Abstract
Moderate stress can increase lifespan by hormesis, a beneficial low-level induction of stress response pathways. 5’-fluorodeoxyuridine (FUdR) is commonly used to sterilize Caenorhabditis elegans in aging experiments. However, FUdR alters lifespan in some genotypes and induces resistance to thermal and proteotoxic stress. We report that hypertonic stress in combination with FUdR treatment or inhibition of the FUdR target thymidylate synthase, TYMS-1, extends C. elegans lifespan by up to 30%. By contrast, in the absence of FUdR, hypertonic stress decreases lifespan. Adaptation to hypertonic stress requires diminished Notch signaling and loss of Notch co-ligands leads to lifespan extension only in combination with FUdR. Either FUdR treatment or TYMS-1 loss induced resistance to acute hypertonic stress, anoxia, and thermal stress. FUdR treatment increased expression of DAF-16 FOXO and the osmolyte biosynthesis enzyme GPDH-1. FUdR-induced hypertonic stress resistance was partially dependent on sirtuins and base excision repair (BER) pathways, while FUdR-induced lifespan extension under hypertonic stress conditions requires DAF-16, BER, and sirtuin function. Combined, these results demonstrate that FUdR, through inhibition of TYMS-1, activates stress response pathways in somatic tissues to confer hormetic resistance to acute and chronic stress. C. elegans lifespan studies using FUdR may need re-interpretation in light of this work.
Keywords: C. elegans, FUdR, hypertonic stress, FOXO, sirtuin, hormesis
1. Introduction
Moderate stress results in adaptive changes that alter physiology, behavior, and longevity. Numerous genes and pathways impact both stress resistance and lifespan. The relationships between these genes and pathways are likely complex; disentangling their interactions is critical for understanding their roles outside of the laboratory. Here, as an example demonstrating these complexities, we examine interactions between hypertonic stress and FUdR-induced DNA damage, and their impact on stress resistance and lifespan in C. elegans.
Stress is a double-edged sword. High levels of stress result in early death, unless damage is repaired. However, low levels of stress are beneficial in specific contexts, leading to dose- and context-dependent hormetic effects. For example, 5’-fluorodeoxyuridine (FUdR), which is commonly used to sterilize self-fertile C. elegans for aging experiments, has a negligible impact on C. elegans lifespan under standard conditions (Mitchell et al., 1979). Several laboratories have reported that FUdR has hormetic effects on C. elegans lifespan in specific genetic backgrounds/culture conditions (Aitlhadj and Stürzenbaum, 2010; Angeli et al., 2013; Mitchell et al., 1979; Van Raamsdonk and Hekimi, 2011). Additionally, FUdR treatment increases C. elegans resistance to environmental and cellular stressors, but the site of FUdR action is disputed and the precise mechanism of FUdR action is unclear (Angeli et al., 2013; Feldman et al., 2014; Mendenhall et al., 2009; Miyata et al., 2008; Rooney et al., 2014).
Like other animals, C. elegans can adapt to hypertonic stress, but this adaptation decreases lifespan under standard culture conditions (Dmitrieva and Burg, 2007). Standard nematode growth medium (NGM) contains 51 mM NaCl as the major osmolyte. Acute exposure to high osmolarity (>400 mM NaCl) is harmful and results in flaccidity, locomotion cessation, and increased internal glycerol (Lamitina et al., 2004) or death. If animals adapt to hypertonic stress, spontaneous locomotion resumes and glycerol concentrations plateau within hours. Exposure of C. elegans to moderate osmotic stress (200 to 300 mM NaCl) is sufficient to induce these adaptive changes, although animals have decreased brood sizes and shorter lifespan (Dmitrieva and Burg, 2007). However, a recent report suggests lifespan is extended by hypertonic stress (Chandler-Brown et al., 2015). The mechanisms by which hypertonic stress impacts lifespan are not clear, but Burg and other suggest these mechanisms may include deleterious effects including double-stranded breaks in DNA (Dmitrieva et al., 2011) and protein misfolding (Burkewitz et al., 2011).
Notch signaling plays a poorly understood role in C. elegans hypertonic stress response. Under normal culture conditions, the activity of Notch co-ligands OSM-7 or OSM-11 prevents inappropriate hypertonic adaptation (Wheeler and Thomas, 2006). Loss of OSM-7 or OSM-11 results in full hypertonic adaptation, including increased glycerol biosynthesis and locomotion, even in the absence of hypertonic stress. Loss of the GLP-1 Notch receptor induces long-term hypertonic stress resistance, though this resistance may be due to germ cell proliferation defects or somatic roles of GLP-1 (Shi and Murphy, 2014).
FOXO family transcription factors are important players in stress resistance. C. elegans FOXO DAF-16 is critical for dietary restriction-induced stress resistance, increased longevity, and UV-induced hormesis (Greer et al., 2007; Zhou et al., 2011). DAF-16 also plays a poorly understood role in C. elegans survival after 24 hours of hypertonic stress (Lamitina and Strange, 2005). Under stress, DAF-16 and sirtuin deacetylases can combine with 14-3-3 proteins, and translocate to the nucleus as a ternary complex resulting in transcriptional activation (Zhou et al., 2011). Sirtuins have been implicated in DNA damage response and some sirtuin family members are actively recruited to sites of DNA damage (Greiss et al., 2008). Roles for sirtuins in stress response and autophagy induction have been established in several organisms; however the requirement for sirtuin activity in extension varies between experimental paradigms (Guarente, 2013; Morselli et al., 2010).
As an exemplar of the complex interactions between stress and lifespan pathways, here we examine the impact of Notch signaling, sirtuins, and DAF-16, on hypertonic stress response and adaptation. We find that FUdR treatment confers hypertonic stress resistance and lifespan extension in the presence of moderate osmotic stress by acting in somatic tissues. We establish that FUdR inhibition of thymidylate synthase is responsible for these adaptive changes and that FUdR treatment activates the C. elegans base-excision repair (BER) DNA repair pathway leading to acute stress resistance and longer lifespan.
2. Materials and methods
2.1 C. elegans strains and culture
All strains were cultured on NGM agar plates, containing 51 mM NaCl, at 15–25 °C. The following strains were used: N2, BS3162 glp-1(ar202) III, CF1038 daf-16(mu86) I, GE24 pha-1(e2123) III, HA1019 osm-11(rt142) X, HA1857 osm-7(tm2256) III, HA1863 osm-7(tm2256) III; osm-11(rt142) X, HA2040 sir-2.4(n5137) I; sir-2.1(ok434) IV; sir-2.2(n5136) X, HA2041 sir-2.4(n5137) I; sir-2.1(ok434) IV; sir-2.3(ok444) X, HA2104 daf-16(mu86) I; osm-7(tm2256) III; osm-11(rt142) X, HA2269 sir-2.2(n5136) X, HA2270 sir-2.4(n5137) I, HA2513 nth-1(ok724) III, HA2604 sir-2.1(ok434) IV, HA2694 sir-2.3(ok444) X, LG394 geIs3[sir-2.1(OE), rol-6], LG398 geIs101[rol-6], MT688 lin-12(n137)/unc-32(e189)III; him-5(e1467)V, SS149 mes-1(bn7) X, TJ356 zIs356[daf-16::GFP, rol-6], DA2123 adIs2122[lgg-1::GFP], VP198 kbIs5[gpdh-1::GFP, rol-6]. LG394 sir-2.1(OE) and LG398 were back-crossed to remove background mutations that impacted lifespan (Viswanathan and Guarente, 2011). HA2269 and HA2270 were 6x backcrossed to the laboratory N2 stock from alleles obtained from the Horvitz laboratory. HA2513 was obtained from RB877 by backcrossing 4 times to N2. HA2604 and HA2694 were obtained from VC199 and RB654, respectively, by backcrossing 6 times to N2.
2.2 Aging experiments
Aging experiments were conducted in 6cm plates containing 10mL standard NGM with 50 or 300 mM NaCl, or other osmolytes as indicated. OP50 was grown in standard LB media and concentrated 5x. Plates were seeded with 200 µL concentrated OP50 containing 0 or 20 mM FUdR, for a final concentration of 400 µM, unless otherwise indicated. Spreading FUdR on NGM plates prior to seeding with OP50 produced effects comparable to adding FUdR with OP50 (data not shown). Where dead bacteria were used as a food source, OP50 was killed by UV exposure and 50 µg/mL kanamycin. Additionally, in experiments using glycerol or sorbitol as the major osmolyte, 50 µg/mL nystatin was added to prevent mold growth. Plates were stored in humidified boxes prior to and during experiments to minimize evaporation and maintain consistent osmolarity.
Synchronous populations were obtained by sodium hypochlorite treatment of gravid hermaphrodites to obtain eggs that were then raised on standard NGM plates. Late L4 or young adult virgin hermaphrodites were transferred to NGM plates with the indicated osmolarity and FUdR concentration, marking day 1 of adult, and survival was scored every 2–3 days. For experiments that included groups with no FUdR, animals in all experimental groups were transferred to fresh plates every other day while any were fertile. Survival was scored at each transfer and every other day for the duration of the experiment. Approximately once per week, or whenever plates had visible evidence of drying, worms were transferred to fresh plates. Animals that were not spontaneously moving and did not respond to gentle prodding were scored as dead. Animals were censored when they died from causes other than age, including internal progeny hatching, extrusion of internal organs, crawling off the agar and dehydrating, or when there was bacterial or fungal contamination.
Kaplan-Meier survival curves and restricted mean lifespans were calculated with package ‘survival’ in R 3.0.2. The log-rank test was used to compare differences in survival between all pairs of groups. Experiment-wise error rate was controlled with the Bonferroni-Holm multiple comparison adjustment.
2.3 Stress assays
Animals were raised on standard NGM at 20 or 25°C and young adults were transferred to RNAi plates and allowed to feed for 48 hours at 20°C prior to stress resistance assays. For FUdR treatment alone, young adult animals were transferred to NGM plates containing 400 µM FUdR and seeded with OP50 for 48 hours at 20°C. RNAi NGM plates were prepared with 50 µg/mL ampicillin, 1 mM IPTG, and 0 or 400 µM FUdR, and seeded with HT115 E. coli containing the indicated RNAi constructs one day before the experiment. The RNAi constructs are osm-11(RNAi) described previously, and ORFeome-RNAi library clone Y110A7A.4 tyms-1(RNAi) (Rual et al., 2004).
