Abstract
Natural product compounds obtained from medicinal plants have been great contributions in the discovery of numerous clinically useful drugs. Markhamia species have been reportedly used by many cultures in human and veterinary traditional medicines. The five identified species of Markhamia, that is, Markhamia lutea, Markhamia obtusifolia, Markhamia stipulata, Markhamia tomentosa, and Markhamia zanzibarica have been the subject of chemical investigations that have led to the characterization of their secondary metabolites. Plants of the genus with the identified phytoconstituents, including phenylpropanoid glycosides (PhGs), terpenoids, phytosterols, lignans, quinones, and flavonoids, have been claimed to possess antiviral, antifungal, antiprotozoal, analgesic, antiinflammatory, and cytotoxic activities. In vitro and in vivo pharmacological research studies have reported the validation of the medicinal properties of plants of this genus. The present review analyzes published data from the ethnomedicinal, phytochemical, and pharmacological studies of plants of the genus Markhamia.
Keywords: Ethnomedicine, ethnopharmacology, Markhamia, phytochemistry
INTRODUCTION
Markhamia (Seemann ex K. Schum) is a genus of flowering plants in the family Bignoniaceae with about 100 genera and 800 species. Markhamia has been reported among other genera of the family in Nigeria and 10 species are widely distributed in tropical Africa and Asia.[1,2] The genus was named by Berthold Seemann, in honor of Sir Clements Robert Markham (1830–1916), who introduced the well-known quinine-yielding Cinchona into India.[3] Plants of this genus are trees or shrubs with opposite, compound imparipinnate leaves and yellow-green flowers grown mostly for social, agrihorticultural, and medicinal purposes.[4] They are mostly found in fringing forests and are drought-resistant. The roots, barks, stems, and leaves of Markhamia species have been used by traditional healers for the treatment of miscellaneous disease conditions such as microbial and parasitic diseases, anemia, diarrhea, backache, sore eyes, intercostal pain, pulmonary troubles, gout, scrotal elephantiasis, rheumatoid arthritis, and external skin diseases.[5,6,7,8,9,10,11] The plant has also been used in the treatment of diarrhea, dysentery, pain, and inflammation in veterinary patients.[12,13]
The therapeutic value of plants used in traditional medicine is due to the presence of phytochemical compounds that are found in parts of the plants; moreover, a medicinal plant is a plant whose biological activity has been ethnobotanically reported and scientifically established.[14,15] Preliminary phytochemical investigations of Markhamia species have shown the presence of biologically active substances such as flavonoids, saponins, steroids, terpenes and terpenoids, phytosterols, tannins, phenols, coumarins, and quinones.[2,16,17] In support of the significance of the genus Markhamia, diverse pharmacological investigations have been reported in the literature.[18,19,20,21] The isolation and identification of various chemical constituents from different plant parts of species including their pharmacological effects have been reported.
This review aims to provide a comprehensive and up-to-date report on species of the genus Markhamia with emphasis on the ethnomedicinal uses, the phytochemical and pharmacological studies, and highlights of research reports on the isolation, characterization, and identification of various active constituents present in the plant.
ETHNOMEDICINAL USES
The medicinal uses of plants range from administration of the various plant parts (alone or in combination with other plant parts) to the use of decoctions and extracts from the plants.[22,23] Plants of the genus Markhamia have been used by different tribes in various parts of African and Asian countries. Details of the uses of Markhamia species and the associated references are indicated in Table 1.
Table 1.
Ethnomedicinal data of plants of the genus Markhamia
PHYTOCHEMISTRY OF MARKHAMIA SPECIES
Chemical investigations of different plant parts of the Markhamia species Markhamia lutea (Benth.) K. Schum [Figure 1], Markhamia obtusifolia (Baker) Sprague [Figure 2], Markhamia stipulata (Wall.) Seem [Figure 3], Markhamia tomentosa (Benth.) K. Schum. ex Engl [Figure 4], and Markhamia zanzibarica (Bojer ex DC.) K. Schum [Figure 5] have led to the characterization of various secondary metabolites. These chemical constituents have been categorized as phenylpropanoid glycosides (PhGs), alkaloids, terpenoids, phytosterols, quinones, lignans, and flavonoids.[7,9,24,25,26,27] Table 2 shows the various chemical constituents isolated from the different plant parts of Markhamia species and the various chromatographic techniques used in the isolation and purification of the compounds.
Figure 1.

Markhamia lutea (Benth.) K.Schum
Figure 2.

Markhamia obtusifolia (Baker) Sprague
Figure 3.

Markhamia stipulata (Wall.) Seem
Figure 4.

Markhamia. tomentosa (Benth.) K.Schum. ex Engl
Figure 5.

Markhamia zanzibarica (Bojer ex DC.) K.Schum
Table 2.
