Summary
Porphyromonas gingivalis is a Gram-negative anaerobe and keystone periodontal pathogen. A mariner transposon insertion mutant library has recently been used to define 463 genes as putatively essential for the in vitro growth of P. gingivalis ATCC 33277 in planktonic culture [Library 1, (Klein et al 2012)]. We have independently generated a transposon insertion mutant library [Library 2] for the same P. gingivalis strain and herein compare genes that are putatively essential for in vitro growth in complex media, as defined by both libraries. 281 genes (61%) identified by Library 1 were common to Library 2. Many of these common genes are involved in fundamentally important metabolic pathways, notably pyrimidine cycling as well as lipopolysaccharide, peptidoglycan, pantothenate and coenzyme A biosynthesis and nicotinate and nicotinamide metabolism. Also in common are genes encoding heat shock protein homologues, sigma factors, enzymes with proteolytic activity and the majority of sec-related protein export genes. In addition to facilitating a better understanding of critical physiological processes, transposon sequencing technology has the potential to identify novel strategies for the control of P. gingivalis infections. Those genes defined as essential by two independently generated TnSeq mutant libraries are likely to represent particularly attractive therapeutic targets.
Keywords: Cigarette smoking, essential genes, KEGG, periodontal diseases, Porphyromonas gingivalis, TnSeq
Introduction
Periodontitis, a microbial-driven, destructive disease of the tissues surrounding the teeth occurs in approximately 50% of the population (Eke et al 2012). Increasing evidence suggests that periodontitis is associated with elevated risk of vascular diseases, including coronary artery disease and stroke, diabetes mellitus, lung diseases such as COPD and pneumonia, rheumatoid arthritis, preterm low birth weight delivery and even some forms of cancer (Chun et al 2005; Kumar 2013; Maddi and Scannapieco 2013; Teng et al 2002; Whitmore and Lamont 2014).
The anaerobic, Gram-negative bacterium, Porphyromonas gingivalis, is an emerging systemic pathogen and a causative agent of chronic periodontitis [70, 71]. P. gingivalis has been proposed as the prototypic oral keystone pathogen (Hajishengallis et al 2012), that is, a bacterium that promotes dysbiosis and inflammation-driven tissue destruction even at low levels of infection. P. gingivalis ATCC 33277 is one of the most commonly employed strains in periodontal research and is pathogenic in experimental animals following oral infection (Wang et al 2009). The P. gingivalis ATCC 33277 genome is comprised of 2155 genes, 2090 of which encode proteins, and there are no plasmids (Chen et al 2004; Klein et al 2012; Naito et al 2008). Recently, Klein et al (Klein et al 2012) generated a Mariner transposon insertion mutant (TnSeq) library (which we denote Library 1) for this P. gingivalis strain and utilized it to identify 463 genes potentially required for in vitro viability. We have independently generated a TnSeq mutant library for P. gingivalis ATCC 33277 (Library 2) building on our prior work on Vibrio and Pseudomonas gene fitness (Dong et al 2013; Skurnik et al 2013a; Skurnik et al 2013b).
There is no fully defined medium for P. gingivalis and different complex media are likely to provide different nutritional environments. Therefore, we hypothesized that multiple genes essential for planktonic growth in complex media would be common (i.e., potentially representing the core genome) and divergent (i.e., environment specific) between Library 1 and Library 2.
Materials and Methods
Materials
Porphyromonas gingivalis ATCC 33277 was cultured in Gifu anaerobe medium (GAM Nissui Pharmaceutical, Tokyo, Japan), and on GAM blood agar. E. coli DH5α and pSAM_Bt were cultured in LB broth and agar from BD Biosciences (San Jose, CA). Sheep blood was from Lampire Biological Laboratories (Pipersville, PA). Gentamicin, erythromycin, ampicillin, dichloromethane, sodium sulfate and analytical nicotine standards were from Sigma-Aldrich (St. Louis, MO). GeneReleaser® came from Bio Ventures, Inc. (Murfreesboro, TN). All primers were from Biosynthesis Inc. (Lewiston, TX). Lonza flash gels came from Lonza (Rockland, ME). Wizard SV and PCR cleanup kit were from Promega (Madison, WI). T4 DNA Ligase and buffer; MmeI; and TAE buffer came from New England Biolabs (Ipswich, MA), while HiFi Hotstart Readymix was from KAPA Biosystems (Wilmington, MA) and PCR SuperMix came from Invitrogen (Carlsbad, CA).
Generation and validation of a P. gingivalis ATCC 33277 transposon sequencing library
A 33277 Mariner transposon insertion library was generated. P. gingivalis was inoculated into GAM without antibiotics; E. coli strain DH5α containing the pSAM_Bt plasmid was grown in LB broth with ampicillin; and a 5:1 ratio of log phase P. gingivalis to E. coli was employed for conjugation. pSAM_Bt was originally employed to generate the first TnSeq library in the porphyromonad-related microbe, Bacteroides thetaiotaomicron (Goodman et al 2009). Gentamicin (E. coli is sensitive; P. gingivalis is naturally resistant) and erythromycin (resistance is transposon-encoded) were used to select for insertion mutants grown on GAM/blood agar plates. Random insertion in the P. gingivalis genome was confirmed by nested semi-random PCR. One hundred randomly selected colonies were harvested to test for hot spots of insertion of the transposon by semi-nested PCR involving two rounds of PCR. DNA was harvested from selected colonies by GeneReleaser® following the manufacturer’s protocol. Round one PCR was performed with Invitrogen Supermix using Round1-pSAM and Round 1-RndomPG primers (Supplemental Table 1) under the following conditions: 94°C for 3 min; 94°C for 30 sec, 50°C for 40 sec, 72°C for 3 min, 10× cycles; 94°C 30 sec, 62°C for 40 sec, 72°C for 3 min, 25× cycles. Round one PCR products were used for round two PCR amplification using Round2-PG and Round2-pSAM primers (Supplemental Table 1) under the following conditions: 94°C for 3 min; 94°C for 30 sec, 57°C for 40 sec, 72°C for 3 min, ×30; 72°C for 5 min. Amplicons were run on a Lonza flashgel system (Allendale, NJ) to verify correct fragment size. 16S rRNA gene specific primers (Supplemental Table 1) were also used to confirm P. gingivalis specificity with the same cycle conditions as round two of the nested PCRs employed.
