Abstract
Study Objectives:
To evaluate the association between early stages of chronic kidney disease (CKD) and sleep disordered breathing (SDB), restless legs syndrome (RLS), and subjective and objective sleep quality (SQ).
Methods:
Cross-sectional analysis of a general population-based cohort (HypnoLaus). 1,760 adults (862 men, 898 women; age 59.3 (± 11.4) y) underwent complete polysomnography at home.
Results:
8.2% of participants had mild CKD (stage 1–2, estimated glomerular filtration rate [eGFR] ≥ 60 mL/min/1.73 m2 with albuminuria) and 7.8% moderate CKD (stage 3, eGFR 30–60 mL/min/1.73 m2). 37.3% of our sample had moderate-to-severe SDB (apnea-hypopnea index [AHI] ≥ 15/h) and 15.3% had severe SDB (AHI ≥ 30/h). SDB prevalence was positively associated with CKD stages and negatively with eGFR. In multivariate analysis, age, male sex, and body mass index were independently associated with SDB (all P < 0.001), but kidney function was not. The prevalence of RLS was 17.5%, without difference between CKD stages. Periodic leg movements index (PLMI) was independently associated with CKD stages. Subjective and objective SQ decreased and the use of sleep medication was more frequent with declining kidney function. Older age, female sex, and the severity of SDB were the strongest predictors of poor SQ in multivariate regression analysis but CKD stage was also independently associated with reduced objective SQ.
Conclusions:
Patients with early stages of CKD have impaired SQ, use more hypnotic drugs, and have an increased prevalence of SDB and PLM. After controlling for confounders, objective SQ and PLMI were still independently associated with declining kidney function.
Citation:
Ogna A, Forni Ogna V, Haba Rubio J, Tobback N, Andries D, Preisig M, Tafti M, Vollenweider P, Waeber G, Marques-Vidal P, Heinzer R. Sleep characteristics in early stages of chronic kidney disease in the HypnoLaus cohort. SLEEP 2016;39(4):945–953.
Keywords: chronic kidney disease, periodic leg movements during sleep, polysomnography, restless legs syndrome, sleep apnea, sleep disordered breathing, sleep quality
Significance.
Analyzing a large population-based cohort by polysomnography, we observed an independent association between declining kidney function, objective sleep quality and periodic leg movements. Conversely, the increased prevalence of sleep disordered breathing in early stages of chronic kidney disease seems to be explained by classic risk factors, such as age, sex, and obesity. These observations provide new information to the current understanding of sleep disturbances in chronic kidney disease patients, suggesting that specific mechanisms appearing late in the course of kidney function decline, such as uremic toxins accumulation and fluid overload, may explain the increased prevalence of sleep disordered breathing in end stage renal disease patients. The causal significance of the described relationships warrants prospective investigation.
INTRODUCTION
Sleep complaints are common in patients with chronic kidney disease (CKD), with up to 80% of patients with end-stage renal disease (ESRD) presenting with poor sleep quality, sleep disordered breathing (SDB), and restless legs syndrome (RLS).1–4
SDB is a disease characterized by repeated episodes of upper airway obstruction during sleep, which cause repetitive decreases in nighttime oxygen saturation and arousals from sleep. Sleep fragmentation and the repeated desaturation episodes cause oxidative stress and stimulate the sympathetic nervous system, leading to hypertension and increased cardiovascular risk.5–7 Hypertension and activation of the neuroendocrine axis are known risk factors for renal function loss and may explain the association between SDB and CKD.8 Conversely, there is some evidence that kidney failure may promote the development of SDB and nocturnal hypoxia, with fluid overload shifting to the upper part of the body in the supine position, leading to an upper airway edema and an increased risk of pharyngeal collapse.9,10
Likewise, RLS has been described to be highly prevalent in hemodialysis patients, possibly due to uremia, iron deficiency, and metabolic imbalance.4,11 Both SDB and RLS, as well as metabolic disturbances and comorbidities, may explain the poor sleep quality of hemodialysis patients.
Even though sleep disturbances in subjects with advanced kidney dysfunction are increasingly studied, only a very limited number of studies have attempted to objectively characterize sleep in early CKD, and no polysomnographic data exist in this population.8 Using nocturnal cardiovascular monitoring in patients from nephrology clinics, Nicholl et al.3 found a higher prevalence of SDB in patients with CKD stage 3–4 when compared to patients without kidney failure. Significantly lower sleep efficiency and higher sleep fragmentation were described by Agarwal and Light12 in 145 patients with CKD stage 3–4, when compared with 19 subjects without kidney disease, assessed by actigraphy.
In this analysis, we aimed to evaluate the association between early stages of CKD and SDB, RLS, as well as subjective and objective sleep quality, in a large population-based sample investigated by polysomnography.
METHODS
Participants
Data of the population-based cross-sectional HypnoLaus study, performed between 2009 and 2013 as an ancillary study of the CoLaus/PsychoLaus cohort, were analyzed. CoLaus/ PsychoLaus is a general-population based cohort, started in 2003 with the primary aim to explore the epidemiology and genetic determinants of cardiovascular risk factors.13 It includes 6,732 participants aged 35–75 y, recruited in the city of Lausanne, Switzerland by simple, nonstratified random selection of the inhabitants.
The HypnoLaus cohort included a subgroup of 2,162 participants to CoLaus/PsychoLaus, randomly selected to be representative of the main cohort and of the general population of Lausanne, which were investigated by home nocturnal polysomnography. Details on participant selection, assessment process, and clinical data measurements have been described previously.14
The study complied with the Declaration of Helsinki and was approved by the local institutional ethics committee. All participants provided written informed consent.
