Abstract
Viruses play an important role in cancerogenesis. It is estimated that approximately 20% of all cancers are linked to infectious agents. The viral genes modulate the physiological machinery of infected cells that lead to cell transformation and development of cancer. One of the important adoptive responses by the cancer cells is their metabolic change to cope up with continuous requirement of cell survival and proliferation. In this review we will focus on how DNA viruses alter the glucose metabolism of transformed cells. Tumor DNA viruses enhance “aerobic” glycolysis upon virus-induced cell transformation, supporting rapid cell proliferation and showing the Warburg effect. Moreover, viral proteins enhance glucose uptake and controls tumor microenvironment, promoting metastasizing of the tumor cells.
1. Introduction
Development of cancer is a multistep process. Cancer cells differ from normal cells by genetic, metabolic, and histological features. Cancer cells have to fulfill their needs for continuous proliferation. Hence, they acquire various hallmarks during the process of tumor progression, such as self-sufficiency in growth signals, insensitivity to growth-inhibitory (antigrowth) signals, evasion of programmed cell death (apoptosis), limitless replicative potential, sustained angiogenesis, and tissue invasion and metastases [1].
Viruses play an important role in cancerogenesis. Globally, it is estimated that approximately 20% of all cancers are linked to infectious agents [2]. The viral genes transcribed or expressed in infected cells modulate the physiological machinery of cells that leads to cell transformation and development of tumor. One of the important adoptive responses by the cancer cells is their metabolic change to cope up with continuous requirement of cell survival and proliferation. In this review, we will focus on how DNA viruses alter the glucose metabolism of cancer cells during carcinogenesis.
2. DNA Tumor Viruses: An Overview
In 1960, Sweet and Hilleman discovered a new virus in cultures of kidney cells of rhesus monkeys, producing vaccines to poliovirus [3]. This virus was named Simian vacuolating virus (SV40). Two years later, the tumorigenic potential of this monkey virus was revealed [4]. At the same time, it was also shown that human adenoviruses could induce tumors in newborn hamsters [5]. For now, many DNA tumor viruses are known; they are grouped in four families, namely, SV40 and polyomavirus, papilloma viruses (HPV), adenoviruses, and herpesviruses. Because of their relatively small genomes and striking biological effects, it is generally assumed that DNA tumor viruses have evolved to target the minimal number of cellular nodes and pathways required for transformation. Studies of DNA viruses have led to the identification of viral genes responsible for cancer induction and paving the way to our current understanding of cancer at the molecular level [2]. In their life cycle, viruses replicate, inducing the cytopathic effect in the host cells and forming new viral particles. Herpesviruses are able to establish persistent infection transforming the host cells. HPV, adenoviruses, and polyomaviruses induce the host cell transformation while infecting nonpermissive cells and integrating into the host genome (see Table 1).
Table 1.
Human tumor DNA viruses.
| Family | Virus | Virus-cell interaction |
Associated disease | Level of association % |
Size of genome kb |
|---|---|---|---|---|---|
| Herpesviruses | Epstein-Barr virus, EBV | Episomal, rarely integrated in transformed cells | Endemic Burkitt's lymphoma (BL) AIDS-asscoiated lymphoma Nasopharengyal carcinoma (NPC) |
98 100 100 |
172 |
| Kaposi sarcoma herpes virus, KSHV | Episomal, rarely integrated in transformed cells | Kaposi's sarcoma | 97 | 165 | |
|
| |||||
| Polyoma viruses | John Cunningham Virus, JCV |
Episomal, rarely integrated in transformed nonpermissive cells | Progressive multifocal leukoencephalopathy | 50–80 | 5.2 |
| Virus of B.K. patient, BKV | Episomal, rarely integrated in transformed nonpermissive cells | Nephropathy Nephritis Hemorrhagic cystitis |
10–20 | 5.2 | |
| SV40 | Episomal, rarely integrated in transformed nonpermissive cells | Mesothelioma | 10–20 cofactor |
5.2 | |
|
