Abstract
Most depressed patients suffer from sleep abnormalities, which are one of the critical symptoms of depression. They are robust risk factors for the initiation and development of depression. Studies about sleep electroencephalograms have shown characteristic changes in depression such as reductions in non-rapid eye movement sleep production, disruptions of sleep continuity and disinhibition of rapid eye movement (REM) sleep. REM sleep alterations include a decrease in REM sleep latency, an increase in REM sleep duration and REM sleep density with respect to depressive episodes. Emotional brain processing dependent on the normal sleep-wake regulation seems to be failed in depression, which also promotes the development of clinical depression. Also, REM sleep alterations have been considered as biomarkers of depression. The disturbances of norepinephrine and serotonin systems may contribute to REM sleep abnormalities in depression. Lastly, this review also discusses the effects of different antidepressants on REM sleep disturbances in depression.
Keywords: Antidepressants, depression, mood disorders, norepinephrine, serotonin, sleep disorders.
1. INTRODUCTION
Currently, depression is a common disease and has become increasingly prevalent in the world [1]. Most depressed people show evidence of one or more changes in sleep neurophysiology; i.e., some complain of insomnia and the others suffer from hypersomnia [2, 3]; therefore, impaired sleep is a critical symptom of depressed patients. Gresham SC et al. [4] found there were more REM sleep alterations in the first third of the night in depressed patients, which was the first study on depression and sleep [5]. Later quantitative electroencephalogram (EEG) studies showed that there were characteristic changes in depression consisting of disturbed sleep continuity, inductions of rapid eye movement (REM) sleep; that is, reductions of REM sleep latency, increases in REM sleep time and REM sleep density, and decreases of non-REM (NREM) sleep [3, 6-8]. Of all the changes, REM sleep abnormalities are key involvements of EEG changes in depression, and they implicate the severity of disease and indicate the effect of individualized antidepressant therapy [8].
To truly understand depression, knowledge of REM sleep is required. Therefore, we reviewed the data on REM sleep disturbances in depression from different aspects. Furthermore, treatments of REM sleep disturbances in depression are also reviewed.
2. OVERVIEW OF REM SLEEP ABNORMALITIES IN DEPRESSION
Although symptoms of depression vary from patient to patient and can overlap through different subtypes [9], sleep disturbances are always one of the most common complaints of depressed patients. Sleep EEG recordings in depression are often characterized by the following [3, 8, 10-13]: decreased sleep efficiency, for example, the time before falling asleep is prolonged and more awakening episodes after falling asleep [14-16]; reduced NREM sleep, disturbed distribution of delta activity, sleep cycle, and a reduced delta ratio (ratio between delta activity in the first and second NREM periods); longer time of REM sleep, such as reduced REM sleep latency and deeper REM sleep density, especially during the first REM sleep period [11, 16-25].
REM sleep disturbances have been considered to be more specific for depression since other sleep disturbances are common in many mental disorders. In 1966, Hartmann E and Green WJ et al. found that the latency of REM sleep was reduced at sleep onset and its percentage was increased [26, 27]. So the latency of REM sleep has been viewed as a marker of depression [28].
Animal models of depression also show changes in sleep. Interestingly, animal models show similar changes of REM sleep, but the other insomnia changes including prolonged wakefulness and shortened NREM sleep are not obvious [29, 30]. REM sleep increased significantly in the light period, especially in the afternoon, in olfactory bulbectomized rats, which is thought to be a classic depressive model [31]. Prenatally stressed rats showed an increased REM sleep, and the change of REM sleep was correlated positively with the increase of corticosterone levels in the plasma [32]. In the other depressive animal models, elevated REM sleep was a landmark [32, 33]. All these studies have suggested that increased REM sleep is supposed to be the characteristic change in depressive animal models.
Therefore, from these studies, we concluded that abnormalities in REM sleep are potential biomarker of depression.
3. THE NEUROBIOLOGICAL BASIS OF REM SLEEP ABNORMALITIES IN DEPRESSION
3.1. The Regulation of REM Sleep
There was the first description about REM sleep more than 60 years ago [34-36]. In both humans and other mammals, REM sleep is used to describe sleep accompanied by REM, fast and desynchronized rhythm in cortical EEG, an activation in autonomic activity and a loss of muscle tone [37]. REM sleep was shown to be regulated through the interplays of cholinergic and monoaminergic neurons in the brainstem, which activate or inactivate during REM sleep [38-43].
Noradrenergic (NE) neurons in the locus coeruleus (LC) have shown a negative regulation to REM sleep [44]. These neurons in the LC exhibit an increased activity during wakefulness, a decreased discharge rate during NREM sleep and a vanished firing during REM sleep [45]. However, others have suggested that cholinergic neurons in LCalpha, peri-LCalpha, laterodorsal (LDT) and pedunculopontine (PPT) tegmental nuclei may be responsible for REM sleep generation [46, 47]. Nucleus points oralis (NPO) has been emphasized to be involved in the coordination of the generation of REM sleep. It had been shown earlier that lesion of the ventral part of the NPO (vNPO) causes a decrease in REM sleep. Injection of carbachol into it could induce REM sleep with a shorter latency [48-51]. The vNPO receives cholinergic afferent fibers from the rostral peri-LCalpha, LDT, PPT and parabrachial nuclei (PB) and γ-aminobutyric acid (GABA)-ergic fibers from the postero-lateral hypothalamus [52]. So the interactions between those two systems play an important role in the regulation of REM sleep [53, 54].
With the development of the techniques to record the activity of single neurons in freely moving animals, it has been possible to assess the effect of pontine nuclei at the neuronal level. The concept of REM-on and REM-off neurons has come into being, and the former means neurons that start firing or increase firing rate significantly during REM sleep, while the latter indicates those stopping firing or decreasing firing rate significantly at the same time [55, 56]. Almost all the REM-off neurons are monoaminergic in the LC as NE-ergic neurons [43, 57, 58], in the dorsal raphe as the serotoninergic (5-HT) neurons [59] and in the tuberomammillary nucleus (TMN) within the hypothalamic region as the histaminergic neurons [60]. However, some non-monoaminergic neurons in the medulla have been demonstrated to be REM-off neurons [61]. The REM-on neurons are chiefly cholinergic and are distributed in the LDT [62, 63] and PPT [64]. Nevertheless, some non-cholinergic REM-on neurons have also been found in the brain [61, 65]. Both the generation and regulation of REM sleep directly depend on the interaction between those two neurons. It is still not clear how REM-off neurons stop firing during REM sleep, which is necessary to generate REM sleep. GABA has been supposed to inhibit REM-off neurons [66]. Lu J et al. proposed a flip–flop switch in brainstem, which involved a mutual inhibition between REM-on and REM-off regions in the mesopontine tegmentum [67]. There were GABAergic neurons in each side that heavily innervated the others. The REM-on and REM-off neurons are summarized in Fig. 1.
Fig. (1).
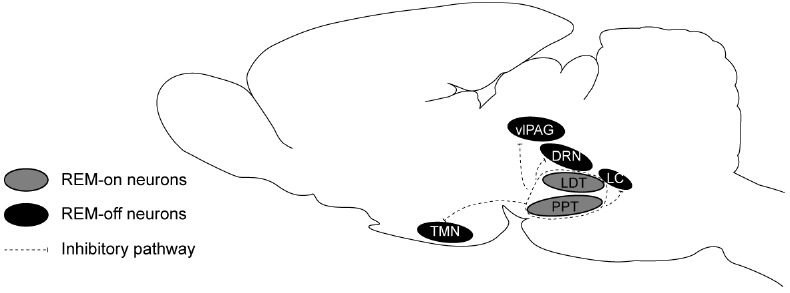
The REM-on and REM-off neurons in REM sleep flip-flop circuit model. DRN: dorsal raphe nucleus; LC: locus coeruleus; LDT: laterodorsal tegmentum; PPT: pedunculopontine tegmentum; TMN: tuberomamillary nucleus; vlPAG: ventrolateral periaqueductal grey.
3.2. The Basis of REM Sleep Dysregulation in Depression
Clinical antidepressant drugs selectively increase the function of monoaminergic systems such as the norepinephrine and serotonin systems. Dysfunction of the monoaminergic system has been demonstrated to have a close relationship with the morbidity of depression [68]. It was found that the depletion of monoamine reserpine was associated with serious depression. Moreover, the inhibitors of monoamine oxidase, which inhibit the degradation of NE and 5-HT, could improve depressive emotions, suggesting that the NE and 5-HT systems play a critical role in the generation process of depression.