Hypertonic stress resistance was measured by transferring animals to NGM plates containing 500 mM NaCl. Animals that moved spontaneously or in response to prodding after 10 minutes were scored as resistant to hypertonic stress. Some non-motile animals subsequently adapt to hypertonic stress. To measure adaptive stress resistance, hypertonic stress resistance was assayed approximately 24 hours after transfer to 500 mM NaCl. For resistance to anoxia, plates were placed in anaerobic Bio-Bag Type A environmental chambers which maintain < 0.1% oxygen concentration, and survival was measured after 24 hours. For thermotolerance, plates were placed at 35° C and survival was measured after 12 hours. Each assay was performed with three or more replicates, and presented here as an average of all assays. Differences in mean survival or resistance were compared with Student’s t-test.
2.4 Transgenic animal generation
To obtain transgenic animals, pha-1(+), dpy-30p::tyms-1(RNAi) or dpy-30p::empty, and elt-2p::GFP plasmids were injected into the gonads of pha-1(e2123) hermaphrodites. Injected animals were raised at 25°C and F1 progeny containing transgenic constructs were used in stress assays. dpy30p::empty was created by cloning 681bp of the dpy-30 promoter sequence into the XmaI and MscI sites of pPD49.26. dpy-30p::tyms-1(RNAi) was created by PCR amplifying the tyms-1 cDNA with primers CCGCggtaccTGGAAGTCATGAACAAAGAAAATATCATCG, containing an added KpnI site, and CGCGCCgctagcAAACAGCCATATCCATTGGGATTTTTG, containing an added NheI site, and inserted into the NheI-KpnI sites of dpy30p::empty.
2.5 Microscopy
Young adult animals were transferred to NGM plates containing 50 or 300 mM NaCl and 0 or 400 µM FUdR for 48 hours. After treatment, animals were mounted on an agarose pad and paralyzed for 5 minutes with 0.03% sodium azide. Images of GFP fluorescence were obtained on a Zeiss AxioImager M2 compound microscope, using 3ms exposures and identical settings for all images in a set. Fluorescent intensity was quantified in a uniform area of the anterior and posterior intestine for each image, using Axiovision 4.8 software. Differences in mean fluorescence intensity were compared with Student’s t-test. LGG-1::GFP puncta were counted in seam cells of 2-day old adults. Differences in numbers of puncta per cell were compared with the Wilcoxon rank-sum test.
3. Results
3.1 C. elegans lifespan extension under hypertonic stress requires FUdR
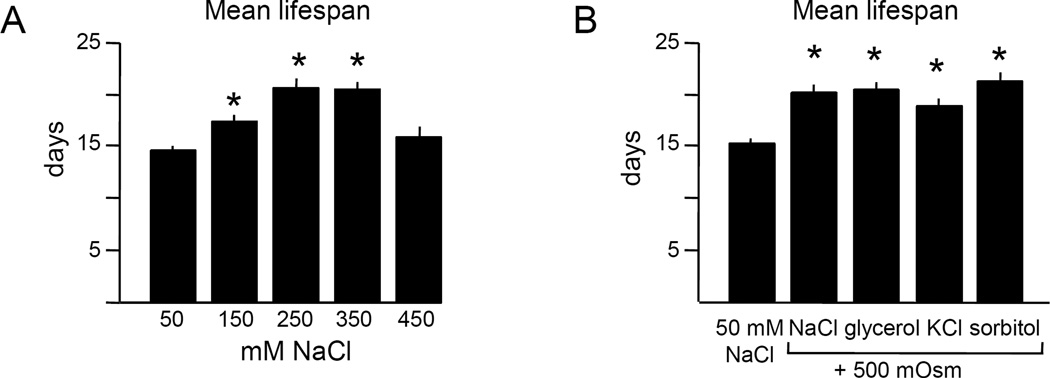
Hypertonic stress can increase lifespan in yeast and C. elegans (Chandler-Brown et al., 2015; Kaeberlein et al., 2002). We examined lifespan under hypertonic stress in adult C. elegans using the standard N2 laboratory strain (referred to as “wild type”), initially with FUdR to prevent progeny growth. Under these conditions, increasing NaCl concentration to 250 or 350 mM maximally increased lifespan (30–40%, Fig. 1A, S1, see Table S1 for summary and statistical analysis of all lifespan experiments). Lifespan was extended by similar amounts by other osmolytes (Fig. 1B), indicating that increased osmolarity was beneficial. We concluded that when FUdR is present, moderate hypertonic stress extends C. elegans lifespan.
Figure 1. Hypertonic stress extends lifespan in the presence of FUdR.
A. Hypertonic stress can extend lifespan at some NaCl concentrations. Restricted mean lifespans for survival curves shown in panel A. Maximal extension was observed at 250 or 350 mM NaCl in the presence of FUdR. Restricted mean lifespans for each group are shown, with n = 48 to 69 animals per condition.
B. Lifespan extension is independent of osmolyte. Osmotic equivalents of NaCl, KCl, glycerol, or sorbitol extend lifespan equivalently in the presence of FUdR. n = 60 to 106 animals per condition.
All experiments were performed at 25°C with 400 µM FUdR.
Error bars indicate S.E.M., * indicates p<0.0001 in log rank test vs. 50 mM NaCl control.
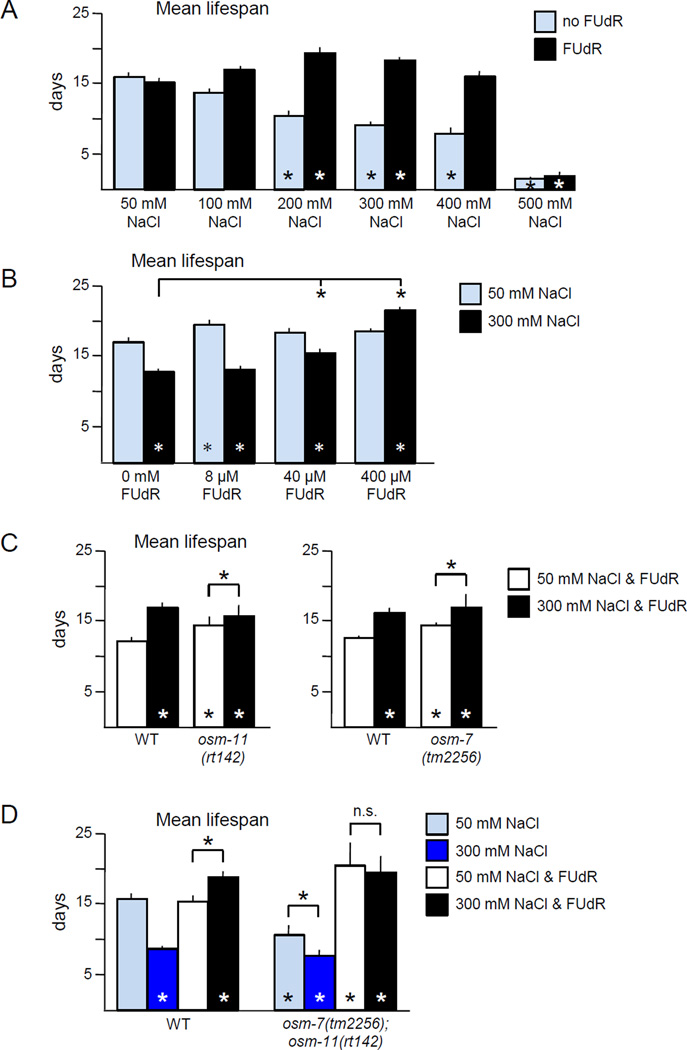
The increased lifespan caused by high concentration NaCl reported here was unexpected given the 50% lifespan reduction reported by Dmitrieva and Burg (2007) and the 35% reduction reported by Chandler-Brown et al. (2015). Two differences between the lifespan protocols were identified: temperature of rearing and use of FUdR. Moderate hypertonicity increased lifespan at 20°C and 25°C, ruling out differences in temperature (Table S1). We found that FUdR altered the lifespan of C. elegans under hypertonic stress. In the absence of FUdR, moderate hypertonic stress (300 mM NaCl) resulted in a 50% decrease in lifespan (Fig. 2A). The effect of hypertonic stress was dose dependent; mean lifespan decreased as osmolarity increased (Fig. 2A). These results were consistent with previous reports of reduced lifespan by 350 mM NaCl (Dmitrieva and Burg, 2007). However, in the presence of FUdR, 300 mM NaCl resulted in a 20%–25% increase in the lifespan of wild type C. elegans (Fig. 2A). Osmotic equivalents of KCl, glycerol, and sorbitol reduced or had no effect on lifespan in the absence of FUdR, but significantly extend lifespan in the presence of FUdR (Fig. S2) consistent with previous observations of lifespan extension by sorbitol in the presence of FUdR (Chandler-Brown et al., 2015). Introduction of FUdR did not extend lifespan when NaCl concentration was excessive (Fig. 2A).
Figure 2. Hypertonic stress lifespan extension is dependent on the concentration of FUdR.
A. Increasing hypertonicity decreased lifespan, but the addition of FUdR in combination with moderate hypertonic stress increased lifespan. Pooled analysis of two independent replicates at 20° C with 0 or 400 µM FUdR, for a total of n ≥ 178 animals per condition.
B. Lifespan extension is dependent on FUdR concentration. Little impact of FUdR was observed at 50 mM NaCl. Under moderate hypertonic stress, lifespan was increased by 40 µM or 400 µM FUdR. Pooled analysis of three independent replicates at 20° C, for a total of n ≥ 226 animals per condition.
C. Loss of Notch co-ligands OSM-7 or OSM-11 increased lifespan on 50 mM NaCl. Three independent replicate experiments were pooled for analysis, for a total of n ≥ 273 animals per condition. Experiments were performed at 25°C with 400 µM FUdR.
D. Lifespan extension due to hypertonic stress or loss of Notch co-ligands is dependent on the presence of FUdR. Hypertonic stress alone decreased wild type (WT) lifespan, while hypertonic stress combined with FUdR increased. Loss of Notch co-ligands OSM-7 and OSM-11 decreased lifespan in the absence of FUdR, but increased in the presence of FUdR, regardless of hypertonic stress. Pooled analysis of two independent replicates at 20° C with 0 or 400 µM FUdR, for a total of n ≥ 140 animals per condition.
Error bars indicate SEM, * indicates p<0.01 in log rank test vs. 50 mM NaCl control or between indicated columns, n.s. indicates p > 0.05.
