lowest ratio) |
| Red and processed meats | All red meat containing products (e.g., sausage, steak, beef, etc.) | Quartiles (+4 points for greatest intake; +1 point for least intake) |
| Fruits | All fruit and fruit-containing products (e.g., fruit juice, avocado, tomato, apple, etc.) | Quartiles (+4 points for greatest intake; +1 point for least intake) |
| Sugar-sweetened beverages | Regular soft drinks, soda, sweetened mineral water (not diet), non-alcoholic beer | Quartiles (+4 points for greatest intake; +1 point for least intake*) |
For yogurt and sugar-sweetened beverage consumption, at least 25% of study participants reported zero consumption. See text for details.
Methods
Participant population
The original MESA cohort consisted of 6,814 men and women of 4 ethnicities (non-Hispanic White, African American, Chinese American, and Hispanic) from 6 centers in the United States and enrolled between July 2000 and August 2002. These individuals were free of clinical cardiovascular disease (history of myocardial infarction, angina pectoris, prior revascularization, heart failure, atrial fibrillation, stroke, or peripheral arterial disease) at enrollment23. Protocols were approved by the Institutional Review Board at each participating institution. All participants provided written informed consent.
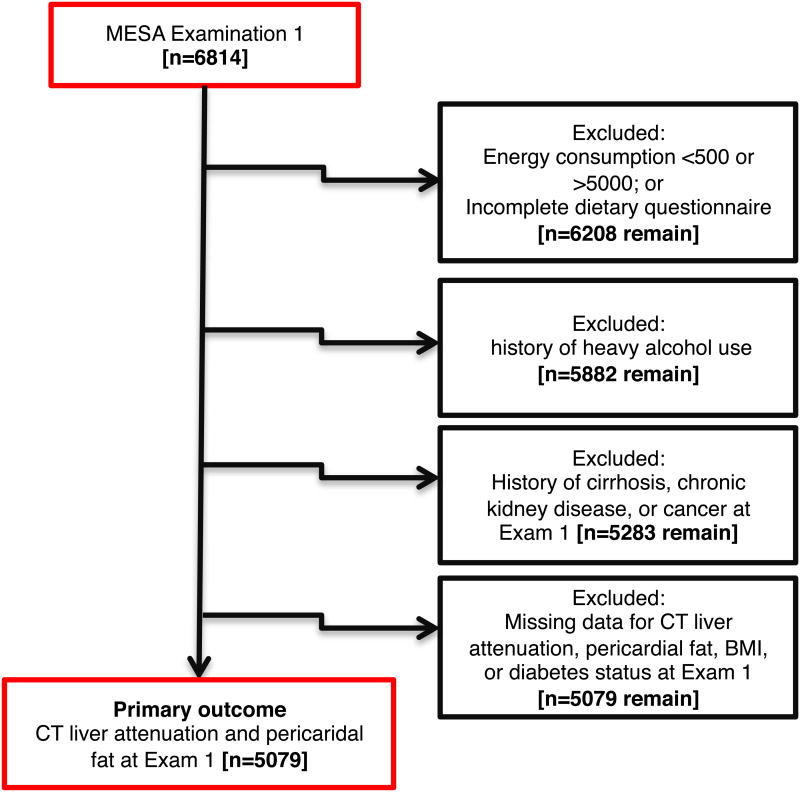
The final analytic cohort for this investigation included 5,079 participants with directly measured liver attenuation (a marker of hepatic steatosis) and pericardial fat volume (our primary outcomes) at baseline (Figure 1). As with prior dietary studies in MESA19, we excluded individuals with unrealistic energy consumption (>5,000 kcal/day or <500 kcal/day), incomplete dietary questionnaires, significant alcohol consumption (>14 drinks/week in men or >7 drinks/week in women), history of end-organ disease (including self-reported cirrhosis, renal disease or cancer), or missing data on body mass index and prevalent diabetes. Relative to those individuals in the overall MESA study but not included in the primary analysis (N=1,735), the final primary analytic cohort (N=5,079) was well-matched by sex, waist circumference, systolic blood pressure, dysglycemia and triglyceride; there were slight differences in age (median 62 vs. 63 years; P=0.0005), with a slightly higher BMI (27.7 vs. 27.2 kg/m2; P=0.002), and lower high-density lipoprotein (48 vs. 50 mg/dl; P<0.0001) in the primary analytic cohort.
Figure 1.
Construction of the study cohort. MESA participants from the baseline examination underwent successive exclusions (as detailed in text) to arrive at a final study population (N</=5,102) for the primary measures of liver attenuation and pericardial fat. In addition, a subgroup of individuals at the second and third MESA study visits underwent abdominal CT imaging for visceral and subcutaneous fat, of which 1,390 were included in this analysis.
In a separate secondary analysis, we examined visceral and subcutaneous fat obtained by computed tomography, starting with a subset of MESA participants at the second and third study visit (between 2002-2005) studied in our prior work in MESA8 (N=1,511); from this initial cohort, we excluded individuals without completed dietary questionnaires, unrealistic energy consumption (as defined above), leaving 1,390 MESA participants for this secondary analysis. Of note, imputation methods were required in 292 subjects (3 for visceral fat and 292 for subcutaneous fat) to account for image truncation using previously described methods8. Methods for pericardial fat24, hepatic attenuation25, and abdominal fat imaging8 have been previously described. A higher hepatic attenuation is evidence for lower hepatic fat content, as in prior published work in MESA25.
