Abstract
Background
Costimulatory blockade with anti-CD40L mAb plus donor-specific splenocyte transfusion (DST) induces alloantigen-specific tolerance. We previously showed that lymphotoxin signaling in the fibroblastic reticular cell (FRC) stromal subset was required for proper lymph node structure and function during tolerization in murine cardiac transplantation. Here we focused on FRC functions and hypothesized that donor-specific splenocyte transfusion and anti-CD40L mAb modulated FRC interactions with CD4+ T cells in mice.
Methods
Mice were immunized or tolerized by DST or DST plus anti-CD40L mAb. FRC were flow-sorted at different time points for characterization and in vitro proliferation and activation assays.
Results
FRC responded rapidly to DST by transcribing inflammatory cytokine and chemokine mRNAs such as CXCL2, CXCL9, CXCL10, and CCL21. Conversely, anti-CD40L mAb inhibited FRC inflammatory responses. CD40 was expressed on FRC and agonistic anti-CD40 mAb activated FRC, which supported CD4+ T cell proliferation, while unstimulated FRC did not. Anti-CD3 mAb activated CD4+ T cells induced inflammatory cytokine and chemokine expression by FRC, which was inhibited by anti-CD40L mAb. Thus, FRC phenotype was altered by interaction with CD4+ T cells through CD40-CD40L, and activated FRC interacted directly with CD4+ T cells to support T cell activation and proliferation in vitro.
Conclusions
Taken together, these results demonstrated that CD40 on FRC facilitated bidirectional communication between FRC and CD4+ T cells via CD40-CD40L, thereby altering FRC gene expression of immune regulatory molecules. Since blockade of CD40-CD40L interactions results in tolerance in mice, identification of FRC-T cell interactions provides a new research target for tolerance induction.
Introduction
Lymph node (LN) structure is dynamic and plays an essential role in regulating immune responses. Lymph node stromal cells (LNSC) support and modulate LN structure and contribute to important aspects of immune responses. For example, LNSC regulate T lymphocyte immunity and tolerance by providing survival factors, and processing and presenting peripheral tissue autologous antigens (1–3). Mice that lack lymphoid architecture have impaired immune responses (4–6). Previously we showed that LNSC, particularly fibroblastic reticular cells (FRC) in the T cell areas of the LN, play an important role in transplant tolerance; and FRC function depended on proper lymphotoxin (LT) signaling. Blocking LTβ receptor (LTβR) signaling with LTβRIg caused dysregulation of FRC chemokine and cytokine expression during tolerance induction. LTβR blockade resulted in increased transcription of inflammatory cytokines and chemokines by FRC in tolerogenic conditions (7).
Transplant tolerance can be induced by costimulatory blockade using anti-CD40L mAb, while LTβRIg inhibits this tolerance (7). LTβR is expressed on follicular DCs (FDC), DC, high endothelial venules, macrophages and stromal cells including FRC (8–10). There is increasing evidence that FRC actively influence the adaptive immune response. FRC constitutively express the chemokines CCL19 and CCL21 to attract and retain T cells and antigen-presenting DC (11). The FRC-rich T cell zones where DC and T cells interact are critical for the choice of immunity vs. tolerance. Fletcher et al. demonstrated that FRC express CD40 and PD-L1, and that FRC directly present peripheral tissue antigens under steady state and inflammatory conditions; however, the role of CD40 in T cell regulation is not fully elucidated (1).
CD40L is expressed on activated T cells, predominantly CD4+ T cells (12), and binds to CD40. CD40 is expressed on B cells, dendritic cells (DC), monocytes, platelets, and macrophages (13). It has also been reported that fibroblasts, epithelial, and endothelial cells may express CD40 and contribute to immunity (13–15). It is well accepted that blocking CD40-CD40L interactions induces tolerance in transplantation; however, the exact mechanisms of tolerance, which include T cell anergy and generation of regulatory T cells or suppressive DC, are not fully elucidated (16, 17). We investigated the hypothesis that CD4+ T cells interact with FRC through CD40L-CD40, and this interaction regulates key elements required for tolerance.
