A MATE transporter is required and sufficient for export of hydroxycinnamic acid amides, which act as antimicrobial compounds on the plant surface.
Abstract
The ability of Arabidopsis thaliana to successfully prevent colonization by Phytophthora infestans, the causal agent of late blight disease of potato (Solanum tuberosum), depends on multilayered defense responses. To address the role of surface-localized secondary metabolites for entry control, droplets of a P. infestans zoospore suspension, incubated on Arabidopsis leaves, were subjected to untargeted metabolite profiling. The hydroxycinnamic acid amide coumaroylagmatine was among the metabolites secreted into the inoculum. In vitro assays revealed an inhibitory activity of coumaroylagmatine on P. infestans spore germination. Mutant analyses suggested a requirement of the p-coumaroyl-CoA:agmatine N4-p-coumaroyl transferase ACT for the biosynthesis and of the MATE transporter DTX18 for the extracellular accumulation of coumaroylagmatine. The host plant potato is not able to efficiently secrete coumaroylagmatine. This inability is overcome in transgenic potato plants expressing the two Arabidopsis genes ACT and DTX18. These plants secrete agmatine and putrescine conjugates to high levels, indicating that DTX18 is a hydroxycinnamic acid amide transporter with a distinct specificity. The export of hydroxycinnamic acid amides correlates with a decreased ability of P. infestans spores to germinate, suggesting a contribution of secreted antimicrobial compounds to pathogen defense at the leaf surface.
INTRODUCTION
Arabidopsis thaliana is not a host plant for Phytophthora infestans, the causal agent of late blight disease of Solanum tuberosum (Kamoun, 2001). This nonhost resistance depends on efficient defense mechanisms at the cell periphery as well as on postinvasion defense responses (Lipka et al., 2005; Westphal et al., 2008; Kopischke et al., 2013; Geissler et al., 2015). An extracellular chemical defense mechanism, identified in genetic screens, involves the myrosinase PEN2, which metabolizes indole glucosinolates to release tryptophan-derived antimicrobial metabolites. These are postulated to be transported out of the cell by the ABC transporter PEN3 to the site of attempted penetration (Bednarek, 2012). Compromised PEN2 or PEN3 functions result in enhanced penetration success of the oomycete (Lipka et al., 2005; Kobae et al., 2006; Stein et al., 2006), suggesting that the export of specific indole derivatives is required for the first layer of nonhost resistance.
Hydroxycinnamic acid amides (HCAAs) are major phytoalexins in leaves of potato, a host plant of P. infestans (Keller et al., 1996; Schmidt et al., 1998). Both infection with the oomycete and treatment with elicitors lead to the accumulation of HCAAs such as coumaroyltyramine and feruloyltyramine (Schmidt et al., 1998). These metabolites are assumed to act as antimicrobial and as cell wall reinforcing compounds. The potato enzyme catalyzing the biosynthesis of coumaroyltyramine is a member of the General Control Non-Repressed Protein5-related N-acyl transferase superfamily (Schmidt et al., 1999). By contrast, the Arabidopsis enzyme catalyzing the biosynthesis of coumaroylagmatine, p-coumaroyl-CoA:agmatine N4-p-coumaroyl transferase (ACT), belongs to the class of BAHD enzymes, which was named after the first characterized enzymes of this family, benzylalcohol O-acetyltransferase, anthocyanin O-hydroxycinnamoyltransferase, N-hydroxycinnamoyl/benzoyltransferase, and deacetylvindoline 4-O-acetyltransferase (D’Auria, 2006; Muroi et al., 2009). Thus, sequence-unrelated enzymes are present in Arabidopsis and potato, which display similar enzyme activities.
Despite detailed studies on the biosynthesis of secondary metabolites, less is known about their subcellular localization and transport within and out of the cell. Multidrug and toxin extrusion (MATE) transporters are membrane-localized proteins that transport metabolites, toxins, or xenobiotics across membranes, presumably in exchange for H+ or Na+ ions. There are 58 MATE transporter genes in Arabidopsis, only a few of which have been functionally characterized. The MATE transporter Enhanced Disease Susceptibility 5 is required for the accumulation of salicylic acid, an important signaling compound in the pathogen defense response of plants (Nawrath et al., 2002). The transporter is localized at the chloroplast envelope and transports salicylic acid from the chloroplast to the cytosol (Serrano et al., 2013; Yamasaki et al., 2013). The Arabidopsis MATE transporter Activated Disease Susceptibility 1 acts as a negative regulator of plant disease resistance (Sun et al., 2011). In addition, other plant MATE transporters have been shown to transport secondary metabolites, such as the tonoplast-localized anthoMATE1 of grapevine (Vitis vinifera), which transports acylated anthocyanins into the vacuole (Gomez et al., 2009), or MATE2, an essential transporter for proanthocyanidin biosynthesis in Medicago truncatula (Zhao et al., 2011).
In this study, the nonhost interaction of Arabidopsis and P. infestans was characterized at the metabolite and transcript level. Metabolite profiling identified the antimicrobial compound coumaroylagmatine, whose biosynthesis is catalyzed by ACT, as the major secreted hydroxycinnamic acid amide. Functional analyses showed that the MATE transporter DETOXIFICATION18 (DTX18) is required for export of coumaroylagmatine to the plant surface, suggesting that biosynthesis and secretion of coumaroylagmatine is part of the chemical defense activated in the nonhost resistance response of Arabidopsis against P. infestans. Gain of function experiments in potato show that DTX18 mediates export of specific HCAAs, leading to an enhanced capacity to inhibit P. infestans spore germination.
RESULTS
Coumaroylagmatine Accumulates Extracellularly in Response to Inoculation by P. infestans in Arabidopsis, but Not in Potato
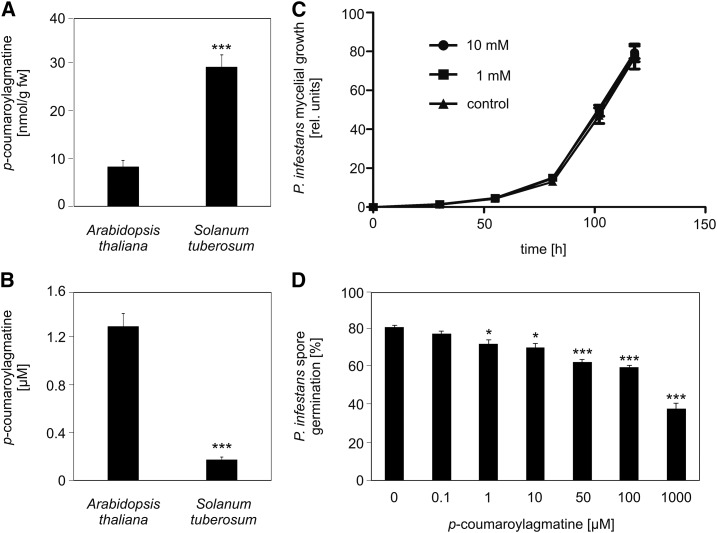
To determine the extent of extracellular chemical defense in the nonhost resistance response of Arabidopsis to P. infestans infection, untargeted metabolite profiling using UPLC-electrospray ionization-quadrupole time-of-flight-mass spectrometry (UPLC-ESI-QTOF-MS) was performed (Böttcher et al., 2008). Arabidopsis plants (Col-0) were drop-inoculated with a P. infestans zoospore solution. The droplets and the inoculated leaves were collected separately 1 d after inoculation and subjected to metabolite analysis. In both inoculated leaves and droplets of the inoculum, the HCAA coumaroylagmatine was detectable (Figures 1A and 1B). Interestingly, P. infestans-infected potato leaves contained significantly higher levels of coumaroylagmatine compared with Arabidopsis; however, in droplets isolated from potato plants, only very low levels of coumaroylagmatine were detectable (Figures 1A and 1B). This observation suggests that both host and nonhost plants accumulate coumaroylagmatine intracellularly but that only Arabidopsis is able to export coumaroylagmatine to the leaf surface efficiently.
Figure 1.
Coumaroylagmatine Accumulates Extracellularly in Arabidopsis, But Not in Potato, and Inhibits P. infestans Spore Germination in Vitro.
(A) and (B) Coumaroylagmatine content of leaves (A) and in droplets of the inoculum (B) from Arabidopsis and potato plants 24 h after inoculation with P. infestans. Data shown were obtained in at least five independent experiments (Arabidopsis, n ≥ 107; potato, n = 56). Error bars represent se. Significance analysis of differences was performed by t test, ***P < 0.001. fw, fresh weight.
(C) Mycelial growth inhibition assays. Coumaroylagmatine was added to GFP-expressing P. infestans (isolate Cra208m2) mycelium at the indicated concentrations. GFP fluorescence was determined at the time points indicated (control, n = 5; 1 mM, n = 10; 10 mM, n = 9). Data shown are representative for at least three independent experiments.
