Abstract
Distinct potassium, anion, and calcium channels in the plasma membrane and vacuolar membrane of plant cells have been identified and characterized by patch clamping. Primarily owing to advances in Arabidopsis genetics and genomics, and yeast functional complementation, many of the corresponding genes have been identified. Recent advances in our understanding of ion channel genes that mediate signal transduction and ion transport are discussed here. Some plant ion channels, for example, ALMT and SLAC anion channel subunits, are unique. The majority of plant ion channel families exhibit homology to animal genes; such families include both hyperpolarization-and depolarization-activated Shaker-type potassium channels, CLC chloride transporters/channels, cyclic nucleotide–gated channels, and ionotropic glutamate receptor homologs. These plant ion channels offer unique opportunities to analyze the structural mechanisms and functions of ion channels. Here we review gene families of selected plant ion channel classes and discuss unique structure-function aspects and their physiological roles in plant cell signaling and transport.
Keywords: guard cells, stomatal regulation, Arabidopsis, comparative genomics
INTRODUCTION
Ion channels have long been known to function in action potential generation in animal and plant algal cells (1–3). In plants the depolarizing phase of action potentials is, however, not mediated by Na+ channels, owing to the lack of a suitable Na+ gradient and to the fact that Na+ ions are toxic to most plant cells. Rather, the depolarizing phase of action potentials is mediated by Cl− efflux channels and by Ca2+ channels (3–6). Recent research has led to initial characterizations of Cl−/anion channel–encoding genes with unique structures (7–11).
In addition to their classical roles in action potential mediation, several classes of plant ion channels function in long-term net uptake or release of ions from cells or organelles (12–23). In this respect, several classes of plant ion channels do not show the typical rapid inactivation of animal ion channels. For example, inward-and outward-rectifying K+ channels in guard cells can remain activated for >30 min (24). This property allows these ion channels to function in long-term ion transport, thus mediating plant movements that are osmotically driven by so-called motor cells, which can show large changes in their turgor (25, 26).
Plant ion channels provide potent models to dissect general properties of ion channels that are presently of great interest and intensely debated. For example, the mechanisms allowing voltage sensor domains to activate and deactivate ion channels can be analyzed in plant ion channels because relatively closely related K+ channels exist with opposite voltage dependencies (27–32). Recent studies have shown that different members of the CLC family of membrane proteins can encode for either Cl− channels or endosomal Cl−/H+ exchangers (33–36). However, the physiological relevance of this Cl−/H+ exchange mechanism remains incompletely understood. Recent research has directly analyzed and demonstrated anion/H+ exchange mediated by an Arabidopsis CLC in the intact large vacuolar organelles of plant cells and has illustrated the physiological necessity of this type of transporter for concentrating nitrate reserves at 50-fold-higher concentrations inside vacuoles than in the cytoplasm (37).
A few studies have characterized depolarization-activated Ca2+-permeable channels in plant cells (38–40), and this type of ion channel has only recently been recorded in Arabidopsis (189). These Ca2+ channels may have specific functions, including the rapid responses of Venus flytrap (Dionaea muscipula) leaves (5). Most plasma membrane Ca2+ channels that have been recorded in plants show increased activity upon hyperpolarization (41–44). Interestingly, the genes that encode these Ca2+ permeable channels have not yet been characterized. Candidate genes in Arabidopsis may be the family of 20 cyclic nucleotide–gated (CNG) channel homologs (45) and 20 genes encoding homologs to animal glutamate receptor channels (46). However, sequenced genomes and EST databases indicate that higher plants may not include TRP channels (47) or ORAI homologs (48) within their genetic repertoires.
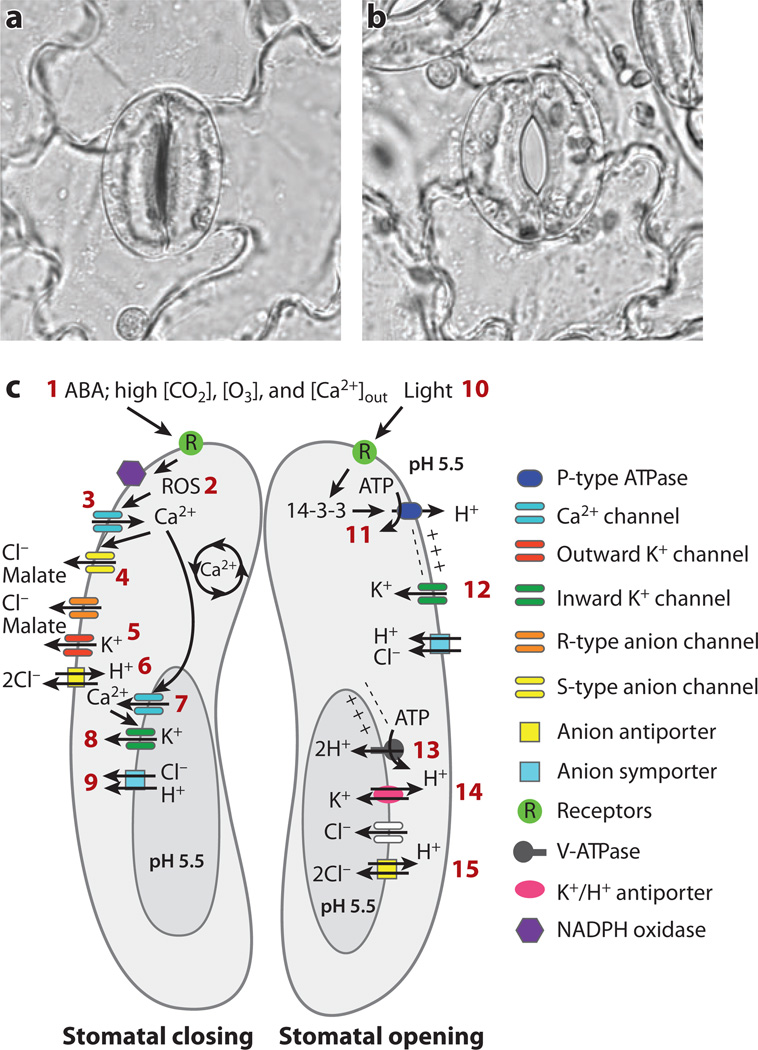
Analyses of cells with specific functions have facilitated the characterization of plant ion channel functions and regulation. Guard cells have been developed as a model system for illuminating plant ion channel function and signaling (Figure 1). Pairs of guard cells in the epidermis of leaves form stomatal pores (Figure 1a,b) that enable plants to take in CO2 from the atmosphere for carbon fixation. However, stomatal pores also provide the major conduits for water loss of plants, via transpiration to the atmosphere. Stomatal pores can close (Figure 1a) and open (Figure 1b), thus regulating the exchange of CO2 and water with the atmosphere. Physiological stimuli such as light and low CO2 concentrations regulate the osmotic opening of stomatal pores by increasing the K+, anion, and organic solute concentrations of guard cells (Figure 1b;c, right). Stomatal closing, in contrast, is mediated by the release of K+ and anions from guard cells, resulting in guard cell turgor reduction (Figure 1a;c, left). Stomatal closing reduces water loss in plants and is triggered by drought conditions, elevated CO2, and darkness. The adaptation of patch clamping and Ca2+-imaging methods to the relatively small guard cells of Arabidopsis (49, 50) opened up the possibility of combining powerful molecular genetic/genomic analyses in Arabidopsis with single-cell ion channel regulation and cell signaling analyses. Analyses of ion channel regulation in Arabidopsis have greatly increased our understanding of ion channel functions and signaling mechanisms in plants. Several reviews are available for background information on upstream signaling mechanisms that regulate plant ion channel activity (25, 26, 51, 52), and recently reported examples of ion channel signaling are reviewed here.
Figure 1.
Model for ion channel and transporter classes and their demonstrated or predicted roles during stomatal movements. (a) A closed stomate in a leaf from Vicia faba (broad bean). (b) An open stomate from Vicia faba. Two guard cells surround the stomatal pore and regulate the aperture of the central pore. (c) Model for regulation and activity of guard cell ion channels and transporters. (Left) (1) Signals that induce stomatal closing include the hormone abscisic acid (ABA), high [CO2], high extracellular [Ca2+], and high ozone [O3]. Many of the receptors involved remain to be identified (see Reference 26). Stomatal closing requires net cellular efflux of solutes, in particular K+, Cl, and malate. ABA induces reactive oxygen species (ROS) production (2), which activates Ca2+-permeable ICa channels (3). Cytosolic Ca2+ concentration ([Ca2+]cyt) is a central regulator of transport mechanisms in guard cells and activates slow/sustained (S-type) anion channels (4) and vacuolar SV (TPC) channels (7) and VK (TPK) channels (8). Cl and malate efflux through S-type and rapid (R-type) anion channels (4) and possibly CLC Cl/H+ antiporters (6) causes depolarization and drives K+ efflux through outward-rectifying K+ channels (5). At the vacuole membrane, SV (TPC1) channels (7) are Ca2+-activated and Ca2+-permeable voltage-dependent channels. VK (TPK) channels (8) are Ca2+ activated, are highly selective for K+, and are proposed to allow K+ release from the vacuole during stomatal closing. (Right) Mechanisms that function in guard cell ion uptake and stomatal opening. Blue light (10) activates phototropins (receptor/kinase), leading to stomatal opening. Signaling results in 14-3-3 binding to plasma membrane proton pumps (11), leading to hyperpolarization and acidification of the extracellular space. Hyperpolarization activates inward-rectifying K+ channels (12). At the vacuolar membrane, proton pumps (13) acidify the vacuole lumen and drive K+/H+ antiporters (14 ). Cl and malate may accumulate in the vacuole through anion channel and anion antiporters (15 ).
GUARD CELLS AS A MODEL SYSTEM TO STUDY PHYSIOLOGICAL FUNCTIONS OF PLANT ION CHANNELS
Guard cells regulate the aperture of stomatal pores in response to many physiological stimuli such as light, CO2 concentration, and hormones (26, 52). Additional features have made guard cells an attractive model system for studies of ion channels: These cells are located in the epidermis and can be easily isolated, they are not connected to adjacent cells by plasmodesmata, and their cellular movements are rapid and driven by osmotic changes. Early work showed that cellular ion flux mediates guard cell osmotic changes (53–55). The inward and outward-rectifying K+ channels (14, 24) and anion channels (15, 16) were characterized and proposed to mediate stomatal movements (Figure 1c). The development of the plant Arabidopsis thaliana as a genetic model organism opened the possibility of combining electrophysiology, cell biology, molecular genetics, and functional genomics to study ion channel function and signal transduction in plants. This has allowed several approaches to relate specific ion channel genes to physiological functions in guard cells. Insertional mutants have been used to identify physiological functions for specific channel genes (10, 21–23, 56). Ion channel activities have been analyzed in Arabidopsis signal transduction mutants to understand how mutations in upstream abscisic acid (ABA) signal transduction components such as protein phosphatases impair ABA regulation of anion channels that control stomatal responses (49). The ability to apply the transgenic cameleon Ca2+ indicator to analyze Arabidopsis signaling mutants has allowed further resolution of the roles of cytosolic Ca2+ in the control of stomatal aperture (50, 57, 58).