Increasing extracellular levels of NE and 5-HT via the use of monoamine reuptake inhibitors could inhibit REM sleep, which could also be helpful for treating depression [69]. However, selective lesions of either the cholinergic or monoaminergic nucleus in the brainstem did not have much influence on REM sleep [70-72]. Furthermore, also some evidence showed no causality between REM sleep abnormalities and depressive behaviors [31]. Therefore, there may be two parallel pathways for the regulation of REM sleep and depression, which have a close relationship with the monoaminergic system.
In addition to monoaminergic system, cholinergic system also plays a critical role in REM sleep abnormalities in depression. Cholinergic agonists such as arecoline and physostigmine shortened REM sleep latency and reduced REM sleep interval times preferentially in patients with depression [73]. Moreover, Prathiba J et al. found that REM sleep deprivation could reverse the sensitivity of central cholinergic receptors in rats given clomipramine neonatally, and the mechanism may be involved in mediating the antidepressant effects of REM sleep deprivation treatment in clomipramine model of depression [74].
Recently, it was found that depression may be related to the dysfunction of a network of structures that also regulate REM sleep, such as the limbic system including the hippocampus, amygdala and medial prefrontal cortex. Posttraumatic stress disorder and major depressive disorder (MDD) are two stress-related disorders that are associated with the disruption of REM sleep [75-79]. In MDD, REM sleep is characterized by activation of limbic and paralimbic brain regions compared to wakefulness. Posttraumatic stress disorder is associated with increased REM sleep limbic and paralimbic metabolism, whereas MDD is associated with wake and REM sleep hypermetabolism in these areas [80]. The hippocampus of the depressed patients was 12%–15% smaller than that of the non-depressed patients [81]. Hegde P et al. reported the effect of chronic immobilization stress on theta oscillations in the hippocampus and amygdala during REM sleep [82]. These studies demonstrated that chronic immobilization stress caused synchronized amygdalo-hippocampal theta activity and enhanced REM sleep duration. Mizuseki K et al. found that theta oscillations, which are the characteristics of REM sleep, decreased spike synchrony in the hippocampus and entorhinal cortex [83]. In addition, others found that stress affected theta activity in limbic networks including the hippocampus [84]. Apart from that, stress could also reduce long-term potentiation and facilitate long term depression, which has extensive effects on anxiety, depression and cognition [68, 85-87]. Therefore, the hippocampus is an important region in regulating REM sleep disturbances in the development of depression. Furthermore, the subregions of the medial prefrontal cortex (mPFC) showed great changes in neural activity in depressed patients. Lesions of the ventral mPFC enhanced REM sleep, reduced REM sleep latency and shortened the immobility time in a forced swimming test. Anatomic tracing studies showed that mPFC projected to the pontine REM-off neurons in the ventrolateral periaqueductal gray and adjacent lateral pontine tegmentum, which interacted with REM-on neurons in the dorsal pons. Therefore, the ventral mPFC may be a critical area for regulating both depression and sleep and it has been suggested to be a critical site for REM sleep abnormalities and other behaviors in depression [88].
The lateral habenula (LHb) is a nucleus that negatively regulates the monoaminergic system in the brain, since activation of LHb inhibits the firing activity of serotonergic neurons in the brainstem [89]. Aizawa H et al. found that the synchronous activity in the LHb was essential for the maintenance of REM sleep via regulation of serotonergic activity, which is also important for the development of depression [90]. The results suggested that the LHb regulates REM sleep abnormalities in depression via serotonergic neurons in the median raphe [91].
4. PHARMACOLOGICAL TREATMENTS FOR DEPRESSION AND REM SLEEP DISTURBANCES
4.1. Effects of Different Antidepressants on REM Sleep
Most antidepressants show suppressive effects on REM sleep, including prolonged REM sleep latency, decreased total duration of REM sleep, and reduced REM sleep density and the number of REM sleep episodes. These drugs include tricyclic antidepressants (TCAs) such as amitriptyline, imipramine and clomipramine [92-99]; tetracyclic antidepressants such as mianserin and maprotiline [98]; monoamine-oxidase inhibitors (MAOIs) such as phenelzine, tranylcypromine, clorgyline [100-103]; selective NE reuptake inhibitors (NARIs) such as desipramine and reboxetine [104, 105]; selective 5-HT reuptake inhibitors (SSRI) such as fluoxetine, paroxetine, zimelidine [106-108]; 5-HT/NE reuptake inhibitors (SNRIs) such as venlafaxine and duloxetine and 5-HT2 receptor antagonists/reuptake inhibitor trazodone [98, 105, 109, 110].
The specific effects of different antidepressants on REM sleep are various. For example, duloxetine, amitriptyline, phenelzine and desipramine could significantly reduce REM sleep time and prolong the onset latency of REM sleep and phenelzine could completely suppress REM sleep after a few weeks of treatment [93, 98, 101, 102, 104, 110]. Clomipramine, imipramine and vilazodone showed profound REM sleep-suppressive effects, and venlafaxine may be the strongest REM sleep inhibitor among all SNRIs [96-98, 107]. Milnacipran, at a therapeutic dose, induced small effects on REM sleep compared with imipramine, paroxetine, and venlafaxine [95]. Moclobemide, a reversible MAOI, showed a less suppressive REM sleep effect than other traditional MAOIs [94]. Mianserin reduced REM sleep only in rats, but not in MDD patients [98]. The suppressive effect of trazodone was also relatively slight [109] and, therefore, this drug was included in the class of antidepressants that could not suppress REM sleep [111].
A REM sleep rebound is very common after a few weeks following withdrawl of most antidepressants, such as amitriptyline, citalopram, clomipramine, phenelzine and tranylcypromine [93, 94, 96, 98]. Nortriptyline showed inhibition of REM sleep, such as increased REM sleep latency and decreased REM sleep time, but after withdrawl, REM sleep rebounded even higher than before [94]. Moreover, no rebound of REM sleep also occurred after the withdrawal of antidepressants milnacipran, imipramine and paroxetine [95].
Although most antidepressants showed a suppressive effect on REM sleep, some antidepressants did not have this effect, or even had the opposite effect. Many studies have demonstrated that trimipramine did not show a REM sleep suppressive effect at lower doses [103, 112], and even one study drew the conclusion that trimipramine could enhance REM sleep [113], which was a typical exception among TCAs. In addition, iprindole, viloxazine, nefazodone and tianeptine also did not have significant suppressive effect on REM sleep [98, 99]. In some studies, nefazodone even could increase the total time and percentage of REM sleep and decrease the latency of REM sleep [98, 108]. The same conclusion was reached for the SNRI bupropion, with which REM sleep latency was reduced, while the REM sleep percentage and time duration increased after treatment in depressed patients [99, 114]. Furthermore, the specific NE and 5-HT antidepressant mirtazapine showed many effects on changing sleep structures in depressed patients, and some studies concluded that it could suppress REM sleep modestly [94]; however, more data showed there was no significant effect on REM sleep when patients were treated with mirtazapine [98, 99, 107, 110]. Escitalopram appeared to be an exception to other SSRIs. It was the only agent that did not show a suppressive effect on REM sleep among all the SSRIs [99]. The reversible MAOI moclobemide showed contradictory results. One study revealed it to be associated with enhanced REM sleep and shorter REM sleep latency, but another study showed an almost opposite result [98].
Interestingly, most antidepressants that showed suppressive effects on REM sleep were also associated with changing the sleep architecture and decreasing restorative sleep, while others that did not elicit this effect tended to improve sleep and return the sleep structure to a restorative function. However, it should be noted that there are also some exceptions. In general, the rebound effect varies for different categories and may be relative to the mechanisms underlying these drugs.
4.2. Effects of Different Antidepressants on other Sleep Architectures
TCAs such as clomipramine, desipramine, amitriptyline and protriptyline, almost all SSRIs (e.g., fluoxetine, paroxetine and sertraline) except for escitalopram, SNRIs such as venlafaxine, duloxetine and zimelidineand, and NARIs such as reboxetine, suppressed REM sleep but elongated sleep latency, increased awakenings, and decreased total sleep time and sleep continuity at the same time [94, 98, 99, 104, 105, 107, 108, 115]. Almost all MAOIs could inhibit REM sleep time, reduce sleep efficiency, increase sleep latency, and induce nocturnal disturbance and other negative influences on sleep efficiency [99, 102]. The SNRI bupropion and SARI nefazodone, which could increase REM sleep, have been revealed to improve overall sleep efficiency and decrease the number and duration of awakenings, similar to mirtazapine, which has not been associated with the changes in REM sleep [98, 99, 108, 116]. A novel antidepressant; i.e., agomelatine, was thought to have a unique mechanism of action. The melatonin MT1 and MT2 receptor agonists and 5-HT2C receptor antagonist were also found to increase sleep efficiency, the duration of NREM and normalize sleep structure, but had no significant influence on REM sleep [95, 117].
