Abstract
Study Objectives:
To examine the association between markers of sleep-disordered breathing (SDB) and white matter hyperintensity (WMH) volume in an elderly, multiethnic, community-dwelling cohort.
Methods:
This is a cross-sectional analysis from the Washington Heights-Inwood Columbia Aging Project (WHICAP), a community-based epidemiological study of older adults. Structural magnetic resonance imaging was obtained starting in 2004; the Medical Outcomes Study-Sleep Scale (MOS-SS) was administered to participants starting in 2007. Linear regression models were used to assess the relationship between the two MOS-SS questions that measure respiratory dysfunction during sleep and quantified WMH volume among WHICAP participants with brain imaging.
Results:
A total of 483 older adults had both structural magnetic resonance imaging and sleep assessment. Self-reported SDB was associated with WMH. After adjusting for demographic and vascular risk factors, WMH volumes were larger in individuals with frequent snoring (β = 2.113, P = 0.004) and among those who reported waking short of breath or with headache (β = 1.862, P = 0.048).
Conclusions:
In community-dwelling older adults, self-reported measures of SDB are associated with larger WMH volumes. The cognitive effects of SDB that are increasingly being recognized may be mediated at the small vessel level.
Citation:
Rostanski SK, Zimmerman ME, Schupf N, Manly JJ, Westwood AJ, Brickman AM, Gu Y. Sleep disordered breathing and white matter hyperintensities in community-dwelling elders. SLEEP 2016;39(4):785–791.
Keywords: cerebrovascular disease, cognition, sleep disordered breathing
Significance.
White matter hyperintensities are a frequent manifestation of subclinical cerebrovascular disease and are linked to dementia. Our study confirms the association between sleep-disordered breathing and white matter hyperintensities in an elderly, community-dwelling cohort. These findings significantly enhance the generalizability of this relationship and may have implications for dementia prevention and treatment.
INTRODUCTION
Sleep disordered breathing (SDB) is a general term that refers to nocturnal apneas, hypopneas, and intermittent hypoxemia, most often due to obstructive sleep apnea (OSA). It is a condition that increases in frequency with advancing age. Estimates of the prevalence of SDB range from 2% to 4% in middle age to 7% to 44% in the elderly, although a prevalence as high as 60% has been reported in some community-dwelling elderly individuals.1,2 Although SDB has a well-established relationship with cardiovascular disease and stroke, the extent to which it is associated with subclinical cerebrovascular disease is less well understood.3,4
Subclinical cerebrovascular disease frequently manifests as white matter hyperintensities (WMH), areas of increased signal on T2-weighted magnetic resonance imaging (MRI) scans. In addition to their association with overt cerebrovascular disease, WMH are linked to cognitive aging and dementia5,6 and are hypothesized to be in the causal pathway to Alzheimer disease.7,8 The identification of reversible risk factors for WMH therefore has important public health implications. To date, few studies have examined the relationship between WMH and SDB.9–14 Those that have shown a positive association are limited in their generalizability because data were ascertained in racially and ethnically homogeneous cohorts9 or were limited to a sleep clinic population.13 We therefore evaluated the association between self-reported measures of SDB and WMH volume in an elderly, multiethnic, community-dwelling cohort using quantified WMH volumes. Our goal was to determine whether or not participants who endorsed symptoms of SDB would have larger WMH volumes.
METHODS
Study Population
This study was a cross-sectional analysis from the ongoing Washington Heights-Inwood Columbia Aging Project (WHICAP), a community-based longitudinal study of aging in older adults of northern Manhattan. Sampling and follow-up methods of the WHICAP study have been previously described in detail.15 Participants received in-home visits approximately every 18 to 24 months, which included physical and neurologic examinations and a detailed neuropsychological test battery. Dementia was diagnosed using standard criteria at a consensus conference of physicians and neuropsychologists with expertise in cognitive aging and dementia as previously described.15 Depressive symptoms were assessed with the 10-item version of the Center for Epidemiologic Studies-Depression (CES-D) scale. The conventional cutoff score of ≥ 4 was used to indicate the presence of depressive symptoms.16 Recruitment, informed consent, and study procedures were approved by the institutional review board of Columbia University Medical Center.
Imaging Acquisition and Processing
Beginning in 2004, high-resolution MRI scans were obtained from WHICAP participants who did not have dementia at the last follow-up visit. The derivation of this imaging cohort has been described elsewhere.17 Images were obtained on a Philips 1.5-Tesla scanner at Columbia University. T2-weighted fluid attenuated inversion recovery (FLAIR) images (repetition time [TR] = 11,000 ms, echo time [TE] = 144.0 ms, inversion time = 2800, field of view 25 cm, 2 nex, 256 × 192 matrix with 3-mm slice thickness) were obtained for quantification of WMH volume. WMH were derived from standard T2-weighted FLAIR images using an intensity-driven algorithm to provide quantitative measurements of WMH volume.18,19 Basal ganglia WMH are excluded in this processing stream. Intracranial volume was calculated from T1-weighted images using FreeSurfer (http://surfer.nmr.mgh.harvard.edu).
