Abstract
As the average world-wide lifespan continues to increase, heart failure (HF) has dramatically increased in incidence leading to the highest degree of mortality and morbidity of any disease presently studied. G protein-coupled receptors (GPCRs) play a prominent role in regulation of cardiovascular function. GPCRs are effectively “turned off” by GPCR kinases (GRKs) in a process known as “desensitization”. GRKs 2 and 5 are highly expressed in the heart, and known to be upregulated in HF. Over the last 20 years, the role of GRK2 in HF has been widely studied. However, until recently, the role of GRK5 in cardiac pathophysiology had yet to be elucidated. In the present review, we will focus on GRK5’s role in the myocardium in normal physiology, and its apparent critical role in the progression of HF. Further, we will also present potential therapeutic strategies (i.e. small molecule inhibition, gene therapy) that may have potential in combating the deleterious effects of GRK5 in HF.
Keywords: G protein-coupled receptor, Hypertrophy, Heart Failure, Myocardium
Introduction to GRKs
Interestingly, 50 years ago many scientists were still skeptical that cell surface receptors existed in a physical form. Receptors were spoken of in an abstract sense to explain the response seen by agonist stimulation [1]. Today, many classes of receptors have been identified, with the G protein-coupled receptors (GPCRs) or seven-transmembrane receptors comprising the largest protein superfamily in the mammalian genome [1, 2]. GPCRs respond to a wide variety of extracellular signals including photons, small molecules, peptides and proteins [1, 3]. This family of receptors is a major target of therapeutic intervention as most physiological processes including vision, smell, taste in addition to neurologic, cardiovascular, endocrine and reproductive function require signaling through GPCRs [4].
Upon ligand binding, GPCRs become activated resulting in downstream intracellular signaling via heterotrimeric G proteins. GPCRs are effectively “turned off” in a process termed “desensitization”, wherein GPCR kinases (GRKs) phosphorylate activated GPCRs resulting in β-arrestin binding and subsequent steric hinderance of further G protein coupling (Figure 1A). The actions of β-arrestin ultimately lead to receptor internalization and degradation or resensitization of the GPCR [5].
Figure 1.
Canonical GRK function and structure. (A) After activation, GPCRs are effectively “turned off” in a process termed “desensitization”, wherein G protein-coupled receptor kinases (GRKs) phosphorylate activated GPCRs resulting in β-arrestin binding and subsequent steric hinderance of further G protein coupling. The actions of β-arrestin ultimately lead to receptor internalization and degradation or resensitization of the GPCR or β-arrestin dependent signaling (B) Structural schematics of GRKs 2 and 5. The catalytic domain of GRKs is strongly conserved and is centrally located in the tri-domain structure. The N-terminal amino acids are necessary for recognition of the activated GPCR. The C-terminal third of GRKs is responsible for membrane localization and is divergent between GRK subfamilies. GRK5 has been found to localize to the nucleus via a nuclear localization sequence (NLS) within its catalytic domain, and also contains a nuclear export sequence (NES) within the centrally conserved kinase domain.
There are seven known GRKs, which have been classified into 3 subfamilies based on sequence and structural similarity: the rhodopsin kinase subfamily (GRK1 and GRK7), the βARK subfamily (GRK2 and GRK3) and the GRK4-like subfamily (GRK4, GRK5, GRK6) [6]. The rhodopsin kinase subfamily is solely expressed in the retina and regulates opsins [7]. GRK2, 3, 5 and 6 are expressed ubiquitously and of interest to this work play a role in GPCR phosphorylation in the cardiovascular system [8]. Alternatively, GRK4 is found primarily in the testes [9].
The GRKs are members of the AGC protein kinase subfamily of serine/threonine kinases, which is so named for PKA, PKG and PKC. The placement into this subgroup is based on the sequence alignment of the kinase domain of these proteins [10]. The catalytic domain of GRKs is strongly conserved and is centrally located in the tri-domain structure (Figure 1B). Likewise, the first 30 N-terminal amino acids are highly conserved within the GRK family and are necessary for recognition of the activated GPCR [11]. The C-terminal third of GRKs is responsible for membrane localization and is divergent between GRK subfamilies [12]. GRK4, 5 and 6 cellular localization is mediated by the interaction with phosphatidylinositol 4,5 bisphosphate (PIP2) within the plasma membrane [13]. The GRK4 family members GRK4 and GRK6 contain a palmitoylation site and the addition of this lipid allows for constitutive membrane association. GRK5 is unique from GRK4 and 6 as it does not contain this palmitoylation site and rather depends on a positively charged lipid-binding elements in the form of an amphipathic helix for membrane association [14, 15]. Recently, it was found that GRK5 can associate with the plasma membrane via N-terminal residues 25–31 [16, 17].
Interestingly, GRKs are known to interact with calcium (Ca2+) binding proteins which can alter their affinity for the receptor [18]. In the visual system, the Ca2+ binding protein recoverin binds to GRK1 and prevents the phosphorylation of rhodopsin by GRK1 [19]. The Ca2+ binding protein calmodulin has also been shown to bind GRK1, 2, 5 and 6 with calmodulin having the greatest affinity for GRK5 [20]. Each of these GRKs was found to have both an N-terminal and C-terminal calmodulin binding site [21]. Initially, it was shown that calmodulin binding to GRK5 leads to autophosphorylation of the C-terminus and reduces the enzyme’s ability to phosphorylate GPCRs, but increases its ability to phosphorylate non-receptor substrates [22]. More recently, as detailed below, calmodulin binding to GRK5 induces its translocation to the nucleus. [23]
Non-canonical localization of GRK5 in the nucleus of cells
While the canonical role of GRKs is to phosphorylate agonist bound GPCRs located within the plasma membrane, members of the GRK4 subfamily have been found to localize to the nucleus via a nuclear localization sequence (NLS) within their catalytic domain that is homologous to homeobox containing transcription factors [24–26]. In addition to the NLS, members of the GRK4 subfamily contain a nuclear export sequence (NES) within the centrally conserved kinase domain[26]. While GRK5 nuclear export is Ca2+ dependent, nuclear export of GRK6 is not[26]. The suggested mechanism of GRK5 nuclear export involves calmodulin binding to the N-terminus of GRK5 and exposing the NES and allowing for nuclear export of the kinase[26].