Note that not all antidepressants abide by this principle. For example, both amitriptyline and doxepin could suppress REM sleep, but they also improved sleep structure with a shorter sleep latency and increased total sleep time, as well as a decreased awakening after sleep onset [99]. Another example was trazodone, which could slightly reduce REM sleep in depressed patients. Trazodone was shown to improve sleep quality and provide restorative sleep with enhanced NREM sleep and increased sleep time [98, 99]. Phenelzine was shown to suppress REM sleep strongly but did not change total sleep time and EEG slow-wave activity in NREM sleep [118]. It was also found with milnacipran and imipramine [95]. Various pathways responsible for different antidepressants taking effect may be the reason for the different relationship between REM sleep suppression and changes in sleep architecture.
4.3. The Relationship between the Effects of Antidepressants on REM Sleep and Depressive Behavior Abnormalities
In terms of REM sleep, suppressive effects appeared with most antidepressants several decades ago and many scientists believed that there must be some inherent association between the suppressive and therapeutic effects of antidepressants. Studies have shown that REM sleep deprivation can improve endogenous depression, measured on Hamiton and Global scales, and the extent of the improvement correlated positively and significantly with REM sleep pressure [110, 119]. Later, scientists proposed that REM sleep deprivation is the mechanism of antidepressant action or the mechanism of the drugs resided in their REM sleep suppressive effects [118, 120]. REM sleep latency can be a psychobiologic marker for depression [121]. These reports indicated that REM sleep suppression at the outset of antidepressant treatment could predict therapeutic effects for a time.
In recent years, more new antidepressants such as nefazodone, bupropion, mirtazapine and escitalopram, which do not suppress REM sleep, appear to be working well in treating depression, and more doubts are put on the hypotheses regarding REM sleep and depression [94, 99]. In one study on the effects of tranylcypromine on sleep in depressed patients, correlation analyses indicated that the antidepressant response was only weakly associated with changes in REM sleep [101]. A study on phenelzine and clomipramine also showed that their use did not rely on the cease of REM sleep or inhibition of NREM sleep [97, 100]. A common current viewpoint is that none of the hypotheses alone on NREM sleep, REM sleep or another sleep index is likely to predict therapeutic responses [122].
REM sleep suppression must be relative to the therapeutic effects of antidepressants more or less, and it should be considered combination with the mechanisms of these medications. Whether REM sleep suppression brought on by antidepressants is simply an epiphenomenon of drug actions or it participates positively in the therapeutic process of antidepressants is unclear and needs more investigation.
4.4. Mechanisms of the Suppressive Effects of Antidepressants on REM Sleep
The mechanisms of antidepressants differ in different classes of antidepressants and with specific medications. In general, the mechanism is associated with 5-HT and NE reuptake inhibition, the affinity or/and number of 5-HT1A and 5-HT2 receptors, α1-, α2-adrenoceptors, and histamine H1 receptors. Most TCAs can inhibit the reuptake of both NE and 5-HT and block histamine H1 receptors (except for lofepramine) and α1-adrenoceptors (except for desipramine) [94]. MAIOs increase the availability of monoamines. NARIs, SSRIs and SNRIs are generally associated with 5-HT and/or NE reuptake inhibition, and some of them also have effects on receptor sites. The SARIs trazodone and nefazodone can block 5-HT reuptake weakly and are α1-adrenoceptor, 5-HT1A and 5-HT2 receptor antagonists. The NE and 5-HT antidepressant mirtazapine also acts as an antagonist of α2-adrenoceptors, 5-HT2 receptors and H1 receptors [111, 123, 124].
The common point of different antidepressant action is to positively modulate the 5-HT and NE systems in the central nervous system. Both of these neurotransmitters, mostly derived from the dorsal raphe and LC, respectively, can inhibit cholinergic REM-on neurons in the LDT/PPT and lead to REM-off and arousal [125]. It may, to some extent, explain the REM sleep suppressive effect, sleep disturbances and fragmentation caused by most antidepressants. This part is also supported by the fact that most antidepressants, which cannot suppress REM sleep, having no potent or direct effects on NE or 5-HT neurotransmission. Others proposed that 5-HT1A stimulation is linked to antidepressant suppression of REM sleep, while 5-HT2 agonism is related to sleep disturbances. Blockade of α1 and α2-adrenoceptors is responsible for sleep promotion and fragmentation of sleep, respectively. Blockage of the histamine H1 receptor is also regarded to have nonspecific sedative effects and may promote sleep [111]. Different subtypes of receptors that antidepressants act on may partially account for their various therapeutic effects.
5. DISCUSSION
As a result, impaired sleep could be common in patients with depression, while it also could be an independent cause to this psychiatric disorder. EEG studies proved that REM sleep disinhibition is the main and key performance of sleep disorder in patients suffering from different depressive disorders.
REM sleep could be modulated by complex neuro-biological process. Cholinergic and monoaminergic neurons in the brainstem are the earliest targets noticed by researchers. Cholinergic neurons in LCalpha, peri-LCalpha, LDT, PPT and NPO tend to induce and sustain REM sleep, while monoaminergic neurons in LC regulating REM sleep negatively. Antidepressants which increase the level of 5-HT and NE, or the affinity of their receptors in synapses are found to influence REM sleep strongly, while those that do not suppress REM sleep or decrease REM sleep moderately have no direct effects on monoaminegic system. GABAergic and histaminergic neurons are also important to regulate REM sleep. Besides, as one of the most important incentives of depression, stress could disturb the function of other REM sleep modulating regions including corticolimbic system such as hippocampus, amygdala and mPFC, and LHb.
This paper discusses the neurobiological basis of REM sleep regulation and REM sleep abnormalities in depressed patients, and explores the mechanisms of antidepressants in regulating REM sleep. It should be noted that most researches in the paper which explore the effect of antidepressants on REM sleep are studies on patients with MDD. In fact, there are still some studies on animals, healthy volunteers or patients with other types of depression that could draw a similar conclusion. An interesting fact is that most antidepressants which suppress REM sleep could weaken sleep efficiency (elongated sleep latency, increased awakenings, decreased total sleep and sleep continuity) while those which do not influence REM sleep or alter it moderately tend to improve overall sleep efficiency. It means there may be relative opposite neurobiological foundation to modulate REM sleep and sleep efficiency, and even antidepressants in same group act in some different ways.
Among other treatments of depression, brain stimulation such as electroconvulsive therapy (ECT) and repetitive magnetic stimulation (rTMS), combined with partial sleep deprivation, are reported to be effective among drug-resistant patients [126, 127]. It is suggested that EEG may be employed to monitor longitudinally the electrophysiological effects of ECT and rTMS [128]. Patients with MDD accepting dorsolateral prefrontal rTMS displayed decreases of the alpha activity during REM sleep and increases of slow-wave activity, which is proportion by clinical outcome, possibly reflecting locally enhanced synaptic plasticity [129, 130].
However, it is still not completely understood how REM sleep is induced and regulated. Some depressed patients, especially patients with atypical depression, do not suffer from REM sleep disturbances. And more and more researches proved that there is no direct and necessary causality between the effects of antidepressants on REM sleep and the prognosis of disease. It still needs more discussion about how important REM sleep abnormalities in depressed patients are and whether they could be an auxiliary index to diagnose disease or evaluate the effect of pharmacological treatments.
CONCLUSIONS
Although current studies have confirmed a strong relationship between the disturbances of REM sleep and depression, the role of REM sleep abnormalities should be better elucidated for understanding psychiatric consequences and treatments of depression.
SPECIFIC AUTHOR CONTRIBUTIONS
Y.Q.W., R.L., M.Q. Z. and Z.Z. wrote most of the sections; and Z.L.H. and W.M.Q. revised the entire manuscript.
Table 1.