(D) Spore germination inhibition assay. Coumaroylagmatine was added to a P. infestans zoospore suspension (105/mL−1) at the indicated concentrations. After 24 h, the samples were evaluated microscopically for spore germination. Data are derived from two independent experiments (n = 4). Significance analysis of differences was performed by t test, *P < 0.05 and ***P < 0.001.
Coumaroylagmatine Inhibits Cyst Germination but Not Mycelial Growth of P. infestans
The pathogen-induced accumulation of coumaroylagmatine in Arabidopsis leaves might indicate a role of this HCAA in defense against the oomycete. To address this, synthesized coumaroylagmatine was tested for antioomycete activity against P. infestans in an in vitro assay (Prost et al., 2005) that determines fluorescence of the GFP-expressing P. infestans isolate Cra208m2 as an indicator for oomycete biomass (Si-Ammour et al., 2003). Even at concentrations of 10 mM, coumaroylagmatine did not affect mycelial growth of P. infestans (Figure 1C). Since extracellular coumaroylagmatine might influence early stages of P. infestans infection prior to penetration, spore germination was analyzed microscopically in the presence and absence of coumaroylagmatine. Germination of P. infestans zoospores was inhibited in a dose-dependent manner, with 50% inhibition at a concentration of 1 mM coumaroylagmatine (Figure 1D). These observations suggest that only extracellular coumaroylagmatine might act as an antimicrobial compound against P. infestans, when spores encounter the compound during germination on the cell surface.
ACT Is Responsible for the P. infestans-Induced Accumulation of Coumaroylagmatine
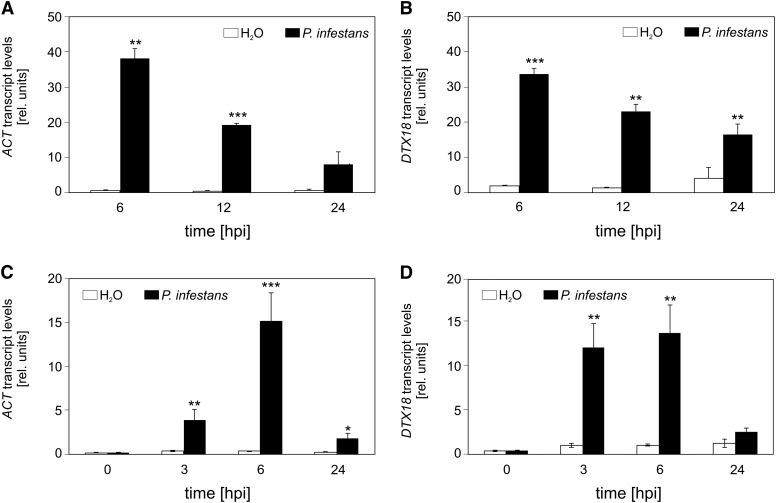
Microarray analyses with RNA from P. infestans-inoculated Col-0 plants (AtGenExpress; www.arabidopsis.org; www.genevestigator.com) were analyzed for genes that were activated in Arabidopsis specifically after inoculation with P. infestans, but not in the interaction with bacterial pathogens. One of these genes, At5g61160, encodes ACT (Muroi et al., 2009). The microarray data demonstrated that ACT transcript levels were more than 60-fold higher in P. infestans-inoculated leaves compared with those of water-treated plants (Figure 2A). The gene At3g23550, annotated to encode the MATE transporter DTX18 (Li et al., 2002), was coexpressed with the ACT gene (Figure 2B). More detailed qRT-PCR analyses revealed that ACT and DTX18 are expressed within 3 h after inoculation with P. infestans (Figures 2C and 2D).
Figure 2.
P. infestans-Induced Expression of ACT and DTX18 in Arabidopsis.
(A) and (B) Microarray analyses. ACT (A) and DTX18 (B) expression in response to inoculation with P. infestans (black bars) or water (white bars). Data were obtained from AtGenExpress (www.arabidopsis.org; n = 3). hpi, hours after inoculation.
(C) and (D) Time course of ACT (C) and DTX18 (D) expression in response to inoculation with P. infestans. qRT-PCR was performed with RNA isolated from P. infestans-inoculated Arabidopsis (black bars) or water-treated plants (white bars) at the time points indicated. At-PP2A transcript levels were used for standardization. Data are derived from three independent experiments (n > 5). Error bars represent se. Significance analysis of differences was performed by t test, *P < 0.05, **P < 0.01, and ***P < 0.001.
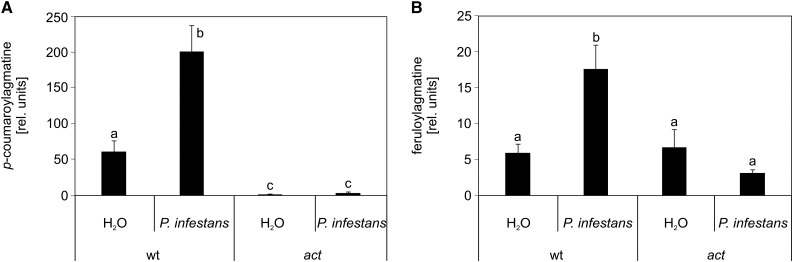
Leaves of homozygous plants of the act T-DNA insertion line SALK_097380 were inoculated with P. infestans and analyzed by untargeted metabolite profiling using UPLC-ESI-QTOF-MS (Böttcher et al., 2008). Under these conditions, coumaroylagmatine and feruloylagmatine were highly decreased in inoculated leaves (Figure 3). These compounds accumulated in wild-type plants in response to inoculation with P. infestans. In the act mutant, coumaroylagmatine levels were close to the detection limit in both water-treated and P. infestans-inoculated leaves (Figure 3A). Feruloylagmatine levels in the act mutant were similar to those in water-treated wild-type leaves, regardless of the treatment (Figure 3B). These results suggest that the P. infestans-induced accumulation of coumaroylagmatine and feruloylagmatine is mediated by ACT.
Figure 3.
Reduced Levels of HCAAs in the act Mutant.
Coumaroylagmatine (A) and feruloylagmatine (B) levels were determined by UPLC-ESI-QTOF-MS in methanolic extracts of leaves of wild-type and act lines 24 h after inoculation with P. infestans or water. Data are derived from four independent experiments (wild type, water, n = 27; P. infestans, n = 95; act, water, n = 12; P. infestans, n = 23). Error bars represent se. Letters indicate statistically different values (two-way ANOVA, P < 0.05).
To characterize ACT enzyme activity, the coding region of At5g61160 was cloned into the bacterial expression vector pDEST17. The recombinant protein was purified from extracts of isopropyl β-d-1-thiogalactopyranoside-treated bacteria via a HisTrap HP column (Supplemental Figure 1). Recombinantly expressed protein converted p-coumaroyl-CoA and feruloyl-CoA with agmatine as amine acceptor (Table 1). Feruloyl-CoA as donor showed better affinity but produced lower turnover velocity with agmatine.
Table 1. Enzymatic Parameters of Arabidopsis ACT Purified after Expression in Escherichia coli.
| Donor | Km (µM) | Vmax (nkat/mg) | Vmax/Km |
|---|---|---|---|
| p-coumaroyl-CoA | 1.13 ± 0.12 | 11.0 ± 0.29 | 9.7 |
| Feruloyl-CoA | 0.61 ± 0.01 | 4.9 ± 0.16 | 8.2 |
| Acceptora | |||
| Agmatine | 0.45 ± 0.16 | 6.06 ± 0.27 | 24.4 |
With p-coumaroyl-CoA as acyl donor.
The Coexpressed Gene At3g23550 Encodes a MATE Transporter
At3g23550 is highly coexpressed with ACT (Figure 2; www.genevestigator.com). The gene is annotated to encode the MATE transporter DTX18 (Li et al., 2002). The encoded protein of 469 amino acids is predicted to contain 12 transmembrane domains (http://www.enzim.hu/hmmtop/) and to be localized to the plasma membrane (http://aramemnon.botanik.uni-koeln.de/).