During stomatal opening, guard cell volume increases by approximately 40% (59, 60). This increase occurs by activation of plasma membrane H+ pumps, K+ uptake through inward-rectifying K+ channels, organic acid (mainly malate) production, and the uptake of inorganic anions (Cl−), presumably through proton-coupled uptake transporters (Figure 1c, right). This lowers the water potential in guard cells and drives osmotic water uptake into guard cells. Most of the volume change in guard cells is accounted for by increased vacuolar volume, with multiple smaller vacuoles fusing to form a large central vacuole (60). K+ uptake into vacuoles during stomatal opening is likely due to H+/K+ antiporter activity (61), whereas anion uptake into vacuoles may occur through a combination of low-affinity anion channels (9, 62, 63) and an active H+/anion exchange mechanism (37).
During stomatal closing, guard cell volume decreases owing to net cellular efflux of solutes. Depolarization due to plasma membrane anion channel activity was proposed to drive K+ efflux through outward-rectifying K+ channels (14–16). The resulting cellular export of K, Cl−, and organic ions such as malate drives water efflux (Figure 1a;c, left). At the vacuole membrane, Ca2+-activated vacuolar K+ (VK) channels are required for K+ release to the cytoplasm (17, 22, 64).
In the following sections, we present an overview of plant ion channel classes, how they relate in structure and activity to channels in other systems, and how they function in plants.
PLANT CHLORIDE/ANION CHANNELS AND TRANSPORTERS
Cl− channels in algae mediate a major component of the depolarizing phase of action potentials, consistent with a downhill Cl−/anion gradient from the cytoplasm to the cell wall space of plants cells that is typically opposite to the gradient found in animal cells (3, 6, 65, 66). In guard cells, roots, and other cell types, anion channels may provide major mechanisms for net anion efflux and transport across the plasma membrane (7, 15, 16, 67) (Figure 1). In addition, many responses in plants, including plant pathogen responses, involve membrane potential depolarization accompanied by Cl− efflux, and anion channel blockers inhibit pathogen-induced signaling, suggesting that anion channels may play roles in these responses as well (68). Indeed, anion channel– mediated depolarization may contribute to the propagation of signals because most plant cells are cytoplasmically connected to one another via plasmodesmata.
Aluminum Tolerance Mediated by ALMT Anion Channels/Transporters
In addition to their proposed functions as major regulators of stomatal closing as discussed above, plant anion channels have been of great interest in understanding how plants can acquire tolerance to aluminum stress. Aluminum is the third most abundant element in the earth’s lithosphere (crust). In acidic soils, which cause solubilization of Al3+, Al3+ is highly toxic to plant growth (69). Research on different plant species has demonstrated that plant cultivars that show tolerance to Al3+ are able to secrete Al3+-chelating organic anions, such as malate and citrate at the root surface, in response to Al3+ exposure (70–72). Electrophysiological studies have shown that Al3+ activates anion channel currents in cortical cells in the root apex (71, 73).
Major genetic Al3+ tolerance loci have been cloned and mapped in several plants, and several of these loci encode members of a family of membrane proteins known as Al3+-activated malate transporters (ALMTs) (Table 1) (7, 8, 74). ALMTs have 6–8 predicted membrane domains. Interestingly, expression of individual ALMTs in Xenopus oocytes is sufficient to mediate Al3+-induced malate currents, suggesting that the ALMT transporters are direct sensors or receptors of Al3+ stress (7, 8, 75). Reversal potential shifts in Xenopus oocytes show Nernstian behavior correlating with the malate gradient (8), leading to the suggestion that ALMTs encode anion channels.
Table 1.
Number of predicted transporter genes in the genomes of Arabidopsis, poplar, rice, and Chlamydomonasa
| A. thaliana | P. trichocarpa | O. sativa | C. reinhardtii | |
|---|---|---|---|---|
| Predicted proteome size | 31,711 | 58,036 | 26,841 | 14,598 |
| Cation transporters | ||||
| KcsA type | 1 | 0 | 0 | 0 |
| Shaker type | 9 | 11 | 6 | 0 |
| TPK/KCO | 5 | 10 | 4/3 | 0 |
| Unclassified 6TM1P | 0 | 0 | 0 | 9 |
| CNGC | 20 | 12 | 10 | 0 |
| TPC | 1 | 1 | 1 | 0 |
| HKT | 1 | 2 | 6 | 0 |
| KUP/HAK | 13 | 28 | 25 | 3 |
| Anion channels | ||||
| CLC | 7 | 8 | 5 | 5 |
| SLAC | 5 | 4 | 7 | 1 |
| ALMT | 13 | 22 | 8 | 0 |
| Calcium channels | ||||
| VDCC | 0 | 0 | 0 | 9 |
| Ins-3P–R | 0 | 0 | 0 | 1 |
| GluR (plant type) | 20 | 61 | 13 | 0 |
The predicted proteomes were screened with HMMer profiles (188) made from homology-reduced sets of representative proteins of each family. See text for abbreviations of family names. The results are affected by differences in sequence coverage and quality of gene predictions between species.
A recent study has shown that the Arabidopsis AtALMT9 protein is targeted to the vacuolar membrane of plant cells and mediates low-affinity malate uptake into plant vacuoles (9). Vacuolar (V-type) proton pumps acidify the lumen of vacuoles and provide the driving force for low- and high-affinity vacuolar malate uptake. Plant vacuoles have malate uptake channels (62, 63, 76). Patch clamp analysis of intact vacuoles has shown that this vacuolar malate current is absent in ALMT9 knockout mutant plants (9). Further structure-function analyses of ALMTs should shed light into the mechanisms of Al3+ activation, anion transport, and selectivity.
CLC Chloride Channels and Proton/Anion Exchangers
In animal cells several classes of Cl− channels are known. These include the CLC family (77), GABA receptor and glycine receptor channels (78), volume-regulated anion channels (VRACs), and bestrophins (79). In plants no bestrophin homologs or direct GABA/glycine receptor homologs have been reported. Small domains with homology to these ion channels have been reported within the 20-member glutamate receptor homolog family (80), although no evidence has been reported that these function as anion channels.
In the case of the membrane protein CLC genes, a family of seven homologs is present in the Arabidopsis genome (Table 1). Several plant CLC proteins are targeted to intracellular organelles (37, 81–83). The first electrophysiologically characterized plant CLC protein was AtCLCa, which is targeted to plant vacuoles (37). Interestingly, AtCLCa mediates proton/anion (nitrate) exchanger activity in the vacuolar membrane, rather than anion channel activity (37). Previous studies of the bacterial ClC-e1 and the endosomal CLC-4 and CLC-5 membrane proteins showed that they encode proton/Cl− exchangers (33–35). However, the physiological relevance of proton/Cl− exchange activity compared with Cl− channel activity remained unclear in bacteria and animal cells. For example, both Cl− channels and proton/Cl− exchangers would be predicted to mediate Cl− uptake into endosomes. Research on plant vacuoles showed that AtCLCa is energetically suited for the physiological accumulation of nitrate into vacuoles at lumenal concentrations that are 50-fold higher than typical cytosolic nitrate concentrations (37). Passive nitrate channels would not allow such physiological nitrate accumulation in plant vacuoles that may generate membrane potentials of –30 mV (on the cytoplasmic membrane side relative to the vacuole lumen).
Analyses of bacterial CLC proton/Cl− exchanger ClC-ec1 have shown that two glutamate residues (E148 and E203) are important for proton/Cl− exchange activity vis-à-vis Cl− channel activity of other CLC proteins (36). Interestingly, four of seven Arabidopsis CLC proteins have glutamate residues at both corresponding positions (e.g., E203 and E270 of AtCLC-a) (37, 84), indicating that only three Arabidopsis CLCs may encode anion channels, whereas most plant CLC transporters may encode proton/anion exchangers.
Expression of a proton/anion exchanger CLC in the plasma membrane of plant cells may be relevant for anion efflux and membrane depolarization (84) because the extracellular space of plant cells has an acidic pH (e.g., pH 5.5 to 6). For example, motor cells that rapidly reduce their turgor via ion release (Figure 1a) could build up high cell wall anion concentrations (25, 84), which would limit the ability of passive anion channels to continue driving turgor reduction (Figure 1c, left). However, proton/anion exchangers would energetically favor continued anion efflux across the plasma membrane and could continue to mediate anion efflux and depolarization, which is required for K+ efflux via K+ channels from motor cells. Further research is needed to determine whether a plant CLC protein is targeted to the plasma membrane and what type of biophysical anion transport mechanism such transporters utilize.
Slow Anion Channels Require the SLAC1 Membrane Protein
Research on guard cells has led to the model that anion channels are critical mechanisms for controlling stomatal closing and anion efflux from plant cells (15, 16). However, apart from genes encoding the ALMT malate channels/transporters, anion channel– encoding genes have remained unknown in plants. Recent genetic screens for ozone sensitivity of leaves and for CO2 insensitivity of leaf temperature have led to isolation of a gene, named SLAC1, that encodes a plasma membrane protein in guard cells (10, 11). Interestingly, slac1 mutant alleles show insensitivity to CO2-, ABA-, and ozone-induced stomatal closing (10, 11). This ozone insensitivity leads to an increased entry of ozone into leaves through open stomata (Figure 1) and thus to enhanced ozone damage in slac1 mutants (10).
Patch clamp analyses of two slac1 mutant alleles showed that slow/sustained (S-type) anion channel currents were greatly impaired, whereas the rapid (R-type) anion channels and the plasma membrane Ca2+-permeable ICa channels were intact in guard cells (10). Thus, these data provide strong genetic evidence for the model that S-type anion channels provide one of the critical mechanisms for signal-induced stomatal closing (15). These analyses do not exclude a role for R-type anion channels in stomatal closing because, for example, light-dark transitions, ABA, and transitions to low humidity showed a slowed or a partial stomatal closing response in slac1 mutant alleles that may be mediated by the remaining R-type anion channels or other anion efflux conductances (10). As discussed above in reference to CLCs, there may be a third type of anion efflux channel/transporter that also contributes to the control of stomatal closing. Furthermore, analyses of slac1 mutants show that R-type and S-type anion channels do not require identical membrane proteins (10), although these findings do not exclude the possibility that other proteins are shared among these two types of plant anion channels (85).
SLAC1 is part of a gene family with five members in the Arabidopsis genome (Table 1). SLAC1 encodes a membrane protein that has nine to ten predicted transmembrane domains with large N- and C-terminal domains. SLAC proteins show a weak homology in their predicted transmembrane domains to a fungal (86) and a bacterial malate transporter in the TDT family. Both the S-type and the R-type anion channels allow Cl− and malate efflux from guard cells (16, 87), which correlates with malate and Cl− efflux from guard cells during stomatal closing (88, 89). Thus, the homology of SLAC1 to malate transporters and the disruption of S-type anion currents in slac1 mutants indicate that SLAC1 is a member of a novel plant anion/Cl− channel family. Further research on SLAC proteins should determine their regulation mechanisms and functions in various plant tissues.