C. elegans lifespan studies commonly use FUdR concentrations between 5µM and 400 µM (Table 1), and doses as high as 6000 µM do not affect wild type lifespan under standard conditions (Mitchell et al., 1979). We examined the dose dependence of the impact of FUdR on lifespan extension under hypertonic stress. FUdR had little impact on lifespan in the absence of hypertonic stress, although at 8 µM FUdR caused a small, significant lifespan increase (Fig. 2B). However, under hypertonic stress, there was a dose dependent response to FUdR (Fig. 2B). In the presence of 300 mM NaCl, 40 µM FUdR extended lifespan by 23% and 400 µM FUdR by 67%. 400 µM FUdR combined with hypertonic stress extended lifespan 25% versus no FUdR and 50 mM NaCl. However, at intermediate doses of FUdR high osmolarity did reduce lifespan, similar to previous reports of 35% reduction by high NaCl and 50 µM FUdR (Chandler-Brown et al., 2015). We also observed that high doses of FUdR combined with low osmolarity cause a large proportion of animals to rupture (see Table S2 for summary of censor causes for experiments in Fig. 2A and 2B). Combined, there is a synergistic interaction between FUdR treatment and hypertonic stress, which can extend lifespan compared to normal culture conditions.
Table 1.
Genes and treatments where lifespan is altered by FUdR treatment
| Genotype/ treatment |
without FUdR |
with FUdR (dose) | Reference |
|---|---|---|---|
| N2 | normal | normal (5–6000 µM), reduced (50,000 µM) | (Mitchell et al., 1979) |
| akt-1 | normal | extended (100 µM) | (Dumas et al., 2010; Hertweck et al., 2004) |
| ash-2 | normal | extended (400 µM1, 2) | (Greer et al., 2010; Lee et al., 2003a) |
| daf-11 | normal | extended (400 µM) | (Hahm et al., 2009; Shen et al., 2010) |
| daf-16 OE | normal | extended (400 µM) | (Qi et al., 2012) |
| daf-2 | long | extended (25 µM) | (Arum and Johnson, 2007) |
| flcn-1 | normal | extended (100–150 µM) | (Possik et al., 2014) |
| frh-1 | short | extended (40 µM3) | (Vázquez-Manrique et al., 2006) |
| gas-1 | short | extended (25–100 µM) | (Van Raamsdonk and Hekimi, 2011) |
| glp-1 | long | extended (25 µM), reduced (400 µM) | (Rooney et al., 2014) |
| mek-1 | short | normal (400 µM) | (Qi et al., 2012) |
| mir-238 | normal | reduced (400 µM4) | (de Lencastre et al., 2010) |
| osm-11 | short | extended (400 µM) | this study |
| osm-7 | short | extended (400 µM) | this study |
| rbr-2 | short | extended (400 µM1, 2) | (Greer et al., 2010; Lee et al., 2003a) |
| sir-2.1; eat-2 | eat-2 | <eat-2 (400 µM) | (Hansen et al., 2007; Wang and Tissenbaum, 2006) |
| sod-1 | short | normal (400 µM) | (Yen et al., 2009) |
| tub-1 | normal | extended (400 µM) | (Aitlhadj and Stürzenbaum, 2010) |
| W09C5.8 | short | extended (400 µM2) | (Lee et al., 2003b; Suthammarak et al., 2009) |
| Y37D8A.14 | short | extended (400 µM2) | (Lee et al., 2003b; Suthammarak et al., 2009) |
| axenic culture | long | extended (400 µM) | (Mitchell et al., 1979) |
| hypertonic NaCl | short | short(40–50 µM), extended (400 µM) | this study, (Chandler-Brown et al., 2015) |
| hypertonic sorbitol | short | extended (50–400 µM) | this study, (Chandler-Brown et al., 2015) |
| hypertonic KCl, glycerol | normal | extended (400 µM) | this study |
| mild heat stress | short | extended (400 µM) | (Angeli et al., 2013) |
| resveratrol | normal | extended (50 µM) | (Chen et al., 2013; Viswanathan et al., 2005) |
| RNAi control conditions | normal | extended (100 µM1) | (Greer et al., 2010) |
A. Brunet, personal communication.
G. Ruvkun, personal communication.
R. Vázquez Manrique, personal communication.
F. Slack, personal communication.
Are known hypertonic stress adaptation pathways were involved in lifespan extension? Complete loss of Notch co-ligands encoded by osm-7 or osm-11 produces many of the same physiological and behavioral consequences as adaptation to environmental hypertonic stress (Wheeler and Thomas, 2006). We confirmed that these two Notch co-ligands act via Notch receptors to modulate hypertonic stress resistance (Fig. S3). When treated with FUdR, animals lacking either osm-7 or osm-11, had longer lifespans than wild type animals (Fig. 2C). However, moderate hypertonic stress can still extend the lifespan of osm-7 or osm-11 animals (Fig. 2C). As osm-7 and osm-11 encode related proteins and may act redundantly, we examined osm-7; osm-11 double mutant animals. On 50 mM NaCl, without FUdR, the lifespan of osm-7; osm-11 animals was reduced, compared to wild type (Fig. 2D). However, FUdR treatment greatly extended the lifespan of osm-7; osm-11 animals on 50 mM NaCl. Hypertonic stress slightly reduced the lifespan of osm-7; osm-11 animals in the absence of FUdR, and had no effect in the presence of FUdR (Fig. 2D). Reduced Notch signaling in adults or environmental hypertonic stress leads to a hypertonic stress response. In the absence of FUdR, this reduces lifespan, but in the presence of FUdR the hypertonic stress response increases lifespan.
3.2 Germ cells and bacteria are likely not the site of FUdR action
FUdR blocks the synthesis of thymidine, preventing DNA replication. While adult C. elegans somatic cells do not divide, some adult cells synthesize DNA (Golden et al., 2007; Lozano et al., 2006). C. elegans germ cells constantly divide and preventing germ cell replication leads to lifespan extension (Arantes-Oliveira et al., 2002). Therefore, FUdR treatment might extend lifespan under hypertonic conditions by inhibiting germ cell DNA replication. To test this, we used mes-1(bn7) animals that have defective germ cell specification. When reared at the restrictive temperature, most animals completely lack germ cells, but germline development is normal in the remaining animals (Arantes-Oliveira et al., 2002). We found that mes-1(bn7) animals with normal germ cells (mes-1 GC+) were indistinguishable from wild type animals in their response to FUdR and hypertonic stress (Fig. S4). As expected, mes-1(bn7) animals lacking germ cells (mes-1 GC-) had increased lifespan under standard norm-osmotic culture conditions, regardless of the presence of FUdR (Fig. S3). Without FUdR, hypertonic stress reduced the lifespan of mes-1 GC- (Fig. S3). Critically, under hypertonic stress conditions addition of FUdR dramatically extended the lifespan of mes-1 GC- animals (Fig. S4), indicating that FUdR can act on somatic tissue. However, in FUdR-treated mes-1 GC- animals hypertonic stress did not further extend lifespan (Fig. S4). This non-additive lifespan extension suggests that some downstream pathways overlap. We conclude that the effects of FUdR on lifespan are primarily germline-independent.
It was possible that FUdR might inhibit bacterial growth or replication in C. elegans cultures, leading to lifespan extension due to decreased infection. To test this, OP50 E. coli was killed with UV and antibiotics and used as food for aging experiments. As expected, dead bacteria increased C. elegans lifespan on 50 mM NaCl plates in the absence of FUdR and hypertonic stress decreased lifespan of animals exposed to live or dead bacteria (Fig. S4). Unexpectedly, FUdR appears to block the beneficial effects of dead bacteria. Bacteria have numerous independent effects on C. elegans lifespan via pathogenicity or bacterial metabolites (Cabreiro et al., 2013; Garigan et al., 2002; Gusarov et al., 2013; Virk et al., 2012); we hesitate to speculate about the unexpected interaction between FUdR, live vs. dead bacteria, and lifespan. However, in the presence of FUdR and dead bacteria, animals on 300 mM NaCl had longer lifespan than animals on 50 mM NaCl (Fig. S4), indicating that FUdR-induced lifespan extension is not dependent on altered bacteria growth. Combined, these results suggest that FUdR and hypertonic stress act synergistically on C. elegans somatic tissues to extend lifespan.
3.3 FUdR likely inhibits tyms-1 thymidylate synthase and activates base excision repair
FUdR metabolites directly inhibit thymidylate synthase resulting in increased 2′-deoxyuridine-5′-monophosphate (dUMP) and decreased 2′-deoxythymidine-5′-monophosphate (dTMP) levels (Wyatt and Wilson, 2009). Consequently, uracil or its analogs can be misincorporated into DNA. The first step in uracil excision from DNA requires uracil-DNA glycosylases, which recognize incorporated uracils and cleave their glycosidic bonds, initiating base excision repair and DNA repair (Fig. S5). To determine if FUdR acts via thymidine synthesis inhibition in C. elegans, we examined the role of thymidylate synthase and base excision repair (BER) induction in FUdR-induced hypertonic lifespan extension.
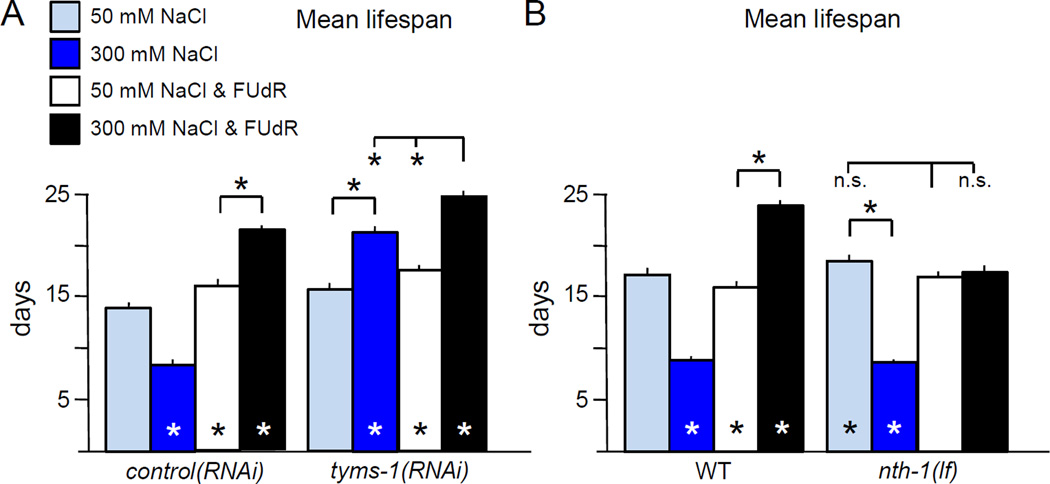
The C. elegans ortholog of thymidylate synthase is encoded by Y110A7A.4, which we designate thymidylate synthase-1 (tyms-1). Complete loss of tyms-1 likely causes lethality, while knockdown of tyms-1 by RNA interference (RNAi) feeding causes sterility and lethality. We used a transgenic RNAi approach to knockdown tyms-1 during longevity studies with FUdR, to avoid possible impact of FUdR on bacteria. The dpy-30 promoter (dpy-30p) was used to drive ubiquitous expression of tyms-1 antisense; F1 progeny of injected animals were examined. dpy-30p::tyms-1(RNAi) and dpy-30p::empty(RNAi) control animals had similar lifespans at 50 mM NaCl, with or without FUdR (Fig. 3A), but dpy-30p::tyms-1(RNAi) animals had extended lifespan on 300 mM NaCl, compared to control animals (Fig. 3A). Addition of FUdR slightly extended the lifespan of tyms-1(RNAi) animals, indicating that FUdR may inhibit residual TYMS-1 activity due to incomplete knockdown; we cannot rule out possible impacts of FUdR on other pathways. We conclude that FUdR-mediated inhibition of TYMS-1 is a major player in lifespan extension under these hypertonic stress conditions.