Identification of essential genes for planktonic growth in GAM
The mutant library was passaged twice in 1 L of GAM broth containing gentamicin (50 µg/ml) and erythromycin (5 µg/ml). Genomic DNA from the mutant library (100 µg) was harvested using the Wizard DNA isolation kit and digested with MmeI (1 h, 37°C; 20 min, 80°C). The DNA digest was run on a 1% agarose gel and the band corresponding to the transposon plus the flanking sequences (~1500) was extracted using a Wizard gel extraction kit, as per the manufacturer’s instructions. A double stranded DNA (dsDNA) adapter was created using the primers LIB_AdaptT_4 and LIB_AdaptB_4 (Supplemental Table 1). The primers were combined and heated at 95°C for 5 min and then allowed to cool to RT. The dsDNA adapter was ligated to the gel purified DNA product using T4 DNA ligase (16 h, 16°C; 10 min, 65°C). Ligation products purified by a Wizard purification kit and amplified by PCR using HiFi Hotstart SuperMix with LIB_PCR_5 and LIB_PCR_3 primers (Supplemental Table 1) under the following conditions: 98°C for 3 min; 98°C for 20 sec; 60°C for 1 min; 72°C for 1 min, 18× cycles; 72°C for 4 min. The PCR product was purified using Wizard purification kit and run on a Lonza flash gel system to verify the 125 bp band. Products were quantified using a NanoDrop ND-1000 spectrophotometer (Wilmington, DE) and sequenced at the University of Michigan DNA Core. The sequences were read by 50 bp single end reads. The prepared mutant library DNA was run on a single flow cell lane on the Illumina HiSeq2000 platform. The resulting reads were analyzed by CLC Genomics Workbench 7.1.1. Adapter sequences were removed along with the sequence corresponding to the end of the transposon. All sequences with reads with a quality score < 0.05 or of < 15 nucleotides were removed. The remaining reads were aligned to the annotated gene list of P. gingivalis strain 33277. To control for variations in gene length and depth of coverage, genes with <0.5 reads per kilobase per million mapped reads (RPKM) were considered essential. The remaining genes were determined to be non-essential for survival in GAM broth.
Cross library comparison of essential genes for planktonic growth in complex media
The genes essential for survival in GAM broth were compared to the previously published list of essential genes for survival in supplemented brain-heart infusion broth (Klein et al 2012). The function and interrelationships of the 281 common inherently essential genes for planktonic growth of P. gingivalis in complex media were investigated in silico by KEGG (www.genome.jp/kegg) database analyses. The common essential genes were mapped to the P. gingivalis ATCC 33277 genome using CGViewer (stothard.afns.ualberta.ca/cgview_server/) (Grant and Stothard 2008) and compared to the Database of Essential Genes (DEG; tubic.tju.edu.cn/deg/, blastx search, <1e−8) (Luo et al 2014).
Results
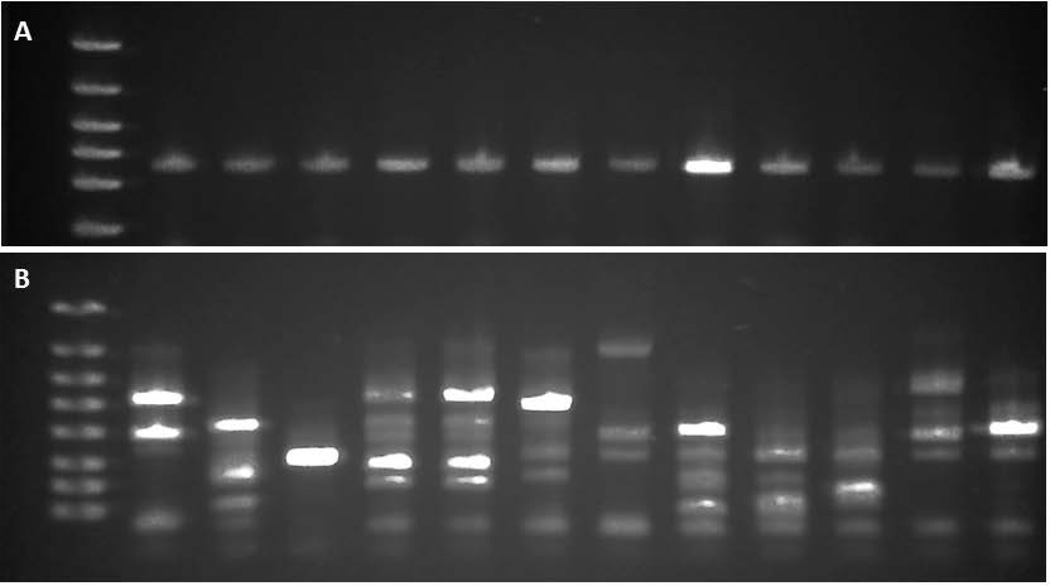
Random insertion in the P. gingivalis 33277 genome, which contains ~117,000 TA sites distributed among 2155 genes, was confirmed by nested semi-random PCR, as shown in Figure 1.