The NLS of homeobox transcription factors is known to bind DNA and this holds true with GRK5 and GRK6. GRK5 and GRK6, but not GRK4 were shown to bind DNA cellulose [26] . In vivo, it was found via chromatin immuno-precipitation (ChIP) that GRK5 binds directly to the Bcl-2 promoter and inhibits transcription of Bcl-2 [27]. The proposed model of GRK5:DNA binding involves GRK5 interacting with DNA via the NLS as well as the N-terminal calmodulin binding domain. Auto-phosphorylation at the C-terminus of GRK5 or binding of calmodulin to the C-terminus of GRK5 reduces the affinity of GRK5 for DNA and allows the enzyme to dissociate from the DNA [26]. As the crystal structure of GRK6 shows dimer formation, Johnson, et al. speculate that GRK5 may form a dimer to wrap itself around DNA [28].
Role of GRK5 in the myocardium
The first GRK identified in the heart was GRK2, originally named β-adrenergic receptor kinase or βARK for its ability to phosphorylate agonist occupied β-adrenergic receptors as well as other GPCRs [29]. GRK5 was later discovered and found to be highly expressed in the heart and other muscle types. It is also expressed in the nervous, respiratory, and immune systems, and this has been confirmed in several studies involving genetically engineered mice with altered levels of the GRKs [7, 30, 31] . Of note, GRK2 and GRK5, the predominant GRKs expressed in the myocardium, are both shown to be up-regulated in heart failure (HF).
The physiological importance of GRKs has been demonstrated via genetic deletion studies in transgenic mice. The homozygous GRK2 knockout mouse is embryonic lethal [32]. These animals die by gestational day 15.5, presumably due to HF, as they exhibit hypoplasia of the ventricular myocardium and a 70% decrease in ejection fraction (EF) [32] . The GRK5 homozygous KO is viable, and animals are born with a normal basal phenotype ([9]). However, studies in zebrafish suggest that GRK5 is important in fine tuning of cardiac development through the mTOR pathway [33, 34]. Zebrafish lacking the GRK5 homologue Grk5l develop asymmetric hearts due to a decrease of early cardiac progenitor cells during heart development [33, 34]. Further, embryonic lethality has been reported in GRK5/GRK6 double knockout mice providing evidence that GRK6 may compensate for the loss of GRK5 in GRK5KO mice, however loss of both of these GRK4 family members results in lethality[33].
GRK5 in Cardiovascular Biology and Pathophysiology
The development of a transgenic mouse with cardiac-specific GRK5 overexpression (TgGRK5) revealed interesting information about the specificity of different GRKs in the heart. The TgGRK5 mouse was found to desensitize the βAR similarly to GRK2; however, these TgGRK5 mice were unable to desensitize the angiotensin receptor as seen in GRK2 overexpressing mice [35]. Interestingly, the TgGRK5 were intolerant to left ventricular (LV) pressure-overload via trans-aortic constriction (TAC) surgery [36]. The exaggerated hypertrophy and early progression to HF seen in the TgGRK5 mice was attributed to GRK5 nuclear accumulation and non-canonical activity (Figure 2). It was shown that following pressure-overload, a Gαq dependent process [37], GRK5 accumulated within the nucleus of cardiomyocytes [36]. Importantly, mice which overexpressed a nuclear deficient mutant of GRK5 (NLS) did not exhibit the same severe pathology following pressure-overload [36]. In fact, these NLS transgenic mice had function and hypertrophy comparable to non-transgenic mice [36]. Interestingly, it was found that GRK5 acts as a class II HDAC kinase, phosphorylating HDAC5 leading to its nuclear export and de-repression of MEF2. Once free of HDAC repression, MEF2 is responsible for the transcription of hypertrophic genes which leads to pathology [36]. This study was important because it placed GRK5 mediated pathology downstream of Gαq signaling and identified a specific pathological role of nuclear GRK5 that was novel and independent of its canonical GRK activity. In fact, studies using a hypertensive model of cardiovascular disease showed that GRK5 partitions to nuclear compartments, which is consistent with these more recent studies in post-TAC mice [24, 38]. A subsequent study investigated the dynamic interplay between GRK5 and Gq in pathological hypertrophy [39], and demonstrated that GRK5 phosphorylates HDAC5 in vivo leading to its nuclear export in mice overexpressing both Gq and GRK5, reversing Gq-mediated nuclear accumulation of HDAC5. Further, deep RNA-sequencing in these mice revealed that GRK5 counter-regulated 96 of 200 genes downregulated by constitutive active Gq signaling suggesting that GRK5’s non-canonical nuclear activity can directly oppose transcriptional repression of a subset of hypertrophy/heart failure regulated genes [39]
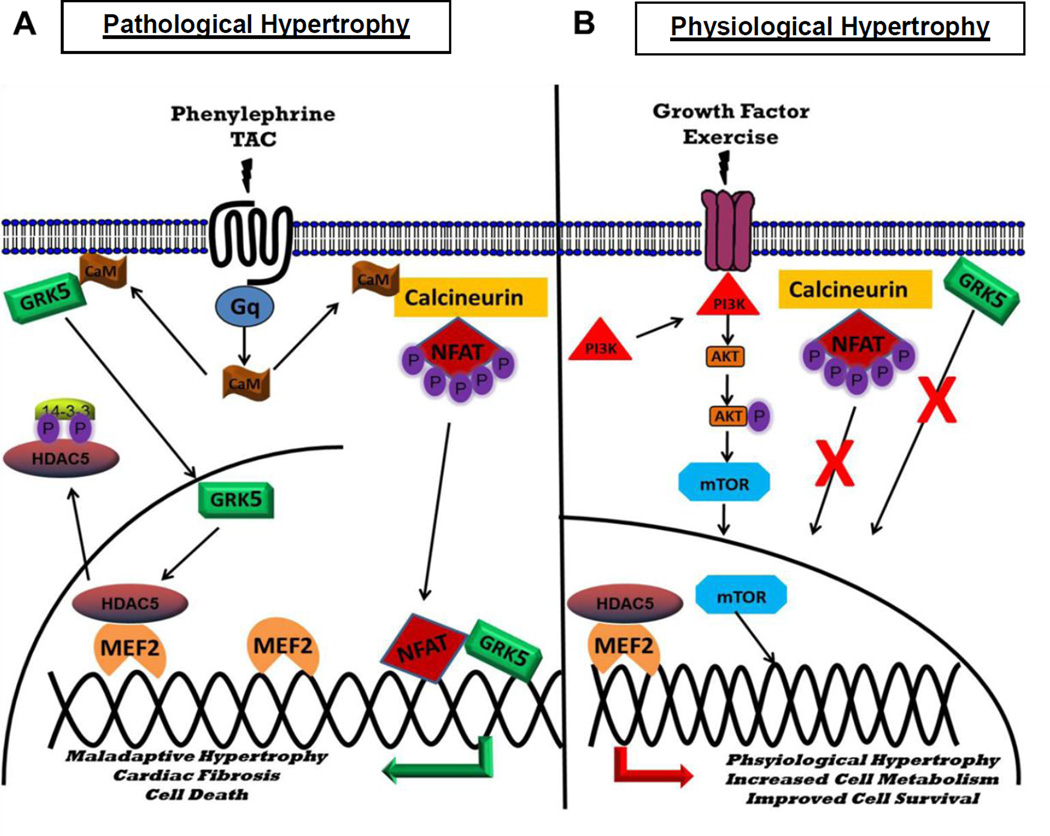
Figure 2.
