Abstract
Intestinal ischemia-reperfusion (I/R) occurs in various clinical situations and causes local and remote organ injury, especially in the lungs, leading to significant morbidity and mortality. Maintenance of mitochondrial biogenesis is essential for cell survival, and is regulated in part by sirtuin 1, an energy-sensing enzyme. We hypothesized that sirtuin 1 activation with SRT1720 would reduce local and remote organ injury after intestinal I/R. Intestinal I/R was induced by occlusion of the superior mesenteric artery of adult male C57BL/6 mice for 45 min, followed by reperfusion for 4 h. SRT1720 or vehicle was injected intravenously at the time of reperfusion. Blood, small intestine, and lung tissues were collected for analysis. SRT1720 treatment of I/R mice resulted in a 57% increase in protein levels of succinate dehydrogenase (SDH), an index of mitochondrial mass, and a 120% increase in mRNA levels of mitochondrial transcription factor A (TFAM), a marker for mitochondrial biogenesis. The microscopic architecture and apoptosis of the gut tissue was improved in the SRT1720-treated I/R mice. SRT1720 decreased intestinal mRNA levels of TNF-α by 60% and inducible nitric oxide synthase (iNOS) to baseline after I/R. Systemic inflammation, as determined by serum IL-6, was reduced in treated mice. Lung injury, as measured by histologic architecture and myeloperoxidase activity, and lung apoptosis were also improved after SRT1720 treatment. SRT1720 preserved mitochondrial biogenesis and mass, leading to inhibition of inflammation and oxidative stress, thereby protecting against intestinal I/R-induced injury. Thus, the sirtuin 1-mediated pathway is a promising target for treatment of intestinal I/R injury.
Keywords: Inflammation, neutrophil infiltration, apoptosis, oxidative stress, energy metabolism, lung injury
INTRODUCTION
Intestinal ischemia is a surgical emergency with mortality rates up to 50-80% (1). The etiology of intestinal ischemia can be classified as obstructive or non-obstructive, with obstruction to the superior mesenteric artery being the most common (1). Restoration of blood flow to the affected bowel is one of the main objectives of therapy, however, this may lead to ischemia-reperfusion (I/R) injury. Intestinal I/R injury is similar to I/R of other organs in cellular pathobiology, which is characterized by cellular energy deficits leading to initial injury, followed by a cascade of inflammatory and oxidative injury (2). In addition, intestinal I/R is characterized by a dramatic systemic inflammatory response and remote organ injury (3). Acute lung injury has been consistently associated with intestinal I/R injury (4, 5). The pathophysiology of lung injury is mediated by systemic inflammation secondary to the initial insult, which in turn causes recruitment of inflammatory cells, local endothelial cell injury, and smooth muscle dysfunction (6, 7). Several of the pathophysiologic features of experimentally induced acute lung injury resemble those of the acute respiratory distress syndrome, which is associated with significant mortality (8).
Ischemic injury is commonly associated with mitochondrial injury which results in persistent energy deficits despite reperfusion (9). The damage to enterocyte mitochondria induced by intestinal I/R can be irreversible despite short periods of ischemia (10). Therefore, a strategy aimed at regenerating the cellular mitochondrial pool, mitochondrial biogenesis, may prove protective in the setting of intestinal I/R.
Sirtuins are seven evolutionarily conserved mammalian proteins homologous to the silence information regulator-2 (Sir-2) enzyme in yeast (11). Sir-2 has been shown to mediate longevity during calorie restriction in yeast (11). Sirtuin 1 (Sirt1) is an energy-sensing deacetylase which depends on NAD+ as a substrate for enzymatic activity (11). It is ubiquitously expressed and is localized to the nucleus and cytosol (11). Sirt1 is therefore thought to mediate cellular survival during conditions of energy scarcity. Among its multiple functions, Sirt1, has been shown to enhance mitochondrial biogenesis and inhibit apoptosis and inflammation (12). Consequently, it has been regarded as a promising therapeutic target in other I/R models (12, 13). The increasing interest in the therapeutic potential of Sirt1 in aging and chronic metabolic diseases has led to the development of the sirtuin activating compounds (STACs) (14), which were demonstrably safe in phase I and II clinical trials (15). SRT1720 was among the first STACs described and has shown marked activation and potency towards Sirt1, compared to the other STACs (14).
In this study, we hypothesized that Sirt1 activation would attenuate local and remote organ injury after intestinal I/R. To test this hypothesis, we used a well-established murine model of superior mesenteric artery occlusion (4). Animals were post-treated with SRT1720 at the time of reperfusion. The effects of SRT1720 on intestinal mitochondrial mass, organ injury, and inflammation were assessed after I/R. Further, we investigated the effects of pharmacologic Sirt1 activation on systemic inflammation and lung injury.