PLANT Ca2+ CHANNELS
Several distinct Ca2+ channel activities have been characterized in the plasma membrane of plant cells. However, to date, the corresponding genes have not been identified. Land plants encode one type of voltage-dependent Ca2+ channel homolog (TPC1) that has been well characterized and is expressed in the vacuolar membrane (see below). Candidate genes that possibly encode plasma membrane Ca2+ channels are a family of 20 glutamate receptor homologs (GluR) (46) and a family of 20 CNG channel homologs (CNGC) (45, 90). Glutamate receptor homologs are required for glutamate-induced depolarization and intracellular [Ca2+] elevation in roots (91, 92). Further voltage clamp recordings of these channels will be valuable for comparisons to known root Ca2+ channels (42, 93). For more information on plant homologs of ionotropic glutamate receptors, see Lacombe et al. (46).
Several studies have identified hyperpolarization-activated Ca2+ channels (41–44). ABA enhances this ICa activity (44) by shifting the activation voltage to more positive potentials (43). ABA regulation of ICa can occur through reactive oxygen species (ROS) production (44, 94), which induces increases in cytosolic Ca2+ concentration ([Ca2+]cyt). ABA induction of ROS and ABA activation of ICa channels are impaired in guard cells in which the plasma membrane NADPH oxidase Atr-BOHD and AtrBOHF genes are disrupted, providing genetic evidence for a role of ROS in ICa regulation by ABA (94). Furthermore, ICa is regulated by Ca2+-dependent protein kinases and protein phosphorylation events (95, 96).
Hyperpolarization-activated Ca2+-permeable channels have also been analyzed in root cells (42, 97). Differences in ROS sensitivity of these channels in mature versus elongating root cells indicates that more than one class of hyperpolarization-activated channel may exist in plants (93). A study in the root hair development mutant rhd2, which has very short root hair cells, identified the rhd2 mutation in the transmembrane NADPH oxidase AtRBOHC (98). This study further showed a defect in rhd2 in establishing a root hair tip–focused [Ca2+]cyt gradient. Application of hydrogen peroxide to cells reestablished Ca2+ influx and Ca2+ channel activation in root hair cells (98; see Note Added in Proof following the Literature Cited).
Depolarization-activated Ca2+ channels have been characterized in plant cells (38–40, 99) but appear to be less ubiquitous than hyperpolarization-activated channels. The relative scarcity of depolarization-activated Ca2+ channels may be because optimal conditions for analyzing these channels have not yet been developed or other plant types and cell systems may need to be analyzed for this purpose. Studies of action potentials in Venus flytrap suggest that depolarization-activated Ca2+ channels may function in this electrically propagated response (5, 100). Plants also have stretch-activated Ca2+ channels (101), for which the genes and regulation mechanisms remain less studied to date. Mechanical stimulation of Arabidopsis roots causes a rapid rise in [Ca2+]cyt (102). This system may be well suited for advancing models of functions, regulation, and genes mediating mechanosensitive [Ca2+]cyt elevations. A recent study identified Arabidopsis Mca1, a potential component of mechanosensitive Ca2+ channels (103; see Note Added in Proof ).
COMPARATIVE GENOMICS OF HIGHER PLANT ION CHANNELS WITH CHLAMYDOMONAS
The unicellular green alga Chlamydomonas reinhardtii has been a model organism primarily for the study of photosynthesis and chloroplast biogenesis and its ability to grow heterotrophically in the dark, allowing for isolation of photosynthetic mutations that would be lethal in vascular plants. Since the completion of the Chlamydomonas genome project (104), C. reinhardtii has been useful for comparative genomics with other members of the kingdom Viridiplantae. These genomic analyses allow extrapolation to the common ancestor of all green plants, a photosynthetic organism that lived more than a billion years ago before the divergence of the chlorophytes (green algae) and the streptophytes (land plants). The C. reinhardtii genome was found to encode a number of ion channels that had been thought to be specific to animals (105; Table 1): an inositol-1,4,5-trisphosphate-gated Ca2+ channel, nine predicted 4 × 6 transmembrane domain–containing Ca2+ channel α subunits, and seven 6TM1P proteins with similarity to hyperpolarization-activated cyclic nucleotide– gated (HCN)-type K+ channels. The physiological function of these ion channels in C. reinhardtii remains to be elucidated. On the bases of these findings and the lack of known close homologs in land plant genomes (Table 1), Chlamydomonas can be considered a “green animal” (104). The presence of such animal-type ion channel genes in a green alga suggests that they had also been part of the ion channel repertoire of the ancestral viridiplant and were subsequently eliminated in the streptophyte lineage (105). Likewise, Na+ and K+ ion transporter/channels such as HKT/Trk or the KUP/HAKs (discussed below), which occur not only in plants but also in fungi and bacteria, may have been present in an ancestor of animals as well and were lost in the course of metazoan evolution. Thus, parsimonious comparison of transporter genes indicates that the common ancestor of plants and animals had a larger repertoire of ion channel classes than did extant members of either kingdom. Presumably such an organism was heterotrophic and unicellular.
POTASSIUM CHANNELS
Cation Channels with Pore Loops
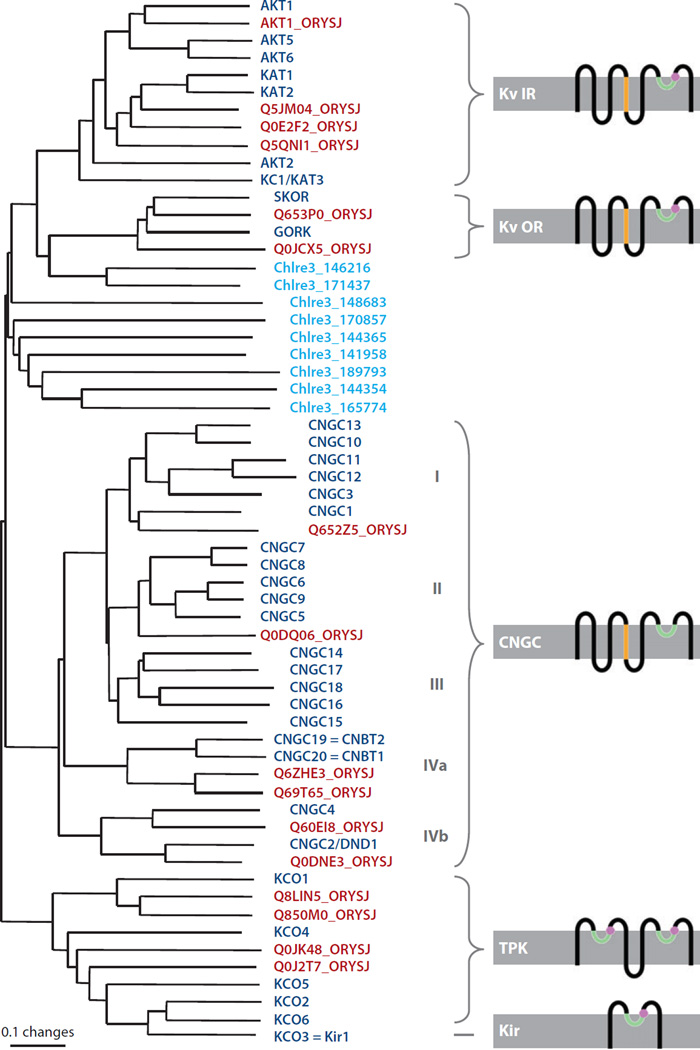
We refer the reader to reviews on the physiology, regulation, and structure-function analyses of plant K+ channels for background information (106, 107, 108). Here we review some of the major and new insights into K+ channel structure and function and also recent advances from functional genetic analyses in Arabidopsis. Loop structures, in contrast to α-helical transmembrane domains, allow channels to engage moieties of the polypeptide backbone in K+ ion binding (109). Thus, the K+ ions inside the pore of the K+ channel interact with the carbonyl oxygens of the filter triad glycine-tyrosine-glycine (GYG) located at the end of the channel’s pore loop (P-loop) (110). Together, the carbonyl groups of the four filters of a tetrameric channel coordinate two K+ ions separated by one molecule of H2O in a similar way to the hydration sphere in water, explaining at once the near diffusion limit Vmax of K+ channels and the strong discrimination against the smaller cation Na+ (110). The GYG triad is characteristic for K+ channels (110); less selective cation channels/transporters also possess P-loops, but with a less stringent filter motif (111–113). Nevertheless, the P-loops are evolutionarily conserved to a degree that renders them readily detectable in silico (Figure 2). To identify all types of P-loop-containing cation channels from plants, we scanned the predicted proteomes of A. thaliana, Populus trichocarpa, Oryza sativa, and C. reinhardtii with a hidden Markov model–based profile for P-loops. The results are shown in Figure 2. Ubiquitous throughout all kingdoms of life are the tetrameric cation channel with cytosolic N and C termini and, per subunit, six transmembrane domains and one P-loop between TM5 and TM6. This topology, 6TM1P, is shared between voltage-gated K+ (Kv) channels, CNG channels, and HCN channels, suggesting a common evolutionary origin of these families. The smallest K+ channel subunits, represented by KcsA from Streptomyces lividans (110), consist of just one P-loop between two transmembrane domains. Other classes of cation channels carry two P-loops per subunit and presumably function as dimers. In plants these are the KCO/TPK (two-pore) K+ channels with four transmembrane domains; the P-loops are located between TM1 and TM2 and between TM3 and TM4. The schematic topologies are depicted in Figure 2.
Figure 2.
Phylogenetic tree of P-loop-containing proteins from Arabidopsis thaliana (dark blue), rice (Oryza sativa; red ), and Chlamydomonas reinhardtii (light blue). Where applicable, schematized membrane topologies are indicated: P-loop, green; transmembrane domain 4 (TM4) including positively charged amino acids, orange; K+ selectivity filter residues, magenta. Cytosolic elements such as long C-terminal domains, cyclic nucleotide (cNMP) binding domains, or ankyrin repeats are not depicted. The photosynthetic alga C. reinhardtii has a unique set of ion channel genes (see Reference 105 and text). The P-loop-containing channels from C. reinhardtii all have a positively charged TM4, but only five (179857, 144365, 189793, 144354, and 165774) have a K+ selectivity filter. Because these do not form a clade, and because the C. reinhardtii channels are poorly resolved in general, they could not be attributed to specific classes. Scale indicates the number of amino acid substitutions per site. Kv IR, inward-rectifying K+ channel; Kv OR, outward-rectifying K+ channel; CNGC, cyclic nucleotide–gated channel; TPK, two-pore K+ channel.
Shaker-Type K+ Channels
Named after the Drosophila shaker mutant, Shaker-type K+ channels are the archetypal voltage-gated K+ channels. Crystal structures have been resolved for the archaebacterial KvAP and the mammalian channel Kv1.2 (110). Shaker channels are activated by depolarization of the membrane potential. The voltage sensitivity is conferred by TM4, which contains a series of positively charged amino acids. Movement inside the membrane of this voltage sensor in response to depolarization opens the channel (114–117). The first depolarization-activated K+ channel described from plants was SKOR, the stelar K+ outward rectifier from A. thaliana (29). SKOR is expressed in the vascular cylinder of the root, where it is thought to mediate the release of K+ into the xylem (conducting tissue) for root-to-leaf transport. This model is in accordance with the lowered K+ content in xylem sap of T-DNA-disrupted skor null mutant Arabidopsis (29). SKOR mainly allows unidirectional K+ efflux from cells because the activation potential of plant outward-rectifying K+ channels shifts to more positive voltages upon an increase in the extracellular K+ concentration (24, 118, 119). A related channel from Arabidopsis, GORK (guard cell outward-rectifying K+ channel) (120), is the major outward-rectifying K+ channel in guard cells and contributes to stomatal closure. Depolarization of guard cells by anion efflux activates GORK, and the resulting K+ efflux further contributes to water loss and stomatal closure. Disruption of GORK by T-DNA insertion impaired stomatal closure (21). GORK is also expressed in root hairs (121). Although there are apparently no further genes for outward-rectifying K+ channels in the Arabidopsis genome, the situation is more complex: When coexpressed in Saccharomyces cerevisiae or Xenopus laevis oocytes, SKOR and GORK subunits are able to form heteromeric channels, and thus heteromeric channels may form in vivo (122).
