Abstract
Background
Montelukast is a cysteinyl-leukotriene type 1 (CysLT1) selective receptor antagonist. In recent years, investigations have shown that montelukast possesses secondary anti-inflammatory activities and also antioxidant effects. For this reason, we aimed to determine the possible effects of montelukast on liver damage in experimental obstructive jaundice.
Methods
30 Wistar-Albino male rats were randomized and divided into three groups of 10 animals each: group I, sham-operated; group II, ligation and division of the common bile duct (BDL) followed by daily intraperitoneal injection of 1 ml of saline; group III, BDL followed by daily intraperitoneal injection of 10 mg/kg montelukast dissolved in saline. The animals were killed on postoperative day 7 by high-dose diethyl ether inhalation. Blood and liver samples were taken for examination.
Results
In this study, liver malondialdehyde (MDA) (p = 0.001), myeloperoxidase (p = 0.003), and total sulfhydryl (SH) (p = 0.009) were found to be significantly different between the BDL + montelukast and the BDL groups. Plasma total SH (p = 0.002) and MDA (p = 0.027) values were also statistically different between these groups. Statistical analyses of histological activity index scores showed that the histopathological damage in the BDL + montelukast group was significantly less than the damage in the control group (p < 0.05 for all pathological parameters).
Conclusion
According to the results of this study, montelukast showed a significant hepatoprotective effect in this experimental obstructive jaundice model, which might be due to its antioxidant and anti-inflammatory activities.
Key Words: Montelukast, Obstructive jaundice, Hepatobiliary diseases, Oxidative stress, Histopathology
Introduction
Patients with jaundice due to extrahepatic biliary obstruction still experience a high rate of complications and death. Many complications are infectious in nature or related to defects in host defense, and others are systemic [1]. The major complications of untreated obstructive jaundice are cholangitis, coagulation defects, and liver damage progressing to biliary fibrosis and cirrhosis [2]. Surgical procedures for the relief of obstructive jaundice are associated with high mortality and morbidity rates, mainly due to postoperative complications such as hepatic failure, sepsis, bleeding, renal failure, and pulmonary dysfunction [3]. Although the mechanisms of liver damage associated with cholestasis are complex and multifactorial, bile acid-mediated hepatotoxicity certainly plays a pivotal role in the pathogenesis of the disease. Oxidative stress and lipid peroxidation are also involved in the pathogenesis of cholestatic liver injury [4].
Montelukast is a prototype, selective, pharmacological antagonist of type 1 cysteinyl-leukotriene receptors (CysLT1Rs). It effectively antagonizes the proasthmatic, proinflammatory, and priming activities of cysteinyl leukotrienes (CysLTs) and forms part of numerous international guidelines for asthma therapy. Interestingly, recent evidence suggests that montelukast possesses a range of secondary anti-inflammatory activities, apparently unrelated to the antagonism of CysLT1Rs, and also shows antioxidant activity [5]. The anti-inflammatory and antioxidant effects of montelukast have been emphasized by many authors. Sener et al. [6] showed that montelukast is protective against oxidative damage resulting from reactive oxygen radicals and lipid peroxidation after thermal injury. Tugtepe et al. [7] concluded that montelukast had protective effects on the kidney by inhibiting neutrophil infiltration, balancing the oxidant-antioxidant status and regulating the generation of inflammatory mediators. Coskun et al. [8] found that montelukast had a marked effect on lung, liver, heart, and kidney tissues by attenuating or decreasing the myeloperoxidase (MPO) levels. Mohamadin et al. [9] demonstrated that the administration of montelukast protects the liver from lipopolysaccharide-induced oxidative damage.
With reference to the antioxidant and anti-inflammatory properties, the effects of montelukast on liver damage were evaluated in this study of an obstructive jaundice model in rats.
Material and Methods
Animals
Thirty Wistar-Albino male rats, weighing 250 ± 25 g, were allowed to adapt to laboratory conditions for 1 week before experimental use. The animals had free access to water and standard laboratory chow. They were individually housed in wire cages under constant temperature (21 ± 2 °C) and under a 12-h light-dark cycle. 12 h before anesthesia, the animals were deprived of food but had free access to water until 2 h before anesthesia. No enteral or parenteral antibiotics were administered at any time. Rats that died during the experiment were excluded from the study and no new rats were included. The procedures in this experimental study were performed in accordance with the National Guidelines for the Use and Care of Laboratory Animals, and approval for the study was granted by the Animal Ethics Committee of Ankara Research and Training Hospital.
Study Groups
The rats were randomized and divided into 3 groups of 10 animals each: group I, sham-operated; group II, ligation and division of the common bile duct (BDL) followed by daily intraperitoneal injection of 1 ml of saline; group III, BDL followed by daily intraperitoneal injection of 10 mg/kg montelukast dissolved in saline. The animals were killed on postoperative day 7 by high-dose diethyl ether inhalation. Liver biopsy and blood samples were taken for examination.
Operative Procedure
The animals were anesthetized by intramuscular injection of 30 mg/kg ketamine hydrochloride (Ketalar®; Parke-Davis, Istanbul, Turkey) and 5 mg/kg xylazine (Rompun®; Bayer, Istanbul, Turkey). A midline laparotomy was performed under sterile conditions. In the sham-operated group (group I), the common bile duct (CBD) was freed from the surrounding soft tissue and manipulated without ligation and transection. In groups II and III, the CBD was identified in each rat, double-ligated with 5-0 silk, and divided between the ligatures. The same surgeon performed all procedures. The abdominal incisions were closed in two layers with continuous 3-0 silk sutures. The animals were allowed to feed after the operation.