Only subjects with complete data allowing kidney function assessment and with an estimated glomerular filtration rate (eGFR) ≥ 30 mL/min were considered for the current analysis. There were 398 subjects excluded because of incomplete data regarding kidney function (2 missing creatinine, 396 missing urinary spot for albuminuria) and 4 because of eGFR < 30 mL/ min/1.73 m2 (Table S1, supplemental material).
Measurements and Equipment
Kidney function was estimated with the CKD-Epidemiology collaboration (CKD-EPI) equation,15 using creatinine from a morning fasting venous blood sample. Albuminuria was measured as albumin-to-creatinine ratio on a morning urinary spot. Laboratory analyses were performed in the CHUV Clinical Laboratory of Lausanne using the Roche method on a MODULAR apparatus (Roche Diagnostics, Rotkreuz, Switzerland) and stringent internal quality controls. Participants were categorized as having either CKD stage 1–2 (eGFR ≥ 60 mL/min/1.73 m2 with albuminuria), CKD stage 3 (eGFR 30–60 mL/min/1.73 m2) or normal kidney function (eGFR ≥ 60 mL/min/1.73 m2 without albuminuria), according to the Kidney Disease - Improving Global Outcomes (KDIGO) classification.16 For the analysis, eGFR was also divided in quartiles: 30 to 72; 72 to 83; 83 to 93, and 93 to 127 mL/min/1.73 m2.
All participants underwent unattended home polysomnography, recorded with a digital system device (Embletta; Embla Systems, Flaga, Reykjavik, Iceland) and manually scored by a trained technician using Somnologica software (Version 5.1.1, by Embla Flaga, Reykjavik, Iceland). Sleep stages and arousals were scored according to the American Academy of Sleep Medicine (AASM) 2007 criteria.17–19 Total sleep time (TST), sleep efficiency (percentage of time in bed spent asleep) and the duration of slow wave sleep (SWS) and rapid eye movement (REM) sleep were used to characterize objective sleep quality. Periodic leg movements during sleep (PLM) were assessed by surface electromyography (right and left anterior tibialis muscles), scored according to the official World Association of Sleep Medicine standards,20 developed in collaboration with the task force from the International Restless Legs Syndrome Study Group (IRLSSG), and expressed as number of PLM per hour of sleep (PLM index, PLMI). Chest and abdominal motion bands, finger pulse oximetry, and a nasal pressure cannula were applied to analyze respiration and to assess SDB according to the AASM 2012 consensus criteria.21
We assessed subjective sleep quality using the following question: “During the past month, how would you rate your sleep quality overall?” coded in four levels (very good, fairly good, fairly bad, very bad), corresponding to the subjective sleep quality item of the Pittsburgh Sleep Quality Index.22 “Fairly bad” and “very bad” answers were considered as poor subjective sleep quality for the analysis. The presence of RLS was assessed by questionnaire, using the criteria proposed by the International RLS study group.23
Data on medication taken before the sleep recording were collected using a questionnaire administered in the morning. Hypnotics and benzodiazepines were considered as sleep medications.
Statistics
Statistical analysis was conducted using Stata 11.0 for Windows (Stata Corp LP, College Station, TX, USA) and R 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria). Mean and standard deviation were used to describe continuous variables, and percentages to describe dichotomous or categorical variables. We used a t-test and a chi-square test to compare the characteristics of groups as appropriate, and univariate, logistic, or linear regression to assess the trend across ordered categorical variables. The factors associated with the presence of each studied sleep disorder were explored by multivariate logistic or linear regression. Statistical significance was established for a two-sided test P < 0.05.
RESULTS
Study Population
There were 862 men and 898 women who were included in the current analysis. Mean age of the population was 56.7 (10.7) y and mean eGFR 81.9 (14.9) mL/min/1.73 m2. There were 282 patients (16.0%) with CKD: 8.2% of stage 1–2 and 7.8% of stage 3. Demographic, anthropometric, and relevant medical data of the study population are detailed in Table 1.
Table 1.
Characteristics of the study population.
Sleep Disordered Breathing
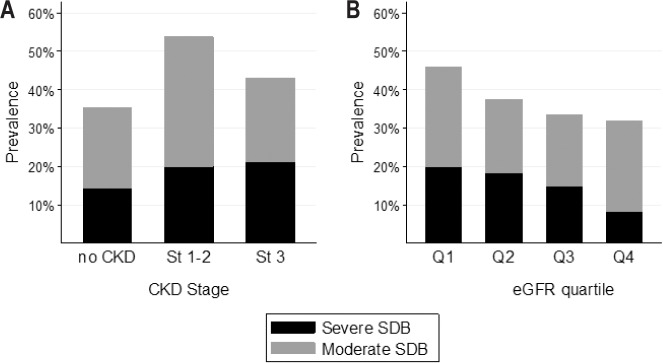
The prevalence of moderate-to-severe SBD (apnea-hypopnea index [AHI] ≥ 15/h) was 35.2% in participants without CKD, 53.8% in CKD stage 1–2, and 43.1% in CKD stage 3. The prevalence of severe SDB (AHI ≥ 30/h) increased progressively across CKD stages: 14.4% in patients without CKD, 20.0% and 21.2% respectively in stages 1–2 and 3 (P < 0.001 for trend). The prevalence of both moderate-to-severe and severe SDB increased significantly with decreasing eGFR (P < 0.001 for trend across eGFR quartiles) (Figure 1).