Secondary metabolites isolated from plants of the genus Markhamia and their phytochemical analyses
CLASS OF SECONDARY METABOLITES COMMON TO MARKHAMIA SPECIES
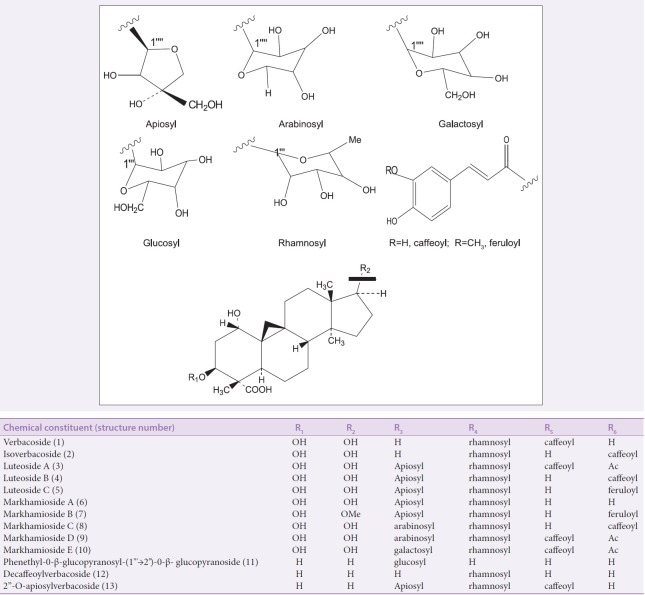
Phenylpropanoid glycosides
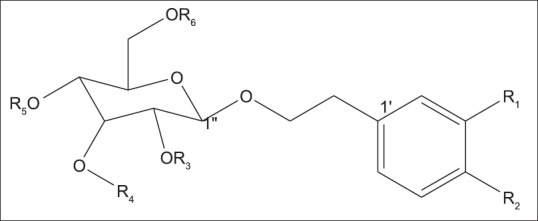
PhGs are acylated glycoconjugates with the core structure [Figure 6] characterized by a hydroxyphenylethyl aglycone linked to a β-glucopyranose through glycosidic linkage. The glucose residue of the core structure is often encircled with substituents such as aromatic acids (cinnamic acid, ferulic acid, isoferulic acid, and caffeic acid) and various sugars (apiose, arabinose, rhamnose, galactose, and xylose) through ester and glycosidic linkages, respectively.[28] Isolation of PhGs from the genus Markhamia was reported for the first time by Kernan et al.[25] The known PhGs verbacoside (1) and isoverbacoside (2) and three new PhGs luteosides A–C (3–5) were isolated from the roots of Markhamia lutea. This was followed by the isolation of five new verbacoside derivatives: Markhamiosides A–E (6–10) and 13 known compounds from the leaves and branches of Markhamia stipulata.[7] The characterization and identification of acteoside, also known as verbacoside (1) and isoacteoside (2), in the ethyl-acetate fraction of the leaves of Markhamia tomentosa have been reported.[29]
Figure 6.
Phenylpropanoid glycosides
Terpenoids and phytosterols
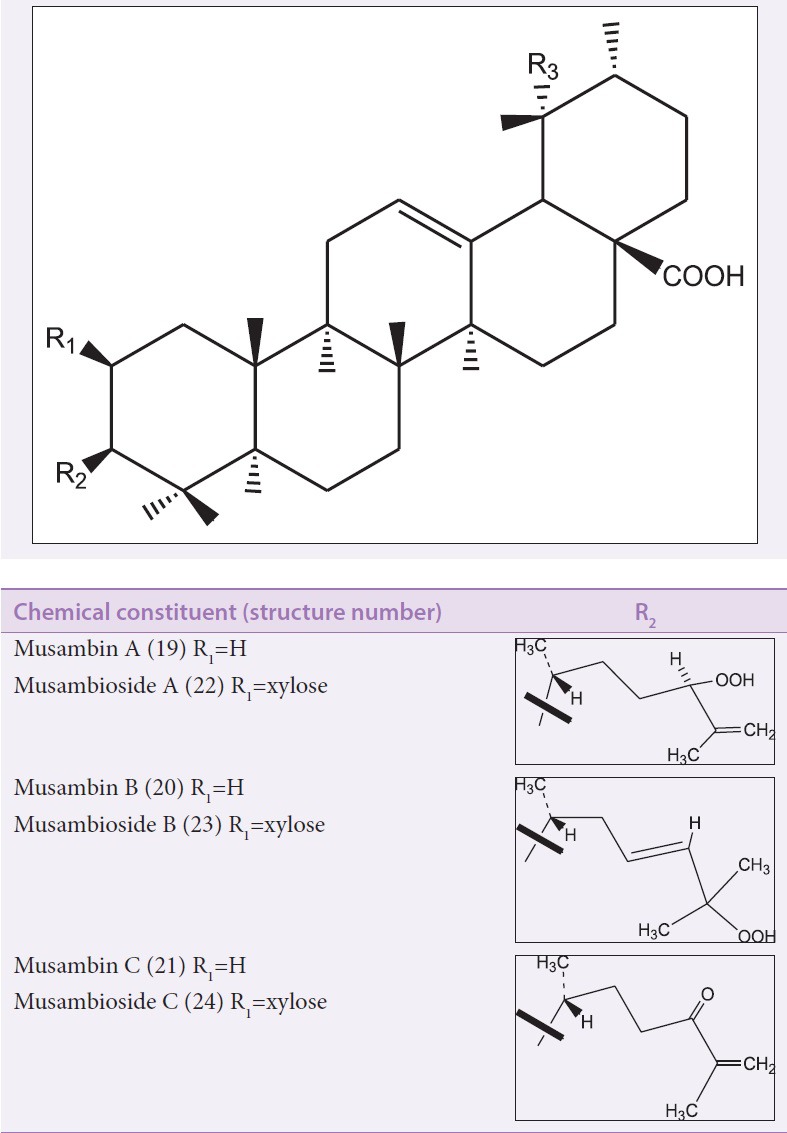
Terpenoids including their oxygenated, hydrogenated, and dehydrogenated derivatives are naturally occurring hydrocarbon molecules that are built up of isoprene units (C5H8) n joined in a head-to-tail fashion. Terpenoids are classified based on the number of isoprene units into monoterpenoids C10, sesquiterpenoids C15, diterpenoids C20, sesterterpenoids C25, triterpenoid C30, and carotenoids C40.