| |||||
| Papilloma viruses | HPV | Episomal, integrated in transformed cells |
Cervical cancer | 71–88 (types 16 and 18) |
8 |
|
| |||||
| Adenoviruses | Integrated in transformed nonpermissive cells | Small cell lung cancer Childhood ALL |
No data | 35 kb (type 11) | |
3. Glucose Metabolism in General
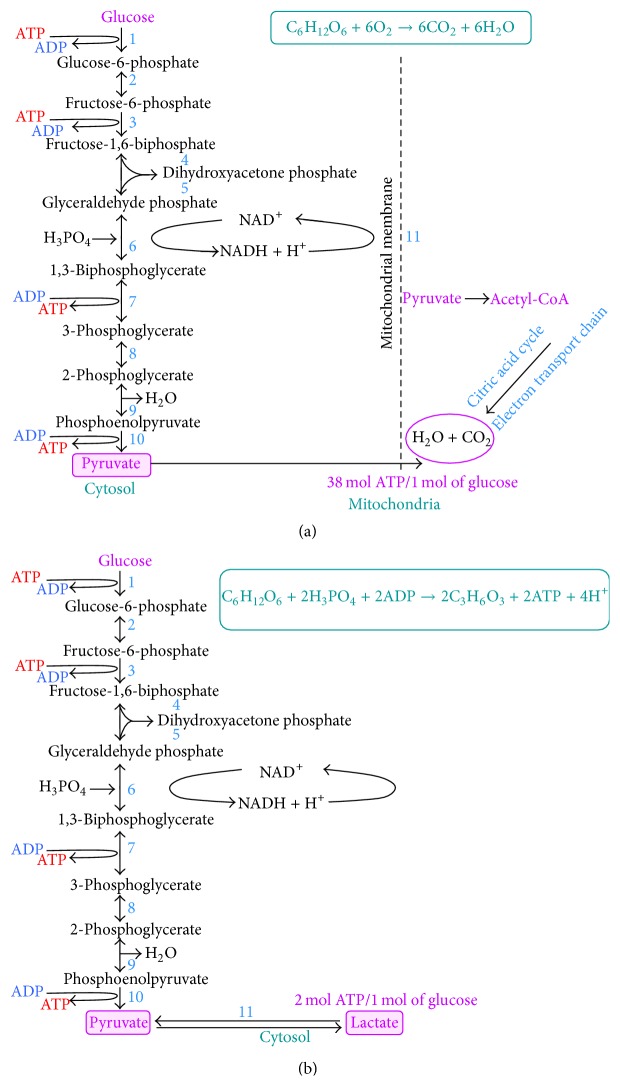
It is well known that tumor cells differ from normal cells by glucose metabolism. At the ordinary physiological conditions, one glucose molecule is converted into two pyruvate molecules. Pyruvate oxidation on mitochondria to CO2 and O2 results in synthesis of 38 ATP molecules per molecule of glucose [6]. When concentration of oxygen is diminished, no pyruvate oxidation is carried out. Pyruvate is converted to lactate; that is, anaerobic glycolysis is activated. This conversion produces NAD+, which is required for glycolysis. Glucose is also used by pentose phosphate pathway to produce nucleic acids and NADPH. NADPH is required for anabolic biosynthetic reactions as well as to neutralize ROS [7].
Cells secrete lactate and produce only 2 ATP molecules during glycolysis as compared to pyruvate oxidation [8]. Noteworthy, cancerous cells under normal conditions (in the presence of abundant oxygen) still convert pyruvate to lactate, in parallel to pyruvate oxidation; that is, the Warburg effect is observed (Figures 1(a) and 1(b)). Despite the fact that only 2 molecules of ATP are produced as a result of so-called “aerobic” glycolysis, the rate of reaction is quite high, compared to ATP synthesis on mitochondria (at least nine reactions should be carried out).
Figure 1.
Glucose metabolism at the normal and hypoxic conditions. (a) Glucose is metabolized to pyruvate; the latter undergoes Crebb's cycle in mitochondria and catabolized to CO2 and oxygen, while 38 molecules of ATP are synthesized. (b) Anaerobic metabolism of glucose, resulting in lactate production and two molecules of ATP. No mitochondria are involved in this process. Cancerous cells use this way of glucose metabolism even at the normal conditions, that is, so-called “aerobic” glycolysis takes place.
Excess lactate production increases the acidity of tumor cell microenvironment and this favors the tumor cell invasion and metastasis [9]. Anaerobic glycolysis is used by tumor cells at hypoxic conditions, which is generally found in solid tumors due to deregulated vasculature. “Aerobic” glycolysis also provides the biosynthetic advantage for tumor cells. Glycolytic intermediates are utilized by proliferating cells to produce fatty acids and nonessential amino acids [10].
In addition to glycolysis, cancer cells exhibit increased gluconeogenesis, glutaminolytic activity, glycerol turnover, pentose phosphate pathway activity, de novo fatty acid synthesis, reduced fatty acid oxidation, and modified amino acid metabolism [11]. We have to emphasize that mitochondrial respiration is not hampered in cancer cells [12] but operates at low capacity [13].
Enhanced glucose uptake has also been exploited in FDG-PET technology used clinically for the tumor detection. There are a lot of studies devoted to target the metabolic pathways as anticancer therapy [14].
4. Regulation of Warburg Effect
Many oncoproteins and tumor suppressor proteins can affect the cancer cell metabolism [15]. Transcription factor HIF1A [16] and MYC oncoprotein [17] are involved in upregulation of glucose transporters and many enzymes involved in glycolysis. MYC can also promote the expression of PKM2, resulting in faster proliferation. Tumor suppressor p53 (TP53) can inhibit glycolysis by inducing TIGAR, a regulator of glycolysis and apoptosis [18]. This can support oxidative phosphorylation by inducing SCO2, which is necessary for the formation of electron transport chain [19]. Downstream signaling molecule of PI3 Kinase, AKT1, can enhance glycolysis by various ways. AKT1 promotes glycolysis by increasing expression and membrane translocation of glucose transporters. It also phosphorylates glycolytic enzymes, such as hexokinase and phosphofructokinase 2. AKT1 stimulates mTOR kinase, which activates transcription factor HIF1A even at the normoxic conditions [15]. Loss of AMPK signaling, which is inhibitor of mTOR, also stimulates glycolysis.
