Abstract
Mitochondria transport and utilize iron for the synthesis of haem and Fe–S clusters. Although many proteins are known to be involved in these processes, additional proteins are likely to participate. To test this hypothesis, in the present study we used a genetic screen looking for yeast mutants that are synthetically lethal with the mitochondrial iron carriers Mrs3 and Mrs4. Several genes were identified, including an isolate mutated for Yfh1, the yeast frataxin homologue. All such triple mutants were complemented by increased expression of Rim2, another mitochondrial carrier protein. Rim2 overexpression was able to enhance haem and Fe–S cluster synthesis in wild-type or Δmrs3/Δmrs4 backgrounds. Conversely Rim2 depletion impaired haem and Fe–S cluster synthesis in wild-type or Δmrs3/Δmrs4 backgrounds, indicating a unique requirement for this mitochondrial transporter for these processes. Rim2 was previously shown to mediate pyrimidine exchange in and out of vesicles. In the present study we found that isolated mitochondria lacking Rim2 exhibited concordant iron defects and pyrimidine transport defects, although the connection between these two functions is not explained. When organellar membranes were ruptured to bypass iron transport, haem synthesis from added iron and porphyrin was still markedly deficient in Rim2-depleted mitochondrial lysate. The results indicate that Rim2 is a pyrimidine exchanger with an additional unique function in promoting mitochondrial iron utilization.
Keywords: haem, iron, iron–sulfur, mitochondrial carrier protein, mitochondrion, pyrimidine
INTRODUCTION
Iron in cells is used as a cofactor for many essential enzymes [1]. For example, haem and Fe–S clusters are iron-containing cofactors that are necessary for the activities of some respiratory complexes and for critical enzymes. In most eukaryotes, iron insertion into these cofactors occurs inside mitochondria [2]. For haem, iron insertion into the porphyrin precursor occurs solely within the membrane-bound compartment of the mitochondrial matrix [3]. Likewise for Fe–S clusters, their biogenesis (including the iron insertion step) often takes place entirely within mitochondria [4]. Iron insertion for maturation of mitochondrial di-iron proteins is also likely to occur inside mitochondria [5]. Mitochondria may store iron bound to proteins, e.g. ferritin [6], or complexed with inorganic ligands, such as phosphate [7]. The mitochondrial inner membrane is impermeable to protons and ions, a property that is necessary for forming the proton gradient [8]. Therefore mitochondrial iron transporters are needed in order to translocate iron from the cytoplasm to the mitochondrial sites of cofactor synthesis and iron storage.
Mitochondrial iron transporters have already been described. These include mitochondrial carrier proteins Mrs3 and Mrs4 in yeast, and the orthologous proteins mitoferrin 1 and 2 in vertebrates [9–11]. Mitochondrial carrier proteins are polytopic proteins located primarily in the inner membrane and constituting a large protein family (35 members in yeast) with high sequence homology. They transport various substrates across the mitochondrial inner membrane, such as amino acids, carboxylic acids, inorganic anions, nucleotides and cofactors [12]. In vertebrates, the knock-down of mitoferrin 1 causes a defect in erythropoiesis, as would be expected from a failure to transport iron into mitochondria for synthesis of haem, a key constituent of red blood cells [10].
Mrs3 and Mrs4 are mitochondrial carrier proteins of the yeast Saccharomyces cerevisiae that have been linked to iron transport by studies in cells, organelles and vesicles [9,13–15]. These proteins exhibit high sequence homology and redundant functions, such that only the double-deletion strain exhibits sensitivity to iron deprivation. However, alternative pathways for iron uptake into mitochondria probably exist, analogous to the multiplicity of iron acquisition pathways deployed by Escherichia coli [16]. Furthermore, genetic and biochemical pathways may influence the biological availability of iron within mitochondria by affecting the protein or chemical ligands of the metal [17,18].
In the present study we characterize various roles of a different mitochondrial carrier protein, Rim2. Rim2 functions as a pyrimidine exchanger of mitochondria, bringing in pyrimidine triphosphates from the cytoplasm in exchange for pyrimidine monophosphates from the mitochondria [19]. Rim2 also functions in iron metabolism, genetically backing up the iron transporters Mrs3 and Mrs4. Finally Rim2 is required for iron utilization for haem and Fe–S cluster synthesis within mitochondria. This unique function of Rim2 in mitochondrial iron use appears to be distinct from its role in pyrimidine transport.
EXPERIMENTAL
Yeast strains and culture conditions
A sectoring screen for synthetic lethality with Δmrs3Δmrs4 has been described previously [20]. Newly identified synthetic lethal mutants included J116 (MATa ura3 his3 leu2 ade2 ade3 cyh2 Δmrs3::TRP1 Δmrs4::HIS3 tsa1-116 [pTSV31A-URA3-2micron-ADE3-MRS4]) and J386 (MATα leu2 ura3 ade2 ade3 cyh2 Δmrs3::TRP1 Δmrs4::HIS3 rad50-386 [pTSV31A-URA3-2micron-ADE3-MRS4]). J116 and J386 were non-viable when streaked on 5-fluoroorotic acid agar plates for counterselection against the URA3-marked MRS4-containing plasmid. J116 and J386 were complemented by genomic clones with TSA1 or RAD50 respectively. The parental strain YPH499, Δmrs3/Δmrs4 and Δmrs3Δmrs4Δyfh1 have been described previously [21]. The latter triple mutant was maintained under low-oxygen conditions by culturing in defined medium bubbled with argon gas. GAL1 promoter swaps were performed using the HIS3 gene from Saccharomyces kluyveri for selection in YPH499 or Δmrs3/Δmrs4 strains [22]. For evaluating mitochondrial iron accumulation during growth, cells from the Gal-Rim2 Δmrs3/Δmrs4 strain were cultured in standard defined medium under inducing conditions (2% raffinose and 0.5% galactose) or non-inducing conditions (2% raffinose) supplemented with 50 μM 55Fe-labelled citrate. After 48 h of growth, mitochondria were harvested, disrupted by sonication in hypotonic buffer [50 mM Hepes/KOH (pH 7.5) and 1 mM sodium citrate] followed by ultracentrifugation (80000 rev./min, TLA 100.3 rotor, for 20 min at 4°C) and scintillation counting of the supernatant and pellet.
Haem synthesis assays
Fluorescent haem synthesis assays were performed using isolated intact mitochondria and added porphyrin precursors. Intact mitochondria equivalent to 200 μg of protein were incubated in buffer [50 mM Tris/HCl (pH 7.5) and 0.6 M sorbitol)] in the presence of 5 mM NADH. Some assays were performed in 50 mM Hepes/KOH (pH 7.5) and 0.6 M sorbitol, with or without the addition of 50 mM NaCl. PPO (protoporphyrinogen; 1 μM final concentration) was added, and formation of fluorescent protoporphyrin IX was followed (excitation at 410 nm and emission at 632 nm). After 100 s, 1 μM iron was added from an anaerobic stock solution of ferrous ammonium sulfate, and recording was continued for a total of 300 s. At this time, an emission scan was collected from 550 to 700 nm. To ascertain the incorporation of radioactive iron into haem, PPO was added to intact mitochondria followed by radioactive iron (1 μM [55Fe]ferrous ascorbate, 100 mCi/mg; CNL Scientific). After a 5 min incubation at 30°C, the sample was acidified with 0.2 M HCl. Radioactive haem was extracted with methyl ethyl ketone, and radioactivity was counted in a Beckman scintillation counter.
Fe–S synthesis assays
The kinetics of Fe–S cluster synthesis under different conditions was ascertained in isolated intact mitochondria by incubation in the presence of [35S]cysteine and nucleotides, with or without added iron (10 μM ferrous ascorbate). The radiolabelled Fe–S cluster of endogenous aconitase was visualized by native gel electrophoresis and radioautography [23].
TTP transport assays
TTP uptake and export were tested in isolated mitochondria in the same buffers and time frames used for measuring haem synthesis. Intact mitochondria were incubated with tritiated TTP ([methyl-3H]TTP, tetrasodium salt, 6 nM final concentration; MP Biomedical) for various times, and mitochondria-associated radioactivity was determined. For export, mitochondria were incubated with radioactive TTP, the buffer was changed, and an additional incubation was performed for 10 min. Supernatant and pellet were separated, and the radioactivity was measured for each fraction.