Summary diagram illustrating the differences between GRK5’s role in physiological and pathological cardiac hypertrophy. With pathological stimuli (Figure 2A:PE,TAC), GRK5 associates with Ca2+-calmodulin leading to translocation to the nucleus, wherein it promotes maladaptive cardiac hypertrophy via association with HDAC5 and NFAT. Conversely, the physiological hypertrophic stimulus of exercise (Figure 2B) does not lead to translocation of GRK5 or NFAT to the nucleus, resulting in a normal adaptive hypertrophic response with GRK5 overexpression.
More recent research has served to more precisely elucidate the mechanism of GRK5 nuclear translocation. GRK5 was found to translocate to the nucleus in a manner which was dependent on calmodulin binding to the N-terminus of GRK5 following the activation of select Gαq coupled receptors [23]. Stimulation of the α1-adrenergic receptor as well as the angiotensin II receptor, both of which GRK5 does not appear to desensitize, led to translocation of GRK5 from the membrane in a calmodulin dependent manner [23]. Surprisingly, stimulation of the endothelin receptor, which is also Gαq coupled, but is desensitized by GRK5 did not lead to nuclear translocation of GRK5. Thus, it appears that GRK5 can translocate to the nucleus when it is not actively engaged with a membrane-bound activated GPCR.
Importantly, GRK5 was shown to be a physiologically relevant HDAC kinase [40]. This was done utilizing GRK5 null mice (GRK5 KO) and subjecting them to LV pressure-overload or chronic administration of phenylephrine. Following pathological stress, GRK5 KO mice exhibited protection from cardiac hypertrophy, attenuation of the fetal gene program and showed decreased HDAC5 phosphorylation [40].
Genetic screening has yet to identify any non-synonymous polymorphisms of GRK2 in the human population; however, four single nucleotide polymorphisms (SNPs) of GRK5 are known [41]. One polymorphism, found primarily in the African American population with approximately a 40% prevalence, leads to a substitution of leucine (GRK5-Leu41) for glutamine at position 41 [39]. This polymorphism appears to be largely unique to the African-American population, as it is present in ~ 2% of caucasians, was not identified in a large Chinese cohort of systolic HF, and no association was found between GRK5-Leu41 and an Australian cohort of Takotsubo cardiomyopathy [41–43]. Interestingly, patients with LV apical ballooning also have displayed an increased prevalence of this polymorphism [44]
It has been shown that the mutant GRK5-Leu41 was able to enhance β1AR desensitization [39]. The activity of GRK5-Leu41 for the soluble substrate rhodopsin was not altered, however. This led to the conclusion that this mutation alters the regulation of GRK5 rather than its kinase activity. Interestingly, in a prospective trial following African American HF patients it was found that this GRK5-Leu41 point mutation protected against death or cardiac transplantation [41, 45]. It was postulated that this point mutation provides a “genetic β-blockade” that leads to the increased survival in this population and may be the cause of conflicting results in clinical trials of β-blockers within the African American population [41]. Surprisingly, a follow-up study in African-American GRK5-Leu41 patients showed that this polymorphism did not affect sensitivity to the β blocker, atenolol, and did not contribute to ethnic differences observed in sensitivity to atenolol [46].
Clinically in humans, myocardial expression of GRK5 has been shown to be elevated in various disease states (i.e. dilated cardiomyopathy, volume overload) [47–50]. Further a direct correlation between GRK5 levels and β1-AR levels has been reported in HF patients prior to transplant [51]. Increased GRK5 expression has also been observed in myocardial biopsies obtained from heart transplant without evidence of acute rejection or echocardiographic dysfunction [52]. Interestingly, a positive correlation has been reported with regard to EF and GRK5 expression in HF, which contrasts greatly with the rodent studies detailed above in disease [49].
In the vasculature, GRK5 has been shown to have a role in regulating blood pressure via the smooth muscle cells (SMC), which control vascular tone. Mice with SMC-specific GRK5 overexpression demonstrate hypertension in a Gi-dependent manner [53]. Interestingly, it was found that male mice had higher blood pressure than their female littermates. In another study of the vasculature, GRK5 KO mice demonstrated greater proliferation of macrophages and SMCs in atherosclerotic lesions through the activation of the NF-κB pathway [54].