Figure 3. Lifespan extension by thymidylate synthase inhibition and osmotic stress is partially dependent on DNA base excision repair.
A. FUdR inhibition of thymidylate synthase increases lifespan under hypertonic stress. Lifespan was determined for transgenic F1 animals carrying ubiquitously expressed control or tyms-1(RNAi) transgenes (dpy-30p::empty or dpy-30p::tyms-1(RNAi)). Hypertonic stress increased dpy-30p::tyms-1(RNAi) lifespan, but not control. FUdR slightly extended lifespan when combined with hypertonic stress in dpy-30p::tyms-1(RNAi) animals. Pooled analysis of 3 independent replicate experiments, for a total of n ≥ 148 animals per condition.
B. Loss of uracil-DNA glycosylase NTH-1 partially abrogated FUdR-mediated lifespan extension under hypertonic stress, but did not completely eliminate the beneficial impact of FUdR under hypertonic stress. Pooled analysis of 3 independent replicate experiments, for a total of n ≥ 313 animals per condition.
Experiments were performed at 20 °C with 0 or 400 µM FUdR. Error bars indicate SEM, * indicates p<0.01 in log rank test vs. 50 mM NaCl, no FUdR or between indicated columns, n.s. indicates p>0.05.
FUdR treatment can cause misincorporation of uracil and uracil analogs in DNA, which are subsequently repaired BER DNA repair pathways (Wyatt and Wilson, 2009). Therefore, we predicted that preventing activation of DNA damage response pathways should abrogate the impact of FUdR. Under standard osmotic conditions, animals completely lacking uracil DNA glycosylase nth-1 function had lifespans comparable to wild- type, with or without FUdR (Fig. 3B). Hypertonic stress shortened nth-1 lifespan as expected (Fig. 3B). However, under moderate hypertonic conditions, the impact of FUdR on lifespan was blunted in animals lacking nth-1 (Fig. 3B). As loss of nth-1 partially abrogates the impact of FUdR treatment, we conclude that DNA damage repair pathways are required for FUdR-induced lifespan extension. Since germ cells are not required for FUdR to extend lifespan in this paradigm, DNA damage repair pathways in somatic tissues are likely responsible. Hypertonic stress induces double stranded breaks (DSBs) in DNA (Dmitrieva et al., 2005). We hypothesize that up-regulation of DNA repair pathways by long-term FUdR treatment may facilitate DSB repair, or combined activation of both BER and DSB response pathways may have synergistic effects that extend lifespan. However, we cannot rule out the possibility that incorporation of FUdR metabolites into somatic RNA also contributes to lifespan extension.
3.4 FUdR treatment induces resistance to environmental stress
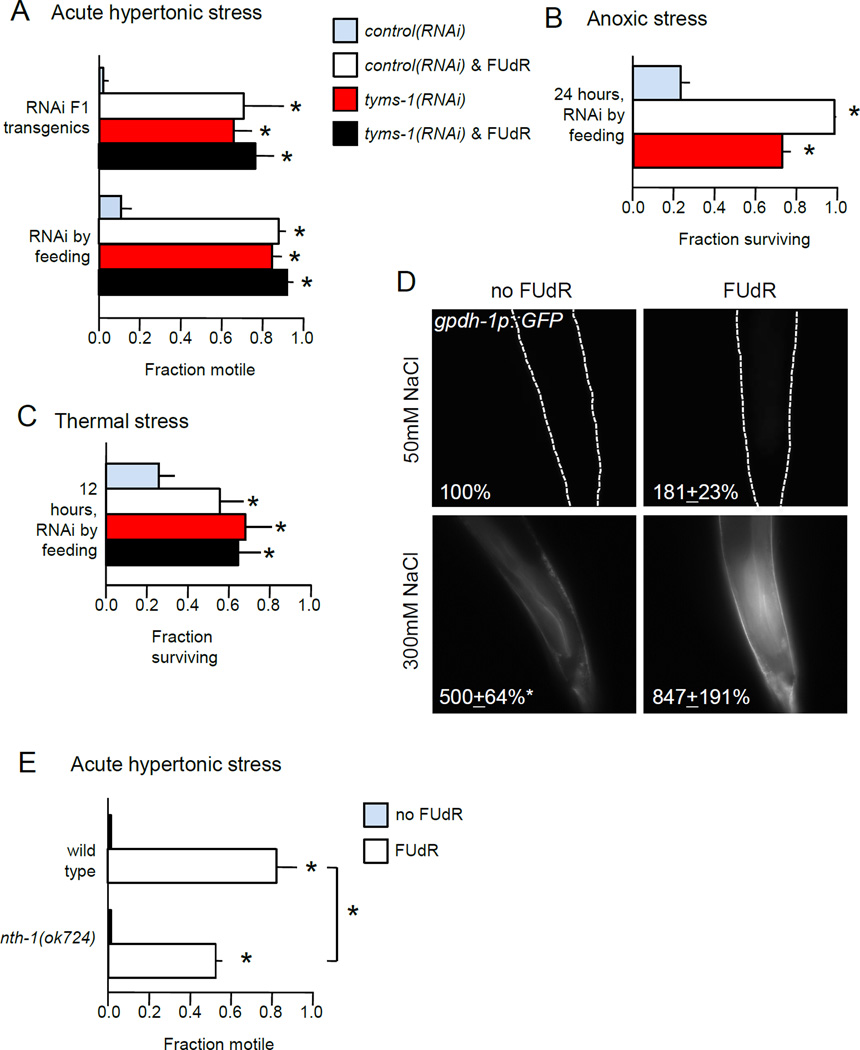
DNA damage engages cellular pathways that establish resistance to various stressors. To determine if FUdR inhibition of tyms-1 induces stress resistance, we examined the ability of FUdR-treated animals and tyms-1(RNAi) animals to survive three environmental stresses: increased osmolarity, anoxia, and increased temperature. In all cases examined, either tyms-1 knockdown and/or 48 hours of FUdR pre-treatment conferred considerable stress resistance. tyms-1(RNAi) by feeding or tyms-1(RNAi) in F1 transgenic animals was sufficient to confer resistance to acute or overnight hypertonic stress on 500 mM NaCl NGM plates (Fig. 4A and not shown). Similarly, ability to survive anoxia for 24 hours was increased by either tyms-1(RNAi) or FUdR pre-treatment (Fig. 4B). Finally, either treatment significantly protected C. elegans against thermal stress at 35 °C (Fig. 4C), consistent with other reports (Angeli et al., 2013). We conclude that FUdR increases stress resistance by inhibiting thymidylate synthase.
Figure 4. Thymidylate synthase inhibition induces broad stress resistance.
A. FUdR or tyms-1(RNAi) increases resistance to acute hypertonic stress. Fraction of motile animals was measured 10 minutes after transfer to 500 mM NaCl plates. n = 3 independent replicate experiments, with 30 animals per replicate.
B. FUdR increases resistance to anoxia. Fraction surviving anoxia in BioBag was assessed at 24 hours. n = 3 independent replicate experiments, with at least 20 animals per replicate.
C. FUdR confers resistance to thermal stress. Fraction surviving was assessed after 12 hours at 35°C. n = 4 independent replicate experiments, with at least 28 animals per replicate.
D. Either FUdR treatment or hypertonic stress induced expression of gpdh-1p::GFP. Average percent change in GFP fluorescence for gpdh-1::GFP at rear of intestine after 48 hours in 3 replicate experiments, with 10 animals per replicate. Dotted lines show outline of animal (top panels).
E. nth-1 loss partially abrogates FUdR-mediated resistance to acute hypertonic stress. n = 4 independent replicate experiments, with 30 animals per replicate.
Young adult animals were pre-treated with 400 µM FUdR or bacteria expressing tyms-1(RNAi) for 48 hours prior to stress assays. Error bars indicate SEM, * indicates p < 0.05, n.s. indicates p > 0.05 in Student’s T-test vs. control or between indicated groups.
C. elegans adapt to hypertonic stress by increasing transcription of glycerol phosphate dehydrogenase gpdh-1, which raises internal glycerol levels (Lamitina et al., 2006). After two days of moderate hypertonic stress, expression of the gpdh-1p::GFP transcriptional reporter increased five-fold in the posterior intestine (Fig. 4D). FUdR treatment for two days approximately doubled expression of gpdh-1p::GFP (Fig. 4D and Fig. S6), suggesting a partial induction of the hypertonic stress response. The impact of FUdR treatment and hypertonic stress was, at a minimum, additive (Fig. 4D). Similar changes were observed in anterior intestine (not shown). The increased lifespan of FUdR-treated C. elegans under hypertonic conditions may result, in part, from up-regulation of stress resistance pathways including increased osmolyte production.
Next, we asked if FUdR acts in somatic tissues to induce hypertonic stress resistance. Loss of nth-1 decreased the fraction of animals resistant to acute hypertonic stress by 30% (Fig. 4E), consistent with a role for the DNA damage response. Additionally, mes-1(bn7) animals with germ cells were not significantly more resistant to acute hypertonic stress than mes-1(bn7) animals lacking germ cells (Fig. S7). These results, combined with the lifespan results in Fig. 3, suggest that FUdR engages DNA damage response pathways in somatic tissues to induce acute and long-term resistance to hypertonic stress.
