Abstract
OBJECTIVES
To determine whether glyoxal can be converted to oxalate in human erythrocytes. Glyoxal synthesis is elevated in diabetes, cardiovascular disease and other diseases with significant oxidative stress. Erythrocytes are a good model system for such studies as they lack intracellular organelles and have a simplified metabolism.
METHODS
Erythrocytes were isolated from healthy volunteers and incubated with varying concentrations of glyoxal for different amounts of time. Metabolic inhibitors were used to help characterize metabolic steps. The conversion of glyoxal to glycolate and oxalate in the incubation medium was determined by chromatographic techniques.
RESULTS
The bulk of the glyoxal was converted to glycolate but ~1% was converted to oxalate. Inclusion of the pro-oxidant, menadione, in the medium increased oxalate synthesis, and the inclusion of disulfiram, an inhibitor of aldehyde dehydrogenase activity, decreased oxalate synthesis.
CONCLUSIONS
The glyoxalase system, which utilizes glutathione as a cofactor, converts the majority of the glyoxal taken up by erythrocytes to glycolate but a small portion is converted to oxalate. A reduction in intracellular glutathione increases oxalate synthesis and a decrease in aldehyde dehydrogenase activity lowers oxalate synthesis and suggests that glyoxylate is an intermediate. Thus, oxidative stress in tissues could potentially increase oxalate synthesis.
Keywords: urolithiasis, oxalate synthesis, glyoxal, erythrocytes
INTRODUCTION
Endogenous oxalate synthesis contributes approximately half of the oxalate excreted in urine and occurs primarily in the liver 1,2. An increased oxalate synthesis is associated with kidney stones and end-stage renal disease in the rare genetic diseases, Primary Hyperoxaluria types 1–3 3,4. Furthermore, it may be associated with the development of idiopathic calcium oxalate stone disease, but its contribution has not been well defined 1,2. We have shown that sources of oxalate in humans include hydroxyproline, glycine and phenylalanine 5,6. Studies with cultured human hepatocytes have also suggested that the two carbon dialdehyde, glyoxal, may be an important source 7. Glyoxal in part is derived from lipid peroxidation and protein glycation 8. Of note, blood levels of glyoxal and urinary oxalate excretion are both higher in diabetics 9,10. Furthermore, diabetes is a risk factor for kidney stone formation 11 and urinary oxalate excretion is higher in stone formers 12, suggesting that increased glyoxal and oxalate synthesis may be a link between these two diseases.
Glyoxylate is recognized as the major precursor of oxalate in mammalian metabolism 1. We have previously hypothesized that the oxidation of glyoxal to glyoxylate may contribute to endogenous oxalate synthesis.13. Erythrocytes lack intracellular organelles and have a simplified metabolism making them a useful model system to examine the pathways involved in the conversion of glyoxal to oxalate. Through the use of inhibitors and the analysis of metabolites we provide support for our previously proposed pathway for the conversion of glyoxal to oxalate using a human erythrocyte model 13.
MATERIALS AND METHODS
Reagent grade chemicals were obtained from either Sigma-Aldrich Chemicals (St Louis, MO) or Fisher Scientific (Pittsburgh, PA). Glyoxal, methylglyoxal, menadione, and disulfiram were purchased from Sigma-Aldrich. Menadione (90mM) and disulfiram (20mM) stocks were prepared in ethanol and DMSO, respectively.
Whole blood was obtained from normal healthy human adult volunteers (n=3) with IRB approval and the informed consent of participants. After removing plasma and the buffy coat, red blood cells (RBC) were washed twice in ice-cold 0.9% saline and subjected to centrifugation at 1500 g for 5min at 4°C. Incubations were performed in triplicate with cells resuspended at 10% hematocrit in Hyclone Hanks Buffered Salt Solution containing calcium, magnesium and 5.6 mM glucose (catalog # 14025-076 Life Technologies, Carlsbad, CA). Aliquots in TPP® polystyrene, flat bottom, 24 well tissue culture plates were rocked gently (ORBI-Shaker JR, model BT300, 80 rpm) at 37°C. When examining the impact of menadione or disulfiram, appropriate ethanol and DMSO control incubations were also performed.