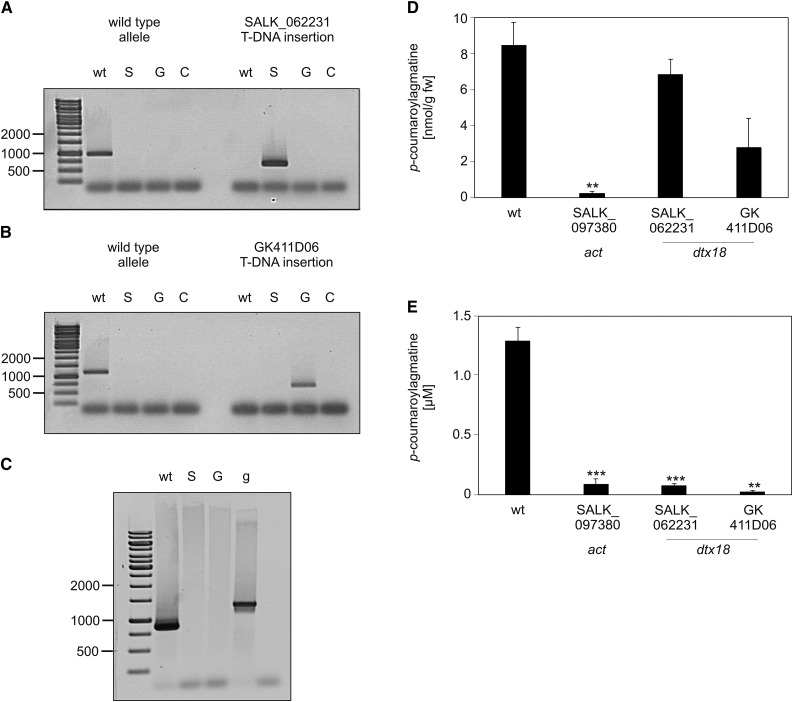
To address the function of DTX18, homozygous plants of the T-DNA insertion line SALK_062231 and of the GABI-KAT line GK411D06 (Kleinboelting et al., 2012) were obtained (Figures 4A and 4B). In contrast to wild-type plants, DTX18 transcripts were below the detection limit 6 h after inoculation with P. infestans (Figure 4C). Methanolic extracts of P. infestans-infected leaves contained similar levels of coumaroylagmatine as P. infestans-infected leaves of wild-type Arabidopsis plants (Figure 4D), suggesting that dtx18 lines retain the ability to biosynthesize coumaroylagmatine. However, coumaroylagmatine was absent in P. infestans droplets incubated on the leaf surface of both dtx18 mutant lines (Figure 4E). Thus, the disruption of the DTX18 gene resulted in an inability to secrete coumaroylagmatine. These results suggest that the MATE transporter encoded by At3g23550 is required for export of coumaroylagmatine.
Figure 4.
dtx18 Knockout Lines Are Unable to Secrete p-Coumaroylagmatine.
(A) and (B) Characterization of T-DNA insertion lines of DTX18. DNA was isolated from leaves of wild-type plants or the T-DNA insertion lines SALK_062231 and GK_411D06. PCR was performed using specific primers for the alleles from the wild type, SALK_062231 (S), or GK_411D06 (G). C, water control.
(C) Loss of DTX18 gene expression. RT-PCR using DTX18-specific primers was performed using RNA from leaves of wild-type plants and the T-DNA insertion lines SALK_062231 (S) and GK_411D06 (G). Genomic DNA (g) was used as a control.
(D) and (E) Reduced extracellular levels of coumaroylagmatine in dtx18 mutant lines. Coumaroylagmatine levels were determined in leaves (D) and in droplets of the inoculum (E) from Arabidopsis wild-type and mutant plants (SALK_062231 and GK_411D06) 24 h after inoculation with P. infestans. Data shown were obtained in at least three independent experiments (wild type, n ≥ 106; act, n = 21; SALK_062231, n = 47; GK_411D06, n = 8). Significance analysis of differences was performed by t test, *P < 0.05, **P < 0.01, and ***P < 0.001. fw, fresh weight.
DTX18 Expression in Potato Results in Extracellular Accumulation of HCAAs
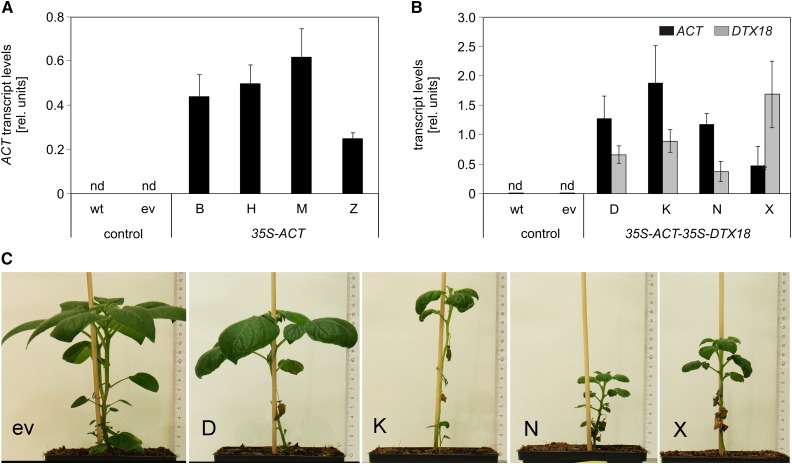
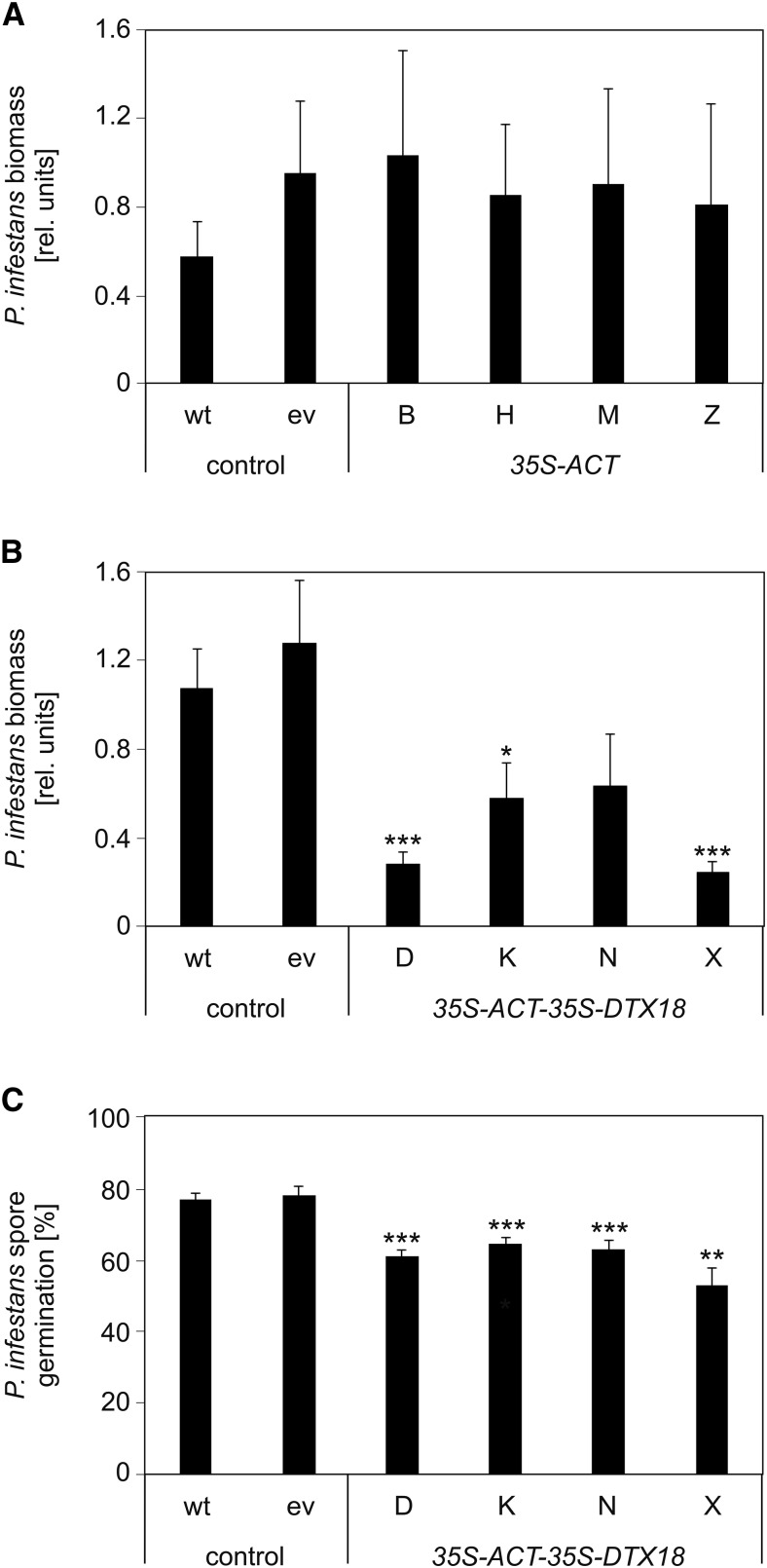
The major phytoalexins of potato are HCAAs such as coumaroyl- and feruloyltyramine, which are exported to the apoplast and incorporated into the cell wall upon pathogen infection (Keller et al., 1996; Schmidt et al., 1999). The low extracellular levels of coumaroylagmatine in potato in response to P. infestans infection (Figure 1B) might be due to a missing or inefficient export system for this compound. To assess whether DTX18 can confer the ability to secrete coumaroylagmatine to potato, transgenic plants were generated which expressed either ACT alone or both ACT and DTX18 under the control of the 35S promoter. Agrobacterium tumefaciens-mediated leaf disk transformation resulted in the regeneration of independently transformed plants, which were tested for the presence of the transgenes by DNA gel blot analyses. Expression of the transgenes was demonstrated by qRT-PCR for four individual lines each for ACT (B, H, M, and Z) and ACT-DTX18 transgenic plants (D, K, N, and X; Figures 5A and 5B). While ACT-expressing potato plants did not show morphological alterations, ACT-DTX18-expressing plants were smaller, had crinkled leaves, and developed spontaneous necroses at later development stages (Figure 5C).