Miscellaneous procedures
PPO was prepared from protoporphyrin by reduction with sodium amalgam [24]. Ferrochelatase was measured in mitochondrial membranes as follows: Rim2-expressing or Rim2-depleted mitochondria (250 μg of protein) were ruptured by sonication. Membranes were recovered and exposed to buffer containing 10 mM EDTA to remove endogenous metals. EDTA was removed by buffer changes. In an anaerobic cuvette, 1 μM protoporphyrin IX was added and formation of zinc protoporphyrin was measured by following the fluorescence emission over time (excitation at 420 nm, emission at 587 nm). Rim2-expressing and Rim2-depleted mitochondria showed equivalent activities. Low temperature spectra analysis ( – 191°C) of whole cells was performed as described previously [25]. An antibody against Rim2 protein was made by injecting rabbits with the unique Rim2 peptide (SIEKFGYQAEGTKSTSEKVKEWC) conjugated to KLH (keyhole-limpet haemocyanin) followed by affinity-purification of the immune serum (Covance).
RESULTS
Genetics
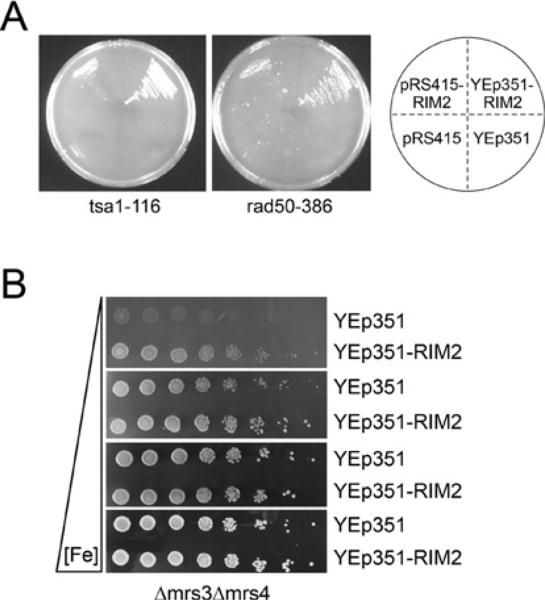
A number of complementation groups were identified in a sectoring screen for mutants that are synthetically lethal with Δmrs3/Δmrs4 double mutants [20]. These included genes involved in iron metabolism (DRE2), in DNA repair (RAD50, RAD55) and oxidative stress protection (TSA1). Curiously, genomic fragments of RIM2 complemented all of these synthetic lethal groups (Figure 1A). The complementing activity was shown to reside in the RIM2 open reading frame and was mediated by low (pRS415) or high (YEp351) copy-number expression of the genomic fragment (Figure 1A). Since these various synthetic lethal mutants shared their relationship to Mrs3 and Mrs4, these genetic data suggested that Rim2 was substituting for Mrs3 and/or Mrs4. Consistent with this idea, the slow growth of the Δmrs3/Δmrs4 mutant on low-iron medium was improved by Rim2 overexpression (Figure 1B). A recently published description of another genetic screen, involving abrogation of iron toxicity in a ccc1 mutant deficient for vacuolar iron import, also identified RIM2, MRS3 and MRS4, again suggesting that they might perform related functions [26].
Figure 1. Synthetic lethality with Δmrs3/Δmrs4 complemented by RIM2.
(A) A sectoring screen was performed to find mutations that conferred synthetic lethality when combined with Δmrs3/Δmrs4 as described previously [20]. The synthetic lethal mutant J116 (tsa1-116 Δmrs3Δmrs4 [pTSV31A-MRS4-URA3]) was complemented by low-(pRS415-RIM2) and high-copy (YEp351-RIM2)-containing plasmids carrying the RIM2 open reading frame and flanking regions. The transformants were streaked on 5-fluoroorotic acid plates to remove the covering copy of MRS4, and only the RIM-containing transformants were viable (left-hand side). Similarly, the synthetic lethal mutant J386 (rad50-386 Δmrs3/Δmrs4 [pTS-V31A-MRS4-URA3]) was analysed and only the RIM2-containing transformants were viable (right-hand side). These mutants were also complemented by TSA1 for J116 and RAD50 for J386 respectively. (B) RIM2 overexpression improves the iron-dependent growth defect of the Δmrs3/Δmrs4 mutant. The Δmrs3/Δmrs4 strain [MATa ura3-52 lys2-801(amber) trp1-Δ1 ade2-101(ochre) his3-Δ200 cyh2 Δmrs4::KAN Δmrs3::URA3] was transformed with a high-copy-number plasmid carrying the genomic fragment of RIM2 or empty plasmid. Serial dilutions of the transformants were spotted on to defined medium plates with different available iron concentrations. All plates contained standard defined medium, 1 mM ferrozine and 50 mM Mes buffer (pH 6.5). In addition, 0, 25, 100 and 350 μM ferrous ammonium sulfate (from top to bottom) were added back.
Rim2 overexpression partially corrects defective iron use in triple-mutant mitochondria (Δmrs3Δmrs4Δyfh1)
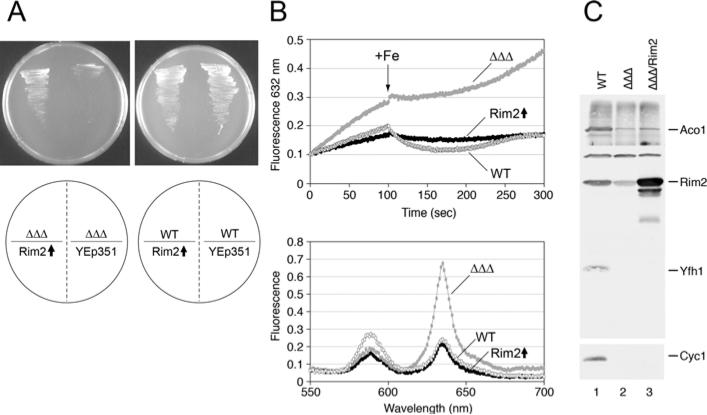
The frataxin mutant Δyfh1 and the Δmrs3/Δmrs4 mutant have been previously shown to exhibit negative synthetic interaction, such that the triple mutant was extremely sick and slow-growing [14,21]. The complex biochemical phenotype of this mutant was characterized by severe deficiency of haem and Fe–S clusters in mitochondria [14,21]. Rim2 overexpression was able to partially rescue the growth defect (Figure 2A). There was also a small correction (~2-fold) of the respiratory defect in oxygen consumption, but only to 3% of WT (wild-type) levels (results not shown). Haem synthesis in isolated mitochondria was assessed using a fluorescence assay [17]. In this assay, isolated intact mitochondria were incubated with non-fluorescent porphyrin precursor, PPO, and the time-dependent appearance of protoporphyrin IX fluorescence was followed. The initial slope of protoporphyrin IX production reflects the amount of endogenous iron available for haem synthesis (iron already present in mitochondria at the time of isolation). A greater slope indicates less available iron and a lesser slope indicates more available iron. The greater slope in the Δmrs3Δmrs4Δyfh1 mutant mitochondria was consistent with a lack of bioavailable iron in this mutant, and a consequent increase in porphyrin fluorescence. Rim2 overexpression was associated with a decrease in the initial slope (Figure 2B, top panel), consistent with more bioavailable iron resulting from Rim2 expression. The effect of iron added from outside mitochondria during the assay was also tested. In the triple mutant, addition of 1 μM iron at 100 s produced a minor deflection of the protoporphyrin IX curve. In the Rim2-overexpressing mutant, there was an immediate and continuous decrease in the fluorescence, reflecting conversion of protoporphyrin IX into haem. The tracings resemble those from assaying mitochondria from the unmanipulated WT strain (Figure 2B, top panel). Emission scans performed after the time course confirmed that less fluorescent protoporphyrin IX remained, indicating more haem synthesis in the mitochondria overexpressing Rim2 (Figure 2B, bottom panel). These experiments were performed several times with similar results, using ferrous ascorbate or ferrous ammonium sulfate at different concentrations (0.5–20 μM). In all cases, Rim2 overexpression enhanced use of both endogenous iron and transported iron for haem synthesis. However, although Rim2 overexpression was able to enhance iron use for haem in isolated mitochondria from this triple-mutant strain, steady-state levels of cytochrome c, a haem protein, remained quite low, as reported for the Δyfh1 single-mutant phenotype (Figure 2C). The discrepancy could be due to a failure of haem attachment or increased haem protein turnover in the cells. Rim2 protein was markedly decreased in the triple mutant compared with WT YPH499 (Figure 2C), suggesting that the protein level is regulated. The mode of regulation (transcriptional, post-transcriptional or protein stability) has not been ascertained.