The comparisions of the effects of different antidepressants on REM sleep.
| Antidepressants | Design | Subjects | n | Doses (per day) | Duration | Reference Treatment | Changes of Polysomnography |
|---|---|---|---|---|---|---|---|
| TCAs | |||||||
| Amitriptyline [93, 131] | EEG recording before, during and after treatment | Depressed inpatients | 6 | 370 nights | Suppressed REM sleep A REM sleep rebound after treatment | ||
| Double-blind randomized trial | Patients with MDD | 30 | 100-225 mg | 6 weeks | Alprazolam 4-9 mg | Suppressed REM sleep | |
| Clomipramine [96, 97] | EEG recording before and after treatment | Healthy man | 1 | Up to 100 mg | 1 month | Placebo | Suppressed REM sleep A REM sleep rebound after withdrawal Increased wakefulness Decreased NREM sleep |
| EEG recording after total sleep deprivation and treatment afterwards | Patients with MDD | 19 days | Suppressed REM sleep | ||||
| Imipramine [132] | Double-blind trial | Unipolar and bipolar patients hospitalized for MDD | 79 | 100-250 mg | 6 weeks | Amitriptyline 100-250 mg | Improvement in insomnia |
| Trimipramine [113, 133] | Double-blind trial | Depressed patients with insomnia and anxiety | 30 | 75-200 mg | 4 weeks | Imipramine 75-200 mg | Improvement in sleep disturbances Increased REM sleep in some cases |
| Double-blind trial | Male patients with MDD | 20 | 50-250 mg | 4 weeks | Imipramine 50-200 mg | Increased REM sleep and NREM sleep | |
| MAOIs | |||||||
| Phenelzine [100, 118] | Open-label trial | Patients with MDD | 11 | 30-90 mg | 5 weeks | Suppressed REM sleep Increased stage 2 NREM sleep | |
| EEG recording before and after treatment | Depressed patients | 3 | 18 months | Suppressed REM sleep initially A REM sleep rebound after 3 to 6 months of medication No change of NREM sleep | |||
| Tranylcypromine [101] | EEG recording before and after treatment | Patients with anergic bipolar depression | 23 | 37 mg (average) | Suppressed REM sleep Decreased total sleep time | ||
| Moclobemide [134, 135] | Double-blind trial | Depressed patients | 4 weeks | Placebo | Improvement in sleep continuity Increased stage 2 NREM sleep and REM sleep | ||
| Patients with MDD | 12 | 450 mg | 6 weeks | REM sleep habituation A slight REM sleep rebound after withdrawal | |||
| SSRIs | |||||||
| Paroxetine [92] | Double-blind randomized trial | Patients with MDD | 40 | 30 mg | 4 weeks for treatment | Amitriptyline 150 mg | Suppressed REM sleep |
| Fluoxetine [136] | Double-blind trial | Patients with MDD | 34 | 60 mg | 42 days for treatment | Amitriptyline 150 mg | Suppressed REM sleep Disrupted sleep continuity |
| SSRIs | |||||||
| Fluvoxamine [137] | Double-blind cross-over study | Normal volunteers | 12 | 100 mg | Dothiepin 100 mg Placebo | Suppressed REM sleep | |
| Clomipramine [138] | Double-blind, randomized, multicenter trial | Depressed patients | 52 | 25-150 mg | 6 weeks | Maprotiline 50-150 mg | Improvement in sleep disturbances |
| Zimelidine [107] | Double-blind trial | Depressed inpatients | 27 | 28 days | Amitriptyline | No improvement, even worsening in sleep continuity Suppressed REM sleep | |
| Nefazodone [108] | Multisite, randomized double-blind trial | Patients with MDD | 125 | 8 weeks | Fluoxetine | Increased sleep efficiency Decreased awakenings Suppressed REM sleep | |
| Citalopram [139] | EEG recording before and after treatment | Depressed patients | 16 | 8 weeks | Placebo | Suppressed REM sleep A REM sleep rebound after withdrawal | |
| SNRIs | |||||||
| Venlafaxine [109] | Double-blind trial | Depressed patients | 24 | Up to 225 mg | 29 days | Placebo | Decreased sleep continuity Suppressed REM sleep |
| Mirtazapin [140] | EEG recording before, during and after treatment | Patients with MDD | 16 | 15-30 mg | 2 weeks | Decreased sleep latency Increased total sleep time and efficiency No change of REM sleep | |
| Milnacipran [141] | EEG recording before and after treatment | Depressed patients | 8 | 100 mg | 1 month | Increased total sleep time and sleep efficiency No change of REM sleep | |
| Duloxetine [110] | EEG recording before and after treatment | Patients with MDD | 10 | 60 mg | 14 days | Increased stage 3 NREM sleep Suppressed REM sleep | |
| NARIs | |||||||
| Desipramine [104] | Double-blind trial | Depressed patients | 17 | 150 mg | 28 days | Placebo | Worsened sleep continuity Suppressed REM sleep |
| Trazodone [142] | EEG recording before, during and after treatment | Depressed patients | 400-600 mg | 1 month | Increased stage 2 NREM sleep Decreased sleep latency and intrasleep awakenings Suppressed REM sleep | ||
ACKNOWLEDGEMENTS
This study was supported in part by grants-in-aid for scientific research from the National Basic Research Program of China (2011CB711000, 2015CB856401), the National Natural Science Foundation of China (81420108015, 31471064, 81471344, 31171010, 31121061, 31271164, J1210041), the Fundamental Research Funds for the Central Universities (10FX041), a Key Laboratory program of the Education Commission of Shanghai Municipality (ZDSYS14005), the Shanghai Committee of Science and Technology (14JC1400900, 13dz2260700, 13140903100), the Shanghai Leading Academic Discipline Project (B119), and the Ph.D. Programs Foundation of Ministry of Education of China (20110071110033).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Bland R.C. Epidemiology of affective disorders: a review. Can. J. Psychiatry. 1997;42(4):367–377. doi: 10.1177/070674379704200403. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins D.R., Taub J.M., Van de Castle R.L. Extended sleep (hypersomnia) in young depressed patients. Am. J. Psychiatry. 1985;142(8):905–910. doi: 10.1176/ajp.142.8.905. [DOI] [PubMed] [Google Scholar]
- 3.Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr. Scand. Suppl. 2007;(433):104–115. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 4.Gresham S.C., Agnew H.W., Jr, Williams R.L. The sleep of depressed patients. An EEG and eye movement study. Arch. Gen. Psychiatry. 1965;13(6):503–507. doi: 10.1001/archpsyc.1965.01730060021003. [DOI] [PubMed] [Google Scholar]
- 5.Gottesmann C., Gottesman I. The neurobiological characteristics of rapid eye movement (REM) sleep are candidate endophenotypes of depression, schizophrenia, mental retardation and dementia. Prog. Neurobiol. 2007;81(4):237–250. doi: 10.1016/j.pneurobio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds C.F., III, Kupfer D.J. Sleep research in affective illness: state of the art circa 1987. Sleep. 1987;10(3):199–215. doi: 10.1093/sleep/10.3.199. [DOI] [PubMed] [Google Scholar]
- 7.Benca R.M., Okawa M., Uchiyama M., Ozaki S., Nakajima T., Shibui K., Obermeyer W.H. Sleep and mood disorders. Sleep Med. Rev. 1997;1(1):45–56. doi: 10.1016/S1087-0792(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 8.Steiger A., Kimura M. Wake and sleep EEG provide biomarkers in depression. J. Psychiatr. Res. 2010;44(4):242–252. doi: 10.1016/j.jpsychires.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Sharpley C.F., Bitsika V. Differences in neurobiological pathways of four "clinical content" subtypes of depression. Behav. Brain Res. 2013;256:368–376. doi: 10.1016/j.bbr.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Wichniak A., Wierzbicka A., Jernajczyk W. Sleep and antidepressant treatment. Curr. Pharm. Des. 2012;18(36):5802–5817. doi: 10.2174/138161212803523608. [DOI] [PubMed] [Google Scholar]
- 11.Wichniak A., Wierzbicka A., Jernajczyk W. Sleep as a biomarker for depression. Int. Rev. Psychiatry. 2013;25(5):632–645. doi: 10.3109/09540261.2013.812067. [DOI] [PubMed] [Google Scholar]
- 12.Palagini L., Baglioni C., Ciapparelli A., Gemignani A., Riemann D. REM sleep dysregulation in depression: state of the art. Sleep Med. Rev. 2013;17(5):377–390. doi: 10.1016/j.smrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Edge L.C. The role of emotional brain processing during sleep in depression. J. Psychiatr. Ment. Health Nurs. 2010;17(10):857–861. doi: 10.1111/j.1365-2850.2010.01598.x. [DOI] [PubMed] [Google Scholar]