Materials and Methods
Mice
C57BL/6 (H-2b), BALB/c (H-2d), and CD40L deficient mice on a C57BL/6 background 8–12 weeks old were purchased from The Jackson Laboratories (Bar Harbor, ME). All mice were housed in a specific pathogen free facility in microisolator cages. All experiments were done using age- and sex- matched mice in accordance with protocols approved by the Institutional Animal Care and Utilization Committee of the University of Maryland (protocol number 0610007).
Treatments
1×106 donor-specific splenocyte transfusion (DST) from BALB/c spleen (immunization) or DST plus anti-CD40L mAb (tolerization) (250μg/dose) (clone MR-1, hamster IgG3 κ, BioXCell, West Lebanon, NH) (18) were administered intravenously to C57BL/6 mice. Hamster IgG3, κ (clone E36-239) is purchased from BD Bioscience (San Jose, CA). FRC were analyzed by flow cytometry or sorted 6–24 hours later. RNA was isolated from sorted FRC for qRT-PCR.
Antibodies
The following antibodies (all from eBioscience, San Diego, CA) were used for flow cytometry and cell sorting: anti-CD45 (clone 30-F11), anti-podoplanin (gp38, clone eBio8.1.1), anti-CD31 (clone 390), anti-CD4 (clone RM4.5), anti-CD69 (clone H1.2F3 BD), anti-CD44 (clone 1M7), anti-B220 (clone RA3-6B2), and streptavidin PE-Cy7.
LNSC sorting
LNSC were isolated as previously described (7). Briefly, LNs from 10 mice (14 LNs/mouse/experiment) were pooled and digested using 2 ml 250 mg/ml Liberase TL enzyme mix (Roche Applied Science, Indianapolis, IN), 0.1 mg/ml DNase I (Sigma-Aldrich, St. Louis, MO) in RPMI medium supplemented with 1% L-glutamine, 1mM sodium pyruvate, 1% penicillin/streptomycin, 1% non-essential amino acid, and 0.05% 2-mercaptoethanol without fetal bovine serum (FBS) at 37 °C for 40 minutes, incubated for an additional five minutes with 10 mM EDTA, and passed through a cell strainer and washed. CD45− cells were enriched with a MACS magnetic column according to the manufacturer's protocol (Miltenyi Biotec, Auburn, CA). FRC were sorted as the CD45−gp38+CD31− population with a FACSAria (BD, Franklin Lakes, NJ), average purity is 97% (SDC Figure 1).
Flow Cytometry
LN from one mouse were digested as above, and stained with the indicated antibodies without CD45 enrichment. Samples from individual mice were kept separate and acquired on an LSR Fortessa (BD, Franklin Lakes, NJ).
T cell activation and proliferation assay
FRC were sorted from naïve mice as described above and used in the following conditions: naïve (sorted on the same day); or stimulated with agonistic anti-CD40 mAb (10 μg/ml, clone FGK4.5) overnight. Naïve CD4+ T cells were isolated from spleen by EasySep CD4+ T cell enrichment kit (STEMCELL Technologies, Vancouver, BC, Canada), labeled with CFSE (5μM) (Molecular Probes/Life Technologies, Grand Island, NY) according to the manufacturer's protocol and added to the FRC. T cell culture conditions were as follows: 1) IL-2 only (20 ng/ml, R&D Systems, Minneapolis, MN); 2) IL-2 and soluble anti-CD3 mAb (eBioscience) (0.3 μg/ml); and 3) IL-2, anti-CD3 and soluble anti-CD28 (eBioscience) (0.3 μg/ml) mAbs. FRC and CD4+ T cells (5 × 104) were cultured in 200μl in 96 well plates. 5 × 104 CD4 depleted, 800 rad irradiated splenocytes were used as control antigen presenting cells (APC). CD4+ T cell proliferation by CSFE dilution was measured on day 3 by flow cytometry.