Figure 2. RIM2 overexpression improves growth of the Δmrs3Δmrs4Δyfh1 triple mutant.
(A) The triple mutant Δmrs3Δmrs4Δyfh1 was maintained anaerobically and transformed with empty plasmid YEp351 or YEp351-RIM2. The WT control strain YPH499 was handled similarly. The transformants were then streaked on to standard defined medium in air. The triple mutant transformed with RIM2 showed enhanced growth compared with empty plasmid (left-hand plate, left side). (B) Rim2 overexpression restores the rate of iron insertion into porphyrin in Δmrs3Δmrs4Δyfh1 mutant mitochondria. Mitochondria (0.5 mg) were treated with 5 mM NADH for 2 min. PPO (2 μM) was added and protoporphyrin IX fluorescence was measured over time (excitation at 410 nm, emission at 632 nm). After 100 s, 1 μM ferrous ammonium sulfate was added and tracing was continued. At the end of the 5 min time trace, fluorescent emissions were scanned from 550 nm to 700 nm (bottom panel). Cells were cultured under low oxygen in argon-bubbled flasks in defined glucose medium and then shifted to air for 4 h in defined raffinose medium. Mitochondria were isolated. white circle, YPH499; black circle, Δmrs3Δmrs4Δyfh1[YEp351-RIM2]; grey circle, Δmrs3Δmrs4Δyfh1. (C) Immunoblot analysis of mitochondrial proteins from: WT YPH499 (lane 1); ΔΔΔ, Δmrs3Δmrs4Δyfh1 (lane 2); ΔΔΔ/Rim2, triple mutant transformed with YEp351-Rim2 (lane 3). Mitochondria (100 μg equivalent) were separated by SDS/PAGE (13% gel) and transferred on to a Protran nitrocellulose membrane for blotting with antibodies as indicated. Aco1, aconitase; Rim2, Yfh1 (yeast frataxin homologue); Cyc1, cytochrome c.
Rim2 effects on cytochromes in the absence of Mrs3 and Mrs4
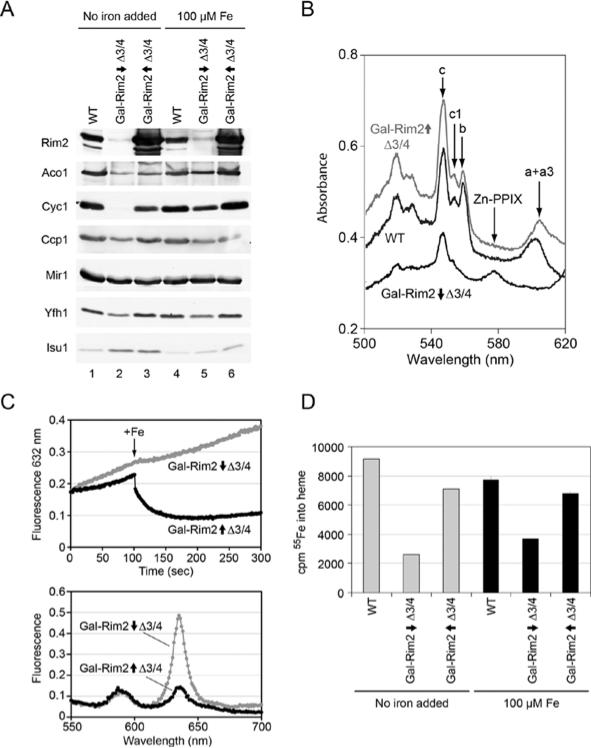
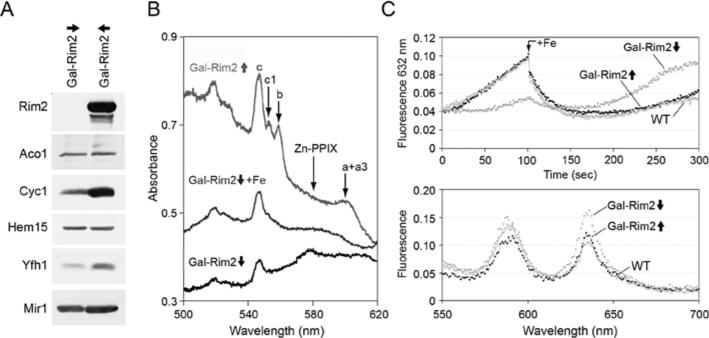
RIM2 deletion was found to be non-viable in some yeast genetic backgrounds (YPH499/500). This is surprising given that the supposed function of the transporter is to deliver pyrimidines into mitochondria for use in transcription and replication [19]. The latter are non-essential functions in yeast since even rho◦ strains completely lacking the mitochondrial genome can grow by fermentation. The essentiality of RIM2 in some yeast genetic backgrounds therefore suggests another more critical function of this protein. To better define the role of RIM2, the open reading frame was placed under the control of the carbon-source-sensitive GAL1 promoter. Initial experiments were performed in the Δmrs3/Δmrs4 background to remove potentially redundant functions. The promoter in this strain, Gal-Rim2, was turned on or off by growth in galactose or raffinose respectively. The raffinose-grown cultures tended to stop growing, owing to effects of Rim2 depletion and iron limitation. Therefore the time course of depletion could not be extended beyond 24 h. Growth in galactose or raffinose respectively for shorter time frames still conferred high-level expression or significant depletion of Rim2 protein (Figure 3A, lanes 1–3). A second set of cells was grown in iron-supplemented medium with Rim2 induced or repressed (Figure 3A, lanes 4–6).
Figure 3. Haem proteins and haem synthesis in Gal-Rim2 Δmrs3/Δmrs4.
(A) Immunoblots of mitochondrial proteins. Mitochondria were isolated from WT grown in raffinose medium (YPH499, lane 1), or from Gal-Rim2 Δmrs3Δmrs4 grown in raffinose medium (Gal-Rim2↓Δ3/4, lane 2) or galactose medium (Gal-Rim2↑Δ3/4, lane 3). A second set of cultures was supplemented with 100 μM ferrous ammonium sulfate (lanes 4–6). Mitochondria were analysed by immunoblotting with antibodies against mitochondrial proteins. (B) Cytochromes in whole cells. Cells from WT (middle trace, YPH499), Rim2-induced (top trace, Gal-Rim2↑Δ3/4) or Rim2-repressed (bottom trace, Gal-Rim2↓Δ3/4) were evaluated for their low temperature (– 191°C) UV–visible spectra [25]. Cells were grown for 48 h in defined medium with raffinose (WT and repressed cells) or raffinose-galactose (induced cells) as the carbon source. Cytochrome peaks (c, c1, b, a + a3) and the zinc protoporphyrin peak (Zn-PPIX) are indicated. (C) A fluorescence assay for haem synthesis from added porphyrin and iron in isolated mitochondria. Rim2-induced (black trace, Gal-Rim2↑Δ3/4) or Rim2-repressed (grey trace, Gal-Rim2↓Δ3/4) mitochondria were evaluated as described in the legend to Figure 2(B). Time traces are shown in the top panel and emission scans are shown in bottom panel. (D) 55Fe incorporation into haem. Mitochondria were isolated from YPH499, Gal-Rim2↓Δ3/4, Gal-Rim2↑Δ3/4 or the same grown in standard defined or iron-supplemented media as described in (A). Haem synthesis was measured as follows: mitochondria (0.2 mg) were incubated with 5 mM NADH and 2 μM PPO for 3 min at room temperature (25°C), followed by the addition of 5 μM ferrous [55Fe]ascorbate. After a 5 min incubation, the reaction was stopped by the addition of hydrochloric acid and haem-associated 55Fe was determined by organic extraction with methyl ethyl ketone and scintillation counting of the radionuclide. Results shown are from three assays, and are means ± S.D.