Dietary quality score
Usual dietary habits were assessed at baseline using a semi-quantitative, 127-item food frequency questionnaire adapted from a questionnaire used in the multi-ethnic Insulin Resistance Atherosclerosis study to capture the dietary habits of self-identified Chinese individuals26. Recognizing several different approaches to defining healthy diet patterns, we created a 11-component dietary metric that closely followed the AHA's Simple 7 goals2 to maximize simplicity and applicability, while also consistent with a Mediterranean-style diet pattern established in prior published work within MESA (Table 1)19. These components included fruits, vegetables (excluding potatoes), whole grains, nuts, legumes, fish, ratio of unsaturated (poly- and monounsaturated) to total saturated fat, red and processed meat, sugar-sweetened beverages, alcohol, and yogurt intake. For each food group, servings per day were calculated as the product of frequency of consumption and serving size (small, 0.5; medium, 1.0; or large, 1.5) for each contributing food item, and summed over all foods contributing to a particular food group.
For approximately 25% of the participants in MESA, a coding error led to data being unavailable on the intake of 14 foods (fruit juice, dark/while grains, fruit, salty snacks, other vegetables, leafy green vegetables, yogurt, potato, red meat, high fat and processed meat, high-fat dairy, desserts, cottage cheese and legumes). As the majority of these participants were from one of two MESA field centers, the data are not missing completely at random (MCAR) and may not be missing at random (MAR). To avoid bias in the sample, for these 14 intakes (servings per day and serving size) for this portion of the MESA population only, imputation was conducted using sequential chained regression implemented in Stata 12 using the MI program specifying multinomial or ordinal regression, with one model for all 14 items. In all cases, the imputation model accounted for basic demographics. Other auxiliary variables were selected on the basis of having a high correlation with the FFQ or serving size items. The subsequent imputed dataset was used for this analysis, and has been approved by the MESA Steering Committee.
The contribution of each food group to the overall dietary score (termed “DietQuality” hereafter) was calculated by dividing the intake of each food group into quartiles, with a score from 1-4 points. Higher scores corresponded to greater intake of each food item. Alcohol consumption was given +4 points for moderate intake (5-15 grams/day) and 0 for alcohol intake outside these bounds. The final score was obtained by summing all salutatory food items (fruit, vegetables, whole grains, nuts, legumes, fish, unsaturated fat ratio, alcohol and yogurt), and subtracting scores from potentially harmful items (red and processed meat, sugar-sweetened beverages). Of note, for most food items (except yogurt and sugar-sweetened beverages), stratification by quartile of intake led to approximately equal number of MESA participants in each quartile; deviations from this pattern (in yogurt and sugar-sweetened beverages) were due to large numbers of individuals with zero intake (for yogurt, 2533 participants; for sugar-sweetened beverages, 2381 participants of our study cohort). For yogurt, +1 point was assigned for the lower two quartiles, and +3 and +4 points for the third and fourth quartile, respectively. Standard scoring was applied to sugar-sweetened beverages.
Statistical analysis
Baseline characteristics across quartiles of DietQuality score were tabulated and compared using Wilcoxon rank-sum testing (for continuous covariates) or chi-square tests (for categorical covariates). Given that hepatic attenuation and pericardial fat volume were measured concurrently with dietary assessment, we treated the association of DietQuality score and pericardial fat volume (indexed to height, in meters) or hepatic attenuation measured in >5,000 MESA participants at the baseline study visit as our primary outcome. Our secondary outcome was the association of DietQuality score with visceral and subcutaneous fat area (indexed to height, in meters) in a subgroup with these indices measured at the second and third MESA study visit.
We tested the form of the relationship between each regional fat depot and DietQuality score (treated as a continuous variable) using general additive models to assess linearity. Using covariates obtained contemporaneously with CT scans for regional adipose tissue measures, we constructed linear models to measure association between each regional fat depot and DietQuality (treated both as a continuous variable and in quartiles), adjusted for age, race/ethnicity, sex, weight, cigarette use, systolic blood pressure, fasting blood glucose, total cholesterol, total intentional exercise, and energy consumption (in kilocalories/day). Energy consumption was included in models to adjust for the underreporting of intake associated with food frequency questionnaire data, and to ensure that analyses were independent of caloric intake. A least squares means approach with Tukey's test was performed to test multiple comparisons of adiposity across quartiles of DietQuality score. Pericardial, visceral, and subcutaneous fat depots were log-transformed to establish linearity in these regressions (liver attenuation has negative values and was not log-transformed). Continuous variables used in adjustments in regression models (e.g., age, cholesterol, etc.) were standardized to a mean of 0 and standard deviation of 1 for inclusion in regression. In addition, we performed multiplicative interaction terms with sex and race to evaluate for effect modification.
Finally, to estimate which food groups were most strongly related to regional ectopic fat distributions, we estimated adjusted associations between each component of diet (treated as servings per day, except for alcohol intake, which was coded as beneficial or non-beneficial, as described above) and regional adiposity (with adjustments for identical covariates as in the linear models above). For the dietary component regression models, adiposity measures (including liver attenuation) were standardized (but not log-transformed) to improve interpretability. SAS 9.3 (SAS, Cary, NC) was used for analyses. Given that 11 different food groups were tested for association with each specific fat depot, we performed a Bonferroni adjustment for 11 tests (0.05/11≈0.0045) to minimize the chance of a type 1 error.