Figure 1. Validation of Tn-Seq Library.
(A) 16s RNA confirmation of P. gingivalis; (B) Random insertion determined by nested PCR (lanes from 100 selected colonies). Approximately 80,000 insertion strains (individual mutants) were generated in the 33277 chromosome.
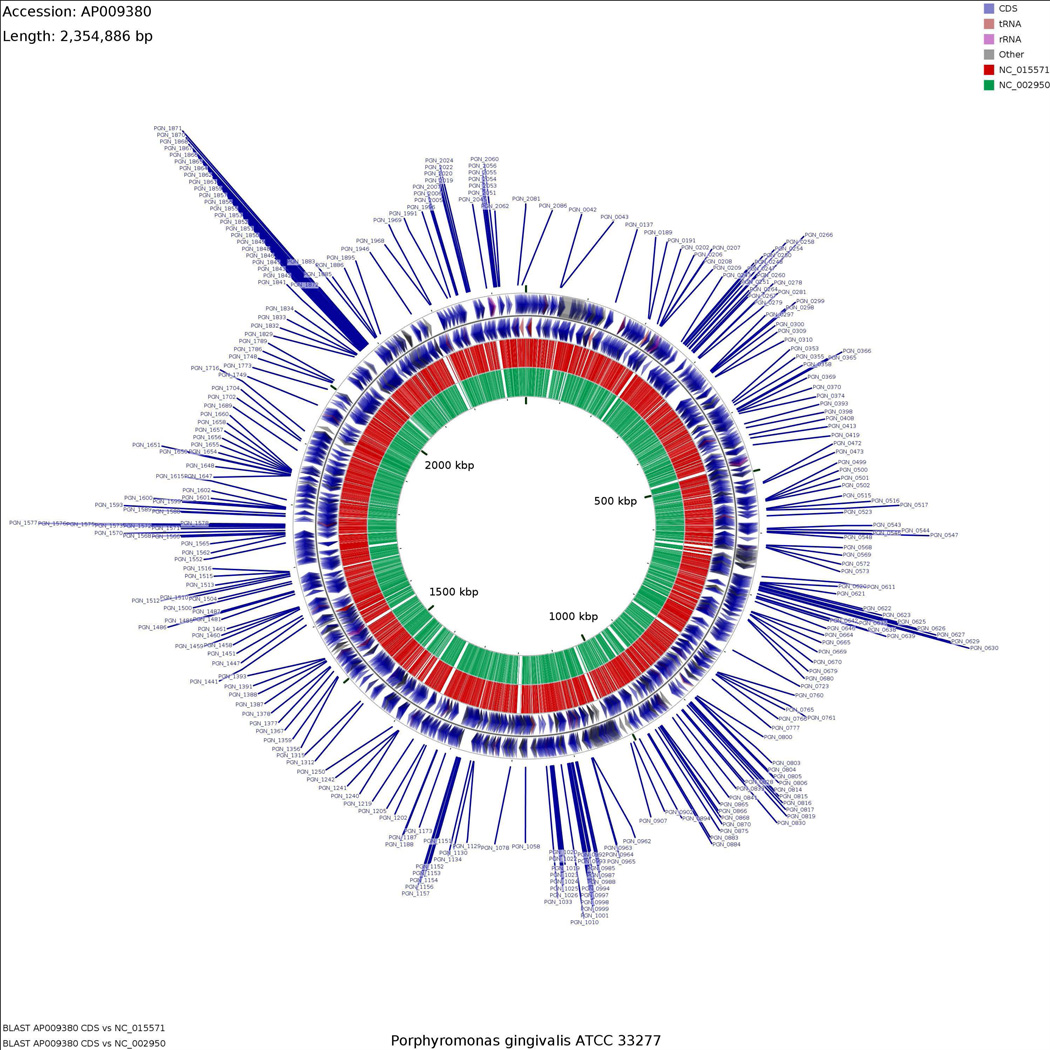
Inherently essential genes common to Library 1 and Library 2 were mapped to the P. gingivalis ATCC 33277 genome using CGViewer, as presented in Figure 2.
Figure 2. Map of putative common inherently essential genes for planktonic growth of P. gingivalis in complex media.
Common essential genes were mapped to the P. gingivalis ATCC 33277 genome using CGviewer (Grant and Stothard 2008). The (blue arrows show the location of the 281 putative common essential genes within the P. gingivalis 33277 chromosome. The adjacent two rings depict gene orientation (outer and inner blue arrows represent the positive and negative strands, respectively). The next two rings represent the coding sequences for P. gingivalis TDC 60 and W83 strains, respectively.
KEGG analysis (www.genome.jp/kegg) was employed in order to functionally classify the same P. gingivalis gene set, as noted in Table 1. The highest percentage of essential genes found within the functional groupings was for those whose products are involved in protein export, 90% being inherently essential. Similar functional groupings were obtained using the DAVID database (david.niaid.nih.gov; data not shown).
Table 1.