In these experiments, the abundance of several proteins involved in iron metabolism was altered, as assessed by immunoblotting. Aconitase, a constituent of the tricarboxylic acid cycle and the most abundant Fe–S cluster protein in mitochondria, was decreased in the Rim2-repressed mitochondria, and was slightly increased in the Rim2-induced mitochondria (Figure 3A, lanes 2 and 3). Iron addition to the growth medium led to further increases of aconitase protein (Figure 3A, lanes 4 and 5). Yfh1, the yeast frataxin homologue, is implicated in Fe–S cluster assembly, and has been shown to be responsive to iron availability via a post-transcriptional mechanism [27]. In this case, Yfh1 protein was decreased only in the Gal-Rim2-repressed Δmrs3/Δmrs4 mitochondria (Figure 3A, lane 2). Isu1/2, the scaffold proteins involved in Fe–S cluster assembly, showed increased levels in standard medium with or without Rim2 expression (Figure 3A, lanes 2 and 3). With regard to haem proteins, cytochrome c was not detected in the Rim2-repressed condition (Figure 3A, lane 2), whereas it was restored almost to WT levels in the Rim2-induced condition (Figure 3A, lane 3). Addition of iron to the growth medium was able to partially, but not fully, restore cytochrome c levels in the absence of Rim2 expression. Ccp1, another haem protein, was also decreased in the Rim2-depleted cells (Figure 3A, lane 2). Thus Rim2 expression in the Δmrs3/Δmrs4 context was associated with various changes of proteins involved in iron metabolism, including haem and Fe–S cluster proteins.
Cytochromes were examined more globally by spectral analysis of whole cells. In the Rim2-depleted Δmrs3/Δmrs4 condition, cytochromes were severely deficient (Figure 3B, bottom trace). The effect was not uniform for all cytochromes. Thus the cytochrome oxidase aa3 signal and cytochrome b signals were undetectable. By contrast, cytochromes c and and c1 were decreased, but still detectable. The appearance of zinc porphyrin (Figure 3B, bottom trace) indicated that the effect was due to iron deprivation. Zinc insertion into porphyrin by ferrochelatase occurs only if the preferred substrate, iron, is totally unavailable [3]. In the Rim2-overexpressing cells (Figure 3B, top trace), even in the absence of Mrs3/Mrs4, there was complete restoration of cytochromes, and the levels were even slightly higher than in the WT with intact MRS3 and MRS4 loci. The aa3 peak was slightly red-shifted compared with the WT for unknown reasons (Figure 3B).
Rim2 promotes haem synthesis in isolated mitochondria in the absence of Mrs3 and Mrs4
In the fluorescence assay, Rim2 (Δmrs3/Δmrs4)-depleted mitochondria were shown to be defective for haem synthesis from endogenous iron, as indicated by the increase in the initial slope of protoporphyrin IX formation (Figure 3C, top compared with bottom tracing). The efficient use of exogenous iron added during the assay was also highly dependent on Rim2 expression. In the absence of Rim2 expression, little change in slope was observed following iron addition (1 μM ferrous ammonium sulfate) after 100 s (Figure 3C, top panel), indicating little haem synthesis, and this was confirmed by a high level of residual protoporphyrin IX detected at the end of the assay (Figure 3C, bottom panel). In the presence of Rim2, by contrast, the addition of iron was associated with a rapid and continuous decrease in protoporphyrin IX fluorescence, consistent with efficient haem synthesis (Figure 3C, top panel), and fluorescent protoporphyrin IX was depleted owing to its conversion into non-fluorescent haem (Figure 3C, bottom panel). To further validate these results, haem synthesis was measured in a different manner. PPO and iron ([55Fe]ferrous ascorbate) were incubated with isolated intact mitochondria, and after several minutes, iron radionuclide incorporated into haem was extracted with an organic solvent. Rim2-depleted (Δmrs3/Δmrs4) mitochondria showed defective haem synthesis compared with the WT. Rim2-overexpressing (Δmrs3Δmrs4) mitochondria recovered significant haem synthesis capability, although not quite to WT levels (Figure 3D). Growth of cells in high iron only minimally altered this pattern. Ferrochelatase measured in mitochondrial membranes from Rim2-induced and -repressed mitochondria was equivalent, and thus the Rim2 effect on haem synthesis was not due to modulation of ferrochelatase activity (results not shown). In summary, haem proteins and haem synthesis were markedly dependent on Rim2 protein levels in the Δmrs3/Δmrs4 background. In terms of cytochrome levels in cells and haem synthesis activity in isolated mitochondria, the high level expression of Rim2 was able to partially, if not completely, bypass the need for Mrs3 and Mrs4.
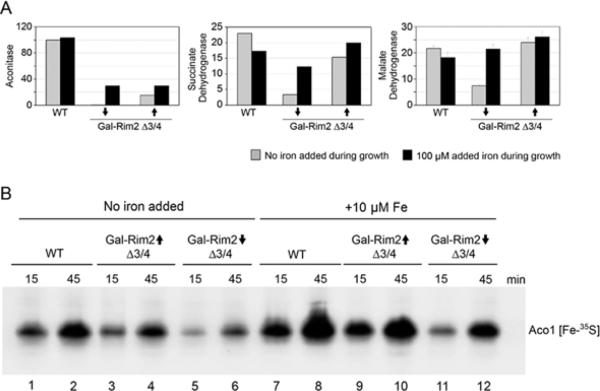
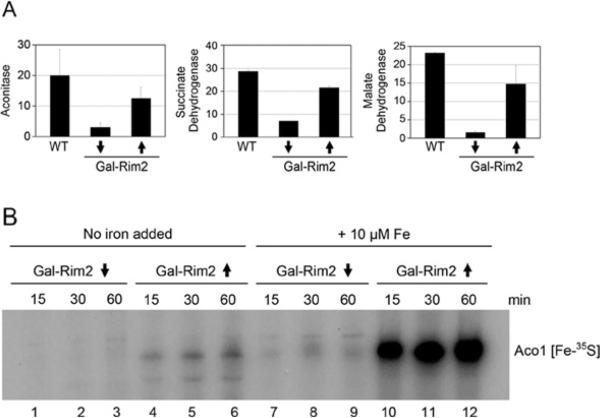
Rim2 promotes Fe–S cluster synthesis in the absence of Mrs3 and Mrs4
Fe–S cluster proteins represent another major destination for iron in mitochondria. Fe–S cluster enzyme activities for aconitase and succinate dehydrogenase were measured in the Gal-Rim2 Δmrs3/Δmrs4 strain under different conditions. For aconitase (Figure 4A, left-hand panel), activity was undetectable in the absence of Rim2 and only a small amount was restored by Rim2 expression. A further increment was observed in the high-iron grown cells, but the activity remained low compared with WT. For succinate dehydrogenase (Figure 4A, middle panel), a protein with haem and Fe–S cofactors, Rim2 expression was correlated with an increase in activity of more than 3-fold, but not quite to WT levels. The synthesis of new Fe–S clusters was then examined. In this assay, [35S]cysteine was added to isolated intact mitochondria, and incorporation into apoaconitase was evaluated to determine the efficiency and kinetics of Fe–S cluster synthesis [23]. In mitochondria lacking Rim2 (Gal-Rim2-depleted Δmrs3/Δmrs4), Fe–S cluster formation was impaired in terms of speed and efficiency compared with the WT (Figure 4B, compare lanes 5 and 6 with lanes 1 and 2). In cells expressing Rim2 (Gal-Rim2-induced Δmrs3/Δmrs4), Fe–S cluster synthesis on aconitase was improved, although not to WT levels (Figure 4B, compare lanes 3 and 4 with lanes 1 and 2). Iron addition to the mitochondria during the assay further enhanced loading, although Rim2-dependence could still be discerned (Figure 4B, compare lanes 9 and 10 with lanes 11 and 12). When high Rim2 expression and iron addition were combined, the level of Fe–S cluster synthesis (Gal-Rim2-induced Δmrs3/Δmrs4) approached WT levels (Figure 4B, compare lanes 9 and 10 with lanes 7 and 8). In summary, Rim2 was shown to promote the efficiency of new Fe–S cluster formation in isolated mitochondria lacking Mrs3 and Mrs4. Similar to the haem experiments, Rim2-dependent Fe–S cluster synthesis was characterized by improved utilization of endogenous iron accumulated during cell growth or exogenous iron added during the assays with isolated mitochondria.
Figure 4. Fe–S cluster proteins and Fe–S cluster synthesis in Gal-Rim2 Δmrs3/Δmrs4.