- 14.Lopes M.C., Quera-Salva M.A., Guilleminault C. Non-REM sleep instability in patients with major depressive disorder: subjective improvement and improvement of non-REM sleep instability with treatment (Agomelatine). Sleep Med. 2007;9(1):33–41. doi: 10.1016/j.sleep.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann R., Hendrickse W., Rush A.J., Armitage R. Slow-wave activity during non-REM sleep in men with schizophrenia and major depressive disorders. Psychiatry Res. 2000;95(3):215–225. doi: 10.1016/S0165-1781(00)00181-5. [DOI] [PubMed] [Google Scholar]
- 16.Olbrich S., Arns M. EEG biomarkers in major depressive disorder: discriminative power and prediction of treatment response. Int. Rev. Psychiatry. 2013;25(5):604–618. doi: 10.3109/09540261.2013.816269. [DOI] [PubMed] [Google Scholar]
- 17.Rotenberg V.S., Shamir E., Barak Y., Indursky P., Kayumov L., Mark M. REM sleep latency and wakefulness in the first sleep cycle as markers of major depression: a controlled study vs. schizophrenia and normal controls. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26(6):1211–1215. doi: 10.1016/S0278-5846(02)00216-6. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds C.F., III, Kupfer D.J., Taska L.S., Hoch C.C., Spiker D.G., Sewitch D.E., Zimmer B., Marin R.S., Nelson J.P., Martin D., et al. EEG sleep in elderly depressed, demented, and healthy subjects. Biol. Psychiatry. 1985;20(4):431–442. doi: 10.1016/0006-3223(85)90045-9. [DOI] [PubMed] [Google Scholar]
- 19.Lauer C.J., Riemann D., Wiegand M., Berger M. From early to late adulthood. Changes in EEG sleep of depressed patients and healthy volunteers. Biol. Psychiatry. 1991;29(10):979–993. doi: 10.1016/0006-3223(91)90355-P. [DOI] [PubMed] [Google Scholar]
- 20.Goetz R.R., Puig-Antich J., Dahl R.E., Ryan N.D., Asnis G.M., Rabinovich H., Nelson B. EEG sleep of young adults with major depression: a controlled study. J. Affect. Disord. 1991;22(1-2):91–100. doi: 10.1016/0165-0327(91)90089-B. [DOI] [PubMed] [Google Scholar]
- 21.Wichniak A., Riemann D., Kiemen A., Voderholzer U., Jernajczyk W. Comparison between eye movement latency and REM sleep parameters in major depression. Eur. Arch. Psychiatry Clin. Neurosci. 2000;250(1):48–52. doi: 10.1007/s004060050009. [DOI] [PubMed] [Google Scholar]
- 22.Jernajczyk W. Latency of eye movement and other REM sleep parameters in bipolar depression. Biol. Psychiatry. 1986;21(5-6):465–472. doi: 10.1016/0006-3223(86)90188-5. [DOI] [PubMed] [Google Scholar]
- 23.Gillin J.C., Duncan W.C., Murphy D.L., Post R.M., Wehr T.A., Goodwin F.K., Wyatt R.J., Bunney W.E., Jr Age-related changes in sleep in depressed and normal subjects. Psychiatry Res. 1981;4(1):73–78. doi: 10.1016/0165-1781(81)90010-X. [DOI] [PubMed] [Google Scholar]
- 24.Foster F.G., Kupfer D.J., Coble P., McPartland R.J. Rapid eye movement sleep density. An objective indicator in severe medical-depressive syndromes. Arch. Gen. Psychiatry. 1976;33(9):1119–1123. doi: 10.1001/archpsyc.1976.01770090109011. [DOI] [PubMed] [Google Scholar]
- 25.Kupfer D.J. REM latency: a psychobiologic marker for primary depressive disease. Biol. Psychiatry. 1976;11(2):159–174. [PubMed] [Google Scholar]
- 26.Hartmann E., Verdone P., Snyder F. Longitudinal studies of sleep and dreaming patterns in psychiatric patients. J. Nerv. Ment. Dis. 1966;142(2):117–126. doi: 10.1097/00005053-196602000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Green W.J., Stajduhar P.P. The effect of ECT on the sleep-dream cycle in a psychotic depression. J. Nerv. Ment. Dis. 1966;143(2):123–134. doi: 10.1097/00005053-196608000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Kupfer D.J., Foster F.G. Interval between onset of sleep and rapid-eye-movement sleep as an indicator of depression. Lancet. 1972;2(7779): 684–686. doi: 10.1016/S0140-6736(72)92090-9. [DOI] [PubMed] [Google Scholar]
- 29.Adrien J., Dugovic C., Martin P. Sleep-wakefulness patterns in the helpless rat. Physiol. Behav. 1991;49(2):257–262. doi: 10.1016/0031-9384(91)90041-L. [DOI] [PubMed] [Google Scholar]
- 30.Grønli J., Murison R., Bjorvatn B., Sørensen E., Portas C.M., Ursin R. Chronic mild stress affects sucrose intake and sleep in rats. Behav. Brain Res. 2004;150(1-2):139–147. doi: 10.1016/S0166-4328(03)00252-3. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y.Q., Tu Z.C., Xu X.Y., Li R., Qu W.M., Urade Y., Huang Z.L. Acute administration of fluoxetine normalizes rapid eye movement sleep abnormality, but not depressive behaviors in olfactory bulbectomized rats. J. Neurochem. 2012;120(2):314–324. doi: 10.1111/j.1471-4159.2011.07558.x. [DOI] [PubMed] [Google Scholar]
- 32.Dugovic C., Maccari S., Weibel L., Turek F.W., Van Reeth O. High corticosterone levels in prenatally stressed rats predict persistent paradoxical sleep alterations. J. Neurosci. 1999;19(19):8656–8664. doi: 10.1523/JNEUROSCI.19-19-08656.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dugovic C., Solberg L.C., Redei E., Van Reeth O., Turek F.W. Sleep in the Wistar-Kyoto rat, a putative genetic animal model for depression. Neuroreport. 2000;11(3):627–631. doi: 10.1097/00001756-200002280-00038. [DOI] [PubMed] [Google Scholar]
- 34.Aserinsky E., Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118(3062):273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- 35.Dement W. The occurrence of low voltage, fast, electroencephalogram patterns during behavioral sleep in the cat. Electroencephalogr. Clin. Neurophysiol. 1958;10(2):291–296. doi: 10.1016/0013-4694(58)90037-3. [DOI] [PubMed] [Google Scholar]
- 36.Jouvet M., Michel F. [Electromyographic correlations of sleep in the chronic decorticate & mesencephalic cat]. C. R. Seances Soc. Biol. Fil. 1959;153(3):422–425. [PubMed] [Google Scholar]
- 37.Fuller P.M., Saper C.B., Lu J. The pontine REM switch: past and present. J. Physiol. 2007;584(3):735–741. doi: 10.1113/jphysiol.2007.140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarley R.W. Mechanisms and models of REM sleep control. Arch. Ital. Biol. 2004;142(4):429–467. [PubMed] [Google Scholar]
- 39.Kayama Y., Ohta M., Jodo E. Firing of ‘possibly’ cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569(2):210–220. doi: 10.1016/0006-8993(92)90632-J. [DOI] [PubMed] [Google Scholar]
- 40.Steriade M., Paré D., Datta S., Oakson G., Curró Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J. Neurosci. 1990;10(8):2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu M.F., John J., Boehmer L.N., Yau D., Nguyen G.B., Siegel J.M. Activity of dorsal raphe cells across the sleep-waking cycle and during cataplexy in narcoleptic dogs. J. Physiol. 2004;554(Pt 1):202–215. doi: 10.1113/jphysiol.2003.052134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hobson J.A., McCarley R.W., Nelson J.P. Location and spike-train characteristics of cells in anterodorsal pons having selective decreases in firing rate during desynchronized sleep. J. Neurophysiol. 1983;50(4):770–783. doi: 10.1152/jn.1983.50.4.770. [DOI] [PubMed] [Google Scholar]
- 43.Aston-Jones G., Bloom F.E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1981;1(8):876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jouvet M. The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. Ergeb. Physiol. 1972;64:166–307. doi: 10.1007/3-540-05462-6_2. [DOI] [PubMed] [Google Scholar]
- 45.Verret L., Fort P., Gervasoni D., Leger L., Luppi P.H. Localization of the neurons active during paradoxical (REM) sleep and projecting to the locus coeruleus noradrenergic neurons in the rat. J. Comp. Neurol. 2006;495(5): 573–586. doi: 10.1002/cne.20891. [DOI] [PubMed] [Google Scholar]
- 46.Vanni-Mercier G., Sakai K., Lin J.S., Jouvet M. Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat. Arch. Ital. Biol. 1989;127(3):133–164. [PubMed] [Google Scholar]
