Abstract
Background
Case reports suggest that children with food-triggered atopic dermatitis (AD) on elimination diets may develop immediate reactions upon accidental ingestion or reintroduction of an avoided food.
Objective
To systematically study the incidence and risk factors associated with these immediate reactions.
Methods
A retrospective chart review of 298 patients presenting to a tertiary-care allergy-immunology clinic based on concern for food-triggered AD was performed. Data regarding triggering foods, laboratory testing and clinical reactions were collected prospectively from the initial visit. Food-triggered AD was diagnosed by an allergist-immunologist with clinical evaluation and laboratory testing. We identified immediate reactions as any reaction to a food for which there was evidence of sIgE and for which patient developed timely allergic signs and symptoms. Differences between children with and without new immediate reaction were determined by Mann-Whitney, Chi-square, or Fisher’s exact test as appropriate.
Results
19% of patients with food-triggered AD and no previous history of immediate reactions developed new immediate food reactions after initiation of an elimination diet. Seventy percent of reactions were cutaneous but 30% were anaphylaxis. Cow’s milk and egg were the most common foods causing immediate-type reactions. Avoidance of a food was associated with increased risk of developing immediate reaction to that food (p<0.01). Risk was not related to specific IgE level nor a specific food.
Conclusion
A significant number of patients with food triggered atopic dermatitis may develop immediate type reactions. Strict elimination diets need to be thoughtfully prescribed as they may lead to decreased oral tolerance.
Keywords: atopic dermatitis, food allergy, elimination diets, anaphylaxis
Introduction
Atopic dermatitis (AD) is a common skin disease with an incidence between 10% to 30% in children (1–4). It is a multifactorial disease caused by a combination of genetic predisposition, impaired skin barrier function, and exposure to environmental triggers including allergens, irritants, and microorganisms. Foods have been shown to be a trigger in about 20–30% cases of moderate-to-severe AD (5–8). Food allergens are more likely to cause eczema in infants and children less than 5 years old whereas aeroallergens are more likely to cause eczema in older children and adults (9, 10). Ingestion of the offending agent can cause both immunoglobulin E (IgE)-mediated immediate-type reactions, such as urticarial, gastrointestinal, respiratory, or anaphylactic reactions, as well as non-IgE or mixed delayed type reactions such as eczema exacerbations that typically occur between 6–48 hours after ingestion (11, 12).
Food-triggered atopic dermatitis is diagnosed through a combination of clinical history and supportive laboratory work-up, including food specific IgE levels, skin-prick test (SPT), and oral food challenge (OFC), and is confirmed through improvement on an elimination diet (13). Ideally, optimal skin care should be performed prior to allergy evaluation as SPT and specific IgE both have low positive predictive values and sub-optimal skin care can confound the diagnosis (14, 15).
After diagnosis of food-triggered atopic dermatitis, patients are typically instructed to begin elimination diets of the offending agent (13). However, case reports have also suggested that after long periods of elimination diets, foods that were previously tolerated can cause immediate reactions including reactions as severe as anaphylaxis (16–18) and death (18). To our knowledge, no large-scale study has been performed to determine the incidence of the development of immediate reactions in children who previously had only delayed-type reactions. It is not yet clear what quantifiable risks are posed by elimination diets for children of this atopic predisposition. Understanding these risks will help determine the need for emergency action plans and prescription for injectable epinephrine. We aimed to determine the frequency and identify the characteristics of patients with food-triggered atopic dermatitis who developed immediate type reactions to food.
Methods
Subject Database
After Institutional Review Board approval, a retrospective chart review was performed. Data was collected from patient records from children who presented to the outpatient allergy-immunology clinic at the Ann and Robert H. Lurie Children’s Hospital of Chicago (formerly Children’s Memorial Hospital) between January 1, 2003 through June 30, 2010. Patient charts were identified using ICD9 codes for food allergy (693.1, 558.3 and 995.6) and for eczema/atopic dermatitis (692.9, 692, 373.31, 691.8, 690.12 and 698.3). Charts were reviewed and enrolled if there was concern in the history of present illness for food-triggered atopic dermatitis. Data was collected from the initial evaluation and subsequent follow-up visits. Demographics, SPT results, food specific IgE levels, reactions to food exposures, and epinephrine auto-injector prescription information were obtained from the initial visit. From subsequent visits, data collected included duration of follow-up from initial visit to final follow-up visit within the study period, eczema severity or improvement, development of reactions to foods, food specific IgE levels, foods that were being avoided, and results of OFC to determine immediate or delayed reactions.
Definitions
All patients with eczema met Hanifan and Reijka criteria for diagnosis (19). Based on practice recommendations, food-triggered AD was defined by positive clinical history of improvement of dermatitis upon food removal or worsening dermatitis upon introduction of causative food and supportive SPT (>3 mm wheal) or specific IgE (>0.35 kU/L) testing (20, 21) to the trigger food. Eczema unrelated to food was defined when either clinical history or laboratory testing were negative. Patients for whom clinical history or laboratory tests were supportive but evidence was lacking in the other category were considered equivocal and not included in further analysis.
Immediate reactions were defined by the timely development of typical signs and symptoms following ingestion of a food. Immediate reaction categories were: cutaneous (hives and non-life-threatening angioedema without other symptoms), gastrointestinal (vomiting, diarrhea, abdominal pain without other symptoms), or anaphylaxis (2 or more organ systems affected)/respiratory (lower airway symptoms such as wheezing). Anaphylaxis and respiratory reactions were grouped together as the most severe category.