Figure 1.
Prevalence of sleep disordered breathing across chronic kidney disease stages and eGFR quartiles. (A) SDB and CKD stages. (B) SDB and eGFR quartiles. CKD, chronic kidney disease; CKD stage 1–2, eGFR ≥ 60 mL/min/1.73 m2 with albuminuria; CKD stage 3, eGFR 30– 60 mL/min/1.73 m2; eGFR, glomerular filtration rate, estimated with the Chronic Kidney Disease Epidemiology Collaboration (CKD–EPI) formula; No CKD, eGFR ≥ 60 mL/min/1.73 m2 without albuminuria; Q1-Q4, quartiles of eGFR (30 to 72; 72 to 83; 83 to 93 and 93 to 127 mL/min/1.73 m2); SDB, sleep disordered breathing.
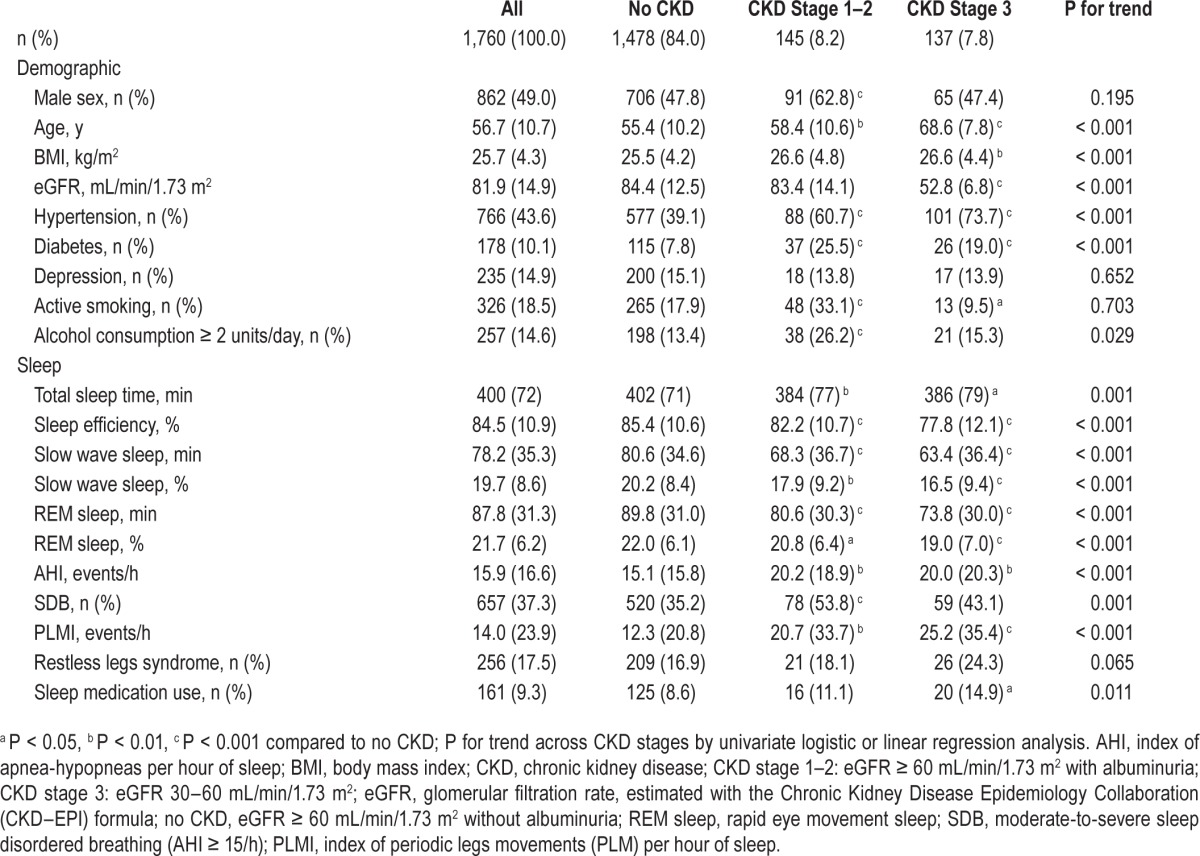
In multivariate analysis, age, male sex, and BMI were the only factors independently associated with SDB, whereas CKD stages and GFR quartiles were not (Table 2).
Table 2.
Factors associated with sleep disordered breathing and restless legs syndrome prevalence and periodic leg movements index in multivariate analysis.