Glycolate and oxalate were measured in the incubation media following removal of erythrocytes by centrifugation at 1500g for 5min at 4°C. Glycolate was determined using ion chromatography (IC) coupled with mass spectroscopy (Thermo Fisher Scientific Inc.). The IC equipment consisted of a DionexTM ICS-5000 system with an AS15, 2 × 150 mm, anion exchange column using a controlled temperature of 30°C and a Dionex ERS 500 anion electrolytically regenerated suppressor. A gradient of KOH from 3 to 80 mM over 30 min at a flow rate of 0.3 ml min−1 was used to separate sample anions. The mass spectrometer (MSQ-PLUS) was operated in ESI negative mode, needle voltage 1.5V, cone voltage 30V, 450°C, and column eluent was mixed with 50% acetonitrile at 0.30 ml/min using a zero dead volume mixing tee prior to entry into the MSQ. Samples were diluted in the same amount of 13C2- glycolate. Selected-ion monitoring (SIM) at the following mass/charge ratios, 12C2- glycolate (SIM75) and 13C2- glycolate (SIM77), was undertaken. This was used to quantify glycolate. Oxalate was determined using an AS22, 2 × 150 mm, anion exchange column at a controlled temperature of 30°C. A 30mM sodium tetraborate eluent at a flow rate of 0.30 ml min−1 was used to separate oxalate from other anions.
Changes in intracellular glyoxylate and glyoxal were measured over time in erythrocytes incubated with 1 mM glyoxal. Samples were centrifuged at 1500g for 5 minutes at 4°C through 1-bromodecane to achieve rapid separation of erythrocytes from media. Intracellular glyoxylate and glyoxal were measured in erythrocytes following extraction with perchloric acid (PCA). Erythrocytes (1 volume) were mixed with Milli-Q water (1 volume) to lyse the cells, and then 1 volume 1.5 M PCA was added. The PCA extract was obtained following centrifugation of the sample at 20,000g for 10 minutes at 4°C to remove precipitated material.
Glyoxylate in extracts was determined by reversed phase HPLC following derivatization with phenylhydrazine, as previously described 14. A Kinetex 100 * 4.6 mm, 2.6 micron, C18, 100A column (Phenomenex Inc, Torrance, CA) was used at a flow rate of 0.4 ml/min, 20°C, with UV detection at 320nm to separate and measure the phenylhydrazone products. The mobile phase contained 0.1M ammonium acetate, 4% acetonitrile and 4% methanol. Assays were incubated at room temperature in the dark for 15 minutes before injection. Glyoxal and methyglyoxal were determined by reversed phase HPLC following derivatization with o-phenylenediamine, as previously described 15. PCA extracts were incubated at 65°C for 1 hour for determination of glyoxal and 4 hours at room temperature for determination of methylglyoxal. Assays contained the internal standards 2,3-hexanedione and 5-methylquinoxaline. A Kinetex 100 * 4.6mm, 2.6micron, C18, 100A column (Phenomenex Inc) was used at a flow rate of 0.7ml/min, 25°C, with UV detection at 316nm. A linear gradient program of 40mM ammonium acetate/acetic acid buffer, pH 4.5 (Eluent A), and 85% acetonitrile (Eluent B) was utilized to separate the quinoxaline and methylquinoxaline products.
The effect of menadione and disulfiram on metabolism of glyoxal to glycolate and oxalate synthesis was analyzed by the Student’s t-test. Data are expressed as mean ± SD. The criterion for statistical significance was P < 0.05.
RESULTS
Freshly isolated erythrocytes contained low levels of glyoxal, methylglyoxal, oxalate, glyoxylate and glycolate as shown in Table 1. While the glycolate and oxalate levels were similar to those reported previously in plasma (11), the levels of glyoxal, methylglyoxal and glyoxylate were higher than their levels in plasma which were reported to be below 300 nM using LC-MS (20).
Table 1.