Results
Clinical and biochemical indices of cardiometabolic risk by quartiles of modified MedDiet score
Baseline clinical and demographic characteristics for the study population are shown in Table 2. The median DietQuality score was 15 (interquartile range IQR 12-19). In crude (unadjusted) analyses, individuals with a higher quality diet were more likely to be older and female with a lower body mass index (all P<0.01). In addition, a greater DietQuality score was associated with a more favorable lipid profile (lower LDL-C and triglycerides, higher HDL-C), lower biomarkers of inflammation (C-reactive protein, interleukin-6) and greater insulin sensitivity (lower HOMA-IR). We further explored DietQuality score and food group constituents across race (Supplemental Table 1). Dietary score was slightly higher in Chinese-Americans (consistent with Table 1) with a more salutatory dietary profile, lower weight, and inflammatory markers relative to other races in MESA, including greater fish intake, more unsaturated fat, vegetables, legumes, and less sugar-sweetened beverage consumption.
Table 2.
Baseline clinical, demographic, biochemical and imaging indices, stratified by DietQuality score in quartiles (where available). A higher quartile signifies a more salutatory diet. Values represent median (interquartile range) or number (percentage). Abbreviations: lbs, pounds; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HOMA-IR, homeostatic model of insulin resistance; HU, Hounsfield units.
| Variable | Dietary Quality Score (quartiles) | P | |||
|---|---|---|---|---|---|
|
| |||||
| Q1 (N=1180) | Q2 (N=1378) | Q3 (N=1335) | Q4 (N=1186) | ||
|
| |||||
| Age (baseline, years) | 60 (52-68) | 61 (53-69) | 63 (54-70) | 63 (54-71) | <0.01 |
|
| |||||
| Race (%) | <0.01 | ||||
| Caucasian | 375 (32%) | 471 (34%) | 465 (35%) | 502 (42%) | |
| Chinese-American | 75 (6%) | 179 (13%) | 248 (19%) | 145 (12%) | |
| African-American | 394 (33%) | 370 (27%) | 335 (25%) | 342 (29%) | |
| Hispanic | 336 (28%) | 358 (26%) | 287 (22%) | 197 (17%) | |
|
| |||||
| Male sex (%) | 615 (52%) | 643 (47%) | 602 (45%) | 516 (44%) | <0.01 |
|
| |||||
| Income | <0.01 | ||||
| $0-$100,000/year | 1016 (91%) | 1192 (90%) | 1106 (86%) | 946 (82%) | |
| >$100,000/year | 105 (9%) | 129 (10%) | 178 (14%) | 207 (18%) | |
|
| |||||
| Educational attainment | <0.01 | ||||
| ≤ High school | 547 (47%) | 570 (41%) | 463 (35%) | 295 (25%) | |
| Some college | 342 (29%) | 404 (29%) | 361 (27%) | 320 (27%) | |
| ≥ 4 years college | 287 (24%) | 402 (29%) | 511 (38%) | 569 (48%) | |
|
| |||||
| Body mass index (kg/m2) | 28.7 (25.7-32.3) | 27.8 (24.7-31.2) | 27.4 (24.6-30.9) | 26.9 (24.2-30.3) | <0.01 |
|
| |||||
| Weight (lbs) | 177 (153-204) | 169 (145-196) | 168 (145-195) | 166 (144-192) | <0.01 |
|
| |||||
| Waist circumference (cm) | 99.8 (90.8-109.5) | 97.0 (88.7-106.5) | 97.0 (88.0-105.5) | 95.7 (86.5-104.0) | <0.01 |
|
| |||||
| Systolic blood pressure (mmHg) | 124 (112-140) | 125 (113-141) | 124 (112-139) | 124 (111-139) | 0.41 |
|
| |||||
| Never smoker (%) | 563 (48%) | 737 (54%) | 733 (55%) | 625 (53%) | <0.01 |
|
| |||||
| Diabetes (%) | 152 (13%) | 204 (15%) | 170 (13%) | 127 (11%) | 0.02 |
|
| |||||
| Hypertension (%) | 516 (44%) | 631 (46%) | 587 (44%) | 530 (45%) | 0.71 |
|
| |||||
| Biomarkers (median, IQR) | |||||
| LDL cholesterol (mg/dl) | 118 (98-138) | 118 (98-138) | 116 (96-136) | 114 (95-134) | <0.01 |
| HDL cholesterol (mg/dl) | 46 (39-56) | 48 (40-58) | 48 (40-58) | 50 (42-60) | <0.01 |
| Triglycerides (mg/dl) | 117 (83-163) | 112 (79-166) | 114 (79-165) | 104 (73-153) | <0.01 |
| Fasting glucose (mg/dl) | 91 (84-101) | 91 (83-100) | 89 (83-100) | 88 (82-97) | <0.01 |
| C-reactive protein (mg/l) | 2.23 (1.02-4.46) | 1.91 (0.86-4.55) | 1.93 (0.86-4.24) | 1.59 (0.70-3.82) | <0.01 |
| Interleukin-6 (pg/ml) | 1.27 (0.84-1.97) | 1.22 (0.78-1.92) | 1.20 (0.78-1.84) | 1.07 (0.69-1.73) | <0.01 |
| HOMA-IR | 2.18 (1.39-3.40) | 1.98 (1.35-3.08) | 1.93 (1.31-2.92) | 1.66 (1.18-2.59) | <0.01 |
P values represent chi-square or Wilcoxon tests, where appropriate.