Inward-Rectifying Shaker K+ Channels
Plants possess members of the 6TM1P super-family that carry all the hallmarks of Shaker channels, including the voltage sensor. These channels function as inward-rectifiers, activated by hyperpolarization of the membrane potential (14, 27, 123). The family of inward-rectifying Shaker channels comprises the first K+ channels identified from plants, Arabidopsis AKT1 and KAT1. Both genes were identified by functional complementation in K+ uptake– deficient yeast selected on limiting [K+] (124, 125).
AKT1 is expressed in Arabidopsis roots. Homozygously disrupted akt1 plants exhibit an impaired growth phenotype on micromolar extracellular [K+], indicating that a channel can contribute to root K+ accumulation from soil provided the membrane potential is large enough to enable concentrative uptake (18). The growth phenotype is apparent only in the presence of NH4+ (18). These data correlate with the finding that ammonium blocks alternative transporters for root K+ uptake (126). AKT1 is regulated by the kinase CIPK23, which in turn is activated by calcineurin B–like Ca2+ sensors (127, 128). The Arabidopsis null mutant cipk23 also exhibits an impaired growth phenotype at low K+ concentrations in the presence of NH4+ (129). Arabidopsis guard cells express several of the inward-rectifying Shaker channels: AKT1, AKT2, KAT1, KAT2, and the regulatory subunit KC1 (19). These channels mediate hyperpolarization-induced K+ influx, resulting in water influx and stomatal opening in response to signals such as sunlight (Figure 1b;c, right). Expression of dominant-negative KAT1 variants suppressed light-induced K+ uptake in guard cells and reduced stomatal opening (20). In accordance with an important role of KAT1 in stomatal opening, KAT1 activity is regulated at multiple levels. Treatment with ABA reduced KAT1 expression in guard cells and triggered endocytosis of the channel (130), whereas auxin enhanced KAT1 expression in seedlings (131). When expressed in heterologous systems, KAT1 was activated by external acidification (132) or coexpression of 14-3-3 proteins (133) and was inhibited by coexpression of the regulatory subunit KC1 (134). Again, the properties of the inward-rectifying Shaker channels in planta may be multiplexed by the formation of heteromeric channels (135–138).
HCN Channels and CNG Channels
HCN and CNG cation channels have a 6TM1Ptopology, with positive charges in TM4 and a C-terminal cyclic nucleotide (cNMP) binding domain, and presumably function as tetramers. Both channel types are allosterically activated by cyclic nucleotides, but only the HCN channels are voltage sensitive, activated by membrane hyperpolarization. In accordance with the less stringent filter sequence, HCN K+ channels are also permeable to Na+. CNG channels are even less selective and also conduct divalent cations. In mammals, HCN channels function as cardiac pacemakers, whereas CNG channels generate electrical signals in olfactory neurons and photoreceptors. Land plants do not appear to possess HCN channels. The photosynthetic green alga C. reinhardtii, however, encodes seven unclassified channels related to HCN channels (Table 1; Figure 2) that represent the predominant GTM1P channels in this organism.
The Arabidopsis genome encodes 20 CNGC genes (45). Forward and reverse genetic studies have identified functions of Arabidopsis CNGC genes. The Arabidopsis dnd1 (defense, no death) mutant has a defect in the CNGC2 gene (139). dnd1 mutants exhibit a reduced localized programmed cell death response to pathogens, termed a hypersensitive response, but have elevated systemic acquired resistance. AtCNGC11 and −12 are important for resistance against an avirulent Hyaloperonospora pathogen (140). These results indicate roles for CNG channels in pathogen defense responses. Furthermore, a recent study showed that null mutations in the Arabidopsis CNGC18 gene abolishes polarized growth of pollen tubes (141). cAMP but not cGMP activates a hypopolarization-dependent Ca2+ current in guard cells (142). Apart from these advances, the in vivo electrophysiological regulation properties of AtCNGCs remain less well understood and will require further analyses.
HKT TRANSPORTERS
Plant HKT (high-affinity K+ transporter) proteins belong to the Trk/HKT superfamily of monovalent cation transporters, together with bacterial TrkH, KtrB, and fungal Trk proteins (111). Trk/HKT transporters carry eight transmembrane domains and four P-loop-like domains in one polypeptide chain and are therefore thought to function as monomers that resemble KcsA-type K+ channel tetramers in structure (111, 143). The first gene cloned from plants was wheat HKT1 (144), which, when expressed in X. laevis oocytes or in S. cerevisiae, functions as a K+/Na+ cotransporter/channel (145). The majority of the subsequently characterized HKTs from plants are permeable to Na+ as well (146–148). HKT transporters in Arabidopsis, rice, and wheat protect plant leaves from over accumulating Na+ to toxic levels (149–151). In plant physiology, Na+ has a dual role: Under K+ starvation, Na+ can replace the nutrient K+ (152) in its nonspecific function as an osmolyte in the vacuole. However, in the cytosol, Na+ competes with K+-specific functions and is toxic to most plants. Arabidopsis AtHKT1 is a single-copy gene that transports Na+ when expressed in oocytes or yeast (146). Site-directed mutagenesis showed that a point mutation in the predicted selectivity filter contributes to determining K+/Na+ selectivity (112). Forward genetic (150, 153–155) as well as reverse genetic (149, 151, 156) approaches have identified AtHKT1 as a key component in protection of plants against salinity stress and in controlling shoot/root Na+ distribution. Arabidopsis athkt1 null mutants exhibit lower root Na+ content and higher shoot Na+ content compared with wild-type plants (149). This indicates that AtHKT1 functions to counteract Na+ accumulation in the shoot, where photosynthetic tissue is particularly Na+ sensitive. AtHKT1 and the rice ortholog OsHKT8 (OsHKT1;5) are expressed in xylem parenchyma cells in leaves (150, 151). Both function in the retrieval of Na+ from the xylem sap (150, 151, 156, 157), thus protecting leaves from overaccumulating Na+. A recent review describes plant HKT proteins in more detail (158).
KUP/HAK/KT K+ TRANSPORTERS
The KUP/HAK/KTs are a family of permeases that are selective for K+ but do not have P-loops. They have a predicted topology of 10 to 14 predicted transmembrane domains (159), but the K+ transport mechanism (channel or other) remains unknown. The founding members of this family were cloned from bacteria (named KUP) (160) and from fungi named HAK (high-affinity K+ uptake mechanism) (161). In plants, the first KUP/HAK/KT transporters were discovered independently by several laboratories from EST sequences and via yeast complementation (126, 162–164). Bacteria and fungi usually have only one or two KUP/HAK/KT genes (if any; S. cerevisiae, for instance, has none), whereas in plants the KUP/HAK/KTs form multigene families, with 13 members in Arabidopsis, 25 in rice, and 24 in poplar (90, 158, 159, 165). C. reinhardtii has three predicted KUP/HAK/KT genes (104). The expansion of the KUP/HAK/KT family in vascular plants is likely to reflect the speciation of individual KUP/HAK/KT paralogs and the importance of K+ transport for many different physiological processes. The physiological functions of individual KUP/HAK/KT members are therefore challenging to assign by reverse genetics and require knowledge of the gene’s expression pattern. The promoter of A. thaliana AtHAK5 is active in the root vasculature and epidermis (166), and AtHAK5 expression is upregulated at low [K+] (166–168). K+ starvation also induced expression of orthologs from rice and barley (165, 169). Genetic disruption of AtHAK5 did not greatly affect plant growth. However, athak5 null mutant plants contained less K+ and displayed impaired Rb+ uptake kinetics. In particular, the K+-starvation inducible high-affinity component of root Rb+/K+ absorption (170) was diminished in athak5 null mutant Arabidopsis (166). For two other KUP/HAK members, a physiological function could be attributed by forward genetics: The Arabidopsis mutant Shy3-1 (short hypocotyl in the dark) results from a point mutation in AtKUP2 (At2g30070) (171), and the mutant Thr1 (tiny root hair) is caused by disruption of AtKUP4 (At4g23640) (172), indicating a role for KUP/HAK transporters in cell expansion. A recent review discusses the KUP/HAKs in more detail (158).
VACUOLAR ION CHANNELS
TPK1 Twin-Pore K+ Channels
VK (vacuolar K+) channels were first identified in the guard cell vacuole membranes and are activated by elevated [Ca2+]cyt in the range of 1 µM and highly selective for K+ (17). VK channels were suggested to function as a pathway for organellar K+ release into the cytosol in response to elevated [Ca2+]cyt during stomatal closure (17). VK channels show a slight rectification that favors K+ efflux from vacuoles, and VK channel activity is also stimulated by low cytosolic pH (17, 173). Expression of an Arabidopsis cDNA TPK1/KCO1 (At5g55630) in yeast revealed vacuolar channel activity consistent with hallmark properties of VK channels (174). Arabidopsis mutants disrupted in TPK1 lacked detectable VK channel activity and exhibited slower stomatal closing in response to ABA (22).
TPK1 is localized in vacuole membranes, and the TPK1 gene is expressed at high levels in most tissues (22, 175). TPK1 is a member of the two-pore-domain K+ channel family, first described in yeast (176; Figure 2). There are five members of this family in Arabidopsis and three in rice, and all share the same topology of four transmembrane spans and two pore domains (Figure 2; Table 1). TPK1 has two EF hand Ca2+ binding domains in the C terminus, which makes TPK1 distinct from animal TPK channels (177) and indicates that Ca2+ binds and activates the channel directly (17, 22).
The KcsA-Type Channel Kir
Consisting of just two transmembrane domains and one P-loop per subunit, the KcsA-type channels are the most rudimentary of the K+ channels (110). The Arabidopsis genome encodes only one KcsA-type channel, Kir1, whereas rice and poplar apparently lack such channels. Kir1 was formerly named KCO3 because it belongs to the KCO family of TPK channels (Figure 2). Thus, Kir1/KCO3 may be the direct descendant of a gene that, upon duplication, became the founding member of the two-pore family. Alternatively, Kir1/KCO3 may be a truncated descendant of that family. The position of Kir1/KCO3 in the phylogenetic tree (Figure 2) does not support the first hypothesis, and the circumstance that other plants lack KcsA-type genes also points toward Kir1/KCO3 being a descendant of the KCO/TPK family. The function of Kir1/KCO3 in Arabidopsis remains to be investigated.
TPC1 Voltage-Dependent Calcium Channel: Slow Vacuolar Channel
The slow vacuolar (SV) channel was shown to be Ca2+ activated in vacuole membranes from beet storage tissue (178) and has subsequently been found in vacuole membranes from many plant cell types. SV channels are activated by Ca2+ and positive voltages on the cytoplasmic membrane side and are large-conductance, nonselective cation channels. In guard cells SV channels were found to be Ca2+-permeable (17), leading to the hypothesis that SV channels participate in Ca2+ signaling by releasing Ca2+ from the vacuole.