5. Virus-Encoded Proteins Play an Important Role in Regulation of “Aerobic” Glycolysis
5.1. Herpesviruses
EBV (HHV4) and KSHV (HHV8) belong to the Herpesviridae family. EBV is associated with BL, a highly aggressive malignancy that is developing from germinal center B-cells [20]. Characteristic of all BL subtypes is enhanced expression of MYC oncoprotein, due to chromosomal rearrangements [21, 22]. Recently we reported that in BL cell lines MYC is the main regulator of “aerobic” glycolysis, while in LCL, with the low expression levels of MYC, HIF1A controls the Warburg effect [23, 24]. HIF1 is a heterodimer consisting of oxygen dependent transcriptional factors HIF1A and ARNT, or HIF1B. The stability of HIF1A is regulated by oxygen level, while HIF1B is constitutively expressed. At the normoxic condition HIF1A is ubiquitinated by VHL (E3 ubiquitin ligase) at the specific proline residues (402 and 564) [25] that are hydroxylated by prolyl hydroxylases (PHDs) [26, 27]. The ubiquitinated HIF1A undergoes intensive proteasomal degradation. PHD enzymes require iron (Fe2+) and ascorbate as cofactors to perform hydroxylation [28]. At hypoxic condition oxygen is not available for hydroxylation of prolines that results in stabilization of HIF1A [29]. The stabilized HIF1A translocates to nucleus where it forms the HIF1A-ARNT heterodimer and activates transcription of a set of genes and also those involved in regulation of the Warburg effect.
We have reported earlier that even under normal oxygen level EBNA3 binds to PHD2 and EBNA5 to PHD1, thus, inactivating both enzymes. As a consequence, ubiquitination of HIF1A is inhibited, and the Warburg effect is activated [24].
Another herpes virus, KSHV, also promotes the Warburg effect. KSHV induces the expression of miRNA clusters that inhibit the expression of gene, encoding the HIF1A prolyl hydroxylase PHD1 and also the mitochondrial heat shock protein HSPA9 [30]. As a result, the HIF1A is stabilized and transactivates responsive genes. It was shown that one-third of the 194 different biochemicals were altered upon infection of endothelial cells with KSHV, compared with the noninfected host cells, using a metabolomics approach. Noteworthy, the number of altered metabolic pathways was similar to that observed for cancer cells. Pathways include amino acid metabolism and many glycolytic intermediates, such as 3-phosphoglycerate and 2-phosphoglycerate and phosphoenolpyruvate. The pentose phosphate pathway intermediates, such as ribose 5-phosphate, ribulose 5-phosphate, and/or xylulose 5-phosphate, were elevated significantly in KSHV infected samples. Metabolites involved in de novo fatty acid synthesis were also increased in KSHV infected cells. Moreover, inhibition of fatty acid synthesis resulted in induction of apoptosis in infected cells [31].
5.2. Polyomaviruses
JCV, SV40, and virus, obtained from a specimen of a renal transplant patient with initials B.K. (BKV), are common polyomaviruses in human populations.
The SV40 transformed rabbit chondrocytes showed alterations in the activities of mitochondria and metabolism. Increases in “aerobic” glycolysis and in activity of glycolytic enzymes were observed in SV40-transformed cells, probably due to chromosomal rearrangements induced by virus [32]. It was also shown, using transfections of primary human fibroblasts with large and small T antigens of SV40 in different combinations along with hTERT and HRAS, that the large T antigen expression leads to decreased dependency of transformed cells on mitochondrial energy production [33]. Noteworthy, the small T antigen of SV40 expression resulted in activation of the AKT signaling, enhancing “aerobic” glycolysis [34, 35]. Interestingly, medulloblastoma cells, expressing the large T antigen of JCV, showed significantly lower mitochondrial respiration and glycolysis. Upon glucose deprivation, T-antigen expression was suppressed due to activation of AMPK, an important sensor of the AMP/ATP ratio in cells. Therefore, the consumption of glutamine increased threefold in cells that expressed the large T of JCV [36]. As was mentioned above, TP53 can inhibit the Warburg effect [37]. It is well known that the large T antigen of polyomaviruses SV40 [38], JCV [39], and BKV [40] binds to TP53 and abolishes functional activity of the latter as transcription factor. Hence, functional inactivation of TP53 not only promotes cell transformation but also induces the metabolic switch.
5.3. Papillomavirus
It was shown that HPV encoded E2 protein is localized predominantly in the nucleus of infected cells. However, in the case of oncogenic (high-risk) strains 18 and 16 the E2 protein can shuttle between cytoplasm and nucleus. It was shown, using mass spectrometry of interactome, that cytoplasmic E2 is associated with the components of respiratory chain in the inner mitochondrial membrane. Electron microscopy showed that E2 alters morphology of cristae and enhances the production of mitochondrial reactive oxygen species (ROS). Such ROS release was found concurrent with stability of HIF1A and increased rate of glycolysis [41]. Another HPV-encoded oncoprotein, E6, also can promote the Warburg effect through inhibiting the binding between HIF1A and VHL. This abolishes VHL-mediated HIF1A ubiquitination, thus stabilizing the latter [42].