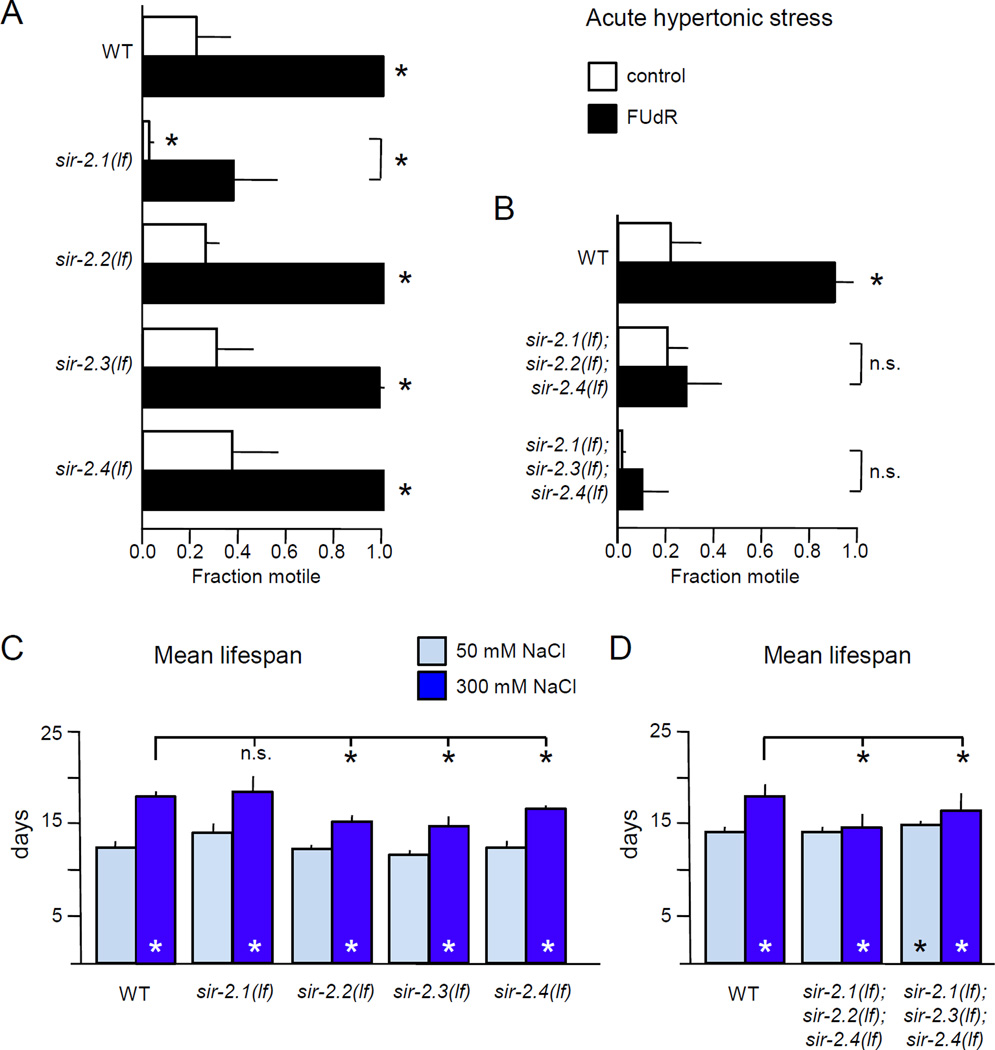
3.5 Sirtuins contribute to FUdR-induced hypertonic stress resistance
Hypertonic stress extends the lifespan of S. cerevisiae in a sirtuin-dependent manner (Kaeberlein et al., 2002). Although this effect is independent of FUdR, we reasoned that conserved osmotic stress response pathways may exist in S. cerevisiae and C. elegans; we investigated the contribution of sirtuins to FUdR-induced hypertonic stress resistance and lifespan extension. The C. elegans genome contains two genes encoding predicted mitochondrial sirtuins, sir-2.2 and sir-2.3, along with two genes encoding sirtuins that act in the cytoplasm and nucleus, sir-2.1 and sir-2.4. We obtained deletion alleles for each sirtuin. None of the sirtuins were required for adaptation to hypertonic stress and incubation on 200 mM NaCl plates overnight conferred resistance to 500 mM NaCl acute hypertonic stress (data not shown). Loss of sir-2.2, sir-2.3, or sir-2.4 alone had no impact on acute hypertonic stress resistance in naïve animals or in animals pre-treated with FUdR for 48 hours (Fig. 5A). However, loss of sir-2.1 increased sensitivity to acute hypertonic stress resistance in naïve animals (Fig. 5A). FUdR treatment of sir-2.1 animals modestly improved resistance to acute hypertonic stress, but did not completely protect (Fig. 5A). This suggests that sir-2.1 function is important for survival under acute hypertonic stress in naïve animals, but sir-2.1 alone is not essential for induction of stress resistance by FUdR. However, other C. elegans sirtuins could contribute redundantly to hypertonic stress response.
Figure 5. Sirtuin function is required for FUdR to induce stress resistance or increase lifespan.
A. sir-2.1 plays a role in FUdR-induced hypertonic stress resistance. Animals lacking sir-2.1 were hypersensitive to acute hypertonic stress; the effect of FUdR pre-treatment was reduced compared to wild type. Hypertonic stress resistance and FUdR response of animals lacking other sirtuin genes was normal. n = 3 independent replicate experiments, with 45 animals per replicate.
B. Simultaneous loss of three sirtuin genes eliminated the ability of FUdR to induce acute hypertonic stress resistance. For either triple mutant strain, FUdR had no impact on hypertonic stress resistance. n = 5 independent replicate experiments, with at least 34 animals per replicate.
C. Loss of sir-2.1, sir-2.2, sir-2.3, or sir-2.4 did not prevent lifespan extension by FUdR and hypertonic stress. Pooled analysis of three independent replicates at 25° with 400 µM FUdR, for a total of n ≥ 253 animals per condition.
D. Sirtuin function is required for FUdR-induced lifespan extension under hypertonic stress. Loss of three sirtuins partially abrogated lifespan extension induced by FUdR and hypertonic stress. Pooled analysis of three independent replicates at 25° with 400 µM FUdR, for a total of n ≥ 193 animals per condition.
For stress resistance experiments, young adult animals were pre-treated for 48 hours with 400 µM FUdR prior to stress assays. Error bars indicate SEM, * indicates p<0.05 vs. control or between designated columns using Student’s T-test for stress assays and the log-rank test for lifespan.
To address this, C. elegans lacking three of the four sirtuin genes were examined. sir-2.2 and sir-2.3 are immediately adjacent on chromosome X, preventing recombination of the respective alleles. FUdR pretreatment did not induce hypertonic stress resistance in animals lacking three sirtuin genes; naïve animals simultaneously lacking sir-2.1, sir-2.3, and sir-2.4 were hypersensitive to acute hypertonic stress (Fig. 5B). However, animals lacking three sirtuin genes were capable of adapting to hypertonic stress; overnight incubation on 200 mM NaCl plates conferred resistance (not shown). We conclude that sirtuins function redundantly in hypertonic stress resistance, that sir-2.1 function is important in naïve animals, and that FUdR requires sirtuin function to induce stress resistance.
Loss of a single C. elegans sirtuin gene does not alter lifespan under standard culture conditions (Wang & Tissenbaum 2006, Fig. 5C). If C. elegans sirtuins act downstream of FUdR and DNA damage under hypertonic stress, then sirtuin loss should decrease lifespan extension under these conditions. We found that loss of a single sirtuin gene did not alter lifespan extension caused by hypertonic stress and FUdR (Fig. 5C, dark blue columns). However, loss of multiple sirtuins did impact lifespan under hypertonic stress conditions. The lifespan of sir-2.1; sir-2.2; sir-2.4 or sir-2.1; sir-2.3; sir-2.4 animals was not different from wild type (Fig. 5D). But, in animals lacking three sirtuin genes, the beneficial impact of FUdR combined with hypertonic stress was greatly reduced (Fig. 5D). Decreased sirtuin function did not completely eliminate the beneficial effects of FUdR, as hypertonic stress did not shorten lifespan compared to standard culture conditions as observed previously (Fig. 2). Overexpression of sir-2.1 was sufficient to confer resistance to acute hypertonic stress, though it does not extend lifespan under standard or hypertonic stress conditions, with or without FUdR (Fig. S8). Furthermore, overexpression of sir-2.1, sir-2.2, and sir-2.3 enhanced adaptation to overnight hypertonic stress, and slightly increased lifespan of animals exposed to hypertonic stress (Fig. S8). Many stress response pathways activate autophagy; in some cases, this up-regulation is dependent on sirtuin function (Morselli et al., 2010). One consequence of autophagy induction is accumulation of Atg8 on the autophagosome (Jenzer et al., 2014). Discrete cytoplasmic puncta of C. elegans Atg8, LGG-1::GFP, were increased in animals treated with FUdR and dramatically increased by hypertonic stress, but no synergistic induction was observed (Fig. S9). We conclude that lifespan extension via FUdR and hypertonic stress is partially dependent on sirtuin function.