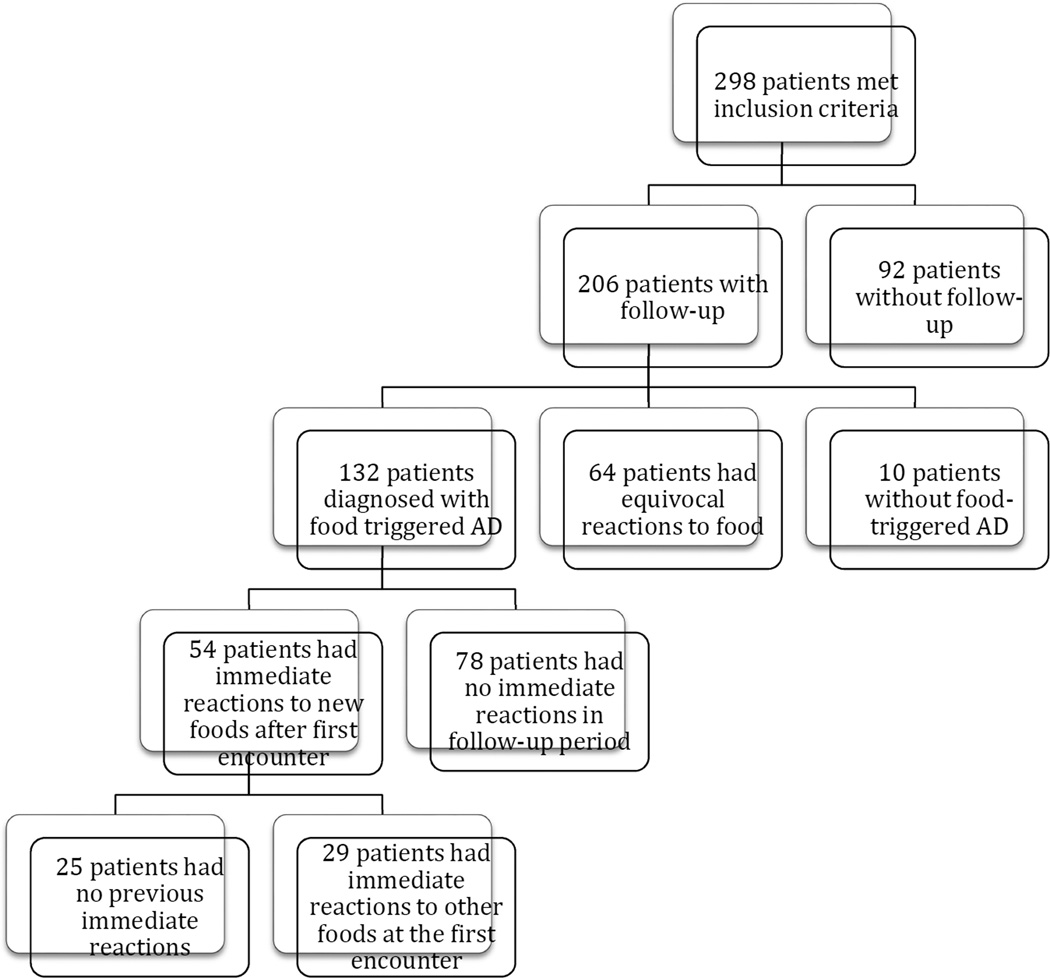
Patient groups are illustrated in figure 1. At the initial evaluation, patients with likely food-triggered AD were subcategorized into food-triggered AD only or food-triggered AD with concurrent immediate reactions to other foods. At follow-up, a new immediate reaction was defined as type I symptoms (cutaneous, gastrointestinal, or anaphylaxis, as defined above) to a food that the patient never previously had an immediate reaction to. Patients who had immediate reactions during the follow-up period were subcategorized into patients with immediate reactions without history of any immediate reactions at the initial evaluation, and patients with immediate reactions with history of immediate reaction at the initial evaluation.
Figure 1. Study Population.
Total number of patients meeting each clinical categoriztion is shown.
Reasons for patient avoidance of particular foods were obtained at interval visits during the follow-up period and categorized as follows: suspicion for AD trigger, previous immediate reaction to that food, previous positive test without prior ingestion, and prophylactic avoidance (no testing completed, but patient avoided the food). Patients were prescribed a diet eliminating a specific food, or in some cases, multiple foods, that were thought to be causing or exacerbating AD, as defined above. Patients were also advised to avoid a food if there was a clear clinical history of ingestion causing immediate reaction. Some younger children were advised to avoid a food if they had a positive SPT or specific IgE without a history of a clear reaction.
Statistical Methods
Mann-Whitney test was used to compare mean differences between 2 independent groups (i.e. patients with follow-up vs. patients without follow-up) when the dependent variable was numerical such as age. For categorical variables, we used a two-tailed Chi-square test or Fisher’s exact test, as appropriate. P<0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary NC).