Sleep Quality Assessment
Sleep assessment using the Medical Outcomes Study – Sleep Scale (MOS-SS) was incorporated into standard WHICAP follow-up of the original cohort starting in 2007. The majority of eligible participants completed the sleep assessment (Figure 1). The MOS-SS is a self-report questionnaire that includes 10 Likert-type questions about different aspects of sleep, and two free-response questions that quantify sleep initiation and duration. Each Likert-type question has six responses indicating frequency of occurrence of the sleep related event (values range from 1 to 6 with higher value indicating lower frequency). The MOS-SS has been validated in several populations and has internal consistencies of 0.83 and 0.78 (Cronbach α) for various indices determined when the scale was being developed for use in the parent Medical Outcomes Study.20
Figure 1.
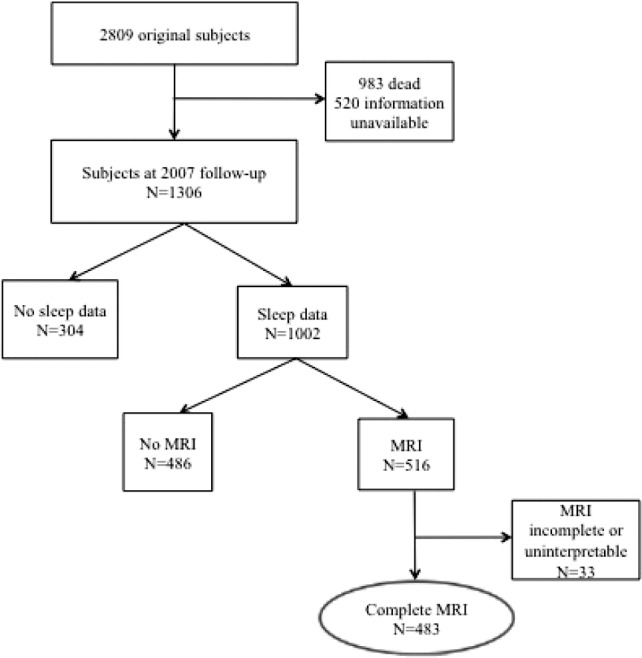
Schematic representation of derived sample with imaging and sleep information.
In our study, we used the two Likert-type MOS-SS questions that measure respiratory dysfunction during sleep: “how often do you snore during your sleep” and “how often do you awake short of breath or with headache”. These self-reported factors have been shown to have a high predictive accuracy for SDB and are incorporated into many SDB screening scales.21,22 We dichotomized the responses to these two questions into high and low frequency, taking into account the distribution of responses to each question in our study population. Specifically, individuals were considered to have a high likelihood of SDB if they reported 1 through 4 to “waking short of breath or with headache” and 1 through 3 to “snoring”. These dichotomized SDB variables were then used in the regression models as described below.
To further assess the likelihood of SDB, we calculated a “four variable” score for each subject following the work of Takagami et al. and Silva et al.23,24 This score assesses risk of SDB using a combination of factors such as sex, blood pressure (BP), body mass index (BMI) and self-reported snoring frequency. Sex was assigned a value of 4 for men and 0 for women; BMI in kg/m2 was assigned a value between 1 and 6 based on the following categories: < 21.0, 21.0–22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, ≥ 30; BP in mmHg was assigned a value between 1 and 4 based on the following categories: systolic BP [SBP] < 140 or diastolic BP [DBP] < 90, SBP 140–159 or DBP 90–99, SBP 160–179 or DBP 100–109, SBP ≥ 180 or DBP ≥ 110; and snoring was assigned a value of 4 if high frequency and 0 if low frequency, as determined by MOS-SS response. These variables were then combined in the following equation: (sex) + (BMI category value) + (BP category value) + (snoring), where the maximum score is 18. We calculated a score for each participant and dichotomized participants into “high likelihood” (score ≥ 11) and “low likelihood” (score < 11) of SDB. A cutoff of 11 had a sensitivity of 0.93 and specificity of 0.66 for moderate to severe SDB confirmed by polysomnography in the derivation cohort.23
Statistical Analysis
Baseline characteristics were compared between participants with and without MRI data. In the subsample of those with both sleep and MRI data, baseline characteristics of those with a high likelihood of SDB were compared to those without. Chi-square tests and analysis of variance were used to compare dichotomous and continuous variables, respectively, across groups.