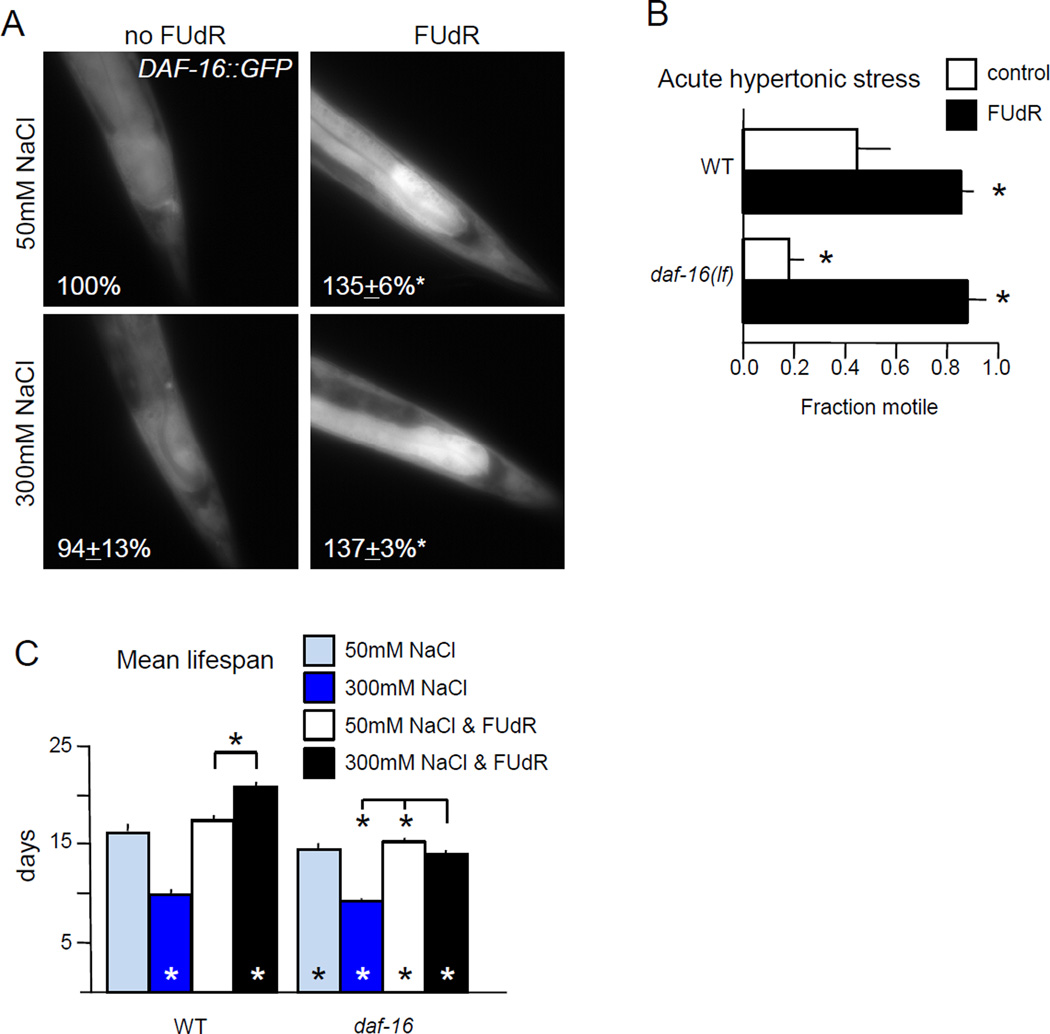
3.6 DAF-16 FoxO is required for FUdR-induced hypertonic lifespan extension
DAF-16 FoxO has been implicated in C. elegans hypertonic stress response (Gerke et al., 2014; Lamitina and Strange, 2005). We examined potential roles for DAF-16 FoxO in FUdR-induced acute stress resistance and lifespan extension, in the context of FUdR treatment. Although DAF-16::GFP nuclear localization increases after exposure to 400 mM NaCl (Gerke et al., 2014), we do not observe changes in its localization after 48 hours on 300 mM NaCl (Fig. 6A). However, 48 hours of FUdR treatment increased total DAF-16::GFP levels by roughly 30%, independent of osmolarity, and the greatest increases were observed in the intestine (Fig. 6A). Increased DAF-16 levels in somatic tissues may contribute to FUdR-induced stress resistance. Naïve animals with decreased daf-16 function are hypersensitive to acute hypertonic stress; but eventually adapt to hypertonic conditions and acquire acute resistance comparable to wild type animals (Lamitina and Strange, 2005). We found that while naïve daf-16(mu86) animals were sensitive to hypertonic stress, FUdR pre-treatment was sufficient to confer resistance to acute hypertonic stress (Fig. 6B). Therefore, DAF-16 function is not required for hypertonic stress resistance conferred by FUdR.
Figure 6. DAF-16 is required for lifespan extension, but not hypertonic stress resistance induced by FUdR.
A. FUdR treatment, but not hypertonic stress, induced expression of daf-16p::DAF-16::GFP. FUdR treatment for 48 hours increased DAF-16::GFP levels by one third, regardless of external osmolarity. Average percent change in GFP fluorescence at rear of intestine after 48 hours in 3 replicate experiments, with 10 animals per replicate.
B. Animals lacking daf-16 were hypersensitive to acute hypertonic stress, but FUdR pre-treatment conferred resistance. n = 5 independent replicate experiments, with at least 21 animals per replicate.
C. DAF-16 FOXO is required for FUdR to extend lifespan under hypertonic conditions. daf-16 loss modestly decreased lifespan under standard culture conditions, regardless of FUdR treatment. In the absence of FUdR, daf-16 loss had no impact on lifespan under hypertonic stress, but loss of daf-16 partially abrogated lifespan extension induced by hypertonic stress with FUdR. Pooled analysis of two independent replicates at 20° with 0 or 400 µM FUdR, for a total of n ≥ 161 animals per condition.
Error bars indicate SEM, * indicates p<0.05 vs. control or between designated columns using Student’s T-test for stress assays and the log-rank test for lifespan.
Is daf-16 required for lifespan extension induced by FUdR and hypertonic stress? The short lifespan of daf-16(mu86) animals under standard conditions of 50 mM NaCl was not altered by FUdR (Fig. 6C). However, daf-16 function was required for full lifespan extension by moderate hypertonic stress and FUdR (Fig. 6C). daf-16 was also required for lifespan extension in FUdR-treated osm-7;osm-11 animals lacking Notch co-ligands (Fig. S10). In both cases, impairing DAF-16 partially abrogated lifespan extension. Combined, these results suggest that daf-16 function is not required for long-term adaptation to hypertonic conditions, induced by either pre-exposure to high osmolarity or FUdR. However, daf-16 plays a role in lifespan extension when animals are challenged with both hypertonic stress and FUdR inhibition of thymidylate synthesis. Increased DAF-16 levels after FUdR treatment may contribute to long-term survival under hypertonic conditions.
4. Discussion
4.1 Hormesis, hypertonic stress and Notch signaling
Lifespan and stress resistance are dramatically altered by both experience and conditions. For example, pre-exposure to moderate hypertonic stress allows animals to survive acute hypertonic challenges (Lamitina et al., 2004). And, moderate doses of FUdR lead to lifespan extension and increased stress resistance. The beneficial consequences of these moderate stresses are consistent with hormesis, an adaptive response to modest challenge that up-regulates stress response pathways. The specificity and level of hormetic protection varies with the stress-inducing agent and the level of challenge. Under standard culture conditions, FUdR blocks reproduction with little impact on wild type C. elegans adult lifespan, but FUdR provides broad protection against a variety of stresses.
By contrast, adaptation to hypertonic stress provides resistance to only a few stresses. Hypertonic stress decreases soluble Notch co-ligand secretion (Singh et al., 2011), but the signaling pathways connecting Notch signaling with hypertonic stress adaptation have not been identified. Either Notch co-ligand loss or hypertonic stress of adult C. elegans increases gpdh-1 transcription and glycerol levels (Wheeler and Thomas, 2006). Here, we show that increasing Notch receptor function slows acquisition of hypertonic stress resistance when co-ligand levels drop. Increased internal osmolyte levels provide protection against hypertonic stress and increase survival after freezing, but not heat stress (Wheeler and Thomas, 2006). There is a price for adaptation to hypertonic stress, even in wild type C. elegans; adaptation alters behavior, decreases progeny number, and shortens lifespan. Some of these changes may be attributable to hypertonic stress impacting autophagy, induced protein misfolding (Burkewitz et al., 2011) or double stranded breaks in DNA (Dmitrieva et al., 2011). However, results presented here suggest that combining hypertonic stress with FUdR can have unexpected consequences.
4.2 FUdR treatment likely engages DNA damage repair pathways
Here, we report that FUdR pre-treatment induces resistance to acute and long-term hypertonic stress. Where direct comparison is possible, the results presented here are consistent with previous reports by other laboratories (Berg, Kaeberlein, Angeli, and others). But, the impact of FUdR was explicitly examined herein. Despite the negligible impact of FUdR on lifespan under normal culture conditions, FUdR has a profound impact on stress resistance even in wild type C. elegans. FUdR pre-treatment ameliorates the impact of anoxic, thermal, hypertonic, and proteotoxic stress. Many of these stresses cause DNA damage. For example, hypertonic stress causes double-stranded breaks in gene-poor regions of eukaryotic DNA that are not repaired until osmolarity returns to normal (Dmitrieva et al., 2011). Loss of the DNA-glycosylase NTH-1 decreased the protection provided by FUdR, suggesting that FUdR-induced stress resistance requires DNA damage response/repair pathways, many of which have been previously implicated in stress resistance. Induction of autophagy by the FUdR-analog 5-FU requires BER and other DNA damage response pathways (SenGupta et al., 2013), and autophagy has been implicated in the hypertonic stress response (Nunes et al., 2013). Intriguingly, Notch signaling reduces the activity of DNA damage response pathways in C. elegans by direct binding and inhibition of ATM kinase (Vermezovic et al., 2015), suggesting that the hypertonic stress response may activate the DNA damage response and facilitate beneficial effects of FUdR.
4.3 Where does FUdR act to increase stress resistance and/or lifespan?
Apart from the germline, cell proliferation does not occur in adult C. elegans, although DNA endoreduplication occurs in somatic and germline tissues (Golden et al., 2007; Lozano et al., 2006). Germ cell loss results in lifespan extension with resistance to thermal and oxidative stress (Arantes-Oliveira et al., 2002). FUdR treatment does not increase the stress resistance of animals lacking oocytes (Angeli et al., 2013); they are already stress resistant due to germ cell loss. This lack of additivity initially suggested that FUdR acts via the germline or that maximal lifespan extension/stress resistance had already been achieved by germ cell loss.
The cellular focus of FUdR action remains unresolved and may differ for acute stress versus lifespan- or for specific stressors. Heat stress or anoxia kill animals within hours or days, respectively; FUdR confers resistance to both. Previous work suggests that FUdR-mediated inhibition of oogenesis or oocyte maturation plays a role in FUdR-induced stress resistance (Angeli et al., 2013). In part, this model is based on the ability of FUdR to partially restore resistance to heat stress only after mating, when sperm transfer triggers meiotic maturation. We found that loss of germline reduced FUdR-induced protection against acute hypertonic stress, suggesting that somatic actions of FUdR also play a major role in acute hypertonic stress resistance.
In the context of lifespan extension, the site of FUdR action may not be the germline. FUdR increases lifespan at higher temperatures in wild type or daf-16(lf) animals, but not in fem-3(lf) animals lacking sperm/maturing oocytes (Angeli et al., 2013). However the presence of sperm causes hypertonic stress susceptibility (along with body length decreases) in aging C. elegans after mating, independent of oocyte maturation and mechanical cuticle damage (Shi and Murphy, 2014). And, FUdR induces thermotolerance in animals lacking germ cells (Feldman et al., 2014). We find that under hypertonic conditions, FUdR induces lifespan extension regardless of the presence of the germline, ruling out FUdR action solely in oocytes under hypertonic stress. Either FUdR acts in both somatic and germline tissues to induce longevity under stress or FUdR acts in different locations for different stresses.
4.4 Sirtuins and DAF-16 FOXO contribute to stress resistance and lifespan extension
Sirtuin function was required for FUdR-induced stress resistance in both acute and long-term paradigms herein. Loss of SIR-2.1 alone was sufficient to abrogate acute hypertonic stress resistance, but FUdR treatment increased resistance. However; loss of multiple sirtuins completely eliminated FUdR-induced resistance. In lifespan studies, loss of multiple sirtuins reduced the beneficial effects of FUdR under hypertonic stress. Previous work has demonstrated that sir-2.1 is required for DNA damage response (Greiss et al., 2008); this is consistent with FUdR induction of nuclear DNA damage response pathways. We note that SIR-2.2 and SIR-2.3 are predicted to act in mitochondria and their functional targets remain unclear. The actions of sirtuins in stress resistance are not limited to DNA damage pathways and we report here that autophagy may be up-regulated. Numerous sirtuin targets likely contribute to stress resistance and longevity.