RLS and PLMS
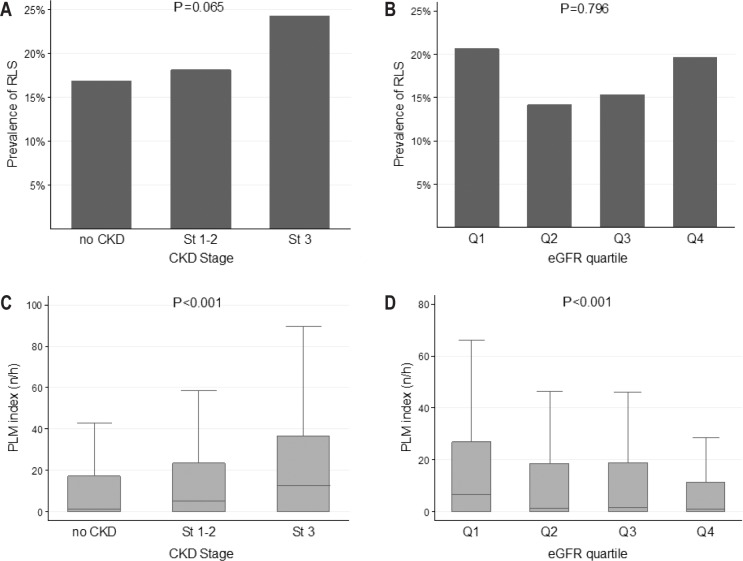
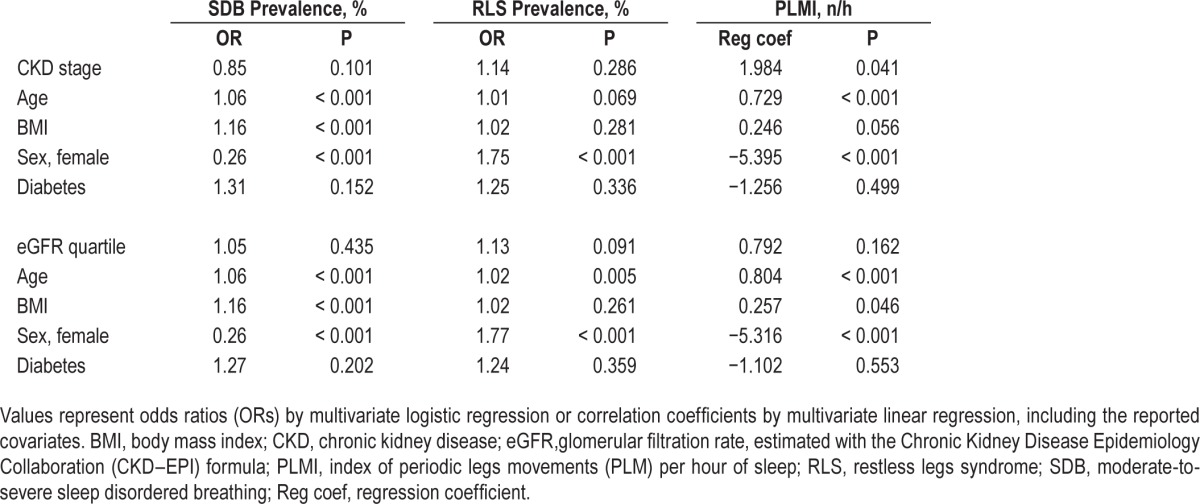
RLS was present in 256 participants, corresponding to 17.5% of the whole sample. Its prevalence tended to increase with CKD stages, being the lowest (16.9%) in subjects without CKD and at highest (24.3%) in CKD stage 3; there was, however, no difference in RLS prevalence between eGFR quartiles (Figure 2). Multivariate analysis showed no independent association between RLS prevalence and CKD stages or eGFR quartiles after adjusting for confounders (Table 2). This lack of association persisted after adding in the model hemoglobin level and red blood cell distribution width as a marker of iron deficiency.
Figure 2.
Prevalence of restless legs syndrome and periodic limb movement index across chronic kidney disease stages and eGFR quartiles. (A,B) RLS and CKD stages/eGFR quartiles. (C,D) PLM index and CKD stages/eGFR quartiles. CKD, chronic kidney disease; CKD stage 1–2, eGFR ≥ 60 mL/min/1.73 m2 with albuminuria; CKD stage 3, eGFR 30–60 mL/min/1.73 m2; eGFR, glomerular filtration rate, estimated with the Chronic Kidney Disease Epidemiology Collaboration (CKD–EPI) formula; No CKD, eGFR ≥ 60 mL/min/1.73 m2 without albuminuria; Q1-Q4, quartiles of eGFR (30 to 72; 72 to 83; 83 to 93 and 93 to 127 mL/min/1.73 m2); PLM index, index of periodic leg movements (PLM) per hour of sleep; RLS, restless legs syndrome.
The PLMI was higher in participants with more advanced CKD stages and lower eGFR (Figure 2). The associations with CKD stages persisted after adjustment for confounders (P = 0.041), but the association with declining eGFR became nonsignificant (Table 2).
Subjective Sleep Quality and Sleep Medication Use
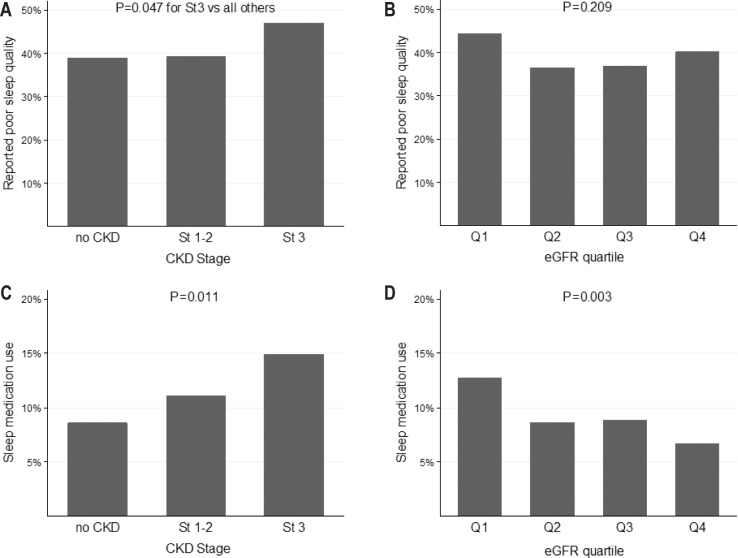
Perceived poor sleep quality was a common complaint in our population, affecting 638 (39.6%) participants. Almost half (47.0%) of the participants with CKD stage 3 reported poor sleep quality, which was significantly higher compared to participants without CKD and those with CKD stage 1–2 (38.9% and 39.3%, respectively, P = 0.05) (Figure 3). Subjective sleep quality was not associated with eGFR decline, neither in unadjusted nor in adjusted regression analysis.