(A) Enzyme activities for aconitase (left-hand panel), succinate dehydrogenase (middle panel) and malate dehydrogenase (right-hand panel) were measured in isolated mitochondria from YPH499, Gal-Rim2↓Δ3/4 or Gal-Rim2↑Δ3/4 cells grown in standard medium (grey bars) or medium supplemented with 100 μM iron (black bars). Results shown are arbitrary units from three assays, and are means ± S.D. (B) Fe–S cluster synthesis. Mitochondria from WT (lanes 1, 2, 7 and 8), Gal-Rim2↑Δ3/4 (lanes 3, 4, 9 and 10) or Gal-Rim2↓Δ3/4 (lanes 5, 6, 11 and 12) were incubated with [35S]cysteine for various times (15 or 45 min), followed by separation of the matrix fraction on a native gel. In some samples (lanes 7–12), iron (10 μM ferrous ascorbate) was added during the labelling reaction. The labelled band, Aco1[Fe-35S], represents newly formed Fe–S clusters on aconitase.
Iron homoeostatic defect
A possible explanation for defective iron metabolism in Rim2-depleted mitochondria could be that iron levels are low or iron solubility is perturbed. To evaluate these possibilities, cells were grown in the presence of radioactive iron, followed by cell fractionation. The depletion of Rim2 (Δmrs3/Δmrs4) was associated with markedly increased cellular iron uptake compared with Rim2-expressing (Δmrs3/Δmrs4) cells (results not shown). Mitochondrial iron accumulation was also increased in the Rim2-depleted cells compared with Rim2-expressing cells (14×103 compared with 2.5×103 c.p.m. per mg of mitochondrial protein). Furthermore, in both cases, a significant portion of the mitochondrial iron was soluble (4.2×103 c.p.m. per mg of mitochondrial protein for Rim2-depleted compared with 1.8 × 103 c.p.m. per mg of mitochondrial protein for Rim2-expressing mitochondria). In this respect, the iron accumulation in the Gal-Rim2-depleted Δmrs3/Δmrs4 mitochondria differs from the accumulation occurring in Fe–S cluster assembly mutants. In the latter case, iron in mitochondria was found to precipitate as iron-phosphate nanoparticles, which exhibit low solubility [17,28]. In summary, mitochondria from the Rim2-depleted Δmrs3/Δmrs4 cells accumulated more iron and more soluble iron than the paired Rim2-expressing sample. However, Rim2-depleted mitochondria failed to utilize the iron efficiently for haem or Fe–S cluster synthesis, suggesting a role for Rim2 in promoting iron utilization.
Unique role for Rim2 expression in a WT context
Rim2 effects were examined in a WT context, that is, in the presence of normal chromosomal loci for MRS3 and MRS4. The initial expectation for these experiments was that, because of overlapping functions with Mrs3 and Mrs4, there would be only minor iron-related defects associated with Rim2 depletion. This was not the case. Rim2-depleted mitochondria exhibited striking abnormalities of iron enzymes and iron use. Immunoblotting of the protein patterns confirmed regulated expression of Rim2 (Figure 5A), and again showed decreased cytochrome c in the Rim2-depleted state. Yfh1 protein abundance was decreased, consistent with functional iron deficiency. The abundance of the aconitase protein and the phosphate carrier protein Mir1 were unchanged (Figure 5A).
Figure 5. Haem proteins and haem synthesis in the Gal-Rim2 strain with intact MRS3 and MRS4.
(A) Mitochondria from Gal-Rim2↓ or Gal-Rim2↑ in a WT context were subjected to immunoblotting for Rim2, aconitase (Aco1), cytochrome c (Cyc1), ferrochelatase (Hem15), yeast frataxin homologue (Yfh1) and phosphate carrier protein (Mir1). (B) Cytochromes in whole cells. Cells from the Rim2-induced (Gal-Rim2↑) or Rim2-repressed (Gal-Rim2↓) cultures were evaluated for their low temperature UV–visible spectra [25]. In a separate culture, the repressed Gal-Rim2 strain was grown in raffinose medium supplemented with 100 μM iron as ferric ammonium sulfate (Gal-Rim2↓ + Fe). Low temperature UV–visible spectra were collected [25]. Cytochrome peaks (c, c1, b, a + a3) and the zinc protoporphyrin peak (Zn-PPIX) are indicated. (C) Fluorescent haem synthesis assay. Mitochondria were incubated with PPO for 100 s followed by iron addition (1 μM ferrous ammonium sulfate) and protoporphyrin IX fluorescence was monitored. Open circle, WT or YPH499; black circle, Gal-Rim2↑; grey circle, Gal-Rim2↓). Assays were performed as described in the legend for Figure 2.
Cytochrome spectra were examined, comparing whole cells with Gal-Rim2-induced or Gal-Rim2-repressed mitochondria. MRS3 and MRS4 were intact. However, Rim2 depletion by itself was associated with marked attenuation of cytochromes, including almost complete disappearance of cytochrome aa3 and cytochrome b signals. Cytochrome c was also diminished, and zinc porphyrin was detected, indicating functional iron deficiency in mitochondria (Figure 5B). If the raffinose culture (Gal-Rim2-repressed) was supplemented with iron, the zinc porphyrin signal disappeared, indicating that less zinc was available or more iron was available to ferrochelatase. However, cytochrome deficiency persisted and was minimally corrected by iron supplementation (Figure 5B).
Mitochondria were isolated from WT, Gal-Rim2-induced and Gal-Rim2-repressed cultures. In the fluorescent porphyrin assay for haem synthesis in isolated mitochondria (Figure 5C), Rim2 overexpression had no discernable effect. By contrast, the protoporphyrin IX tracing from the Rim2-depleted mitochondria responded briefly to added iron and then resumed an upward trend (Figure 5C), indicating a problem with iron use. The radioactive haem extraction assay confirmed defective haem formation in isolated mitochondria lacking Rim2 (as shown in Figure 8). Similarly Fe–S cluster protein activities (succinate dehydrogenase and aconitase) were very low in the Rim2-depleted cells and partially restored in Rim2-overexpressing cells (Figure 6A). In the radionuclide assay for Fe–S cluster loading of aconitase in isolated mitochondria, both Rim2 and iron effects were discerned (Figure 6B). In the Rim2-depleted mitochondria with MRS3 and MRS4 intact, there was virtually no detectable radiolabelled Fe–S cluster formed on aconitase (Figure 6B, lanes 1–3). This result is consistent with lack of aconitase activity in these mitochondria, although the protein was present in normal amounts. Expression of Rim2 (Figure 6B, lanes 4–6) or addition of iron during the assay restored a small amount of Fe–S cluster-loading activity (Figure 6B, lanes 7–9). However, in the presence of Rim2 expression and added iron, there was extremely rapid and efficient loading, such that the initial time point at 15 min showed the maximal effect (Figure 6B, lanes 10–12). This highly efficient Fe–S cluster formation was enhanced even compared with mitochondria from a WT strain (results not shown). Taken together, the results indicate that Rim2 serves a unique function in Fe–S cluster synthesis in mitochondria. Rim2 expression influenced Fe–S cluster synthesis from both endogenous iron and exogenous iron added during in vitro assays with isolated mitochondria. As in the case of haem synthesis, Rim2-depleted cells and isolated mitochondria were markedly deficient for Fe– S cluster synthesis. Furthermore, this deficiency was observed despite intact loci for MRS3 and MRS4.
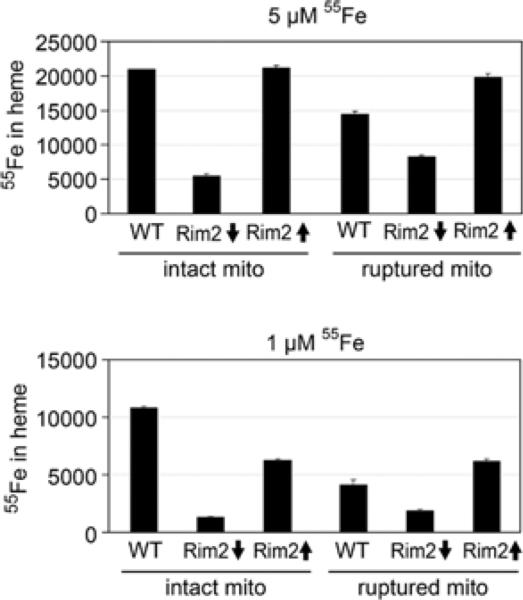
Figure 8. Iron incorporation into haem in intact mitochondria or mitochondrial lysate.