Functional classification of inherently essential genes of Porphyromonas gingivalis common to two transposon sequencing libraries.
| Cell Envelope | 32/108 (29.6%) |
| Fatty Acid and Phospholipid | 3/15 (20.0%) |
| Protein Fate | 17/77 (22.1%) |
| Protein Synthesis | 60/110 (54.5%) |
| Purines, pyrimidines | 13/45 (28.9%) |
| Regulatory functions | 4/32 (12.5%) |
| Signal transduction | 1/9 (11.1%) |
| Transcription | 10/34 (29.4%) |
| Cell wall biogenesis | 8/15 (53.3%) |
| Lipopolysaccharide biosynthesis | 8/18 (44.4%) |
| Protein export | 8/9 (89%)* |
| Riboflavin | 2/5 (40.0%) |
| Ribosomal | 32/54 (59.3%) |
| tRNA synthetase | 15/24 (62.5%) |
The essential protein export genes, as determined by KEGG analysis, were secY (PGN_1848, PG1918, PGTDC60_0188); secE (PGN_1577, PGTDC60_1503); secG (PGN_0258, PGTDC60_0422); secD/F (PGN_1702, PG1762, PGTDC60_1374); yajC (PGN_1485, PG0485, PGTDC60_1601); SecA (PGN_1458, PG0514, PGTDC60_1633); SRP54/ffh (PGN_1205, PG1115, PGTDC60_1100); ftsY (PGN_0264, PG0151, PGTDC60_0428). yidC (PGN_1446, PG0526, PGTDC60_1645) was found to be non-essential. The orthologues in P. gingivalis W83 and P. gingivalis TDC60, respectively, are also presented.
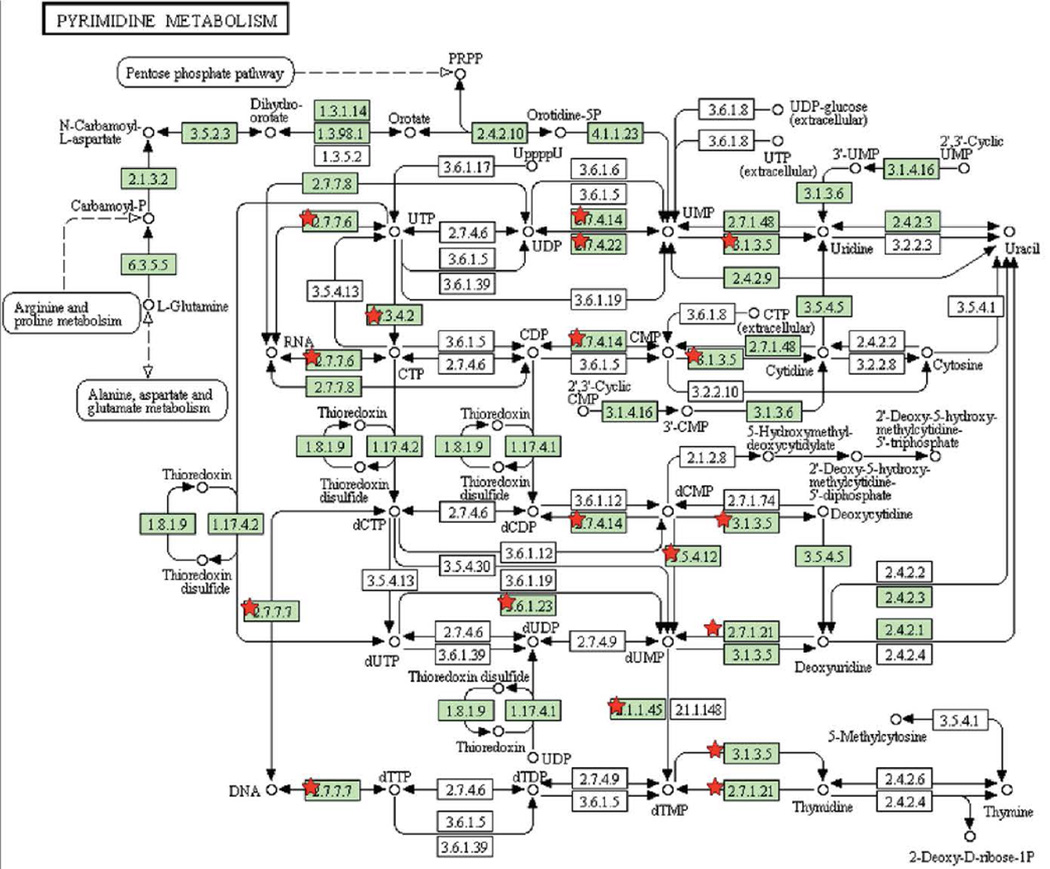
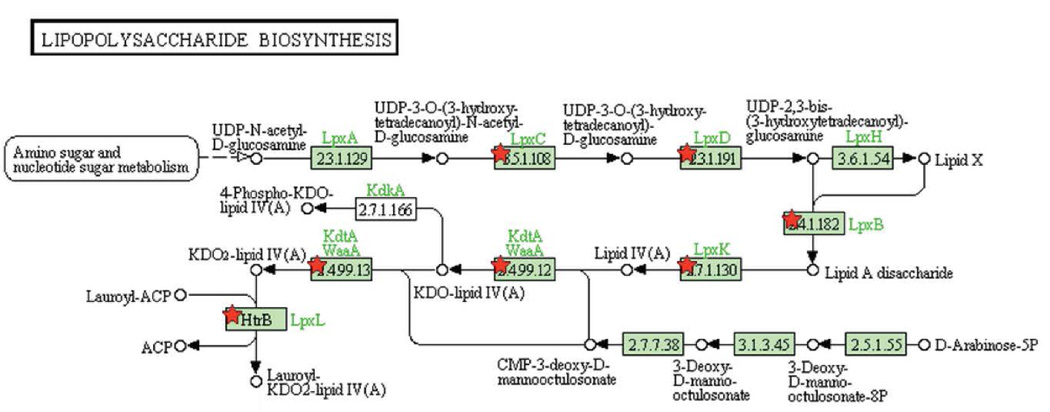
Functional relationships between the common essential genes were determined, using KEGG, as shown in Figure 3A–E.
