Abstract
Enveloped viruses enter cells by fusion of their own membrane with a cellular membrane. Incorporation of inverted-cone-shaped lipids such as lysophosphatidylcholine (LPC) into the outer leaflet of target membranes has been shown previously to impair fusion mediated by class I viral fusion proteins, e.g., the influenza virus hemagglutinin. It has been suggested that these results provide evidence for the stalk-pore model of fusion, which involves a hemifusion intermediate (stalk) with highly bent outer membrane leaflets. Here, we investigated the effect of inverted-cone-shaped LPCs and the cone-shaped oleic acid (OA) on the membrane fusion activity of a virus with a class II fusion protein, the flavivirus tick-borne encephalitis virus (TBEV). This study included an analysis of lipid mixing, as well as of the steps preceding or accompanying fusion, i.e., binding to the target membrane and lipid-induced conformational changes in the fusion protein E. We show that the presence of LPC in the outer leaflet of target liposomes strongly inhibited TBEV-mediated fusion, whereas OA caused a very slight enhancement, consistent with a fusion mechanism involving a lipid stalk. However, LPC also impaired the low-pH-induced binding of a soluble form of the E protein to liposomes and its conversion into a trimeric postfusion structure that requires membrane binding at low pH. Because inhibition is already observed before the lipid-mixing step, it cannot be determined whether impairment of stalk formation is a contributing factor in the inhibition of fusion by LPC. These data emphasize, however, the importance of the composition of the target membrane in its interactions with the fusion peptide that are crucial for the initiation of fusion.
Fusion of viral membranes with cellular membranes is a key step in the entry of enveloped viruses into cells. It requires energy to overcome repulsive forces between the opposing membranes and to locally disrupt the original lipid bilayer structures. This energy is provided by structural changes and protein-protein, as well as protein-lipid, interactions of specific viral envelope proteins, designated viral fusion proteins. In infectious virions these proteins exist in a metastable state and respond to a specific fusion trigger (receptor binding or acidic pH) by conformational changes that lead to the exposure of the fusion peptide and the formation of an energetically more stable postfusion structure (23, 25, 49).
So far, two structurally different classes of viral fusion proteins have been identified (30). Class I is represented by the spike-like envelope proteins found in orthomyxo-, paramyxo-, retro-, filo-, and coronaviruses. They are characterized by amino-terminal or amino-proximal fusion peptides, as well as the formation of a hairpin-like postfusion structure with a central trimeric coiled coil. In this postfusion form the membrane-inserted N and C termini are located at the same end of a very stable protein rod (11, 51). The class II fusion proteins of flaviviruses and alphaviruses are completely unrelated structurally to class I viral fusion proteins. They are oriented parallel to the lipid membrane, possess internal fusion peptide loops, and form part of a metastable icosahedral network in the virion envelope (reviewed in references 19 and 27). Recently, the postfusion structures of the class II proteins from both flavi- and alphaviruses were determined by X-ray crystallography (4, 16, 35). These new structures suggest that the fusion processes mediated by both classes of fusion proteins—despite their structural unrelatedness—follow conceptually related pathways.
It is thought that insertion of fusion peptides disrupts the membrane locally, leading to the formation of an initial local lipid connection (lipid stalk) in which only the outer leaflets of the two membranes have merged (8, 16). This lipid stalk is then believed to expand into a hemifusion diaphragm whose rupture would finally generate a fusion pore (8, 16). The formation of a lipid stalk as proposed requires strong bending into a negative curvature of the outer leaflets, which have already fused at this stage. In addition to theoretical considerations (29, 31, 34, 41, 42), experimental support for this stalk-pore model of fusion has been provided for class I viral fusion systems by using lipids that either impair or promote the bending of lipid membranes (reviewed in reference 8). Specifically, it has been shown that the presence of lipids with an inverted-cone shape (which induce positive curvature) such as lysophosphatidylcholine (LPC) in the outer leaflet of target membranes impaired fusion, whereas fusion was promoted at least in some instances by cone-shaped lipids (which induce negative curvature) such as oleic acid (OA). Although the data obtained have been consistent with the lipid stalk model, alternative explanations have also been discussed that relate to an influence of these lipids on the interaction of the fusion peptides with the target membrane (17, 18, 32, 37, 49).
Since data on the influence of lipids that alter membrane curvature are not yet available for class II viral fusion proteins, we have investigated the effect of such molecules on fusion and on steps preceding fusion of the flavivirus tick-borne encephalitis virus (TBEV), for which atomic structures of the fusion protein E in its pre- and postfusion conformations are known (4, 40). This protein exists as a metastable homodimer on the surface of infectious virions and is converted into a stable homotrimer upon exposure to acidic pH (postfusion structure) (1). It was shown previously that a C-terminally truncated form of the E protein dimer (sE dimer) can be used to study the early stages of fusion protein-membrane interactions in the absence of fusion (46). At low pH in the presence of membranes the sE dimers dissociate into monomers, bind to the target membrane via their internal fusion peptide loops, and form stable trimers corresponding to the postfusion structure (46, 47). Cholesterol (CH) in the target membrane has a strong promoting effect on membrane binding and trimerization of sE, and it was shown by the use of CH analogs that the underlying interactions involve the 3β-hydroxyl group at C-3 (48).