Results
Two hundred ninety-eight patients met inclusion criteria for concern for food-triggered AD. Sixty-four percent of the cohort was male. The mean age at first encounter was 1.82 years (SD 1.63). The majority of the patients were Caucasian (54%) and had a family history of atopy (77%). Two hundred six (69%) patients had follow-up. Mean length of follow-up was 1.98 years (SD 1.64). Patients were more likely to follow-up if they had asthma (p=0.004) or allergic rhinitis (p=0.03). Demographics are shown in Table 1.
Table 1.
Demographics
| All patients | Patients with follow-up |
Patients without follow-up | p value | |
|---|---|---|---|---|
| Number | 298 | 206 (69%) | 92 (31%) | |
| Mean Age at first encounter* | 1.82 SD 1.63 | 1.67 SD 1.61 | 2.16 SD 1.65 | 0.0002 |
| Age Range at first encounter | 0–10.98 | 0–10.98 | 0.16–10.24 | |
| Males: Females | 192:106 (64% M) | 137:69 (66% M) | 55:37 (59.8% M) | 0.26 |
| Race: | ||||
| · White/Caucasian | 161 (54.0%) | 118 (57.0%) | 43 (46.7%) | 0.20 |
| · Black/AA | 29 (9.7%) | 18 (8.7%) | 11 (12.0%) | |
| · Hispanic/Latino | 36 (12.1%) | 19 (9.2%) | 17 (18.5%) | |
| · Asian/PI/I | 34 (11.3%) | 24 (11.7%) | 10 (10.9%) | |
| · Other | 3 (1.0%) | 3 (1.5%) | 0 | |
| · Unknown | 35 (11.7%) | 24 (11.6%) | 11 (12.0%) | |
| Family history of atopy | ||||
| · Yes | 230 (77.0%) | 159 (77.0%) | 71 (77.2%) | 0.17 |
| · No | 64 (21.5%) | 46 (22.3%) | 18 (20.0%) | |
| · Unknown | 4 (1.3%) | 1 (0.5%) | 3 (3.3%) | |
| Sibling with food allergy | ||||
| · Yes | 43 (14.0%) | 32 (15.5%) | 11 (12.0%) | 0.13 |
| · No | 251 (84.0%) | 173 (84.0%) | 78 (84.8%) | |
| · Unknown | 4 (1.3%) | 1 (0.5%) | 3 (3.3%) | |
| Personal history of asthma* | ||||
| · Yes | 104 (34.9%) | 83 (40.3%) | 21 (22.8%) | 0.0035 |
| · No | 194 (65.1%) | 123 (59.7%) | 71 (77.2%) | |
| Personal history of allergic rhinitis* | ||||
| · Yes | 108 (36.2%) | 83 (40.3%) | 25 (27.2%) | 0.0295 |
| · No | 190 (63.8%) | 123 (59.7%) | 67 (72.8%) | |
| Average follow-up length | --- | 1.98 SD 1.64 | --- | |
| (0.04 – 7.23) | ||||
p<0.05, comparing patients with and without follow-up
We first sought to determine the prevalence of suspected food triggered atopic dermatitis in children referred to the allergy clinic for evaluation of food-triggered atopic dermatitis. At the initial visit, 183 of 298 (61.4%) patients were diagnosed with probable food-triggered AD while 19 patients (6.4%) were thought to have eczema unrelated to food and 96 patients (32.2%) had eczema with an equivocal relation to food (Table 2). The average age of patients with likely food-triggered AD was significantly higher than those who had eczema that was not related to food, although both groups of children were less than 2 years old (1.96 years vs. 1.32 years, p=0.02). The most common foods attributed to eczema flares were milk (57.5% of patients), egg (30.6%), and soy (21.0%).
Table 2.
Initial visit data (n=298)
| Patients with eczema likely related to food | 183 (61.4%) | |
| Patients with eczema NOT related to food | 19 (6.4%) | |
| Patients with eczema and equivocal relation to food | 96 (32.2%) | |
| Average age of patients with eczema related to food (years) | 1.96 SD 1.65 | *p=0.02 |
| Average age of patients with eczema NOT related to food (years) | 1.32 SD 1.22 | |
| Average age of patients with eczema and equivocal relation to food (years) | 1.66 SD 1.67 | |
| Foods attributed to eczema flares | Milk: 105 (57.5% pts with food related eczema) | |
| Soy: 39 (21%) | ||
| Egg: 56 (30.6%) | ||
| Wheat: 22 (12.0%) | ||
| Fish: 5 (2.7%) | ||
| Shellfish: 6 (3.3%) | ||
| Peanut: 24 (13.1%) | ||
| Tree nut: 10 (5.5%) | ||
| Other: 60 (32.8%) | ||
| Patients who had immediate reactions documented at first visit | 112 (37.5%) | |
| Patients with cutaneous immediate reactions | 63 (56.2%) | |
| Patients with GI immediate reactions | 23 (20.5%) | |
| Patients with respiratory/anaphylaxis immediate reactions as the most severe reaction | 26 (23.2%) | |
| Patients given epinephrine auto-injectors | 228 (76.5%) | |
p<0.05, compared average age of patients with eczema related to food with average age of patients with eczema NOT related to food
At the initial visit, 112 of 298 (37.5%) patients had documented immediate reactions that were described as cutaneous (hives or angioedema) and did not include AD (56.2%), gastrointestinal (20.5%) and respiratory/anaphylaxis (23.2%) (Table 2). Patients with likely food-triggered AD with immediate reactions at the initial visit had a higher mean age of 2.35 years compared to those who only had likely food-triggered AD (p<0.01) and were more likely to have allergic rhinitis (p<0.01) (Table 3). Other demographic characteristics or trigger foods were not different between children with immediate reactions at diagnosis compared to those with food-triggered eczema only.