Mitochondria were isolated from WT, Gal-Rim2↓ or Gal-Rim2↑ cultures. In some cases, mitochondria were ruptured by sonication in hypotonic buffer [50 mM Tris/HCl (pH 7.5)]. Top panel: haem synthesis was measured following the addition of 2 μM PPO and 5 μM [55Fe]ferrous ascorbate to intact mitochondria or mitochondrial lysate. After a 5 min incubation at room temperature (25°C), radiolabelled haem was extracted and counted in a scintillation counter. Bottom panel: haem synthesis was measured as described above, except that the iron concentration for the assay was 1 μM [55Fe]ferrous ascorbate.
Figure 6. Fe–S cluster proteins and Fe–S cluster synthesis in Gal-Rim2 with intact MRS3 and MRS4.
(A) Enzyme activities for aconitase (left-hand panel), succinate dehydrogenase (middle panel) and malate dehydrogenase (right-hand panel) were measured for mitochondria from Gal-Rim2↓ or Gal-Rim2↑. Results shown are arbitrary units from three assays, and are means ± S.D. (B) Fe–S cluster synthesis. Mitochondria from Gal-Rim2↓ (lanes 1–3 and 7–9) or Gal-Rim2↑ (lanes 4–6 and 10–12), were examined for Fe–S cluster synthesis activity by labelling with [35S]cysteine and separation of the matrix fraction on a native gel. In some samples (lanes 7–12), 10μM ferrous ascorbate was added during the labelling reaction. Labelling reactions were performed for 15, 30 or 60 min in each case. The labelled band, Aco1[Fe-35S], represents newly formed Fe–S clusters on aconitase.
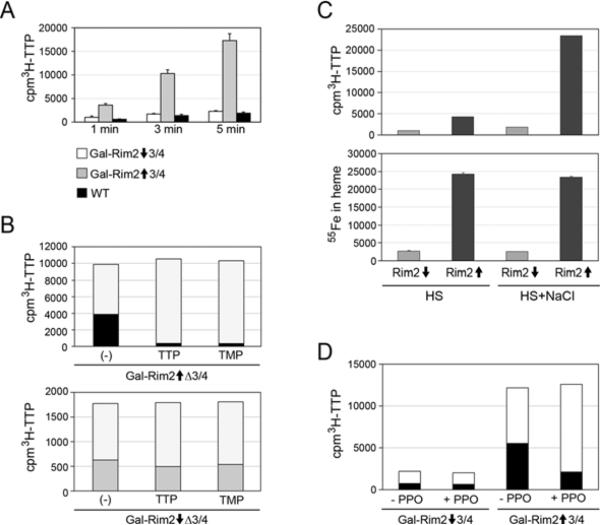
Pyrimidine import and export (exchange) by Rim2 in isolated mitochondria
In a previously published study [19], Rim2 protein incorporated into vesicles was shown to mediate exchange of various pyrimidine nucleotides and deoxynucleotides. To confirm the role of Rim2 as a pyrimidine transporter, uptake of [3H]TTP into isolated mitochondria was examined. In the same Gal-Rim2 Δmrs3/Δmrs4 mitochondria that exhibited large changes in haem and Fe–S cluster synthesis dependent upon Rim2 expression, pyrimidine uptake was affected in a concordant manner. In a 5 min time course, TTP uptake was promoted 8-fold in Rim2-expressing compared with Rim2-depleted mitochondria (Figure 7A). Rim2-dependent pyrimidine export was also detected. If intact mitochondria were allowed to take up radiolabelled TTP and then incubated in buffer, release of radiolabel was enhanced in the Rim2-expressing mitochondria (Figure 7B, white portion of the histograms represents exported TTP, compare the top and bottom panels). Consistent with an antiport function for Rim2, export of radiolabelled TTP from mitochondria was enhanced by addition of pyrimidine nucleotides to the assay buffer (Figure 7B, top panel). The nucleotide-dependent exchange did not occur in mitochondria lacking Rim2 expression (Figure 7B, bottom panel).
Figure 7. Rim2 acts as a pyrimidine exchanger in mitochondria.
(A) TTP uptake. Mitochondria energized with NADH were incubated with 0.4 μCi (6 nM final) of [3H]TTP for 5 min at room temperature (25°C). After washes in buffer [50 mM Tris/HCl (pH 7.5) and 0.6 M sorbitol], the radioactivity of the mitochondrial pellet was measured in a scintillation counter. White bars, Gal-Rim2↓Δ3/4; grey bars, Gal-Rim2↑Δ3/4; and black bars, YPH499. (B) [3H]TTP exchange. Gal-Rim2↑Δ3/4 or Gal-Rim2↓3/4 mitochondria were labelled with [3H]TTP and unincorporated radioactivity was removed. Mitochondria were resuspended in 100 μl of buffer [50 mM Tris/HCl (pH 7.5) and 0.6 M sorbitol] with no nucleotides, or with 1 mM TTP or 1 mM TMP. After 10 min incubation at room temperature, samples were centrifuged to separate mitochondria and the supernatant, and the radioactivity was determined by scintillation counting. Top panel, Gal-Rim2↑Δ3/4 mitochondria, white boxes indicate the supernatant, black boxes indicate the mitochondrial pellet. Bottom panel, Gal-Rim2↓Δ3/4 mitochondria, white boxes indicate supernatant, grey boxes indicate the mitochondrial pellet. TTP- or TMP-dependent export of [3H]TTP radionuclide occurs only in the setting of Rim2 expression. (C) [3H]TTP uptake and 55Fe incorporation into haem in parallel experiments. Top panel, [3H]TTP uptake. Gal-Rim2↑ or Gal-Rim2↓ mitochondria were tested for radionuclide TTP uptake in HS buffer [50 mM Hepes/KOH and 0.6 M sorbitol] or the same buffer with 50 mM NaCl added (HS + NaCl). Uptake was allowed to proceed for 5 min at room temperature. Mitochondrial-associated [3H]TTP was determined in triplicate assays. Bottom panel, 55Fe incorporation into haem. Iron (5 μM [55Fe]ascorbate) and porphyrin (2 μM PPO) were added to mitochondria in HS buffer or HS + NaCl. Uptake was allowed to proceed for 5 min and haem was extracted. (D) Porphyrin stimulation of Rim2-dependent TTP exchange in mitochondria. Gal-Rim2↑Δ3/4 or Gal-Rim2↓Δ3/4 mitochondria were incubated with [3H]TTP to load them and unincorporated radioactivity was removed. The mitochondria were resuspended in buffer [50 mM Tris/HCl (pH 7.5) and 0.6 M sorbitol] with or without 1 μM PPO. Exchanges was allowed to proceed for 10 min, and supernatant and mitochondria were separated and subjected to scintillation counting. White boxes, supernatants; black boxes, mitochondrial pellets.
Thus the same mitochondria that exhibited deficient iron utilization for haem and Fe–S cluster synthesis, owing to lack of Rim2, also exhibited deficient pyrimidine uptake. A connection between iron and pyrimidine functions of Rim2 was investigated. Mitochondria from Rim2-depleted or Rim2-overexpressing cells were pre-incubated with pyrimidines (1 mM TTP, TMP or ATP) for 5 min. The mitochondria were recovered by centrifugation and incubated with [55Fe]ascorbate and porphyrin (2 μM PPO), prior to extraction of radiolabelled haem. There was no effect of the nucleotide exposure on iron incorporation into haem or on the Rim2-dependence for this activity (results not shown).
On the other hand, we sought to inhibit Rim2 activity. An accidental observation was that Rim2-mediated pyrimidine exchange was highly dependent on the presence of chloride in the assay buffer. In Hepes/KOH buffer in the absence of NaCl, Rim2 pyrimidine transport activity was decreased by 4–5-fold compared with the same buffer in the presence of NaCl. However, haem synthesis in isolated mitochondria was equally dependent on Rim2 and was unaffected by these manipulations of chloride in the buffer (Figure 7C). These measurements were not dependent on porphyrin concentrations (from 2–10 μM PPO) because iron was limiting for haem synthesis under these conditions. In summary, buffer chloride had a pronounced effect in promoting TTP exchange, and yet there was little or no effect on iron uptake and utilization, suggesting that these two functions of Rim2 are independent.