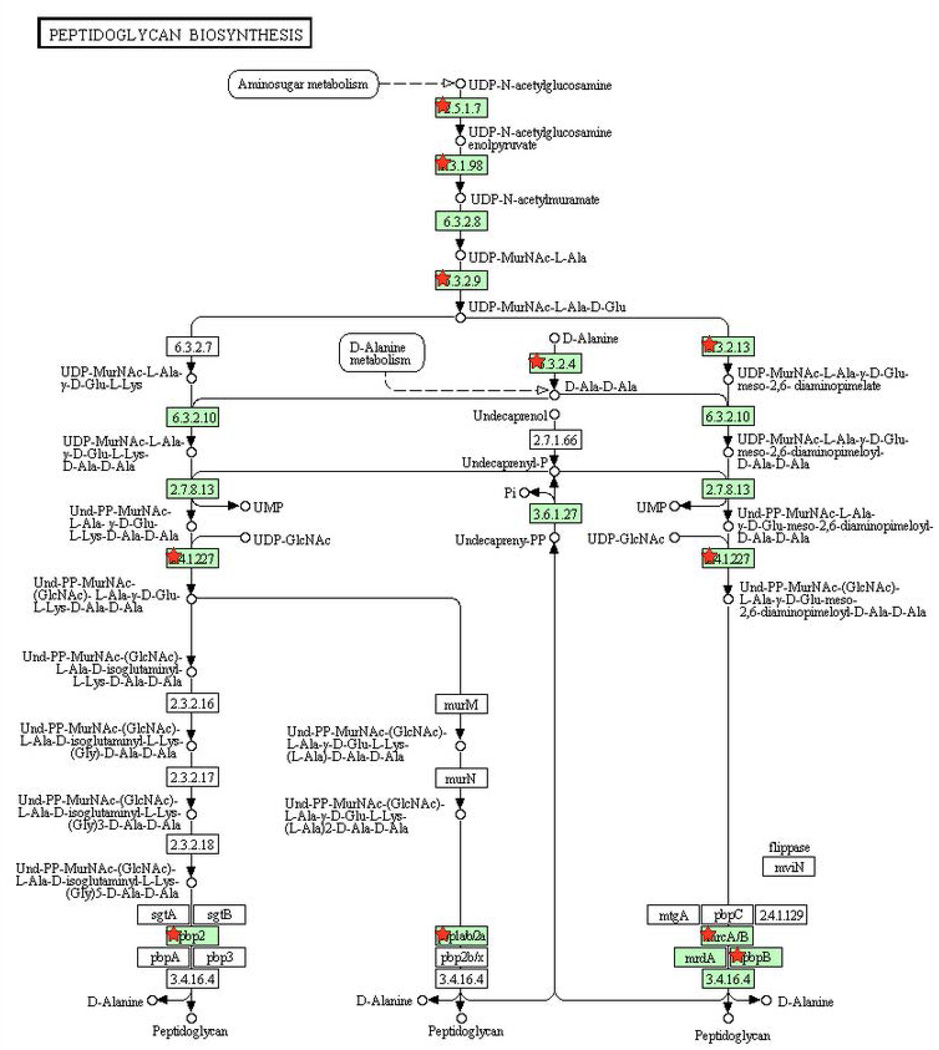
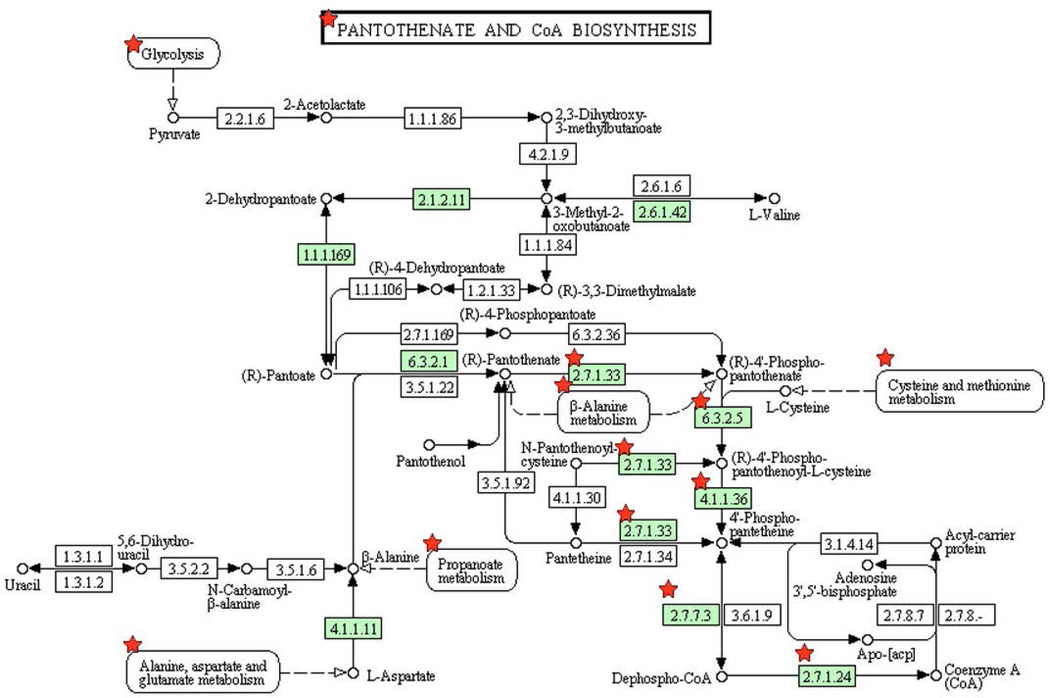
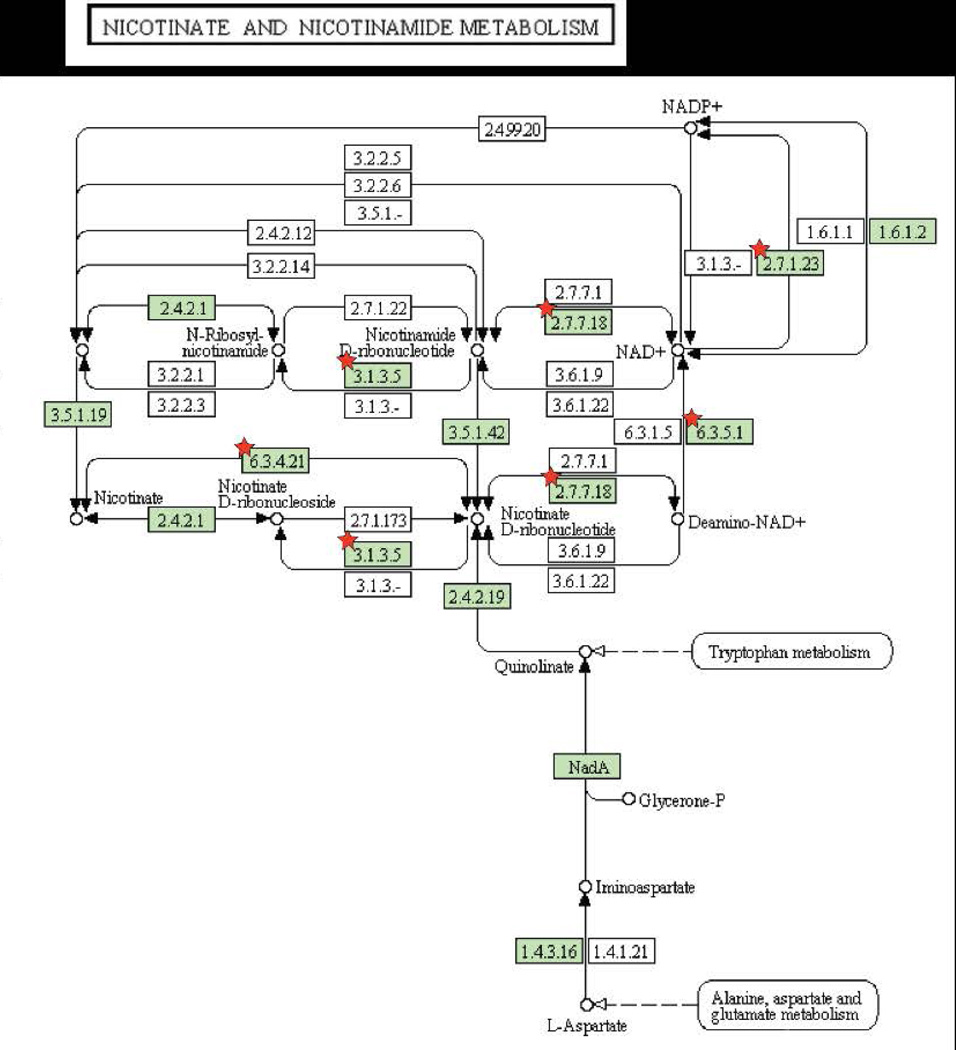
Figure 3. Pathways enriched among inherently essential P. gingivalis genes (A) pyrimidine metabolism, (B) lipopolysaccharide biosynthesis, (C) peptidoglycan biosynthesis, (D) pantothenate and CoA biosynthesis and (E) Nicotinate and nicotinamide metabolism.
KEGG pathways (www.genome.jp/kegg) are presented with the proteins catalyzing each step shown by their enzyme commission number and substrates/products. Green shading indicates the presence of homologs in P. gingivalis. Essential genes are denoted with a red star. The PGN assignments of the essential metabolic genes are provided in Supplemental Table 4.
Finally, the complete data base of 281 common genes is provided in Supplemental Table 2. These have been entered into the Database of Essential Genes (Luo et al 2014).
Discussion
A comparison of two independently generated TnSeq libraries for P. gingivalis ATCC 33277 suggests that 281 common genes are essential for in vitro growth in complex media. This minimal P. gingivalis genome is rich in genes performing critical physiological processes, particularly pyrimidine metabolism as well as lipopolysaccharide, peptidoglycan and CoA biosynthesis. Those genes that encode established virulence factors, such as gingipains (Guo et al 2010), fimbriae (Daep et al 2011), capsule (Singh et al 2011), and RagB (Hutcherson et al 2014), are under-represented in this overlapping gene set, likely because as there is no immune or competing microbial challenge to be met. In other words, they are likely to be conditionally essential rather than inherently essential. Hence it could be reasoned that, while targeting P. gingivalis components that drive pathogenesis in vivo is a