Non-canonical novel mechanisms of GRK5-mediated Cardiac Pathology
Data supporting that only nuclear accumulation of GRK5 can induce pathology was surprising, but pointed towards novel, non-canonical actions of this kinase. Indeed, initial reports demonstrated that nuclear GRK5 acts as a Class II HDAC kinase and participates in the de-repression of MEF2 leading to pathological hypertrophic gene transcription [23, 36]. However, recently we have shown a second novel action of GRK5 in the nucleus of myocytes. It was found that GRK5 interacts with the NFAT-pathway in the nucleus during pathological hypertrophy [55]. First, utilizing cardiac-specific NFAT luciferase reporter mice it was shown that GRK5 overexpression leads to an increase in NFAT activity while GRK5 KO mice exhibited less NFAT transcriptional activity [55]. Interestingly, double transgenic mice with cardiac-specific GRK5 over-expression and deletion of NFATc3 were protected from the early progression to HF observed with increased cardiac GRK5 levels following TAC[55]. Molecular studies ascertained that GRK5 was able to enhance the NFAT pathway via GRK5’s known property of DNA binding [55]. Therefore, this non-canonical action of GRK5 in the nucleus during pathological hypertrophy is kinase-independent. This creates an interesting avenue for future research into the role GRK5:DNA binding in physiology as well as pathology.
The NF-κB pathway is also a nuclear target for GRK5. Originally, it was shown that NF-κB subunit p105 was a substrate for GRK5 [56]. It was later shown that GRK2 is also able to regulate p105 as well [57]. Interestingly, GRK5 as well as GRK2 have been shown to activate the NF-κB pathway through the direct phosphorylation of I-κBα leading to increased inflammation [58, 59]. This appears to be evolutionarily conserved as the Drosophila melanogaster GRK5 homologue Gprk2 is able to interact with the I-κBα homologue Cactus and activate NF-κB signaling. This was confirmed in HeLa cells using GRK5 knockdown [60]. The activation of the NF-κB pathway by GRK5 was shown by our laboratory as well resulting from the upregulation of NF-κB subunits p50 and p65 [61]. This is in opposition to the results published by Sorriento, et. al. which show that GRK5 inhibits NF-κB signaling [62]. Importantly, an NF-κB binding element exists in the GRK5 promoter and NF-κB has been shown to regulate GRK5 expression in cardiomyocytes [63]. Clearly, GRK5 and the NF-κB pathway are intertwined; however, further studies are needed to clarify the extent and direction of this interaction.
While most studies demonstrate that GRK5 is involved in maladaptive signaling, it has also been shown to play a role in the transactivation of β1-AR and EGFRs and subsequent activation of mitogenic and cell survival signaling pathways [64]. With activation of pathological pathways by GRK5 occurring within the nucleus of cardiomyocytes, this establishes an interesting dichotomy in which membrane signaling by GRK5 is protective while the nuclear function of GRK5 appears to be detrimental. By modulating the cellular localization of GRK5 we may be able to decrease its deleterious effects and promote myocyte cell survival.
GRK5 in Physiological Hypertrophy
Pressure-overload is known to produce maladaptive hypertrophy and subsequent HF; however, another form of hypertrophy, physiological hypertrophy, can also occur in the heart. Physiological hypertrophy occurs during pregnancy and after exercise (EX) leading to more uniform growth, with proportional increases in myocyte cell length and width [65, 66]. These adaptations directly oppose those present in the development of pathological hypertrophy, where myocyte width increases disproportionately to myocyte cell length resulting in loss of chamber size and wall and septal thickening [65]. Most importantly, EX-induced hypertrophy does not lead to maladaptation and HF.
Due to GRK5’s established role in pathological hypertrophy, a recent study evaluated GRK5’s role in physiological hypertrophy induced by swim training, a standard protocol to study this form of adaptive growth [67, 68]. Swim training produces similar levels of cardiac hypertrophy in TgGRK5, GRK5 KO and their corresponding non-littermate control (NLC) mice [69]. Further, GRK5 nuclear localization, which is the triggering mechanism for pathological hypertrophy induced by GRK5 after pressure-overload, was unaltered after EX in TgGRK5 mice. This finding is not surprising in that an established co-factor of GRK5 in pathological hypertrophy, NFAT, has been reported to not be involved in physiological hypertrophy such that NFAT activity does not increase with EX [67]. This conclusion is most supported by data that exercise does not induce the nuclear translocation and accumulation of GRK5, which prevents GRK5 from interacting with NFAT and its DNA binding targets (Figure 2).
The association of Ca2+ binding protein calmodulin plays a critical role in the translocation of GRK5 to the nucleus, and subsequently its non-canonical nuclear activity [70]. Further, it is well established that mediators of pathological hypertrophy (i.e. PE, AngII) are known to increase intracellular Ca2+; however, it is important to note that regulators of physiological hypertrophy (i.e. IGF-1) also have been shown to increase [Ca2+]I [71]. Thus, it is likely that the Ca2+ pool activated in exercise, does not interact with GRK5. Accordingly, pathological hypertrophic stimuli (i.e. Gq activation, PE), may activate a pool of Ca2+ unique from that activated in physiological hypertrophy, ultimately leading to GRK5 nuclear translocation and its subsequent deleterious nuclear signaling paradigm. Taken together, it is not surprising that GRK5 nuclear translocation, a downstream consequence of α-1 adrenergic and angiotensin II receptor activation and therefore Ca2+-calmodulin association via hypertrophic stimuli, is unaltered with exercise training.
Unlike pathological hypertrophy, physiological hypertrophy is considered to be beneficial resulting in favorable cardiac adaptations (i.e. anti-apoptotic, stimulation of myocyte renewal) [65]. Prior studies have demonstrated that physiological hypertrophy exerts its beneficial effects, in part via activation of the insulin-like growth factor-1 (IGF-1)/PI3K/AKT/PKB/mTOR signaling axis [72], and can prolong lifespan, preserve cardiac function, and reduce cardiac fibrosis in a mouse model of dilated cardiomyopathy [73]. Subsequent studies have demonstrated that the beneficial effects of EX are cardioprotective in various cardiac insults such as ischemia-reperfusion injury (IR) and pressure-overload [74–76]. Interestingly in the aforementioned study on EX in TgGRK5 mice, it was found that EX ameliorated cardiac dysfunction in these mice after pressure-overload, but not cardiac growth [69]. Thus, it can be inferred from this observation that the beneficial effects seen in EX trained pressure-overload GRK5 overexpressing mice must be due to a non-GRK5 event. These findings present a promising route by which the deleterious cardiac effects of GRK5 up-regulation can be slowed; however, further studies are warranted to elucidate the mechanism by which cardiac function is preserved in trained pressure-overload TgGRK5 mice.