Linear regression models were used to evaluate the relationship between WMH volume (as the outcome variable) and dichotomized responses to the two MOS-SS SDB questions and the four-variable score. A series of models was used to adjust for potential relevant variables. Model 1 adjusted only for intracranial volume (ICV); model 2 included ICV and demographic factors including race/ethnicity (white race as the reference), age (as a continuous variable), sex (male as the reference); model 3 included ICV, demographic factors, and history of hypertension (as a dichotomous variable, no hyper-tension as the reference) and apolipoprotein E (APOE) ε4 genotype (as a dichotomous variable, no ε4 alleles as the reference). This analysis was then repeated excluding participants who met criteria for dementia at the time of sleep data acquisition. A stratified analysis was conducted to evaluate whether the relationship between WMH and dichotomized responses to the two MOS-SS SDB questions and the four-variable score varied by race/ethnicity. As years of education were associated with snoring in univariate testing, we did a post hoc analysis to assess whether the association between snoring and WMH would remain after adjusting for years of education. All analyses were conducted with SPSS Statistics version 21.0 (IBM, Armonk, NY). All P values were based on two-sided tests with the significance level set at 0.05.
RESULTS
Missing Data Analysis
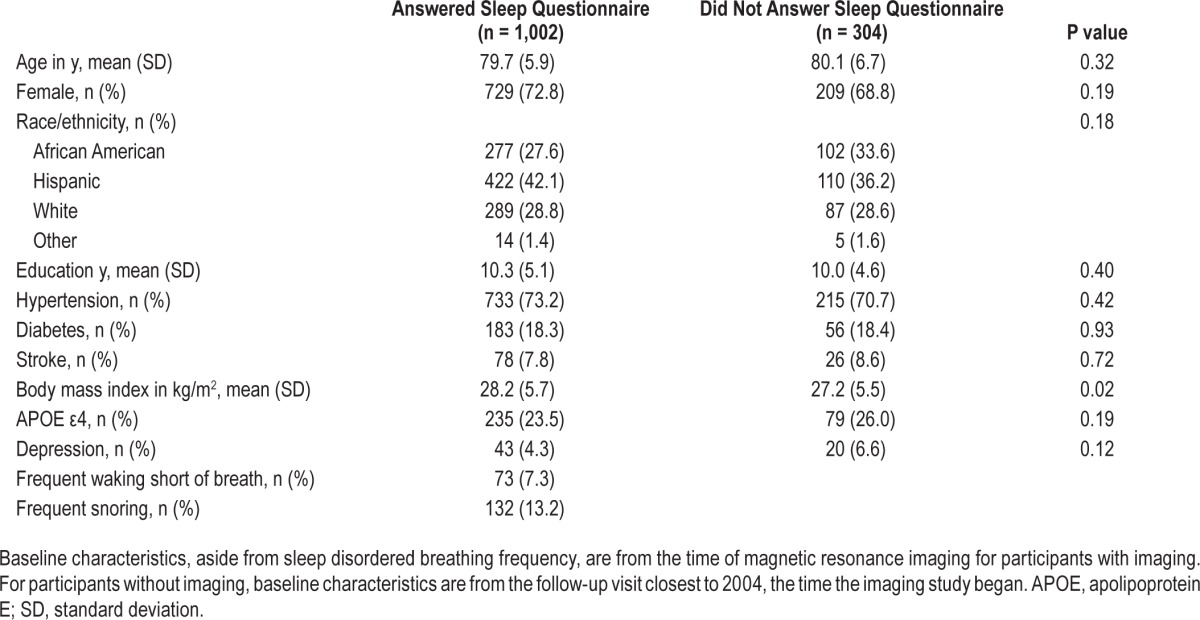
Of the 1,306 individuals eligible for follow-up in 2007 when the MOS-SS was introduced, 1,002 completed the questionnaire (Figure 1). Compared with those without sleep data (n = 304), those with sleep information had a higher mean BMI; other characteristics did not differ (Table 1).
Table 1.
Demographic and clinical characteristics of sleep-eligible subjects.