Association between greater dietary quality and pericardial, visceral, subcutaneous and liver fat
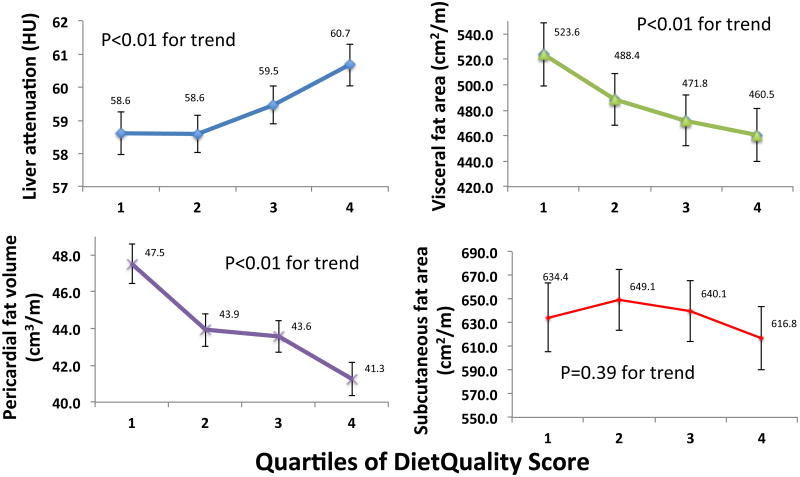
After adjustment for major demographic and cardiometabolic risk factors (as specified in Methods), a higher DietQuality score was associated with lesser hepatic fat content, and lower pericardial, subcutaneous and visceral fat content (Table 3). When DietQuality score was treated as a continuous variable, a higher DietQuality score was associated with lower hepatic fat content, greater visceral and pericardial fat, but was not associated with subcutaneous fat (Table 3). Least squares means for hepatic fat content and height-indexed pericardial fat volume (primary outcomes), as well as height-indexed subcutaneous fat and visceral fat area (secondary outcomes) as a function of DietQuality quartile (fully adjusted for covariates specified in Methods) are shown in Figure 2. We observed significantly lower hepatic fat content in individuals with a healthier diet pattern, as well as lower pericardial and visceral fat with higher diet quality score. There was no significant difference in subcutaneous fat area.
Table 3.
Regional adipose tissue quantity as a function of DietQuality score. As noted in Methods, all adipose tissue depots (except for liver attenuation) were log-transformed for regression analyses. Values presented are least squares means of each regional fat depot at each quartile of DietQuality score, fully adjusted for demographic and cardiometabolic covariates specified in Methods. P values for quartile analyses correspond to P values for DietQuality score in multivariable linear regression. In addition, DietQuality score was treated as a continuous variable (in columns marked “Multivariable Adjusted Regression”), and beta coefficients and P values for DietQuality score (per an increment of +1 in score) are shown. Abbreviations: HU, Hounsfield units; GLM, general linear model.
| Regional adiposity | Dietary Quality Score (in quartiles) | Multivariable Adjusted Regression | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Q1 | Q2 | Q3 | Q4 | P (from GLM) | β | P | |
|
| |||||||
| Liver attenuation (HU) | 58.6 (58.0-59.2) | 58.6 (58.0-59.2) | 59.5 (58.9-60.0) | 60.7 (60.0-61.3) | <0.01 | 0.15 | <0.01 |
| Visceral fat area (cm2/m) | 523.6 (498.6-549.8) | 488.4 (468.2-509.5) | 471.8 (451.8-492.7) | 460.5 (439.8-482.2) | <0.01 | -0.009 | <0.01 |
| Subcutaneous fat area (cm2/m) | 634.4 (605.5-664.7) | 649.1 (623.4-675.8) | 640.1 (614.2-667.2) | 616.8 (590.3-644.5) | 0.39 | -0.003 | 0.17 |
| Pericardial fat volume (cm3/m) | 47.5 (46.4-48.6) | 43.9 (43.0-44.8) | 43.6 (42.7-44.5) | 41.3 (40.4-42.2) | <0.01 | -0.009 | <0.01 |
Figure 2.
Adjusted regional adiposity measures (mean with 95% confidence limits) across quartiles of DietQuality score. Means listed are least-squares means from general linear regression models, adjusted for age, race/ethnicity, sex, weight, cigarette use, systolic blood pressure, fasting blood glucose, total cholesterol, total intentional exercise, and energy consumption (in kilocalories/day).
We next sought to understand whether there was a difference in relationship between DietQuality and regional adiposity measures by sex or race. We used multiplicative interaction terms between DietQuality and sex or race (included in the same model for adiposity measures). There was no evidence of effect modification in the association between dietary quality (in quartiles) and regional fat quantity by sex or race (non-significant interaction term P values for each adiposity depot; visualized graphically in Supplementary Figure 1). Nevertheless, given the differences in adherence to a salutatory dietary pattern by race and potential differences in adherence to a Mediterranean-style diet by culture (see Supplemental Table 1), we performed a sensitivity analysis by excluding Chinese-Americans (who had the greatest adherence to the salutatory dietary pattern). When we considered non-Hispanic White, African American, and Hispanic Americans, a higher DietQuality score (treated in quartiles) was associated with lower hepatic fat content (P<0.01), lower pericardial fat (P<0.01), lower visceral fat (P<0.01), but no difference in subcutaneous fat (P=0.18), after full adjustment.