In the present study we investigated the effect of inverted-cone-shaped (LPCs) and cone-shaped (OA) lipids on the fusion of TBEV with liposomes, as well as on the steps preceding or accompanying fusion, i.e., fusion peptide loop-mediated binding of sE to the target membrane and lipid-induced trimerization of sE. Similar to what has been observed with class I viral fusion proteins and consistent with a fusion mechanism that involves a lipid stalk, the presence of LPC in the outer leaflet of liposomes strongly inhibited TBEV-mediated fusion, whereas fusion was slightly enhanced by OA. However, we also show that these lipids strongly influence the efficiency of the initial interaction of the fusion peptide loop with the target membrane and the generation of the trimeric postfusion structure of sE. Because inhibition already occurred before the lipid-mixing step, it was not possible to demonstrate a role of stalk formation in the TBEV fusion mechanism. The data do indicate, however, that early interactions of TBEV class II fusion protein E with membranes are very sensitive to the target membrane composition.
MATERIALS AND METHODS
Lipids.
Phosphatidylcholine (PC) from egg yolk, phosphatidylethanolamine (PE; prepared by transphosphatidylation of egg PC), lyso-1-stearoyl-sn-3-PC (LSPC), lyso-1-palmitoyl-sn-3-PC (LPPC), lyso-1-myristoyl-sn-3-PC (LMPC), and lyso-1-lauroyl-sn-3-PC (LLPC) were purchased form Avanti Polar Lipids (Alabaster, Ala.). CH, cholesteryl methyl ether (CM), and OA were purchased from Sigma Chemical Co., and 1-pyrenehexadecanoic acid was from Molecular Probes (Leiden, The Netherlands).
Virus growth and purification.
The TBEV prototype strain Neudoerfl was grown in primary chicken embryo cells, harvested 48 h after infection, and purified by two cycles of sucrose density gradient centrifugation (20). For membrane fusion assays, the virions were metabolically labeled with 1-pyrenehexadecanoic acid as described previously (14).
Preparation of sE dimers.
sE dimers were generated by limited trypsin digestion of purified virions at 0°C as described previously (21). The residual particles were removed by ultracentrifugation, and purification of the sE dimers was performed by anion-exchange chromatography (21).
Liposomes.
For the preparation of standard liposomes, PC, PE, and CH were mixed at a molar ratio of 1:1:2 from stock solutions in chloroform (48). The mixture was dried to a thin film with a rotary evaporator and then dried further in a high vacuum for at least 1.5 h. The lipid film was hydrated in liposome buffer (10 mM triethanolamine, 140 mM NaCl, pH 8.0) and subjected to five cycles of freeze-thawing, followed by 21 cycles of extrusion through two polycarbonate membranes with a pore size of 200 nm with a Liposofast syringe-type extruder (Avestin, Ottawa, Ontario, Canada).
Incorporation of LPC or OA into liposomes.
Stock solutions of 1 mM LPC were prepared as aqueous dispersions in liposome buffer (10 mM triethanolamine, 140 mM NaCl, pH 8.0); those of 10 mM OA were prepared as an ethanolic solution. Liposomes were mixed with LPC or OA in a molar ratio of 15:1 and incubated for 3 min at 37°C. For the removal of LPC from outer membranes, fatty-acid-free bovine serum albumin (BSA; Sigma Chemical Co.) was added to the liposome-LPC mixture to a final concentration of 10 mg/ml and further incubated for 5 min at 37°C.
Fusion assay.
Fusion of pyrene-labeled virions with liposomes was measured by monitoring the decrease in pyrene excimer fluorescence caused by the dilution of pyrene-labeled phospholipids in the viral membrane into the unlabeled liposome membrane (14, 45). Fluorescence was recorded continuously for 60 s at 480 nm with a Perkin-Elmer LS 50B fluorescence spectrophotometer at an excitation wavelength of 343 nm. For determining the effect of LPC or OA on fusion, pyrene-labeled virions (0.5 to 1 μM) were mixed with 0.3 mM liposomes containing LPC or OA in a continuously stirred fluorimeter cuvette at 37°C and acidified to pH 5.4 by the addition of 300 mM morpholineethanesulfonic acid (MES). For controls at pH 8.0, the same amount of liposome buffer (pH 8.0) was added. The initial excimer fluorescence after mixing was defined as 0% fusion. To determine the residual excimer fluorescence at infinite dilution of the probe (defined as 100% fusion for calculating the fusion extents), the detergent octaethyleneglycol-monododecylether (C12E8) was added to a final concentration of 10 mM to disperse the viral and liposomal membranes.
Coflotation of virions with liposomes.
Virions were mixed with liposomes containing LPC or OA in a ratio of 1 μg of E protein to 300 nmol of lipid. The samples were acidified with 300 mM MES, incubated for 10 min at 37°C at pH 5.4, back neutralized, and adjusted to a final volume of 2 ml of 20% (wt/wt) sucrose in TAN buffer (pH 8.0) as described previously (46). The 2-ml virus-liposome mixture was then applied to a 50% cushion and overlaid with 1 ml of 5% (wt/wt) sucrose. Centrifugation was carried out for 1.5 h at 50,000 rpm and 4°C in a Beckman SW55 rotor, and fractions of 200 μl were collected by upward displacement. The amount of E protein in each fraction was determined by a quantitative four-layer enzyme-linked immunosorbent assay (ELISA) after denaturation of the samples with 0.4% sodium dodecyl sulfate (SDS) at 65°C (22).