FRC activation assay
Five × 104 naïve splenic CD4+ T cells from wild type (WT) or CD40L knock out (KO) were left unstimulated or activated with plates coated with 5μg/ml anti-CD3 mAb for 3 to 5 hours. Sorted naïve FRC (5 × 104) were added to unstimulated or activated CD4+ T cells with or without anti-CD40L mAb. FRC and T cells were re-isolated by FACS Aria after 18 hours. RNA was isolated from FRC for qPCR analysis, and CD4+ T cells were analyzed by flow cytometry.
qRT-PCR
Total RNA was extracted using Trizol Reagent (Life Technologies, Grand Island, NY) or RNeasy kit (Quiagen, Valencia, CA). All primers were from Sigma (St. Louis, MO). Reverse transcription by oligo dT primer using 1 μg RNA and the Omniscript Reverse transcriptase (Qiagen) was performed according to the manufacturer's instructions. mRNA expression levels were quantitated by real-time PCR with SYBR Green PCR kit (Qiagen) with ABI Prism (Life Technologies). PCR consisted of a 15 minute 95 °C activation step, followed by 45 cycles of 15 seconds at 95 °C, 20 seconds at 56 °C, and 25 seconds at 72 °C. Normalized values for specific gene mRNA expression were calculated as follows: 2cycle threshold [Ct] control − Ct gene using cyclophilin A as an endogenous control. Primer sequences are summarized in SDC Table 1.
Statistical analysis
The GraphPad Prism software version 5 (GraphPad Software Inc., La Jolla, CA) was used for the two-tailed unpaired Student's t-test.
Results
FRC respond to allogeneic stimulation in vivo
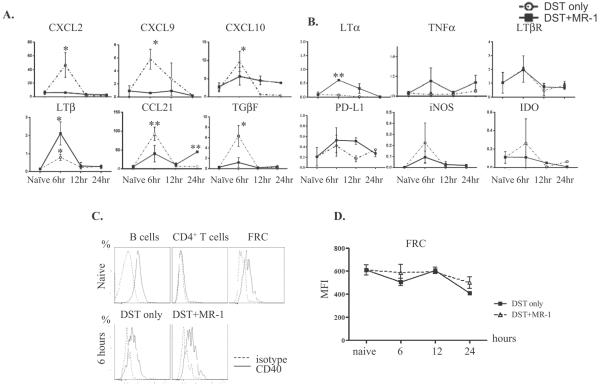
Our previous work showed that FRC were immunologically active five days after cardiac allografting in that cytokines and chemokines with important immunoregulatory functions were induced. To study the mechanisms of FRC regulation in tolerance and immunity further, we first determined if FRC respond to allogeneic stimulation. Naïve mice were stimulated by allogeneic DST administration intravenously. Compared to naïve FRC, FRC from DST-treated mice up-regulated various inflammatory cytokines and chemokines, including CXCL2, CXCL9, CXCL10, LTβ, CCL21 and TGFβ, as determined by qRT-PCR (Figure 1A). This up-regulation occurred rapidly, within 6 hours of DST administration, and expression decreased by 12 hours. There was no significant change in mRNA expression of LTα, TNFα, LTβR, PD-L1, iNOS and IDO (Figure 1B) at the time points examined after DST treatment. We next examined if blocking CD40-CD40L interactions altered FRC responses. Tolerance was induced by the combination of alloantigen stimulation with DST along with costimulatory blockade by anti-CD40L mAb (17, 18). In contrast to alloantigen stimulation alone that resulted in FRC expression of numerous inflammatory molecules, under costimulatory blockade most of these inflammatory genes did not increase in response to DST (Figure 1A). Interestingly, LTα increased at 6 hours, and LTβ and CCL21 increased at 6 and 24 hours, under costimulatory blockade, and these molecules all have homeostatic roles in the LN (10, 11). CCL19 mRNA was constitutively highly expressed as expected, and IL-7 mRNA expression was increased with DST treatment (SDC Figure 2). These findings suggested that DST stimulation of FRC responses required an interaction between CD40-CD40L. Thus, FRC were immunologically active and responded rapidly to allogeneic stimulation in vivo by differentially expressing key cytokines and chemokines important in immune regulation.
Figure 1. FRC respond to allogeneic stimulation in vivo in a CD40L dependent manner.