Future Directions
It is evident from the studies detailed above that GRK5 acts as a hypertrophic facilitator only under pathological conditions and does not affect physiological growth of the heart even when robustly overexpressed [69]. Due to the deleterious non-canonical action of GRK5 in the nucleus of cardiomyocytes and the fact that this kinase is up-regulated in diseased myocardium it could be a therapeutic target in various hypertrophic cardiomyopathies. That is with direct GRK5 inhibition, physiological hypertrophy will continue while only pathological hypertrophy would be affected.
Accordingly, selective small molecule inhibitors of GRK5 as well as GRK2 have begun to emerge as an exciting avenue of therapeutic treatment in HF[77]. Like GRK5, GRK2 has been well characterized in disease [78], and in a recent study, small molecule therapy has been utilized to inhibit GRK2 in HF[79]. That is, paroxetine preserved cardiac function and reduced induction of markers of HF via selective GRK2 inhibition [79]. Pharmacological screens have also been conducted to evaluate potential GRK5 inhibitors, and a recent study demonstrated that a small molecule, amlexanox, was able to inhibit GRK5, thus blocking myocyte MEF2 activation after PE stimulation, which is consistent with an involvement of GRK5 in this aspect of pathological hypertrophy [80]. Further studies are needed to evaluate in vivo if compounds such as amlexanox are able to ameliorate the deleterious effects associated with hypertrophic stimulus in TgGRK5 mice. Taken together, the therapeutic potential of these small molecule GRK inhibitors is promising. Moving forward, it will be important to determine which GRKs should be inhibited in HF. Given that GRK2 has been shown to exhibit far-reaching effects on various deleterious signaling pathways ranging from reactive oxygen species generation to promotion of cell death [81, 82] while GRK5 has implicated solely as a facilitator of pathological hypertrophy[69], it appears that GRK2 inhibition may yield greater therapeutic benefit versus simultaneous inhibition of GRKs 2 and 5 or GRK5 alone. However, further studies will be needed to confirm this hypothesis.
GRK5’s nuclear non-canonical activity has been largely associated with its HDAC5 kinase activity as well as its interaction with NFAT. It is well established that GRK5 is capable of binding DNA itself; therefore, it is likely that GRK5 has more binding partners when it interacts with DNA that also may play a role in the maladaptive phenotype observed with cardiac elevation of GRK5 [26] . Thus, if therapeutics are to be designed to combat the deleterious effects associated with GRK5 in HF, it will be beneficial to determine which non-canonical activity of GRK5 (i.e. HDAC5 kinase activity or DNA binding ability) is more important to its detrimental pathological effects. With this said, studies are needed to evaluate additional GRK5 DNA binding partners to further characterize the detrimental effects of nuclear GRK5
Highlights.
GRK5 acts as a hypertrophic facilitator only under pathological conditions.
The beneficial effects of physiological hypertrophy are due to a non-GRK5 event.
Inhibition of GRK5 nuclear targeting may be a novel therapeutic strategy in disease.
Acknowledgments
W.J.K is the W.W. Smith Chair in Cardiovascular Medicine at Temple University School of Medicine. This project was supported by National Institute of Health (NIH) grant P01 HL091799. W.J.K. is also supported by NIH grants R37 HL061690, R01 HL085503, P01 HL075443 (Project 2) and P01 HL108806 (Project 3). C.J.T. was supported by the National Institutes of Health T32 HL091804-CJT
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES:
None.
Literature Cited
- 1.Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf) 2007;190:9–19. doi: 10.1111/j.1365-201X.2007.01693.x. [DOI] [PubMed] [Google Scholar]
- 3.Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol. 2013;53:531–556. doi: 10.1146/annurev-pharmtox-032112-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 5.Pierce KL, Lefkowitz RJ. Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci. 2001;2:727–733. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- 6.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 7.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- 8.Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res. 2006;99:570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- 9.Gainetdinov RR, Bohn LM, Walker JK, Laporte SA, Macrae AD, Caron MG, et al. Muscarinic supersensitivity and impaired receptor desensitization in G protein-coupled receptor kinase 5-deficient mice. Neuron. 1999;24:1029–1036. doi: 10.1016/s0896-6273(00)81048-x. [DOI] [PubMed] [Google Scholar]
- 10.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 11.Homan KT, Tesmer JJ. Structural insights into G protein-coupled receptor kinase function. Curr Opin Cell Biol. 2014;27C:25–31. doi: 10.1016/j.ceb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang ZM, Gold JI, Koch WJ. G protein-coupled receptor kinases in normal and failing myocardium. Front Biosci (Landmark Ed) 2011;16:3047–3060. doi: 10.2741/3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitcher JA, Fredericks ZL, Stone WC, Premont RT, Stoffel RH, Koch WJ, et al. Phosphatidylinositol 4,5-bisphosphate (PIP2)-enhanced G protein-coupled receptor kinase (GRK) activity. Location, structure, and regulation of the PIP2 binding site distinguishes the GRK subfamilies. J Biol Chem. 1996;271:24907–24913. doi: 10.1074/jbc.271.40.24907. [DOI] [PubMed] [Google Scholar]
- 14.Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther. 2012;133:40–69. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiyagarajan MM, Stracquatanio RP, Pronin AN, Evanko DS, Benovic JL, Wedegaertner PB. A predicted amphipathic helix mediates plasma membrane localization of GRK5. J Biol Chem. 2004;279:17989–17995. doi: 10.1074/jbc.M310738200. [DOI] [PubMed] [Google Scholar]