This experimental model of obstructive jaundice is the most preferred model which considerably mimics the clinical features of complete obstructive jaundice in humans. Although there are some conflicts regarding the similarity of the model to the clinical situation, this model is widely used all over the world for preclinical experimental studies evaluating the effects of different treatment modalities on the relief of obstructive jaundice. Even though the mechanism of liver damage associated with cholestasis is complex and multifactorial, oxidative stress and inflammation are two main mechanisms that influence the degree of liver damage during cholestasis. Thus, oxidative stress parameters and the degree of pathological inflammation were examined as possible responsible factors of liver damage in the current study.
Evaluation of Oxidative Stress
The evaluation of oxidative stress parameters was performed in the Department of Biochemistry of the Ankara Education and Research Hospital. Liver biopsy samples were stored at a temperature of −80 °C until the assays. Tissue malondialdehyde (MDA), total sulfhydryl (SH) levels, and MPO enzyme activities were measured. Plasma MDA and total SH levels were also evaluated.
MDA is an indicator of lipid peroxidation and is known to be an index of tissue injury. MDA levels were calculated by the fluorometric method, as described by Wasowicz et al. [10]. After the reaction of thiobarbituric acid (TBA) with MDA, the reaction product was extracted in butanol and measured spectrofluorometrically at wavelengths of 525 nm for excitation and 547 nm for emission. 0-5 μmol/l 1,1,3,3-tetraethoxypropane solution was used as a standard. For the measurement of tissue MDA levels, 50 μl of homogenate were added and introduced into 10-ml glass tubes containing 1 ml of distilled water. After the addition of 1 ml of the solution containing 29 mmol/l TBA in acetic acid and mixing, the samples were placed in a water bath and heated for 1 h at 95-100 °C. The samples were then cooled, 25 μl of 5 mol/l HCL were added, and the reaction mixture was extracted by agitation for 5 min with 3.5 ml of n-butanol. After separation of the butanol phase by centrifugation at 1500 × g for 10 min, the fluorescence of the butanol extract was measured with a fluorometer (HITACHI F-2500) at wavelengths of 525 nm for excitation and 547 nm for emission. 0-5 μmol/l 1,1,3,3-tetraethoxypropane solutions were used as a standard. MDA levels were given as µmol/g wet tissue.
Total SH levels are found to be lower in injured tissues when compared with normal tissues. Total SH groups were measured spectrophotometrically using the method of Sedlak and Lindsay [11]. Aliquots of 250 μl of the supernatant fraction of the tissue homogenate were mixed in 5-ml test tubes with 750 μl of 0.2 M Tris buffer, pH 8.2, and 50 μl of 0.01 M 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB). The mixture was brought up to 5 ml with 3,950 μl of absolute methanol. A reagent blank (without sample) and a sample blank (without DTNB) were prepared in a similar manner. The test tubes were stoppered with rubber caps, the color was developed for 15 min, and the reaction mixtures were centrifuged at approximately 3,000 × g at room temperature for 15 min. The absorbance of supernatant fractions was given at 412 nm in a spectrophotometer.
MPO is an enzyme that has specific activity in neutrophils and is widely used as an enzyme marker for the degree of neutrophil infiltration. MPO activity was assayed spectrophotometrically by determining the decomposition of hydrogen peroxide, using o-dianisidine as the hydrogen donor. Tissue samples of approximately 50 mg were taken, weighed, and homogenized three times for 30 s at 4 °C in 1 ml of ice-cold 0.5% hexadecyltrimethylammonium bromide in 50 mmol/l phosphate buffer (pH 6). The homogenate was subjected to three freeze/thaw cycles and centrifuged for 15 min at 40,000 × g. MPO activity was determined by the addition of 0.1 ml of the supernatant to 2.9 ml of 50 mmol/l phosphate buffer containing 0.167 mg/ml o-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. The change in absorbance at 460 nm over a 5-min period was measured at 25 °C. The data were expressed as the change in absorbance/min/g wet weight [12].
Histopathological Examination
The histopathological analyses were performed in the Department of Pathology of Aksaray Hospital. For light microscopy analyses, the samples were obtained from the liver and fixed in 10% neutral buffered formaline solution for 2 days. The tissues were washed in running water and dehydrated with increasing concentrations of ethanol (50, 75, 96, 100%). After dehydration, the specimens were put into xylene to obtain transparency and then infiltrated with and embedded in paraffin. The embedded tissues were cut into 5-µm thick sections using a Leica RM 2125 RT microtome and stained with hematoxylin & eosin (H & E) and trichrome. The histopathological examinations were performed with a light microscope (Olympus, BX51TF) by a pathologist blinded to the study design. Inflammatory activities and fibrosis were evaluated semiquantitatively according to the modified histological activity index (HAI) described by Ishak [13,14].
The scoring scales of the pathological findings are given in table 1.
Table 1.
Scoring scale of fibrosis and liver damage
| Pathological findings | Scores |
|---|---|
| No fibrosis | 0 |
| Fibrosis of some portal areas without fibrous septae | 1 |
| Fibrosis of some portal areas with fibrous septae | 2 |
| Fibrosis in portal areas, sparse porto-portal (P-P) bridging fibrosis | 3 |
| Fibrosis in portal areas and dense (P-P) and porto-central (P-C) bridging fibrosis | 4 |
| Dense P-P and P-C bridging fibrosis and sparse nodules (incomplete cirrhosis) | 5 |
| Complete cirrhosis | 6 |
| Severity of liver damage | Scores |
| No damage | 0 |
| Mild | 1 |
| Moderate | 2 |
| Severe | 3 |
Statistical Analysis
Data analysis was performed using the Statistical Package for Social Sciences (SPSS) version 15.0 for Windows (SPSS Inc., Chicago, IL, USA). All variables were normally distributed around the mean. Data were presented as mean ± standard deviation. Differences between the groups were evaluated by one-way analysis of variance (ANOVA) or Kruskal-Wallis variance analysis, whichever was appropriate. When the p values from the variance analysis were statistically significant, the Tukey's honestly significant difference test or Kruskal-Wallis multiple comparison test was used to determine which group was different from the others. A p value of <0.05 was considered to be statistically significant.