[30] Phytosterols are among the subclass of terpenoids and are derived from tetracyclic triterpenes. Six cycloartane triterpenoids [Figure 7], that is, musambins A–C (19–21) and their 3-O-xyloside derivatives musambiosides A–C (22–24), along with other with pentacyclic triperpenes [Figure 8], that is, 2-epi-tormentic acid (25) and arjunic acid (26), were reportedly isolated from the ethylacetate leaf extract of Markhamia lutea. Three bioactive pentacyclic triterpenoids [Figure 8], that is, epi-tormentic acid (25), ursolic acid (29), and pomolic acid (30) were isolated from the leaves of Markhamia obtusifolia.[31] Gamma-sitosterol (38), campesterol (39), and tritriacontane (40) were isolated from the root, stem bark, and leaves of Markhamia zanzibarica, respectively.[26] Additionally, the isolation of pentacyclic triterpenoids such as pomolic acid (30), oleanolic acid (33), tormentic acid (35), and β-sitosterol (28) and its derivatives has been reported from the stem bark of Markhamia tomentosa.[9] Ajugol (31), tormentic acid (35), carnasol (36), and oxopomolic acid (37) were identified in the leaves of M. tomentosa.[29] The structures of the compounds were established by proton nuclear magnetic resonance (1H-NMR) and carbon-13 nuclear magnetic resonance (13C-NMR)—including one- and two- dimensional techniques—spectroscopy and mass spectrometry.
Figure 7.
Cycloartane triterpenoids
Figure 8.
Pentacyclic triterpenoids
Lignans
Lignans are dimeric compounds formed by the union of two molecules of a phenylpropene derivative.[32] The lignans paulownin (41) and palmitone (42), as well as palustrine, have been isolated from the stem heartwood of Markhamia stipulata[33] and Markhamia tomentosa, respectively.[24]
Quinones
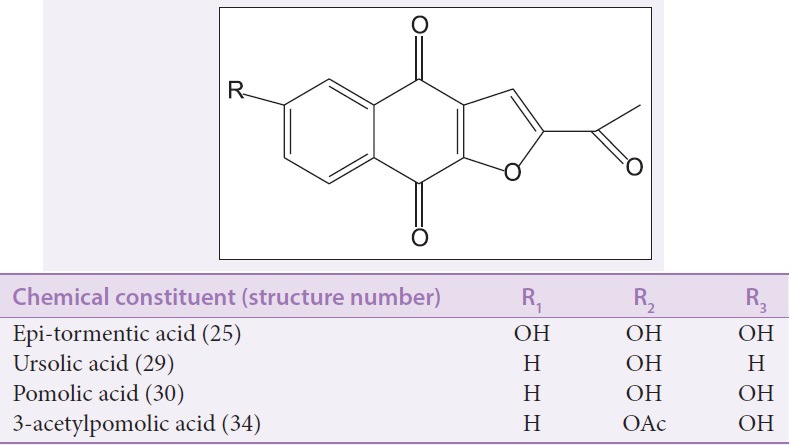
Quinones are derived from benzoquinone, naphthoquinone, or anthraquinone structural moieties. Four lapachol-type naphthoquinones (43–46) and markhamioside F (48) were isolated from the stem heartwood of Markhamia stipulata.[33] Two bioactive naphtho[2,3-b] furan-4,9-diones [Figure 9a], that is, 2-acetylnaphtho[2,3-b] furan-4,9-dione (49) and 2-acetyl-6- methoxy-naphtho[2,3-b] furan-4,9-dione (50) were reported to have been isolated from the stem bark of Markhamia tomentosa.[9] In addition, dilapachone (51) [Figure 9b] was identified in the ethyl-acetate fraction of the leaves of Markhamia tomentosa.[29]
Figure 9a.
Naphtho [2,3-b] furan-4,9 –dione
Figure 9b.