valid approach, the rather more mundane set of essential genes identified herein may be prove to be more efficacious in controlling P. gingivalis infections. It should be noted that the ‘essential’ gene set can change depending on the growth conditions. Some genes will likely be required in all conditions (e.g., rpoN and lepB, identified herein) but the importance of others will depend on the nutritional content of the medium, i.e., they will be conditionally essential genes. In the long-term, then, a comparison of inherently essential genes and genes essential in multiple conditions will facilitate optimization of potential therapeutic targets.
In addition to the elucidation of genes grouped into metabolic pathways, there are other essential genes that are interesting at the individual level. The rpoN gene (PGN_1202, 33277; PG1105, W83 orthologue; PGTDC60_1103, TDC60 orthologue), for example, encodes the alternative sigma factor, σ54. Bacteroides spp are rich in extracytoplasmic sigma factors compared to other Bacteroidetes (Karlsson et al 2011). These sigma factors form part of the RNA polymerase holoenzyme and control transcriptional specificity. σ54 is commonly used for large scale transcriptional changes by binding to different and more highly conserved consensus sequences than those for σ70 (Bush and Dixon 2012). Furthermore, while there are multiple members of the σ70 family - and the σ70 factor rpoD (PGN_0638; PG0594; PGTDC60_1719) is also essential - there is only one member of σ54. Interestingly, rpoN is not typically an essential gene in most systems. The only described instance where rpoN has been shown to be essential is in the soil bacterium, Myxococcus xanthus (Keseler and Kaiser 1997). Why the P. gingivalis rpoN gene is essential growth remains to be determined.