Coflotation of sE with liposomes.
sE dimers were mixed with liposomes containing LPC or OA in a ratio of 1 μg of sE protein (11 pmol) to 15 nmol of lipid. The samples were acidified with 300 mM MES, incubated for 20 min at 37°C and pH 5.4, back neutralized, and adjusted to a final volume of 0.6 ml of 20% (wt/wt) sucrose in TAN buffer (pH 8.0) as described previously (46). The 0.6-ml sE protein-liposome mixture was then applied to a 50% cushion overlaid with 1.6 ml of 15% (wt/wt) sucrose and 0.8 ml of 5% (wt/wt) sucrose. Centrifugation was carried out as described above for coflotation of virus with liposomes.
Sedimentation analysis.
The conversion of sE dimers into sE trimers was measured by sedimentation analysis in sucrose gradients as described previously (48). sE dimers were mixed with liposomes containing LPC or OA in a ratio of 1 μg of sE protein to 15 nmol of lipid and incubated for 5 min at 37°C. The samples were acidified with 300 mM MES, incubated for 30 min at 37°C at pH 5.4, back neutralized, solubilized with 1.5% n-octylglucoside (n-OG), and applied to 7 to 20% sucrose gradients in TAN buffer (pH 8.0) containing 0.8% n-OG. Samples were centrifuged for 20 h in an SW40 rotor (Beckman) at 38,000 rpm and 15°C. Fractions were collected by upward displacement, and E protein was quantitated by four-layer ELISA after denaturation with 0.4% SDS at 65°C (22).
RESULTS
Effect of inverted-cone-shaped and cone-shaped lipids on fusion of TBEV with liposomes.
The effect of inverted-cone-shaped LPCs with different chain lengths (LLPC-C12, LMPC-C14, LPPC-C16, and LSPC-C18) and the cone-shaped OA molecule on TBEV fusion was studied with an in vitro fusion assay with liposomes into which LPCs or OA had been incorporated into the outer leaflet of the membrane as described in Materials and Methods.
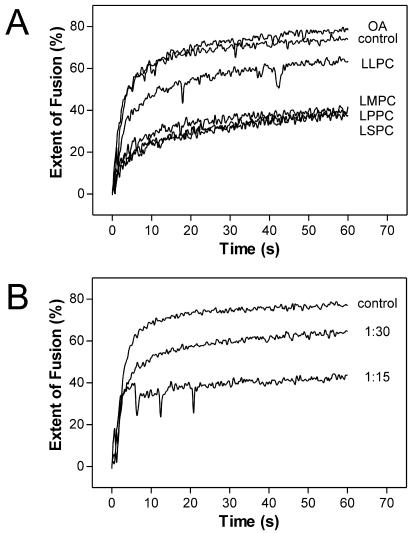
As shown in Fig. 1A, low-pH-induced fusion of TBEV with liposomes consisting of PC, PE, and CH (standard liposomes) is very fast and efficient. Incorporation of LMPC-C14, LPPC-C16, or LSPC-C18 resulted in a strong reduction of the extent of fusion. With LLPC-C12 only a slight reduction was observed, and this could be due to the less efficient incorporation of LPC with a shorter aliphatic side chain into lipid membranes (9). In contrast to the LPCs, the cone-shaped OA molecule caused a very slight but consistent increase in fusion efficiency (Fig. 1A and 2B).
FIG. 1.
(A) Fusion of pyrene-labeled TBEV with liposomes into which LPCs with aliphatic side chains of different lengths (LSPC-C18, LPPC-C16, LMPC-C14, and LLPC-C12) and OA were incorporated into the outer leaflet at a molar ratio of liposomal lipid to LPC of 15:1. After acidification, the change in pyrene excimer fluorescence was monitored continuously for 60 s. (B) Concentration dependence of LSPC-mediated inhibition of TBEV fusion. Incorporation of LSPC into standard liposomes was carried out at molar ratios of 1:30 and 1:15.
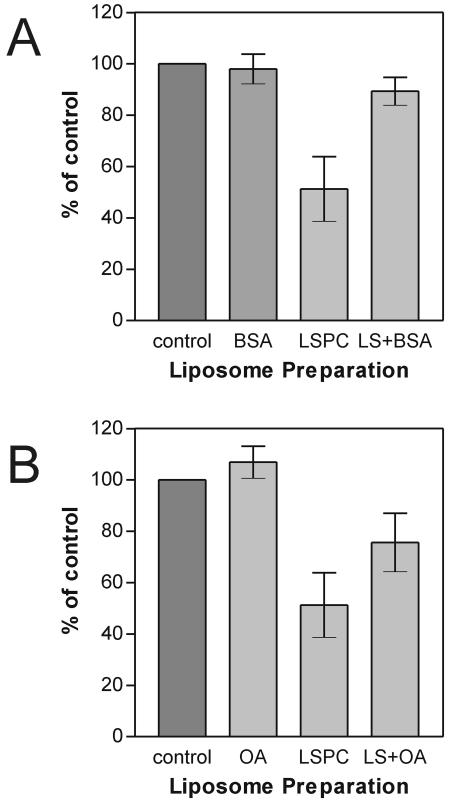
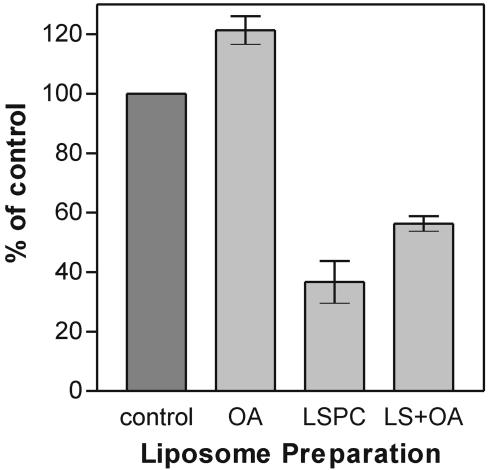
FIG. 2.