Dilapachone
Flavonoids
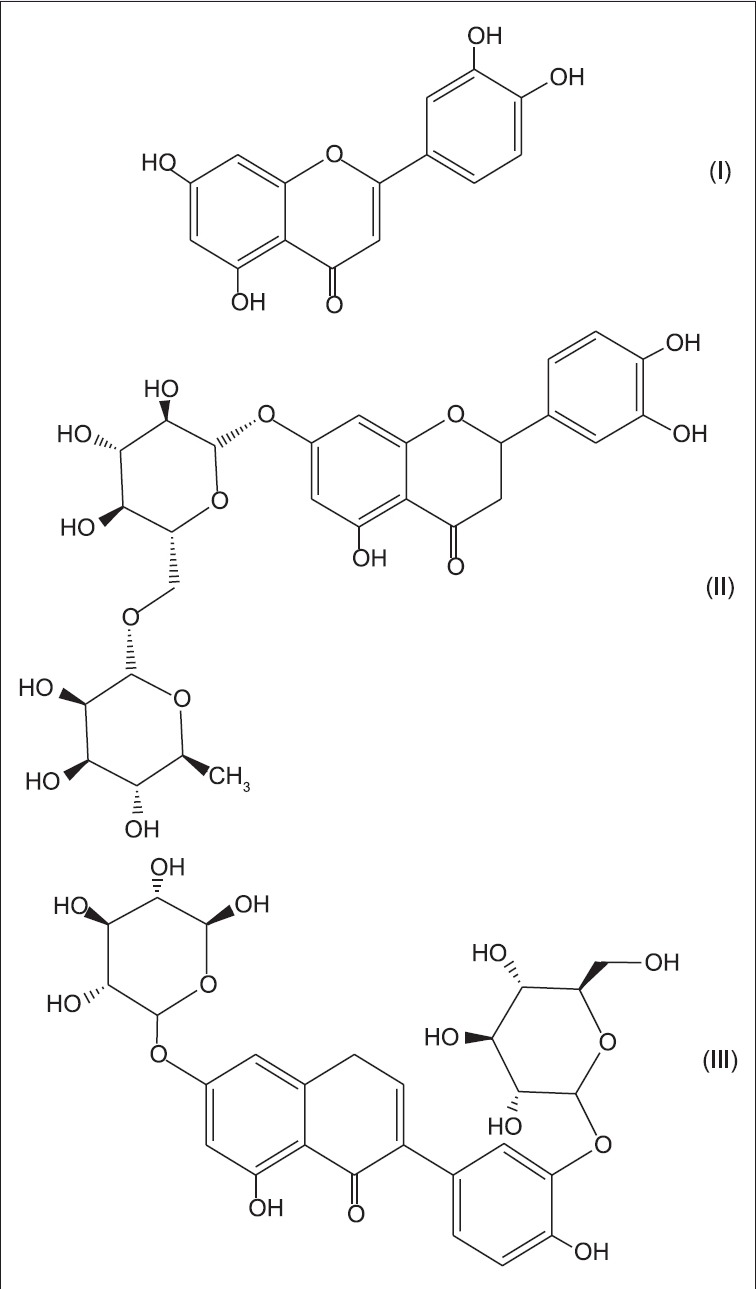
The identification of luteolin (52), luteolin-7-rutinoside (53), and luteolin-3’,7-di-O-glucoside (54) [Figure 10] from the ethyl-acetate fraction of the leaves of Markhamia tomentosa has been reported.[29]
Figure 10.
(I): Luteolin; (II): Luteolin-7-rutinoside; (III): Luteolin-3’,7-di-O-glucoside
ETHNOPHARMACOLOGICAL ACTIVITY
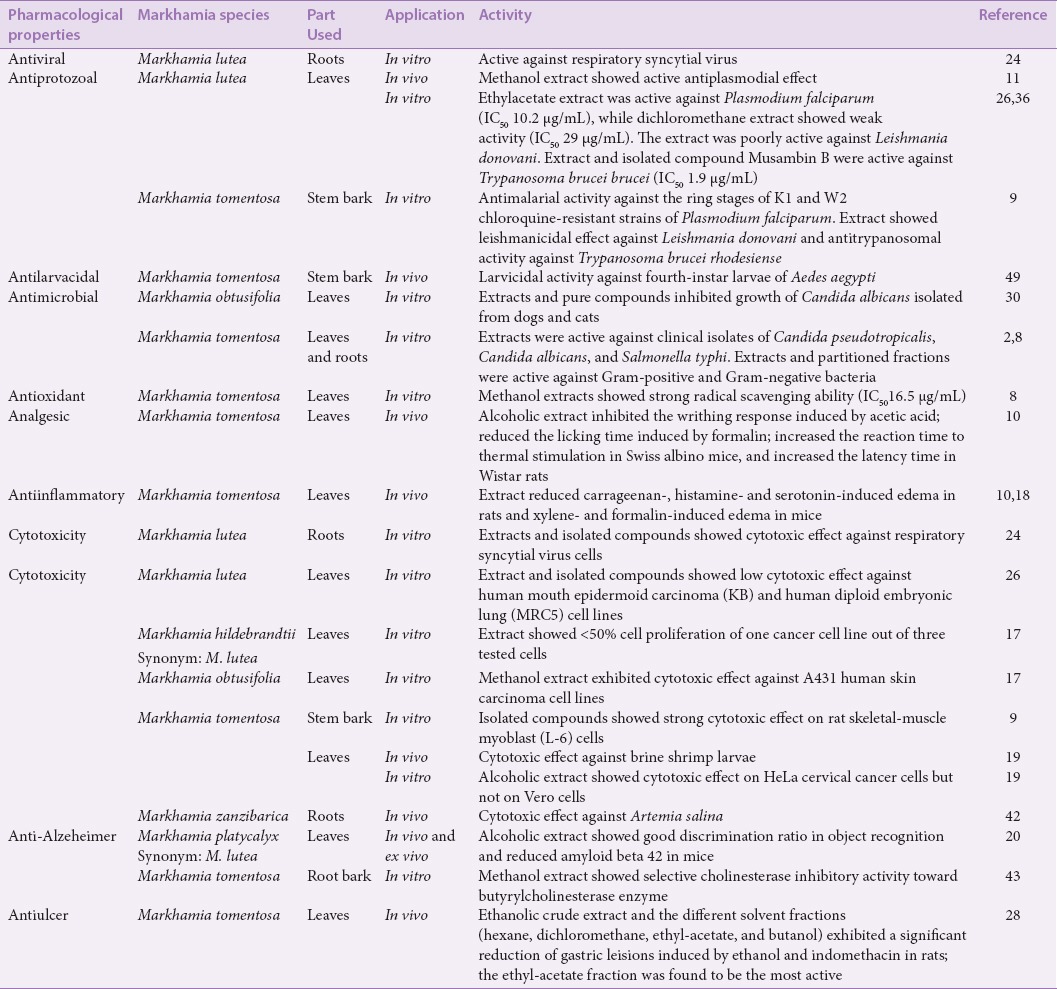
The primary metabolites are mainly important to the plants, while the secondary metabolites are of medicinal value for humans.[34] The medicinal plants of the genus Markhamia have emerged as a good source of medicines. Researchers have carried out various in vitro and in vivo screenings on the extracts and isolated compounds from members of the genus to authenticate their use in traditional medicine. Plants of this genus have demonstrated a wide spectrum of pharmacological profiles such as antiulcer, antioxidant, antimicrobial, antiinflammatory, analgesic, and antiviral activities. In our earlier work,[20] we reported the cytotoxicity and the antiproliferative and apoptosis-inducing activity of one member of the genus Markhamia against brine shrimp larvae and HeLa cervical cancer cell lines. The following section presents a review of ethnopharmacological uses of Markhamia species. More details of the pharmacological properties of these species and the associated references are shown in Table 3.