- 47.Sakai K., Crochet S., Onoe H. Pontine structures and mechanisms involved in the generation of paradoxical (REM) sleep. Arch. Ital. Biol. 2001;139(1-2):93–107. [PubMed] [Google Scholar]
- 48.Gutiérrez-Rivas E., de Andrés I., Gómez-Montoya J., Reinoso-Suárez F. The influence of the rostropontine-ventrolateral region on the sleep-wakefulness cycle. Experientia. 1978;34(1):61–62. doi: 10.1007/BF01921902. [DOI] [PubMed] [Google Scholar]
- 49.De Andrés I., Gómez-Montoya J., Gutiérrez-Rivas E., Reinoso-Suárez F. Differential action upon sleep states of ventrolateral and central areas of pontine tegmental field. Arch. Ital. Biol. 1985;123(1):1–11. [PubMed] [Google Scholar]
- 50.Garzón M., De Andrés I., Reinoso-Suárez F. Sleep patterns after carbachol delivery in the ventral oral pontine tegmentum of the cat. Neuroscience. 1998;83(4):1137–1144. doi: 10.1016/S0306-4522(97)00494-6. [DOI] [PubMed] [Google Scholar]
- 51.Reinoso-Suárez F., De Andrés I., Rodrigo-Angulo M.L., Rodríguez-Veiga E. Location and anatomical connections of a paradoxical sleep induction site in the cat ventral pontine tegmentum. Eur. J. Neurosci. 1994;6(12):1829–1836. doi: 10.1111/j.1460-9568.1994.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 52.De La Roza C., Martínez-Mena J., Sánchez-Valle M.E., Reinoso-Suárez F. Projections from the cat posterior lateral hypothalamus to the ventral part of the oral pontine reticular nucleus contain a GABAergic component. Brain Res. 2004;1020(1-2):118–129. doi: 10.1016/j.brainres.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 53.Xi M.C., Fung S.J., Yamuy J., Morales F.R., Chase M.H. Hypocretinergic facilitation of synaptic activity of neurons in the nucleus pontis oralis of the cat. Brain Res. 2003;976(2):253–258. doi: 10.1016/S0006-8993(03)02566-6. [DOI] [PubMed] [Google Scholar]
- 54.Xi M.C., Morales F.R., Chase M.H. Interactions between GABAergic and cholinergic processes in the nucleus pontis oralis: neuronal mechanisms controlling active (rapid eye movement) sleep and wakefulness. J. Neurosci. 2004;24(47):10670–10678. doi: 10.1523/JNEUROSCI.1987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCarley R.W., Hobson J.A. Single neuron activity in cat gigantocellular tegmental field: selectivity of discharge in desynchronized sleep. Science. 1971;174(4015):1250–1252. doi: 10.1126/science.174.4015.1250. [DOI] [PubMed] [Google Scholar]
- 56.Chu N., Bloom F.E. Norepinephrine-containing neurons: changes in spontaneous discharge patterns during sleeping and waking. Science. 1973;179(4076):908–910. doi: 10.1126/science.179.4076.908. [DOI] [PubMed] [Google Scholar]
- 57.Hobson J.A., McCarley R.W., Wyzinski P.W. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189(4196):55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- 58.Jacobs B.L. Single unit activity of locus coeruleus neurons in behaving animals. Prog. Neurobiol. 1986;27(2):183–194. doi: 10.1016/0301-0082(86)90008-0. [DOI] [PubMed] [Google Scholar]
- 59.McGinty D.J., Harper R.M. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101(3):569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 60.Sherin J.E., Elmquist J.K., Torrealba F., Saper C.B. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J. Neurosci. 1998;18(12):4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakai K., Kanamori N. Are there non-monoaminergic paradoxical sleep-off neurons in the brainstem? Sleep Res. Online. 1999;2(3):57–63. [PubMed] [Google Scholar]
- 62.el Mansari M., Sakai K., Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp. Brain Res. 1989;76(3):519–529. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- 63.Sakai K. Executive mechanisms of paradoxical sleep. Arch. Ital. Biol. 1988;126(4):239–257. [PubMed] [Google Scholar]
- 64.Datta S., Siwek D.F. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J. Neurosci. Res. 2002;70(4):611–621. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- 65.Sakai K., Koyama Y. Are there cholinergic and non-cholinergic paradoxical sleep-on neurones in the pons? Neuroreport. 1996;7(15-17):2449–2453. doi: 10.1097/00001756-199611040-00009. [DOI] [PubMed] [Google Scholar]
- 66.Mallick B.N., Kaur S., Jha S.K., Siegel J.M. Possible role of GABA in the regulation of REM sleep with special reference to REM-OFF neurons. Rapid Eye Movement Sleep; 1999. pp. 153–166. [Google Scholar]
- 67.Lu J., Sherman D., Devor M., Saper C.B. A putative flip-flop switch for control of REM sleep. Nature. 2006;441(7093):589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 68.Naughton M., Mulrooney J.B., Leonard B.E. A review of the role of serotonin receptors in psychiatric disorders. Hum. Psychopharmacol. 2000;15(6):397–415. doi: 10.1002/1099-1077(200008)15:6<397::AID-HUP212>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 69.Wilson S., Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65(7):927–947. doi: 10.2165/00003495-200565070-00003. [DOI] [PubMed] [Google Scholar]
- 70.Jones B.E., Harper S.T., Halaris A.E. Effects of locus coeruleus lesions upon cerebral monoamine content, sleep-wakefulness states and the response to amphetamine in the cat. Brain Res. 1977;124(3):473–496. doi: 10.1016/0006-8993(77)90948-9. [DOI] [PubMed] [Google Scholar]
- 71.Mouret J., Coindet J. Polygraphic evidence against a critical role of the raphe nuclei in sleep in the rat. Brain Res. 1980;186(2):273–287. doi: 10.1016/0006-8993(80)90975-0. [DOI] [PubMed] [Google Scholar]
- 72.Shouse M.N., Siegel J.M. Pontine regulation of REM sleep components in cats: integrity of the pedunculopontine tegmentum (PPT) is important for phasic events but unnecessary for atonia during REM sleep. Brain Res. 1992;571(1):50–63. doi: 10.1016/0006-8993(92)90508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perlis M.L., Smith M.T., Orff H.J., Andrews P.J., Gillin J.C., Giles D.E. The effects of an orally administered cholinergic agonist on REM sleep in major depression. Biol. Psychiatry. 2002;51(6):457–462. doi: 10.1016/S0006-3223(01)01287-2. [DOI] [PubMed] [Google Scholar]
- 74.Prathiba J., Kumar K.B., Karanth K.S. Effects of REM sleep deprivation on cholinergic receptor sensitivity and passive avoidance behavior in clomipramine model of depression. Brain Res. 2000;867(1-2):243–245. doi: 10.1016/S0006-8993(00)02248-4. [DOI] [PubMed] [Google Scholar]
- 75.Benca R.M., Obermeyer W.H., Thisted R.A., Gillin J.C. Sleep and psychiatric disorders. A meta-analysis. Arch. Gen. Psychiatry. 1992;49(8):651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 76.Ross R.J., Ball W.A., Sullivan K.A., Caroff S.N. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am. J. Psychiatry. 1989;146(6):697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- 77.Newman A.B., Spiekerman C.F., Enright P., Lefkowitz D., Manolio T., Reynolds C.F., Robbins J. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. 2000. [DOI] [PubMed]
- 78.Lustberg L., Reynolds C.F. Depression and insomnia: questions of cause and effect. Sleep Med. Rev. 2000;4(3):253–262. doi: 10.1053/smrv.1999.0075. [DOI] [PubMed] [Google Scholar]
- 79.Adrien J. Neurobiological bases for the relation between sleep and depression. Sleep Med. Rev. 2002;6(5):341–351. doi: 10.1016/S1087-0792(01)90200-X. [DOI] [PubMed] [Google Scholar]
- 80.Ebdlahad S., Nofzinger E.A., James J.A., Buysse D.J., Price J.C., Germain A. Comparing neural correlates of REM sleep in posttraumatic stress disorder and depression: a neuroimaging study. Psychiatry Res. 2013;214(3):422–428. doi: 10.1016/j.pscychresns.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sheline Y.I., Wang P.W., Gado M.H., Csernansky J.G., Vannier M.W. Hippocampal atrophy in recurrent major depression. Proc. Natl. Acad. Sci. USA. 1996;93(9):3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hegde P., Jayakrishnan H.R., Chattarji S., Kutty B.M., Laxmi T.R. Chronic stress-induced changes in REM sleep on theta oscillations in the rat hippocampus and amygdala. 2011. [DOI] [PubMed]