Table 3.
Risk factors for having only food related eczema vs immediate reactions at initial visit
| Food related eczema only |
Both food related eczema and immediate reactions |
p values | |
|---|---|---|---|
| Total number of patients | 95 | 88 | - |
| Race | |||
| · White/Caucasian | 49 (51.6%) | 49 (55.7%) | 0.95 |
| · Black/AA | 9 (9.5%) | 10 (11.4%) | |
| · Hispanic/Latino | 11 (11.6%) | 9 (10.2%) | |
| · Asian/PI/I | 11 (11.6%) | 10 (11.4%) | |
| · Other | 2 (2.1%) | 1 (1.1%) | |
| · Unknown | 13 (13.7%) | 9 (10.2%) | |
| Males: Females | 59:36 (62.1% M) | 57:31(64.8% M) | 0.71 |
| Mean age | 1.59 SD 1.51 | 2.35 SD 1.7 | <0.01 |
| Family history of atopy | |||
| · Yes | 72 (75.8%) | 70 (79.6%) | 0.8 |
| · No | 21 (22.1%) | 16 (18.2%) | |
| · Unknown | 2 (2.1%) | 2 (2.3%) | |
| Sibling with food allergy | |||
| · Yes | 16 (16.7%) | 6 (6.82%) | 0.11 |
| · No | 77 (81.0%) | 80 (90.9%) | |
| · Unknown | 2 (2.1%) | 2 (2.3%) | |
| Personal history of asthma | |||
| · Yes | 33 (34.7%) | 40 (45.5%) | 0.14 |
| · No | 62 (65.3%) | 48 (54.6%) | |
| Personal history of allergic rhinitis | |||
| · Yes | 27 (28.4%) | 44 (50%) | <0.01 |
| · No | 68 (71.6%) | 44 (50%) | |
| Foods causing immediate and delayed type reactions | Milk: 46 (28.8%) | Milk: 32 (24.6%) | |
| Soy: 17 (10.6%) | Soy: 6 (4.6%) | ||
| Egg: 31 (19.4%) | Egg: 28 (2.2%) | ||
| Wheat: 12 (7.5%) | Wheat: 5 (3.8%) | ||
| Fish: 3 (1.9%) | Fish: 8 (6.2%) | ||
| Shellfish: 3 (1.9%) | Shellfish: 2 (1.5%) | ||
| Peanut: 12 (7.5%) | Peanut: 22 (16.9%) | ||
| Tree nut: 4 (2.5%) | Tree nut: 7 (5.4%) | ||
| Other: 32 (20%) | Other: 20 (15.4%) | ||
Because it has been reported that some children with food-triggered AD can develop new type 1 immediate reactions, we next investigated the frequency and risk factors associated with the development of new type 1 immediate-type reactions during follow-up after diagnosis of food-triggered eczema. Of the 206 patients with follow-up, 132 of the patients were diagnosed with food triggered atopic dermatitis (Figure 1). Among 54 patients (40.9% of those with follow up, 18.1% of the total initial cohort) there were 60 immediate reactions upon accidental ingestion or oral food challenge in the clinic during the follow-up period (Table 4). Alarmingly, 25 patients (19.8% of these with follow up, 8.4% of the entire cohort) had no previous history of immediate reactions at their initial presentation, but developed a total of 31 immediate reactions during follow-up (Table 6, Table 7, Figure 1). Milk (n=21), egg (n=16), and peanut (n=9) caused the most immediate reactions in follow-up (Table 6). Of the 60 reactions that occurred during follow-up, 11 (18.3%) occurred to foods that patients were previously eating ad lib and 49 (81.7%) occurred to foods that patients were avoiding (p<0.001, Table 8).
Table 4.
Follow-up visit data (n=206)
| Patients with eczema likely related to food | 132 (64.1%) | |
| Patients with eczema NOT related to food | 10 (4.9%) | |
| Patients with eczema and equivocal relation to food | 64 (31.1%) | |
| Average age of patients with eczema related to food (years)* | 1.8 SD 1.5 | *p=0.02 |
| Average age of patients with eczema NOT related to food (years) | 1.0 SD 1.0 | |
| Average age of patients with eczema and equivocal relation to food (years) | 1.5 SD 1.8 | |
| Foods attributed to eczema flares | Milk: 70(53.0%) | |
| Soy: 22 (16.7%) | ||
| Egg: 38 (28.8%) | ||
| Wheat: 17 (12.9%) | ||
| Fish: 4 (3.0%) | ||
| Shellfish: 6 (4.5%) | ||
| Peanut: 18 (13.6%) | ||
| Tree nut: 7 (5.3%) | ||
| Other: 36 (27.2%) | ||
| Patients with immediate reactions documented at follow-up | 54 (40.9%) | |
| Total number of immediate reactions documented at follow-up | 60 | |
| Cutaneous immediate reactions | 41 (68.3%) | |
| GI immediate reactions | 5 (8.3%) | |
| Respiratory/anaphylaxis immediate reactions | 14 (23.3%) | |
p<0.05, compared average age of patients with eczema related to food with average age of patients with eczema NOT related to food
Table 6.