Dietary components and regional adiposity
After adjustment for multiple comparisons (as specified in Methods), we found that greater intake of vegetables, whole grains, and fish was associated with less hepatic fat and greater red/processed meat intake associated with greater hepatic fat (Table 4; all P<0.05 after adjustment). Similarly, greater fruit, vegetable, whole grain, seeds/nuts and yogurt intake were associated with decreased pericardial fat, while increased red/processed meat and sugar-sweetened beverage intake was associated with greater pericardial fat. In terms of our secondary outcomes (visceral and subcutaneous fat), greater sugar-sweetened beverage intake was associated with greater subcutaneous far. Greater whole grain intake and consuming a greater proportion of fat as unsaturated was associated with less visceral fat, while red/processed meat intake was associated with greater visceral fat.
Table 4.
Linear models for the association between each food group with regional adiposity. Each model was adjusted for age, race/ethnicity, sex, weight, cigarette use, systolic blood pressure, fasting blood glucose, total cholesterol, total intentional exercise, and energy consumption. Fat depots (except for liver attenuation) were normalized to height (as described in Methods). Each food item was treated in servings per day, except for alcohol (treated as moderate vs. non-moderate intake) and fat ratio (a ratio of unsaturated to saturated fat).
| Food group | Liver attenuation | Pericardial fat | Subcutaneous fat | Visceral fat | ||||
|---|---|---|---|---|---|---|---|---|
| Beta | p | Beta | p | Beta | p | Beta | p | |
| Fruit | 0.001 | 0.87 | -0.028 | <0.01* | -0.008 | 0.42 | -0.023 | 0.03 |
| Vegetable | 0.054 | <0.01* | -0.028 | <0.01* | 0.007 | 0.68 | -0.026 | 0.12 |
| Whole grains | 0.072 | <0.01* | -0.118 | <0.01* | -0.057 | 0.06 | -0.114 | <0.01* |
| Fish | 0.123 | <0.01* | -0.056 | 0.11 | 0.015 | 0.82 | -0.037 | 0.58 |
| Unsaturated/Saturated Fat | ||||||||
| Ratio | 0.065 | 0.01 | -0.068 | <0.01* | -0.040 | 0.3 | -0.113 | <0.01* |
| Legumes | -0.001 | 0.97 | 0.028 | 0.15 | -0.054 | 0.12 | 0.042 | 0.26 |
| Seeds/Nuts | 0.020 | 0.54 | -0.117 | <0.01* | 0.071 | 0.15 | -0.058 | 0.26 |
| Yogurt | 0.143 | 0.02 | -0.164 | <0.01* | -0.047 | 0.57 | 0.025 | 0.77 |
| Alcohol (moderate intake) | 0.087 | 0.02 | -0.066 | 0.04 | -0.056 | 0.29 | 0.016 | 0.78 |
| Red and processed meats | -0.092 | <0.01* | 0.118 | <0.01* | 0.022 | 0.62 | 0.171 | <0.01* |
| Sugar-sweetened beverages | -0.039 | 0.01 | 0.045 | <0.01* | 0.074 | <0.01* | 0.027 | 0.30 |
Indicates that the corresponding p-value was significant after Bonferroni correction (as specified in Methods). “Beta” refers to beta coefficient in regression.
Discussion
In this large multi-racial/ethnic cohort of adult Americans, we demonstrated associations between dietary quality (composed of features consistent with AHA goals and a Mediterranean-style diet) and specific regional adiposity phenotypes. Specifically, individuals who had a higher quality diet had higher hepatic attenuation (lower hepatic fat content), and lower pericardial and visceral (but not subcutaneous) fat. These associations were independent of age, race, sex, weight, and markers of cardiometabolic risk. These results provide a large-scale, imaging-based assessment of the relationship between regional adiposity and diet quality across multiple ethnicities.
Mechanisms by which improved dietary quality may impact cardiometabolic risk factors and outcomes have been the focus of intense investigation. A variety of potential pathways that may mediate the relationship between diet and cardiometabolic risk have been identified, including decreased oxidative stress15, epigenetic alterations27, 28, and gut microbial diversity29. Given an increasing recognition that specific fat depots (i.e., pericardial, hepatic, and visceral) may promote metabolic dysfunction5, establishing a link between diet quality and adipocyte quantity/function is imperative to clarify mechanisms that bridge diet and cardiometabolic disease. Indeed, both regional fat accumulation and diet influence a common set of cardiometabolic physiologies, including hypertension, dyslipidemia, endothelial function, oxidative stress, and epigenetics5. On a molecular level, diet may influence fat accumulation and function: for example, fructose may promote de novo lipogenesis and alter global transcriptional profiles within adipose tissue, leading to hepatic steatosis30, and high-fat Western diets promote adipose tissue inflammation and dysfunction in mice31. In addition, dietary poly-unsaturated fats (PUFAs; e.g., n-3 PUFAs) may favorably influence adipocyte hypertrophy and inflammation32, 33, key features of dysfunctional adipose tissue structure. While these findings suggest an intimate connection between diet quality, adiposity, and downstream cardiometabolic risk, evidence from large, multi-racial populations on dietary patterns, constituents, and regional adipose tissue quantity have not been reported.