FUdR provides acute stress resistance independent of DAF-16 FOXO function under all paradigms tested thus far: osmotic stress (Fig. 6B) and thermal stress (Angeli et al., 2013). However, DAF-16 FOXO plays a role in FUdR-induced lifespan extension. Loss of daf-16 decreases lifespan under standard conditions, but does not further shorten lifespan under hypertonic stress. But, loss of daf-16 diminished the beneficial impact of FUdR under hypertonic stress conditions. A comparable observation was made for lifespan extension with FUdR in the hypertonic stress susceptibility post-mating paradigm; FUdR treatment increases lifespan in stressed daf-16 animals, but FUdR is much more effective in lengthening lifespan in wild type animals (Shi and Murphy, 2014). FUdR treatment increased DAF-16::GFP total protein level without altering nuclear localization. Similarly, some methods of dietary restriction require daf-16 for lifespan extension, but do not alter DAF-16::GFP nuclear localization (Greer et al., 2007). Overall, our results suggest that increased DAF-16 levels play a role in lifespan extension caused by hypertonic stress and FUdR, but other pathways likely contribute.
4.5 Use of FUdR in C. elegans lifespan studies
Thymidylate synthase inhibitors, including FUdR and 5-FU, are among the most successful chemotherapeutic agents available. Rapidly replicating cells are most sensitive to these drugs, which accounts for their utility in C. elegans lifespan studies. FUdR treatment blocks germ cell proliferation in self-fertilizing C. elegans hermaphrodites and prevents growth of progeny, who could easily be mistaken for parents in longevity assays. FUdR has no overt impact on the lifespan of wild type C. elegans under standard culture conditions and the use of FUdR in lifespan studies rapidly gained acceptance. Concentrations of FUdR ranging from 16 µM to 400 µM were used, with the presumption that FUdR acted solely to prevent germ cell proliferation and progeny over-growth. We and others find that FUdR treatment results in resistance to stress (Angeli et al., 2013; Brunquell et al., 2014; Mendenhall et al., 2009; Miyata et al., 2008; Rooney et al., 2014). A number of C. elegans studies have reported that FUdR dramatically impacted lifespan for specific genotypes or conditions. Other studies reported conflicting results for specific genotypes/stresses, which might now be explained by differing FUdR usage (summarized in Table 1). Lifespan can be extended by FUdR treatment, even in long-lived genotypes. FUdR increases the lifespan of animals lacking the DAF-2 insulin receptor function and FUdR alters metabolism in these animals (Arum and Johnson, 2007; Davies et al., 2012). In light of this evidence, most recent lifespan studies replicate their critical results with and without FUdR. The text of many papers is ambiguous regarding FUdR use, with no explicit indication of FUdR use and/or citing papers with conflicting methods; this complicates interpretation of the literature. Studies that allow explicit comparison of lifespan between animals tested with or without FUdR are listed in Table 1.
The impact of FUdR is generally “beneficial”; in the presence of this compound and certain genotypes or environmental conditions, lifespan is increased. Note, however, that FUdR treatment can be harmful at higher concentrations with 400 µM reducing glp-1 lifespan and 50,000 µM reducing wild-type lifespan (Mitchell et al., 1979; Rooney et al., 2014). It is difficult to predict which conditions/genotypes will be dramatically impacted by FUdR based on examination of Table 1, although DNA damage may be a common thread. SOD1 loss, hypertonic stress, or loss of mitochondrial complex 1 proteins likely cause DNA damage (Van Raamsdonk and Hekimi, 2011; Vázquez-Manrique et al., 2006; Woodruff et al., 2004). But, to our knowledge, DNA damage has not been reported/examined for other genes listed in Table 1 and FUdR treatment does not cause substantial wide-spread DNA damage (Rooney et al., 2014). Lacking a rubric for predicting FUdR sensitive pathways/genes/conditions, we suggest that explicitly stating the FUdR dose used, stating the genotypes/treatments tested, and publishing these methods should be required for publication in the future.
4.6 Conclusions
We report that FUdR acts by inhibition of TYMS-1 to induce acute stress resistance. Additionally, when hypertonic stress response is induced by diminished Notch pathway signaling, FUdR inhibition of TYMS-1 dramatically increases lifespan. FUdR-induced lifespan extension under hypertonic stress is concentration dependent, does not require the presence of germ cells or oocytes, and is dependent on sirtuins, FOXO and DNA damage repair pathways. FUdR-induced acute stress resistance is also dependent on sirtuins and DNA damage repair, but not FOXO function. Combined with other recent studies (Aitlhadj and Stürzenbaum, 2010; Angeli et al., 2013; Van Raamsdonk and Hekimi, 2011), these results make a compelling case against the unfettered use of FUdR in C. elegans lifespan studies.
Supplementary Material
Highlights.
FUdR extends lifespan of C. elegans adapted to hypertonic stress conditions.
FUdR increases resistance to acute hypertonic stress, thermal stress, and anoxia.
Effects of FUdR are primarily somatic in this context.
FUdR acts via thymidylate synthase inhibition and impacts FOXO, sirtuins, and DNA repair pathways.
FUdR use should be avoided in C. elegans stress or aging studies, or when different genotypes are compared
Acknowledgments
We thank K. Blackwell for discussion, M. Hoh, G. Somers and E. Beaumont for assisting with experiments, and A. Walker and B. Horvitz for providing strains and reagents. Strains were obtained from the CGC and generated by the OMRF KO consortium. Funding was provided by the Ellison Medical Foundation and the National Institutes of Health under grant numbers R01GM78171, R01NS055813, and P01NS66888.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
All authors designed/analyzed experiments and approved the final manuscript. EA, MC, JL, KS, SP, AS, TT, and HH performed experiments. EA, MD, and AH wrote the manuscript.
Contributor Information
Edward N Anderson, Email: edward_anderson@brown.edu.
Mark E Corkins, Email: markcork@yahoo.com.
Jia-Cheng Li, Email: jia-cheng_li@brown.edu.
Komudi Singh, Email: komudis@gmail.com.
Sadé Parsons, Email: sade.parsons@gmail.com.
Tim M Tucey, Email: timothy.tucey@monash.edu.
Altar Sorkaç, Email: altar_sorkac@brown.edu.
Huiyan Huang, Email: huiyan_huang@brown.edu.
Maria Dimitriadi, Email: m.dimitriadi@herts.ac.uk.
David A Sinclair, Email: david_sinclair@hms.harvard.edu.
Anne C Hart, Email: anne_hart@brown.edu.
References
- Aitlhadj L, Stürzenbaum SR. The use of FUdR can cause prolonged longevity in mutant nematodes. Mech. Ageing Dev. 2010;131:364–365. doi: 10.1016/j.mad.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Angeli S, Klang I, Sivapatham R, Mark K, Zucker D, Bhaumik D, Lithgow GJ, Andersen JK. A DNA synthesis inhibitor is protective against proteotoxic stressors via modulation of fertility pathways in Caenorhabditis elegans . Aging (Albany. NY) 2013;5:759–769. doi: 10.18632/aging.100605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans . Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- Arum O, Johnson TE. Reduced Expression of the Caenorhabditis elegans p53 Ortholog cep-1 Results in Increased Longevity. Journals Gerontol. Ser. A Biol. Sci. Med. Sci. 2007;62:951–959. doi: 10.1093/gerona/62.9.951. [DOI] [PubMed] [Google Scholar]
- Brunquell J, Bowers P, Westerheide SD. Fluorodeoxyuridine enhances the heat shock response and decreases polyglutamine aggregation in an HSF-1-dependent manner in Caenorhabditis elegans . Mech. Ageing Dev. 2014:8–11. doi: 10.1016/j.mad.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Burkewitz K, Choe K, Strange K. Hypertonic stress induces rapid and widespread protein damage in C. elegans . Am. J. Physiol. Cell Physiol. 2011;301:C566–C576. doi: 10.1152/ajpcell.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F, Au C, Leung K-Y, Vergara-Irigaray N, Cochemé HM, Noori T, Weinkove D, Schuster E, Greene NDE, Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler-Brown D, Choi H, Park S, Ocampo BR, Chen S, Le A, Sutphin GL, Shamieh LS, Smith ED, Kaeberlein M. Sorbitol treatment extends lifespan and induces the osmotic stress response in Caenorhabditis elegans . Front. Genet. 2015;6:1–9. doi: 10.3389/fgene.2015.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Rezaizadehnajafi L, Wink M. Influence of resveratrol on oxidative stress resistance and life span in Caenorhabditis elegans . J. Pharm. Pharmacol. 2013;65:682–628. doi: 10.1111/jphp.12023. [DOI] [PubMed] [Google Scholar]
- Davies SK, Leroi AM, Bundy JG. Fluorodeoxyuridine affects the identification of metabolic responses to daf-2 status in Caenorhabditis elegans. Mech. Ageing Dev. 2012;133:46–49. doi: 10.1016/j.mad.2011.11.002. [DOI] [PubMed] [Google Scholar]
- de Lencastre A, Pincus Z, Zhou K, Kato M, Lee SS, Slack FJ. MicroRNAs both promote and antagonize longevity in C. elegans . Curr. Biol. 2010;20:2159–2168. doi: 10.1016/j.cub.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva NI, Burg MB. High NaCl Promotes Cellular Senescence. Cell Cycle. 2007;6:3108–3113. doi: 10.4161/cc.6.24.5084. [DOI] [PubMed] [Google Scholar]
- Dmitrieva NI, Celeste A, Nussenzweig A, Burg MB. Ku86 preserves chromatin integrity in cells adapted to high NaCl. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10730–10735. doi: 10.1073/pnas.0504870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva NI, Cui K, Kitchaev Da, Zhao K, Burg MB. DNA double-strand breaks induced by high NaCl occur predominantly in gene deserts. Proc. Natl. Acad. Sci. U. S. A. 2011;108:20796–20801. doi: 10.1073/pnas.1114677108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas KJ, Guo C, Wang X, Burkhart KB, Adams EJ, Alam H, Hu PJ. Functional divergence of dafachronic acid pathways in the control of C. elegans development and lifespan. Dev. Biol. 2010;340:605–612. doi: 10.1016/j.ydbio.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman N, Kosolapov L, Ben-Zvi A. Fluorodeoxyuridine improves Caenorhabditis elegans proteostasis independent of reproduction onset. PLoS One. 2014;9:e85964. doi: 10.1371/journal.pone.0085964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garigan D, Hsu AL, Fraser AG, Kamath RS, Abringet J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: A role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke P, Keshet A, Mertenskötter A, Paul RJ. The JNK-Like MAPK KGB-1 of Caenorhabditis Elegans Promotes Reproduction, Lifespan, and Gene Expressions for Protein Biosynthesis and Germline Homeostasis but Interferes with Hyperosmotic Stress Tolerance. Cell. Physiol. Biochem. 2014;34:1951–1973. doi: 10.1159/000366392. [DOI] [PubMed] [Google Scholar]