Patients with follow-up and food-triggered AD (n=132) who developed immediate reactions
| Patients with immediate reactions at follow up |
Patients with new immediate reactions without prior history of any immediate reactions |
Patients with immediate reactions with prior history of immediate reactions |
p values+ |
|
|---|---|---|---|---|
| All patients who developed immediate reaction | 54 (40.9%) | 25 (18.9%) | 29 (22.0%) | |
| · Patients with cutaneous reactions | 35 (64.8%) | 16 (64%) | 19 (64.3%) | 0.05 |
| · Patients with GI reactions | 5 (9.3%) | 0 | 5 (17.9%) | |
| · Patients with respiratory/anaphylaxis reactions | 14 (25.9%) | 9 (36%) | 5 (17.9%) | |
| Avoidance of food causing reaction | ||||
| · Not previously avoiding | 11 (20.4%) | 7 (28.0%) | 4 (13.8%) | <0.01 |
| · Previously avoiding | 43 (79.6%) | 18 (72.0%) | 25 (86.2%) | |
| Foods causing immediate reactions | ||||
| · Milk: | 21 (39%) | 9 (36%) | 12 (37.9%) | 0.88 |
| · Soy: | 1 (1.9%) | 0 | 1 (3.5%) | 1 |
| · Egg: | 16 (29.6%) | 7 (28%) | 9 (31%) | 0.81 |
| · Wheat: | 3 (5.6%) | 1 (4%) | 2 (6.7%) | 1 |
| · Fish: | 1 (1.9%) | 0 | 1 (3.5%) | 1 |
| · Shellfish: | 2 (3.7%) | 0 | 2 (6.9%) | 0.49 |
| · Peanut: | 9 (16.7%) | 5 (20%) | 4 (13.8%) | 0.54 |
| · Tree nut: | 4 (7.4%) | 1 (4%) | 3 (10.3%) | 0.61 |
| · Other: | 15 (27.8%) | 9 (36%) | 6 (20.7%) | 0.21 |
p value compares the group of patients with new immediate reactions without prior history of any immediate reactions vs. the group of patients with immediate reactions with prior history of immediate reactions
Table 7.
25 patients with food-triggered AD without a history of immediate reactions at the initial visit developed 31 new immediate reactions during follow-up
| n | Food causing immediate reaction |
Reaction severity | Average length of time to reaction |
% Food triggering AD |
|---|---|---|---|---|
| 9 | Cow’s milk | 4 Anaphylaxis | 1.0y (SD 0.4y) | 7/9 (78%) |
| 5 Cutaneous | ||||
| 7 | Egg | 2 Anaphylaxis | 1.1y (SD 0.8y) | 5/7 (71%) |
| 5 Cutaneous | ||||
| 5 | Peanut | 2 Anaphylaxis | 2.4y (SD 1.4y) | 2/5 (40%) |
| 3 Cutaneous | ||||
| 10 | Other | 1 Anaphylaxis | 1.7y (SD 0.6y) | 2/10 (20%) |
| 9 Cutaneous | ||||
Table 8.
Description of 54 patients with 60 immediate reactions during follow-up and description of previous tolerance and elimination reasons
| Avoiding | Reason for Eliminating from Diet | Previous Tolerance | Causative Food |
|---|---|---|---|
| No | 11 not eliminated | 11 eating ad lib | 8 other |
| 1 milk | |||
| 1 egg | |||
| 1 soy | |||
| Yes | 7 prophylactic avoidance | 7 never ingested | 3 peanut |
| 2 tree nut | |||
| 2 fish/shellfish | |||
| 14 previous type 1 reaction | 14 not eating at enrollment due to previous reaction | 7 milk | |
| 4 egg | |||
| 2 peanut | |||
| 1 other | |||
| 9 positive SPT only | 6 never ingested | 2 egg | |
| 2 milk | |||
| 1 peanut | |||
| 1 shellfish | |||
| 3 eating in breast milk, removed after testing | 2 egg | ||
| 1 wheat | |||
| 19 food triggered AD | 12 eating ad lib, removed for AD | 7 milk | |
| 3 egg | |||
| 2 other | |||
| 7 eating in breast milk, removed for AD | 3 egg | ||
| 2 milk | |||
| 1 peanut | |||
| 1 soy | |||
Given the frequency of type 1 reactions, we next sought to characterize the severity of these reactions and to describe differences in children with and without prior history of type 1 reactions. Of the 31 new immediate reactions seen in 25 patients without any prior history of any immediate reaction, 22 (70.0%) reactions were hives and 9 (30.0%) reactions were anaphylaxis (Table 7). A more detailed description of these children and the triggering food is listed (Supplemental Table 1). For this group, the mean time to reaction from the initial visit was 1.4 years (range 0.2–4.7 years). For children with new immediate reactions without a prior history of immediate reactions, the majority of reactions (77.4%) occurred to foods that the patients were avoiding. The ingestion history and reason for avoidance for all patients who developed immediate reactions in follow-up are listed in table 8. The severity of reaction and triggering foods were not different between children with a history of previous reaction compared to children without a history of previous reaction.