The HSP60 homologue, groEL (PGN_1452; PG0520; PGTDC_1639), is an emergent P. gingivalis virulence factor that has been proposed to promote cytokine production and inflammation, to enhance osteoclastogenesis, to aid in the vascularization of tumors and, perhaps, to play a role in atherosclerosis (Argueta et al 2006; Hagiwara et al 2014; Lin et al 2014a; Lin et al 2014b). While PGN_1452 is not itself essential, the groEL co-chaperone, groES, (PGN_1451; PG0520; PGTDC60_1640) is required for growth in complex media. dnaJ (PGN_1716; PG1776; PGTDC60_1360), another heat shock protein family member, and the gene encoding the DnaK repressor (PGN_0516; PG1597; PGTDC_0704) are further common essential genes.
There are several proteolytic genes common to Library 1 and Library 2. These are genes encoding: signal peptidase I (lepB; PGN_1946; PG1598; PGTDC60_0703); signal peptidase II (lspA; PGN_0515; PG2001; PGTDC60_0274); as well as an aminoacyl-histidine dipeptidase (PGN_0250; PG0137; PGTDC60_0414); and a leucine aminopeptidase (PGN_0202; PG2157; PGTDC60_1640) that is related to M28 group of metalloproteinases. P. gingivalis contains only a single copy each of lepB and lspA, which are required for protein translocation across cell membranes, consistent with the finding that these signal peptidases form part of the core genome. Additionally, the majority of sec-related protein export genes were also essential (secY, secE, secG, secD/F, yajC, SecA, SRP54/ffh and ftsY but not yidC).
Multiple genes that control bacterial shape, including those encoding RodA (PGN_0626; PG703; PGTDC60_0579 and PGN_0870; PG1392; PGTDC60_2072) as well as MreB (PGN_0866; PG1396; PGTDC60_2068), are also common inherently essential genes. Interestingly, mreB is not usually an essential gene for most rod-shaped bacteria. Perhaps the essentiality of mreB in P. gingivalis in lab media suggests a need to maintain a rod shape in order to keep the surface area –to–volume ratio lower than that for a coccus.
Upon interrogation of the Database of Essential Genes, it is clear that some P. gingivalis ATCC 33277 genes have common essential homologues while others are unique (n = 54; 19%) to this bacterium. Eighty-three (30%) genes have ≤ 10 essential homologues, while 11 (n = 4%) have ≥ 50 homologues. While it must be acknowledged that the current database of known essential prokaryotic genes is limited to 36 bacterial species other than P. gingivalis - due to the recent emergence of TnSeq technology - the combination of common and unique required genes suggests that, in the long term, it may be possible to generate both broad spectrum and P. gingivalis-specific therapeutic targets for the control of bacterial infections.
Differential phenotypes are sometimes reported for the same bacterial strains. While this can be partly explained, for example, by different model systems or the propagation of relevant mutant genes within laboratory collections, the comparison of Library 1 and Library 2 raises another possibility. Despite the fact that both libraries are P. gingivalis ATCC 33277-specific, approximately half of the genes identified in Library 1 were not common to Library 2. Considering that both libraries lack transposon insertion hot spots and contain large mutant numbers (54000 and 80000 colonies, respectively), the major difference between the experimental setups is the composition of the complex growth media employed (Supplemental Table 3). As different nutritional and other needs may met by each of these complex media, this may be sufficient to induce considerable differences in the adaptive response to such growth conditions, reflected in the different profiles of essential genes. Performing similar analyses on other bacterial pathogens to distinguish between inherently essential versus conditionally essential genes may reveal additional targets for new therapeutics.
In summary, we have identified 281 genes that are essential for in vitro survival of the periodontal pathogen, P. gingivalis, as defined by two independently generated TnSeq mutant libraries. It is expected, following validation of these genes by determining the growth characteristics of essential gene deletion mutants and their complements, that some of these required genes will represent novel therapeutic targets for the efficient control of P. gingivalis.
Supplementary Material
Acknowledgments
The authors acknowledge the support of NIDCR (DE023193 [MW], DE012505 [RJL], DE011111 [RJL], DE014372 [MH] and DE019826 [DAS]).