In a seminal investigation of 2,926 mainly Caucasian participants in the Framingham Heart Study, Molenaar and colleagues demonstrated an association between a composite dietary quality metric (the 2005 Dietary Guidelines Adherence Index) and decreased subcutaneous and visceral adiposity, providing important initial evidence that dietary quality may impact regional adiposity16. In this report, the authors called for studies in multi-racial groups and with specific dietary food groups to further investigate these relationships. In response, several recent reports have addressed the role for sugar-sweetened beverages on directly measured adiposity depots17, 18, 34 using CT methods similar to those employed in MESA. Our study directly addresses these important areas in nutritional research by examining quantitative CT adiposity phenotypes in a multi-racial/multi-ethnic cohort, focusing on both guideline-recommended diet patterns as well as dietary constituents. In addition to identifying a direct relationship between a more salutatory diet pattern and regional adiposity, we identified fruits, vegetables, legumes, fish (among others) as associated with a beneficial adiposity profile, while red and processed meats and sugar-sweetened beverages were associated with poorer adiposity profiles. These conclusions are consistent with reduction in abdominal fat mass with a Mediterranean diet (relative to a carbohydrate-rich alternative), alongside improvements in insulin resistance35, and the landmark, randomized PREDIMED trial that demonstrated long-term reduction in waist circumference with traditional Mediterranean-type diets rich in extra virgin olive oil or nuts36.
The strengths of our study lie in the diverse adult population comprised of multi-racial and multi-ethnic individuals, and detailed CT-based phenotyping of regional adiposity. Certainly, the use of dietary scores (as opposed to single dietary components) is critical to limit nutritional confounding, to provide an integrated landscape of dietary habits across multiple nutrients, and to closely reflect the actual consumption patterns of individuals37-39. Nevertheless, the results of our study should be viewed in the context of its design. While our study is cross-sectional, with imaging performed at three different MESA study visits, the associations we observed are based on a large sample size, precise imaging quantification, and multivariable adjustment, which may limit the effects of residual confounding. Nevertheless, it is still possible that adherence to a higher quality diet may be associated with unmeasured, salutatory health behaviors that promote reduced generalized and regional adiposity. Furthermore, hepatic attenuation is only one marker of liver triglyceride content, and future work employing more specific methods (e.g., MRI spectroscopy) and CT or MRI imaging of visceral or subcutaneous fat at the time of the dietary assessment may be useful to further elucidate these relationships. We recognize that the expanded DietQuality score has several modifications over a traditional Mediterranean diet (including inclusion of yogurt and sugar-sweetened beverages and exclusion of dairy), and has not been directly externally validated; however, yogurt has proven benefits on cardiometabolic health20, 21, and reduced sugar-sweetened beverages are part of the American Heart Association Simple 7 dietary guidelines2. In addition, we did not have access to glycemic indices of different foods in this study, though previous investigations in MESA have noted relationships between a Mediterranean diet and insulin resistance19. Finally, future studies involving both serial imaging and serial questionnaire and molecular assessment of nutrition (e.g., metabolite profiling) specifically across different races/ethnicities may be warranted to further explore these associations.
In conclusion, using computed tomography, we observed an association between diet quality and regional adiposity. Specifically, a higher quality diet was associated with lower hepatic fat content and lower visceral and pericardial fat. In addition, specific foods had an impact on regional adiposity phenotypes independent of weight, energy consumption, or favorable behaviors (e.g., physical activity), suggesting that dietary quality may have an independent impact on regional adipose tissue stores. Collectively, these data demonstrate a link between adiposity in specific anatomic locations and dietary patterns in a large population of multi-ethnic Americans. These findings provide the basis for future prospective studies that investigate the effect of diet on metabolic and molecular consequences of regional adiposity.
Supplementary Material
Supplementary Figure 1. Least-squares means (with 95% confidence limits) for primary outcome measures (hepatic attenuation and pericardial fat) by quartiles of DietQuality score, stratified by sex. Interaction P values were not significant. Models adjusted as in Figure 2.
Highlights.
Dietary quality is a major focus of cardiometabolic disease prevention worldwide
A dietary score consisting of constituents of a Mediterranean-style diet was associated with decreased adiposity by computed tomography
This association was independent of age, sex, or race
Specific food groups had beneficial (and deleterious) association with regional adiposity
Dietary composition may be critical to determining adipose tissue distributions relevant to cardiometabolic risk
Acknowledgments
We acknowledge the participants in the MESA study and local site coordinators for their tireless efforts in the prevention of cardiometabolic disease. This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. In addition, adiposity assessments were supported by grants R01-HL085323 (to JD), R01-HL088451 (to MA), and R01-HL071739 (to MB). Dr. Shah is supported by an American Heart Association Fellow-to-Faculty Award.
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding: This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-RR-025005 from NCRR. Adiposity assessments were supported by grants R01-HL085323 (to JD), R01-HL088451 (to MA), and R01-HL071739 (to MB). Dr. Shah is supported by an American Heart Association Fellow-to-Faculty Award.
Footnotes
Conflict of Interest: No authors have any relevant disclosures.