Figure 5.
Expression of ACT and DTX18 in Transgenic Potato Plants.
(A) and (B) qRT-PCR analysis for ACT (black bars) or DTX18 expression (gray bars) was performed with RNA from leaves of wild-type and transgenic potato plants carrying an empty vector construct (ev) or expressing 35S-ACT (lines B, H, M, and Z) (A) or 35S-ACT-35S-DTX18 (lines D, K, N, and X) (B). Expression of EF1a was used as a reference. Data are derived from two independent experiments ([A]: wild type, n = 7; empty vector, n = 5; B, M, H, and Z, n = 3; [B]: wild type, n = 8; empty vector, n = 6; D, K, N, and X, n = 3). Error bars represent se. nd, not detectable.
(C) Phenotype of 35S-ACT-35S-DTX18 potato plants.
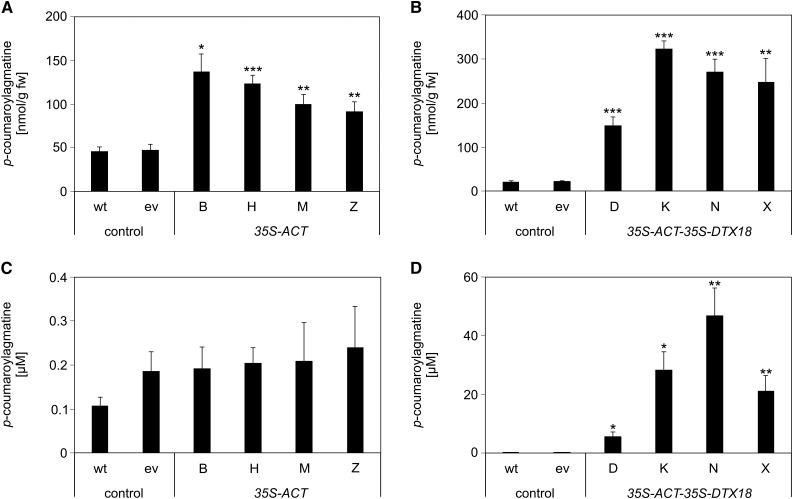
Metabolite analyses revealed enhanced levels of coumaroylagmatine in leaf disks of P. infestans inoculation sites in all lines, with highest levels detected in ACT-DTX18-expressing lines (Figures 6A and 6B). However, infection droplets on ACT-expressing plants contained very low levels of coumaroylagmatine, comparable to those of wild-type and empty vector plants (Figure 6C). Thus, despite significantly higher levels of coumaroylagmatine in the leaves, coumaroylagmatine was not secreted in the ACT-expressing lines. These results suggest that coumaroylagmatine levels are not the limiting factor for export in potato. By contrast, extracellular accumulation of coumaroylagmatine was observed for all four ACT-DTX18-expressing lines, reaching more than 40 µM for line N (Figure 6D). Compared with the extracellular coumaroylagmatine levels in Arabidopsis, roughly 25-fold higher levels were obtained in ACT-DTX18-expressing potato plants. These observations suggest that expression of DTX18 is required and sufficient for export of coumaroylagmatine.
Figure 6.
Characterization of ACT- and ACT-DTX18-Expressing Potato Plants.
Coumaroylagmatine levels were determined in leaves ([A] and [B]) and in droplets of the inoculum ([C] and [D]) from wild-type and transgenic potato plants carrying an empty vector construct (ev) or 35S-ACT (lines B, H, M, and Z) ([A] and [C]) or 35S-ACT-35S-DTX18 (lines D, K, N, and X) ([B] and [D]). Significance analysis of differences was performed by t test, *P < 0.05, **P < 0.01, and ***P < 0.001.
(A) Two independent experiments; wild type, empty vector, n = 10; B, n = 5; H, M, and Z, n = 6.
(B) Three independent experiments; wild type, empty vector, n = 18; D2, n = 7; K3, n = 6; N3, n = 7; X2, n = 8.
(C) Two independent experiments; wild type, empty vector, n = 10; lines B, H, M, and Z, n = 6.
(D) Three independent experiments; wild type, empty vector, n = 18; D2, n = 7; K3, n = 5; N3, n = 6; X2, n = 7.
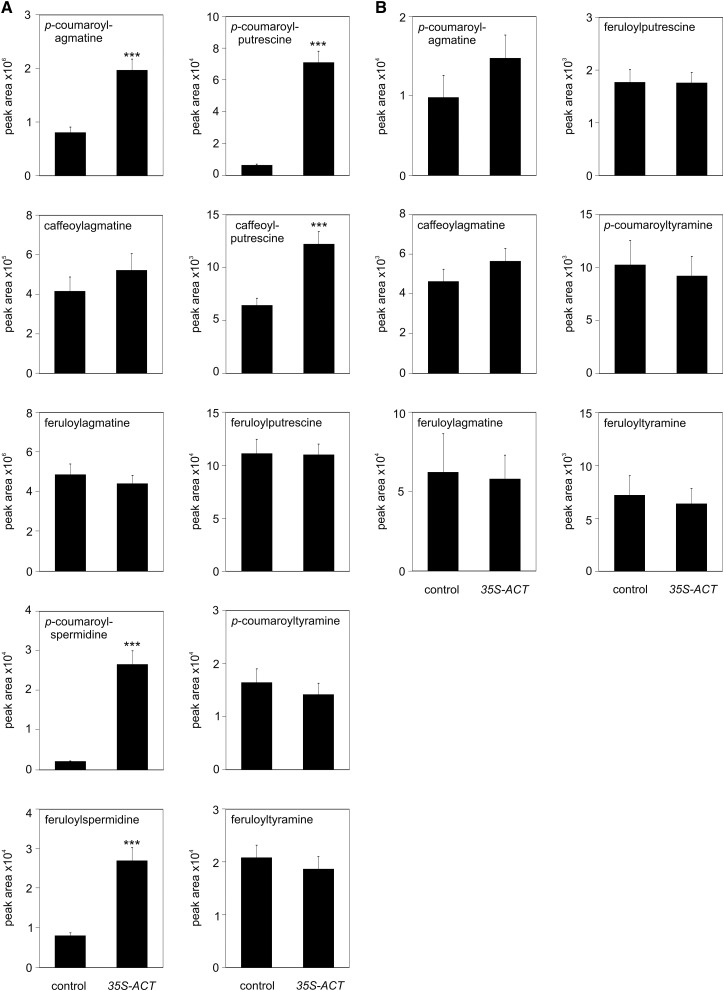
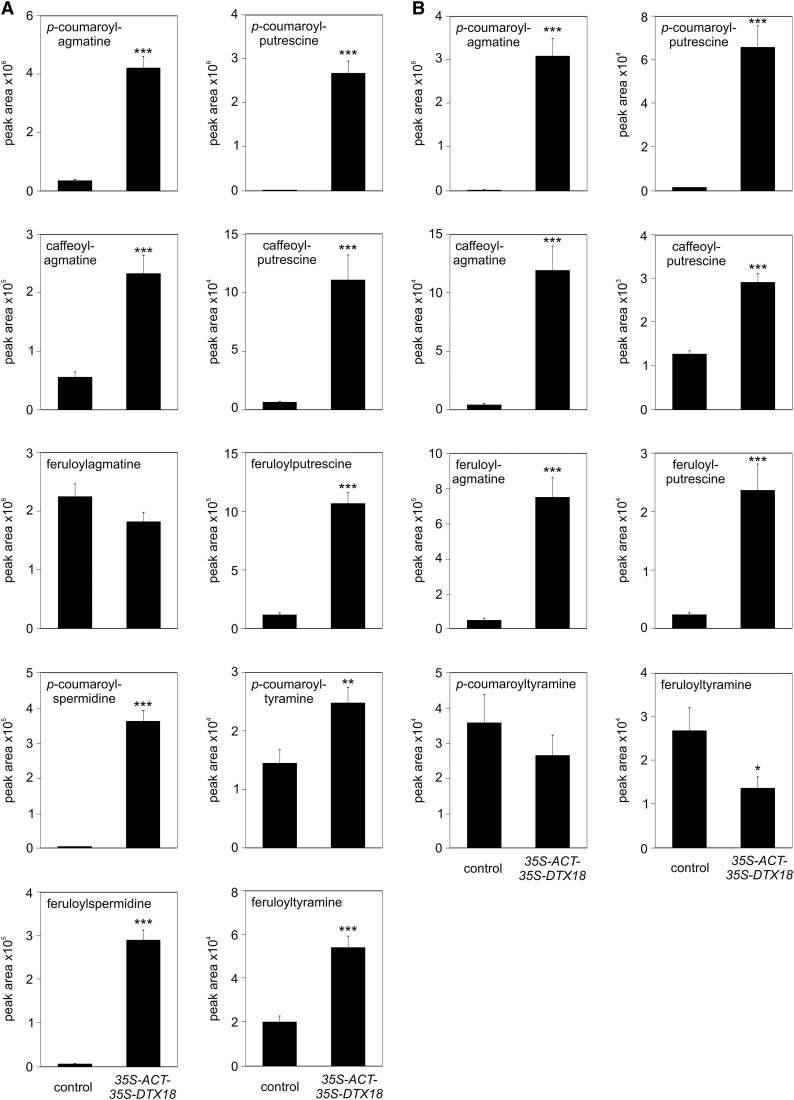
Untargeted metabolite profiling revealed additional differences in the metabolite pattern of control, ACT, and ACT-DTX18 plants in response to P. infestans infection. Among the differentially accumulating compounds (Supplemental Figure 2), other HCAAs were identified based on tandem mass spectrometry analyses (Supplemental Figure 3). In addition to coumaroylagmatine, ACT-expressing plants displayed more than 10-fold higher levels of coumaroylputrescine and coumaroylspermidine, as well as a 3-fold increase in feruloylspermidine and a 2-fold increase in caffeoylputrescine (Figure 7A). Despite the increased intracellular levels of these compounds, no changes in their extracellular abundance were observed (Figure 7B). Apparently, intracellular levels of these HCAAs are not limiting for their export. By contrast, when DTX18 was expressed in addition to ACT in potato, the intra- and extracellular HCAA patterns changed dramatically. Intracellular levels of coumaroylagmatine, coumaroylputrescine, caffeoylputrescine, as well as coumaroyl- and feruloylspermidine were 10-fold to more than 300-fold higher than in P. infestans-infected control plants (Figure 8A). Importantly, ACT-DTX18 plants secreted high amounts of feruloyl- and caffeoylagmatine, in addition to coumaroylagmatine, as well as the respective putrescine conjugates into the inoculum (Figure 8B). Interestingly, despite 100- and 50-fold enhanced intracellular levels of coumaroyl- and feruloylspermidine, respectively (Figure 8A), these compounds were not detectable in the inoculum. Moreover, the extracellular abundance of coumaroyl- and feruloyltyramine was not enhanced in ACT-DTX18-expressing plants (Figure 8B). These results indicate that the transporter protein DTX18 selectively exports putrescine- and agmatine-conjugated hydroxycinnamic acids, but no tyramine nor spermidine HCAA derivatives.