Finally, as development of immediate reactions was more common than suspected, we next sought to describe risk factors for the development of immediate reactions. There was no difference in race, sex, age, family history, or other atopic disease status, although, a personal history of asthma trended towards increased risk of immediate reaction at follow up (55.6% vs 39.7%, p <0.07, Table 5). Importantly, relative abundance of food triggers did not differ between children with and without immediate reactions, with milk, egg and peanut being the most common in both groups (Table 6). Avoidance of the food was associated with development of an immediate reaction (Table 6).
Table 5.
Patients with follow-up and food-triggered AD (n=132)
| Patients with immediate reactions |
Patients without immediate reactions |
p values | |
|---|---|---|---|
| Total number of patients | 54 | 78 | -- |
| Race | |||
| · White/Caucasian | 31 (57.4%) | 44 (56.4%) | 0.77 |
| · Black/AA | 6 (11.1%) | 6 (7.7%) | |
| · Hispanic/Latino | 5 (9.3%) | 8 (10.3%) | |
| · Asian/PI/I | 6 (11.1%) | 8 (10.3%) | |
| · Other | 2 (3.7%) | 1 (1.3%) | |
| · Unknown | 4 (7.4%) | 11 (14.1%) | |
| Males: Females | 36:18 (66.7% M) | 52:26(66.7% M) | 1 |
| Mean age | 1.69 (SD 1.5) | 1.87 (SD 1.54) | 0.4 |
| Family history of atopy | |||
| · Yes | 44 (81.5%) | 57 (73.1%) | 0.45 |
| · No | 10 (18.5%) | 20 (25.6%) | |
| · Unknown | 0 | 1 (1.3%) | |
| Sibling with food allergy | |||
| · Yes | 5 (9.3%) | 9 (11.5%) | 0.87 |
| · No | 49 (90.7%) | 68 (87.2%) | |
| · Unknown | 0 | 1 (1.3%) | |
| Personal history of asthma | |||
| · Yes | 30 (55.6%) | 31 (39.7%) | 0.07 |
| · No | 24 (44.4%) | 47 (60.3%) | |
| Personal history of allergic rhinitis | |||
| · Yes | 27 (50%) | 30 (38.5%) | 0.19 |
| · No | 27 (50%) | 48 (61.5%) | |
| Food causing immediate reactions | |||
| · Milk: | 21 (39%) | n/a | n/a |
| · Soy: | 1 (1.9%) | ||
| · Egg: | 16 (29.6%) | ||
| · Wheat: | 3 (5.6%) | ||
| · Fish: | 1 (1.9%) | ||
| · Shellfish: | 2 (3.7%) | ||
| · Peanut: | 9 (16.7%) | ||
| · Tree nut: | 4 (7.4%) | ||
| · Other: | 15 (27.8%) | ||
Discussion
Food allergy is an important etiology to consider in children with moderate-to-severe atopic dermatitis recalcitrant to typical topical therapy (5–8). Our study sought to determine the incidence of the development of immediate reactions in patients with food triggered atopic dermatitis and to identify risk factors for the development of type 1 food allergy. With regards to the diagnosis of food-triggered AD, of the 298 children with atopic dermatitis who were referred for suspicion of food allergy, 183 (61%) were diagnosed with food-triggered atopic dermatitis. The high incidence in our population compared to approximately 30% previously reported (5, 6) is likely due to the broad inclusion criteria used in our study. We selected charts using ICD-0 codes for food allergy and atopic dermatitis, rather than selecting for moderate-to-severe eczema only.
For patients diagnosed with food triggered AD, clinical practice varies with regards to injectable epinephrine prescription, emergency action plan distribution, and even avoidance strategies. Therefore, we next attempted to determine which children were at risk for developing type 1 immediate reactions to foods which patients were previously avoiding or instructed to eliminate after the initial visit. Surprisingly, our study determined that 18.9% of patients with likely food triggered atopic dermatitis and no prior immediate reactions developed immediate reactions during the time they were followed for their atopic dermatitis. The most common foods that triggered immediate reactions (milk and egg) were also the most common foods that triggered AD. Thus, one type of food is not more likely to cause immediate reactions than others. Additionally, new immediate reactions were as severe as anaphylaxis in close to one-third of patients. Therefore, once a diagnosis of food triggered AD is made, our study suggests that follow-up should be arranged shortly after to ensure that the elimination diet is effective. If it is deemed effective, then the patient should be monitored at least annually for repeat IgE testing and food re-introduction, when appropriate, should occur in a monitored setting. Given the risk for the development of a type 1 reaction upon re-introduction, or with accidental ingestion, these patients may benefit from food allergy emergency action plans and injectable epinephrine. If elimination diet is not deemed effective, our data suggest that the food or foods should be reintroduced into the diet rather than avoided.
Food avoidance is clearly a risk factor for developing immediate reactions to food, as 24 of 31 new reactions (77.4%) occurred to foods that patients were avoiding (p<0.01). Our study suggests that complete avoidance may not be the best management strategy in high-risk children even with food triggered AD. Reactions occurred across a range of sIgE levels. In a case series, Flinterman et al (16) documented 11 patients who had initially tolerated cow’s milk without developing immediate reactions and who were prescribed elimination diets for suspected cow’s milk triggered atopic dermatitis. Upon re-introduction in a subsequent double-blind placebo-controlled cow’s milk challenge, all of the patients developed immediate reactions. There have also been other case series of patients who developed immediate reactions following long periods of elimination diets (17, 18). Our findings are consistent with the results from the recent LEAP study, which found that early introduction and frequent ingestion of peanuts decreased the development of peanut allergy among atopic children (22).