(A, B) C57BL/6 mice untreated or treated with DST or DST plus anti-CD40L mAb intravenously, and FRC flow sorted 6, 12 and 24 hours later. DST increased some inflammatory cytokines (A), but did not alter others (B), and anti-CD40L mAb inhibited the inflammatory cytokine response. FRC were sorted as the CD45−gp38+CD31− population, RNA isolated to make cDNA, and qRT-PCR performed for the indicated primers. Results from 3 to 5 samples at each time point, and each sample from 10 mice pooled. * p<0.05, ** p<0.005 vs. naïve. (C.) Surface CD40 stained on CD19+ B220+ naïve B cells, CD4+ T cells and FRC (top). FRC stained 6, 12, and 24 hours after DST or DST plus anti-CD40L mAb administration. Shown here is 6 hours (bottom). (D.) CD40 mean intensity in FRC for each time point after DST (square) or DST plus anti-CD40L (triangle) administration. Results for C and D from 2 to 4 samples per time point.
Since allogeneic tolerance is induced by anti-CD40L mAb and others have reported that FRC express CD40, we next determined if FRC express CD40 and under what conditions. FRC surface CD40 expression was analyzed by flow cytometry. Naïve FRC expressed substantial quantities of CD40 on the cell surface (Figure 1C); however, the surface expression did not change after DST or DST plus anti-CD40L mAb administration up to 24 hours (Figure 1D). These results indicated that although FRC express CD40, the surface expression level did not change in response to DST within 24 hours in vivo. This agrees with Fletcher et al. who showed CD40 surface expression on FRC, which did not change under naïve or TLR3 stimulatory conditions (1).
FRC stimulate T cells in a CD40-dependent manner
Figure 1 showed that FRC expressed CD40 and responded to alloantigen stimulation, and stimulation was inhibited by costimulatory blockade in vivo. Since the CD40-CD40L costimulatory interaction is important for CD4+ T cell responses, we tested if FRC could stimulate CD4+ T cell proliferation, and if this response could be influenced by FRC activation with agonistic anti-CD40L mAb.
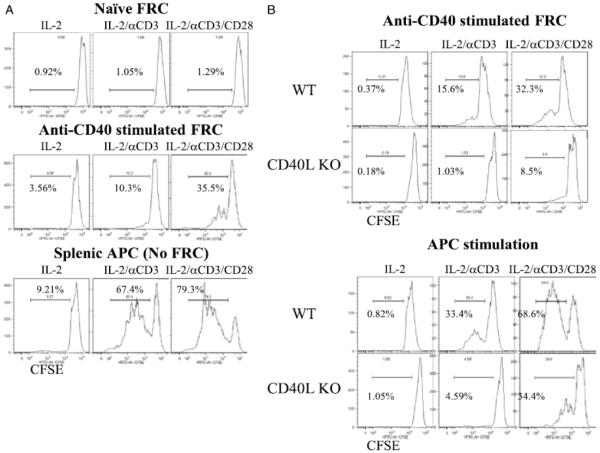
Freshly isolated naïve FRC (Figure 2A, top) were tested for their ability to stimulate CD4+ T cell proliferation along with suboptimal doses of IL-2, anti-CD3 mAb, and/or anti-CD28 mAb. We chose combinations of IL-2, anti-CD3 mAb and anti-CD28 mAb to reflect the variety of commonly used T cell stimuli. Suboptimal dose was used to determine the subtle effects since these are potent T cell stimuli. Naïve FRC did not induce any T cell proliferation under any of the stimulatory conditions. In contrast, FRC stimulated with anti-CD40 mAb induced moderate levels of CD4+ T cell proliferation (Figure 2A, middle). As expected, CD4+ T cell proliferation supported by non-FRC, irradiated, T cell-depleted splenocytes as control APC was robust under these conditions (Figure 2A, bottom).
Figure 2. Activation status of FRC affects their ability to stimulate T cells.
(A.) Flow sorted naïve FRC (top), FRC activated by anti-CD40 mAb FGK4.5 for 12 hours and washed (middle), or CD4 depleted non-FRC APC (bottom) plus CFSE labeled CD4+ T cells added on the same day to cultures containing IL-2 (20 ng/ml), IL-2 + soluble anti-CD3 (0.3 μg/ml), or IL-2 + soluble anti-CD3 + anti-CD28 mAbs (0.3 μg/ml). (B.) Flow sorted FRC activated by anti-CD40 mAb FGK4.5 for 12 hours, FRC washed, and CFSE labeled WT or CD40L KO CD4+ T cells then added to culture (top). CFSE labeled WT or CD40L KO CD4+ T cells cultured with WT CD4-depleted, non-FRC, splenic APC (bottom). CD4+ T cells assayed 3 days later. Histograms gated on CD4+ T cells and the percent of proliferating cells shown. CD4 purity 90 to 97%. Representative of 3 independent experiments. FRC from 10 mice pooled for each experiment.