MATERIALS AND METHODS
Experimental animals
Adult male C57BL/6 mice (age 8–10 weeks, 20–25g, Taconic Biosciences, Albany, NY) were housed in a temperature-controlled room on a 12-h light-dark cycle. They were fed a standard laboratory diet. This study was approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research and experiments were performed in accordance with National Institute of Health guidelines for the use of experimental animals.
Animal model of intestinal I/R
Mice were randomly assigned to three groups: control, vehicle (normal saline with 10% DMSO) or SRT1720 treatment (n=4–7/group). Intestinal I/R was performed as previously described (4). Briefly, animals were placed on a heating pad and anesthetized with 2.5% inhalational isoflurane. The abdomen was shaved and cleaned with 10% povidone-iodine wash followed by 70% ethanol. A 2–3 cm upper midline incision was made, the bowel was eviscerated, and the superior mesenteric artery (SMA) was dissected at its take-off from the aorta. A microvascular clip was placed on the SMA for 45 min, during which time the internal jugular vein was exposed and prepared for injection. After 45 min, the clip was removed, allowing reperfusion. Vehicle or SRT1720 (20 mg/kg body weight) was immediately injected via the internal jugular vein. A 0.5 ml bolus of normal saline was given i.p. before closure of the abdomen. Control mice did not undergo any intervention. Four hours after reperfusion, intestine and lung tissues were collected and blood was aspirated by inferior vena cava puncture. All samples were stored at −80°C before use.
Western blot analysis
Gut and lung tissue samples were homogenized and lysed using a sonic dismembrator in lysis buffer (10 mM Tris-HCl pH 7.5, 120 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate) containing a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Samples were centrifuged at 12,000 rpm for 15 min at 4°C and the supernatant was collected. The sample protein concentration was measured by Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). Samples (50 μg/well) were separated on 4% to 12% Bis-Tris gels and transferred to nitrocellulose membranes. Membranes were incubated with primary antibody against cleaved caspase-3 (Cell Signaling Technology, Beverly, MA), succinyl dehydrogenase (SDH), or β-actin (Santa Cruz Biotechnologies, Santa Cruz, CA). All protein bands were detected by species-specific fluorescence-labeled secondary antibodies and analyzed by the LI-COR Odyssey Fc Imager (LI-COR, Lincoln, NE).
Real-time polymerase chain reaction (qPCR) analysis
Total RNA was extracted from lung and gut tissue samples by homogenizing with a sonic dismembrator in TRIzol reagent (Invitrogen, Carlsbad, CA). The total RNA (3 μg) then underwent reverse transcription using murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA). A PCR reaction was carried out in a 25 μL final volume containing 0.5 μL of each forward and reverse primer, 5 μL cDNA, 6.5 μL H2O, and 12.5 μL SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). An Applied Biosystems 7300 real-time PCR machine was used for amplification under the thermal profile of 50°C for 2 min and 95°C for 10 min, followed by 45 cycles of 95°C for 15 sec and 60°C for 1 min. Mouse β-actin mRNA levels were used for normalization. Relative expression of mRNA was calculated by the 2−ΔΔCt method, and results were expressed as fold change in comparison to control group. The sequences for the primers used in this study are as follows: mouse TFAM 5′-AACGCCTAAAGAAGAAAGCACAA-3′ (forward) and 5′-GGTCTTTTTGGTTTTCCAAGCA-3′ (reverse); mouse TNF-α 5′-AGACCCTCACACTCAGATCATCTTC-3′ (forward) and 5′-TTGCTACGACGTGGGCTACA-3′ (reverse); mouse iNOS, 5′-GGCAAACCCAAGGTCTACGTT-3′ (forward) and 5′-GAGCACGCTGAGTACCTCATTG-3′ (reverse); mouse β-actin, 5′-CGTGAAAAGATGACCCAGATCA-3′ (forward) and 5′-TGGTACGACCAGAGGCATACAG-3′ (reverse).