As children with AD are often significantly atopic, differentiating food sensitization (due to their atopic predisposition) from true food allergy is crucial. Exclusion diets need to be thoughtfully prescribed as they can inadvertently lead to loss of tolerance of foods and increase the risk of immediate reactions. Although this is a decision shared between providers and families, providers should be aware of the pitfalls associated with elimination diets so that proper anticipatory guidance may be provided. The risk of an immediate reaction must always be considered when re-introducing a food that had been eliminated as a treatment for a child’s atopic dermatitis. These patients can develop new immediate reactions at any time. These patients will continue to require follow-up as we continue to identify which patients are at risk, and may benefit from dietary inclusion of small amounts of food that are tolerated. Further prospective research in these areas is required to clarify these questions.
Consistent with prior studies milk, egg, and peanut were the most common foods contributing to AD (7, 13). These foods were also the most common causes of immediate reactions. Thus, it does not appear that any one food is more likely to result in an immediate reaction compared to food triggered AD, and this possibility should be considered regardless of implicated foods.
The study has limitations as a retrospective chart review and lacks a case control group of atopic dermatitis patients without dietary restrictions. Practices have evolved since 2003–2010, the period from which charts were reviewed. New guidelines for the management of food allergy and atopic dermatitis have resulted in changes, including performing more oral food challenges for diagnosis of food-triggered AD (21, 25). Families are no longer instructed to prophylactically avoid other common allergenic foods simply because they have had an immediate reaction to another food. There has also been an evolution in practice in regards to the introduction of baked or extensively heated forms of foods such as milk and egg, which if tolerated, may provide a path towards tolerance of unheated forms of these foods in some individuals (26). There is also a possible referral bias given that the study was performed at a tertiary care referral center that frequently manages food allergy, including severe cases, and may explain why many children were diagnosed with food-triggered AD and developed immediate reactions.
Future prospective studies are required to determine if keeping tolerable amounts of allergenic foods in the diet of children with food-triggered atopic dermatitis would decrease the development of immediate reactions. Additionally, studies to investigate the underlying mechanism that causes some patients to change from a delayed type to an immediate of IgE-mediated Type 1 reaction would be informative.
In this large-scale study, we have tried to determine the incidence of development of immediate food reactions in children previously avoiding foods due to atopic dermatitis. We found that 18.9% of children with no previous history of any immediate reaction developed a new immediate reaction to food. One clear risk factor for developing immediate reactions was avoidance of the culprit food. Careful consideration is necessary before prescribing strict elimination diets which may increase the likelihood of developing immediate reactions in the future, and patients may benefit from keeping tolerable amounts of a triggering food in their diet. Finally, our data also suggest that patients with food-triggered AD warrant an emergency action plan and self-injectable epinephrine.
Supplementary Material
Highlights Box.
-
What is already known about this topic?
Food allergy is a known trigger in 20–30% of patients with moderate-to-severe atopic dermatitis. Elimination diets are often instituted once the diagnosis is made in order to improve the disease course.
-
What does this article add to our knowledge?
The risk of developing immediate type reactions in children avoiding food due to (food-triggered) atopic dermatitis has previously not been systematically studied. Here, we report that approximately 19% of patients may develop type 1 reactions, suggesting that these patients must be followed closely.
-
How does this study impact current management guidelines?
Strict elimination diets for children with food-triggered atopic dermatitis should be recommended with caution, as these children may develop type I IgE-mediated food reactions. Close follow-up of response to diet elimination vs inclusion and monitoring for type 1 IgE-mediated reactions is essential. Future studies of the possibility of keeping small amounts of the offending agent in the diet as tolerated may help clarify risk factors in the development of new IgE-mediated food allergies.
Acknowledgements
AMS is funded in part by:
NIAID K23 100995, Thrasher Pediatric Fund, Blowitz-Ridgeway Respiratory Health Association Award, Bunning Food Allergy Initiative
Abbreviations
- AD
Atopic dermatitis
- SPT
Skin prick test
- OFC
Oral food challenge
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Angela Chang, Email: angelalinchang@gmail.com.
Rachel Robison, Email: rrobison@luriechildrens.org.
Miao Cai, Email: cindycm51@msn.com.
Anne Marie Singh, Email: anne-singh@northwestern.edu.