These data showed that FRC stimulated T cell proliferation depending on their activation status, and anti-CD40 activated FRC to support T cell proliferation. Soluble anti-CD3 requires presentation by FcγR on APC to stimulate T cells. FRC express five different FcγR (FcγR1, FcγR2b, FcγR3, FcγR4, and FcγRT) (19). We did not observe CD4+ T cells proliferation with soluble IL-2/anti-CD3/anti-CD28 (SDC Figure 3). To further investigate CD40-CD40L FRC-CD4+ T cell interactions, CD40L KO CD4+ T cells were used. We observed impaired proliferation and activation of CD40L KO CD4+ T cells by anti-CD40 activated FRC (Figure 2B top). These results demonstrated that FRC interact with T cells through CD40-CD40L to support T cell activation and proliferation. CD40L KO CD4+ T cells did not proliferate as well as WT CD4+ T cells, so there is an intrinsic defect of CD40L KO CD4+ T cells, which has been reported. However, APC induced greater proliferation of CD40L KO CD4+ T cells compared to FRC (Figure 2B bottom).
Activated CD4+ T cells stimulate FRC in a CD40L-dependent manner
We next investigated the ability of activated CD4+ T cells to stimulate naïve FRC through CD40-CD40L. CD4+ T cells were pre-activated with plate-bound anti-CD3 mAb to increase CD40L expression (20), and then added to sorted naïve FRC, with or without anti-CD40L mAb. After 18 hours, FRC and CD4+ T cells were re-isolated, FRC were analyzed for activation by expression of cytokine and chemokine mRNA, and CD4+ T cells analyzed for activation by staining for the surface activation markers CD44 and CD69.
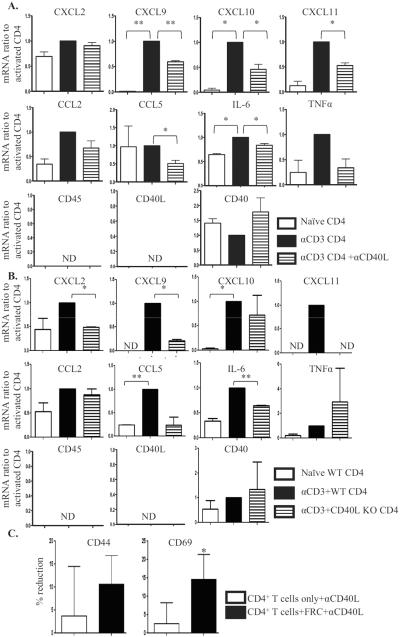
FRC cultured with pre-activated CD4+ T cells expressed markedly increased transcripts for several cytokines and chemokines. Further, many of these transcripts were reduced significantly when CD40-CD40L interactions were inhibited by blocking mAb (Figure 3A). There was no detectable CD45 or CD40L in the FRC preparations, demonstrating that there was no lymphocyte contamination; and isotype control did not have non-specific inhibitory effects (not shown). CD40L KO CD4+ T cells were less able than WT CD4+ T cells to stimulate FRC responses (Figure 3B), and the pattern of FRC responses was similar to that with anti-CD40L mAb treatment. The additional differences between anti-CD40L treated WT compared to CD40L KO cells might derive from intrinsic defects during development in CD40L KO mice, so that CD40L KO CD4+ T cells are not fully normal (21) and at least minor differences were expected in comparison to using a blocking mAb on normal cells.
Figure 3. Activated CD4+ T cells stimulate FRC responses and FRC modulate T cell responses in a CD40L dependent manner.