Metabolite levels in freshly isolated erythrocytes.
| Metabolite | Concentration (μmol/L hemolysate) |
|---|---|
| Glyoxal | 1.4 ± 0.6 |
| Methylglyoxal | 0.7 ± 0.4 |
| Oxalate | 2.9 ± 1.1 |
| Glyoxylate | 2.3 ± 0.1 |
| Glycolate | 8.1 ± 2.6 |
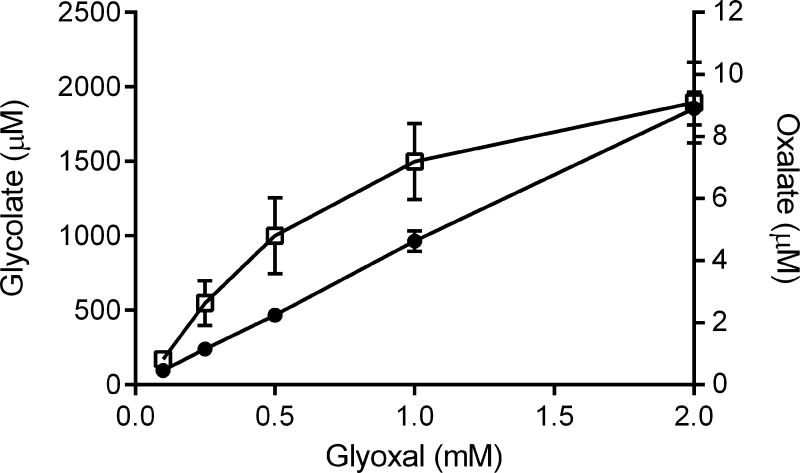
Erythrocytes effectively metabolized exogenous glyoxal to glycolate, as shown in Figures 1 and 2. This conversion was dependent on glyoxal concentration (Fig. 1A) and time of incubation (Fig. 1B). This metabolism is consistent with the activity of the glyoxalase system which consists of 2 enzymes, glyoxalase-1 and glyoxalase-2, using glutathione as a cofactor 16. With 1 mM glyoxal in the incubation medium, approximately 90% of it was converted to glycolate in 3 hours (Fig. 1B), suggesting that glyoxal freely permeated into the cell. Small amounts of the glyoxal, ~1.0%, were converted to oxalate.
Fig. 1.
Relationship of glyoxal concentration (A) and time (B) to the conversion of glyoxal to glycolate and oxalate in erythrocytes. Media concentrations of oxalate (□) and glycolate (●) were determined by IC/MS after incubation with erythrocytes (10% hematocrit).
Fig. 2.
Time dependence of the changes in the intracellular levels of glyoxal and glyoxylate. PCA extracts were obtained at varying time intervals after the incubation of erythrocytes (10% hematocrit) with 1mM glyoxal to measure glyoxal (■) and glyoxylate (△) by HPLC.
As glyoxylate is the major precursor of oxalate, the levels of glyoxylate within cells were also examined. The time dependent increase in intracellular glyoxal and glyoxylate is shown in Fig.2. After 20 minutes incubation with 1mM glyoxal, intracellular glyoxal and glyoxylate concentrations increased ~5 fold and ~2 fold, respectively. The level of glyoxal within cells peaked at 10 minutes and glyoxylate at 20 minutes.
To provide support for the pathway we previously proposed to account for the conversion of glyoxal to oxalate in cells 7, we incubated cells with menadione, a pro-oxidant, which decreases the GSH concentration in erythrocytes 17 and as a result will decrease glyoxalase-1 activity. The results in Fig. 3 show that menadione decreased the amount of glycolate formed by 75% and doubled the amount of oxalate produced. (p values) Disulfiram treatment, which inhibits aldehyde dehydrogenase activity 18 and therefore the oxidation of glyoxal to glyoxylate 19, did not alter glycolate synthesis (P = 0.30), but reduced oxalate synthesis by ~60% (Fig. 3).
Fig. 3.
Impact of menadione and disulfiram on metabolism of glyoxal to glycolate and oxalate in erythrocytes. Erythrocytes (10% hematocrit) were incubated for 1 hour with 1mM glyoxal and inhibitor, and media harvested for metabolite measurements. Media harvested from erythrocytes without inhibitor and with inhibitor are represented by the black bars, (■), and open bars, (□), respectively. * indicates a significant difference (P < 0.05).
COMMENT
Our previous experiments with HepG2 cells, indicated that they were capable of converting the dialdehyde, glyoxal, to the dicarboxylic acid, oxalate 7. The pathway accounting for this synthesis was not elucidated. Erythrocytes, lacking intracellular organelles, have a much simpler metabolism than other cells and are attractive models for analyzing metabolic pathways. They have also been reported to contain glyoxal which increases in the plasma of individuals with diabetes and other disorders associated with metabolic stress 9,10. The results reported here with intact human erythrocytes suggest that glyoxal is metabolized in these cells by the pathways previously postulated 13. These pathways consisted of the glyoxalase system (GLO), which converts the bulk of the glyoxal to glycolate, and a minor pathway involving aldehyde dehydrogenase (ALDH) that converted some glyoxal to glyoxylate which could then be converted to glycolate by glyoxylate reductase (GR) or to oxalate by lactate dehydrogenase (LDH).