Results
General
Two rats from group II (BDL) and 2 from group III (BDL + montelukast) died during the study period.
Oxidative Stress
The mean levels of the oxidative stress parameters of the liver (MDA, MPO, and total SH) and plasma (MDA and total SH) are summarized in table 2. Liver MDA (p = 0.001), MPO (p = 0.003), and total SH (p = 0.009) were significantly different between the BDL + montelukast and the BDL groups. Plasma total SH (p = 0.002) and MDA (p = 0.027) values were also statistically different between these groups. When oxidative stress parameters were evaluated, there was no significant difference between the sham and the BDL + montelukast groups (p > 0.05).
Table 2.
Oxidative stress parameters of liver and plasma
| Group I (sham) (n = 10) | Group II (BDL) (n = 8) | Group III (BDL + montelukast) (n =8) | |
|---|---|---|---|
| MDA, tissue (µmol/g tissue) | 43.92 ± 9.69a | 115.14 ± 12.74a,b | 64.51 ± 11.03b |
| Total SH, tissue (µmol/g protein) | 52.78 ± 9.18a | 29.36 ± 6.17a,b | 48.46 ± 10.22b |
| MPO, tissue | 0.04 ± 0.20a | 0.12 ± 0.06a,b | 0.06 ± 0.02b |
| MDA, plasma (µmol/l) | 2.09 ± 0.31a | 4.09 ± 1.22a,b | 2.63 ± 0.86b |
| Total SH, plasma (µmol/l) | 54.55 ± 10.45a | 21.32 ± 6.44a,b | 50.48 ± 8.65b |
Significantly different, sham vs. BDL group.
Significantly different, BDL vs. BDL + montelukast group.
BDL = Bile duct ligated; MDA = malondialdehyde; MPO = myeloperoxidase; SH = sulfhydryl.
Histopathological Results
The typical histopathological changes are presented in figures 1, 2, 3. For each group, one demonstrative subject was selected and different panels of the figures (a-c or a-d) were achieved from different areas of the same subject with different magnifications.
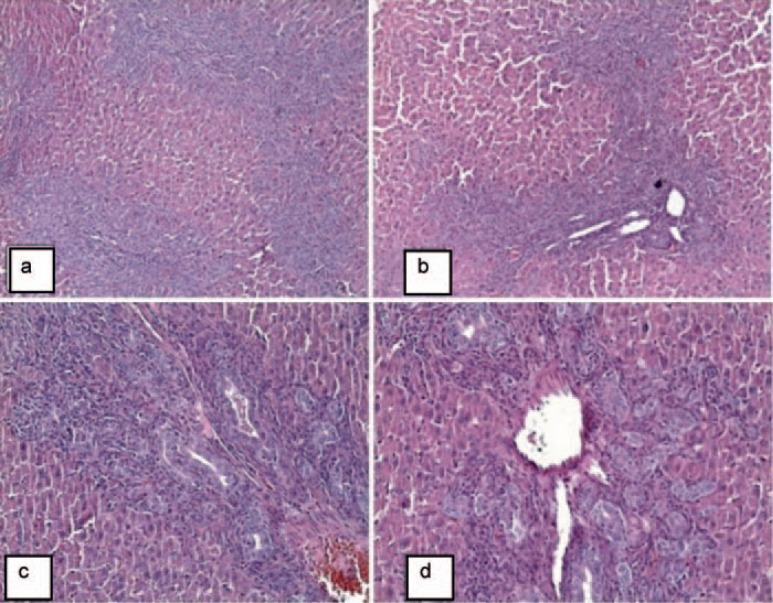
Fig. 1.

Group I: Terminal branch of the portal vein (pv), terminal branch of the hepatic artery (a), bile duct (marked with arrow). a, b Portal area: Bile ducts and branches of the hepatic artery located at the periphery of the portal vein. c Hepatocytes with one or two polygonal nuclei (H & E, ×400).
Fig. 2.
Group II: Histopathological changes in the portal areas (a-d). Significant bile duct proliferation in the portal areas, swelling of hepatocytes, and sinusoidal dilatation (H & E, ×100, ×100, ×200, ×200).
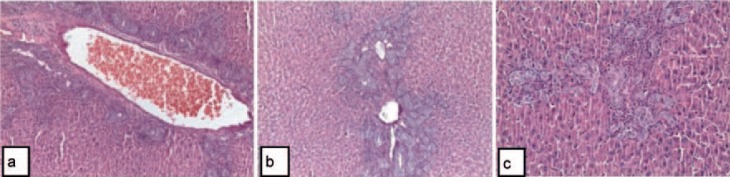
Fig. 3.
Group III: Histopathological changes in the portal areas (a-c). Bile duct proliferation in the portal areas, swelling of hepatocytes, and congestion of portal veins (H & E, ×100, ×200, ×400).
In the sham group (group I), hepatocytes were extended radially from the central vein to the periphery and had one or two polygonal nuclei. The sinusoids between the hepatocytes were normal and the bile ducts were localized in the periphery of the portal area (fig. 1).