Disclosures: There are no disclosures relevant to the content of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shikany JM, Safford MM, Newby PK, Durant RW, Brown TM, Judd SE. Southern Dietary Pattern is Associated with Hazard of Acute Coronary Heart Disease in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.014421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C and Stroke Statistics S. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 3.Chao TF, Hung CL, Tsao HM, Lin YJ, Yun CH, Lai YH, Chang SL, Lo LW, Hu YF, Tuan TC, Chang HY, Kuo JY, Yeh HI, Wu TJ, Hsieh MH, Yu WC, Chen SA. Epicardial adipose tissue thickness and ablation outcome of atrial fibrillation. PLoS One. 2013;8:e74926. doi: 10.1371/journal.pone.0074926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbasi SA, Hundley WG, Bluemke DA, Jerosch-Herold M, Blankstein R, Petersen SE, Rider OJ, Lima JA, Allison MA, Murthy VL, Shah RV. Visceral adiposity and left ventricular remodeling: The Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 2015;25:667–76. doi: 10.1016/j.numecd.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–73. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, Vega GL, Khera A, McGuire DK, Grundy SM, de Lemos JA. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring) 2013;21:E439–47. doi: 10.1002/oby.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. Jama. 2012;308:1150–9. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, Jerosch-Herold M, Lima JA, Ding J, Allison MA. Visceral Adiposity and the Risk of Metabolic Syndrome Across Body Mass Index: The MESA Study. JACC Cardiovasc Imaging. 2014;7:1221–35. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romaguera D, Norat T, Mouw T, May AM, Bamia C, Slimani N, Travier N, Besson H, Luan J, Wareham N, Rinaldi S, Couto E, Clavel-Chapelon F, Boutron-Ruault MC, Cottet V, Palli D, Agnoli C, Panico S, Tumino R, Vineis P, Agudo A, Rodriguez L, Sanchez MJ, Amiano P, Barricarte A, Huerta JM, Key TJ, Spencer EA, Bueno-de-Mesquita HB, Buchner FL, Orfanos P, Naska A, Trichopoulou A, Rohrmann S, Kaaks R, Bergmann M, Boeing H, Johansson I, Hellstrom V, Manjer J, Wirfalt E, Uhre Jacobsen M, Overvad K, Tjonneland A, Halkjaer J, Lund E, Braaten T, Engeset D, Odysseos A, Riboli E, Peeters PH. Adherence to the Mediterranean diet is associated with lower abdominal adiposity in European men and women. The Journal of nutrition. 2009;139:1728–37. doi: 10.3945/jn.109.108902. [DOI] [PubMed] [Google Scholar]
- 10.Asghari G, Mirmiran P, Rashidkhani B, Asghari-Jafarabadi M, Mehran M, Azizi F. The association between diet quality indices and obesity: Tehran Lipid and Glucose Study. Archives of Iranian medicine. 2012;15:599–605. [PubMed] [Google Scholar]
- 11.Funtikova AN, Benitez-Arciniega AA, Gomez SF, Fito M, Elosua R, Schroder H. Mediterranean diet impact on changes in abdominal fat and 10-year incidence of abdominal obesity in a Spanish population. The British journal of nutrition. 2014;111:1481–7. doi: 10.1017/S0007114513003966. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Gonzalez MA, Salas-Salvado J, Estruch R, Corella D, Fito M, Ros E, Predimed I. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Progress in cardiovascular diseases. 2015;58:50–60. doi: 10.1016/j.pcad.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Lasa A, Miranda J, Bullo M, Casas R, Salas-Salvado J, Larretxi I, Estruch R, Ruiz-Gutierrez V, Portillo MP. Comparative effect of two Mediterranean diets versus a low-fat diet on glycaemic control in individuals with type 2 diabetes. European journal of clinical nutrition. 2014;68:767–72. doi: 10.1038/ejcn.2014.1. [DOI] [PubMed] [Google Scholar]
- 14.Casas R, Sacanella E, Urpi-Sarda M, Chiva-Blanch G, Ros E, Martinez-Gonzalez MA, Covas MI, Rosa Ma LR, Salas-Salvado J, Fiol M, Aros F, Estruch R. The effects of the mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PloS one. 2014;9:e100084. doi: 10.1371/journal.pone.0100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamora-Ros R, Serafini M, Estruch R, Lamuela-Raventos RM, Martinez-Gonzalez MA, Salas-Salvado J, Fiol M, Lapetra J, Aros F, Covas MI, Andres-Lacueva C, Investigators PS. Mediterranean diet and non enzymatic antioxidant capacity in the PREDIMED study: evidence for a mechanism of antioxidant tuning. Nutr Metab Cardiovasc Dis. 2013;23:1167–74. doi: 10.1016/j.numecd.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Molenaar EA, Massaro JM, Jacques PF, Pou KM, Ellison RC, Hoffmann U, Pencina K, Shadwick SD, Vasan RS, O'Donnell CJ, Fox CS. Association of lifestyle factors with abdominal subcutaneous and visceral adiposity: the Framingham Heart Study. Diabetes Care. 2009;32:505–10. doi: 10.2337/dc08-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma J, Sloan M, Fox CS, Hoffmann U, Smith CE, Saltzman E, Rogers GT, Jacques PF, McKeown NM. Sugar-sweetened beverage consumption is associated with abdominal fat partitioning in healthy adults. The Journal of nutrition. 2014;144:1283–90. doi: 10.3945/jn.113.188599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odegaard AO, Choh AC, Czerwinski SA, Towne B, Demerath EW. Sugar-sweetened and diet beverages in relation to visceral adipose tissue. Obesity (Silver Spring) 2012;20:689–91. doi: 10.1038/oby.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abiemo EE, Alonso A, Nettleton JA, Steffen LM, Bertoni AG, Jain A, Lutsey PL. Relationships of the Mediterranean dietary pattern with insulin resistance and diabetes incidence in the Multi-Ethnic Study of Atherosclerosis (MESA) The British journal of nutrition. 2013;109:1490–7. doi: 10.1017/S0007114512003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tremblay A, Doyon C, Sanchez M. Impact of yogurt on appetite control, energy balance, and body composition. Nutrition reviews. 2015;73(Suppl 1):23–7. doi: 10.1093/nutrit/nuv015. [DOI] [PubMed] [Google Scholar]