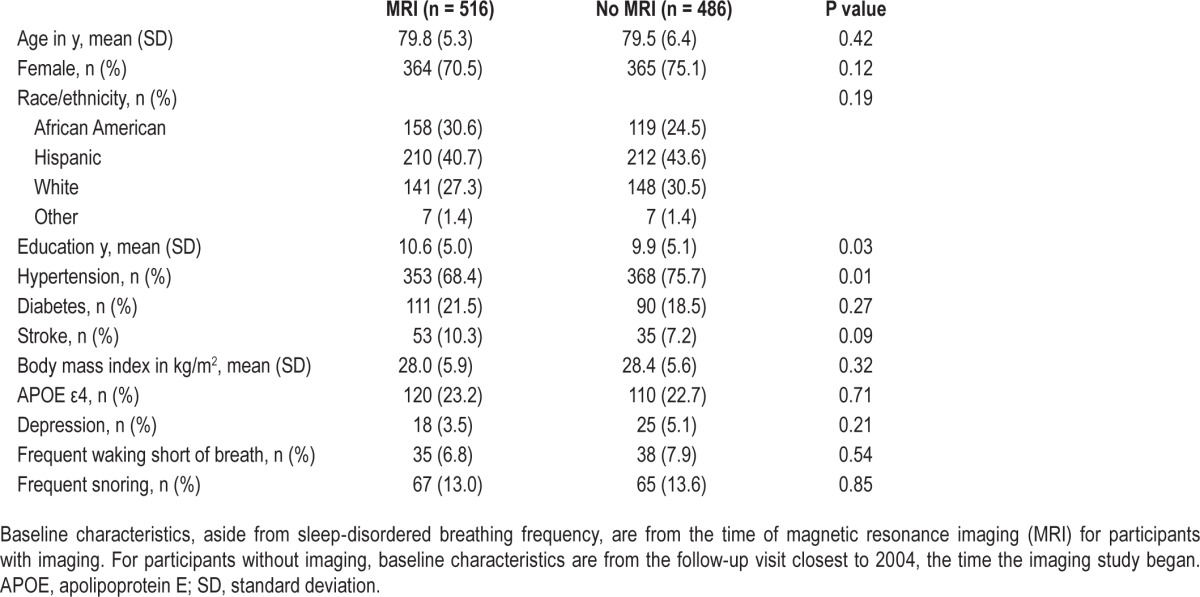
Of the 1,002 individuals with sleep information, MRI scans were obtained from 516 (Table 2). Compared with those who did not undergo MRI (n = 486), those who did were more likely to be African American, less likely to have hypertension, and had more years of education. There were no other significant differences between the groups.
Table 2.
Baseline demographic and clinical characteristics for the sleep cohort.
Subject Characteristics
Four hundred eighty-three of the 516 participants who underwent MRI (94%) had complete FLAIR and T1 imaging and served as the basis for our analysis. On average, sleep data were collected 3.4 y (standard deviation [SD] 1.4 y) after the time of MRI. The average age of the sample at the time of sleep data collection was 83 y (SD 5.3 y). At the time of MRI acquisition, 19 participants met diagnostic criteria for dementia; dementia developed in an additional 37 participants in the interval between MRI and sleep data collection.
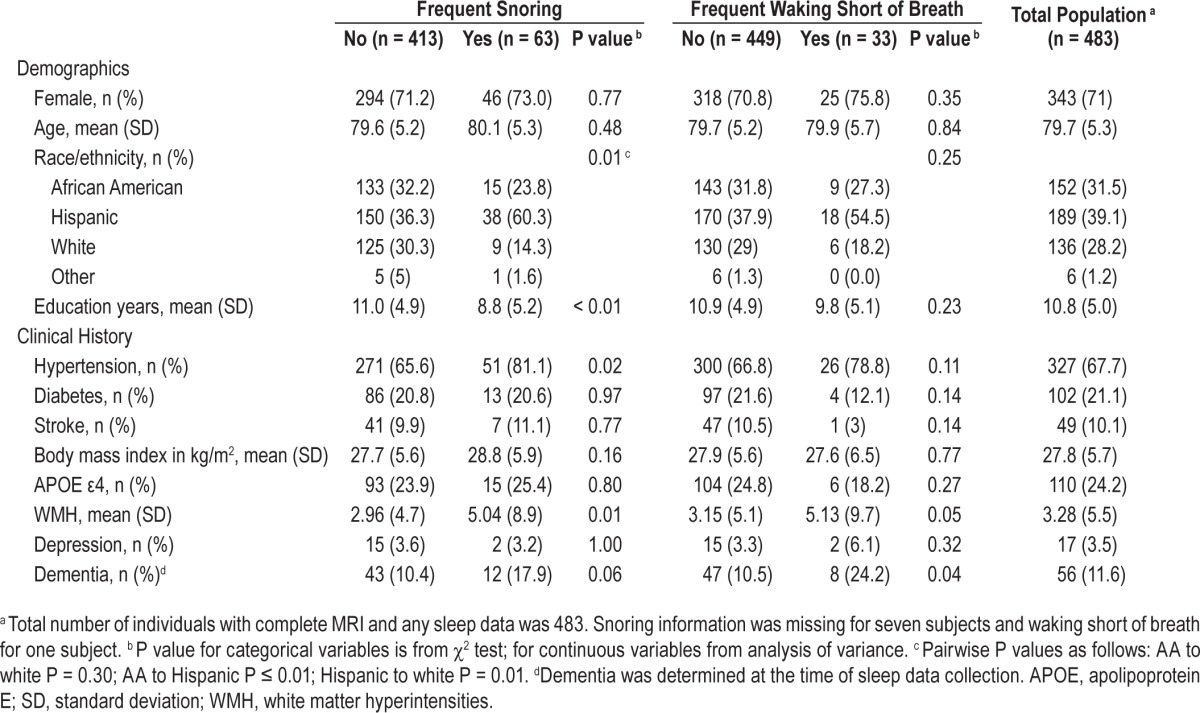
Detailed baseline characteristics at the time of MRI for those with and without SDB as determined by responses to MOS-SS are presented in Table 3. Individuals with frequent snoring had larger WMH volumes. Additionally, individuals with frequent snoring were more likely to be Hispanic, had a history of hypertension, and had fewer years of education than those with infrequent snoring. Individuals with frequent waking short of breath were more likely to have dementia than those with infrequent waking short of breath. The remaining baseline characteristics did not differ between the two groups.