In the control group (group II), inflammation of portal areas, focal inflammation of lobular areas, scattered necrosis, confluent necrosis, and proliferation of bile ducts were very prominent (fig. 2).
In the BDL + montelukast group (group III), although there was a proliferation of bile ducts in portal areas, this proliferation was significantly less than that detected in group II. Focal inflammation areas, inflammation of portal areas, and necrotic areas occurred to a much lesser extent (fig. 3).
The mean scores of the HAI of the groups are given in table 3. When statistical analyses of the HAI scores were performed, it was found that the histopathological damage in the BDL + montelukast group was significantly less than the damage in the control group (p < 0.05 for all pathological parameters).
Table 3.
Mean pathological scores of the groups
| Pathological findings | Group I (sham) (n = 10) | Group II (BDL) (n = 8) | Group III (BDL + montelukast) (n = 8) |
|---|---|---|---|
| Periportal/periseptal interface hepatitis | 0.00 ± 0.00a | 2.62 ± 1.06a,b | 0.87 ± 0.33b |
| Confluent necrosis | 0.00 ± 0.00a | 3.25 ± 0.46a,b | 1.25 ± 0.81b |
| Focal lytic necrosis/apoptosis | 0.00 ± 0.00a | 2.87 ± 0.64a,b | 1.12 ± 0.69b |
| Portal inflammation | 0.30 ± 0.08a | 2.62 ± 0.74a,b | 1.50 ± 0.72b |
| Fibrosis | 0.00 ± 0.00a | 4.37 ± 0.74a,b | 2.25 ± 1.28b |
| Liver damage | 0.30 ± 0.00a | 3.00 ± 0.92a,b | 0.87 ± 0.35b |
Significantly different, sham vs. BDL group.
Significantly different, BDL vs. BDL + montelukast group.
Discussion
Leukotrienes (LTs) are bioactive proinflammatory molecules that are produced by the 5-lipoxygenase pathway from arachidonic acid in many cell types, including epithelial cells, fibroblasts, myoblasts, smooth muscle cells, basophils, eosinophils, neutrophils, macrophages, and lymphocytes [15]. The liver plays a key role in the synthesis and metabolism of LTs. Human studies have shown that CysLTs are involved in the pathogenesis of alcohol intoxication, bile duct obstruction, hepatitis B, hepatorenal syndrome, liver cirrhosis, and other diseases. Furthermore, experimental data indicate that CysLTs production is increased in CCl4-induced hepatopathy, alcoholic hepatopathy, lipopolysaccharide-induced liver injury, hepatic ischemia/reperfusion (I/R) injury, liver cirrhosis, and liver allograft rejection [16].
In recent years, several lines of evidence have emerged implicating CysLTs as possible mediators of cholestasis. It was originally proposed that the ability of CysLTs to increase vascular permeability in microcirculation caused hepatic edema, leading to cholestasis due to an increased resistance to bile flow. This is supported by the observation that in guinea pigs, the CysLTs cause plasma extravasation around the bile ducts. In rats, a similar effect is produced by the synergistic action of leukotriene D4 (LTD4) and prostaglandin E2 (PGE2). At high doses, leukotriene C4 (LTC4) exerts a cholestatic effect on the isolated rat liver, whereas LTC4 increases bile salt excretion at low doses [17].
LT modifiers are a new class of drugs that include 5-lipoxygenase selective inhibitors (zileuton) and CysLT1 receptor antagonists (such as montelukast, zafirlukast, pranlukast) which were introduced into clinical practice in 1995. CysLT1 receptor antagonists are now largely used in the treatment of asthma and allergic pathologies. Among them, montelukast has shown the best efficacy and safety profile, and has become the most widely studied anti-LT compound [15].
Montelukast is a prototype, selective, pharmacological antagonist of CysLT1Rs. It effectively antagonizes the proasthmatic, proinflammatory, and priming activities of CysLTs and forms a part of numerous international guidelines for asthma therapy [5]. In particular, LTD4 is the most potent bronchoconstricting agent on a molar basis, but CysLTs also have chemoattractant properties for many inflammatory cells (mainly eosinophils), effects on vascular permeability, mucous secretions, and sensory nerve activation, and are responsible for a part of the pathophysiology of asthma [18].
Interestingly, recent evidence suggests that montelukast possesses a range of secondary anti-inflammatory activities, apparently unrelated to the antagonism of CysLT1Rs. These include inhibition of the enzymes 5-lipoxygenase, histone acetyltransferase (HAT), and adenosine 3',5'-cyclic monophosphate (cAMP) phosphodiesterase, as well as interference with purinergic P2Y receptors and inhibition of eosinophil adhesion to vascular endothelium and migration. These CysLT1R-independent anti-inflammatory mechanisms of action of montelukast may be particularly effective in controlling the corticosteroid-insensitive neutrophils [5].
Hepatic inflammation is an important feature of cholestatic liver disease in both humans and experimental animals. The inflammatory features of obstructive cholestasis include portal tract edema, neutrophil infiltration in the portal tracts, proliferation of the biliary epithelial cells, and portal tract fibrosis [19]. Moreover, biliary obstruction in rats has been reported to result in a significant depression of the phagocytic function of the reticuloendothelial system [20,21]. When histopathological analyses were made in the current study, it was found that inflammation of portal areas, focal inflammation of lobular areas, piecemeal necrosis, confluent necrosis, and proliferation of bile ducts were very prominent in the control group (fig. 2). In the BDL + montelukast group (group 3), although there was a proliferation of bile ducts in the portal areas, this proliferation was significantly less than that detected in group 2. Focal inflammation areas, inflammation of portal areas, and necrotic areas were much less (fig. 3). Statistical analyses of HAI scores showed that the histopathological damage in the BDL + montelukast group was significantly less than the damage in the control group (p < 0.05 for all pathological parameters). These results demonstrated that treatment with montelukast ameliorated the negative effects of obstructive jaundice on liver histology.