In 2005, Peiter et al. (56) identified the gene encoding SV channels in Arabidopsis as TPC1 (At4g03560). TPC1 is a unique gene in plants, the only homolog of voltage-dependent Ca2+ channels in the Arabidopsis genome. Similar to TPC channels in animals (179), TPC1 in plants has two pore domains rather than the four pore domains usually associated with voltage-dependent Ca2+ channels. In plants, TPC1 is highly expressed in all tissues, consistent with the ubiquity of SV channel activity in plant vacuoles. The voltage dependence of SV channels limits vacuolar Ca2+ release, although recordings of deactivating (tail) currents show a clear Ca2+ permeation toward the cytoplasmic membrane side (17, 180). For physiological Ca2+ release (181), it has been proposed that the voltage dependence of SV channels would need to be shifted by modulators, as was recently found in Arabidopsis mesophyll vacuoles (182). An Arabidopsis TPC1 knockout mutant was defective in the inhibition of stomatal opening by external Ca2+ (56). This finding is consistent with a function for SV channels in guard cell Ca2+ signaling. However, ABA inhibited stomatal opening in the mutant similarly to wild type (56). The TPC1 mutant was, however, less sensitive to ABA inhibition of seed germination, indicating that SV channels participate in a subset of ABA signaling pathways in plants. Now that TPC1 has been identified as the SV channel–encoding gene (56), the physiological functions of this ubiquitous channel can be analyzed in diverse tissue types and under diverse conditions.
Elevated extracellular Ca2+ causes cytoplasmic [Ca2+] elevations in plant guard cells (57, 183). The CAS1 Ca2+ receptor is required for this response, triggering [Ca2+]cyt oscillations that then inhibit stomatal opening and induce stomatal closing (184, 185). CAS1 has a single putative transmembrane domain and binds Ca2+ ions with a low affinity (184). No CAS1 homologs have been identified in nonplant species. CAS1 has been localized to the plasma membrane (184) and to thylakoid membranes in chloroplasts (185). More work will be required to understand how extracellular Ca2+ causes CAS1-dependent intracellular [Ca2+] elevations (184, 185).
Fast Vacuolar Channels
The fast vacuolar (FV) channel is a low-conductance, nonselective cation channel that is inhibited by elevated [Ca2+]cyt. FV channels were first identified in vacuole membranes from beet storage tissue (178). FV channels were subsequently analyzed in guard cells (173). The genes encoding FV channels have not been identified.
In guard cell vacuoles, if K+ accumulates relative to the cytoplasm during stomatal opening, then FV channels should be downregulated, allowing K+/H+ antiporters such as CHX20 (61) to generate a K+ gradient. FV channels can be very active at resting [Ca2+]cyt. However, physiological concentrations of Mg2+ inhibit FV channels (186), and FV channels are voltage dependent, with lower open probabilities at membrane potentials close to 0 mV (178, 186). Elevated vacuolar K+ causes an increase in the open probability of FV channels (187). FV channels may function as a pathway for K+ flux from the vacuole into the cytoplasm during proposed Ca2+-independent stomatal closing.
CONCLUSIONS
Detailed studies have revealed the activities of K+, anion, and Ca2+ channels and transporters in plant cells. Recent advances, especially patch clamping of Arabidopsis cell types, the sequencing of the Arabidopsis genome, and the development of genomic approaches in Arabidopsis, have facilitated the identification of genes encoding specific ion channel and transporter activities. The availability of mutants with defects in signal transduction pathways combined with electrophysiological assays have allowed rapid progress in understanding physiological functions of specific ion channels, as exemplified for guard cell ion channels. Guard cells have been developed as model plant cells for the study of ion channels because they respond to many physiological stimuli, are easily observed and isolated, and undergo osmotically driven reversible cellular movements requiring ion channel activity. Notably, related ion channels are found in most other cell types and are important for many other physiological processes, including mineral nutrition, signal transduction, tropisms, cell elongation, the export of toxic ions and organic acids, and interactions with pathogens and symbiotic organisms.
The finding that most plant ion channels have homologs in animals has increased interest in plants as model systems. Analysis of differences in ion channel gating, kinetics, selectivity, and regulation is contributing to understanding the structure-function relation of ion channels in general. In addition, plant cells provide advantages for analysis of intracellular channels: The plant vacuole is large and can be directly patch clamped, whereas the equivalent endosomes or lysosomes in animal cells are not as readily accessible.
Understanding the genetic bases of ion channels raises the possibility of engineering new traits of importance for agriculture and the environment. For example, ALMT anion channels function in the export of organic acids that allow plants to survive toxic Al3+ concentrations that are a major global problem in acidic soils. ALMT activity or expression could be modified in agricultural crops to allow food production on marginal land. Another example is the recent identification of SLAC1. SLAC1 has a central role in regulating stomatal aperture, which controls CO2 uptake and water loss. Water use efficiency and resistance to drought are increasingly important in agriculture. Atmospheric CO2 concentrations are predicted to continue to increase. Modification of SLAC1 may provide a mechanism to limit water loss and improve drought resistance while maintaining high carbon fixation rates. Thus, many new and unexpected properties of plant ion channels of importance to humans and the environment are undoubtedly awaiting discovery.
Acknowledgments
We thank colleagues for providing preprints of publications. We thank Glenn Wheeler and Colin Brownlee for sharing results prior to publication. We apologize to those authors whose work we did not cite owing to length and reference limitations. Research in the authors’ laboratories was supported by grants from the DOE (FG03-94-ER20148), NIH (R01GM60396 P42 ES01 0337), and NSF (MCB 0417118) to J.I.S., from the NSF (IOS-0419695) to J.W. and J.I.S., and from the Swiss NSF to P.M.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
NOTE ADDED IN PROOF
Concerning Ca2+ influx and tip growth: Cytoplasmic [Ca2+] oscillates in root hair tips with the same period as oscillations in root hair growth (Monhausen et al. 2008), and the timing of Ca2+ influx indicates a function in limiting root hair growth.
Concerning mechanosensitive ion channels: MscS genes have also recently been characterized as required for plant stretch–activated channels (Haswell et al. 2008).
Monshausen GB, Messerli MA, Gilroy S. 2008. Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 147:1690–98
Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM. 2008. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr. Biol. 18:730–34
LITERATURE CITED
- 1.Cole KS, Curtis HJ. Electrical impedance of Nitella during activity. J. Gen. Physiol. 1938;22:37–64. doi: 10.1085/jgp.22.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodgkin AL, Huxley AF. The components of membrane conductance in the giant axon of Loligo. J. Physiol. 1952;116:473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaffey CT, Mullins LJ. Ion fluxes during the action potential in Chara . J. Physiol. 1958;144:505–524. doi: 10.1113/jphysiol.1958.sp006116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hope AB, Findlay GP. The action potential in Chara. Plant Cell Physiol. 1962;5:377–379. [Google Scholar]
- 5.Hodick D, Sievers A. The action potential of Dionaea muscipula Ellis. Planta. 1988;174:8–18. doi: 10.1007/BF00394867. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko T, Saito C, Shimmen T, Kikuyama M. Possible involvement of mechanosensitive Ca2+ channels of plasma membrane in mechanoperception in Chara . Plant Cell Physiol. 2005;46:130–135. doi: 10.1093/pcp/pci004. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, et al. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004;37:645–653. doi: 10.1111/j.1365-313x.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoekenga OA, Maron LG, Pineros MA, Cancado GM, Shaff J, et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis . Proc. Natl. Acad. Sci. USA. 2006;103:9738–9743. doi: 10.1073/pnas.0602868103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovermann P, Meyer S, Hortensteiner S, Picco C, Scholz-Starke J, et al. The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J. 2007;52:1169–1180. doi: 10.1111/j.1365-313X.2007.03367.x. [DOI] [PubMed] [Google Scholar]
- 10.Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452:487–491. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, et al. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature. 2008;452:483–486. doi: 10.1038/nature06720. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder JI, Hedrich R, Fernandez JM. Potassium-selective single channels in guard cell protoplasts of Vicia faba . Nature. 1984;312:361–362. [Google Scholar]
- 13.Moran N, Ehrenstein G, Iwasa K, Bare C, Mischke C. Ion channels in the plasmalemma of wheat protoplasts. Science. 1984;226:835–537. doi: 10.1126/science.6093255. [DOI] [PubMed] [Google Scholar]
- 14.Schroeder JI, Raschke K, Neher E. Voltage dependence of K+ channels in guard cell protoplasts. Proc. Natl. Acad. Sci. USA. 1987;84:4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder JI, Hagiwara S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature. 1989;338:427–430. [Google Scholar]
- 16.Keller BU, Hedrich R, Raschke K. Voltage-dependent anion channels in the plasma membrane of guard cells. Nature. 1989;341:250–253. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward JM, Schroeder JI. Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell. 1994;6:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch RE, Lewis BD, Spalding EP, Sussman MR. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- 19.Szyroki A, Ivashikina N, Dietrich P, Roelfsema MR, Ache P, et al. KAT1 is not essential for stomatal opening. Proc. Natl. Acad. Sci. USA. 2001;98:2917–2921. doi: 10.1073/pnas.051616698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwak JM, Murata Y, Baizabal-Aguirre VM, Merrill J, Wang M, et al. Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis . Plant Physiol. 2001;127:473–485. [PMC free article] [PubMed] [Google Scholar]
- 21.Hosy E, Vavasseur A, Mouline K, Dreyer I, Gaymard F, et al. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA. 2003;100:5549–5554. doi: 10.1073/pnas.0733970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gobert A, Isayenkov S, Voelker C, Czempinski K, Maathuis FJ. The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc. Natl. Acad. Sci. USA. 2007;104:10726–10731. doi: 10.1073/pnas.0702595104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebaudy A, Vavasseur A, Hosy E, Dreyer I, Leonhardt N, et al. Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc. Natl. Acad. Sci. USA. 2008;105:5271–5276. doi: 10.1073/pnas.0709732105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroeder JI. K+ transport properties of K+ channels in the plasma membrane of Vicia faba guard cells. J. Gen. Physiol. 1988;92:667–683. doi: 10.1085/jgp.92.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran N. Osmoregulation of leaf motor cells. FEBS Lett. 2007;581:2337–2347. doi: 10.1016/j.febslet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Pandey S, Zhang W, Assmann SM. Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett. 2007;581:2325–2336. doi: 10.1016/j.febslet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Schachtman DP, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y, Crawford NM, Schroeder JI. Amino terminus and the first four membrane-spanning segments of the Arabidopsis K+ channel KAT1 confer inward-rectification property of plant-animal chimeric channels. J. Biol. Chem. 1995;270:17697–17701. [PubMed] [Google Scholar]