The importance of the glyoxalase system in erythrocytes to detoxify glyoxal and methylglyoxal has been recognized for some time. Attention has focused on the known production of methylglyoxal during glycolysis, the major source of energy in the erythrocyte, as a side reaction of the activity of triose phosphate isomerase 20. The formation of glyoxal, which may be derived from lipid peroxidation or glucose autoxidation 21, has attracted less attention. Erythrocytes have been reported to contain lipid peroxides suggesting that the oxidation of unsaturated fatty acids could be a glyoxal source in these cells.
Erythrocytes have been reported to contain aldehyde dehydrogenase activity but the isoforms present has not been determined 18,22. ALDH1A1 is the only isoform identified by proteomics 23, but it has been reported to have a low affinity for short chain aldehydes 24. Glyoxylate reductase appears to be ubiquitously expressed in cells and it has been confirmed as part of the erythrocyte proteome 25. We have further confirmed its presence in erythrocytes by Western blotting and the analusis of its enzymatic activity (unpublished results). This enzyme, which is NADPH dependent, has a higher affinity for glyoxylate than LDH and will preferentially convert the glyoxylate formed from glyoxal to glycolate 26. LDH is still capable of converting a small portion of the glyoxylate to oxalate, however. We have observed with a number of substrates that are metabolized to glyoxylate when fed to HepG2 cells, including glycine and hydroxyproline, that 5 –10% of the glyoxylate formed is converted to oxalate and the rest to glycolate. A similar equilibrium may occur in erythrocytes.
The pathway we have proposed for the conversion of glyoxal to oxalate 13 is supported by the response of the metabolism to inhibitors. Oxalate synthesis increased when the conversion of glyoxal to glycolate through the glyoxalase system was reduced by menadione which is known to lower glutathione concentrations. The potential role of aldehyde dehydrogenase is supported by the inhibition of oxalate synthesis by disulfiram, an inhibitor of aldehyde dehydrogenase activity. The formation of oxalate via this pathway in healthy individuals may be limited but could be enhanced in individuals with diabetes and possibly other metabolic diseases including Primary Hyperoxaluria and calcium oxalate stone disease. Chronic oxidative stress due to hyperglycemia, hyperoxaluria or calcium oxalate crystalluria could deplete antioxidant defenses within cells, including GSH, which could potentially increase the production of both glyoxal and oxalate in susceptible cells. It could directly contribute to the increased incidence of calcium oxalate stone disease observed in diabetic individuals 11. Individuals with Primary Hyperoxaluria Type 1 could be especially affected due to glycolate cycling in hepatocytes as glycolate is oxidized in peroxisomes to glyoxylate by glycolate oxidase which in turn is converted back to glyoxylate in the cytosol by glyoxylate reductase. The hydrogen peroxide generated with this cycling could exacerbate the oxidative stress.
It will be possible to confirm the enzymatic steps proposed in this pathway by examining glyoxal metabolism in cultured cells and genetically modified mice where the expression of the proposed enzymes can be manipulated by various techniques. The process by which glyoxal enters cells is not known and warrants further investigation. In a fully hydrated form it is possible that it shares structural similarities with glycerol and is transported by an aquaglyceroporin 27. The use of these model systems may also lead to novel therapies that suppress glyoxal formation and reduce this source of oxalate.
The origin of the relevant acids and aldehydes identified within erythrocytes in Table 1 is of interest. The oxalate and glycolate concentrations are similar to plasma concentrations and may represent equilibrium values between extracellular and intracellular concentrations. Glyoxylate, glyoxal and methylglyoxal are higher within these cells suggesting they may be derived from red cell metabolism. Methylglyoxal may be formed primarily by the secondary activity of triose phosphate isomerase during glycolysis and levels in the range of 100 – 400 nM have been reported 28,29. The concentration of glyoxal in Table 1 is higher but lower than that of malondialdehyde, a dialdehyde also formed by lipid peroxidation, which has been reported to be 13.2 μM in erythrocytes 30.