LSPC-mediated inhibition of fusion of TBEV with liposomes, its reversibility by BSA (A), and its counteraction by OA (B). The bar graph shows the extent of fusion of TBEV with the liposome preparations after 60 s in the pyrene excimer fusion assay. Error bars represent the standard deviation of at least six experiments. (A) Control, standard liposomes; BSA, standard liposomes plus BSA at 10 mg/ml; LSPC, LSPC-treated standard liposomes; LS+BSA, LSPC-treated standard liposomes plus BSA at 10 mg/ml. (B) Control, standard liposomes; OA, OA-treated standard liposomes; LSPC, LSPC-treated standard liposomes; LS+OA, LSPC and OA-treated standard liposomes.
On the basis of the results shown in Fig. 1 and because it can be incorporated with high efficiency (3), LSPC was used in all further experiments. As shown in Fig. 1B, the inhibition of fusion by LSPC was clearly concentration dependent. In order to ascertain whether or not the observed fusion inhibition was indeed caused by membrane-incorporated rather than residual free LSPC, two sets of fusion experiments were carried out with (i) liposomes pretreated with LSPC that were purified via gel filtration and (ii) LSPC-modified liposomes that were treated with BSA, which is known to remove lysolipids from lipid membranes (9). No difference in the degree of fusion impairment was observed between purified and unpurified LSPC-containing liposomes (data not shown). The addition of BSA to LSPC-containing liposomes, however, almost completely reversed the LSPC-mediated fusion inhibition, consistent with the extraction of membrane-inserted LSPC (Fig. 2A).
We also analyzed whether the effect on fusion of the inverted-cone-shaped LSPC molecule could be counteracted by the cone-shaped OA molecule. As shown in Fig. 2B, addition of OA (at the same molar ratio as LSPC) to LSPC-containing liposomes indeed resulted in increased fusion activity, reaching about 75% of control levels (Fig. 2B).
Effect of LSPC on TBEV-lipid interactions preceding fusion.
The flavivirus fusion reaction is a multistep process that includes the low-pH-induced dissociation of the E dimer, interaction of the fusion peptide loop with the target membrane, and conformational changes that convert the E protein into a trimeric postfusion structure (19). We have made use of experimental tools that allowed us to investigate the effects of LSPC in the target membrane—not only with respect to overall fusion but also specifically on the binding and E protein trimerization steps.
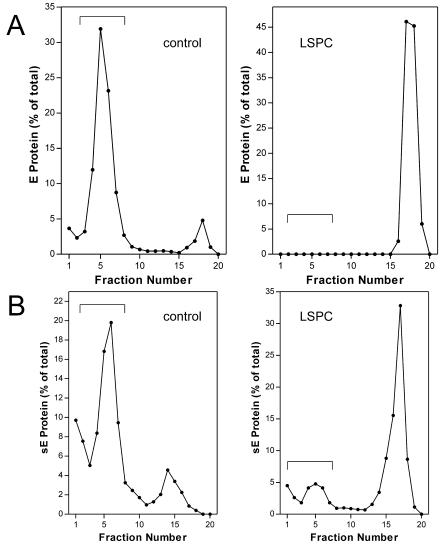
The membrane-binding step was first analyzed by liposome coflotation analysis with whole virions. As shown in the control, the acidification of virions in the presence of standard liposomes led to an almost quantitative association with the target membranes (Fig. 3A, left side). In contrast, the virus-liposome association was completely abolished when LSPC-treated liposomes were used (Fig. 3A, right side), suggesting that LSPC already prevented the binding of the fusion peptide loop to the target membrane.
FIG. 3.
Coflotation analysis demonstrating the impairment of binding of virions (A) and sE proteins (B) to liposomes containing LSPC. Control liposomes and LSPC-treated liposomes were mixed with virions (A) or sE proteins (B), acidified to pH 5.4, back neutralized, and subjected to centrifugation in sucrose step gradients as described in Materials and Methods. The gradients were fractionated, and the amount of E protein in each fraction was determined by a quantitative four-layer ELISA after denaturation of the samples with 0.4% SDS. The top fractions containing the liposomes and bound E protein are indicated by a bracket.
This binding step can be specifically uncoupled from fusion and analyzed by the use of isolated sE instead of whole virions (46). The results of such experiments are shown in Fig. 3B. The controls in which sE and standard liposomes were used yielded results similar to those obtained with whole virions (Fig. 3B, left side). When LSPC-containing liposomes were used, however, the binding of sE was strongly reduced (Fig. 3B, right side), consistent with the suggested impairment of the fusion peptide loop-mediated attachment to the modified liposomes.