Table 3.
Pharmacological Investigation of Markhamia species
M. lutea (Benth.) K. Schum
The roots of Markhamia lutea are soaked in cold water for 30 min and the resulting tea is used to reduce symptoms of watery and bloodless diarrhea.[25] The aqueous extract of the root bark is used in the treatment of anemia and diarrhea.[6] Markhamia lutea and Markhamia tomentosa are both used to cure various parasitic and microbial diseases.[11] In ethnoveterinary medicine, the plant is eaten by primates such as chimpanzees and red and black-and-white colobus monkeys.[17,35,36] The presence of phytoconstituents such as flavonoids, saponins, terpenoids, phytosterols, quinones, and coumarins in the different solvent extracts of M. lutea have been reported.[17] Several in vitro and in vivo studies have so far been carried out to validate the use of this plant. The commonly occurring PhGs including verbacoside (1) and isoverbacoside (2) and new PhGs such as luteosides A, B, and C (1–3) isolated from the root of M. lutea showed activity against respiratory syncytial virus.[24] The bioactive compounds musambins A, B, and C (19–21) isolated from the leaves of the plant exhibited mild antileishmanial and antitrypanosomal activities.[27] Dichloromethane leaf extract of the plant showed weak antiplasmodial activity with a half maximal inhibitory concentration (IC50) value of 29 µg/mL.[37] The cytotoxic potential of the methanolic root extract of Markhamia hildebrandtii (synonym of Markhamia lutea) was investigated against cervical carcinoma, colon adenocarcinoma, and skin carcinoma.[18] In vivo pharmacological screening of the leaf extract of Markhamia platycalyx (synonym of Markhamia lutea) provided evidence that the plant has high potential as an anti-Alzheimer's disease drug lead due to its high phenolic content.[21]
M. obtusifolia (Baker) Sprague
The root of Markhamia obtusifolia is used in folk medicine to treat tuberculosis infection of lymph nodes in the neck,[38] convulsion in children,[18] and hookworm infestation.[39] The roots, barks, and leaves are boiled with other plants and used as an inhalant for the treatment of colds. In ethnoveterinary medicine, the leaves and fruits of this species are consumed as fodder by goats.[40] The methanolic root extract of M. obtusifolia exhibited minimal cytotoxic effect (<50% cell proliferation) against A431 skin carcinoma at 100 µg/mL.[18] The antifungal activity of three isolated triterpenoids (25, 29, and 30) from the acetone extract of M. obtusifolia has been reported.[31] The claimed anthelminthic activity of this plant species has been confirmed in vitro.[39] Further research is required to confirm the folk uses of the plant in treating other disease conditions.
M. stipulata Seem. ex K. Schum
The leaves and barks of Markhamia stipulata are used externally for the treatment of skin diseases and internally as an analgesic [Table 1]. Bioactive chemical compounds including quinones, phytosterols, lignans, and PhGs have been isolated from different parts of the plant.[7,33] Although the pharmacological activity of the compounds isolated from the plant has not been investigated, the pharmacological activities of verbacoside derivatives have been reported to have antifungal, antibacterial, antiviral, and analgesic effects.[25,41,42]