5.4. Adenoviruses
The ability of adenoviruses to perform the metabolic shift was demonstrated by infection of primary rat embryonic fibroblasts (REFs) with the oncogenic adenovirus type 12, in comparison with nononcogenic types 3 and 6. REFs, infected with type 12 virus, intensively used glucose at the ordinary conditions; both, “aerobic” glycolysis and pyruvate oxidation, took place. Similar metabolic switch was observed in the hamster sarcoma cells infected with type 12 adenovirus [43].
Recently it was shown that the adenovirus encoded oncogene E4ORF1 can induce MYC that plays an important role in glycolysis. Transcriptional activity of the MYC protein is enhanced by E4ORF1. Moreover, the expression levels of enzymes involved in “aerobic” glycolysis, such as hexokinase 2 (HK2), phosphofructokinase 1 (PFKM), GAPDH, and LDHA, are increased [44]. As was discussed earlier, these enzymes are encoded by the MYC-dependent genes.
6. Involvement of DNA Tumor Viruses in Glucose Transport
As was discussed above, glucose is the preferential source of energy for cancer cells; therefore, they need massive supply of glucose compared to normal cells [45]. By hijacking glucose transport system, DNA tumor viruses are able to deliver huge amounts of glucose for proliferating cells, enhancing their tumorigenic capacity [2]. Several viral proteins can facilitate the glucose transport in cancer cells.
Notably, the rise in glucose transport in cancer cells is not due to de novo synthesis of a delivery system but by alteration of already existing glucose transport system of cells [46]. Different hypotheses have been proposed to explain this phenomenon, including not sufficient glucose dephosphorylation dependent on glucose-6-phosphatase, increase of HK expression, and/or the overexpression of glucose transporter (GLUT) proteins [47].
GLUTs are a group of membrane proteins that facilitate the transport of glucose across the plasma membrane. Human genome encodes 14 isoforms of GLUT protein, and GLUT-1,-3,-4 and -12 are involved in cancerogenesis [48]. Expression of GLUT is under the control of activated HIF1A [49]. Infection with DNA tumor viruses leads to elevated expression of GLUT proteins, increasing the glucose uptake. We have shown earlier that expression of GLUT-1 at mRNA level was induced in EBV positive LCLs and BL cell lines, compared with EBV negative cells [23, 24]. Upon latent infection of human monocytic cell lines with KSHV, GLUT1, and HK expression are increased at the protein level [50].
Not only are the levels of glucose transporter molecules elevated, the trafficking mechanism is also altered to ensure ample supply of glucose. Virus encoded proteins enhance the translocation of GLUT molecules to a cell surface; hence, there is another strategy to increase the glucose uptake. For example, AKT hyperphosphorylation upon KSHV infection correlates with plasma membrane exposure of GLUT1 [51]. Similarly, EBV also induces the translocation of GLUT1 via protein kinase IKKB-AKT pathway [52].
The HPV18-encoded protein E6 participates in stimulation of the SGLT1 activity. By this way, E6 accomplish cellular glucose uptake through Na+-coupled glucose transport mediated by SGLT1 [53]. It is noteworthy that in SV40 transformed mouse 3T3 cells hexose transporters are relocated from microsomal membranes to plasma membrane, suggesting that oncogenic DNA viruses utilize not only transcriptional regulation of glucose transport but also alterations in transporter trafficking during transformation [54].
7. Effect of DNA Oncoviruses on Secretion of Aerobic Glycolytic Waste
Lactate secreted into an extracellular matrix plays an important role in tumor metastasizing. This process is promoted by lactate-induced secretion of the hyaluronic acid by cancer-associated fibroblasts, thus generating an environment favorable for migration of tumor cells [55]. Moreover, lactate produced by tumor cells helps them to evade immune system by modulating dendritic cell activation and antigen expression that mediate the T cell responses [56, 57].
Activated T cells themselves use glycolysis as a main source of energy [58–60]. Importantly, the immune cells are struggling to get rid of lactate produced by themselves: cellular lactate transport depends on the ratio between the intra- and extracellular concentrations of lactate. Ultimately, leukocytes may be asphyxiated by lactate [61]. Cancerous cells of solid tumors ensure sufficient supply of nutrient and oxygen for rapid proliferation via lactate mediated upregulation of VEGF, thus inducing the angiogenesis [62]. Lactate stimulates the angiogenesis also via PI3K/AKT pathway [63].
The major transporter molecules of lactate in cells are monocarboxylate transporters (MCTs). MCT family consists of 14 members that are encoded by SLC16A gene family. The four MCTs (MCT1, MCT2, MCT3, and MCT4) are responsible for proton-linked transport of metabolically important monocarboxylates such as lactate, pyruvate, and ketone bodies [64–67]. MCTs carry 12 transmembrane domains with intracellular N- and C-termini and a large intracellular loop between transmembrane domains 6 and 7. MCT1 and MCT4 require a monotopic ancillary protein, CD147, for plasma membrane expression and function [68]. CD147 is a multifunctional glycoprotein expressed at higher levels by cancer cells and stromal cells in the tumor microenvironment [69]. KSHV-encoded latency associated nuclear antigen LANA either induces CD147 directly, binding to gene promoter, or transactivates CD147 upon interactions with specificity protein 1 or early growth response protein 2 [70, 71]. Upregulation of MCT4 and CD147 has been also reported in HPV-induced squamous cell carcinoma of the uterine cervix [72]. Importantly, in BL cells MCT4 was also greatly upregulated [23].