Obstruction of the biliary tree, either intrahepatic or extrahepatic, induces a characteristic pattern of early and late liver morphological features that can be attributed to the evolution of an inflammatory response [19]. In addition to the changes in bilirubin and bile salt metabolism as well as in the enterohepatic circulation, there are several alterations in other critical functions of the liver after biliary obstruction [4]. Although the mechanism of the bile salt-induced damage has not been elucidated exactly, inflammatory cell infiltration, accumulation of hydrophobic bile acids, endotoxemia, changes of the mitochondrial permeability transition, and the deleterious effect of oxygen free radicals are possible factors responsible for cholestatic liver injury [1].
Reactive oxygen species include superoxide anions, hydroxyl radicals, hydrogen peroxide, and hydroxyethyl radicals, and are generated from a variety of insults such as drug and toxin metabolites, I/R, cholestasis, and alcohol metabolism. Reactive oxygen species are involved in the necrosis and apoptosis of hepatocytes, and contribute to hepatic stellate cell activation. Bile acids cause oxidative damage by stimulating the generation of oxygen free radicals from mitochondria as well as promoting their release from neutrophils and macrophages [4]. Padillo et al. [22] found that bile duct obstruction induced intense oxidative stress with depletion of different molecules and enzymes with antioxidant properties, and that melatonin reduced the liver injury observed in experimental cholestasis. Baron et al. [23] concluded that the detergent action of bile salts was responsible for the solubility of plasma membranes and cell death, which in turn might lead to oxidative stress, glutathione (GSH) oxidation, and lipid peroxidation. Ljubuncic et al. [24] evaluated the extrahepatic tissue damage in cholestatic liver disease and found that experimental cholestasis was associated with increased lipid peroxidation in the kidney, brain, and heart.
In the present study, tissue MDA, total SH levels, and MPO enzyme activities and also plasma MDA and total SH levels were evaluated for the determination of oxidative stress and the degree of neutrophil infiltration.
MDA is widely used as a marker for the peroxidation of ω3 and ω6 fatty acids, measured by the chemical determination of TBA reactive substances (TBARS). It is an indicator of lipid peroxidation and known to be an index of tissue injury [25]. In the current study, liver and plasma MDA levels were higher in the control group than in the sham and montelukast groups. These results show that montelukast reduced tissue injury and lipid peroxidation.
MPO, a member of the heme peroxidase-cyclooxygenase superfamily, is abundantly expressed in neutrophils and to a lesser extent in monocytes and a certain type of macrophages. Evidence has emerged that MPO-derived oxidants contribute to tissue damage as well as to the initiation and propagation of acute and chronic vascular inflammatory disease. MPO is an enzyme that has a specific activity in neutrophils and is widely used as an enzyme marker for the degree of neutrophil infiltration [26]. In the current study, tissue MPO activity was high in the control group when compared with the sham and montelukast groups. In other words, the treatment with montelukast reduced the tissue MPO activity, and this process might be considered as a lesser degree of tissue injury and less neutrophil infiltration.
GSH is a cysteine-containing tripeptide that is abundant in most eukaryotic cells. Most of the functions of GSH depend upon its cysteine residue, which participates in several types of reactions including displacement, nucleophilic addition, and thiol-disulfide exchange. GSH helps to maintain cellular SH residues in a reduced state. GSH also reacts with free radicals generating glutathionyl. Although the physiological significance of protein glutathionylation has not been fully assessed, it is currently believed that the addition of GSH to protein SH prevents excessive oxidation and thereby preserves protein integrity and function under conditions of oxidative stress. GSH and total SH levels are found to be lower in injured tissues when compared with normal tissues [27]. Similarly, in the current study, total SH levels were low in the control group and high in the montelukast group.
When the oxidative stress parameters analyzed in the current study are evaluated globally, it can be concluded that montelukast has evident antioxidant properties and that the beneficial effects of montelukast on liver injury might be partly due to its antioxidant effects.
The anti-inflammatory and antioxidant effects of montelukast have been emphasized by many authors. Sener et al. [6] showed that montelukast is protective by means of a neutrophil-dependent mechanism against oxidative damage resulting from reactive oxygen radicals and lipid peroxidation after thermal injury. Tugtepe et al. [7] concluded that montelukast had protective effects on the kidney tissue of pyelonephritic rats by inhibiting neutrophil infiltration, balancing the oxidant-antioxidant status, and regulating the generation of inflammatory mediators.
Coskun et al. [8] investigated the effects of montelukast on antioxidant enzymes and proinflammatory cytokines on the heart, liver, lungs, and kidneys in a rat model of cecal ligation and puncture-induced sepsis. Montelukast had a marked effect on attenuating or decreasing the MPO level of lung, liver, heart, and kidney tissues dose-dependently and on decreasing the lipid peroxide (LPO) levels of these tissues except kidney tissue. The lung tissue was most protected by montelukast under sepsis conditions.
Mohamadin et al. [9] demonstrated that the administration of montelukast protects the liver from lipopolysaccharide-induced oxidative damage, as evidenced by decreased liver marker enzymes, LPO, protein oxidation, and neutrophilic infiltration markers as well as an increased antioxidant cascade.