CD4+ T cells unstimulated or stimulated in plates coated with 5μg/ml anti-CD3 mAb for 3 to 5 hours. Flow sorted FRC added to the CD4+ T cells for 18 hours, with or without anti-CD40L mAb (2 μg/ml). FRC and T cells re-sorted, and FRC mRNA isolated for qRT-PCR. (A.) mRNA expression in FRC activated by CD4+ T cells as a baseline (black), ratio of naïve FRC to baseline (white), and ratio of FRC activated by CD4+ T cells with anti-CD40L mAb to baseline (pattern). (B.) WT or CD40L KO CD4+ T cells unstimulated or stimulated in plates coated with 5 μg/ml anti-CD3 mAb for 3 to 5 hours. Flow sorted FRC added to the T cells for 18 hours. FRC and T cells re-sorted, and FRC mRNA isolated for qRT-PCR. mRNA expression in FRC activated by WT CD4+ T cells as baseline (black), ratio of naïve FRC to baseline (white), and ratio of FRC activated by CD40L KO CD4+ T cells to baseline (pattern). Results are from 3 independent experiments, each experiment from 10 mice pooled. ND: not detected. (C.) CD4+ T cells treated as above, CD4+ T cells only or CD4+ T cells with naïve FRC present were re-sorted for surface activation phenotype analysis by flow. Effect of anti-CD40L mAb was calculated as percent reduction of activation marker expression compared to population without anti-CD40L. Reduction by anti-CD40L in CD4+ T cells only (white) or in CD4+ T cells with FRC (black). Results from 4 independent experiments. Gated on CD4+ T cells. * p<0.05, ** p<0.005.
CD4+ T cells pre-activated by anti-CD3 mAb had markedly increased surface expression of CD44 and CD69, as expected (16). Adding naïve FRC alone did not have significant effects on activation marker expression (SDC Figure 4 left). However, when anti-CD40L mAb was also added, the CD44 and CD69 high populations were reduced about 15% and 20%, respectively (SDC Figure 4 right and Figure 3C). In contrast, when FRC were not present, anti-CD40L mAb reduced the CD44 and CD69 high populations by only about 4% and 3%, respectively (SDC Figure 4 right and Figure 3C). These results showed that pre-activated CD4+ T cells and naïve FRC interacted via CD40L-CD40. The decreased T cell surface activation phenotype during CD40L blockade likely indicated direct suppressive effects of naïve FRC on T cell responses (22, 23).
Taken together, these results demonstrated that TCR-activated CD4+ T cells activated FRC as measured by chemokine and cytokine induction; FRC, depending on their activation status, then subsequently modified CD4+ T cell activation, and interactions depended on CD40-CD40L interactions between CD4+ T cells and FRC.
Discussion
In this report, we demonstrated that FRC interacted with CD4+ T cells through CD40-CD40L and functioned as innate immune cells. FRC were stimulated through CD40, either by anti-CD40 mAb or by activated T cells. We chose to use agonistic anti-CD40 mAb to stimulate FRC in vitro to simplify the complex in vivo system and investigate CD4+ T cell-FRC interaction by CD40L-CD40. Agonist anti-CD40 mAb stimulated FRC induced T cell proliferation while naïve FRC did not, the mechanism of which depended on T cell CD40L expression. Activated CD4+ T cells induced an inflammatory phenotype in FRC so that they expressed cytokines and chemokines. Thus, FRC and CD4+ T cells affected each other bidirectionally, and differentially depending on their activation status. This bidirectional interaction may be an important mechanism regulating tolerance versus immunity in addition to the potent and well-characterized APC-CD4+ T cell interactions, and to our knowledge this is the first report demonstrating the function of CD40 on FRC. The FRC response to allogeneic stimulation, which occurred in vivo within 6 hours, and the ability of anti-CD40L to prevent stimulation, suggests that CD40L blockade prevents a stimulatory and perhaps even induces a tolerant phenotype in FRC. Indeed, we observed a slight increase in PD-L1 on FRC after CD40L blockade (Figure 1B), and others reported naïve FRC suppressive function (22, 23). Among the inflammatory chemokines increased by DST, we have shown that CXCL2 is responsible for neutrophil infiltration into the rejecting grafts and blocking CXCL2 restored tolerance (7).
Bidirectional interactions between FRC and T cells could influence diverse aspects of immune regulation. During homeostasis FRC provide the T cell survival factor IL-7, while T cells provide LT signals to maintain FRC structure (24). Abrogation of FRC-T cell interactions by collagen deposition results in loss of both FRC and T cells (25). FRC present tissue antigens under homeostatic conditions and participate in peripheral tolerance induction of CD8+ T cells (1, 26). During acute inflammation, FRC respond to proinflammatory cytokines produced by CD8+ T cells to transiently induce nitric oxide that suppresses neighboring CD8+ T cell proliferation as a negative feedback (22, 23). Thus, these studies support the importance of T cell-FRC bidirectional interactions for homeostasis and tolerance.