The carbon source for glyoxal formation is not known with any certainty. The peroxidation of polyunsaturated fatty acids, the autooxidation of glucose, and the metabolism of glycated amino acid residues in proteins are believed to be potential sources. Without this knowledge it is difficult to discern how much glyoxal is produced in the body each day. It is very possible that a conversion rate of 1% of the glyoxal formed to oxalate is quite significant.
This study has limitations. The reactions were observed in vitro and may not reflect what occurs under in vivo conditions with physiological concentrations of glyoxal. The metabolism was studied in erythrocytes from a small number of normal individuals and more variable results may be observed in a larger and diseased populations. Some carbonylation of lysine residues in proteins by glyoxal may have occurred and could have diminished the metabolism that was observed. Whether this pathway in erythrocytes reflects metabolism in other cells and tissues remains to be resolved.
CONCLUSIONS
Erythrocytes contain low levels of glyoxal, methylglyoxal, oxalate glycolate and glyoxylate. They take up exogenous glyoxal and convert it primarily to glycolate. Approximately 1% is converted to oxalate. Inhibition of oxalate synthesis by disulfiram suggests that glyoxylate is an intermediate and that aldehyde dehydrogenase converts a small fraction of the glyoxal to oxalate. Depleting intracellular glutathione decreases glycolate formation from glyoxal and increases the amount of oxalate formed. This pathway for the formation of oxalate may be enhanced in diabetes and other disease with increased oxidative stress.
Acknowledgments
Support/Financial Disclosures:
This work was supported by the National Institutes of Health grant DK73732.
Footnotes
Conflict of Interest The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holmes RP, Assimos DG. Glyoxylate synthesis, and its modulation and its influence on oxalate synthesis. J Urol. 1998;160:1617–1624. [PubMed] [Google Scholar]
- 2.Holmes RP, Goodman HO, Assimos DG. Contribution of dietary oxalate to urinary oxalate excretion. Kid Intl. 2001;59:270–276. doi: 10.1046/j.1523-1755.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- 3.Rumsby G, Cochat P. Primary hyperoxaluria. New Engl J Med. 2013;369:2163. doi: 10.1056/NEJMc1311606. [DOI] [PubMed] [Google Scholar]
- 4.Salido E, Pey AL, Rodriguez R, et al. Primary hyperoxalurias: disorders of glyoxylate detoxification. Biochim Biophys Acta. 2012;1822:1453–1464. doi: 10.1016/j.bbadis.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Knight J, Assimos DG, Callahan MF, et al. Metabolism of primed, constant infusions of [1,2-(13)C(2)] glycine and [1-(13)C(1)] phenylalanine to urinary oxalate. Metabol. 2011;60:950–956. doi: 10.1016/j.metabol.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knight J, Jiang J, Assimos DG, et al. Hydroxyproline ingestion and urinary oxalate and glycolate excretion. Kid Intl. 2006;70:1929–1934. doi: 10.1038/sj.ki.5001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight J, Assimos DG, Easter L, et al. Metabolism of fructose to oxalate and glycolate. Horm Metab Res. 2010;42:868–873. doi: 10.1055/s-0030-1265145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabbani N, Thornalley PJ. Dicarbonyls (glyoxal, methylglyoxal, and 3-deoxyglucosone) John Wiley & Sons; 2012. pp. 177–192. [Google Scholar]
- 9.Han Y, Randell E, Vasdev S, et al. Plasma methylglyoxal and glyoxal are elevated and related to early membrane alteration in young, complication-free patients with Type 1 diabetes. Mol Cell Biochem. 2007;305:123–131. doi: 10.1007/s11010-007-9535-1. [DOI] [PubMed] [Google Scholar]
- 10.Lapolla A, Flamini R, Dalla Vedova A, et al. Glyoxal and methylglyoxal levels in diabetic patients: quantitative determination by a new GC/MS method. Clinical Chem Lab Med. 2003;41:1166–1173. doi: 10.1515/CCLM.2003.180. [DOI] [PubMed] [Google Scholar]