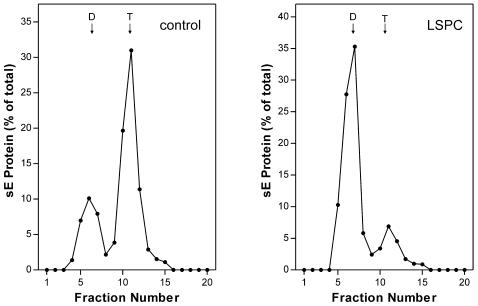
Previously we showed that the low-pH-mediated conversion of the sE dimers into the stable trimeric postfusion structure requires its interaction with membranes (46). To assess the effect of LSPC on this oligomeric transition, the sE preparations used for the coflotation experiments shown in Fig. 3B were solubilized with detergent and analyzed by sedimentation in sucrose gradients to determine the extent of trimer formation. The results in Fig. 4 show that trimer formation at low pH was also reduced in the presence of LSPC-containing liposomes (Fig. 4, right side) compared to the control (Fig. 4, left side).
FIG. 4.
Sedimentation analysis demonstrating the impairment of lipid-induced trimerization of sE with liposomes containing LSPC. sE proteins were mixed with standard liposomes or LSPC-treated liposomes, exposed to acidic pH, solubilized with 1.5% n-OG, and centrifuged in 7 to 20% sucrose gradients containing 0.8% n-OG as described in Materials and Methods. The gradients were fractionated, and the amount of E protein in each fraction was determined by a quantitative four-layer ELISA after denaturation of the samples with 0.4% SDS. The sedimentation direction is from left to right, and the dimer (D) and trimer (T) positions are indicated.
The effect of LSPC on trimerization of sE can either be due to the impaired binding of sE to liposomes or due to an effect of LSPC on the trimerization process itself. To distinguish between these two possibilities we performed the same experiments as described above with whole virions and LSPC-containing liposomes. In contrast to sE, the low-pH-induced formation of trimers in the context of whole virions also occurs in the absence of target membranes as shown previously (1), and consistent with this, the membrane-independent, low-pH-induced trimerization of E on the virion surface was not affected by LSPC (data not shown).
Effect of LSPC on interactions of TBEV with CM-containing liposomes.
As shown previously, the fusion peptide loop-mediated association with liposomes and the subsequent trimerization of sE are facilitated by CH and its 3β-hydroxyl group (48). Since specific interactions of LSPC with this group have been described (5, 6) it is possible that the observed effects of LSPC are indirect and reflect the masking of the hydroxyl group. We therefore carried out a set of experiments in which CH was replaced with CM, which possesses a methyl group instead of a hydroxyl group. Although the fusion activity with CM-containing liposomes was somewhat reduced (48), it was still significant and sufficient for investigating the effect of LSPC.
As shown in Fig. 5, the results obtained were very similar to those obtained with CH-containing liposomes (Fig. 2B). LSPC incorporation caused a strong decrease in the extent of fusion with virus, and this could be partially counteracted by OA (Fig. 5). Similarly, when the same liposome preparations were used for measuring lipid binding and the concomitant trimerization of sE, these processes were significantly reduced by LSPC (data not shown).
FIG. 5.
LSPC-mediated inhibition of the fusion of pyrene-labeled TBEV with CM containing liposomes and its partial reversal by OA. The bar graph shows the extent of fusion of TBEV with the liposome preparations after 60 s. Error bars represent the standard deviation of at least three experiments. Control, CM-containing liposomes; OA, OA-treated CM-containing liposomes; LSPC, LSPC-treated CM-containing liposomes; LS+OA, LSPC and OA treated CM-containing liposomes.
These results suggest that the LSPC-mediated inhibition of TBEV fusion is not due to masking of the 3β-hydroxyl group of CH but is a direct result of membrane modification by LSPC.
DISCUSSION
The increasing knowledge of the structures of viral fusion proteins in their native and postfusion conformations has provided much insight into the processes of fusion between viral and cellular membranes (11, 26). The recent determination of the postfusion structures of the class II viral fusion proteins from flavi- and alphaviruses has formed the basis for proposing that class I and II viral fusion machineries are mechanistically similar, even though they have unrelated protein architectures. Despite the precise knowledge of these protein structures and their fusion-associated conformational changes, the membrane intermediates proposed to be generated during the pathway of membrane fusion are much less well defined. The most prominent model involves the formation of an initial local lipid connection (lipid stalk) with a net negative curvature in which only the outer leaflets of the two membranes have merged and are strongly bent (8). This model was first established for the influenza virus fusion mechanism but since then has been extended to other class I fusion proteins (8) and also to class II viral fusion proteins (16). Consistent with the requirement for strong bending of the outer membrane leaflets for stalk formation, our data have shown that incorporation of inverted-cone-shaped lipids (positive curvature) such as LPC in this leaflet of the target membrane had a strong inhibitory effect on fusion, whereas it was slightly enhanced when cone-shaped lipids (negative curvature) were used. Similar inhibitory effects of inverted-cone-shaped lipids that could be related to membrane curvature effects have been observed in a number of viral and nonviral fusion systems, including influenza virus (3, 9), baculovirus (7), human immunodeficiency virus (33), rabies virus (15), cortical granule exocytosis (50), and microsome fusion (10).