References
- Argueta JG, Shiota S, Yamaguchi N, Masuhiro Y, Hanazawa S. Induction of Porphyromonas gingivalis GroEL signaling via binding to Toll-like receptors 2 and 4. Oral Microbiol Immunol. 2006;21:245–251. doi: 10.1111/j.1399-302X.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- Bush M, Dixon R. The role of bacterial enhancer binding proteins as specialized activators of sigma54-dependent transcription. Microbiol Mol Biol Rev. 2012;76:497–529. doi: 10.1128/MMBR.00006-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Hosogi Y, Nishikawa K, Abbey K, Fleischmann RD, Walling J, Duncan MJ. Comparative whole-genome analysis of virulent and avirulent strains of Porphyromonas gingivalis. J Bacteriol. 2004;186:5473–5479. doi: 10.1128/JB.186.16.5473-5479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun YH, Chun KR, Olguin D, Wang HL. Biological foundation for periodontitis as a potential risk factor for atherosclerosis. J Periodontal Res. 2005;40:87–95. doi: 10.1111/j.1600-0765.2004.00771.x. [DOI] [PubMed] [Google Scholar]
- Daep CA, Novak EA, Lamont RJ, Demuth DR. Structural dissection and in vivo effectiveness of a peptide inhibitor of Porphyromonas gingivalis adherence to Streptococcus gordonii. Infect Immun. 2011;79:67–74. doi: 10.1128/IAI.00361-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong TG, Ho BT, Yoder-Himes DR, Mekalanos JJ. Identification of T6SS-dependent effector and immunity proteins by Tn-seq in Vibrio cholerae. Proc Natl Acad Sci U S A. 2013;110:2623–2628. doi: 10.1073/pnas.1222783110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Thornton-Evans G, Dye B, Genco R. Advances in Surveillance of Periodontitis: The Centers for Disease Control and Prevention Periodontal Disease Surveillance Project. J Periodontol. 2012;83:1337–1342. doi: 10.1902/jop.2012.110676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AL, McNulty NP, Zhao Y, et al. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe. 2009;6:279–289. doi: 10.1016/j.chom.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JR, Stothard P. The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res. 2008;36:W181–W184. doi: 10.1093/nar/gkn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Nguyen KA, Potempa J. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins. Periodontol. 2010;54:15–44. doi: 10.1111/j.1600-0757.2010.00377.x. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M, Kurita-Ochiai T, Kobayashi R, Hashizume-Takizawa T, Yamazaki K, Yamamoto M. Sublingual vaccine with GroEL attenuates atherosclerosis. J Dent Res. 2014;93:382–387. doi: 10.1177/0022034514523784. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcherson JA, Bagaitkar J, Nagano K, Yoshimura F, Wang H, Scott DA. Porphyromonas gingivalis RagB is a proinflammatory signal transducer and activator of transcription 4 agonist. Mol Oral Microbiol. 2014;30:242–252. doi: 10.1111/omi.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson FH, Ussery DW, Nielsen J, Nookaew I. A closer look at bacteroides: phylogenetic relationship and genomic implications of a life in the human gut. Microb Ecol. 2011;61:473–485. doi: 10.1007/s00248-010-9796-1. [DOI] [PubMed] [Google Scholar]
- Keseler IM, Kaiser D. sigma54, a vital protein for Myxococcus xanthus. Proc Natl Acad Sci U S A. 1997;94:1979–1984. doi: 10.1073/pnas.94.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein BA, Tenorio EL, Lazinski DW, Camilli A, Duncan MJ, Hu LT. Identification of essential genes of the periodontal pathogen Porphyromonas gingivalis. BMC Genomics. 2012;13:578. doi: 10.1186/1471-2164-13-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PS. Oral microbiota and systemic disease. Anaerobe. 2013;24:90–93. doi: 10.1016/j.anaerobe.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Lin FY, Hsiao FP, Huang CY, et al. Porphyromonas gingivalis GroEL induces osteoclastogenesis of periodontal ligament cells and enhances alveolar bone resorption in rats. PLoS One. 2014a;9:e102450. doi: 10.1371/journal.pone.0102450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FY, Huang CY, Lu HY, et al. The GroEL protein of Porphyromonas gingivalis accelerates tumor growth by enhancing endothelial progenitor cell function and neovascularization. Mol Oral Microbiol. 2014b;30:198–216. doi: 10.1111/omi.12083. [DOI] [PubMed] [Google Scholar]
- Luo H, Lin Y, Gao F, Zhang CT, Zhang R. DEG 10, an update of the database of essential genes that includes both protein-coding genes and noncoding genomic elements. Nucleic Acids Res. 2014;42:D574–D580. doi: 10.1093/nar/gkt1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddi A, Scannapieco FA. Oral biofilms, oral and periodontal infections, and systemic disease. Am J Dent. 2013;26:249–254. [PubMed] [Google Scholar]
- Naito M, Hirakawa H, Yamashita A, et al. Determination of the genome sequence of Porphyromonas gingivalis strain ATCC 33277 and genomic comparison with strain W83 revealed extensive genome rearrangements in P. gingivalis. DNA Res. 2008;15:215–225. doi: 10.1093/dnares/dsn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Wyant T, Anaya-Bergman C, et al. The Capsule of Porphyromonas gingivalis leads to a reduction in the host inflammatory response, evasion of phagocytosis, and increase in virulence. Infect Immun. 2011;79:4533–4542. doi: 10.1128/IAI.05016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik D, Roux D, Aschard H, Cattoir V, Yoder-Himes D, Lory S, Pier GB. A comprehensive analysis of in vitro and in vivo genetic fitness of Pseudomonas aeruginosa using high-throughput sequencing of transposon libraries. PLoS Pathog. 2013a;9:e1003582. doi: 10.1371/journal.ppat.1003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik D, Roux D, Cattoir V, et al. Enhanced in vivo fitness of carbapenem-resistant oprD mutants of Pseudomonas aeruginosa revealed through high-throughput sequencing. Proc Natl Acad Sci U S A. 2013b;110:20747–20752. doi: 10.1073/pnas.1221552110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YT, Taylor GW, Scannapieco F, Kinane DF, Curtis M, Beck JD, Kogon S. Periodontal health and systemic disorders. J Can Dent Assoc. 2002;68:188–192. [PubMed] [Google Scholar]
- Wang M, Liang S, Hosur KB, Domon H, Yoshimura F, Amano A, Hajishengallis G. Differential virulence and innate immune interactions of Type I and II fimbrial genotypes of Porphyromonas gingivalis. Oral Microbiol Immunol. 2009;24:478–484. doi: 10.1111/j.1399-302X.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore SE, Lamont RJ. Oral bacteria and cancer. PLoS Pathog. 2014;10:e1003933. doi: 10.1371/journal.ppat.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.