Finally, regulation of Rim2 pyrimidine uptake was tested. Iron itself did not alter pyrimidine transport activity. However, porphyrin added as PPO to isolated mitochondria expressing Rim2 was associated with enhanced Rim2-exchange activity. Rim2-expressing mitochondria preloaded with [3H]TTP exported more radiolabelled nucleotide when exposed to the porphyrin (Figure 7D). The enhanced export was observed only when chloride was present in the assay buffer (results not shown). Thus there was a positive regulatory effect of the porphyrin precursor in enhancing Rim2-dependent pyrimidine exchange in isolated mitochondria.
Rim2-depleted mitochondria exhibit a defect in iron use in lysed mitochondria
Mitochondria were ruptured and substrates were added directly to the lysate in order to bypass potential transport defects. Rim2-overexpressing or -depleted mitochondria were ruptured by sonication, and 55Fe and porphyrin precursor were added to the lysate followed by organic extraction of haem. The haem synthesis activities in intact or ruptured mitochondria were compared (Figure 8). As observed previously, intact mitochondria lacking Rim2 showed deficient haem synthesis as compared with Rim2-expressing mitochondria. Surprisingly, the defect in haem synthesis persisted in the Rim2-depleted mitochondria even following rupture. Lysate from Rim2-overexpressing mitochondria demonstrated increased haem synthetic activity compared with the WT lysate, perhaps suggesting accumulation of a factor in this lysate that promotes iron use. Although these results do not rule out a function for Rim2 in transporting iron in some form, they show that Rim2 has another function in mediating iron use in mitochondria.
DISCUSSION
Rim2 is a mitochondrial carrier protein which has been shown in studies with reconstituted vesicles to act as an exchanger for pyrimidines and deoxypyrimidines. Rim2 is thought to bring pyrimidine triphosphates from the cytoplasm into mitochondria in exchange for pyrimidine monophosphates that move in the opposite direction [19]. Pyrimidine triphosphates are needed for maintenance of the mitochondrial genome and its functions in replication, transcription and translation. The surprising finding is that Rim2, in addition to its role in pyrimidine transport, also functions in iron metabolism. Isolated mitochondria depleted of Rim2 simultaneously manifest a defect in pyrimidine transport and a defect in iron utilization. Perhaps this is the explanation for the essentiality of the gene in most yeast genetic backgrounds. Fe–S and haem are essential cofactors and a problem with their formation could lead to a lethal phenotype. In contrast, mitochondrial pyrimidines should be dispensable for viability, since yeast may grow by fermentation and the mitochondrial genome encodes non-essential functions in this organism. Rim2 was originally identified as MRS12, a high-copy suppressor of a mitochondrial RNA splicing defect, and a link to metal transport (in that case magnesium) was proposed [29]. Genetic data show that the mitochondrial iron transporters Mrs3 and Mrs4 are functionally backed up by Rim2. Thus Rim2 corrects various synthetic lethal combinations with Δmrs3/Δmrs4, and Rim2 expression improves iron-dependent growth defects of Δmrs3/Δmrs4 mutants. Another genetic approach also pointed to a role for Rim2 in iron metabolism and a link with Mrs3 and Mrs4. A screen was performed for genes that reverse the iron sensitivity of a mutant lacking the vacuolar iron transporter Ccc1. In this case, overexpression of Rim2, Mrs3 or Mrs4 was able to overcome the iron toxicity [26].
Biochemical experiments portray a more complex picture of the relationship of Rim2 and Mrs3/Mrs4. In the Δmrs3/Δmrs4 background, a defect in mitochondrial iron uptake and utilization exists, and thus under some growth conditions (in defined or iron-depleted media) mitochondrial iron proteins are deficient [10,30]. Rim2 when expressed at a high level bypassed these deficiencies to some extent. Rim2 overexpression restored cytochromes in cells and a high rate of iron insertion into porphyrin in isolated Δmrs3/Δmrs4 mitochondria. Fe–S cluster protein activities and synthesis were similarly affected. Since these mitochondria lack Mrs3 and Mrs4, the data demonstrate some level of functional redundancy between Rim2 and Mrs3/Mrs4. However, Rim2 apparently also performs a unique function distinct from that of Mrs3 and Mrs4. This is shown by experiments in cells with intact MRS3 and MRS4. Here too, depletion of Rim2 was associated with deficient haem and Fe–S cluster proteins, and deficient haem and Fe–S cluster synthesis in isolated mitochondria.
A reasonable hypothesis could be that Rim2 acts as an iron transporter, with pyrimidine transport as a secondary function. However, we were unable to show consistent differences in iron uptake in in vitro uptake assays with isolated mitochondria with or without Rim2, using various iron substrates (results not shown). If Rim2 is an iron transporter, these negative data might only mean that we have not identified the correct or physiological iron-binding Rim2 substrate. The experiments with ruptured mitochondria, showing a defect in haem formation in mitochondrial lysate lacking Rim2, suggest that there is an additional iron utilization function of Rim2 or a Rim2 transport substrate. The basis for this defect in iron utilization in the Rim2-depleted mitochondria cannot directly be attributed to pyrimidines, since addition of pyrimidines did not rescue the defect. Conversely, Rim2-depleted cells with intact MRS3 and MRS4 still manifested significant iron usage problems in mitochondria. If Mrs3 and Mrs4 are indeed mitochondrial iron transporters, their presence should mediate iron transport in the Rim2-depleted mitochondria, and yet these mitochondria still exhibited defective iron usage. This again would point to an additional iron utilization function of Rim2 or a Rim2 transport substrate.
What is the basis for the unique requirement of Rim2 for iron utilization in mitochondria? Rim2 is a mitochondrial carrier protein, and as such it is expected to transport or exchange substrates across the mitochondrial inner membrane. Rim2 functions in mitochondria as a pyrimidine exchanger. A unifying hypothesis then might suggest that pyrimidines are involved in some step of iron transport or use. However, no pyrimidine-requiring step in haem [31] or Fe–S cluster synthesis [32] has been described, and furthermore, addition of pyrimidines to mitochondria or mitochondrial lysate did not reverse the Rim2 defect in iron use. If pyrimidine transport is not the means by which Rim2 mediates mitochondrial iron use, then perhaps Rim2 transports some other substrate that mediates iron utilization. A candidate substrate would be some type of endogenously produced siderophore. The idea has been proposed that such a molecule related to bacterial enterobactin is synthesized by eukaryotes, including yeast, and serves to bind iron in the cytoplasm and target it to mitochondria [33]. Perhaps this or some other biological iron ligand is the relevant Rim2 transport substrate needed to promote iron use within mitochondria. A mitochondrial iron use defect that resembles the Rim2-depleted mitochondria has been recently described for mitochondria from the Δgrx3/Δgrx4 mutant [5]. In this case, the lack of these cytoplasmic glutaredoxins is associated with a global iron utilization defect, and the mitochondria also exhibit deficient haem and Fe–S cluster formation, similar to the defect in Rim2-depleted mitochondria. The basis for this functional defect in mitochondrial iron use is unknown, but the suggestion has been made that the glutaredoxins could synthesize or provide a key iron trafficking intermediate to the mitochondria [5]. Further genetic and biochemical studies will be needed to establish the relevant substrate for Rim2 transport that promotes mitochondrial iron utilization.
ACKNOWLEDGEMENT
We are grateful to Jerry Kaplan for discussions about Rim2.
FUNDING
This work was supported by the National Institutes of Health [grant number R37DK053953 (to A.D)]; the National Institute on Aging [grant number AG030504]; and the American Heart Association [grant number 09GRNT2260364 (to D.P.)].
Abbreviations used
- PPO
protoporphyrinogen
- WT
wild-type
Footnotes
AUTHOR CONTRIBUTION
Heeyong Yoon performed the iron and nucleotide transport experiments. Yan Zhang performed the genetic screen and iron transport experiments. Jayashree Pain performed Fe–S cluster loading assays. Elise Lyver constructed plasmids and yeast strains. Emmanuel Lesuisse performed low-temperature cytochrome spectral analysis, iron transport assays and ferrochelatase assays. Debkumar Pain and Andrew Dancis designed the experiments and wrote the paper.