Modification of the lipid composition of the target membrane, however, can also have an influence on the initial interaction with fusion peptides or other structural elements of fusion proteins that are required for driving fusion (17, 18, 32, 37, 49). They can also potentially affect the way the fusion peptide is inserted into the membrane, thereby modifying local membrane disruptions that are required for promoting fusion (37). We have indeed found that the presence of inverted-cone-shaped LPCs in the outer leaflet of liposomes strongly impairs the low-pH-induced interaction of TBEV and its fusion protein with liposomes, suggesting that the internal fusion peptide loop might interact differently with modified membranes. With a soluble form of the fusion protein we also showed that the concomitant conversion into the trimeric postfusion structure was prevented. Similar effects have also been observed in other membrane fusion systems and could be related to lipid-dependent conformational changes that appear to regulate fusion activity (37). Interestingly, certain bacterial toxins that require lipid interactions to form oligomeric pores in membranes have also been shown to depend on the shape of certain lipids in the membrane. Specifically, the interactions of Vibrio cholerae cytolysin with liposomes were enhanced by the insertion of cone-shaped lipids (52), whereas the pore formation by aerolysin of Aeromonas hydrophila was blocked when inverted-cone-shaped lipids such as LPC were present in the membrane (2).
Independent of curvature effects, the importance of specific protein-lipid interactions for the prefusion stages of viral membrane fusion has been highlighted previously for both class I and II systems. The presence of negatively charged lipids in the target membrane enhanced the binding of the fusion peptide and the efficiency of fusion of influenza virus (24, 44) and human immunodeficiency virus (12, 39). An even more dramatic lipid dependence has been described for alphavirus membrane interactions (reviewed in reference 28). In this case target membrane binding is absolutely dependent on the presence of CH (38, 43), whereas the subsequent merger of the membranes is regulated by sphingolipids (13, 36, 43). Although the alpha- and flavivirus fusion proteins are structurally very similar, we do not observe an absolute requirement for CH in fusion experiments with TBEV (14, 48). However, both membrane attachment and fusion are significantly enhanced by the presence of CH and these effects appear to be dependent on its 3β-hydroxyl group (48). Since it has been reported that the cone-shaped lipids we used in the present investigation (LPCs) can interact with CH in membranes via this hydroxyl group (5, 6), we also carried out experiments with a CH analog that lacks this group. Our data show that the LPC-mediated impairment of fusion-associated processes was independent of such an interaction and was most likely due to direct effects of LPC, such as changes in membrane curvature. This is supported by the finding that the LPC effect on fusion and binding (data not shown) could be counteracted by the simultaneous incorporation of the cone-shaped OA molecule.
Since the inverted-cone-shaped lipids used in this study were shown to already have such profound effects on the initial membrane attachment step, the data presented cannot be taken as evidence for or against the lipid stalk model as an intermediate in membrane fusion by this virus. They do, however, strongly support the notion that the interactions of fusion peptides with target membranes are quite specific and probably control fusion by causing local lipid disturbances and/or protein conformational changes that are essential for the further steps in the fusion process. On the basis of studies with the influenza virus hemagglutinin and lipid-induced conformational changes of its fusion peptide, Tamm et al. have recently proposed the so-called “lipid-mixer” model of membrane fusion, which does not require the formation of a lipid stalk at the stage of the hemifusion intermediate (49). It has not been possible to distinguish between these two models, but both the new structural data (4, 16, 35) and the increasing data on the influence of specific lipids on fusion steps corroborate the notion that the mechanisms of class I and II viral fusion are probably fundamentally similar.
Acknowledgments
We thank Steven Allison for helpful discussions and critical reading of the manuscript and Walter Holzer for technical assistance.
This work was supported in part by the Austrian Fonds zur Förderung der wissenschaftlichen Forschung, FWF project P16535-B09.