Figure 3.
Subjective sleep quality and sleep medication use across CKD stages and eGFR quartiles. (A,B) Sleep quality and CKD stages/eGFR quartiles. (C,D) Sleep medication use and CKD stages/eGFR quartiles. CKD, chronic kidney disease; CKD stage 1–2, eGFR ≥ 60 mL/min/1.73 m2 with albuminuria; CKD stage 3, eGFR 30–60 mL/min/1.73 m2; eGFR, glomerular filtration rate, estimated with the Chronic Kidney Disease Epidemiology Collaboration (CKD–EPI) formula; No CKD, eGFR ≥ 60 mL/min/1.73 m2 without albuminuria; Q1-Q4, quartiles of eGFR (30 to 72; 72 to 83; 83 to 93 and 93 to 127 ml/ min/1.73 m2).
The use of sleep medications was more frequent in participants with more advanced CKD stages and lower eGFR (Figure 3), but both these associations became nonsignificant after adjustment for confounders, with older age and female sex being the only independent determinants.
Objective Sleep Quality
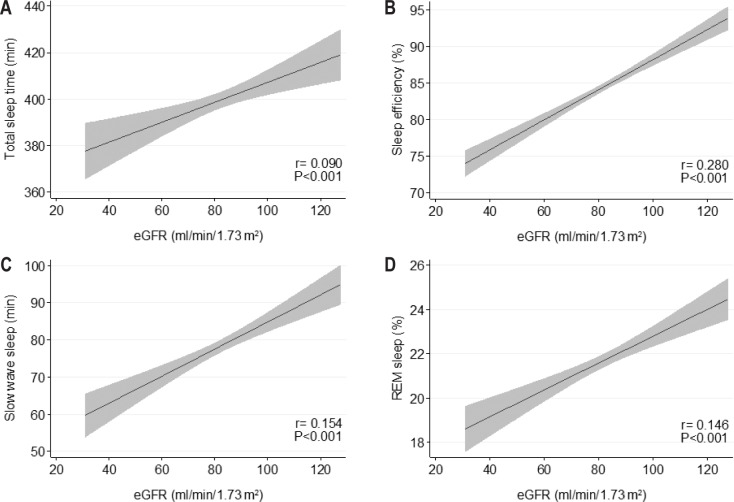
All four parameters (TST, sleep efficiency, and duration of SWS and REM sleep) used to assess objective sleep quality showed a significant trend toward poorer sleep quality with declining eGFR in unadjusted analysis (Table 1 and Figure 4).
Figure 4.
Variations in objective sleep quality parameters according to eGFR values. (A) Total sleep time, (B) sleep efficiency, (C) slow wave sleep, and (D) REM sleep. r represent correlation coefficients. eGFR, glomerular filtration rate, estimated with the Chronic Kidney Disease Epidemiology Collaboration (CKD–EPI) formula.
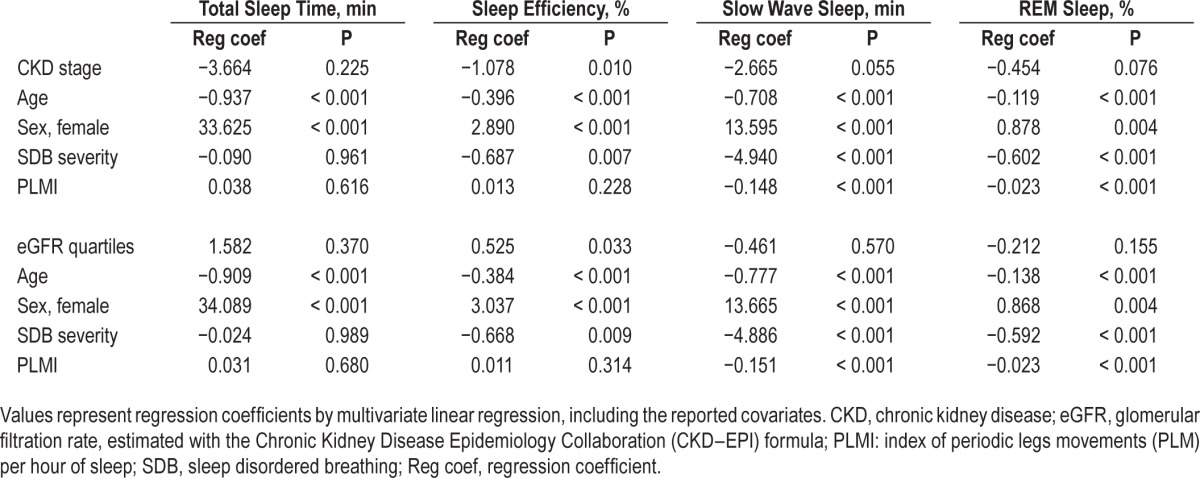
In multivariate analysis, higher CKD stages showed an independent association with lower sleep efficiency and a trend for shorter SWS and REM sleep duration, whereas declining eGFR was associated with lower sleep efficiency (Table 3). Age, sex, SDB severity, and the PLMI during sleep were independent determinants of almost all the objective sleep quality parameters.