- 16.Ding B, Glukhova A, Sobczyk-Kojiro K, Mosberg HI, Tesmer JJ, Chen Z. Unveiling the membrane-binding properties of N-terminal and C-terminal regions of G protein-coupled receptor kinase 5 by combined optical spectroscopies. Langmuir. 2013;30:823–831. doi: 10.1021/la404055a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang P, Glukhova A, Tesmer JJ, Chen Z. Membrane orientation and binding determinants of G protein-coupled receptor kinase 5 as assessed by combined vibrational spectroscopic studies. PLoS One. 2013;8:e82072. doi: 10.1371/journal.pone.0082072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penn RB, Pronin AN, Benovic JL. Regulation of G protein-coupled receptor kinases. Trends Cardiovasc Med. 2000;10:81–89. doi: 10.1016/s1050-1738(00)00053-0. [DOI] [PubMed] [Google Scholar]
- 19.Chen CK, Inglese J, Lefkowitz RJ, Hurley JB. Ca(2+)-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem. 1995;270:18060–18066. doi: 10.1074/jbc.270.30.18060. [DOI] [PubMed] [Google Scholar]
- 20.Pronin AN, Satpaev DK, Slepak VZ, Benovic JL. Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. J Biol Chem. 1997;272:18273–18280. doi: 10.1074/jbc.272.29.18273. [DOI] [PubMed] [Google Scholar]
- 21.Levay K, Satpaev DK, Pronin AN, Benovic JL, Slepak VZ. Localization of the sites for Ca2+-binding proteins on G protein-coupled receptor kinases. Biochemistry. 1998;37:13650–13659. doi: 10.1021/bi980998z. [DOI] [PubMed] [Google Scholar]
- 22.Pronin AN, Morris AJ, Surguchov A, Benovic JL. Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J Biol Chem. 2000;275:26515–26522. doi: 10.1074/jbc.M003542200. [DOI] [PubMed] [Google Scholar]
- 23.Gold JI, Martini JS, Hullmann J, Gao E, Chuprun JK, Lee L, et al. Nuclear translocation of cardiac G protein-Coupled Receptor kinase 5 downstream of select Gq-activating hypertrophic ligands is a calmodulin-dependent process. PLoS One. 2013;8:e57324. doi: 10.1371/journal.pone.0057324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi XP, Gerdes AM, Li F. Myocyte redistribution of GRK2 and GRK5 in hypertensive, heart-failure-prone rats. Hypertension. 2002;39:1058–1063. doi: 10.1161/01.hyp.0000019130.09167.3b. [DOI] [PubMed] [Google Scholar]
- 25.Johnson LR, Scott MG, Pitcher JA. G protein-coupled receptor kinase 5 contains a DNA-binding nuclear localization sequence. Mol Cell Biol. 2004;24:10169–10179. doi: 10.1128/MCB.24.23.10169-10179.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson LR, Robinson JD, Lester KN, Pitcher JA. Distinct structural features of G protein-coupled receptor kinase 5 (GRK5) regulate its nuclear localization and DNA-binding ability. PLoS One. 2013;8:e62508. doi: 10.1371/journal.pone.0062508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu P, Wang X, Gao N, Zhu H, Dai X, Xu Y, et al. G protein-coupled receptor kinase 5, overexpressed in the alpha-synuclein up-regulation model of Parkinson's disease, regulates bcl-2 expression. Brain Res. 2010;1307:134–141. doi: 10.1016/j.brainres.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 28.Lodowski DT, Tesmer VM, Benovic JL, Tesmer JJ. The structure of G protein-coupled receptor kinase (GRK)-6 defines a second lineage of GRKs. J Biol Chem. 2006;281:16785–16793. doi: 10.1074/jbc.M601327200. [DOI] [PubMed] [Google Scholar]
- 29.Dorn GW., 2nd GRK mythology: G-protein receptor kinases in cardiovascular disease. J Mol Med (Berl) 2009;87:455–463. doi: 10.1007/s00109-009-0450-7. [DOI] [PubMed] [Google Scholar]
- 30.Kunapuli P, Benovic JL. Cloning and expression of GRK5: a member of the G protein-coupled receptor kinase family. Proc Natl Acad Sci U S A. 1993;90:5588–5592. doi: 10.1073/pnas.90.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Premont RT, Koch WJ, Inglese J, Lefkowitz RJ. Identification, purification, and characterization of GRK5, a member of the family of G protein-coupled receptor kinases. J Biol Chem. 1994;269:6832–6841. [PubMed] [Google Scholar]
- 32.Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, et al. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci U S A. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burkhalter MD, Fralish GB, Premont RT, Caron MG, Philipp M. Grk5l controls heart development by limiting mTOR signaling during symmetry breaking. Cell Rep. 2013;4:625–632. doi: 10.1016/j.celrep.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Philipp M, Berger IM, Just S, Caron MG. Overlapping and opposing functions of G protein-coupled receptor kinase 2 (GRK2) and GRK5 during heart development. J Biol Chem. 2014;289:26119–26130. doi: 10.1074/jbc.M114.551952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rockman HA, Choi DJ, Rahman NU, Akhter SA, Lefkowitz RJ, Koch WJ. Receptor-specific in vivo desensitization by the G protein-coupled receptor kinase-5 in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:9954–9959. doi: 10.1073/pnas.93.18.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martini JS, Raake P, Vinge LE, DeGeorge BR, Jr, Chuprun JK, Harris DM, et al. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc Natl Acad Sci U S A. 2008;105:12457–12462. doi: 10.1073/pnas.0803153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 38.Yi XP, Zhou J, Baker J, Wang X, Gerdes AM, Li F. Myocardial expression and redistribution of GRKs in hypertensive hypertrophy and failure. Anat Rec A Discov Mol Cell Evol Biol. 2005;282:13–23. doi: 10.1002/ar.a.20143. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Matkovich SJ, Duan X, Gold JI, Koch WJ, Dorn GW., 2nd Nuclear effects of G-protein receptor kinase 5 on histone deacetylase 5-regulated gene transcription in heart failure. Circ Heart Fail. 2011;4:659–668. doi: 10.1161/CIRCHEARTFAILURE.111.962563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gold JI, Gao E, Shang X, Premont RT, Koch WJ. Determining the absolute requirement of G protein-coupled receptor kinase 5 for pathological cardiac hypertrophy: short communication. Circ Res. 2013;111:1048–1053. doi: 10.1161/CIRCRESAHA.112.273367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liggett SB, Cresci S, Kelly RJ, Syed FM, Matkovich SJ, Hahn HS, et al. A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14:510–517. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Figtree GA, Bagnall RD, Abdulla I, Buchholz S, Galougahi KK, Yan W, et al. No association of G-protein-coupled receptor kinase 5 or beta-adrenergic receptor polymorphisms with Takotsubo cardiomyopathy in a large Australian cohort. Eur J Heart Fail. 2013;15:730–733. doi: 10.1093/eurjhf/hft040. [DOI] [PubMed] [Google Scholar]
- 43.Kang S, Hong X, Ruan CW, Yu P, Yu SS, Chen M, et al. Effects of GRK5 and ADRB1 polymorphisms influence on systolic heart failure. J Transl Med. 2015;13:44. doi: 10.1186/s12967-015-0402-7. 10.1186/s12967-015-0402-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spinelli L, Trimarco V, Di Marino S, Marino M, Iaccarino G, Trimarco B. L41Q polymorphism of the G protein coupled receptor kinase 5 is associated with left ventricular apical ballooning syndrome. Eur J Heart Fail. 2010;12:13–16. doi: 10.1093/eurjhf/hfp173. [DOI] [PubMed] [Google Scholar]
- 45.Eijgelsheim M, Visser LE, Uitterlinden AG, Stricker BH. Protective effect of a GRK5 polymorphism on heart failure and its interaction with beta-adrenergic receptor antagonists. Pharmacogenomics. 2008;9:1551–1555. doi: 10.2217/14622416.9.10.1551. [DOI] [PubMed] [Google Scholar]
- 46.Kurnik D, Cunningham AJ, Sofowora GG, Kohli U, Li C, Friedman EA, et al. GRK5 Gln41Leu polymorphism is not associated with sensitivity to beta(1)-adrenergic blockade in humans. Pharmacogenomics. 2009;10:1581–1587. doi: 10.2217/pgs.09.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dzimiri N, Muiya P, Andres E, Al-Halees Z. Differential functional expression of human myocardial G protein receptor kinases in left ventricular cardiac diseases. Eur J Pharmacol. 2004;489:167–177. doi: 10.1016/j.ejphar.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Dzimiri N, Basco C, Moorji A, Afrane B, Al-Halees Z. Characterization of lymphocyte beta 2-adrenoceptor signalling in patients with left ventricular volume overload disease. Clin Exp Pharmacol Physiol. 2002;29:181–188. doi: 10.1046/j.1440-1681.2002.03625.x. [DOI] [PubMed] [Google Scholar]
- 49.Monto F, Oliver E, Vicente D, Rueda J, Aguero J, Almenar L, et al. Different expression of adrenoceptors and GRKs in the human myocardium depends on heart failure etiology and correlates to clinical variables. Am J Physiol Heart Circ Physiol. 2012;303:H368–H376. doi: 10.1152/ajpheart.01061.2011. [DOI] [PubMed] [Google Scholar]
- 50.Aguero J, Almenar L, Monto F, Oliver E, Sanchez-Lazaro I, Vicente D, et al. Myocardial G protein receptor-coupled kinase expression correlates with functional parameters and clinical severity in advanced heart failure. J Card Fail. 2012;18:53–61. doi: 10.1016/j.cardfail.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Aguero J, Almenar L, D'Ocon P, Oliver E, Monto F, Moro J, et al. Correlation between beta-adrenoceptors and G-protein-coupled receptor kinases in pretransplantation heart failure. Transplant Proc. 2008;40:3014–3016. doi: 10.1016/j.transproceed.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Aguero J, Almenar L, D'Ocon P, Oliver E, Monto F, Rueda J, et al. Myocardial and peripheral lymphocytic transcriptomic dissociation of beta-adrenoceptors and G protein-coupled receptor kinases in heart transplantation. J Heart Lung Transplant. 2009;28:1166–1171. doi: 10.1016/j.healun.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Keys JR, Zhou RH, Harris DM, Druckman CA, Eckhart AD. Vascular smooth muscle overexpression of G protein-coupled receptor kinase 5 elevates blood pressure, which segregates with sex and is dependent on Gi-mediated signaling. Circulation. 2005;112:1145–1153. doi: 10.1161/CIRCULATIONAHA.104.531657. [DOI] [PubMed] [Google Scholar]
- 54.Wu JH, Zhang L, Fanaroff AC, Cai X, Sharma KC, Brian L, et al. G protein-coupled receptor kinase-5 attenuates atherosclerosis by regulating receptor tyrosine kinases and 7-transmembrane receptors. Arterioscler Thromb Vasc Biol. 2012;32:308–316. doi: 10.1161/ATVBAHA.111.239608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hullmann JE, Grisanti LA, Makarewich CA, Gao E, Gold JI, Chuprun JK, et al. GRK5-Mediated Exacerbation of Pathological Cardiac Hypertrophy Involves Facilitation of Nuclear NFAT Activity. Circ Res. 2014;20:304475. doi: 10.1161/CIRCRESAHA.116.304475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parameswaran N, Pao CS, Leonhard KS, Kang DS, Kratz M, Ley SC, et al. Arrestin-2 and G protein-coupled receptor kinase 5 interact with NFkappaB1 p105 and negatively regulate lipopolysaccharide-stimulated ERK1/2 activation in macrophages. J Biol Chem. 2006;281:34159–34170. doi: 10.1074/jbc.M605376200. [DOI] [PubMed] [Google Scholar]