- 22.Tremblay A, Gilbert JA. Milk products, insulin resistance syndrome and type 2 diabetes. Journal of the American College of Nutrition. 2009;28(Suppl 1):91S–102S. doi: 10.1080/07315724.2009.10719809. [DOI] [PubMed] [Google Scholar]
- 23.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multiethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 24.Ong KL, Ding J, McClelland RL, Cheung BM, Criqui MH, Barter PJ, Rye KA, Allison MA. Relationship of pericardial fat with lipoprotein distribution: The Multi-Ethnic study of atherosclerosis. Atherosclerosis. 2015;241:664–70. doi: 10.1016/j.atherosclerosis.2015.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah RV, Allison MA, Lima JA, Abbasi SA, Mongraw-Chaffin M, Jerosch-Herold M, Ding J, Budoff MJ, Murthy VL. Liver steatosis and the risk of albuminuria: the multi-ethnic study of atherosclerosis. Journal of nephrology. 2015 doi: 10.1007/s40620-015-0177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Annals of epidemiology. 1999;9:314–24. doi: 10.1016/s1047-2797(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 27.Corella D, Sorli JV, Estruch R, Coltell O, Ortega-Azorin C, Portoles O, Martinez-Gonzalez MA, Bullo M, Fito M, Aros F, Lapetra J, Asensio EM, Saez GT, Serra-Majem L, Munoz-Bravo C, Ruiz-Gutierrez V, Fiol M, Vinyoles E, Pinto X, Richardson K, Ros E, Ordovas JM. MicroRNA-410 regulated lipoprotein lipase variant rs13702 is associated with stroke incidence and modulated by diet in the randomized controlled PREDIMED trial. Am J Clin Nutr. 2014;100:719–31. doi: 10.3945/ajcn.113.076992. [DOI] [PubMed] [Google Scholar]
- 28.Corella D, Ordovas JM. How does the Mediterranean diet promote cardiovascular health? Current progress toward molecular mechanisms: gene-diet interactions at the genomic, transcriptomic, and epigenomic levels provide novel insights into new mechanisms. BioEssays : news and reviews in molecular, cellular and developmental biology. 2014;36:526–37. doi: 10.1002/bies.201300180. [DOI] [PubMed] [Google Scholar]
- 29.Del Chierico F, Vernocchi P, Dallapiccola B, Putignani L. Mediterranean diet and health: food effects on gut microbiota and disease control. International journal of molecular sciences. 2014;15:11678–99. doi: 10.3390/ijms150711678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore JB, Gunn PJ, Fielding BA. The role of dietary sugars and de novo lipogenesis in non-alcoholic fatty liver disease. Nutrients. 2014;6:5679–703. doi: 10.3390/nu6125679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coenen KR, Gruen ML, Chait A, Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56:564–73. doi: 10.2337/db06-1375. [DOI] [PubMed] [Google Scholar]
- 32.Awada M, Meynier A, Soulage CO, Hadji L, Geloen A, Viau M, Ribourg L, Benoit B, Debard C, Guichardant M, Lagarde M, Genot C, Michalski MC. n-3 PUFA added to high-fat diets affect differently adiposity and inflammation when carried by phospholipids or triacylglycerols in mice. Nutrition & metabolism. 2013;10:23. doi: 10.1186/1743-7075-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulange A, Vidal H, Slama G, Clement K, Guerre-Millo M, Rizkalla SW. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86:1670–9. doi: 10.1093/ajcn/86.5.1670. [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Fox CS, Jacques PF, Speliotes EK, Hoffmann U, Smith CE, Saltzman E, McKeown NM. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. Journal of hepatology. 2015;63:462–9. doi: 10.1016/j.jhep.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paniagua JA, Gallego de la Sacristana A, Romero I, Vidal-Puig A, Latre JM, Sanchez E, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care. 2007;30:1717–23. doi: 10.2337/dc06-2220. [DOI] [PubMed] [Google Scholar]
- 36.Babio N, Toledo E, Estruch R, Ros E, Martinez-Gonzalez MA, Castaner O, Bullo M, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Sorli JV, Salas-Salvado J, Investigators PS. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2014;186:E649–57. doi: 10.1503/cmaj.140764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Gonzalez MA, Sanchez-Villegas A. The emerging role of Mediterranean diets in cardiovascular epidemiology: monounsaturated fats, olive oil, red wine or the whole pattern? European journal of epidemiology. 2004;19:9–13. doi: 10.1023/b:ejep.0000013351.60227.7b. [DOI] [PubMed] [Google Scholar]
- 38.Panagiotakos DB, Pitsavos C, Stefanadis C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis. 2006;16:559–68. doi: 10.1016/j.numecd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Gonzalez MA, Fernandez-Jarne E, Serrano-Martinez M, Wright M, Gomez-Gracia E. Development of a short dietary intake questionnaire for the quantitative estimation of adherence to a cardioprotective Mediterranean diet. European journal of clinical nutrition. 2004;58:1550–2. doi: 10.1038/sj.ejcn.1602004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Least-squares means (with 95% confidence limits) for primary outcome measures (hepatic attenuation and pericardial fat) by quartiles of DietQuality score, stratified by sex. Interaction P values were not significant. Models adjusted as in Figure 2.