Figure 7.
Expression of ACT Leads to Alterations in HCAA Patterns in P. infestans-Infected Potato Leaves.
HCAA levels were determined in leaves (A) and in the inoculum (B) 24 h after infection of leaves of control (wild-type and empty vector-carrying plants) or 35S-ACT plants (lines B, H, M, and Z). Data are derived from three independent experiments ([A]: n ≥ 32, except for spermidine derivatives, n ≥ 15; [B]: n ≥ 32, except for feruloyltyramine, n ≥ 20, and caffeoylagmatine, n ≥ 8). Significance analysis of differences was performed by t test, *P < 0.05, **P < 0.01, and ***P < 0.001.
Figure 8.
Expression of ACT and DTX18 Leads to Extracellular Accumulation of HCAAs in P. infestans-Infected Potato Leaves.
HCAA levels were determined in leaves (A) and in the inoculum (B) 24 h after infection of leaves of control (wild-type and empty vector-carrying plants) or 35S-ACT-35S-DTX18-expressing plants (lines D, K, N, and X). Data are derived from three independent experiments ([A]: n ≥ 44; [B]: n ≥ 36, except for feruloyltyramine and caffeoylputrescine, n ≥ 24). Significance analysis of differences was performed by t test, *P < 0.05, **P < 0.01, ***P < 0.001.
Secretion of HCAAs in Potato Contributes to Defense against P. infestans
The efficient secretion of the HCAAs in ACT-DTX18 potato plants raised the question of whether these plants display enhanced resistance against P. infestans. Pathogen biomass was determined in infected leaves by qPCR with primers directed at highly repetitive sequences in the P. infestans genome (Judelson and Tooley, 2000). Significantly reduced growth of P. infestans compared with control plants was observed upon infection of three of the four ACT-DTX18-expressing potato lines, but not in any of the ACT-expressing plants (Figures 9A and 9B). Since the transgenic line with the highest level of extracellular coumaroylagmatine did not show significantly reduced pathogen biomass (Figure 9B) and since ACT-DTX18-expressing plants displayed major changes in morphology compared with control and ACT-expressing plants (Figure 5C), reduction of pathogen biomass might be caused by secondary effects.
Figure 9.
Defense of ACT and ACT-DTX18-Expressing Potato Plants against P. Infestans.
(A) and (B) Determination of pathogen biomass. Wild-type and transgenic potato plants carrying an empty vector construct (ev), 35S-ACT (lines B, H, M, and Z) (A), or 35S-ACT-35S-DTX18 (lines D, K, N, and X) (B) were inoculated with P. infestans. Pathogen biomass was determined 3 d after inoculation. Error bars represent se. Significance analysis of differences was performed by t test, *P < 0.05 and ***P < 0.001. P values for comparison of the ACT-DTX18-expressing lines: D-K, 0.098; D-N, 0.17; D-X, 0.57; K-N, 0.84; K-X, 0.06; N-X, 0.13.
(A) Three independent experiments; wild type, empty vector, n = 24; B, H, M, and Z, n = 12.
(B) Five independent experiments; wild type, n = 35; empty vector, n = 43; D, n = 17; K, n = 13; N, n = 10; X, n = 18.
(C) Spore germination inhibition assay. P. infestans inoculum incubated on wild-type and transgenic potato plants carrying an empty vector construct (ev) or expressing 35S-ACT-35S-DTX18 (lines D, K, N, and X) were collected after 24 h, sterile filtrated, and added to a zoospore suspension of P. infestans. Inhibitory activity was determined by microscopy analyses. Data were obtained in three independent experiments (n = 9). At least 1300 spores were evaluated for each line. Significance analysis of differences was performed by t test, *P < 0.05, **P < 0.01, ***P < 0.001.
Therefore, to assess the importance of secretion of HCAAs, the inoculation droplets were collected from transgenic and control plants after 24 h, sterile filtrated, and incubated with a zoospore suspension of P. infestans. Microscopy analyses of spore germination revealed a slight, but highly significant, inhibitory effect of droplets collected from all four transgenic ACT-DTX18-expressing lines compared with those from wild-type and empty vector plants (Figure 9C). These observations point to a contribution of DTX18-mediated secretion of HCAAs to defense against P. infestans.
DISCUSSION
This study has identified coumaroylagmatine as a secondary metabolite that accumulates extracellularly on Arabidopsis leaves in response to P. infestans inoculation. Loss- and gain-of-function experiments in Arabidopsis and potato, respectively, indicate that the MATE transporter DTX18 is required and sufficient for the export of the HCAA, coumaroylagmatine, to the leaf surface.
HCAAs are biosynthesized via the phenylpropanoid pathway and have been reported to act as antimicrobial compounds against bacteria, such as Staphylococcus aureus and Streptococcus pyogenes (Georgiev et al., 2013). Antifungal effects of different HCAAs were tested using Saccharomyces cerevisiae, Trichoderma beigelii, and Candida albicans (Lee et al., 2004), as well as mycorrhizal fungi (Grandmaison et al., 1993). A limited number of reports exists for the effect of HCAAs on phytopathogenic fungi, such as the cereal fungal pathogen Fusarium culmorum (Fattorusso et al., 1999), the causal agent of brown rot in stone fruit, Monilinia fructicola (Venis, 1969), and the Arabidopsis pathogen Alternaria brassicicola (Muroi et al., 2009). Interestingly, while coumaroylagmatine inhibited both spore germination and hyphal growth in A. brassicicola (Muroi et al., 2009), only spore germination was affected for P. infestans (Figures 1C and 1D).