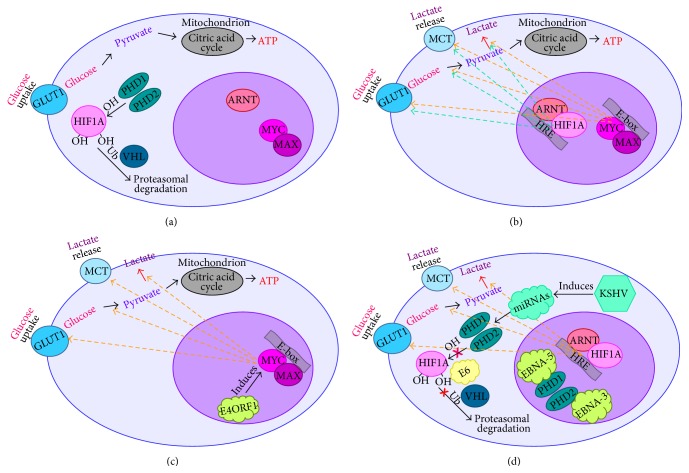
In conclusion, tumor DNA viruses modify metabolism of the transformed cells, supporting their rapid proliferation and showing the Warburg effect (summarized on Figure 2). Moreover, viral proteins enhance glucose uptake and controls tumor microenvironment, promoting metastasizing of the tumor cells.
Figure 2.
Tumor DNA viruses modify metabolism of the transformed cells. (a) Glucose is metabolized to pyruvate; the latter undergoes Crebb's cycle in mitochondria. (b) “Aerobic” glycolysis takes place, resulting in lactate production. HIF1A-ARNT and MYC-MAX heterodimers induce expression of a set of genes that are involved in glycolysis. (c) E4ORF1 encoded by adenoviruses induces MYC that lead to activation of glycolysis upon infection. (d) HPV-encoded E6 prevents ubiquitination of HIF1A by VHL protein; EBV-encoded EBNA-3 and EBNA-5 bind to PHD2 and PHD2, correspondingly, leading to inhibition of HIF1A hydroxylation; upon infection with KSHV a cluster of miRNAs is activated, resulting in inactivation of PHDs. This leads to stabilization of the HIF1A protein and, hence, to activation of “aerobic” glycolysis.
Acknowledgments
Our work was supported by the Swedish Cancer Society, by matching grants from the Concern Foundation (Los Angeles) and the Cancer Research Institute (New York), and by Karolinska Institutet.
Abbreviations
- AKT1:
RAC-alpha serine/threonine-protein kinase 1
- AMPK:
Adenosine monophosphate kinase
- ARNT:
Aryl hydrocarbon receptor nuclear translocator
- ATP:
Adenosine triphosphate
- BKV:
BK virus
- BL:
Burkitt lymphoma
- CD147:
Extracellular matrix metalloproteinase inducer
- E4ORF1:
Early Region 4 Open Reading Frame 1
- EBNA:
EBV-encoded nuclear antigen
- EBV:
Epstein-Barr virus
- FDG-PET:
Fluorodeoxyglucose (18F) positron emission tomography
- GAPDH:
Glyceraldehyde-3-phosphate dehydrogenase
- GLUT:
Glucose transporter
- HIF1A:
Hypoxia inducible factor 1A
- HK:
Hexokinase
- HPV:
Human papilloma virus
- HRAS:
H-rat sarcoma
- HSPA9:
Heat shock protein-A9
- hTERT:
Human telomerase reverse transcriptase
- IKKB:
Inhibitor of nuclear factor kappa-B kinase subunit beta
- JCV:
John Cunningham virus
- KSHV:
Kaposi sarcoma herpes virus
- LANA:
Latency-associated nuclear antigen
- LDHA:
Lactate dehydrogenase A
- MCT:
Monocarboxylate transporter
- mTOR:
Mechanistic target of rapamycin
- NAD:
Nicotinamide adenine dinucleotide
- NADPH:
Nicotinamide adenine dinucleotide phosphate
- PFKM:
Phosphofructokinase 1
- PHD:
Prolyl hydroxylase domain-containing protein
- PI3K:
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- PKM2:
Pyruvate kinase M2
- REF:
Rat embryonic fibroblast
- ROS:
Reactive oxygen specie
- SCO2:
Cytochrome c oxidase 2
- SGLT1:
Sodium-glucose transport protein 1
- SLC16A:
Family of proton coupled MCTs
- SV40:
Simian vacuolating virus
- TIGAR:
TP53-inducible glycolysis and apoptosis regulator
- TP53 or p53:
Tumor suppressor p53
- VEGF:
Vascular endothelial growth factor
- VHL:
Von Hippel–Lindau.
Conflict of Interests
Authors declare no competing interests.