FRC positively regulate T cell responses by enhancing the crosstalk between DC and T cells. The three-dimensional network and chemokines produced by the stroma supports the movements and interactions of T cells and DC (5, 6) and promotes the maturation of DC (27). Here we provided direct evidence that FRC supported T cell proliferation via CD40-CD40L interactions. Ng et al. showed that FRC and CD4+ T cells interact through MHC-TCR and PD-L1-PD-1 and cause activation or suppression, respectively (28). This is commensurate with our findings that FRC have different functions depending on their activation state and that of the responding T cells. FRC did not increase T cell activation markers compared to T cells alone, but when CD40L was blocked activation markers decreased. This implies both positive and negative competing signals arising from FRC at the same time. Since FRC and T cells behave differently in naïve or activated states, it is important to elucidate the complex interactions between FRC and T cells, which likely depends on FRC density, environmental factors, strength and type of T cell responses.
Taken together, these studies demonstrate that FRC have the capacity to fine-tune the immune system in the LN. Our findings expand the role of CD40-CD40L costimulation from DC-CD4+ T cells to FRC-CD4+ T cells and define a role for FRC as innate immune cells. Fibroblast activation protein and CCL19 promoters have been used to target FRC in vivo (29, 30), demonstrating that FRC actively participate in adaptive immunity. Our study provides direct evidence of CD40 related stromal activation by both specific mAb and activated CD4+ T cells; therefore, the interaction between FRC and CD4+ T cells via CD40-CD40L presents new therapeutic possibilities for transplantation and inflammatory diseases.
Supplementary Material
Acknowledgement
Flow core facility of the University of Maryland for excellent technical support. Flow cytometry analyses were performed at the University of Maryland Marlene and Stewart Greenebaum Cancer Center Flow Cytometry Shared Service.
This work was supported by the CVID Training grant: 5T32HL007698 [to CCB] and the National Institutes of Health grants number RO1 AI062765 and R56 AI72039 [to JSB].
Abbreviations
- APC
antigen presenting cell
- DC
dendritic cell
- DST
donor specific transfusion
- FDC
follicular dendritic cell
- FRC
fibroblastic reticular cell
- i.v.
intravenous
- KO
knock out
- LN
lymph node
- LNSC
lymph node stromal cell
- LT
lymphotoxin
- LTβR
LTβ receptor
- mAb
monoclonal antibody
- TCR
T cell receptor
- WT
wild type
Footnotes
The authors declare no conflicts of interest.
References
- 1.Fletcher AL, Lukacs-Kornek V, Reynoso ED, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207:689. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fletcher AL, Malhotra D, Turley SJ. Lymph node stroma broaden the peripheral tolerance paradigm. Trends Immunol. 2011;32:12. doi: 10.1016/j.it.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynoso ED, Lee JW, Turley SJ. Peripheral tolerance induction by lymph node stroma. Adv Exp Med Biol. 2009;633:113. doi: 10.1007/978-0-387-79311-5_10. [DOI] [PubMed] [Google Scholar]
- 4.Berger DP, Naniche D, Crowley MT, Koni PA, Flavell RA, Oldstone MB. Lymphotoxin-beta-deficient mice show defective antiviral immunity. Virology. 1999;260:136. doi: 10.1006/viro.1999.9811. [DOI] [PubMed] [Google Scholar]
- 5.Gunn MD, Kyuwa S, Tam C, et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller SN, Matloubian M, Clemens DM, et al. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci U S A. 2007;104:15430. doi: 10.1073/pnas.0702579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama Y, Bromberg JS. Lymphotoxin-beta receptor blockade induces inflammation and fibrosis in tolerized cardiac allografts. Am J Transplant. 2012;12:2322. doi: 10.1111/j.1600-6143.2012.04090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehlers S, Holscher C, Scheu S, et al. The lymphotoxin beta receptor is critically involved in controlling infections with the intracellular pathogens Mycobacterium tuberculosis and Listeria monocytogenes. J Immunol. 2003;170:5210. doi: 10.4049/jimmunol.170.10.5210. [DOI] [PubMed] [Google Scholar]