Table 3.
Factors associated with objective sleep quality parameters in multivariate analysis.
DISCUSSION
Analyzing a large population-based cohort, we found that subjects with early stages of CKD have impaired sleep quality, use more sleep medication, and have an increased prevalence of SDB and PLM. However, although objective sleep quality and PLMI seem to be independently associated with declining kidney function, the increased prevalence of SDB seems to be linked to classic risk factors, such as age, sex, and obesity.
Sleep Disordered Breathing
There is growing evidence that SDB is more common in patients with advanced CKD than in the general population,2,3 and, inversely, subjects with SDB have a higher prevalence of non-dialysis CKD as compared to the general population.24 A recent Canadian cross-sectional study found that the prevalence of SDB increased as GFR declined, being 41% in CKD stage 3–4 patients and reaching 57% in hemodialysis patients.3 A similar high prevalence (56%) was found by our group assessing a Swiss hemodialysis population.25
Two possible pathophysiologic mechanisms have been suggested to explain the association between SBD and CKD: on the one hand, SDB could influence the time course of CKD through its associated nocturnal hypoxemia and its negative effect on the cardiovascular system, by generating oxidative stress through the reactive oxygen species,26–29 promoting inflammation,30,31 inducing endothelial cell dysfunction,32 and stimulating the sympathetic nervous system5 and the reninangiotensin-aldosterone system.6 All of these factors have been previously shown to be intimately involved in CKD progression.33,34
On the other hand, the decline in kidney function may promote SDB and nocturnal hypoxia. One possible mechanism of this reverse causality relationship could be the CKD-induced fluid overload, with upper airway collapse being amplified by overnight fluid displacement to the soft tissues of the neck. Indeed, an overnight fluid shift from the lower part of the body toward the neck has been documented by our group in hemodialysis patients with SDB,9 yet it is less certain whether fluid shifts occur in the earlier stages of CKD.
In the current observation, we could not find any independent association between prevalence of SDB and early kidney function decline to lend support to the proposed mechanisms, whether assessing kidney function as eGFR or as CKD stages (which include microalbuminuria as an early marker of kidney disease). These results were confirmed when the oxygen de-saturation index instead of SDB prevalence was included in the model, as a direct marker of nocturnal intermittent hypoxia (data not shown). This observation would suggest that the increased prevalence of SDB in patients with end-stage renal disease (ESRD) could be linked to specific mechanisms appearing late in the course of kidney function decline, such as uremic toxins accumulation and fluid overload. However, it is possible that the negative metabolic and neurohormonal consequences of SDB could play a greater role in the progression of established CKD than in the initiation of kidney damage, explaining the appearance of an association only at a later time course of the kidney disease. The prospective follow-up of our cohort will probably give us some elements to disentangle this question in the future.
RLS and PLMS
Our second finding was an independent association between PLM severity and declining kidney function in early CKD. The potential causality of this relationship deserves some discussion: PLM could be explained by metabolic disturbances induced by renal disease, but PLM could also represent a risk factor for progressing CKD. In two previous prospective studies, PLM severity was found to be an independent predictor of cardiovascular events and mortality in hemodialysis patients.35,36 The link between PLM and cardiovascular events lies possibly in the association between PLM episodes and repetitive sleep-time elevations in blood pressure and sleep-time hypertension, a phenomenon that has been described in general population studies37,38 and that may contribute to the progression of CKD. In contrast to PLM, we found no association between RLS and early CKD, suggesting that PLMI may be a more sensitive marker of this phenomenon. Although RLS has been reported to be highly prevalent in ESRD, this complaint has been poorly studied in patients with earlier stages of CKD, and our data are in line with the results of a previous study, showing no association between RLS prevalence and progressing CKD in 500 patients from nephrology clinics.4
Sleep Quality
Perceived poor sleep quality and sleep medication use were frequent in our general population sample, affecting almost 40% and 10% of the participants, respectively. These results are in line with previous descriptions, although being in the upper range of the reported prevalences,39–42 and worsened further in the presence of renal impairment.
Interestingly, even after correction for classic confounders such as SDB and PLM, objective sleep quality was independently associated with declining kidney function in early stages of CKD. Lower TST and sleep efficiency have been previously described in hemodialysis patients and in patients with advanced CKD2,12 when compared to the general population, and sleep quality predicted mortality risk in hemodialysis patients,43,44 but we show here for the first time that sleep quality is decreased even in early CKD stages. The association we observed between CKD and sleep quality fits into the growing body of evidence showing sleep as a key determinant of long-term health, similar to physical activity and nutrition. Sleep duration has been previously associated with all-cause mortality and cardiovascular events in the general population, with a U-shaped relationship, even if most of the existing evidence is based on subjective sleep quality assessment and reported sleep duration.45–51 Short sleep duration and poor sleep quality have also been associated with CKD risk factors, such as metabolic syndrome, diabetes, hypertension, and obesity8 and with the development of proteinuria.52 The link between sleep and progressing CKD could be due to the aforementioned risk factors, with the sleep-wake cycle modulating key hormones that regulate body fluid balance and blood pressure.53,54 For example, the physiologic blood pressure dipping during normal sleep, resulting from reduced sympathetic activity and increased vagal tone, is reduced by experimental SWS suppression in healthy young adults.55
Strengths and Limitations
Our results should be interpreted in light of the study strengths and limitations: despite the use of a gold-standard sleep assessment technique on a large general population-based sample, allowing objective measurement of SDB, PLM, and sleep quality, the main limitation of our study is due to its cross-sectional design, which does not allow us to conclude on the causal association between the studied sleep parameters and the kidney disease progression.