REFERENCES
- 1.Allison, S. L., J. Schalich, K. Stiasny, C. W. Mandl, C. Kunz, and F. X. Heinz. 1995. Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J. Virol. 69:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso, A., F. M. Goni, and J. T. Buckley. 2000. Lipids favoring inverted phase enhance the ability of aerolysin to permeabilize liposome bilayers. Biochemistry 39:14019-14024. [DOI] [PubMed] [Google Scholar]
- 3.Baljinnyam, B., B. Schroth-Diez, T. Korte, and A. Herrmann. 2002. Lysolipids do not inhibit influenza virus fusion by interaction with hemagglutinin. J. Biol. Chem. 277:20461-20467. [DOI] [PubMed] [Google Scholar]
- 4.Bressanelli, S., K. Stiasny, S. L. Allison, E. A. Stura, S. Duquerroy, J. Lescar, F. X. Heinz, and F. A. Rey. 2004. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 23:728-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatelain, P., and R. Brasseur. 1991. A conformational analysis study of the interaction of amiodarone and cholesterol with lysophosphatidylcholine. Biochem. Pharmacol. 41:1639-1647. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan, V. P., L. S. Ramsammy, and H. Brockerhoff. 1984. Molecular interactions in the hydrogen belts of membranes. Glucose-6-phosphatase, lysophosphatidylcholine, and cholesterol. Biochim. Biophys. Acta 772:239-243. [DOI] [PubMed] [Google Scholar]
- 7.Chernomordik, L., E. Leikina, M. S. Cho, and J. Zimmerberg. 1995. Control of baculovirus gp64-induced syncytium formation by membrane lipid composition. J. Virol. 69:3049-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chernomordik, L. V., and M. M. Kozlov. 2003. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 72:175-207. [DOI] [PubMed] [Google Scholar]
- 9.Chernomordik, L. V., E. Leikina, V. Frolov, P. Bronk, and J. Zimmerberg. 1997. An early stage of membrane fusion mediated by the low pH conformation of influenza hemagglutinin depends upon membrane lipids. J. Cell Biol. 136:81-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernomordik, L. V., S. S. Vogel, A. Sokoloff, H. O. Onaran, E. A. Leikina, and J. Zimmerberg. 1993. Lysolipids reversibly inhibit Ca2+-, GTP- and pH-dependent fusion of biological membranes. FEBS Lett. 318:71-76. [DOI] [PubMed] [Google Scholar]
- 11.Colman, P. M., and M. C. Lawrence. 2003. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 4:309-319. [DOI] [PubMed] [Google Scholar]
- 12.Contreras, L. M., F. J. Aranda, F. Gavilanes, J. M. Gonzalez-Ros, and J. Villalain. 2001. Structure and interaction with membrane model systems of a peptide derived from the major epitope region of HIV protein gp41: implications on viral fusion mechanism. Biochemistry 40:3196-3207. [DOI] [PubMed] [Google Scholar]
- 13.Corver, J., L. Moesby, R. K. Erukulla, K. C. Reddy, R. Bittman, and J. Wilschut. 1995. Sphingolipid-dependent fusion of Semliki Forest virus with cholesterol-containing liposomes requires both the 3-hydroxyl group and the double bond of the sphingolipid backbone. J. Virol. 69:3220-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corver, J., A. Ortiz, S. L. Allison, J. Schalich, F. X. Heinz, and J. Wilschut. 2000. Membrane fusion activity of tick-borne encephalitis virus and recombinant subviral particles in a liposomal model system. Virology 269:37-46. [DOI] [PubMed] [Google Scholar]
- 15.Gaudin, Y. 2000. Rabies virus-induced membrane fusion pathway. J. Cell Biol. 150:601-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbons, D. L., M. C. Vaney, A. Roussel, A. Vigouroux, B. Reilly, J. Lepault, M. Kielian, and F. A. Rey. 2004. Conformational change and protein-protein interactions of the fusion protein of Semliki Forest virus. Nature 427:320-325. [DOI] [PubMed] [Google Scholar]
- 17.Gunther-Ausborn, S., A. Praetor, and T. Stegmann. 1995. Inhibition of influenza-induced membrane fusion by lysophosphatidylcholine. J. Biol. Chem. 270:29279-29285. [DOI] [PubMed] [Google Scholar]
- 18.Gunther-Ausborn, S., and T. Stegmann. 1997. How lysophosphatidylcholine inhibits cell-cell fusion mediated by the envelope glycoprotein of human immunodeficiency virus. Virology 235:201-208. [DOI] [PubMed] [Google Scholar]
- 19.Heinz, F. X., and S. L. Allison. 2001. The machinery for flavivirus fusion with host cell membranes. Curr. Opin. Microbiol. 4:450-455. [DOI] [PubMed] [Google Scholar]
- 20.Heinz, F. X., and C. Kunz. 1981. Homogeneity of the structural glycoprotein from European isolates of tick-borne encephalitis virus: comparison with other flaviviruses. J. Gen. Virol. 57:263-274. [DOI] [PubMed] [Google Scholar]
- 21.Heinz, F. X., C. W. Mandl, H. Holzmann, C. Kunz, B. A. Harris, F. Rey, and S. C. Harrison. 1991. The flavivirus envelope protein E: isolation of a soluble form from tick-borne encephalitis virus and its crystallization. J. Virol. 65:5579-5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinz, F. X., K. Stiasny, G. Puschner-Auer, H. Holzmann, S. L. Allison, C. W. Mandl, and C. Kunz. 1994. Structural changes and functional control of the tick-borne encephalitis virus glycoprotein E by the heterodimeric association with protein prM. Virology 198:109-117. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 24.Hinterdorfer, P., G. Baber, and L. K. Tamm. 1994. Reconstitution of membrane fusion sites. A total internal reflection fluorescence microscopy study of influenza hemagglutinin-mediated membrane fusion. J. Biol. Chem. 269:20360-20368. [PubMed] [Google Scholar]
- 25.Jahn, R., T. Lang, and T. C. Sudhof. 2003. Membrane fusion. Cell 112:519-533. [DOI] [PubMed] [Google Scholar]
- 26.Jardetzky, T. S., and R. A. Lamb. 2004. Virology: a class act. Nature 427:307-308. [DOI] [PubMed] [Google Scholar]