- 9.Force WR, Walter BN, Hession C, et al. Mouse lymphotoxin-beta receptor. Molecular genetics, ligand binding, and expression. J Immunol. 1995;155:5280. [PubMed] [Google Scholar]
- 10.Ware CF, VanArsdale TL, Crowe PD, Browning JL. The ligands and receptors of the lymphotoxin system. Current topics in microbiology and immunology. 1995;198:175. doi: 10.1007/978-3-642-79414-8_11. [DOI] [PubMed] [Google Scholar]
- 11.Siegert S, Luther SA. Positive and negative regulation of T cell responses by fibroblastic reticular cells within paracortical regions of lymph nodes. Front Immunol. 2012;3:285. doi: 10.3389/fimmu.2012.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane P, Traunecker A, Hubele S, Inui S, Lanzavecchia A, Gray D. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur J Immunol. 1992;22:2573. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- 13.van Kooten C, Banchereau J. CD40-CD40 ligand. Journal of leukocyte biology. 2000;67:2. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 14.Banchereau J, Dubois B, Fayette J, et al. Functional CD40 antigen on B cells, dendritic cells and fibroblasts. Adv Exp Med Biol. 1995;378:79. doi: 10.1007/978-1-4615-1971-3_16. [DOI] [PubMed] [Google Scholar]
- 15.Mach F, Schonbeck U, Libby P. CD40 signaling in vascular cells: a key role in atherosclerosis? Atherosclerosis. 1998;137(Suppl):S89. doi: 10.1016/s0021-9150(97)00309-2. [DOI] [PubMed] [Google Scholar]
- 16.Burrell BE, Bromberg JS. Fates of CD4+ T cells in a tolerant environment depend on timing and place of antigen exposure. Am J Transplant. 2012;12:576. doi: 10.1111/j.1600-6143.2011.03879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wekerle T, Kurtz J, Bigenzahn S, Takeuchi Y, Sykes M. Mechanisms of transplant tolerance induction using costimulatory blockade. Curr Opin Immunol. 2002;14:592. doi: 10.1016/s0952-7915(02)00378-3. [DOI] [PubMed] [Google Scholar]
- 18.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 19.Jojic V, Shay T, Sylvia K, et al. Identification of transcriptional regulators in the mouse immune system. Nat Immunol. 2013;14:633. doi: 10.1038/ni.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castle BE, Kishimoto K, Stearns C, Brown ML, Kehry MR. Regulation of expression of the ligand for CD40 on T helper lymphocytes. J Immunol. 1993;151:1777. [PubMed] [Google Scholar]
- 21.Renshaw BR, Fanslow WC, 3rd, Armitage RJ, et al. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan O, Headley M, Gerard A, Wei W, Liu L, Krummel MF. Regulation of T cell priming by lymphoid stroma. PLoS One. 2011;6:e26138. doi: 10.1371/journal.pone.0026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegert S, Huang HY, Yang CY, et al. Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PLoS One. 2011;6:e27618. doi: 10.1371/journal.pone.0027618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Link A, Vogt TK, Favre S, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 25.Zeng M, Smith AJ, Wietgrefe SW, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fletcher AL, Malhotra D, Acton SE, et al. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front Immunol. 2011;2:35. doi: 10.3389/fimmu.2011.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsland BJ, Battig P, Bauer M, et al. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity. 2005;22:493. doi: 10.1016/j.immuni.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Ng CT, Nayak BP, Schmedt C, Oldstone MB. Immortalized clones of fibroblastic reticular cells activate virus-specific T cells during virus infection. Proc Natl Acad Sci U S A. 2012;109:7823. doi: 10.1073/pnas.1205850109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cremasco V, Woodruff MC, Onder L, et al. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol. 2014 doi: 10.1038/ni.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denton AE, Roberts EW, Linterman MA, Fearon DT. Fibroblastic reticular cells of the lymph node are required for retention of resting but not activated CD8+ T cells. Proc Natl Acad Sci U S A. 2014;111:12139. doi: 10.1073/pnas.1412910111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.