CONCLUSION
In conclusion, subjects with early stages of CKD have impaired sleep quality, use more sleep medication, and have an increased prevalence of SDB and PLM. After controlling for confounders, objective sleep quality and PLMI were independently associated with declining kidney function, whereas SDB was not. This suggests that the increased prevalence of SDB previously reported in patients with ESRD is probably due to factors appearing only late in the course of GFR decline. The significance of the described relationships should be prospectively investigated.
DISCLOSURE STATEMENT
The HypnoLaus study was supported by grants from the Swiss National Science Foundation, the Leenaards Foundation, the Ligue Pulmonaire Vaudoise, the CIRS, the Lancardis Foundation, and GlaxoSmithKline. The CoLaus/PsyCoLaus study was supported by the Faculty of Biology and Medicine of Lausanne, the Swiss National Science Foundation (grants 3200B0–105993, 3200B0-118308, 33CSCO-122661, 33CS30-139468 and 33CS30-148401) and two unrestricted grants from GlaxoSmithKline. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Dr. Preisig received a research grant from GlaxoSmithKline, related to the current work and receives royalties from Lundbeck and Eli Lilly, not related to the current study. Dr. Tafti received Research Support from UCB Pharma and acted as consultant for UCB Pharma and Jazz Pharmaceuticals, unrelated to the present work. Dr. Vollenweider received a research grant from GlaxoSmithKline, not related to the current work. Dr. Waeber received research grants from the Swiss National Science Foundation and GlaxoSmithKline, related to the current work. Dr. Heinzer acted as consultant for Night balance, without relationship to the current work. The other authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AHI
index of apnea-hypopneas per hour of sleep
- BMI
body mass index
- CKD
Chronic kidney disease
- CKD–EPI
Chronic Kidney Disease Epidemiology Collaboration
- eGFR
estimated glomerular filtration rate
- ESRD
end stage renal disease
- KDIGO
Kidney Disease - Improving Global Outcomes
- PLM
periodic leg movements
- PLMI
index of periodic leg movements per hour of sleep
- PSG
polysomnography
- RAAS
renin-angiotensin-aldosterone system
- REM sleep
rapid eye movement sleep
- RLS
restless legs syndrome
- SDB
sleep disordered breathing
- SQ
sleep quality
- SWS
slow wave sleep
- TST
total sleep time
REFERENCES
- 1.Merlino G, Piani A, Dolso P, et al. Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transplant. 2006;21:184–90. doi: 10.1093/ndt/gfi144. [DOI] [PubMed] [Google Scholar]
- 2.Roumelioti ME, Buysse DJ, Sanders MH, Strollo P, Newman AB, Unruh ML. Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin J Am Soc Nephrol. 2011;6:986–94. doi: 10.2215/CJN.05720710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholl DD, Ahmed SB, Loewen AH, et al. Declining kidney function increases the prevalence of sleep apnea and nocturnal hypoxia. Chest. 2012;141:1422–30. doi: 10.1378/chest.11-1809. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Nicholl DD, Ahmed SB, et al. The prevalence of restless legs syndrome across the full spectrum of kidney disease. J Clin Sleep Med. 2013;9:455–9. doi: 10.5664/jcsm.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilmartin GS, Lynch M, Tamisier R, Weiss JW. Chronic intermittent hypoxia in humans during 28 nights results in blood pressure elevation and increased muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2010;299:H925–31. doi: 10.1152/ajpheart.00253.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ. Intermittent hypoxia increases arterial blood pressure in humans through a renin-angiotensin system-dependent mechanism. Hypertension. 2010;56:369–77. doi: 10.1161/HYPERTENSIONAHA.110.152108. [DOI] [PubMed] [Google Scholar]
- 7.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 8.Turek NF, Ricardo AC, Lash JP. Sleep disturbances as nontraditional risk factors for development and progression of CKD: review of the evidence. Am J Kidney Dis. 2012;60:823–33. doi: 10.1053/j.ajkd.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogna A, Forni Ogna V, Mihalache A, et al. Obstructive sleep apnea severity and overnight body fluid shift before and after hemodialysis. Clin J Am Soc Nephrol. 2015;10:1002–10. doi: 10.2215/CJN.08760914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elias RM, Bradley TD, Kasai T, Motwani SS, Chan CT. Rostral overnight fluid shift in end-stage renal disease: relationship with obstructive sleep apnea. Nephrol Dial Transplant. 2012;27:1569–73. doi: 10.1093/ndt/gfr605. [DOI] [PubMed] [Google Scholar]
- 11.Aurora RN, Kristo DA, Bista SR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults--an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep. 2012;35:1039–62. doi: 10.5665/sleep.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal R, Light RP. Sleep and activity in chronic kidney disease: a longitudinal study. Clin J Am Soc Nephrol. 2011;6:1258–65. doi: 10.2215/CJN.10581110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firmann M, Mayor V, Vidal PM, et al. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord. 2008;8:6. doi: 10.1186/1471-2261-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–8. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrassy KM. Comments on KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;84:622–3. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Kales A. Los Angeles, CA: UCLA Brain Information Service/Brain Research Institute; 1968. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- 18.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 19.Iber C, et al. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 20.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 24.Iseki K, Tohyama K, Matsumoto T, Nakamura H. High prevalence of chronic kidney disease among patients with sleep related breathing disorder (SRBD) Hypertens Res. 2008;31:249–55. doi: 10.1291/hypres.31.249. [DOI] [PubMed] [Google Scholar]