M. tomentosa (Benth.) K. Schum. ex Engl.
Of all the members of the Markhamia genus, the traditional use of the different plant parts of Markhamia tomentosa is the most reported [Table 1]. The species has found use in both human folk and ethnoveterinary medicines.[43,44] The plant is used in ethnoveterinary medicine to control gastrointestinal ailment and in pain management.[12,13] Preliminary phytochemical investigations of the leaves revealed the presence of major classes of bioactive compounds including saponins, flavonoids, terpenes, steroids, and phenolic nuclei.[2,16] A number of in vitro and in vivo studies have been carried out to validate the activity of the plant. Two naphthoquinone [Figure 9] compounds (49–50) isolated from the stem bark of M. tomentosa exhibited potent antiprotozoal activity against Plasmodium falciparum, Leishmania donovani, and Trypanosoma brucei rhodesiense.[9] The leaf extract of the plant was reported to possess strong antimicrobial and antioxidant effects.[8] The inhibition of Escherichia coli by the hexane and ethylacetate extracts of M. tomentosa justifies the traditional use of the plant in the management of dysentery and diarrhea.[2] Although hepatoprotective activity has not been reported for this plant, there has been a report on the prophylactic and therapeutic activities of a member of the family Bignoniaceae against paracetamol-induced liver damage in rats.[45] Alcoholic extracts of the leaves of M. tomentosa were shown to have potent analgesic and antiinflammatory effects[10,19] on rats and mice. The selective inhibition of butyrylcholinesterase enzymes by the root bark of this species in the management of Alzheimer'sdisease has also been reported.[46,47] Ethanol crude extract and the different solvent fractions of M. tomentosa leaves were reported to prevent gastric mucosal ulceration in the stomachs of rats.[29] In our earlier work,[20] we reported the cytotoxicity activity and underlying mechanisms of Markhamia tomentosa leaf extract on brine shrimp larvae, HeLa and MCF-7 cancer cell lines, and noncancerous Vero cell lines. In view of the wide application of this plant species and the tendency for prolonged intake, we are currently investigating the dose- and time-dependent chronic toxicity effects of Markhamia tomentosa in rodents (not published).
M. zanzibarica (Bojer ex DC.) K. Schum.
Markhamia zanzibarica is widely distributed in tropical Africa and Asia. In India, the plant is the second most reported Markhamia species after Markhamia lutea.[3,48] The plant is used to treat toothache, headache, and general pains[Table 1]. The cytotoxic effect of this species on Artemia salina has been investigated[49] and the activity was attributed to the bioactive gamma-sitosterol (38) compound isolated from the root of the species.[26]
CONCLUSION
This review summarizes information on the plants of the genus Markhamia with emphasis on their ethnomedicinal uses, isolated phytoconstituents, and ethnopharmacological studies on them. Species of this genus have been useful in the management of various disease conditions in both human and veterinary traditional medicines. Some of the claimed traditional uses have been validated through phytochemical and pharmacological studies of the genus. On preliminary phytochemical screening of plants of this genus, the presence of a wide range of secondary metabolites was reported. However, the major reported class of phytoconstituents, isolated through various separation and purification techniques from M. lutea, M. obtusifolia, M. stipulata, M. tomentosa, and M. zanzibarica, were PhGs, terpenoids, phytosterols, lignans, quinones, and flavonoids. The isolated compounds were identified on analysis of their spectroscopic and chemical data, which were consistent with values reported in the literature. A number of in vitro and in vivo pharmacological studies have confirmed that the plant extracts and isolated compounds possess significant antiviral, antiprotozoal, antimicrobial, antioxidant, analgesic, antiinflammatory, anti-Alzheimer, antiulcer, and cytotoxic activities. It may be concluded that plants of this genus hold great potential as a source of new drugs. Thus, further studies aimed at the proper documentation of folk uses, validation of the claimed bioactivities, and isolation and identification of the bioactive compounds of species of the genus are required.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Mutiat Bolanle Ibrahim
Mutiat Bolanle Ibrahim, (Mrs) Department of Pharmacognosy, Faculty of Pharmacy, University of Lagos, College of Medicine campus, Idi-araba, Lagos, Nigeria.

Nutan Kaushik
Nutan Kaushik, (PhD) Senior Fellow and Area Convenor. Plant Biotechnology, Environmental and Industrial Biotechnology Division, The Energy and Resources Institute (TERI), Darbari Seth Block, India Habitat Centre, Lodhi Road, New Delhi 110 003, India.

Abimbola Adepeju Sowemimo
Abimbola Adepeju Sowemimo, (PhD) Sub-dean, Faculty of Pharmacy, University of Lagos. Department of Pharmacognosy, Faculty of Pharmacy, University of Lagos, College of Medicine campus, Idi-araba, Lagos, Nigeria.

Olukemi A. Odukoya
Olukemi A. Odukoya, (PhD) Professor of Pharmacognosy Department of Pharmacognosy, Faculty of Pharmacy, University of Lagos, College of Medicine campus, Idi-araba, Lagos, Nigeria.