- 27.Kielian, M. 2002. Structural surprises from the flaviviruses and alphaviruses. Mol. Cell 9:454-456. [DOI] [PubMed] [Google Scholar]
- 28.Kielian, M., P. K. Chatterjee, D. L. Gibbons, and Y. E. Lu. 2000. Specific roles for lipids in virus fusion and exit. Examples from the alphaviruses. Subcell. Biochem. 34:409-455. [DOI] [PubMed] [Google Scholar]
- 29.Kozlovsky, Y., and M. M. Kozlov. 2002. Stalk model of membrane fusion: solution of energy crisis. Biophys. J. 82:882-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lescar, J., A. Roussel, M. W. Wien, J. Navaza, S. D. Fuller, G. Wengler, G. Wengler, and F. A. Rey. 2001. The fusion glycoprotein shell of Semliki Forest virus. An icosahedral assembly primed for fusogenic activation at endosomal pH. Cell 105:137-148. [DOI] [PubMed] [Google Scholar]
- 31.Markin, V. S., and J. P. Albanesi. 2002. Membrane fusion: stalk model revisited. Biophys. J. 82:693-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin, I., M. C. Dubois, T. Saermark, R. M. Epand, and J. M. Ruysschaert. 1993. Lysophosphatidylcholine mediates the mode of insertion of the NH2-terminal SIV fusion peptide into the lipid bilayer. FEBS Lett. 333:325-330. [DOI] [PubMed] [Google Scholar]
- 33.Martin, I., and J. M. Ruysschaert. 1995. Lysophosphatidylcholine inhibits vesicles fusion induced by the NH2-terminal extremity of SIV/HIV fusogenic proteins. Biochim. Biophys. Acta 1240:95-100. [DOI] [PubMed] [Google Scholar]
- 34.May, S. 2002. Structure and energy of fusion stalks: the role of membrane edges. Biophys. J. 83:2969-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2004. Structure of the dengue virus envelope protein after membrane fusion. Nature 427:313-319. [DOI] [PubMed] [Google Scholar]
- 36.Nieva, J. L., R. Bron, J. Corver, and J. Wilschut. 1994. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO J. 13:2797-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pecheur, E. I., I. Martin, A. Bienvenue, J. M. Ruysschaert, and D. Hoekstra. 2000. Protein-induced fusion can be modulated by target membrane lipids through a structural switch at the level of the fusion peptide. J. Biol. Chem. 275:3936-3942. [DOI] [PubMed] [Google Scholar]
- 38.Phalen, T., and M. Kielian. 1991. Cholesterol is required for infection by Semliki Forest virus. J. Cell Biol. 112:615-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rafalski, M., J. D. Lear, and W. F. DeGrado. 1990. Phospholipid interactions of synthetic peptides representing the N-terminus of HIV gp41. Biochemistry 29:7917-7922. [DOI] [PubMed] [Google Scholar]
- 40.Rey, F. A., F. X. Heinz, C. Mandl, C. Kunz, and S. C. Harrison. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 375:291-298. [DOI] [PubMed] [Google Scholar]
- 41.Siegel, D. P. 1993. Energetics of intermediates in membrane fusion: comparison of stalk and inverted micellar intermediate mechanisms. Biophys. J. 65:2124-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegel, D. P. 1999. The modified stalk mechanism of lamellar/inverted phase transitions and its implications for membrane fusion. Biophys. J. 76:291-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smit, J. M., R. Bittman, and J. Wilschut. 1999. Low-pH-dependent fusion of Sindbis virus with receptor-free cholesterol- and sphingolipid-containing liposomes. J. Virol. 73:8476-8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stegmann, T., S. Nir, and J. Wilschut. 1989. Membrane fusion activity of influenza virus. Effects of gangliosides and negatively charged phospholipids in target liposomes. Biochemistry 28:1698-1704. [DOI] [PubMed] [Google Scholar]
- 45.Stiasny, K., S. L. Allison, C. W. Mandl, and F. X. Heinz. 2001. Role of metastability and acidic pH in membrane fusion by tick-borne encephalitis virus. J. Virol. 75:7392-7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stiasny, K., S. L. Allison, J. Schalich, and F. X. Heinz. 2002. Membrane interactions of the tick-borne encephalitis virus fusion protein E at low pH. J. Virol. 76:3784-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stiasny, K., S. Bressanelli, J. Lepault, F. A. Rey, and F. X. Heinz. 2004. Characterization of a membrane-associated trimeric low-pH-induced form of the class II viral fusion protein from tick-borne encephalitis virus and its crystallization. J. Virol. 78:3178-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stiasny, K., C. Koessl, and F. X. Heinz. 2003. Involvement of lipids in different steps of the flavivirus fusion mechanism. J. Virol. 77:7856-7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamm, L. K., J. Crane, and V. Kiessling. 2003. Membrane fusion: a structural perspective on the interplay of lipids and proteins. Curr. Opin. Struct. Biol. 13:453-466. [DOI] [PubMed] [Google Scholar]
- 50.Vogel, S. S., E. A. Leikina, and L. V. Chernomordik. 1993. Lysophosphatidylcholine reversibly arrests exocytosis and viral fusion at a stage between triggering and membrane merger. J. Biol. Chem. 268:25764-25768. [PubMed] [Google Scholar]
- 51.Weissenhorn, W., A. Dessen, L. J. Calder, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1999. Structural basis for membrane fusion by enveloped viruses. Mol. Membr. Biol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 52.Zitzer, A., R. Bittman, C. A. Verbicky, R. K. Erukulla, S. Bhakdi, S. Weis, A. Valeva, and M. Palmer. 2001. Coupling of cholesterol and cone-shaped lipids in bilayers augments membrane permeabilization by the cholesterol-specific toxins streptolysin O and Vibrio cholerae cytolysin. J. Biol. Chem. 276:14628-14633. [DOI] [PubMed] [Google Scholar]