References
- 1.Hanahan D., Weinberg R. A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Ríhová B. Infectious causes of cancer: targets for intervention J.J. Goedert (Ed.) Folia Microbiologica. 2000;45(3):279–280. doi: 10.1007/BF02908960. [DOI] [Google Scholar]
- 3.Sweet B. H., Hilleman M. R. The vacuolating virus, S.V. 40. Proceedings of the Society for Experimental Biology and Medicine. 1960;105:420–427. doi: 10.3181/00379727-105-26128. [DOI] [PubMed] [Google Scholar]
- 4.Girardi A. J., Sweet B. H., Slotnick V. B., Hilleman M. R. Development of tumors in hamsters inoculated in the neonatal period with vacuolating virus, SV40 . Experimental Biology and Medicine. 1962;109(3):649–660. doi: 10.3181/00379727-109-27298. [DOI] [PubMed] [Google Scholar]
- 5.Yabe Y., Trentin J. J., Taylor G. Cancer induction in hamsters by human type 12 adenovirus. Effect of age and of virus dose. Experimental Biology and Medicine. 1962;111(2):343–344. doi: 10.3181/00379727-111-27786. [DOI] [PubMed] [Google Scholar]
- 6.Heiden M. G. V., Cantley L. C., Thompson C. B. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan C., Ding A. SnapShot: Reactive Oxygen Intermediates (ROI) Cell. 2010;140(6):p. 951.e2. doi: 10.1016/j.cell.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 8.DeBerardinis R. J., Lum J. J., Hatzivassiliou G., Thompson C. B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metabolism. 2008;7(1):11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Gillies R. J., Robey I., Gatenby R. A. Causes and consequences of increased glucose metabolism of cancers. Journal of Nuclear Medicine. 2008;49(supplement 2):24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- 10.Fritz V., Fajas L. Metabolism and proliferation share common regulatory pathways in cancer cells. Oncogene. 2010;29(31):4369–4377. doi: 10.1038/onc.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dakubo G. D. Mitochondrial Genetics and Cancer. Springer Science & Business Media; 2010. [Google Scholar]
- 12.Moreno-Sánchez R., Rodríguez-Enríquez S., Marín-Hernández A., Saavedra E. Energy metabolism in tumor cells. The FEBS Journal. 2007;274(6):1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 13.Bellance N., Benard G., Furt F., et al. Bioenergetics of lung tumors: alteration of mitochondrial biogenesis and respiratory capacity. The International Journal of Biochemistry & Cell Biology. 2009;41(12):2566–2577. doi: 10.1016/j.biocel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Vander Heiden M. G. Targeting cancer metabolism: a therapeutic window opens. Nature Reviews Drug Discovery. 2011;10(9):671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 15.Cairns R. A., Harris I. S., Mak T. W. Regulation of cancer cell metabolism. Nature Reviews Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 16.Semenza G. L. HIF-1: upstream and downstream of cancer metabolism. Current Opinion in Genetics & Development. 1994;20(1):51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dang C. V. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Research. 2010;70(3):859–862. doi: 10.1158/0008-5472.can-09-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bensaad K., Tsuruta A., Selak M. A., et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 19.Matoba S., Kang J.-G., Patino W. D., et al. p53 regulates mitochondrial respiration. Science. 2006;312(5780):1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 20.Klein G., Klein E., Kashuba E. Interaction of Epstein-Barr virus (EBV) with human B-lymphocytes. Biochemical and Biophysical Research Communications. 2010;396(1):67–73. doi: 10.1016/j.bbrc.2010.02.146. [DOI] [PubMed] [Google Scholar]
- 21.Hecht J. L., Aster J. C. Molecular biology of Burkitt's lymphoma. Journal of Clinical Oncology. 2000;18(21):3707–3721. doi: 10.1200/JCO.2000.18.21.3707. [DOI] [PubMed] [Google Scholar]
- 22.ar-Rushdi A., Nishikura K., Erikson J., Watt R., Rovera G., Croce C. M. Differential expression of the translocated and the untranslocated c-myc oncogene in Burkitt lymphoma. Science. 1983;222(4622):390–393. doi: 10.1126/science.6414084. [DOI] [PubMed] [Google Scholar]
- 23.Mushtaq M., Darekar S., Klein G., Kashuba E. Different mechanisms of regulation of the Warburg effect in lymphoblastoid and Burkitt lymphoma cells. PLoS ONE. 2015;10(8) doi: 10.1371/journal.pone.0136142.e0136142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darekar S., Georgiou K., Yurchenko M., et al. Epstein-barr virus immortalization of human B-cells leads to stabilization of hypoxia-induced factor 1 alpha, congruent with the Warburg effect. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0042072.e42072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maxwell P. H., Wiesener M. S., Chang G. W., et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 26.Jaakkola P., Mole D. R., Tian Y.-M., et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 27.Ivan M., Kondo K., Yang H., et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 28.McDonough M. A., Li V., Flashman E., et al. Cellular oxygen sensing: crystal structure of hypoxia-inducible factor prolyl hydroxylase (PHD2) Proceedings of the National Academy of Sciences of the United States of America. 