Gideroglu et al. [28] investigated the role of montelukast sodium on neutrophil infiltration and flap survival. Improved flap survival was observed in the rat group that had been treated with montelukast, and this result was associated with decreased neutrophil infiltration and a balance in the oxidant-antioxidant activity status.
There are a number of prior studies that have shown the potential beneficial effect of montelukast in various liver disease models. Ozkan et al. [29] assessed the possible protective effect of montelukast on hepatic I/R injury in rats. They found that the administration of montelukast alleviated the I/R-induced liver injury and improved the hepatic structure and function. They concluded that montelukast might have a potential therapeutic value with its anti-inflammatory and antioxidant properties in protecting the liver against oxidative injury due to I/R [29]. Similarly, in the present study it was shown that montelukast had a hepatoprotective effect in an obstructive jaundice model, and it was concluded that this effect of montelukast might be due to its antioxidant and anti-inflammatory effects. In another study, Steib et al. [30] stated that treatment with montelukast for 10 days resulted in an impressive reduction in the basal portal pressure and an attenuation of the Kupffer cell-dependent increase in portal pressure. According to these results, they decided that montelukast might be of therapeutic benefit for patients with portal hypertension.
Apart from these studies on the beneficial effects of montelukast on hepatic I/R injury and portal hypertension models, montelukast was also used for the improvement of hepatic fibrosis in cholestatic rats. Wasowicz et al. [10] induced cholestasis and subsequent fibrosis by CBD ligation and resection in rats for 5 weeks. Montelukast (10 mg/kg) was administered orally for 34 days. The authors showed that a treatment with montelukast reversed the pathological biochemical parameters and ameliorated the histopathological changes that were induced by bile duct ligation. Although this study investigated the effects of montelukast in obstructive jaundice, the focus of the present article was slightly different. In the present study, the effects of montelukast were investigated in an acute period of obstructive jaundice whereas Wasowicz et al. [10] investigated the effects of montelukast on hepatic fibrosis in cholestatic rats, i.e. the chronic phase of obstructive jaundice. In the long-term evolution, experimental cholestasis models develop hepatomegaly with a marked ductular proliferation and fibrosis; however, loss of normal liver architecture, typical of cirrhosis, is seldom found [19]. In contrast, in the present study the antioxidant and anti-inflammatory effects of montelukast were investigated in the early stage of obstructive jaundice. This is the reason of the relatively short observation period (7 days) of the present study.
Immediately after complete bile duct obstruction in rats, an intense increase (60%) in biliary ductal pressure is produced, and this is quickly followed by pathological extracellular matrix changes. The cholestatic liver involves both parenchymal (hepatocytes and cholangiocytes) and non-parenchymal cells (sinusoidal endothelial cells, Kupffer cells, and hepatic myofibroblasts), as well as blood cells that migrate to the liver interstitium. Neutrophils are key components of the initial inflammatory response to liver cholestatic injury. In experimental extrahepatic cholestasis, neutrophil interstitial infiltration occurs in early phases, 3 days after BDL [19].
In the early stage of BDL, the insufficient supply of oxygen suffered by the liver, related to hemodynamic alterations, as well the incorrect use of oxygen derived from bile salt hepatotoxicity constitute the essential factors that induce the reduction of hepatic energy metabolism. It is clearly known that there is a strong correlation between experimental obstructive jaundice and oxidative stress [19].
The experimental obstructive jaundice model used in the present study is an irreversible obstruction model which does not fully simulate the clinical course of obstructive jaundice in humans. However, this model is widely used in the literature for the evaluation of pathophysiology and the treatment of experimental obstructive jaundice [31,32,33,34]. Thus, we preferred to use this model in the current study. This choice might be accepted as the main limitation of our study.
In the study performed by Wasowicz et al. [10,] montelukast was administered orally for 34 days. In our experience, some difficulties, such as daily orogastric intubation or standardization of drug doses, might be encountered during oral administration of the drugs to the rats. Although the oral administration of montelukast might be more realistic, we preferred to give montelukast intraperitoneally due to the above mentioned difficulties. In the literature, there are some studies to be found in which montelukast was given intraperitoneally [6,16]. However, this administration route might be accepted as another limitation of the present study.
The results of the present study showed that montelukast alleviated the secondary effects (due to oxidative stress and inflammation) of obstructive jaundice but it had no effect on the alleviation of jaundice itself.
Montelukast can be used for treatment in different organ disorders through the independent mechanisms of its action. In the current study, montelukast showed a significant hepatoprotective effect in the experimental obstructive jaundice model, which can be considered to be due to its antioxidant and anti-inflammatory activities. However, further studies are needed to evaluate the exact mechanism of the hepatoprotective effect of montelukast.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgement
SK and KK were responsible for the study design and data analysis. AMB and ST were responsible for the study concept and interpretation of data. PC made the histopathological examination. HS and EO performed the biochemical analyses. SK and KK were responsible for article drafting and critical appraisal. EE was responsible for the revision of the article and critical appraisal.
Imprint
ISSN Print Edition: 1662-6664
ISSN Online Edition: 1662-6672
Journal Homepage: http://www.karger.com/vim
Publication Data: Volume 31, 2015 of ‘Viszeralmedizin’ appears with 6 issues.
Copyright: © 2015 by S. Karger Verlag für Medizin und Naturwissenschaften GmbH, Freiburg (Germany). All rights reserved. No part of the journal may be reproduced in any form without the written permission of the publisher. This includes digitalisation and any further electronic computing, like saving, copying, printing or electronic transmission of digitalized material from this journal (online or offline). Authorization to photocopy items for internal or personal use of specific clients is granted by Karger.