REFERENCES
- 1.Hutchinson J, Dalziel JM. Vol. 2. London: Crown Agents for Oversea Government and Administrations; 1954. Flora of West Tropical African. Part I; pp. 383–8. [Google Scholar]
- 2.Ugbabe GE, Ayodele AE, Ajoku GA, Kunle OF, Kolo I, Okogun JI. Preliminary phytochemical and antimicrobial analyses of the leaves of Nigerian Bignoniaceae Juss. Global Res J. 2010;1:1–5. [Google Scholar]
- 3.Mohammed I, Malik V, Pranita Markhamia zanzibarica (Bojer ex DC.) K. Schum A new exotic beauty for India. Species. 2013;5:16–7. [Google Scholar]
- 4.Burkill HM. Vol. 1. England: Royal Botanical Gardens, Kew; 1985. The Useful Plant of West Tropical African; pp. 252–8. [Google Scholar]
- 5.Bouquet A, Debray M. Paris: ORSTOM (Spanish); 1974. Plantes M ˈ dicinales de la Cˈte dˈIvoire; pp. 50–2. [Google Scholar]
- 6.Kerharo J. Historic and Ethnopharmacognosic Review on the Belief and Traditional Practices in the Treatment of sleeping sickness in West Africa. Bull Soc Med Afr Noire Lang FR. 1974;19:400. [PubMed] [Google Scholar]
- 7.Kanchanapoom T, Kasai R, Yamasaki K. Phenolic glycosides from Markhamia stipulata. Phytochemistry. 2002;59:557–63. doi: 10.1016/s0031-9422(01)00466-6. [DOI] [PubMed] [Google Scholar]
- 8.Aladesanmi AJ, Iwalewa EO, Adebajo AC, Akinkunmi EO, Taiwo BJ, Olorunmola FO, et al. Antimicrobial and antioxidant activities of some Nigerian medicinal plants. Afr J Tradit Complement Altern Med. 2007;4:173–84. doi: 10.4314/ajtcam.v4i2.31206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tantangmo F, Lenta BN, Boyom FF, Ngouela S, Kaiser M, Tsamo E, et al. Antiprotozoal activities of some constituents of Markhamia tomentosa (Bignoniaceae) Ann Trop Med Parasitol. 2010;104:391–8. doi: 10.1179/136485910X12743554760180. [DOI] [PubMed] [Google Scholar]
- 10.Temdie RJ, Fotio LA, Dimo T, Beppe JG, Tsague M. Analgesic and anti-inflammatory effects of extracts from the leaves of Markhamia tomentosa (Benth.) K. Schum (Bignoniaceae) Pharmacol. 2012;3:565–73. [Google Scholar]
- 11.Adjanohoun EJ, Aboubakar N, Dramane K, Ebat ME, Ekpere JE, Enow-orock EG, et al. Contribution to Ethnobotanical and Floristic Studies in Cameroon. Yaounde’: Commission Scientifique Techniqueet de la Recherche. 1996:423–64. [Google Scholar]
- 12.De Villiers BJ, Van Vuuren SF, Van Zyl RL, Van Wyk BE. Antimicrobial and antimalarial activity of Cussonia species (Araliaceae) J Ethnopharmacol. 2010;129:189–96. doi: 10.1016/j.jep.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Stark TD, Mtui DJ, Balemba OB. Ethnopharmacological survey of plants use in the traditional treatment of gastrointestinal pain, inflammation and diarrhea in Africa: Future perspectives for integration in modern medicine. Animals. 2013;3:158–227. doi: 10.3390/ani3010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elujoba AA. The role of pharmacognosy in phytotherapy, the challenges of our time. Nigerian J Nat Prod and Med. 1998;2:5–8. [Google Scholar]
- 15.Ayodele SQ. Lagos, Nigeria: University of Lagos; 2003. The Effects of Herbal Remedies. Paper Presented at the 12th Annual Conference of the Botanical Society of Nigeria (BOSON) pp. 21–9. [Google Scholar]
- 16.Borokini TI, Omotayo F. Phytochemical and ethnobotanical study of some selected medicinal plants from Nigeria. J Med Plant Res. 2012;6:1106–18. [Google Scholar]
- 17.Joselin J, Brintha TS, Florence AR, Jeeva S. Phytochemical evaluation of Bignoniaceae flowers. J Chem Pharm Res. 2013;5:106–11. [Google Scholar]
- 18.Kamuhabwa A, Nshimo C, de Witte P. Cytotoxicity of some medicinal plant extracts used in Tanzanian traditional medicine. J Ethnopharmacol. 2000;70:143–9. doi: 10.1016/s0378-8741(99)00161-0. [DOI] [PubMed] [Google Scholar]
- 19.Sowemimo A, Samuel F, Fageyinbo MS. Anti-inflammatory activity of Markhamia tomentosa (Benth.) K. Schum. Ex Engl. ethanolic leaf extract. J Ethnopharmacol. 2013;149:191–4. doi: 10.1016/j.jep.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim B, Sowemimo A, Spies L, Koekomoer T, van de Venter M, Odukoya OA. Antiproliferative and apoptosis inducing activity of Markhamia tomentosa leaf extract on HeLa cells. J Ethnopharmacol. 2013;149:745–9. doi: 10.1016/j.jep.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 21.Hassaan Y, Handoussa H, El-Khatib AH, Linscheid MW, El Sayed N, Ayoub N. Evaluation of plant phenolic metabolites as a source of Alzheimer's drug leads. Biomed Res Int 2014. 2014:843263. doi: 10.1155/2014/843263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogbulie JN, Ogueke CC, Okorundu S. Antibacterial properties of A. cordifolia, M. florum, U. chaeme, B. pinnatum, C. albidem, and A. cilata on some hospital isolates. Nigerian J Microbiol. 2004;18:249–55. [Google Scholar]
- 23.Halde UK, Wake R, Patil N. Genus Sida - The plants with ethno medicinal and therapeutic potential. Golden Res Thoughts. 2011;1:1–4. [Google Scholar]