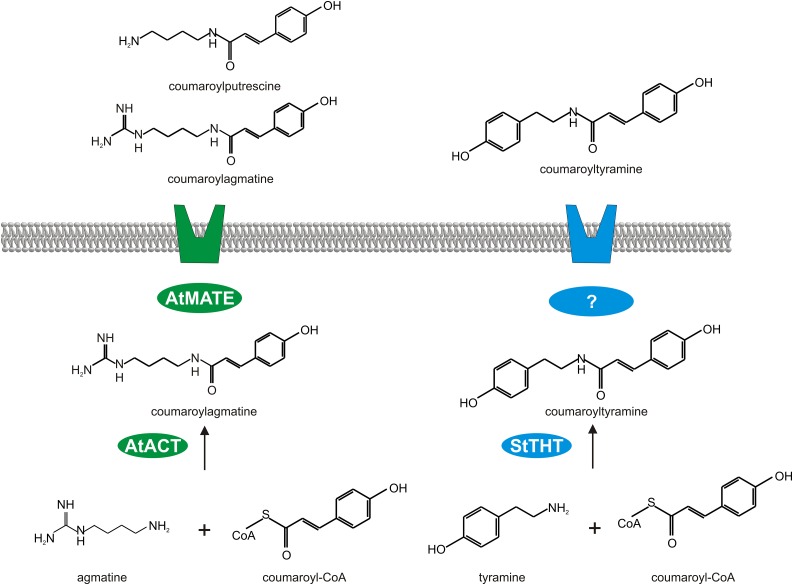
The finding that coumaroylagmatine inhibits spore germination, but not mycelial growth, of the oomycete P. infestans implies that an inhibitory effect of this compound requires its presence at early stages of the infection process and, thus, its occurrence on the plant surface. While this is the case for the nonhost plant Arabidopsis, potato is apparently not able to secrete coumaroylagmatine efficiently (Figure 1B). Other HCAAs, such as coumaroyl- and feruloyltyramine, accumulate extracellulary in elicitor-treated cultured potato cells (Schmidt et al., 1998), suggesting the existence of a functional export system for these HCAAs in potato. Apparently, coumaroylagmatine cannot be transported by this system, not even if intracellular levels are increased (Figures 6A and 6C). This suggests that the existing HCAA export system in potato has a distinct specificity for HCAAs containing tyramine but not agmatine (Figure 10).
Figure 10.
Hypothetical Model of DTX18-Mediated Coumaroylagmatine Secretion.
Potato responds to infection by P. infestans with the biosynthesis of coumaroyl- and feruloyltyramine by THT (Schmidt et al., 1998) and their export by an unknown transport system. The extracellular accumulation of novel HCAAs in transgenic potato plants expressing DTX18 from Arabidopsis, predicted to encode a plasma membrane-localized MATE transporter, suggests that DTX18 is able to export coumaroyl, caffeoyl, and feruloyl conjugates of both agmatine and putrescine.
Our results strongly suggest that DTX18 is a transporter for coumaroylagmatine. First, loss-of-function experiments revealed no extracellular accumulation of coumaroylagmatine in two independent Arabidopsis dtx18 mutants, despite wild-type levels of coumaroylagmatine in leaf extracts (Figures 4D and 4E). Second, the inability of potato plants to efficiently export coumaroylagmatine is overcome by expression of DTX18 in transgenic plants (Figure 6D). The extracellular coumaroylagmatine levels obtained in plants expressing both ACT and DTX18 is more than 100-fold higher than those in transgenic lines expressing only ACT, and, interestingly, even higher than those in Arabidopsis (Figure 1B).
Due to the different HCAA repertoire of potato, additional potential substrates of DTX18 could be postulated. Untargeted metabolite profiling of ACT-DTX18-expressing plants revealed high levels of extracellular coumaroyl, feruloyl, and caffeoyl conjugates of agmatine and putrescine. By contrast, tyramine and spermidine conjugates apparently were not exported by DTX18, since their extracellular levels were not altered or not detectable in DTX-overexpressing plants, although higher intracellular abundances were observed. Therefore, it was possible to assign a specificity to DTX18 as a HCAA transporter of agmatine and putrescine conjugates. Alternatively, the increased export of coumaroylagmatine by DTX18 might induce other metabolic mechanisms like the activation of an endogenous transporter for putrescine conjugates. Since putrescine conjugates of hydroxycinnamic acids were not detectable in Arabidopsis, analysis of the dtx18 mutant cannot clarify this issue. Similarly, whether DTX18 is able to transport sinapoyl derivatives cannot be addressed here, since these compounds were not detectable in leaf extracts or the inoculum droplets.
Both the expression of the biosynthetic enzyme and the transporter resulted in major alterations in intracellular HCAA levels. Consistent with ACT’s ability to take not only agmatine but also putrescine as an acceptor (Muroi et al., 2009), ACT-expressing potato plants contained higher levels of coumaroyl- and caffeoylputrescine. The enhanced presence of spermidine conjugates, moreover, suggests that ACT is also able to use this amine as an acceptor. By contrast, the expression of a maize (Zea mays) putrescine hydroxycinnamoyl transferase in rice (Oryza sativa; Wen et al., 2014) led to increased levels of agmatine and putrescine conjugates, as observed in ACT-expressing potato plants, but unchanged or even decreased amounts of spermidine conjugates.
Even more drastic changes in intracellular HCAA levels were observed in ACT-DTX18-expressing potato plants. Compared with ACT-expressing plants, nearly all analyzed HCAAs were present at higher levels in ACT-DTX18 lines. The selective removal of specific HCAAs from the intracellular metabolic system apparently leads to enhanced biosynthesis and accumulation of HCAAs in the cell. Most strikingly, coumaroyl- and feruloylspermidine levels in ACT-DTX18-expressing plants are 100-fold higher than in control plants, but this does not correlate with any export of these compounds. Feedback mechanisms might occur as a response to the depletion of agmatine and putrescine conjugates, resulting in the accumulation of spermidine derivatives, which are biosynthesized by either ACT or a yet unidentified enzyme.
Interestingly, the expression of the transporter, but not of the biosynthetic enzyme, leads to major morphological alterations in transgenic plants, since the phenotype of ACT-expressing plants does not differ from that of wild-type plants. Possibly, the export of HCAAs from the cell leads to a depletion of metabolites of the phenylpropanoid and other pathways, which are important for cell growth and development. Moreover, since HCAAs are incorporated into cell walls (Schmidt et al., 1998; Burhenne et al., 2003), distinct mechanical cell wall properties and expansion flexibility might be affected, which could explain the smaller leaves of the transgenic plants. Alternatively, secondary effects such as membrane destabilization due to high DTX18 expression might be responsible for the alterations in plant morphology. Developmental defects have also been observed in Arabidopsis upon overexpression of other MATE transporters, such as Os-MATE1, Os-MATE2 (Tiwari et al., 2014), and ZRZ (Burko et al., 2011), indicating the potential importance of proper localization of secondary metabolites.
The secretion of antimicrobial secondary metabolites is considered to be of importance for control of pathogen entry. In Arabidopsis, currently unidentified indole derivatives, produced by the degradation of indole glucosinolates via the PEN2 pathway (Bednarek, 2012), are believed to be transported out of the cell by the ABC transporter PEN3. Lack of transport results in enhanced penetration rates of several filamentous pathogens, implying that the occurrence of these indolic compounds in the apoplast contributes to penetration resistance (Stein et al., 2006). Similarly, the Nicotiana plumbaginifolia ABC transporter Np-PDR1 has been suggested to be responsible for the secretion of the antifungal terpenoid sclareol (Jasiński et al., 2001). Transgenic plants with reduced Np-PDR1 expression displayed an enhanced susceptibility to infection by Botrytis cinerea (Stukkens et al., 2005). In our study, we correlate the secretion of the antimicrobial compound coumaroylagmatine to significantly reduced growth of P. infestans in three out of four transgenic potato lines expressing Arabidopsis ACT and DTX18 (Figure 9B). Moreover, all four lines secreted coumaroylagmatine to levels that were inhibitory to P. infestans spore germination in vitro (Figure 9C). These results suggest the importance of secreted secondary metabolites for pathogen entry control by inhibiting early pathogen development.
METHODS
Plant Growth Conditions and Infection Experiments
The Arabidopsis thaliana T-DNA insertion mutants SALK_097380, SALK_062231, and GK411D06 were obtained from NASC (Alonso et al., 2003; Kleinboelting et al., 2012). Arabidopsis plants were grown on steam-sterilized soil in a mix with vermiculite (3:1) in a phytochamber with 8 h light (∼150 µE m−2 s−1) at 22°C and 16 h dark at 20°C. Plants were inoculated by placing 10 μL droplets of a Phytophthora infestans zoospore suspension (5 × 105 mL−1) onto the adaxial side of the leaves. Subsequently, plants were placed in a phytochamber with 16 h light (∼150 µE m−2 s−1, metal halide lamp HQI-T; Osram) at 20°C and 8 h dark at 18°C. Potato plants (Solanum tuberosum cv Désirée) derived from sterile explants were grown in soil in a phytochamber with 16 h of light (140 µE) at 20°C and 60% humidity for 4 weeks. Plants were inoculated by placing 10-μL droplets of a P. infestans zoospore suspension (105 mL−1) onto the abaxial side of the leaves. Leaves were subsequently covered with plastic bags to ensure high humidity.
P. infestans Inhibition Assays
Spore germination and mycelial growth inhibition were assayed as described (Prost et al., 2005; Eschen-Lippold et al., 2009). Spore germination inhibition assays were performed at 15°C.