Table 3.
Demographic and clinical characteristics of the study population at the time of magnetic resonance imaging divided by sleep disordered breathing likelihood.
Association between SDB and WMH Volume
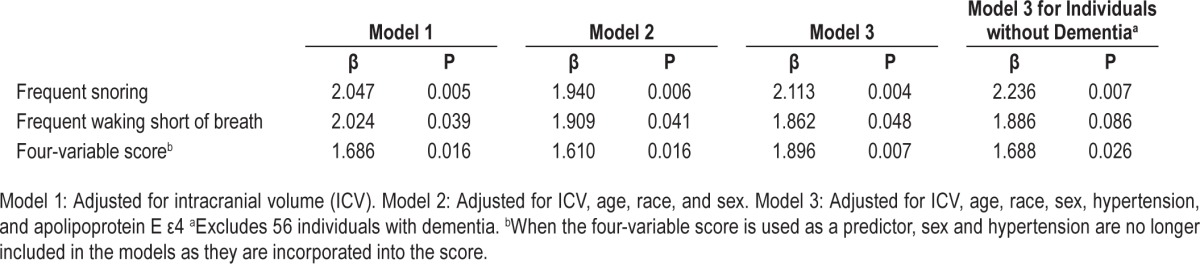
Across all three models, individuals with frequent waking short of breath and frequent snoring had larger WMH volumes than those with infrequent events. Individuals with a high likelihood of SDB by four-variable score had a larger volume of WMH compared with those with a low likelihood (Table 4).
Table 4.
Association between sleep disordered breathing and white matter hyperintensity volume.
Sensitivity Analyses
When participants with dementia at the time of sleep data acquisition (n = 56) were excluded from the analysis, the results remained similar. In the fully adjusted model, those who reported frequent snoring or a high likelihood of SDB by four-variable score had larger WMH volumes (Table 4). However, there was only a trend toward significance for those with frequent waking short of breath. We additionally adjusted for years of education in the model as years of education were associated with snoring in univariate analyses (Table 3); the association between snoring and WMH remained (β = 2.093, P = 0.005) after adjustment.
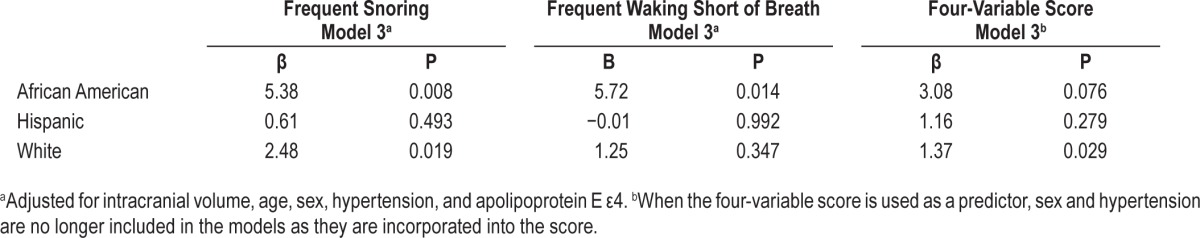
In stratified analyses, the association between SDB and WMH volume varied by race/ethnicity (Table 5). The association was strongest in Afrcian Americans (frequent snoring β = 5.38, P = 0.008; frequent waking short of breath β = 5.71, P = 0.014), and was not significant in Hispanic participants (frequent snoring β = 0.61, P = 0.493; frequent waking short of breath β = −0.01, P = 0.992). In white participants, only the association between snoring and WMH was significant (frequent snoring β = 2.48, P = 0.019; frequent waking short of breath β = 1.25, P = 0.347). When likelihood of SDB was approximated by the four-variable score, the association with WMH volume also differed across race/ethnicity groups. Statistical significance was seen among white participants (β = 1.99, P = 0.003) with a trend toward significance in African Americans (β = 3.08, P = 0.076). The association was not significant among Hispanics (β = 1.16, P = 0.279). The association between between SDB and WMH volume did not differ between men and women.