2006;103(26):9814–9819. doi: 10.1073/pnas.0601283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page E. L., Chan D. A., Giaccia A. J., Levine M., Richard D. E. Hypoxia-inducible factor-1alpha stabilization in nonhypoxic conditions: role of oxidation and intracellular ascorbate depletion. Molecular Biology of the Cell. 2008;19(1):86–94. doi: 10.1091/mbc.e07-06-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yogev O., Lagos D., Enver T., Boshoff C., Cullen B. R. Kaposi's sarcoma herpesvirus microRNAs induce metabolic transformation of infected cells. PLoS Pathogens. 2014;10(9) doi: 10.1371/journal.ppat.1004400.e1004400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delgado T., Carroll P. A., Punjabi A. S., Margineantu D., Hockenbery D. M., Lagunoff M. Induction of the Warburg effect by Kaposi's sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proceedings of the National Academy of Sciences. 2010;107(23):10696–10701. doi: 10.1073/pnas.1004882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bravard A., Beaumatin J., Luccioni C., et al. Chromosomal, mitochondrial and metabolic alterations in SV40-transformed rabbit chondrocytes. Carcinogenesis. 1992;13(5):767–772. doi: 10.1093/carcin/13.5.767. [DOI] [PubMed] [Google Scholar]
- 33.Ramanathan A., Wang C., Schreiber S. L. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(17):5992–5997. doi: 10.1073/pnas.0502267102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Viciana P., Collins C., Fried M. Polyoma and SV40 proteins differentially regulate PP2A to activate distinct cellular signaling pathways involved in growth control. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(51):19290–19295. doi: 10.1073/pnas.0609343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elstrom R. L., Bauer D. E., Buzzai M., et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Research. 2004;64(11):3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 36.Noch E., Sariyer I. K., Gordon J., Khalili K. JC virus T-antigen regulates glucose metabolic pathways in brain tumor cells. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035054.e35054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madan E., Gogna R., Bhatt M., Pati U., Kuppusamy P., Mahdi A. A. Regulation of glucose metabolism by p53: emerging new roles for the tumor suppressor. Oncotarget. 2011;2(12):948–957. doi: 10.18632/oncotarget.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali S. H., DeCaprio J. A. Cellular transformation by SV40 large T antigen: interaction with host proteins. Seminars in Cancer Biology. 2001;11(1):15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]
- 39.Staib C., Pesch J., Gerwig R., et al. p53 inhibits JC virus DNA replication in vivo and interacts with JC virus large T-antigen. Virology. 1996;219(1):237–246. doi: 10.1006/viro.1996.0241. [DOI] [PubMed] [Google Scholar]
- 40.Shivakumar C. V., Das G. C. Interaction of human polyomavirus BK with the tumor-suppressor protein p53. Oncogene. 1996;13(2):323–332. [PubMed] [Google Scholar]
- 41.Lai D., Tan C. L., Gunaratne J., et al. Localization of HPV-18 E2 at mitochondrial membranes induces ROS release and modulates host cell metabolism. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0075625.e75625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Y., Meng X., Ma J., et al. Human papillomavirus 16 E6 contributes HIF-1alpha induced Warburg effect by attenuating the VHL-HIF-1alpha interaction. International Journal of Molecular Sciences. 2014;15(5):7974–7986. doi: 10.3390/ijms15057974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ageyenko A., Knm I., Rimofeyev V. T., Kogan I. Ya., Saprin A. N. Glycolysis in Adenovirus Infected Rat Cell Cultures and in Adenovirus Type 12 Induced Hamster Sarcoma Cells. National Aeronautics and Space Administration; 1971. [Google Scholar]
- 44.Thai M., Graham N. A., Braas D., et al. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metabolism. 2014;19(4):694–701. doi: 10.1016/j.cmet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gatenby R. A., Gillies R. J. Why do cancers have high aerobic glycolysis? Nature Reviews Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 46.Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. The Journal of Biological Chemistry. 1973;248(9):2978–2983. [PubMed] [Google Scholar]
- 47.Calvo M. B., Figueroa A., Pulido E. G., Campelo R. G., Aparicio L. A. Potential role of sugar transporters in cancer and their relationship with anticancer therapy. International Journal of Endocrinology. 2010;2010:14. doi: 10.1155/2010/205357.205357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barron C., Tsiani E., Tsakiridis T. Expression of the glucose transporters GLUT1, GLUT3, GLUT4 and GLUT12 in human cancer cells. BMC Proceedings. 2012;6(supplement 3):p. P4. doi: 10.1186/1753-6561-6-s3-p4. [DOI] [Google Scholar]
- 49.Chen C., Pore N., Behrooz A., Ismail-Beigi F., Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. Journal of Biological Chemistry. 2001;276(12):9519–9525. doi: 10.1074/jbc.m010144200. [DOI] [PubMed] [Google Scholar]