REFERENCES
- 1.Kaplan CD, Kaplan J. Iron acquisition and transcriptional regulation. Chem. Rev. 2009;109:4536–4552. doi: 10.1021/cr9001676. [DOI] [PubMed] [Google Scholar]
- 2.Richardson DR, Lane DJ, Becker EM, Huang ML, Whitnall M, Rahmanto YS, Sheftel AD, Ponka P. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10775–10782. doi: 10.1073/pnas.0912925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labbe-Bois R, Camadro JM. Ferrochelatase in Saccharomyces cerevisiae. In: Winkelmann G, Winge DR, editors. Metal Ions in Fungi. Marcel Dekker; New York: 1994. pp. 413–454. [Google Scholar]
- 4.Amutha B, Gordon DM, Gu Y, Lyver ER, Dancis A, Pain D. GTP is required for iron-sulfur cluster biogenesis in mitochondria. J. Biol. Chem. 2008;283:1362–1371. doi: 10.1074/jbc.M706808200. [DOI] [PubMed] [Google Scholar]
- 5.Muhlenhoff U, Molik S, Godoy JR, Uzarska MA, Richter N, Seubert A, Zhang Y, Stubbe J, Pierrel F, Herrero E, et al. Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab. 2010;12:373–385. doi: 10.1016/j.cmet.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levi S, Arosio P. Mitochondrial ferritin. Int. J. Biochem. Cell Biol. 2004;36:1887–1889. doi: 10.1016/j.biocel.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Garber Morales J, Holmes-Hampton GP, Miao R, Guo Y, Munck E, Lindahl PA. Biophysical characterization of iron in mitochondria isolated from respiring and fermenting yeast. Biochemistry. 2010;49:5436–5444. doi: 10.1021/bi100558z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wohlrab H. Transport proteins (carriers) of mitochondria. IUBMB Life. 2009;61:40–46. doi: 10.1002/iub.139. [DOI] [PubMed] [Google Scholar]
- 9.Muhlenhoff U, Stadler JA, Richhardt N, Seubert A, Eickhorst T, Schweyen RJ, Lill R, Wiesenberger G. A specific role of the yeast mitochondrial carriers MRS3/4p in mitochondrial iron acquisition under iron-limiting conditions. J. Biol. Chem. 2003;278:40612–40620. doi: 10.1074/jbc.M307847200. [DOI] [PubMed] [Google Scholar]
- 10.Shaw GC, Cope JJ, Li L, Corson K, Hersey C, Ackermann GE, Gwynn B, Lambert AJ, Wingert RA, Traver D, et al. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440:96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- 11.Paradkar PN, Zumbrennen KB, Paw BH, Ward DM, Kaplan J. Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol. Cell. Biol. 2009;29:1007–1016. doi: 10.1128/MCB.01685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson AJ, Kunji ER. Mitochondrial carriers in the cytoplasmic state have a common substrate binding site. Proc. Natl. Acad. Sci. U.S.A. 2006;103:2617–2622. doi: 10.1073/pnas.0509994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Murdock G, Bagley D, Jia X, Ward DM, Kaplan J. Genetic dissection of a mitochondria-vacuole signaling pathway in yeast reveals a link between chronic oxidative stress and vacuolar iron transport. J. Biol. Chem. 2010;285:10232–10242. doi: 10.1074/jbc.M109.096859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Lyver ER, Knight SA, Pain D, Lesuisse E, Dancis A. Mrs3p, Mrs4p, and frataxin provide iron for Fe-S cluster synthesis in mitochondria. J. Biol. Chem. 2006;281:22493–22502. doi: 10.1074/jbc.M604246200. [DOI] [PubMed] [Google Scholar]
- 15.Froschauer EM, Schweyen RJ, Wiesenberger G. The yeast mitochondrial carrier proteins Mrs3p/Mrs4p mediate iron transport across the inner mitochondrial membrane. Biochim. Biophys. Acta. 2009;1788:1044–1050. doi: 10.1016/j.bbamem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Skaar EP. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 2010;6:e1000949. doi: 10.1371/journal.ppat.1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesuisse E, Santos R, Matzanke BF, Knight SA, Camadro JM, Dancis A. Iron use for haeme synthesis is under control of the yeast frataxin homologue (Yfh1). Hum. Mol. Genet. 2003;12:879–889. doi: 10.1093/hmg/ddg096. [DOI] [PubMed] [Google Scholar]
- 18.Yang M, Cobine PA, Molik S, Naranuntarat A, Lill R, Winge DR, Culotta VC. The effects of mitochondrial iron homeostasis on cofactor specificity of superoxide dismutase 2. EMBO J. 2006;25:1775–1783. doi: 10.1038/sj.emboj.7601064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marobbio CM, Di Noia MA, Palmieri F. Identification of a mitochondrial transporter for pyrimidine nucleotides in Saccharomyces cerevisiae: bacterial expression, reconstitution and functional characterization. Biochem. J. 2006;393:441–446. doi: 10.1042/BJ20051284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Lyver ER, Nakamaru-Ogiso E, Yoon H, Amutha B, Lee DW, Bi E, Ohnishi T, Daldal F, Pain D, Dancis A. Dre2, a conserved eukaryotic Fe/S cluster protein, functions in cytosolic Fe/S protein biogenesis. Mol. Cell. Biol. 2008;28:5569–5582. doi: 10.1128/MCB.00642-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Lyver ER, Knight SA, Lesuisse E, Dancis A. Frataxin and mitochondrial carrier proteins, Mrs3p and Mrs4p, cooperate in providing iron for heme synthesis. J. Biol. Chem. 2005;280:19794–19807. doi: 10.1074/jbc.M500397200. [DOI] [PubMed] [Google Scholar]
- 22.Longtine MS, McKenzie A, III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Amutha B, Gordon DM, Dancis A, Pain D. Nucleotide-dependent iron-sulfur cluster biogenesis of endogenous and imported apoproteins in isolated intact mitochondria. Methods Enzymol. 2009;456:247–266. doi: 10.1016/S0076-6879(08)04414-5. [DOI] [PubMed] [Google Scholar]
- 24.Camadro JM, Chambon H, Jolles J, Labbe P. Purification and properties of coproporphyrinogen oxidase from the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 1986;156:579–587. doi: 10.1111/j.1432-1033.1986.tb09617.x. [DOI] [PubMed] [Google Scholar]
- 25.Lesuisse E, Labbe P. Reductive and non-reductive mechanisms of iron assimilation by the yeast Saccharomyces cerevisiae. J. Gen. Microbiol. 1989;135:257–263. doi: 10.1099/00221287-135-2-257. [DOI] [PubMed] [Google Scholar]
- 26.Lin H, Li L, Jia X, Ward DM, Kaplan J. Genetic and biochemical analysis of high iron toxicity in yeast: iron toxicity is due to the accumulation of cytosolic iron and occurs under both aerobic and anaerobic conditions. J. Biol. Chem. 2011;286:3851–3862. doi: 10.1074/jbc.M110.190959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puig S, Askeland E, Thiele DJ. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell. 2005;120:99–110. doi: 10.1016/j.cell.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 28.Miao R, Martinho M, Morales JG, Kim H, Ellis EA, Lill R, Hendrich MP, Munck E, Lindahl PA. EPR and Mossbauer spectroscopy of intact mitochondria isolated from Yah1p-depleted Saccharomyces cerevisiae. Biochemistry. 2008;47:9888–9899. doi: 10.1021/bi801047q. [DOI] [PubMed] [Google Scholar]
- 29.Van Dyck E, Jank B, Ragnini A, Schweyen RJ, Duyckaerts C, Sluse R, Foury F. Replicatie deoxyribonucleic acid synthesis in isolated mitochondria from Saccharomyces cerevisiae. Mol. Gen. Genet. 1995;246:426–436. doi: 10.1007/BF00290446. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Kaplan J. A mitochondrial-vacuolar signaling pathway in yeast that affects iron and copper metabolism. J. Biol. Chem. 2004;279:33653–33661. doi: 10.1074/jbc.M403146200. [DOI] [PubMed] [Google Scholar]
- 31.Ajioka R, Phillips J, Kushner J. Biosynthesis of heme in mammals. Biochim. Biophys. Acta. 2006;1763:723–736. doi: 10.1016/j.bbamcr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Muhlenhoff U, Lill R. Biogenesis of iron-sulfur proteins in eukaryotes: a novel task of mitochondria that is inherited from bacteria. Biochim. Biophys. Acta. 2000;1459:370–382. doi: 10.1016/s0005-2728(00)00174-2. [DOI] [PubMed] [Google Scholar]
- 33.Devireddy LR, Hart DO, Goetz DH, Green MR. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell. 2010;141:1006–1017. doi: 10.1016/j.cell.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]