References
- 1.Kvenshagen B, Jacobsen M, Halvorsen R. Atopic dermatitis in premature and term children. Archives of Disease in Childhood. 2009;94:202–205. doi: 10.1136/adc.2008.142869. [DOI] [PubMed] [Google Scholar]
- 2.Beasley R The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351:1225–1232. [PubMed] [Google Scholar]
- 3.Roduit C, Frei R, Loss G, Buchele G, Weber J, Depner M, et al. Development of atopic dermatitis according to age of onset and association with early-life exposures. The Journal of Allergy and Clinical Immunology. 2012;130(1):130–136 e5. doi: 10.1016/j.jaci.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 4.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children's Health. The Journal of Investigative Dermatology. 2011;131(1):67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forbes LR, Saltzman RW, Spergel JM. Food allergies and atopic dermatitis: Differentiating Myth from Reality. Pediatric Annals. 2009;38(2):84–90. doi: 10.3928/00904481-20090201-05. [DOI] [PubMed] [Google Scholar]
- 6.Eigenmann P, Sicherer S, Borkowski T, Cohen B, Sampson H. Prevalence of IgE-Mediated Food Allergy Among Children With Atopic Dermatitis. Pediatrics. 1998;101(3):E8. doi: 10.1542/peds.101.3.e8. [DOI] [PubMed] [Google Scholar]
- 7.Eigenmann PC, Calza AM. Diagnosis of IgE-mediated food allergy among Swiss children with atopic dermatitis. Pediatric Allergy and Immunology. 2000;11:95–100. doi: 10.1034/j.1399-3038.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- 8.Burks AW, James JM, Hiegel A, Wilson G, Wheeler JG, Jones SM, et al. Atopic dermatitis and food hypersensitivity reactions. The Journal of Pediatrics. 1998;132(1) doi: 10.1016/s0022-3476(98)70498-6. [DOI] [PubMed] [Google Scholar]
- 9.Werfel T, Bruer K. Role of food allergy in atopic dermatitis. Cur Opin Allergy Clin Immunol. 2004;4:379–385. doi: 10.1097/00130832-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Eller E, Kjaer HF, Host A, Andersen KE, Bindslev-Jensen C. Food allergy and food sensitization in early childhood: results from the DARC cohort. Allergy. 2009;64(7):1023–1029. doi: 10.1111/j.1398-9995.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- 11.Niggemann B, Reibel S, Roehr CC, Felger D, Ziegert M, Sommerfeld C, et al. Predictors of positive food challenge outcome in non-IgE-mediated reactions to food in children with atopic dermatitis. The Journal of Allergy and Clinical Immunology. 2001;108(6):1053–1058. doi: 10.1067/mai.2001.120192. [DOI] [PubMed] [Google Scholar]
- 12.Breuer K, Heratizadeh A, Wulf A, Baumannw UC A, Tetau D, Kapp A, et al. Late eczematous reactions to food in children with atopic dermatitis. Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology. 2004;34(5):817–824. doi: 10.1111/j.1365-2222.2004.1953.x. [DOI] [PubMed] [Google Scholar]
- 13.Sicherer SH, Sampson HA. Food Hypersensitivity and atopic dermatitis: Pathophysiology, epidemiology, diagnosis, and management. The Journal of Allergy and Clinical Immunology. 1999;104(3) doi: 10.1016/s0091-6749(99)70053-9. [DOI] [PubMed] [Google Scholar]
- 14.Bergmann MM, Caubet JC, Boguniewicz M, Eigenmann PA. Evaluation of food allergy in patients with atopic dermatitis. The Journal of Allergy and Clinical Immunology: In Practice. 2013;1(1):22–28. doi: 10.1016/j.jaip.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Sampson HA. The evaluation and management of food allergy in atopic dermatitis. Clinics in Dermatology. 2003;21(3):183–192. doi: 10.1016/s0738-081x(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 16.Flinterman AE, Knulst AC, Meijer Y, Bruijnzeel-Koomen CA, Pasmans SG. Acute allergic reactions in children with AEDS after prolonged cow's milk elimination diets. Allergy. 2006;61(3):370–374. doi: 10.1111/j.1398-9995.2006.01018.x. [DOI] [PubMed] [Google Scholar]
- 17.David TJ. Anaphylactic shock during elimination diets for severe atopice czema. Archives of Disease in Childhood. 1984;59:983–986. doi: 10.1136/adc.59.10.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbi E, Gerarduzzi T, Longo G, Ventura A. Fatal Allergy as a possible consequence of long-term elimination diet. Allergy. 2004;59:668–669. doi: 10.1111/j.1398-9995.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 19.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermato-Venereologica. 1980;92(suppl):44–47. [Google Scholar]
- 20.Lio PA, Lee M, LeBovidge J, Timmons KG, Schneider L. Clinical management of atopic dermatitis: practical highlights and updates from the atopic dermatitis practice parameter 2012. The Journal of Allergy and Clinical Immunology: In Practice. 2014;2(4):361–369. doi: 10.1016/j.jaip.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Schneider L, Tilles S, Lio P, Boguniewicz M, Beck L, LeBovidge J, et al. Atopic dermatitis: a practice parameter update 2012. The Journal of Allergy and Clinical Immunology. 2013;131(2):295–299. e1–e27. doi: 10.1016/j.jaci.2012.12.672. [DOI] [PubMed] [Google Scholar]
- 22.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. The New England Journal of Medicine. 2015;372(9):803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohle B. The impact of pollen-related food allergens on pollen allergy. Allergy. 2007;62(1):3–10. doi: 10.1111/j.1398-9995.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- 24.Breuer K, Wulf A, Constien A, Tetau D, Kapp A, Werfel T. Birch pollen-related food as a provocation factor of allergic symptoms in children with atopic eczema/dermatitis syndrome. Allergy. 2004;59(9):998–994. doi: 10.1111/j.1398-9995.2004.00493.x. [DOI] [PubMed] [Google Scholar]
- 25.Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. The Journal of Allergy and Clinical Immunology. 2010;126(6 Suppl):S1–S58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JS, Nowak-Wegrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow's milk allergy in children. The Journal of Allergy and Clinical Immunology. 2011;128(1):125–131 e2. doi: 10.1016/j.jaci.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.