Table 5.
Association between sleep disordered breathing and white matter hyperintensity volume, stratified by race/ethnicity.
DISCUSSION
We found that individuals who reported respiratory impairment during sleep had larger WMH volumes. Similarly, WMH volumes were larger in those with a higher likelihood of SDB as determined by the four-variable score. These associations remained significant when controlling for WMH risk factors, including hypertension and age, and after a sensitivity analysis excluding participants with dementia. These findings suggest a relationship between SDB and WMH.
Our study adds to the small but emerging body of literature on SDB and WMH, which has shown mixed results. A relationship between WMH and obstructive sleep apnea (OSA) measured by polysomnography was previously reported in a middle-aged Korean population.9 Similarly, an association was demonstrated between polysomnography-confirmed OSA and visually rated WMH in a middle-aged Japanese group referred to a sleep clinic.13 In a subset of the Sleep Heart Health Study, a predominantly white cohort, WMH were associated only with central but not with obstructive apneic events.14 However, some investigations have failed to show an association between WMH and SDB11 or are limited by small sample size.12,25 It is important to note that prior studies of SDB and WMH used visual rating scales to measure WMH. We use a fully automated quantification method,18 which makes direct comparison between the WMH volumes we describe and those of prior studies difficult. Furthermore, because our sample was older than that of prior studies of imaging and SDB, we would expect our WMH volumes to be larger as age is the strongest predictor of WMH.26
To our knowledge, our study is the first to examine the association between SDB and quantified WMH volumes in an elderly, multiethnic, community-dwelling cohort. Although there was a suggestion of a difference in association between SDB and WMH by race/ethnicity in our study, the overall number of participants with SDB within each racial group is small, raising issues of power in the stratified analysis. In addition, those with and without sleep data were not evenly matched in terms of the proportion of African American and Hispanic participants; thus, there may be inherent selection bias in this sample underlying the race/ethnic differences. Interpretation of these stratified results requires further study.
The association between SDB and WMH that we observed may be particularly relevant as an explanatory factor for sleep related cognitive changes. The cognitive effects of SDB are an active area of investigation. In two recent prospective studies on cognition and SDB, measures of hypoxia were associated with the development of incident cognitive impairment over the study period.27,28 Because of the known association between WMH and cognitive impairment,29 we speculate that the cognitive effects of SDB may be a function of small-vessel cerebrovascular disease, with hypoxia as a potential mediator.30 Altered cerebral autoregulation may offer a mechanistic link. Cerebral autoregulation is the brain's ability to maintain constant blood flow across a wide range of perfusion pressures and is essential for normal brain function. Several studies have shown that cerebral autoregulation is altered in SDB.31,32 This autoregulatory dysfunction may render the brain, particularly the white matter, vulnerable to hypoperfusion and hypoxia, which may manifest as WMH.33
Alternatively, SDB may mediate small-vessel cerebrovascular disease by increasing cardiovascular risk factors including hypertension. Interestingly, the association between WMH volume and SDB remained significant after adjusting for hypertension, suggesting that blood pressure alone is not the primary mediator of the association. Because of the emerging relationship between Alzheimer disease and WMH,8 and specific data linking APOE ε4 carriers to larger WMH volumes,34 we adjusted for APOE ε4 to remove variance that could be related to biological risk factors for Alzheimer disease. In addition, as APOE ε4 has also been associated with SDB in two large sleep cohorts,35,36 we adjusted for it to account for a possible shared genetic basis for SDB and WMH. Adjusting for APOE ε4 had no effect on our results. Although the exact pathophysiologic relationship remains to be clarified, the role that WMH play in the cognitive effects of SDB is an emerging area of study.
Strengths and Limitations
The major limitation of this study is the use of self-reported sleep data, which risks inaccurate approximation of SDB. Without overnight polysomnography, the gold standard for SDB diagnosis,37 we cannot verify the true prevalence of SDB. Our SDB prevalence of 13% is lower than that of another community-based study of elderly individuals where SDB diagnosis was made with polysomnogram.2 Our prevalence of SDB may be lower because reports of snoring decrease with age due to less accurate reporting of snoring by elderly bed partners.3
Our prevalence of SDB is also lower compared with a racial and ethnically diverse community-based study where frequent snoring was reported.38 The proportion of men in our study is smaller than in this other community-based study. Given rates of SDB are two to three times higher in men compared with women,3 this difference in sex distribution may have contributed to the lower prevalence of SDB we observed. We also do not have information on treatment for SDB so cannot exclude the possibility that some participants may have been treated.