- 25.Forni Ogna V, Ogna A, Pruijm M, et al. Prevalence and diagnostic approach to sleep apnea in hemodialysis patients: a population study. Biomed Res Int. 2015;2015:103686. doi: 10.1155/2015/103686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng YJ, Yuan G, Ramakrishnan D, et al. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–16. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol. 2011;226:2925–33. doi: 10.1002/jcp.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamauchi M, Nakano H, Maekawa J, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127:1674–9. doi: 10.1378/chest.127.5.1674. [DOI] [PubMed] [Google Scholar]
- 29.Pialoux V, Hanly PJ, Foster GE, et al. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am J Respir Crit Care Med. 2009;180:1002–9. doi: 10.1164/rccm.200905-0671OC. [DOI] [PubMed] [Google Scholar]
- 30.Minoguchi K, Yokoe T, Tazaki T, et al. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:625–30. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 31.Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J. 2009;33:1195–205. doi: 10.1183/09031936.00111208. [DOI] [PubMed] [Google Scholar]
- 32.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–53. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal R. Proinflammatory effects of oxidative stress in chronic kidney disease: role of additional angiotensin II blockade. Am J Physiol Renal Physiol. 2003;284:F863–9. doi: 10.1152/ajprenal.00385.2002. [DOI] [PubMed] [Google Scholar]
- 34.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–5. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 35.Jung HH, Lee JH, Baek HJ, Kim SJ, Lee JJ. Nocturnal hypoxemia and periodic limb movement predict mortality in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2010;5:1607–13. doi: 10.2215/CJN.08881209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benz RL, Pressman MR, Hovick ET, Peterson DD. Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis. 2000;35:1052–60. doi: 10.1016/s0272-6386(00)70039-4. [DOI] [PubMed] [Google Scholar]
- 37.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–8. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 38.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–30. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34:1487–92. doi: 10.5665/sleep.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helbig AK, Stockl D, Heier M, Ladwig KH, Meisinger C. Symptoms of insomnia and sleep duration and their association with incident strokes: findings from the population-based MONICA/KORA Augsburg cohort study. PloS One. 2015;10:e0134480. doi: 10.1371/journal.pone.0134480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doi Y, Minowa M, Uchiyama M, Okawa M. Subjective sleep quality and sleep problems in the general Japanese adult population. Psychiatry Clin Neurosci. 2001;55:213–5. doi: 10.1046/j.1440-1819.2001.00830.x. [DOI] [PubMed] [Google Scholar]
- 42.Chong Y, Fryer CD, Gu Q. Prescription sleep aid use among adults: United States, 2005-2010. NCHS Data Brief. 2013;(127):1–8. [PubMed] [Google Scholar]
- 43.Elder SJ, Pisoni RL, Akizawa T, et al. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2008;23:998–1004. doi: 10.1093/ndt/gfm630. [DOI] [PubMed] [Google Scholar]
- 44.Brekke FB, Waldum B, Amro A, et al. Self-perceived quality of sleep and mortality in Norwegian dialysis patients. Hemodial Int. 2014;18:87–94. doi: 10.1111/hdi.12066. [DOI] [PubMed] [Google Scholar]
- 45.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 46.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 47.Ikehara S, Iso H, Date C, et al. Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep. 2009;32:295–301. doi: 10.1093/sleep/32.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meisinger C, Heier M, Lowel H, Schneider A, Doring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Sleep. 2007;30:1121–7. doi: 10.1093/sleep/30.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shankar A, Koh WP, Yuan JM, Lee HP, Yu MC. Sleep duration and coronary heart disease mortality among Chinese adults in Singapore: a population-based cohort study. Am J Epidemiol. 2008;168:1367–73. doi: 10.1093/aje/kwn281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rod NH, Kumari M, Lange T, Kivimaki M, Shipley M, Ferrie J. The joint effect of sleep duration and disturbed sleep on cause-specific mortality: results from the Whitehall II cohort study. PloS One. 2014;9:e91965. doi: 10.1371/journal.pone.0091965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai H, Shu XO, Xiang YB, et al. Sleep duration and mortality: a prospective study of 113,138 middle-aged and elderly chinese men and women. Sleep. 2015;38:529–36. doi: 10.5665/sleep.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamoto R, Nagasawa Y, Iwatani H, et al. Self-reported sleep duration and prediction of proteinuria: a retrospective cohort study. Am J Kidney Dis. 2012;59:343–55. doi: 10.1053/j.ajkd.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 53.Charloux A, Gronfier C, Lonsdorfer-Wolf E, Piquard F, Brandenberger G. Aldosterone release during the sleep-wake cycle in humans. Am J Physiology. 1999;276:E43–9. doi: 10.1152/ajpendo.1999.276.1.E43. [DOI] [PubMed] [Google Scholar]
- 54.Brandenberger G, Follenius M, Goichot B, et al. Twenty-four-hour profiles of plasma renin activity in relation to the sleep-wake cycle. J Hypertens. 1994;12:277–83. [PubMed] [Google Scholar]
- 55.Sayk F, Teckentrup C, Becker C, et al. Effects of selective slow-wave sleep deprivation on nocturnal blood pressure dipping and daytime blood pressure regulation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R191–7. doi: 10.1152/ajpregu.00368.2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.