- 50.Yu Y., Maguire T. G., Alwine J. C. Human cytomegalovirus activates glucose transporter 4 expression to increase glucose uptake during infection. Journal of Virology. 2011;85(4):1573–1580. doi: 10.1128/jvi.01967-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonnella R., Santarelli R., Farina A., et al. Kaposi sarcoma associated herpesvirus (KSHV) induces AKT hyperphosphorylation, bortezomib-resistance and GLUT-1 plasma membrane exposure in THP-1 monocytic cell line. Journal of Experimental & Clinical Cancer Research. 2013;32, article 79 doi: 10.1186/1756-9966-32-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sommermann T. G., O'Neill K., Plas D. R., Cahir-McFarland E. IKKκ and NF-κB transcription govern lymphoma cell survival through AKT-induced plasma membrane trafficking of GLUT1. Cancer Research. 2011;71(23):7291–7300. doi: 10.1158/0008-5472.can-11-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leiprecht N., Munoz C., Alesutan I., et al. Regulation of Na+-coupled glucose carrier SGLT1 by human papillomavirus 18 E6 protein. Biochemical and Biophysical Research Communications. 2011;404(2):695–700. doi: 10.1016/j.bbrc.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 54.Kitagawa K., Nishino H., Iwashima A. Analysis of hexose transport in untransformed and sarcoma virus-transformed mouse 3T3 cells by photoaffinity binding of cytochalasin B. Biochimica et Biophysica Acta (BBA)–Biomembranes. 1985;821(1):63–66. doi: 10.1016/0005-2736(85)90153-1. [DOI] [PubMed] [Google Scholar]
- 55.Stern R., Shuster S., Neudecker B. A., Formby B. Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Experimental Cell Research. 2002;276(1):24–31. doi: 10.1006/excr.2002.5508. [DOI] [PubMed] [Google Scholar]
- 56.Nasi A., Fekete T., Krishnamurthy A., et al. Dendritic cell reprogramming by endogenously produced lactic acid. The Journal of Immunology. 2013;191(6):3090–3099. doi: 10.4049/jimmunol.1300772. [DOI] [PubMed] [Google Scholar]
- 57.Gottfried E., Kunz-Schughart L. A., Ebner S., et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107(5):2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- 58.Brand K., Williams J. F., Weidemann M. J. Glucose and glutamine metabolism in rat thymocytes. Biochemical Journal. 1984;221(2):471–475. doi: 10.1042/bj2210471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brand K., Leibold W., Luppa P., Schoerner C., Schulz A. Metabolic alterations associated with proliferation of mitogen-activated lymphocytes and of lymphoblastoid cell lines: evaluation of glucose and glutamine metabolism. Immunobiology. 1986;173(1):23–34. doi: 10.1016/s0171-2985(86)80086-9. [DOI] [PubMed] [Google Scholar]
- 60.Brand K., Netzker R., Aulwurm U., et al. Control of thymocyte proliferation via redox-regulated expression of glycolytic genes. Redox Report. 2000;5(1):52–54. doi: 10.1179/rer.2000.5.1.52. [DOI] [PubMed] [Google Scholar]
- 61.Fischer K., Hoffmann P., Voelkl S., et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109(9):3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 62.Beckert S., Farrahi F., Aslam R. S., et al. Lactate stimulates endothelial cell migration. Wound Repair and Regeneration. 2006;14(3):321–324. doi: 10.1111/j.1743-6109.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 63.Ruan G.-X., Kazlauskas A. Lactate engages receptor tyrosine kinases Axl, Tie2, and vascular endothelial growth factor receptor 2 to activate phosphoinositide 3-kinase/AKT and promote angiogenesis. The Journal of Biological Chemistry. 2013;288(29):21161–21172. doi: 10.1074/jbc.m113.474619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deuticke B. Monocarboxylate transport in erythrocytes. The Journal of Membrane Biology. 1982;70(2):89–103. doi: 10.1007/bf01870219. [DOI] [PubMed] [Google Scholar]
- 65.Bonen A., Tonouchi M., Miskovic D., Heddle C., Heikkila J. J., Halestrap A. P. Isoform-specific regulation of the lactate transporters MCT1 and MCT4 by contractile activity. American Journal of Physiology—Endocrinology and Metabolism. 2000;279(5):E1131–E1138. doi: 10.1152/ajpendo.2000.279.5.E1131. [DOI] [PubMed] [Google Scholar]
- 66.Bröer S., Bröer A., Schneider H.-P., Stegen C., Halestrap A. P., Deitmer J. W. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochemical Journal. 1999;341, part 3:529–535. doi: 10.1042/0264-6021:3410529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grollman E. F., Philp N. J., McPhie P., Ward R. D., Sauer B. Determination of transport kinetics of chick MCT3 monocarboxylate transporter from retinal pigment epithelium by expression in genetically modified yeast. Biochemistry. 2000;39(31):9351–9357. doi: 10.1021/bi000464. [DOI] [PubMed] [Google Scholar]
- 68.Halestrap A. P., Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Archiv. 2004;447(5):619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 69.Amit-Cohen B., Rahat M. M., Rahat M. A. Tumor cell-macrophage interactions increase angiogenesis through secretion of EMMPRIN. Frontiers in Physiology. 2013;4, article 178 doi: 10.3389/fphys.2013.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dupin N., Fisher C., Kellam P., et al. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(8):4546–4551. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barbera A. J., Chodaparambil J. V., Kelley-Clarke B., et al. The nucleosomal surface as a docking station for Kaposi's sarcoma herpesvirus LANA. Science. 2006;311(5762):856–861. doi: 10.1126/science.1120541. [DOI] [PubMed] [Google Scholar]
- 72.Pinheiro C., Garcia E. A., Morais-Santos F., et al. Lactate transporters and vascular factors in HPV-induced squamous cell carcinoma of the uterine cervix. BMC Cancer. 2014;14, article 751 doi: 10.1186/1471-2407-14-751. [DOI] [PMC free article] [PubMed] [Google Scholar]