- 24.Adesanya SA, Nia R. Palustrine from Markhamia tomentosa. Nigerian J Nat Prod Med. 1997;1:39–40. [Google Scholar]
- 25.Kernan MR, Amarquaye A, Chen JL, Chan J, Sesin DF, Parkinson N, et al. Antiviral phenylpropanoid glycosides from the medicinal plant Markhamia lutea. J Nat Prod. 1998;61:564–70. doi: 10.1021/np9703914. [DOI] [PubMed] [Google Scholar]
- 26.Khan MR, Mlungwana SM. γ-sitosterol, a cytotoxic sterol from Markhamia zanzibarica and Kigelia Africana. Fitoter. 1999;70:96–7. [Google Scholar]
- 27.Lacroix D, Prado S, Deville A, Krief S, Dumontet V, Kasenene J, et al. Hydroperoxy-cycloartane triterpenoids from the leaves of Markhamia lutea, a plant ingested by wild chimpanzees. Phytochemistry. 2009;70:1239–45. doi: 10.1016/j.phytochem.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Fu GM, Pang HH, Wong YH. Naturally occurring phenylethanoid glycosides: Potential leads for new therapeutics. Curr Med Chem. 2008;15:2592–613. doi: 10.2174/092986708785908996. [DOI] [PubMed] [Google Scholar]
- 29.Sofidiya MO, Agunbiade FO, Koorbanally NA, Sowemimo A, Soesan D, Familusi T. Antiulcer activity of the ethanolic extract and ethyl acetate fraction of the leaves of Markhamia tomentosa in rats. J Ethnopharmacol. 2014;157:1–6. doi: 10.1016/j.jep.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Dillard CJ, German JB. Phytochemicals: Nutraceuticals and Human Health. J Sci Food Agric. 2000;80:1744–56. [Google Scholar]
- 31.Nchu F, Aderogba MA, Mdee LK, Eloff JN. Isolation of anti-Candida albicans compounds from Markhamia obtusifolia (Baker) Sprague (Bignoniaceae) S Afr J Bot. 2010;76:54–7. [Google Scholar]
- 32.Mohammed A. Vol. 1. New Delhi (India): Satish Kumar Jain for CBS Publisher & Distributors; 2008. Pharmacognosy (Pharmacognosy and Phytochemistry) pp. 189–96. [Google Scholar]
- 33.Joshi KC, Singh P, Pardasani RT. Chemical constituents of the stem heart wood of Markhamia stipulata. Planta Medica. 1978;34:219–21. [Google Scholar]
- 34.Trease GE, Evans WC. 14th ed. London: WB Saunders; 1989. Textbook of Pharmacognosy; pp. 13–53. [Google Scholar]
- 35.Onderdonk DA, Chapman CA. Coping with forest fragmentation: The primates of Kibale National Park, Uganda. Int J Primatol. 2000;21:587–611. [Google Scholar]
- 36.Chapman CA, Chapman LJ, Rode KD, Hauck EM, McDowell LR. Variation in the nutritional value of primate foods: Among trees, time periods, and areas. Int J Primatol. 2003;24:317–33. [Google Scholar]
- 37.Muganga R, Angenot L, Tits M, Frédérich M. Antiplasmodial and cytotoxic activities of Rwandan medicinal plants used in the treatment of malarial. J Ethnopharmacol. 2010;128:52–7. doi: 10.1016/j.jep.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 38.Chhabra SC, Mahunnah RL. Plants used in traditional medicine by Hayas of the Kagera Region, Tanzania. Econ Bot. 1994;48:121–9. [Google Scholar]
- 39.Nchu F, Githiori JB, McGaw LJ, Eloff JN. Anthelminthic and cytotoxic activities of extracts of Markhamia obtusifolia Sprague (Bignoniaceae) Vet Parasitol. 2011;183:184–8. doi: 10.1016/j.vetpar.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Kokuraro JO. 2nd ed. Nairobi: East Africa Literature Bureas; Kokwaro; 1976. Medicinal Plants of East Africa; p. 384. [Google Scholar]
- 41.Cometa F, Tomassini L, Nicoletti M, Pieretti S. Phenylpropanoid glycosides: Distribution and pharmacological activity. Fitoterapia. 1993;64:195–217. [Google Scholar]
- 42.Jiménez C, Riguera R. Phenylethanoid glycosides in Plants: Structure and biological activity. Nat Prod Rep. 1994;11:591–606. doi: 10.1039/np9941100591. [DOI] [PubMed] [Google Scholar]
- 43.Khan MR. Cytotoxicity assay of some Bignoniaceae. Fitoterapia. 1998;69:538–40. [Google Scholar]
- 44.Arnold TH, De Wet BC. South Africa: Botanical Survey of South Africa; 1993. Plants of SOUTHERN Africa: Names and Distribution; p. 62. [Google Scholar]
- 45.Shabana MH, Hashem FA, Singab A, Khaled S, Farrag A. Protective and therapeutic activities of Mayodendron ignem Kurz against paracetamol induced liver toxicity in rats and its bioactive constituents. J Applied Pharma Sci. 2013;3:147–55. [Google Scholar]
- 46.Elufioye TO, Obuotor EM, Sennuga AT, Agbedahunsi JM, Adesanya SA. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some selected Nigerian medicinal plants. Braz J Pharmacogn. 201;0:472–7. [Google Scholar]
- 47.Hoang VS, Nanthavong K, Kessler PJ. Trees of Laos and Vietnam: A field guide to 100 economically and ecologically important species. Blumea. 2004;49:201–349. [Google Scholar]
- 48.Arbonnier M. France: CIRAD, MNHN, Margraf Publishers GmBH; 2004. Trees, Shrubs and Lianas of West Africa Dry Zones; p. 573. [Google Scholar]
- 49.Adebajo AC, Famuyiwa FG, John JD, Idem ES, Adeoye AO. Activities of some Nigeria Medicinal Plants against Aedes aegypti. Chinese Med. 2012;3:151–6. [Google Scholar]