Photocopying: This journal has been registered with the Copyright Clearance Center (CCC), as indicated by the code appearing on the first page of each article. For readers in the US, this code signals consent for copying of articles for personal or internal use, or for the personal or internal use of specific clients, provided that the stated fee is paid per copy directly to Copyright Clearance Center Inc., 222 Rosewood Drive, Danvers, MA 01923 (USA).
A copy of the first page of the article must accompany payment. Consent does not extend to copying for general distribution, for promotion, for creating new works, or for resale. In these cases, specific written permission must be obtained from the copyright owner, S. Karger GmbH, Wilhelmstraße 20A, 79098 Freiburg (Germany).
Disclaimer: The statements and data contained in this publication are solely those of the individual authors and contributors and not of the publisher and the editor(s). The appearance of advertisements in the journal is not a warranty, endorsement, or approval of the products or services advertised or of their effectiveness, quality or safety. The publisher and the editor(s) disclaim responsibility for any injury to persons or property resulting form any ideas, methods, instructions or products referred to in the content or advertisements.
Distribution and Subscription: Karger offers three types of subscription: Print Only, Online Only and the combined Print + Online. The basic annual subscription rate is the same for all three delivery forms; however, a fee for the combined print and online subscription is levied, and there is a postage and handling charge for Print Only and Print + Online. Subscriptions run for a full calendar year. Prices are given per volume.
Print subscription: EUR 197.− + postage and handling.
Online subscription: EUR 197.−.
Combined (print + online) subscription: EUR 247.− + postage and handling.
For customers in Germany: Please turn to your bookshop or to S. Karger Verlag für Medizin und Naturwissenschaften GmbH Wilhelmstr. 20A, 79098 Freiburg (Germany) Tel. +49 761 45 20 70, Fax +49 761 45 20 714 E-mail information@karger.com
For customers in all other countries: Please contact your bookshop or S. Karger AG, Allschwilerstr. 10, 4009 Basel (Switzerland) Tel. +41 61 3 06 11 11, Fax +41 61 3 06 12 34 E-mail karger@karger.com
Advertising: Correspondence should be addressed to the publisher. S. Karger Verlag für Medizin und Naturwissenschaften GmbH Attn. Ellen Zimmermann (Head of Marketing) E-mail e.zimmermann@karger.com
Price list No. 22 of January 1, 2015 is effective.
V.i.S.d.P. (Person responsible according to the German Press Law): Sibylle Gross
Type setting and printing: Kraft Druck GmbH, 76275 Ettlingen, Germany.
Bibliographic Services:
EMBASE / Excerpta Medica
Reference Update
Science Citation Index Expanded
ISBN 978-3-318-03037-2
e-ISBN 978-3-318-03038-9
References
- 1.Kismet K, Sabuncuoğlu MZ, Kilicoglu SS, et al. Effect of propolis on oxidative stress and histomorphology of liver tissue in experimental obstructive jaundice. Eur Surg Res. 2008;41:231–237. doi: 10.1159/000136479. [DOI] [PubMed] [Google Scholar]
- 2.Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott-Conner CEH, Grogan JB. The pathophysiology of biliary obstruction and its effect on phagocytic and immune function. J Surg Res. 1994;57:316–336. doi: 10.1006/jsre.1994.1151. [DOI] [PubMed] [Google Scholar]
- 4.Kilicoglu B, Gencay C, Kismet K, et al. The ultrastructural research of liver in experimental obstructive jaundice and effect of honey. Am J Surg. 2008;195:249–256. doi: 10.1016/j.amjsurg.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Tintinger GR, Feldman C, Theron AJ, Anderson R. Montelukast: more than a cysteinyl leukotriene receptor antagonist? Sci World J. 2010;10:2403–2413. doi: 10.1100/tsw.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sener G, Kabasakal L, Cetinel S, Contuk G, Gedik N, Yegen BC. Leukotriene receptor blocker montelukast protects against burn-induced oxidative injury of the skin and remote organs. Burns. 2005;31:587–596. doi: 10.1016/j.burns.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Tugtepe H, Sener G, Cetinel S, Velioglu-Ogunc A, Yegen BC. Oxidative renal damage in pyelonephritic rats is ameliorated by montelukast, a selective leukotriene CysLT1 receptor antagonist. Eur J Pharmacol. 2007;557:69–75. doi: 10.1016/j.ejphar.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Coskun AK, Yigiter M, Oral A, et al. The effects of montelukast on antioxidant enzymes and proinflammatory cytokines on the heart, liver, lungs, and kidneys in a rat model of cecal ligation and puncture-induced sepsis. Sci World J. 2011;11:1341–1356. doi: 10.1100/tsw.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohamadin AM, Elberry AA, Elkablawy MA, Gawad HS, Al-Abbasi FA. Montelukast, a leukotriene receptor antagonist abrogates lipopolysaccharide-induced toxicity and oxidative stress in rat liver. Pathophysiology. 2011;18:235–242. doi: 10.1016/j.pathophys.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Wasowicz W, Neve J, Peretz A. Optimized steps in fluorometric determination of thiobarbituric acid-reactive substance in serum: importance of extraction pH and influence of sample preservation and storage. Clin Chem. 1993;39:2522–2526. [PubMed] [Google Scholar]