- Golden TR, Beckman KB, Lee AHJ, Dudek N, Hubbard A, Samper E, Melov S. Dramatic age-related changes in nuclear and genome copy number in the nematode Caenorhabditis elegans . Aging Cell. 2007;6:179–188. doi: 10.1111/j.1474-9726.2007.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans . Curr. Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Maures TJ, Hauswirth AG, Green EM, Leeman DS, Maro GS, Han S, Banko MR, Gozani O, Brunet A. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans . Nature. 2010;466:383–387. doi: 10.1038/nature09195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiss S, Hall J, Ahmed S, Gartner A. C. elegans SIR-2.1 translocation is linked to a proapoptotic pathway parallel to cep-1/p53 during DNA damage-induced apoptosis. Genes Dev. 2008;22:2831–2842. doi: 10.1101/gad.482608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Calorie restriction and sirtuins revisited. Genes Dev. 2013;27:2072–2085. doi: 10.1101/gad.227439.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusarov I, Gautier L, Smolentseva O, Shamovsky I, Eremina S, Mironov A, Nudler E. Bacterial nitric oxide extends the lifespan of C. elegans . Cell. 2013;152:818–830. doi: 10.1016/j.cell.2012.12.043. [DOI] [PubMed] [Google Scholar]
- Hahm J-H, Kim S, Paik Y-K. Endogenous cGMP regulates adult longevity via the insulin signaling pathway in Caenorhabditis elegans . Aging Cell. 2009;8:473–483. doi: 10.1111/j.1474-9726.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee S-J, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans . Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- Hertweck M, Göbel C, Baumeister R. C. elegans SGK-1 Is the Critical Component in the Akt/PKB Kinase Complex to Control Stress Response and Life Span. Dev. Cell. 2004;6:577–588. doi: 10.1016/s1534-5807(04)00095-4. [DOI] [PubMed] [Google Scholar]
- Jenzer C, Simionato E, Legouis R. Tools and methods to analyze autophagy in C. elegans . Methods. 2014;75:162–171. doi: 10.1016/j.ymeth.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Andalis AA, Fink GR, Guarente L. High Osmolarity Extends Life Span in Saccharomyces cerevisiae by a Mechanism Related to Calorie Restriction. Mol. Cell. Biol. 2002;22:8056–8066. doi: 10.1128/MCB.22.22.8056-8066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamitina ST, Morrison R, Moeckel GW, Strange K. Adaptation of the nematode Caenorhabditis elegans to extreme osmotic stress. Am. J. Physiol. Cell Physiol. 2004;286:C785–C791. doi: 10.1152/ajpcell.00381.2003. [DOI] [PubMed] [Google Scholar]
- Lamitina ST, Strange K. Transcriptional targets of DAF-16 insulin signaling pathway protect C. elegans from extreme hypertonic stress. Am. J. Physiol. Cell Physiol. 2005;288:C467–C474. doi: 10.1152/ajpcell.00451.2004. [DOI] [PubMed] [Google Scholar]
- Lamitina T, Huang CG, Strange K. Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. Proc. Natl. Acad. Sci. 2006;103:12173–12178. doi: 10.1073/pnas.0602987103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Kennedy S, Tolonen AC, Ruvkun G. DAF-16 target genes that control C. elegans life-span and metabolism. Science. 2003a;300:644–647. doi: 10.1126/science.1083614. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lee RYN, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat. Genet. 2003b;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- Lozano E, Sáez AG, Flemming AJ, Cunha A, Leroi AM. Regulation of growth by ploidy in Caenorhabditis elegans . Curr. Biol. 2006;16:493–498. doi: 10.1016/j.cub.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Mendenhall AR, LeBlanc MG, Mohan DP, Padilla Pa. Reduction in ovulation or male sex phenotype increases long-term anoxia survival in a daf-16-independent manner in Caenorhabditis elegans . Physiol. Genomics. 2009;36:167–178. doi: 10.1152/physiolgenomics.90278.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DH, Stiles JW, Santelli J, Sanadi DR. Synchronous Growth and Aging of Caenorhabditis elegans in the Presence of Fluorodeoxyuridine. J Gerontol. 1979;34:28–36. doi: 10.1093/geronj/34.1.28. [DOI] [PubMed] [Google Scholar]
- Miyata S, Begun J, Troemel ER, Ausubel FM. DAF-16-dependent suppression of immunity during reproduction in Caenorhabditis elegans . Genetics. 2008;178:903–918. doi: 10.1534/genetics.107.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes P, Ernandez T, Roth I, Qiao X, Strebel D, Bouley R, Charollais A, Ramadori P, Foti M, Meda P, Féraille E, Brown D, Hasler U. Hypertonic stress promotes autophagy and microtubule-dependent autophagosomal clusters. Autophagy. 2013;9:550–567. doi: 10.4161/auto.23662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possik E, Jalali Z, Nouët Y, Yan M, Gingras M-C, Schmeisser K, Panaite L, Dupuy F, Kharitidi D, Chotard L, Jones RG, Hall DH, Pause A. Folliculin regulates ampk-dependent autophagy and metabolic stress survival. PLoS Genet. 2014;10:e1004273. doi: 10.1371/journal.pgen.1004273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Huang X, Neumann-Haefelin E, Schulze E, Baumeister R. Cell-nonautonomous signaling of FOXO/DAF-16 to the stem cells of Caenorhabditis elegans . PLoS Genet. 2012;8:e1002836. doi: 10.1371/journal.pgen.1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney JP, Luz aL, González-Hunt CP, Bodhicharla R, Ryde IT, Anbalagan C, Meyer JN. Effects of 5’-fluoro-2-deoxyuridine on mitochondrial biology in Caenorhabditis elegans . Exp. Gerontol. 2014;56:69–76. doi: 10.1016/j.exger.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual J-F, Ceron J, Koreth J, Hao T, Nicot A-S, Hirozane-Kishikawa T, Vandenhaute J, Orkin SH, Hill DE, van den Heuvel S, Vidal M. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 2004;14:2162–2168. doi: 10.1101/gr.2505604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SenGupta T, Torgersen ML, Kassahun H, Vellai T, Simonsen A, Nilsen H. Base excision repair AP endonucleases and mismatch repair act together to induce checkpoint-mediated autophagy. Nat. Commun. 2013;4 doi: 10.1038/ncomms3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L-L, Du M, Lin X-F, Cai T, Wang D-Y. Genes required for the functions of olfactory AWA neuron regulate the longevity of Caenorhabditis elegans in an insulin/IGF signaling-dependent fashion. Neurosci. Bull. 2010;26:91–103. doi: 10.1007/s12264-010-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Murphy CT. Mating induces shrinking and death in Caenorhabditis mothers. Science. 2014;343:536–540. doi: 10.1126/science.1242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Chao MY, Somers Ga, Komatsu H, Corkins ME, Larkins-Ford J, Tucey T, Dionne HM, Walsh MB, Beaumont EK, Hart DP, Lockery SR, Hart AC. C. elegans Notch signaling regulates adult chemosensory response and larval molting quiescence. Curr. Biol. 2011;21:825–834. doi: 10.1016/j.cub.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthammarak W, Yang Y-Y, Morgan PG, Sedensky MM. Complex I function is defective in complex IV-deficient Caenorhabditis elegans . J. Biol. Chem. 2009;284:6425–6435. doi: 10.1074/jbc.M805733200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Hekimi S. FUdR causes a twofold increase in the lifespan of the mitochondrial mutant gas-1 . Mech. Ageing Dev. 2011;132:519–521. doi: 10.1016/j.mad.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Manrique RP, González-Cabo P, Ros S, Aziz H, Baylis Ha, Palau F. Reduction of Caenorhabditis elegans frataxin increases sensitivity to oxidative stress, reduces lifespan, and causes lethality in a mitochondrial complex II mutant. FASEB J. 2006;20:172–174. doi: 10.1096/fj.05-4212fje. [DOI] [PubMed] [Google Scholar]
- Vermezovic J, Adamowicz M, Santarpia L, Rustighi A, Forcato M, Lucano C, Massimiliano L, Costanzo V, Bicciato S, Del Sal G, d’Adda di Fagagna F. Notch is a direct negative regulator of the DNA-damage response. Nat. Struct. Mol. Biol. 2015:1–11. doi: 10.1038/nsmb.3013. [DOI] [PubMed] [Google Scholar]
- Virk B, Correia G, Dixon DP, Feyst I, Jia J, Oberleitner N, Briggs Z, Hodge E, Edwards R, Ward J, Gems D, Weinkove D. Excessive folate synthesis limits lifespan in the C. elegans: E. coli aging model. BMC Biol. 2012;10:67. doi: 10.1186/1741-7007-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M, Guarente L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature. 2011;477:E1–E2. doi: 10.1038/nature10440. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev. Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech. Ageing Dev. 2006;127:48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Wheeler JM, Thomas JH. Identification of a novel gene family involved in osmotic stress response in Caenorhabditis elegans . Genetics. 2006;174:1327–1336. doi: 10.1534/genetics.106.059089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff RC, Phillips JP, Hilliker aJ. Increased spontaneous DNA damage in Cu/Zn superoxide dismutase (SOD1) deficient Drosophila . Genome. 2004;47:1029–1035. doi: 10.1139/g04-083. [DOI] [PubMed] [Google Scholar]
- Wyatt MD, Wilson DM. Participation of DNA repair in the response to 5-fluorouracil. Cell. Mol. Life Sci. 2009;66:788–799. doi: 10.1007/s00018-008-8557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen K, Patel HB, Lublin AL, Mobbs CV. SOD isoforms play no role in lifespan in ad lib or dietary restricted conditions, but mutational inactivation of SOD-1 reduces life extension by cold. Mech. Ageing Dev. 2009;130:173–178. doi: 10.1016/j.mad.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Zhou KI, Pincus Z, Slack FJ. Longevity and stress in Caenorhabditis elegans . Aging (Albany. NY) 2011;3:733–753. doi: 10.18632/aging.100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.