Expression Analyses
RNA was isolated from potato plants or P. infestans-inoculated Arabidopsis plants as described previously (Halim et al., 2004). cDNA was synthesized after DNase I digestion (RNase-free DNase Set; Qiagen) using RevertAid H Minus first-strand cDNA synthesis kit (Thermo Fischer Scientific) according to the manufacturer’s instructions. Quantitative PCR was performed with Maxima Probe qPCR MasterMix (Thermo Fischer Scientific) and analyzed with an Mx3000P qPCR system (Agilent). The following probes and primers were used for amplification: Roche Universal Probe Library probe #131 (5′-ACCACCAG-3′) for ACT (At5g61160) with the primers 5′-CGCTGCTGATTTTAGGAACC-3′and 5′-CACAAGTCCCGAAGTATGTCAC-3′; Roche Universal Probe Library probe #15 (5′-GAGCAGGA-3′) for DTX18 (At3g23550) with the primers 5′-GGTAGCTATATGCGTCAACACG-3′and 5′-CCTTCTTTGCGCCTTTTACA-3′; Roche Universal Probe Library probe #29 (5′-GGCAGAAG-3′) for the housekeeping gene AtPP2A A3 (At1g13320) with the primers 5′-GACCGGAGCCAACTAGGAC-3′ and 5′-AAAACTTGGTAACTTTTCCAGCA-3′; and Roche Universal Probe Library probe #162 for potato EF1α with the primers 5′-CACTGCCCAGGTCATCATC-3′ and 5′-GTCGAGCACTGGTGCATATC-3′. Relative transcript levels were determined using MxPro qPCR software (Agilent).
Expression of ACT in Escherichia coli and Enzyme Activity Assays
The coding region of ACT was amplified from Col-0 genomic DNA using the primers 5′-CACCATGGCGTTAAAGGTGATCAAG-3′ and 5′-GACCGTACGTTTTCAGTTTCCAATC-3′, cloned into pENTR, and subsequently transferred to the vector pDEST17 by LR recombination (Life Technologies). Recombinant protein was produced after induction with isopropyl β-d-1-thiogalactoside and purified from extracts of pDEST17-ACT-carrying Rosetta gami cells (Novagen) via HisTrap HP columns (GE Healthcare) with the ÄKTA explorer 100 protein chromatography system (GE Healthcare) according to the manufacturer’s instructions. ACT activity assays and kinetic measurements were performed in a standard assay reaction mixture containing 10 mM Tris-HCl, pH 8.0, 0.1 mM EDTA, 5 mM substrate (acceptor), 2 µg purified recombinant enzyme, and 40 µM CoA donor. The assay was incubated for 30 min at 30°C in the dark and the reaction stopped by adding 52.5 μL 50% trichloroacetic acid. For each substrate concentration, three samples were analyzed in parallel. Substrate affinities (Km) and velocities (Vmax) were calculated using SigmaPlot 10 (Systat Software).
Liquid Chromatography-Mass Spectrometry Measurements
Arabidopsis and potato plants were inoculated with 30 droplets of 10 μL each of a P. infestans zoospore suspension (5 × 105 and 1 × 105 spores mL−1, respectively). After 24 h, droplets were removed, pooled, and immediately frozen in liquid nitrogen. Droplet samples were thawed and centrifuged for 10 min at 21,000g at 4°C and the supernatants were directly transferred to HPLC vials. Methanolic extracts were prepared from inoculated leaves of Arabidopsis or from potato leaf disks covering the inoculation sites as described (Böttcher et al., 2009).
Chromatographic separations were performed as described (Landgraf et al., 2014) with the following modifications: The binary gradient was applied at a flow rate of 150 µL/min for 0 to 1 min, isocratic 95% A (water/formic acid, 99.9/0.1 [v/v]), 5% B (acetonitrile/formic acid, 99.9/0.1 [v/v]); 1 to 5 min, linear from 5 to 25% B; 5 to 10 min, linear to 95% B; 10 to 12 min, isocratic 95% B; 12 to 15 min, isocratic 5% B.
Eluting compounds were detected from m/z 80 to 1000 using a micrOTOF-Q II hybrid quadrupole time-of-flight mass spectrometer (Bruker Daltonics) equipped with an Apollo II electrospray ion source in positive and negative ion mode using the following instrument settings: nebulizer gas, nitrogen, 1.4 bar; dry gas, nitrogen, 6 L min−1, 190°C; capillary, –5000 V; end plate offset, −500 V; funnel 1 RF, 200 V; funnel 2 RF, 200 V; in-source CID energy, 0 V; hexapole RF, 100 V; quadrupole ion energy, 5 eV; collision gas, nitrogen; collision energy, 7 eV; collision RF 150/350 V (timing 50/50); transfer time, 70 µs; prepulse storage, 5 µs; pulser frequency, 10 kHz; spectra rate, 3 Hz. Mass spectra were acquired in centroid mode. Mass calibration of individual raw data files was performed on lithium formate cluster ions obtained by automatic infusion of 20 μL 10 mM lithium hydroxide in isopropanol/water/formic acid, 49.9/49.9/0.2 (v/v/v) at a gradient time of 12 min using a diverter valve.
Raw data files were converted to mzData format using the vendor-specific software CompassXport (http://www.bdal.de/) and processed using the XCMS package (http://bioconductor.org/packages/release/bioc/html/xcms.html). XCMS settings for processing liquid chromatography-mass spectrometry data with findPeaks.centWave() were: prefilter = (3200); snthr = 5; ppm = 25; peak width = (5,12); scan range (50, 1020). For alignment group density function with parameters minfrac = 0.75 and bw = 5, mzwid = 0.05, max = 20 was used.
Identification of the compounds was based on manual interpretation of tandem mass spectra. For comparison with different mass spectra databases, the open source metabolite identification software MetFrag (http://msbi.ipb-halle.de/MetFrag/MetFragIPB.iface) was used. A synthetic standard of p-coumaroylagmatine was used for identification and quantification.
Generation of 35S-ACT and 35S-ACT-35S-DTX18 Transgenic Potato Plants
The coding regions of ACT and DTX18 (including the first intron) were cloned into the binary vector pICH52292 via Golden Gate Cloning (Weber et al., 2011). Potato plants were transformed and analyzed by DNA gel blots as described (Feltkamp et al., 1995; Schmidt et al., 1999).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: At5g61160 (ACT) and At3g23550 (DTX18).
Supplemental Data
Supplemental Figure 1. Purification of Recombinant ACT.
Supplemental Figure 2. HPLC Chromatograms of Leaves and Droplets of Control and ACT- and ACT-DTX18-Expressing Potato Plants.
Supplemental Figure 3. MS/MS of Hydroxyl Cinnamic Acid Derivatives.
Supplementary Material
Acknowledgments
We thank Thomas Vogt for the gift of hydroxycinnamoyl-CoAs and helpful discussions. We also thank Aline Jakobitz, Ben Meinen, and Annett Weichert for help with cloning and expression analyses and Dierk Scheel for continuous support. This work was funded by the IZN (Interdisciplinary Center for Crop Plant Research) and by the Deutsche Forschungsgemeinschaft (SPP1212 Plant Micro).
AUTHOR CONTRIBUTIONS
M.D., T.L., L.E.-L., E.B., and A.M. performed research. S.M., C.B., and K.G. analyzed data. B.D. and S.R. designed research. S.R. wrote the article.
Glossary
- HCAA
hydroxycinnamic acid amide
- UPLC-ESI-QTOF-MS
UPLC-electrospray ionization-quantitative time-of-flight-mass spectrometry
Footnotes
Articles can be viewed online without a subscription.