- 83.Mizuseki K., Buzsaki G. Theta oscillations decrease spike synchrony in the hippocampus and entorhinal cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369(1635):20120530. doi: 10.1098/rstb.2012.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jacinto L.R., Reis J.S., Dias N.S., Cerqueira J.J., Correia J.H., Sousa N. Stress affects theta activity in limbic networks and impairs novelty-induced exploration and familiarization. Front. Behav. Neurosci. 2013;7:127. doi: 10.3389/fnbeh.2013.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berk M. Sleep and depression - theory and practice. Aust. Fam. Physician. 2009;38(5):302–304. [PubMed] [Google Scholar]
- 86.Shors T.J., Seib T.B., Levine S., Thompson R.F. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science. 1989;244(4901):224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- 87.Xu L., Holscher C., Anwyl R., Rowan M.J. 1998. [DOI] [PMC free article] [PubMed]
- 88.Chang C.H., Chen M.C., Qiu M.H., Lu J. Ventromedial prefrontal cortex regulates depressive-like behavior and rapid eye movement sleep in the rat. Neuropharmacology. 2014;86:125–132. doi: 10.1016/j.neuropharm.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang R.Y., Aghajanian G.K. Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science. 1977;197(4298):89–91. doi: 10.1126/science.194312. [DOI] [PubMed] [Google Scholar]
- 90.Aizawa H., Yanagihara S., Kobayashi M., Niisato K., Takekawa T., Harukuni R., McHugh T.J., Fukai T., Isomura Y., Okamoto H. The synchronous activity of lateral habenular neurons is essential for regulating hippocampal theta oscillation. J. Neurosci. 2013;33(20):8909–8921. doi: 10.1523/JNEUROSCI.4369-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aizawa H., Cui W., Tanaka K., Okamoto H. Hyperactivation of the habenula as a link between depression and sleep disturbance. Front. Hum. Neurosci. 2013;7:826. doi: 10.3389/fnhum.2013.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Staner L., Kerkhofs M., Detroux D., Leyman S., Linkowski P., Mendlewicz J. Acute, subchronic and withdrawal sleep EEG changes during treatment with paroxetine and amitriptyline: a double-blind randomized trial in major depression. Sleep. 1995;18(6):470–477. [PubMed] [Google Scholar]
- 93.Gillin J.C., Wyatt R.J., Fram D., Snyder F. The relationship between changes in REM sleep and clinical improvement in depressed patients treated with amitriptyline. Psychopharmacology (Berl.) 1978;59(3):267–272. doi: 10.1007/BF00426633. [DOI] [PubMed] [Google Scholar]
- 94.Mayers A.G., Baldwin D.S. Antidepressants and their effect on sleep. Hum. Psychopharmacol. 2005;20(8):533–559. doi: 10.1002/hup.726. [DOI] [PubMed] [Google Scholar]
- 95.Gervasoni D., Panconi E., Henninot V., Boissard R., Barbagli B., Fort P., Luppi P.H. Effect of chronic treatment with milnacipran on sleep architecture in rats compared with paroxetine and imipramine. Pharmacol. Biochem. Behav. 2002;73(3):557–563. doi: 10.1016/S0091-3057(02)00812-2. [DOI] [PubMed] [Google Scholar]
- 96.Steiger A. Effects of clomipramine on sleep EEG and nocturnal penile tumescence: a long-term study in a healthy man. 1988. [DOI] [PubMed]
- 97.Riemann D., Berger M. The effects of total sleep deprivation and subsequent treatment with clomipramine on depressive symptoms and sleep electroencephalography in patients with a major depressive disorder. Acta Psychiatr. Scand. 1990;81(1):24–31. doi: 10.1111/j.1600-0447.1990.tb06444.x. [DOI] [PubMed] [Google Scholar]
- 98.Le Bon O. Contribution of sleep research to the development of new antidepressants. Dialogues Clin. Neurosci. 2005;7(4):305–313. doi: 10.31887/DCNS.2005.7.4/olebon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Holshoe J.M. Antidepressants and sleep, a review. 2009. [DOI] [PubMed]
- 100.Landolt H.P., Raimo E.B., Schnierow B.J., Kelsoe J.R., Rapaport M.H., Gillin J.C. Sleep and sleep electroencephalogram in depressed patients treated with phenelzine. Arch. Gen. Psychiatry. 2001;58(3):268–276. doi: 10.1001/archpsyc.58.3.268. [DOI] [PubMed] [Google Scholar]
- 101.Jindal R.D., Fasiczka A.L., Himmelhoch J.M., Mallinger A.G., Thase M.E. Effects of tranylcypromine on the sleep of patients with anergic bipolar depression. Psychopharmacol. Bull. 2003;37(3):118–126. [PubMed] [Google Scholar]
- 102.Sharpley A.L., Attenburrow M.E., Hafizi S., Cowen P.J. Olanzapine increases slow wave sleep and sleep continuity in SSRI-resistant depressed patients. J. Clin. Psychiatry. 2005;66(4):450–454. doi: 10.4088/JCP.v66n0407. [DOI] [PubMed] [Google Scholar]
- 103.Steiger A., von Bardeleben U., Guldner J., Lauer C., Rothe B., Holsboer F. The sleep EEG and nocturnal hormonal secretion studies on changes during the course of depression and on effects of CNS-active drugs. 1993. [DOI] [PubMed]
- 104.Shipley J.E., Kupfer D.J., Griffin S.J., Dealy R.S., Coble P.A., McEachran A.B., Grochocinski V.J., Ulrich R., Perel J.M. Comparison of effects of desipramine and amitriptyline on EEG sleep of depressed patients. Psychopharmacology (Berl.) 1985;85(1):14–22. doi: 10.1007/BF00427316. [DOI] [PubMed] [Google Scholar]
- 105.Sánchez C., Brennum L.T., Stórustovu Sí., Kreilgård M., Mørk A. Depression and poor sleep: the effect of monoaminergic antidepressants in a pre-clinical model in rats. Pharmacol. Biochem. Behav. 2007;86(3):468–476. doi: 10.1016/j.pbb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 106.von Bardeleben U., Steiger A., Gerken A., Holsboer F. Effects of fluoxetine upon pharmacoendocrine and sleep-EEG parameters in normal controls. Int. Clin. Psychopharmacol. 1989;4(Suppl. 1):1–5. [PubMed] [Google Scholar]
- 107.Shipley J.E., Kupfer D.J., Dealy R.S., Griffin S.J., Coble P.A., McEachran A.B., Grochocinski V.J. Differential effects of amitriptyline and of zimelidine on the sleep electroencephalogram of depressed patients. Clin. Pharmacol. Ther. 1984;36(2):251–259. doi: 10.1038/clpt.1984.171. [DOI] [PubMed] [Google Scholar]
- 108.Rush A.J., Armitage R., Gillin J.C., Yonkers K.A., Winokur A., Moldofsky H., Vogel G.W., Kaplita S.B., Fleming J.B., Montplaisir J., Erman M.K., Albala B.J., McQuade R.D. Comparative effects of nefazodone and fluoxetine on sleep in outpatients with major depressive disorder. Biol. Psychiatry. 1998;44(1):3–14. doi: 10.1016/S0006-3223(98)00092-4. [DOI] [PubMed] [Google Scholar]
- 109.Luthringer R., Toussaint M., Schaltenbrand N., Bailey P., Danjou P.H., Hackett D., Guichoux J.Y., Macher J.P. A double-blind, placebo-controlled evaluation of the effects of orally administered venlafaxine on sleep in inpatients with major depression. Psychopharmacol. Bull. 1996;32(4):637–646. [PubMed] [Google Scholar]
- 110.Kluge M., Schüssler P., Steiger A. Duloxetine increases stage 3 sleep and suppresses rapid eye movement (REM) sleep in patients with major depression. Eur. Neuropsychopharmacol. 2007;17(8):527–531. doi: 10.1016/j.euroneuro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 111.Thase M.E. Depression and sleep: pathophysiology and treatment. Dialogues Clin. Neurosci. 2006;8(2):217–226. doi: 10.31887/DCNS.2006.8.2/mthase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carney R.M., Rich M.W., Freedland K.E., Saini J., teVelde A., Simeone C., Clark K. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom. Med. 1988;50(6):627–633. doi: 10.1097/00006842-198811000-00009. [DOI] [PubMed] [Google Scholar]
- 113.Sonntag A., Rothe B., Guldner J., Yassouridis A., Holsboer F., Steiger A. Trimipramine and imipramine exert different effects on the sleep EEG and on nocturnal hormone secretion during treatment of major depression. Depression. 1996;4(1):1–13. doi: 10.1002/(SICI)1522-7162(1996)4:1<1::AID-DEPR1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 114.Nofzinger E.A., Reynolds C.F., III, Thase M.E., Frank E., Jennings J.R., Fasiczka A.L., Sullivan L.R., Kupfer D.J. REM sleep enhancement by bupropion in depressed men. Am. J. Psychiatry. 1995;152(2):274–276. doi: 10.1176/ajp.152.2.274. [DOI] [PubMed] [Google Scholar]