Additionally, the inclusion of participants with dementia may have affected the accuracy of sleep questionnaire responses. However, half of these participants had assistance from family in answering the questions and our results remained when participants with dementia were excluded. Another important limitation stems from the fact that our sample was one of convenience from the WHICAP cohort. To address this limitation we provide detailed comparisons between those with and without sleep data as well as those who had and who had not undergone MRI. However, there are differences between the groups that may have contributed to selection bias in our sample. Most importantly, we may have oversampled participants with SDB, as those with sleep data had a higher BMI than those lacking sleep data.
In terms of our imaging data, we do not have regional distributions of WMH in our cohort. However, in our data in general, we see no notable differences in clinical outcome as a function of periventricular versus deep distribution and, consistent with other investigators, view the distinction as arbitrary.39 In addition, our WMH processing stream excludes WMH in the basal ganglia.
Last, the cross-sectional design limits our ability to infer causality in the observed associations. We cannot rule out the possibility that existing WMH actually lead to SDB, especially because most MRI scans were obtained prior to sleep data collection.
The main strength of this study is our ability to evaluate the relationship between WMH and SDB in a large, multiethnic, elderly, community-dwelling population. As prior studies have not included a diverse, community-dwelling population, our results significantly enhance the generalizability of the association between WMH and SDB among African American and white individuals and emphasize the need for additional study in multiethnic cohorts given the suggestion of differences by race/ ethnicity that we observed. Furthermore, using rigorously quantified WMH allows more accurate lesion measurements and better potential for comparison with future studies. Although we used self-reported sleep measures, our results remained when we used the four-variable score, a validated SDB screening tool.
Our results indicate that self-reported SDB is associated with larger WMH volumes among the elderly. Given emerging data on the cognitive effects of SDB, we suggest that this relationship may be mediated at the small-vessel level. We hope that our results create a useful framework for future study.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by the Henry and Marilyn Taub Foundation and the National Institutes of Health/ National Institute on Aging: K99AG042483, R01AG034189, R01 AG037212 and P01AG007232. Dr. Brickman is on the Scientific Advisory Boards and serves as a paid consultant for ProPhase, LLC and Keystone Heart, LLC. He serves on the Board of Directors of the International Neuropsychological Society, which has paid for his travel to annual meetings. He is supported by grants from NIH, the Groff Foundation, Mars Inc, and Columbia University. The other authors have indicated no financial conflicts of interest. All work was carried out at Columbia University Medical Center, New York, NY
ACKNOWLEDGMENTS
The authors thank R. Mayeux for helpful feedback on an earlier version of the manuscript.
REFERENCES
- 1.Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med. 2008;2:349–64. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 5.Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24:109–17. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61:1531–4. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- 7.Brickman AM, Muraskin J, Zimmerman ME. Structural neuroimaging in Altheimer's disease: do white matter hyperintensities matter? Dialogues Clin Neurosci. 2009;11:181–90. doi: 10.31887/DCNS.2009.11.2/ambrickman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brickman AM. Contemplating Alzheimer's disease and the contribution of white matter hyperintensities. Curr Neurol Neurosci Rep. 2013;13:415. doi: 10.1007/s11910-013-0415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H, Yun CH, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep. 2013;36:709–15. doi: 10.5665/sleep.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins J, Redline S, Ervin A, Walsleben JA, Ding J, Nieto FJ. Associations of sleep-disordered breathing and cerebral changes on MRI. J Clin Sleep Med. 2005;1:159–65. [PubMed] [Google Scholar]
- 11.Schulz UG, Mason RH, Craig SE, et al. Leukoaraiosis on MRI in patients with minimally symptomatic obstructive sleep apnoea. Cerebrovasc Dis. 2013;35:363–9. doi: 10.1159/000348845. [DOI] [PubMed] [Google Scholar]
- 12.Davies CW, Crosby JH, Mullins RL, et al. Case control study of cerebrovascular damage defined by magnetic resonance imaging in patients with OSA and normal matched control subjects. Sleep. 2001;24:715–20. doi: 10.1093/sleep/24.6.715. [DOI] [PubMed] [Google Scholar]