- 29.Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, et al. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell. 1998;94:647–655. doi: 10.1016/s0092-8674(00)81606-2. [DOI] [PubMed] [Google Scholar]
- 30.Latorre R, Olcese R, Basso C, Gonzalez C, Muñoz F, et al. Molecular coupling between voltage sensor and pore opening in the Arabidopsis inward rectifier K+ channel KAT1. J. Gen. Physiol. 2003;122:459–469. doi: 10.1085/jgp.200308818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai HC, Grabe M, Jan YN, Jan LY. The S4 voltage sensor packs against the pore domain in the KAT1 voltage-gated potassium channel. Neuron. 2005;47:395–406. doi: 10.1016/j.neuron.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Grabe M, Lai HC, Jain M, Jan YN, Jan LY. Structure prediction for the down state of a potassium channel voltage sensor. Nature. 2007;445:550–553. doi: 10.1038/nature05494. [DOI] [PubMed] [Google Scholar]
- 33.Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 2004;427:803–807. doi: 10.1038/nature02314. [DOI] [PubMed] [Google Scholar]
- 34.Picollo A, Pusch M. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature. 2005;436:420–423. doi: 10.1038/nature03720. [DOI] [PubMed] [Google Scholar]
- 35.Scheel O, Zdebik AA, Lourdel S, Jentsch TJ. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 2005;436:424–427. doi: 10.1038/nature03860. [DOI] [PubMed] [Google Scholar]
- 36.Miller C. ClC chloride channels viewed through a transporter lens. Nature. 2006;440:484–489. doi: 10.1038/nature04713. [DOI] [PubMed] [Google Scholar]
- 37.De Angeli A, Monachello D, Ephritikhine G, Frachisse JM, Thomine S, et al. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature. 2006;442:939–942. doi: 10.1038/nature05013. [DOI] [PubMed] [Google Scholar]
- 38.Huang JW, Grunes DL, Kochian LV. Voltage-dependent Ca2+ influx into right-side-out plasma membrane vesicles isolated from wheat roots: characterization of a putative Ca2+ channel. Proc. Natl. Acad. Sci. USA. 1994;91:3473–3477. doi: 10.1073/pnas.91.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thuleau P, Ward JM, Ranjeva R, Schroeder JI. Voltage-dependent calcium-permeable channels in the plasma membrane of a higher plant cell. EMBO J. 1994;13:2970–2975. doi: 10.1002/j.1460-2075.1994.tb06595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marshall J, Corzo A, Leigh RA, Sanders D. Membrane potential-dependent calcium transport in right-side-out plasma membrane vesicles from Zea mays L. roots. Plant J. 1994;5:683–694. [Google Scholar]
- 41.Gelli A, Blumwald E. Hyperpolarization-activated Ca2+-permeable channels in the plasma membrane of tomato cells. J. Membr. Biol. 1997;155:35–45. doi: 10.1007/s002329900156. [DOI] [PubMed] [Google Scholar]
- 42.Very AA, Davies JM. Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc. Natl. Acad. Sci. USA. 2000;97:9801–9806. doi: 10.1073/pnas.160250397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton DW, Hills A, Kohler B, Blatt MR. Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc. Natl. Acad. Sci. USA. 2000;97:4967–4972. doi: 10.1073/pnas.080068897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan B, Sherman T, Fromm H. Cyclic nucleotide-gated channels in plants. FEBS Lett. 2007;581:2237–2246. doi: 10.1016/j.febslet.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 46.Lacombe B, Becker D, Hedrich R, DeSalle R, Hollmann M, et al. The identity of plant glutamate receptors. Science. 2001;292:1486–1487. doi: 10.1126/science.292.5521.1486b. [DOI] [PubMed] [Google Scholar]
- 47.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 48.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 49.Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, et al. Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 1999;19:735–747. doi: 10.1046/j.1365-313x.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- 51.Blatt MR. Cellular signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 2000;16:221–241. doi: 10.1146/annurev.cellbio.16.1.221. [DOI] [PubMed] [Google Scholar]
- 52.Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- 53.Humble GD, Raschke K. Stomatal opening quantitatively related to potassium transport: evidence from electron probe analysis. Plant Physiol. 1971;48:447–453. doi: 10.1104/pp.48.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Kirk CA, Raschke K. Presence of chloride reduces malate production in epidermis during stomatal opening. Plant Physiol. 1978;61:361–364. doi: 10.1104/pp.61.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacRobbie EAC. Ion fluxes in ‘isolated’ guard cells of Commelina communis L. J. Exp. Bot. 1981;32:545–562. [Google Scholar]
- 56.Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, et al. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- 57.Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, et al. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science. 2000;289:2338–2342. doi: 10.1126/science.289.5488.2338. [DOI] [PubMed] [Google Scholar]
- 58.Young JJ, Mehta S, Israelsson M, Godoski J, Grill E, Schroeder JI. CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proc. Natl. Acad. Sci. USA. 2006;103:7506–7511. doi: 10.1073/pnas.0602225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shope JC, DeWald DB, Mott KA. Changes in surface area of intact guard cells are correlated with membrane internalization. Plant Physiol. 2003;133:1314–1321. doi: 10.1104/pp.103.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao XQ, Li CG, Wei PC, Zhang XY, Chen J, Wang XC. The dynamic changes of tonoplasts in guard cells are important for stomatal movement in Vicia faba . Plant Physiol. 2005;139:1207–1216. doi: 10.1104/pp.105.067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Padmanaban S, Chanroj S, Kwak JM, Li X, Ward JM, Sze H. Participation of endomembrane cation/H+ exchanger AtCHX20 in osmoregulation of guard cells. Plant Physiol. 2007;144:82–93. doi: 10.1104/pp.106.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pei ZM, Ward JM, Harper JF, Schroeder JI. A novel chloride channel in Vicia faba guard cell vacuoles activated by the serine/threonine kinase, CDPK. EMBO J. 1996;15:6564–6574. [PMC free article] [PubMed] [Google Scholar]
- 63.Hafke JB, Hafke Y, Smith JA, Luttge U, Thiel G. Vacuolar malate uptake is mediated by an anion-selective inward rectifier. Plant J. 2003;35:116–128. doi: 10.1046/j.1365-313x.2003.01781.x. [DOI] [PubMed] [Google Scholar]
- 64.MacRobbie EA. Signal transduction and ion channels in guard cells. Philos. Trans. R. Soc. London Ser. B. 1998;353:1475–1488. doi: 10.1098/rstb.1998.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Homann U, Thiel G. Cl− and K+ channel currents during the action potential in Chara. Simultaneous recording of membrane voltage and patch currents. J. Membr. Biol. 1994;141:297–309. doi: 10.1007/BF00235139. [DOI] [PubMed] [Google Scholar]
- 66.Shimmen T. Studies on mechano-perception in Characeae: effects of external Ca2+ and Cl− . Plant Cell Physiol. 1997;38:691–697. [Google Scholar]
- 67.Kohler B, Wegner LH, Osipov V, Raschke K. Loading of nitrate into the xylem: Apoplastic nitrate controls the voltage dependence of X-QUAC, the main anion conductance in xylem-parenchyma cells of barley roots. Plant J. 2002;30:133–142. doi: 10.1046/j.1365-313x.2002.01269.x. [DOI] [PubMed] [Google Scholar]
- 68.Jabs T, Tschope M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2 − from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc. Natl. Acad. Sci. USA. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delhaize E, Ryan PR. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995;107:315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delhaize E, Ryan PR, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 1993;103:695–702. doi: 10.1104/pp.103.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pineros MA, Kochian LV. A patch-clamp study on the physiology of aluminum toxicity and aluminum tolerance in maize. Identification and characterization of Al3+-induced anion channels. Plant Physiol. 2001;125:292–305. doi: 10.1104/pp.125.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delhaize E, Gruber BD, Ryan PR. The roles of organic anion permeases in aluminium resistance and mineral nutrition. FEBS Lett. 2007;581:2255–2262. doi: 10.1016/j.febslet.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 73.Kollmeier M, Dietrich P, Bauer CS, Horst WJ, Hedrich R. Aluminum activatesacitrate-permeable anion channel in the aluminum-sensitive zone of the maize root apex. A comparison between an aluminum-sensitive and an aluminum-resistant cultivar. Plant Physiol. 2001;126:397–410. doi: 10.1104/pp.126.1.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ligaba A, Katsuhara M, Ryan PR, Shibasaka M, Matsumoto H. The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiol. 2006;142:1294–1303. doi: 10.1104/pp.106.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pineros MA, Cancado GM, Maron LG, Lyi SM, Menossi M, Kochian LV. Not all ALMT1-type transporters mediate aluminum-activated organic acid responses: the case of ZmALMT1—an anion-selective transporter. Plant J. 2008;53:352–367. doi: 10.1111/j.1365-313X.2007.03344.x. [DOI] [PubMed] [Google Scholar]
- 76.Pantoja O, Smith JA. Sensitivity of the plant vacuolar malate channel to pH, Ca2+ and anion-channel blockers. J. Membr. Biol. 2002;186:31–42. doi: 10.1007/s00232-001-0132-z. [DOI] [PubMed] [Google Scholar]
- 77.Jentsch TJ. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit. Rev. Biochem. Mol. Biol. 2008;43:3–36. doi: 10.1080/10409230701829110. [DOI] [PubMed] [Google Scholar]
- 78.Colquhoun D, Sivilotti LG. Function and structure in glycine receptors and some of their relatives. Trends Neurosci. 2004;27:337–344. doi: 10.1016/j.tins.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 79.Hartzell HC, Qu Z, Yu K, Xiao Q, Chien LT. Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol. Rev. 2008;88:639–672. doi: 10.1152/physrev.00022.2007. [DOI] [PubMed] [Google Scholar]
- 80.Brenner ED, Martinez-Barboza N, Clark AP, Liang QS, Stevenson DW, Coruzzi GM. Arabidopsis mutants resistant to S(+)-β-methyl-α, β-diaminopropionic acid, a cycad-derived glutamate receptor agonist. Plant Physiol. 2000;124:1615–1624. doi: 10.1104/pp.124.4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hechenberger M, Schwappach B, Fischer WN, Frommer WB, Jentsch TJ, Steinmeyer K. A family of putative chloride channels from Arabidopsis and functional complementation of a yeast strain with a CLC gene disruption. J. Biol. Chem. 1996;271:33632–33638. doi: 10.1074/jbc.271.52.33632. [DOI] [PubMed] [Google Scholar]