- 11.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kid Intl. 2005;68:1230–1235. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 12.Eisner BH, Porten SP, Bechis SK, et al. Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. J Urol. 2010;183:2244–2248. doi: 10.1016/j.juro.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Lange JN, Wood KD, Knight J, et al. Glyoxal formation and its role in endogenous oxalate synthesis. Adv Urol. 2012;2012:819202. doi: 10.1155/2012/819202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knight J, Holmes RP. Mitochondrial hydroxyproline metabolism: implications for primary hyperoxaluria. Amer J Nephrol. 2005;25:171–175. doi: 10.1159/000085409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornalley PJ, Rabbani N. Assay of methylglyoxal and glyoxal and control of peroxidase interference. Biochem Soc Trans. 2014;42:504–510. doi: 10.1042/BST20140009. [DOI] [PubMed] [Google Scholar]
- 16.Rabbani N, Thornalley PJ. The critical role of methylglyoxal and glyoxalase 1 in diabetic nephropathy. Diabetes. 2014;63:50–52. doi: 10.2337/db13-1606. [DOI] [PubMed] [Google Scholar]
- 17.Rossi R, Milzani A, Dalle-Donne I, et al. Different metabolizing ability of thiol reactants in human and rat blood: biochemical and pharmacological implications. J Biol Chem. 2001;276:7004–7010. doi: 10.1074/jbc.M005156200. [DOI] [PubMed] [Google Scholar]
- 18.Helander A, Lowenmo C, Johansson M. Distribution of acetaldehyde in human blood: effects of ethanol and treatment with disulfiram. Alcohol Alcoholism. 1993;28:461–468. [PubMed] [Google Scholar]
- 19.Kraemer RJ, Deitrich RA. Isolation and characterization of human liver aldehyde dehydrogenase. J Biol Chem. 1968;243:6402–6408. [PubMed] [Google Scholar]
- 20.Orosz F, Olah J, Ovadi J. Triosephosphate isomerase deficiency: new insights into an enigmatic disease. Biochim Biophys Acta. 2009;1792:1168–1174. doi: 10.1016/j.bbadis.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. 2005;35:609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- 22.Inoue K, Fukunaga M, Yamasawa K. Correlation between human erythrocyte aldehyde dehydrogenase activity and sensitivity to alcohol. Pharmacol Bochem Behav. 1980;13:295–297. doi: 10.1016/0091-3057(80)90087-8. [DOI] [PubMed] [Google Scholar]
- 23.Kakhniashvili DG, Bulla LA, Jr, Goodman SR. The human erythrocyte proteome: analysis by ion trap mass spectrometry. Mol Cell Proteom. 2004;3:501–509. doi: 10.1074/mcp.M300132-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Wang MF, Han CL, Yin SJ. Substrate specificity of human and yeast aldehyde dehydrogenases. Chemicobiol Interact. 2009;178:36–39. doi: 10.1016/j.cbi.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Lange PF, Huesgen PF, Nguyen K, et al. Annotating N termini for the human proteome project: N termini and Nalpha-acetylation status differentiate stable cleaved protein species from degradation remnants in the human erythrocyte proteome. J Proteome Res. 2014;13:2028–2044. doi: 10.1021/pr401191w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mdluli K, Booth MP, Brady RL, et al. A preliminary account of the properties of recombinant human Glyoxylate reductase (GRHPR), LDHA and LDHB with glyoxylate, and their potential roles in its metabolism. Biochim Biophys Acta. 2005;1753:209–216. doi: 10.1016/j.bbapap.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Mukhopadhyay R, Bhattacharjee H, Rosen BP. Aquaglyceroporins: generalized metalloid channels. Biochim Biophys Acta. 2014;1840:1583–1591. doi: 10.1016/j.bbagen.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karg E, Papp F, Tassi N, et al. Enhanced methylglyoxal formation in the erythrocytes of hemodialyzed patients. Metabol. 2009;58:976–982. doi: 10.1016/j.metabol.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 29.McLellan AC, Phillips SA, Thornalley PJ. The assay of methylglyoxal in biological systems by derivatization with 1,2-diamino-4,5-dimethoxybenzene. Anal Biochem. 1992;206:17–23. doi: 10.1016/s0003-2697(05)80005-3. [DOI] [PubMed] [Google Scholar]
- 30.Egert S, Lindenmeier M, Harnack K, et al. Margarines fortified with alpha-linolenic acid, eicosapentaenoic acid, or docosahexaenoic acid alter the fatty acid composition of erythrocytes but do not affect the antioxidant status of healthy adults. J Nutr. 2012;142:1638–1644. doi: 10.3945/jn.112.161802. [DOI] [PubMed] [Google Scholar]