- 11.Sedlak J, Lindsay RH. Estimation of total, protein bound, and non-protein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 12.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 13.Ishak K. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 14.Ishak KG. Pathologic features of chronic hepatitis. A review and update. Am J Clin Pathol. 2000;113:40–55. doi: 10.1309/42D6-W7PL-FX0A-LBXF. [DOI] [PubMed] [Google Scholar]
- 15.Koca G, Gültekin SS, Ünsal H, Kuru S, Demirel K, Korkmaz M. The efficacy of montelukast as a protective agent against 131I-induced salivary gland damage in rats: scintigraphic and histopathological findings. Nucl Med Commun. 2013;34:507–517. doi: 10.1097/MNM.0b013e32835ffecd. [DOI] [PubMed] [Google Scholar]
- 16.Cuciureanu M, Căruntu ID, Păduraru O, et al. The protective effect of montelukast sodium on carbon tetrachloride induced hepatopathy in rat. Prostaglan Other Lipid Mediat. 2009;88:82–88. doi: 10.1016/j.prostaglandins.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Farzaneh-Far R, Moore K. Cysteinyl-leukotrienes and the liver. Prostaglan Other Lipid Mediat. 2003;72:35–50. doi: 10.1016/s1098-8823(03)00076-5. [DOI] [PubMed] [Google Scholar]
- 18.Paggiaro P, Bacci E. Montelukast in asthma: a review of its efficacy and place in therapy. Ther Adv Chronic Dis. 2011;2:47–58. doi: 10.1177/2040622310383343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aller MA, Arias JL, García-Domínguez J, Arias JI, Durán M, Arias J. Experimental obstructive cholestasis: the wound-like inflammatory liver response. Fibrogenesis Tissue Repair. 2008;1:6. doi: 10.1186/1755-1536-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechner AJ, Velasquez A, Knudsen KR, et al. Cholestatic liver injury increases circulating TNF-α and IL-6 and mortality after Escherichia coli endotoxemia. Am J Respir Crit Care Med. 1998;157:1550–1558. doi: 10.1164/ajrccm.157.5.9709067. [DOI] [PubMed] [Google Scholar]
- 21.Ding JW, Andersson R, Soltesz V, et al. Obstructive jaundice impairs reticuloendothelial function and promotes bacterial translocation in the rat. J Surg Res. 1994;57:238–245. doi: 10.1006/jsre.1994.1138. [DOI] [PubMed] [Google Scholar]
- 22.Padillo FJ, Cruz A, Navarrete C, et al. Melatonin prevents oxidative stress and hepatocyte cell death induced by experimental cholestasis. Free Radic Res. 2004;38:697–704. doi: 10.1080/10715760410001705131. [DOI] [PubMed] [Google Scholar]
- 23.Baron V, Muriel P. Role of glutathione, lipid peroxidation and antioxidants on acute bile duct obstruction in the rat. Biochim Biophys Acta. 1999;1472:173–180. doi: 10.1016/s0304-4165(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 24.Ljubuncic P, Tanne Z, Bomzon A. Evidence of a systemic phenomenon for oxidative stress in cholestatic liver disease. Gut. 2000;47:710–716. doi: 10.1136/gut.47.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pizzimenti S, Ciamporcero E, Daga M, et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front Physiol. 2013;4:242. doi: 10.3389/fphys.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malle E, Furtmuller PG, Sattler W, Obinger C. Myeloperoxidase: a target for new drug development? Br J Pharmacol. 2007;152:838–854. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill BG, Ramana KV, Jian Cai J, Bhatnagar A, Srivastava SK. Measurement and identification of s-glutathiolated proteins. Methods Enzymol. 2010;473:179–197. doi: 10.1016/S0076-6879(10)73009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gideroglu K, Yilmaz F, Aksoy F, Bugdayci G, Saglam I, Yilmaz F. Montelukast protects axial pattern rat skin flaps against ischemia/reperfusion injury. J Surg Res. 2009;157:181–186. doi: 10.1016/j.jss.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 29.Ozkan E, Yardimci S, Dulundu E, Topaloğlu U, Sehirli O, Ercan F, Velioğlu-Oğünç A, Sener G. Protective potential of montelukast against hepatic ischemia/reperfusion injury in rats. J Surg Res. 2010;159:588–594. doi: 10.1016/j.jss.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Steib CJ, Bilzer M, op den Winkel M, Pfeiler S, Hartmann AC, Hennenberg M, Göke B, Gerbes AL. Treatment with the leukotriene inhibitor montelukast for 10 days attenuates portal hypertension in rat liver cirrhosis. Hepatology. 2010;51:2086–2096. doi: 10.1002/hep.23596. [DOI] [PubMed] [Google Scholar]
- 31.Alturfan AA, Aytaç E, Emekli-Alturfan E, Yarat A, Sarıbeyoğlu K, Pekmezci S, Seymen O. Serum total sialic acid as a novel complementary candidate marker of hepatic damage in obstructive jaundice. Ann Clin Lab Sci. 2014;44:56–61. [PubMed] [Google Scholar]
- 32.Wang G, Xiu P, Li F, Xin C, Li K. Vitamin A supplementation alleviates extrahepatic cholestasis liver injury through Nrf2 activation. Oxid Med Cell Longev. 2014;2014:273692. doi: 10.1155/2014/273692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosar NM, Tosun M, Polat C, Kahraman A, Arikan Y. Hepatocyte apoptotic index and p53 expression in obstructive jaundice rats. Bratisl Lek Listy. 2014;115:352–356. doi: 10.4149/bll_2014_069. [DOI] [PubMed] [Google Scholar]
- 34.Zhou YX, Ni Y, Liu YB, Liu X. Histone preconditioning protects against obstructive jaundice-induced liver injury in rats. Exp Ther Med. 2014;8:15–20. doi: 10.3892/etm.2014.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]