- 82.Teardo E, Frare E, Segalla A, De Marco V, Giacometti GM, Szabo I. Localization of a putative ClC chloride channel in spinach chloroplasts. FEBS Lett. 2005;579:4991–4996. doi: 10.1016/j.febslet.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 83.von der Fecht-Bartenbach J, Bogner M, Krebs M, Stierhof YD, Schumacher K, Ludewig U. Function of the anion transporter AtCLC-d in the trans-Golgi network. Plant J. 2007;50:466–474. doi: 10.1111/j.1365-313X.2007.03061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schroeder JI. Physiology: nitrate at the ion exchange. Nature. 2006;442:877–878. doi: 10.1038/nature04999. [DOI] [PubMed] [Google Scholar]
- 85.Linder B, Raschke K. A slow anion channel in guard cells, activating at large hyperpolarization, may be principal for stomatal closing. FEBS Lett. 1992;313:27–30. doi: 10.1016/0014-5793(92)81176-m. [DOI] [PubMed] [Google Scholar]
- 86.Camarasa C, Bidard F, Bony M, Barre P, Dequin S. Characterization of Schizosaccharomyces pombe malate permease by expression in Saccharomyces cerevisiae . Appl. Environ. Microbiol. 2001;67:4144–4151. doi: 10.1128/AEM.67.9.4144-4151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmidt C, Schroeder JI. Anion selectivity of slow anion channels in the plasma membrane of guard cells (large nitrate permeability) Plant Physiol. 1994;106:383–391. doi: 10.1104/pp.106.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Kirk CA, Raschke K. Release of malate from epidermal strips during stomatal closure. Plant Physiol. 1978;61:474–475. doi: 10.1104/pp.61.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.MacRobbie EAC. Effects of ABA on ‘isolated’ guard cells of Commelina communis L. J. Exp. Bot. 1981;32:563–572. [Google Scholar]
- 90.Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, et al. Phylogenetic relationships within cation transporter families of Arabidopsis . Plant Physiol. 2001;126:1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qi Z, Stephens NR, Spalding EP. Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol. 2006;142:963–971. doi: 10.1104/pp.106.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stephens NR, Qi Z, Spalding EP. Glutamate receptor subtypes evidenced by differences in desensitization and dependence on the GLR3.3 and GLR3.4 genes. Plant Physiol. 2008;146:529–538. doi: 10.1104/pp.107.108134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Demidchik V, Shabala SN, Davies JM. Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J. 2007;49:377–386. doi: 10.1111/j.1365-313X.2006.02971.x. [DOI] [PubMed] [Google Scholar]
- 94.Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, et al. NADPHoxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis . EMBO J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kohler B, Blatt MR. Protein phosphorylation activates the guard cell Ca2+ channel and is a prerequisite for gating by abscisic acid. Plant J. 2002;32:185–1894. doi: 10.1046/j.1365-313x.2002.01414.x. [DOI] [PubMed] [Google Scholar]
- 96.Mori IC, Murata Y, Yang Y, Munemasa S, Wang YF, et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 2006;4:e327. doi: 10.1371/journal.pbio.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Demidchik V, Bowen HC, Maathuis FJ, Shabala SN, Tester MA, et al. Arabidopsis thaliana root nonselective cation channels mediate calcium uptake and are involved in growth. Plant J. 2002;32:799–808. doi: 10.1046/j.1365-313x.2002.01467.x. [DOI] [PubMed] [Google Scholar]
- 98.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 99.Ranjeva R, Graziana A, Mazars C, Thuleau PP. Putative L-type calcium channels in plants: biochemical properties and subcellular localisation. In: Cooke DT, Clarkson DT, editors. Transport and Receptor Proteins of Plant Membranes. New York: Plenum; 1992. pp. 145–153. [Google Scholar]
- 100.Shimmen T. Involvement of receptor potentials and action potentials in mechano-perception in plants. Aust. J. Plant Physiol. 2001;28:567–576. [Google Scholar]
- 101.Cosgrove DJ, Hedrich R. Stretch-activated chloride, potassium, and calcium channels coexisting in plasma membranes of guard cells of Vicia faba L. Planta. 1991;186:143–153. doi: 10.1007/BF00201510. [DOI] [PubMed] [Google Scholar]
- 102.Legue V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997;114:789–800. doi: 10.1104/pp.114.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA. 2007;104:3639–3644. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wheeler GL, Brownlee C. Ca2+ signalling in plants and green algae: changing channels. Trends Plant Sci. 2008;13:506–514. doi: 10.1016/j.tplants.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 106.Zimmermann S, Sentenac H. Plant ion channels: from molecular structures to physiological functions. Curr. Opin. Plant Biol. 1999;2:477–482. doi: 10.1016/s1369-5266(99)00020-5. [DOI] [PubMed] [Google Scholar]
- 107.Lebaudy A, Very AA, Sentenac H. K+ channel activity in plants: genes, regulations and functions. FEBS Lett. 2007;581:2357–2366. doi: 10.1016/j.febslet.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 108.Gambale F, Uozumi N. Properties of shaker-type potassium channels in higher plants. J. Membr. Biol. 2006;210:1–19. doi: 10.1007/s00232-006-0856-x. [DOI] [PubMed] [Google Scholar]
- 109.MacKinnon R. Pore loops: an emerging theme in ion channel structure. Neuron. 1995;14:889–892. doi: 10.1016/0896-6273(95)90327-5. [DOI] [PubMed] [Google Scholar]
- 110.MacKinnon R. Potassium channels. FEBS Lett. 2003;555:62–65. doi: 10.1016/s0014-5793(03)01104-9. [DOI] [PubMed] [Google Scholar]
- 111.Durell SR, Guy HR. Structural models of the KtrB, TrkH, and Trk1,2 symporters based on the structure of the KcsA K+ channel. Biophys J. 1999;77:789–807. doi: 10.1016/S0006-3495(99)76932-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mäser P, Hosoo Y, Goshima S, Horie T, Eckelman B, et al. Glycine residues in potassium channellike selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants. Proc. Natl. Acad. Sci. USA. 2002;99:6428–6433. doi: 10.1073/pnas.082123799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tholema N, Vor der Bruggen M, Mäser P, Nakamura T, Schroeder JI, et al. All four putative selectivity filter glycine residues in KtrB are essential for high affinity and selective K+ uptake by the KtrAB system from Vibrio alginolyticus . J. Biol. Chem. 2005;280:41146–41154. doi: 10.1074/jbc.M507647200. [DOI] [PubMed] [Google Scholar]
- 114.Papazian DM, Timpe LC, Jan YN, Jan LY. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature. 1991;349:305–310. doi: 10.1038/349305a0. [DOI] [PubMed] [Google Scholar]
- 115.Cha A, Snyder GE, Selvin PR, Bezanilla F. Atomic scale movement of the voltage-sensing region in a potassium channel measured via spectroscopy. Nature. 1999;402:809–813. doi: 10.1038/45552. [DOI] [PubMed] [Google Scholar]
- 116.Chanda B, Asamoah OK, Blunck R, Roux B, Bezanilla F. Gating charge displacement in voltagegated ion channels involves limited transmembrane movement. Nature. 2005;436:852–856. doi: 10.1038/nature03888. [DOI] [PubMed] [Google Scholar]
- 117.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 118.Vergani P, Miosga T, Jarvis SM, Blatt MR. Extracellular K+ and Ba2+ mediate voltage-dependent inactivation of the outward-rectifying K+ channel encoded by the yeast gene TOK1 . FEBS Lett. 1997;405:337–344. doi: 10.1016/s0014-5793(97)00211-1. [DOI] [PubMed] [Google Scholar]
- 119.Johansson I, Wulfetange K, Poree F, Michard E, Gajdanowicz P, et al. External K+ modulates the activity of the Arabidopsis potassium channel SKOR via an unusual mechanism. Plant J. 2006;46:269–281. doi: 10.1111/j.1365-313X.2006.02690.x. [DOI] [PubMed] [Google Scholar]
- 120.Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema MR, Hedrich R. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett. 2000;486:93–98. doi: 10.1016/s0014-5793(00)02248-1. [DOI] [PubMed] [Google Scholar]
- 121.Ivashikina N, Becker D, Ache P, Meyerhoff O, Felle HH, Hedrich R. K+ channel profile and electrical properties of Arabidopsis root hairs. FEBS Lett. 2001;508:463–469. doi: 10.1016/s0014-5793(01)03114-3. [DOI] [PubMed] [Google Scholar]
- 122.Dreyer I, Poree F, Schneider A, Mittelstadt J, Bertl A, et al. Assembly of plant Shaker-like Kout channels requires two distinct sites of the channel α-subunit. Biophys. J. 2004;87:858–872. doi: 10.1529/biophysj.103.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kourie J, Goldsmith MH. K channels are responsible for an inwardly rectifying current in the plasma membrane of mesophyll protoplasts of Avena sativa . Plant Physiol. 1992;98:1087–1097. doi: 10.1104/pp.98.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon J-M, et al. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- 125.Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae . Proc. Natl. Acad. Sci. USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Santa-Maria GE, Rubio F, Dubcovsky J, Rodriguez-Navarro A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell. 1997;9:2281–2289. doi: 10.1105/tpc.9.12.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu J, Li HD, Chen LQ, Wang Y, Liu LL, et al. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis . Cell. 2006;125:1347–1360. doi: 10.1016/j.cell.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 128.Lee SC, Lan WZ, Kim BG, Li L, Cheong YH, et al. A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proc. Natl. Acad. Sci. USA. 2007;104:15959–15964. doi: 10.1073/pnas.0707912104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheong YH, Pandey GK, Grant JJ, Batistic O, Li L, et al. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis . Plant J. 2007;52:223–239. doi: 10.1111/j.1365-313X.2007.03236.x. [DOI] [PubMed] [Google Scholar]
- 130.Sutter JU, Sieben C, Hartel A, Eisenach C, Thiel G, Blatt MR. Abscisic acid triggers the endocytosis of the Arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Curr. Biol. 2007;17:1396–1402. doi: 10.1016/j.cub.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 131.Philippar K, Ivashikina N, Ache P, Christian M, Luthen H, et al. Auxin activates KAT1 and KAT2, two K+-channel genes expressed in seedlings of Arabidopsis thaliana . Plant J. 2004;37:815–827. doi: 10.1111/j.1365-313x.2003.02006.x. [DOI] [PubMed] [Google Scholar]
- 132.Very AA, Gaymard F, Bosseux C, Sentenac H, Thibaud JB. Expression of a cloned plant K+ channel in Xenopus oocytes: analysis of macroscopic currents. Plant J. 1995;7:321–332. doi: 10.1046/j.1365-313x.1995.7020321.x. [DOI] [PubMed] [Google Scholar]
- 133.Sottocornola B, Visconti S, Orsi S, Gazzarrini S, Giacometti S, et al. The potassium channel KAT1 is activated by plant and animal 14-3-3 proteins. J. Biol. Chem. 2006;281:35735–35741. doi: 10.1074/jbc.M603361200. [DOI] [PubMed] [Google Scholar]
- 134.Duby G, Hosy E, Fizames C, Alcon C, Costa A, et al. AtKC1, a conditionally targeted Shaker-type subunit, regulates the activity of plant K+ channels. Plant J. 2008;53:115–123. doi: 10.1111/j.1365-313X.2007.03324.x. [DOI] [PubMed] [Google Scholar]
- 135.Dreyer I, Antunes S, Hoshi T, Muller-Rober B, Palme K, et al. Plant K+ channel α-subunits assemble indiscriminately. Biophys. J. 1997;72:2143–2150. doi: 10.1016/S0006-3495(97)78857-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Obrdlik P, El-Bakkoury M, Hamacher T, Cappellaro C, Vilarino C, et al. K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc. Natl. Acad. Sci. USA. 2004;101:12242–12247. doi: 10.1073/pnas.0404467101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pilot G, Gaymard F, Mouline K, Cherel I, Sentenac H. Regulated expression of Arabidopsis Shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol. Biol. 2003;51:773–787. doi: 10.1023/a:1022597102282. [DOI] [PubMed] [Google Scholar]