- 57.Patial S, Saini Y, Parvataneni S, Appledorn DM, Dorn GW, 2nd, Lapres JJ, et al. Myeloid-specific GPCR kinase-2 negatively regulates NF-kappaB1p105-ERK pathway and limits endotoxemic shock in mice. J Cell Physiol. 2011;226:627–637. doi: 10.1002/jcp.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patial S, Luo J, Porter KJ, Benovic JL, Parameswaran N. G-protein-coupled-receptor kinases mediate TNFalpha-induced NFkappaB signalling via direct interaction with and phosphorylation of IkappaBalpha. Biochem J. 2010;425:169–178. doi: 10.1042/BJ20090908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patial S, Shahi S, Saini Y, Lee T, Packiriswamy N, Appledorn DM, et al. G-protein coupled receptor kinase 5 mediates lipopolysaccharide-induced NFkappaB activation in primary macrophages and modulates inflammation in vivo in mice. J Cell Physiol. 2011;226:1323–1333. doi: 10.1002/jcp.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valanne S, Myllymaki H, Kallio J, Schmid MR, Kleino A, Murumagi A, et al. Genome-wide RNA interference in Drosophila cells identifies G protein-coupled receptor kinase 2 as a conserved regulator of NF-kappaB signaling. J Immunol. 2010;184:6188–6198. doi: 10.4049/jimmunol.1000261. [DOI] [PubMed] [Google Scholar]
- 61.Islam KN, Bae JW, Gao E, Koch WJ. Regulation of nuclear factor kappaB (NF-kappaB) in the nucleus of cardiomyocytes by G protein-coupled receptor kinase 5 (GRK5) J Biol Chem. 2013;288:35683–35689. doi: 10.1074/jbc.M113.529347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sorriento D, Ciccarelli M, Santulli G, Campanile A, Altobelli GG, Cimini V, et al. The G-protein-coupled receptor kinase 5 inhibits NFkappaB transcriptional activity by inducing nuclear accumulation of IkappaB alpha. Proc Natl Acad Sci U S A. 2008;105:17818–17823. doi: 10.1073/pnas.0804446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Islam KN, Koch WJ. Involvement of nuclear factor kappaB (NF-kappaB) signaling pathway in regulation of cardiac G protein-coupled receptor kinase 5 (GRK5) expression. J Biol Chem. 2012;287:12771–12778. doi: 10.1074/jbc.M111.324566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, et al. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest. 2013;123:37–45. doi: 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang CY, Yang AL, Lin YM, Wu FN, Lin JA, Chan YS, et al. Anti-apoptotic and prosurvival effects of exercise training on hypertensive hearts. 1985) 2012;112:883–891. doi: 10.1152/japplphysiol.00605.2011. [DOI] [PubMed] [Google Scholar]
- 67.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Wisloff U, Kemi OJ. Animal models in the study of exercise-induced cardiac hypertrophy. Physiol Res. 2010;59:633–644. doi: 10.33549/physiolres.931928. [DOI] [PubMed] [Google Scholar]
- 69.Traynham CJ, Cannavo A, Zhou Y, Vouga A, Woodall BP, Hullmann JE, et al. Differential Role of G Protein-Coupled Receptor Kinase 5 in Physiological Versus Pathological Cardiac Hypertrophy. Circ Res. 2015;29:306961. doi: 10.1161/CIRCRESAHA.115.306961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 71.Ibarra C, Estrada M, Carrasco L, Chiong M, Liberona JL, Cardenas C, et al. Insulin-like growth factor-1 induces an inositol 1,4,5-trisphosphate-dependent increase in nuclear and cytosolic calcium in cultured rat cardiac myocytes. J Biol Chem. 2004;279:7554–7565. doi: 10.1074/jbc.M311604200. [DOI] [PubMed] [Google Scholar]
- 72.McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J Biol Chem. 2004;279:4782–4793. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- 73.McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, et al. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci U S A. 2007;104:612–617. doi: 10.1073/pnas.0606663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Budiono BP, See Hoe LE, Peart JN, Sabapathy S, Ashton KJ, Haseler LJ, et al. Voluntary running in mice beneficially modulates myocardial ischemic tolerance, signaling kinases, and gene expression patterns. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1091–R1100. doi: 10.1152/ajpregu.00406.2011. [DOI] [PubMed] [Google Scholar]
- 75.Konhilas JP, Watson PA, Maass A, Boucek DM, Horn T, Stauffer BL, et al. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ Res. 2006;98:540–548. doi: 10.1161/01.RES.0000205766.97556.00. [DOI] [PubMed] [Google Scholar]
- 76.Moreira-Goncalves D, Henriques-Coelho T, Fonseca H, Ferreira RM, Amado F, Leite-Moreira A, et al. Moderate exercise training provides left ventricular tolerance to acute pressure overload. Am J Physiol Heart Circ Physiol. 2011;300:H1044–H1052. doi: 10.1152/ajpheart.01008.2010. [DOI] [PubMed] [Google Scholar]
- 77.Homan KT, Larimore KM, Elkins JM, Szklarz M, Knapp S, Tesmer JJ. Identification and structure-function analysis of subfamily selective g protein-coupled receptor kinase inhibitors. ACS Chem Biol. 2015;10:310–319. doi: 10.1021/cb5006323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schumacher-Bass SM, Traynham CJ, Koch WJ. G protein-coupled Receptor Kinase 2 as a Therapeutic Target for Heart Failure. Drug Discov Today Ther Strateg. 2012;9:e155–e162. doi: 10.1016/j.ddstr.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schumacher SM, Gao E, Zhu W, Chen X, Chuprun JK, Feldman AM, et al. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa0154. 277ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Homan KT, Wu E, Cannavo A, Koch WJ, Tesmer JJ. Identification and characterization of amlexanox as a G protein-coupled receptor kinase 5 inhibitor. Molecules. 2014;19:16937–16949. doi: 10.3390/molecules191016937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen M, Sato PY, Chuprun JK, Peroutka RJ, Otis NJ, Ibetti J, et al. Prodeath signaling of G protein-coupled receptor kinase 2 in cardiac myocytes after ischemic stress occurs via extracellular signal-regulated kinase-dependent heat shock protein 90-mediated mitochondrial targeting. Circ Res. 2013;112:1121–1134. doi: 10.1161/CIRCRESAHA.112.300754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sato PY, Chuprun JK, Ibetti J, Cannavo A, Drosatos K, Elrod JW, et al. GRK2 compromises cardiomyocyte mitochondrial function by diminishing fatty acid-mediated oxygen consumption and increasing superoxide levels. J Mol Cell Cardiol. 2015;89:360–364. doi: 10.1016/j.yjmcc.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]