References
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. [DOI] [PubMed] [Google Scholar]
- Bednarek P. (2012). Sulfur-containing secondary metabolites from Arabidopsis thaliana and other Brassicaceae with function in plant immunity. ChemBioChem 13: 1846–1859. [DOI] [PubMed] [Google Scholar]
- Böttcher C., von Roepenack-Lahaye E., Schmidt J., Schmotz C., Neumann S., Scheel D., Clemens S. (2008). Metabolome analysis of biosynthetic mutants reveals a diversity of metabolic changes and allows identification of a large number of new compounds in Arabidopsis. Plant Physiol. 147: 2107–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher C., Westphal L., Schmotz C., Prade E., Scheel D., Glawischnig E. (2009). The multifunctional enzyme CYP71B15 (PHYTOALEXIN DEFICIENT3) converts cysteine-indole-3-acetonitrile to camalexin in the indole-3-acetonitrile metabolic network of Arabidopsis thaliana. Plant Cell 21: 1830–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhenne K., Kristensen B.K., Rasmussen S.K. (2003). A new class of N-hydroxycinnamoyltransferases. Purification, cloning, and expression of a barley agmatine coumaroyltransferase (EC 2.3.1.64). J. Biol. Chem. 278: 13919–13927. [DOI] [PubMed] [Google Scholar]
- Burko Y., Geva Y., Refael-Cohen A., Shleizer-Burko S., Shani E., Berger Y., Halon E., Chuck G., Moshelion M., Ori N. (2011). From organelle to organ: ZRIZI MATE-Type transporter is an organelle transporter that enhances organ initiation. Plant Cell Physiol. 52: 518–527. [DOI] [PubMed] [Google Scholar]
- D’Auria J.C. (2006). Acyltransferases in plants: a good time to be BAHD. Curr. Opin. Plant Biol. 9: 331–340. [DOI] [PubMed] [Google Scholar]
- Eschen-Lippold L., Draeger T., Teichert A., Wessjohann L., Westermann B., Rosahl S., Arnold N. (2009). Antioomycete activity of γ-oxocrotonate fatty acids against P. infestans. J. Agric. Food Chem. 57: 9607–9612. [DOI] [PubMed] [Google Scholar]
- Fattorusso E., Lanzotti V., Taglialatela-Scafati O. (1999). Antifungal N-feruloyl amides from roots of two Allium species. Plant Biosyst. 133: 199–203. [Google Scholar]
- Feltkamp D., Baumann E., Schmalenbach W., Masterson R., Rosahl S. (1995). Expression of the mannopine synthase promoter in roots is dependent on the mas elements and correlates with high transcript levels of mas-binding factor. Plant Sci. 109: 57–65. [Google Scholar]
- Geissler K., Eschen-Lippold L., Naumann K., Schneeberger K., Weigel D., Scheel D., Rosahl S., Westphal L. (2015). Mutations in the EDR1 gene alter the response of Arabidopsis thaliana to Phytophthora infestans and the bacterial PAMPs flg22 and elf18. Mol. Plant Microbe Interact. 28: 122–133. [DOI] [PubMed] [Google Scholar]
- Georgiev L., Chochkova M., Totseva I., Seizova K., Marinova E., Ivanova G., Ninova M., Najdenski H., Milkova T. (2013). Anti-tyrosinase, antioxidant and antimicrobial activities of hydroxycinnamoylamides. Med. Chem. Res. 22: 4173–4182. [Google Scholar]
- Gomez C., Terrier N., Torregrosa L., Vialet S., Fournier-Level A., Verriès C., Souquet J.M., Mazauric J.P., Klein M., Cheynier V., Ageorges A. (2009). Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters. Plant Physiol. 150: 402–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandmaison J., Olah G.M., Vancalsteren M.R., Furlan V. (1993). Characterization and localization of plant phenolics likely involved in the pathogen resistance expressed by endomycorrhizal roots. Mycorrhiza 3: 155–164. [Google Scholar]
- Halim V.A., Hunger A., Macioszek V., Landgraf P., Nürnberger T., Scheel D., Rosahl S. (2004). The oligopeptide elicitor Pep-13 induces salicylic acid-dependent and -independent defense reactions in potato. Physiol. Mol. Plant Pathol. 64: 311–318. [Google Scholar]
- Jasiński M., Stukkens Y., Degand H., Purnelle B., Marchand-Brynaert J., Boutry M. (2001). A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion. Plant Cell 13: 1095–1107. [PMC free article] [PubMed] [Google Scholar]
- Judelson H.S., Tooley P.W. (2000). Enhanced polymerase chain reaction methods for detecting and quantifying Phytophthora infestans in plants. Phytopathology 90: 1112–1119. [DOI] [PubMed] [Google Scholar]
- Kamoun S. (2001). Nonhost resistance to Phytophthora: novel prospects for a classical problem. Curr. Opin. Plant Biol. 4: 295–300. [DOI] [PubMed] [Google Scholar]
- Keller H., Hohlfeld H., Wray V., Hahlbrock K., Scheel D., Strack D. (1996). Changes in the accumulation of soluble and cell wall-bound phenolics in elicitor-treated cell suspension cultures and fungus-infected leaves of Solanum tuberosum. Phytochemistry 42: 389–396. [Google Scholar]
- Kleinboelting N., Huep G., Kloetgen A., Viehoever P., Weisshaar B. (2012). GABI-Kat SimpleSearch: new features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Res. 40: D1211–D1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobae Y., Sekino T., Yoshioka H., Nakagawa T., Martinoia E., Maeshima M. (2006). Loss of AtPDR8, a plasma membrane ABC transporter of Arabidopsis thaliana, causes hypersensitive cell death upon pathogen infection. Plant Cell Physiol. 47: 309–318. [DOI] [PubMed] [Google Scholar]
- Kopischke M., et al. (2013). Impaired sterol ester synthesis alters the response of Arabidopsis thaliana to Phytophthora infestans. Plant J. 73: 456–468. [DOI] [PubMed] [Google Scholar]
- Landgraf R., et al. (2014). The ABC transporter ABCG1 is required for suberin formation in potato tuber periderm. Plant Cell 26: 3403–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.G., Park Y., Kim M.R., Jung H.J., Seu Y.B., Hahm K.S., Woo E.R. (2004). Anti-fungal effects of phenolic amides isolated from the root bark of Lycium chinense. Biotechnol. Lett. 26: 1125–1130. [DOI] [PubMed] [Google Scholar]
- Li L., He Z., Pandey G.K., Tsuchiya T., Luan S. (2002). Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J. Biol. Chem. 277: 5360–5368. [DOI] [PubMed] [Google Scholar]
- Lipka V., et al. (2005). Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310: 1180–1183. [DOI] [PubMed] [Google Scholar]
- Muroi A., Ishihara A., Tanaka C., Ishizuka A., Takabayashi J., Miyoshi H., Nishioka T. (2009). Accumulation of hydroxycinnamic acid amides induced by pathogen infection and identification of agmatine coumaroyltransferase in Arabidopsis thaliana. Planta 230: 517–527. [DOI] [PubMed] [Google Scholar]
- Nawrath C., Heck S., Parinthawong N., Métraux J.P. (2002). EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost I., et al. (2005). Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol. 139: 1902–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Grimm R., Schmidt J., Scheel D., Strack D., Rosahl S. (1999). Cloning and expression of a potato cDNA encoding hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl)transferase. J. Biol. Chem. 274: 4273–4280. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Scheel D., Strack D. (1998). Elicitor-stimulated biosynthesis of hydroxycinnamoyltyramines in cell suspension cultures of Solanum tuberosum. Planta 205: 51–55. [Google Scholar]
- Serrano M., Wang B., Aryal B., Garcion C., Abou-Mansour E., Heck S., Geisler M., Mauch F., Nawrath C., Métraux J.P. (2013). Export of salicylic acid from the chloroplast requires the multidrug and toxin extrusion-like transporter EDS5. Plant Physiol. 162: 1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si-Ammour A., Mauch-Mani B., Mauch F. (2003). Quantification of induced resistance against Phytophthora species expressing GFP as a vital marker: beta-aminobutyric acid but not BTH protects potato and Arabidopsis from infection. Mol. Plant Pathol. 4: 237–248. [DOI] [PubMed] [Google Scholar]
- Stein M., Dittgen J., Sánchez-Rodríguez C., Hou B.H., Molina A., Schulze-Lefert P., Lipka V., Somerville S. (2006). Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 18: 731–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukkens Y., Bultreys A., Grec S., Trombik T., Vanham D., Boutry M. (2005). NpPDR1, a pleiotropic drug resistance-type ATP-binding cassette transporter from Nicotiana plumbaginifolia, plays a major role in plant pathogen defense. Plant Physiol. 139: 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Gilroy E.M., Chini A., Nurmberg P.L., Hein I., Lacomme C., Birch P.R., Hussain A., Yun B.W., Loake G.J. (2011). ADS1 encodes a MATE-transporter that negatively regulates plant disease resistance. New Phytol. 192: 471–482. [DOI] [PubMed] [Google Scholar]
- Tiwari M., Sharma D., Singh M., Tripathi R.D., Trivedi P.K. (2014). Expression of OsMATE1 and OsMATE2 alters development, stress responses and pathogen susceptibility in Arabidopsis. Sci. Rep. 4: 3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venis M.A. (1969). Similarities in action between streptomycin and hordatine an antifungal factor in barley. Phytochemistry 8: 1193. [Google Scholar]
- Weber E., Engler C., Gruetzner R., Werner S., Marillonnet S. (2011). A modular cloning system for standardized assembly of multigene constructs. PLoS One 6: e16765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W., Li D., Li X., Gao Y., Li W., Li H., Liu J., Liu H., Chen W., Luo J., Yan J. (2014). Metabolome-based genome-wide association study of maize kernel leads to novel biochemical insights. Nat. Commun. 5: 3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal L., Scheel D., Rosahl S. (2008). The coi1-16 mutant harbors a second site mutation rendering PEN2 nonfunctional. Plant Cell 20: 824–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K., Motomura Y., Yagi Y., Nomura H., Kikuchi S., Nakai M., Shiina T. (2013). Chloroplast envelope localization of EDS5, an essential factor for salicylic acid biosynthesis in Arabidopsis thaliana. Plant Signal. Behav. 8: e23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Huhman D., Shadle G., He X.Z., Sumner L.W., Tang Y., Dixon R.A. (2011). MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula. Plant Cell 23: 1536–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.