- 115.Kuenzel H.E., Murck H., Held K., Ziegenbein M., Steiger A. Reboxetine induces similar sleep-EEG changes like SSRI’s in patients with depression. Pharmacopsychiatry. 2004;37(5):193–195. doi: 10.1055/s-2004-827242. [DOI] [PubMed] [Google Scholar]
- 116.Aslan S., Isik E., Cosar B. The effects of mirtazapine on sleep: a placebo controlled, double-blind study in young healthy volunteers. Sleep. 2002;25(6):677–679. [PubMed] [Google Scholar]
- 117.Schmid D.A., Wichniak A., Uhr M., Ising M., Brunner H., Held K., Weikel J.C., Sonntag A., Steiger A. Changes of sleep architecture, spectral composition of sleep EEG, the nocturnal secretion of cortisol, ACTH, GH, prolactin, melatonin, ghrelin, and leptin, and the DEX-CRH test in depressed patients during treatment with mirtazapine. Neuropsychopharmacology. 2006;31(4):832–844. doi: 10.1038/sj.npp.1300923. [DOI] [PubMed] [Google Scholar]
- 118.Landolt H.P., de Boer L.P. Effect of chronic phenelzine treatment on REM sleep: report of three patients. Neuropsychopharmacology. 2001;25(5) Suppl.:S63–S67. doi: 10.1016/S0893-133X(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 119.Murck H., Frieboes R.M., Antonijevic I.A., Steiger A. Distinct temporal pattern of the effects of the combined serotonin-reuptake inhibitor and 5-HT1A agonist EMD 68843 on the sleep EEG in healthy men. Psychopharmacology (Berl.) 2001;155(2):187–192. doi: 10.1007/s002130100703. [DOI] [PubMed] [Google Scholar]
- 120.Ruigt G.S., Kemp B., Groenhout C.M., Kamphuisen H.A. Effect of the antidepressant Org 3770 on human sleep. Eur. J. Clin. Pharmacol. 1990;38(6):551–554. doi: 10.1007/BF00278580. [DOI] [PubMed] [Google Scholar]
- 121.Smith M.I., Piper D.C., Duxon M.S., Upton N. Effect of SB- 243213, a selective 5-HT(2C) receptor antagonist, on the rat sleep profile: a comparison to paroxetine. Pharmacol. Biochem. Behav. 2002;71(4):599–605. doi: 10.1016/s0091-3057(01)00702-x. [DOI] [PubMed] [Google Scholar]
- 122.Argyropoulos S.V., Hicks J.A., Nash J.R., Bell C.J., Rich A.S., Nutt D.J., Wilson S. Redistribution of slow wave activity of sleep during pharmacological treatment of depression with paroxetine but not with nefazodone. J. Sleep Res. 2009;18(3):342–348. doi: 10.1111/j.1365-2869.2008.00724.x. [DOI] [PubMed] [Google Scholar]
- 123.Sato H., Ito C., Tashiro M., Hiraoka K., Shibuya K., Funaki Y., Iwata R., Matsuoka H., Yanai K. Histamine H₁ receptor occupancy by the new-generation antidepressants fluvoxamine and mirtazapine: a positron emission tomography study in healthy volunteers. Psychopharmacology (Berl.) 2013;230(2):227–234. doi: 10.1007/s00213-013-3146-1. [DOI] [PubMed] [Google Scholar]
- 124.Haddjeri N., Blier P., de Montigny C. Noradrenergic modulation of central serotonergic neurotransmission: acute and long-term actions of mirtazapine. Int. Clin. Psychopharmacol. 1995;10(Suppl. 4):11–17. doi: 10.1097/00004850-199512004-00003. [DOI] [PubMed] [Google Scholar]
- 125.Houghton W.C., Scammell T.E., Thorpy M. Pharmacotherapy for cataplexy. Sleep Med. Rev. 2004;8(5):355–366. doi: 10.1016/j.smrv.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 126.He M.L., Gu Z.T., Wang X.Y., Shi H.P. Treatment of depression using sleep electroencephalogram modulated repetitive transcranial magnetic stimulation. Chin. Med. J. (Engl.) 2011;124(12):1779–1783. doi: 10.1016/s0924-9338(11)72847-4. [DOI] [PubMed] [Google Scholar]
- 127.Krstić J., Buzadžić I., Milanović S.D., Ilić N.V., Pajić S., Ilić T.V. Low-frequency repetitive transcranial magnetic stimulation in the right prefrontal cortex combined with partial sleep deprivation in treatment-resistant depression: a randomized sham-controlled trial. J. ECT. 2014;30(4):325–331. doi: 10.1097/YCT.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 128.Casarotto S., Canali P., Rosanova M., Pigorini A., Fecchio M., Mariotti M., Lucca A., Colombo C., Benedetti F., Massimini M. Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain Topogr. 2013;26(2):326–337. doi: 10.1007/s10548-012-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pellicciari M.C., Cordone S., Marzano C., Bignotti S., Pellicciari M.C., Cordone S., Marzano C., Bignotti S., Gazzoli A., Miniussi C., De Gennaro L. Dorsolateral prefrontal transcranial magnetic stimulation in patients with major depression locally affects alpha power of REM sleep. Front. Hum. Neurosci. 2013;7:433. doi: 10.3389/fnhum.2013.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saeki T., Nakamura M., Hirai N., Noda Y., Hayasaka S., Iwanari H., Hirayasu Y. 2013. [DOI] [PubMed]
- 131.Hubain P.P., Castro P., Mesters P., De Maertelaer V., Mendlewicz J. Alprazolam and amitriptyline in the treatment of major depressive disorder: a double-blind clinical and sleep EEG study. 1990. [DOI] [PubMed]
- 132.Casper R.C., Katz M.M., Bowden C.L., Davis J.M., Koslow S.H., Hanin I. The pattern of physical symptom changes in major depressive disorder following treatment with amitriptyline or imipramine. J. Affect. Disord. 1994;31(3):151–164. doi: 10.1016/0165-0327(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 133.Ware J.C., Brown F.W., Moorad P.J., Jr, Pittard J.T., Cobert B. Effects on sleep: a double-blind study comparing trimipramine to imipramine in depressed insomniac patients. Sleep. 1989;12(6):537–549. doi: 10.1093/sleep/12.6.537. [DOI] [PubMed] [Google Scholar]
- 134.Monti J.M. Effect of a reversible monoamine oxidase-A inhibitor (moclobemide) on sleep of depressed patients. Br. J. Psychiatry Suppl. 1989;(6):61–65. [PubMed] [Google Scholar]
- 135.Minot R., Luthringer R., Macher J.P. Effect of moclobemide on the psychophysiology of sleep/wake cycles: a neuroelectrophysiological study of depressed patients administered with moclobemide. Int. Clin. Psychopharmacol. 1993;7(3-4):181–189. doi: 10.1097/00004850-199300730-00009. [DOI] [PubMed] [Google Scholar]
- 136.Kerkhofs M., Rielaert C., de Maertelaer V., Linkowski P., Czarka M., Mendlewicz J. Fluoxetine in major depression: efficacy, safety and effects on sleep polygraphic variables. Int. Clin. Psychopharmacol. 1990;5(4):253–260. doi: 10.1097/00004850-199010000-00002. [DOI] [PubMed] [Google Scholar]
- 137.Wilson S.J., Bailey J.E., Alford C., Nutt D.J. Sleep and daytime sleepiness the next day following single night-time dose of fluvoxamine, dothiepin and placebo in normal volunteers. 2000. [DOI] [PubMed]
- 138.Eberhard G., von Knorring L., Nilsson H.L., Sundequist U., Björling G., Linder H., Svärd K.O., Tysk L. A double-blind randomized study of clomipramine versus maprotiline in patients with idiopathic pain syndromes. Neuropsychobiology. 1988;19(1):25–34. doi: 10.1159/000118429. [DOI] [PubMed] [Google Scholar]
- 139.Beersma D.G., Beersma D.G., Van Den Hoofdakker R.H., Van Bemmel AL. Van Den Hoofdakker RH Changes in EEG power density of NREM sleep in depressed patients during treatment with citalopram. J. Sleep Res. 1993;2(3):156–162. doi: 10.1111/j.1365-2869.1993.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 140.Winokur A., Sateia M.J., Hayes J.B., Bayles-Dazet W., MacDonald M.M., Gary K.A. Acute effects of mirtazapine on sleep continuity and sleep architecture in depressed patients: a pilot study. 2000. [DOI] [PubMed]
- 141.Lemoine P., Faivre T. Subjective and polysomnographic effects of milnacipran on sleep in depressed patients. Hum. Psychopharmacol. 2004;19(5):299–303. doi: 10.1002/hup.600. [DOI] [PubMed] [Google Scholar]
- 142.Mouret J., Lemoine P., Minuit M.P., Benkelfat C., Renardet M. Effects of trazodone on the sleep of depressed subjects--a polygraphic study. Psychopharmacology (Berl.) 1988;95(Suppl.):S37–S43. doi: 10.1007/BF00172629. [DOI] [PubMed] [Google Scholar]