- 13.Nishibayashi M, Miyamoto M, Miyamoto T, Suzuki K, Hirata K. Correlation between severity of obstructive sleep apnea and prevalence of silent cerebrovascular lesions. J Clin Sleep Med. 2008;4:242–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Ding J, Nieto FJ, Beauchamp NJ, Jr, et al. Sleep-disordered breathing and white matter disease in the brainstem in older adults. Sleep. 2004;27:474–9. doi: 10.1093/sleep/27.3.474. [DOI] [PubMed] [Google Scholar]
- 15.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 16.Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Arch Intern Med. 1999;159:1701–4. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- 17.Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65:1053–61. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brickman AM, Sneed JR, Provenzano FA, et al. Quantitative approaches for assessment of white matter hyperintensities in elderly populations. Psychiatry Res. 2011;193:101–6. doi: 10.1016/j.pscychresns.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brickman AM, Zahodne LB, Guzman VA, et al. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer's disease incidence. Neurobiol Aging. 2015;36:27–32. doi: 10.1016/j.neurobiolaging.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hays RD, Sherbourne CD, Mazel R. Santa Monica, CA: Rand Corporation; 1995. User's manual for the Medical Outcomes Study (MOS) core measures of health-related quality of life. [Google Scholar]
- 21.Kapuniai LE, Andrew DJ, Crowell DH, Pearce JW. Identifying sleep apnea from self-reports. Sleep. 1988;11:430–6. doi: 10.1093/sleep/11.5.430. [DOI] [PubMed] [Google Scholar]
- 22.Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57:423–38. doi: 10.1007/s12630-010-9280-x. [DOI] [PubMed] [Google Scholar]
- 23.Takegami M, Hayashino Y, Chin K, et al. Simple four-variable screening tool for identification of patients with sleep-disordered breathing. Sleep. 2009;32:939–48. [PMC free article] [PubMed] [Google Scholar]
- 24.Silva GE, Vana KD, Goodwin JL, Sherrill DL, Quan SF. Identification of patients with sleep disordered breathing: comparing the four-variable screening tool, STOP, STOP-Bang, and Epworth Sleepiness Scales. J Clin Sleep Med. 2011;7:467–72. doi: 10.5664/JCSM.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng CY, Tsai CF, Wang SJ, Hsu CY, Fuh JL. Sleep disturbance correlates with white matter hyperintensity in patients with subcortical ischemic vascular dementia. J Geriatr Psychiatry Neurol. 2013;26:158–64. doi: 10.1177/0891988713493503. [DOI] [PubMed] [Google Scholar]
- 26.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blackwell T, Yaffe K, Laffan A, et al. Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2015;63:453–61. doi: 10.1111/jgs.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Flier WM, van Straaten EC, Barkhof F, et al. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005;36:2116–20. doi: 10.1161/01.STR.0000179092.59909.42. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman ME, Aloia MS. Sleep-disordered breathing and cognition in older adults. Curr Neurol Neurosci Rep. 2012;12:537–46. doi: 10.1007/s11910-012-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasr N, Traon AP, Czosnyka M, Tiberge M, Schmidt E, Larrue V. Cerebral autoregulation in patients with obstructive sleep apnea syndrome during wakefulness. Eur J Neurol. 2009;16:386–91. doi: 10.1111/j.1468-1331.2008.02505.x. [DOI] [PubMed] [Google Scholar]
- 32.Urbano F, Roux F, Schindler J, Mohsenin V. Impaired cerebral autoregulation in obstructive sleep apnea. J Appl Physiol. 2008;105:1852–7. doi: 10.1152/japplphysiol.90900.2008. [DOI] [PubMed] [Google Scholar]
- 33.Brickman AM, Guzman VA, Gonzalez-Castellon M, et al. Cerebral autoregulation, beta amyloid, and white matter hyperintensities are interrelated. Neurosci Lett. 2015;592:54–8. doi: 10.1016/j.neulet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schilling S, DeStefano AL, Sachdev PS, et al. APOE genotype and MRI markers of cerebrovascular disease: systematic review and meta-analysis. Neurology. 2013;81:292–300. doi: 10.1212/WNL.0b013e31829bfda4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottlieb DJ, DeStefano AL, Foley DJ, et al. APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health Study. Neurology. 2004;63:664–8. doi: 10.1212/01.wnl.0000134671.99649.32. [DOI] [PubMed] [Google Scholar]
- 36.Kadotani H, Kadotani T, Young T, et al. Association between apolipoprotein E epsilon4 and sleep-disordered breathing in adults. JAMA. 2001;285:2888–90. doi: 10.1001/jama.285.22.2888. [DOI] [PubMed] [Google Scholar]
- 37.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 38.Ramos AR, Wohlgemuth WK, Dong C, et al. Race-ethnic differences of sleep symptoms in an elderly multi-ethnic cohort: the Northern Manhattan Study. Neuroepidemiology. 2011;37:210–5. doi: 10.1159/000334315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–5. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]