- 138.Xicluna J, Lacombe B, Dreyer I, Alcon C, Jeanguenin L, et al. Increased functional diversity of plant K+ channels by preferential heteromerization of the Shaker-like subunits AKT2 and KAT2. J. Biol. Chem. 2007;282:486–494. doi: 10.1074/jbc.M607607200. [DOI] [PubMed] [Google Scholar]
- 139.Clough SJ, Fengler KA, Yu IC, Lippok B, Smith RK, Jr, Bent AF. The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotidegated ion channel. Proc. Natl. Acad. Sci. USA. 2000;97:9323–9328. doi: 10.1073/pnas.150005697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yoshioka K, Moeder W, Kang HG, Kachroo P, Masmoudi K, et al. The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. Plant Cell. 2006;18:747–763. doi: 10.1105/tpc.105.038786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, et al. A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc. Natl. Acad. Sci. USA. 2007;104:14531–14536. doi: 10.1073/pnas.0701781104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lemtiri-Chlieh F, Berkowitz GA. Cyclic adenosine monophosphate regulates calcium channels in the plasma membrane of Arabidopsis leaf guard and mesophyll cells. J. Biol. Chem. 2004;279:35306–35312. doi: 10.1074/jbc.M400311200. [DOI] [PubMed] [Google Scholar]
- 143.Kato Y, Sakaguchi M, Mori Y, Saito K, Nakamura T, et al. Evidence in support of a four transmembrane-pore-transmembrane topology model for the Arabidopsis thaliana Na+/K+ translocating AtHKT1 protein, a member of the superfamily of K+ transporters. Proc. Natl. Acad. Sci. USA. 2001;98:6488–6493. doi: 10.1073/pnas.101556598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schachtman DP, Schroeder JI. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature. 1994;370:655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- 145.Rubio F, Gassmann W, Schroeder JI. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- 146.Uozumi N, Kim EJ, Rubio F, Yamaguchi T, Muto S, et al. The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae . Plant Physiol. 2000;122:1249–1259. doi: 10.1104/pp.122.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fairbairn DJ, Liu W, Schachtman DP, Gomez-Gallego S, Day SR, Teasdale RD. Characterisation of two distinct HKT1-like potassium transporters from Eucalyptus camaldulensis . Plant Mol. Biol. 2000;43:515–525. doi: 10.1023/a:1006496402463. [DOI] [PubMed] [Google Scholar]
- 148.Horie T, Yoshida K, Nakayama H, Yamada K, Oiki S, Shinmyo A. Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa . Plant J. 2001;27:129–138. doi: 10.1046/j.1365-313x.2001.01077.x. [DOI] [PubMed] [Google Scholar]
- 149.Mäser P, Eckelman B, Vaidyanathan R, Horie T, Fairbairn DJ, et al. Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Lett. 2002;531:157–161. doi: 10.1016/s0014-5793(02)03488-9. [DOI] [PubMed] [Google Scholar]
- 150.Ren ZH, Gao JP, Li LG, Cai XL, Huang W, et al. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005;37:1141–1146. doi: 10.1038/ng1643. [DOI] [PubMed] [Google Scholar]
- 151.Sunarpi, Horie T, Motoda J, Kubo M, Yang H, et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005;44:928–938. doi: 10.1111/j.1365-313X.2005.02595.x. [DOI] [PubMed] [Google Scholar]
- 152.Horie T, Costa A, Kim TH, Han MJ, Horie R, et al. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBOJ. 2007;26:3003–3014. doi: 10.1038/sj.emboj.7601732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Berthomieu P, Conejero G, Nublat A, Brackenbury WJ, Lambert C, et al. Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J. 2003;22:2004–2014. doi: 10.1093/emboj/cdg207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gong JM, Waner DA, Horie T, Li SL, Horie R, et al. Microarray-based rapid cloning of an ion accumulation deletion mutant in Arabidopsis thaliana . Proc. Natl. Acad. Sci. USA. 2004;101:15404–15409. doi: 10.1073/pnas.0404780101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rus A, Baxter I, Muthukumar B, Gustin J, Lahner B, et al. Natural variants of AtHKT1 enhance Na+ accumulation in two wild populations of Arabidopsis . PLoS Genet. 2006;2:e210. doi: 10.1371/journal.pgen.0020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Horie T, Horie R, Chan WY, Leung HY, Schroeder JI. Calcium regulation of sodium hypersensitivities of sos3 and athkt1 mutants. Plant Cell Physiol. 2006;47:622–633. doi: 10.1093/pcp/pcj029. [DOI] [PubMed] [Google Scholar]
- 157.Davenport RJ, Muñoz-Mayor A, Jha D, Essah PA, Rus A, Tester M. The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis . Plant Cell Env. 2007;30:497–507. doi: 10.1111/j.1365-3040.2007.01637.x. [DOI] [PubMed] [Google Scholar]
- 158.Gierth M, Mäser P. Potassium transporters in plants—involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett. 2007;581:2348–2356. doi: 10.1016/j.febslet.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 159.Schwacke R, Schneider A, Van Der Graaff E, Fischer K, Catoni E, et al. ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant Physiol. 2003;131:16–26. doi: 10.1104/pp.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schleyer M, Bakker EP. Nucleotide sequence and 3′-end deletion studies indicate that the K+-uptake protein kup from Escherichia coli is composed of a hydrophobic core linked to a large and partially essential hydrophilic C terminus. J. Bacteriol. 1993;175:6925–6931. doi: 10.1128/jb.175.21.6925-6931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Banuelos MA, Klein RD, Alexander-Bowman SJ, Rodriguez-Navarro A. A potassium transporter of the yeast Schwanniomyces occidentalis homologous to the Kup system of Escherichia coli has a high concentrative capacity. EMBO J. 1995;14:3021–3027. doi: 10.1002/j.1460-2075.1995.tb07304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Quintero FJ, Blatt MR. A new family of K+ transporters from Arabidopsis that are conserved across phyla. FEBS Lett. 1997;415:206–211. doi: 10.1016/s0014-5793(97)01125-3. [DOI] [PubMed] [Google Scholar]
- 163.Fu HH, Luan S. AtKuP1: a dual-affinity K+ transporter from Arabidopsis . Plant Cell. 1998;10:63–73. doi: 10.1105/tpc.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim EJ, Kwak JM, Uozumi N, Schroeder JI. AtKUP1: an Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell. 1998;10:51–62. doi: 10.1105/tpc.10.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Banuelos MA, Garciadeblas B, Cubero B, Rodriguez-Navarro A. Inventory and functional characterization of the HAK potassium transporters of rice. Plant Physiol. 2002;130:784–795. doi: 10.1104/pp.007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gierth M, Mäser P, Schroeder JI. The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol. 2005;137:1105–1114. doi: 10.1104/pp.104.057216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shin R, Schachtman DP. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc. Natl. Acad. Sci. USA. 2004;101:8827–8832. doi: 10.1073/pnas.0401707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Armengaud P, Breitling R, Amtmann A. The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol. 2004;136:2556–2576. doi: 10.1104/pp.104.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rubio F, Santa-María GE, Rodríguez-Navarro A. Cloning of Arabidopsis and barley cDNAs encoding HAK potassium transporters in root and shoot cells. Physiol. Plant. 2000;109:34–43. [Google Scholar]
- 170.Epstein E, Rains DW, Elzam OE. Resolution of dual mechanisms of potassium absorption by barley roots. Proc. Natl. Acad. Sci. USA. 1963;49:684–692. doi: 10.1073/pnas.49.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Elumalai RP, Nagpal P, Reed JW. A mutation in the Arabidopsis KT2/KUP2 potassium transporter gene affects shoot cell expansion. Plant Cell. 2002;14:119–131. doi: 10.1105/tpc.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rigas S, Debrosses G, Haralampidis K, Vicente-Agullo F, Feldmann KA, et al. TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell. 2001;13:139–151. doi: 10.1105/tpc.13.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Allen GJ, Sanders D. Control of ionic currents in guard cell vacuoles by cytosolic and luminal calcium. Plant J. 1996;10:1055–1069. doi: 10.1046/j.1365-313x.1996.10061055.x. [DOI] [PubMed] [Google Scholar]
- 174.Bihler H, Eing C, Hebeisen S, Roller A, Czempinski K, Bertl A. TPK1 is a vacuolar ion channel different from the slow-vacuolar cation channel. Plant Physiol. 2005;139:417–424. doi: 10.1104/pp.105.065599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Czempinski K, Frachisse JM, Maurel C, Barbier-Brygoo H, Mueller-Roeber B. Vacuolar membrane localization of the Arabidopsis ‘two-pore’ K+ channel KCO1. Plant J. 2002;29:809–820. doi: 10.1046/j.1365-313x.2002.01260.x. [DOI] [PubMed] [Google Scholar]
- 176.Ketchum KA, Joiner WJ, Sellers AJ, Kaczmarek LK, Goldstein SA. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature. 1995;376:690–695. doi: 10.1038/376690a0. [DOI] [PubMed] [Google Scholar]
- 177.Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am. J. Physiol. Renal Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- 178.Hedrich R, Neher E. Cytoplasmic calcium regulates voltage dependent ion channels in plant vacuoles. Nature. 1987;329:833–835. [Google Scholar]
- 179.Ishibashi K, Suzuki M, Imai M. Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem. Biophys. Res. Commun. 2000;270:370–376. doi: 10.1006/bbrc.2000.2435. [DOI] [PubMed] [Google Scholar]
- 180.Ward JM, Pei ZM, Schroeder JI. Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell. 1995;7:833–844. doi: 10.1105/tpc.7.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bewell MA, Maathuis FJ, Allen GJ, Sanders D. Calcium-induced calcium release mediated by a voltage-activated cation channel in vacuolar vesicles from red beet. FEBS Lett. 1999;458:41–44. doi: 10.1016/s0014-5793(99)01109-6. [DOI] [PubMed] [Google Scholar]
- 182.Scholz-Starke J, Carpaneto A, Gambale F. On the interaction of neomycin with the slow vacuolar channel of Arabidopsis thaliana . J. Gen. Physiol. 2006;127:329–340. doi: 10.1085/jgp.200509402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McAinsh MR, Webb A, Taylor JE, Hetherington AM. Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell. 1995;7:1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Han S, Tang R, Anderson LK, Woerner TE, Pei ZM. A cell surface receptor mediates extracellular Ca2+ sensing in guard cells. Nature. 2003;425:196–200. doi: 10.1038/nature01932. [DOI] [PubMed] [Google Scholar]
- 185.Nomura H, Komori T, Kobori M, Nakahira Y, Shiina T. Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J. 2008;53:988–998. doi: 10.1111/j.1365-313X.2007.03390.x. [DOI] [PubMed] [Google Scholar]
- 186.Pei ZM, Ward JM, Schroeder JI. Magnesium sensitizes slow vacuolar channels to physiological cytosolic calcium and inhibits fast vacuolar channels in fava bean guard cell vacuoles. Plant Physiol. 1999;121:977–986. doi: 10.1104/pp.121.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pottosin II, Martinez-Estevez M. Regulation of the fast vacuolar channel by cytosolic and vacuolar potassium. Biophys. J. 2003;84:977–986. doi: 10.1016/S0006-3495(03)74914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Eddy SR. Profile hidden Markov models. Bioinformatics. 1998;14:755–763. doi: 10.1093/bioinformatics/14.9.755. [DOI] [PubMed] [Google Scholar]
- 189.Miedema H, Demidchik V, Very AA, Bothwell JH, Brownlee C, Davies JM. Two voltage-dependent calcium channels co-exist in the apical plasma membrane of Arabidopsis thaliana root hairs. New Phytol. 2008;179:378–385. doi: 10.1111/j.1469-8137.2008.02465.x. [DOI] [PubMed] [Google Scholar]