Histological evaluation
Segments of proximal jejunum and lung tissue were collected after 4 h of reperfusion and stored in 10% formalin before fixing in paraffin. The gut and lung biopsies were sectioned into 4 μm cuts and stained with hematoxylin-eosin (H&E). Tissue injury was assessed in a blinded fashion using a semi-quantitative light microscopy evaluation. The severity of gut injury was scored by assessing villus-to-crypt ratio (normal ratio, 5:1), lymphocytic infiltration, epithelial degeneration/necrosis, erosion, glandular dilatation, and transmural changes (4). Three separate cross-sections from the intestines of 3 mice in each treatment group were scored. Each section contained 20–25 villi. The severity of lung injury was scored from 0 to 4 based on the presence of exudates, hyperemia/congestion, neutrophil infiltration, intra-alveolar hemorrhage/debris, and cellular hyperplasia (4).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Gut tissue sections were de-paraffinized and incubated with proteinase K. Slides were stained using a TUNEL kit (Roche Diagnostics, Indianapolis, IN), counterstained with 4′,6-diamidino-2-phenylindole (DAPI, blue) and examined under a fluorescence microscope. Apoptotic cells fluoresced green and were counted per villus at 200× magnification.
Myeloperoxidase (MPO) activity assay
Lung tissue samples were homogenized in KPO4 buffer containing 0.5% hexa-decyl-trimethyl-ammonium bromide. After centrifugation the supernatant was diluted in reaction solution, and the rate of change in optimal density for 30 seconds was measured at 460 nm to calculate MPO activity.
Measurements of transaminases, lactate dehydrogenase, and interleukin 6 (IL-6)
Whole blood samples were centrifuged at 7,000 rpm for 10 min and the serum was aspirated and stored at -80°C. Commercial assay kits from Pointe Scientific (Lincoln Park, MI) were used to measure the activity levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH). Serum IL-6 levels were determined by an enzyme-linked immunosorbent assay (ELISA) kit specific for mouse IL-6 (BD Biosciences, San Diego, CA). The assays were carried out according to the manufacturer’s instructions.
Statistical analysis
All data are expressed as a mean ± standard error (SE) and compared by one-way analysis of variance (ANOVA) and the Student-Newman-Keuls (SNK) test. Differences in values were considered significant if P < 0.05.
RESULTS
SRT1720 increases mitochondrial biogenesis after intestinal I/R
We first determined the effect of SRT1720 treatment on the mitochondrial mass in the gut after I/R. SDH is an enzyme located at the inner mitochondrial membrane, which can be used as a surrogate for mitochondrial mass. The level of SDH protein in the gut was reduced by 28% at 4 h after I/R, compared to the control (Fig. 1A). Treatment with SRT1720 increased SDH levels by 57% in comparison to the vehicle (Fig. 1A). We also examined the expression of mitochondrial transcription factor A (TFAM) which is a DNA binding protein that drives mitochondrial genome replication and is a downstream target of Sirt1. TFAM mRNA expression in the gut was not significantly changed after I/R, however administration of SRT1720 increased TFAM mRNA expression by 120%, even compared to the control (Fig. 1B). Thus, SRT1720 treatment effectively enhanced mitochondrial biogenesis in the gut after I/R.
Figure 1. Effect of SRT1720 on gut mitochondrial biogenesis after intestinal I/R.
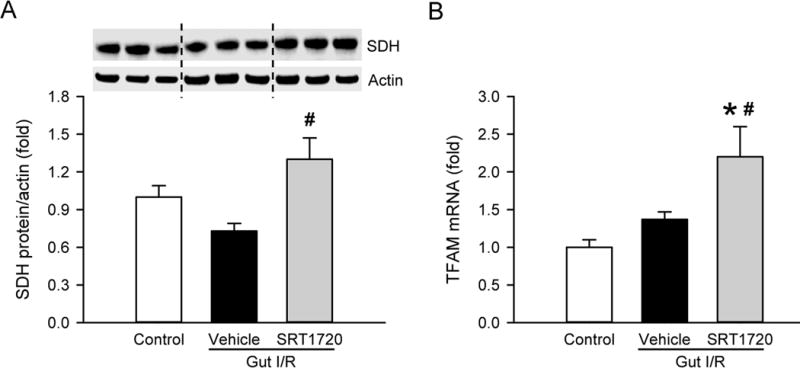
Gut tissue from control, vehicle and SRT1720 treatment groups were collected 4 h after intestinal I/R to measure the expression of (A) succinate dehydrogenase (SDH) protein by Western blot and (B) mitochondrial transcription factor A (TFAM) mRNA by RT-PCR. Expression levels were normalized to β-actin, and the value in the control group is designated as 1 for comparison. Data are presented as mean ± SE (n=4–7/group) and compared by one-way ANOVA and SNK. *P < 0.05 vs. control; #P < 0.05 vs. vehicle.
SRT1720 decreases gut damage after intestinal I/R
We then examined whether stimulation of mitochondrial biogenesis by SRT1720 was associated with reduced gut injury induced by I/R. At 4 h after intestinal I/R, the gut histology showed mucosal damage with lifting of the villus epithelium and collapse of small vessels in comparison to the control (Fig. 2A). After treatment with SRT1720, the integrity of morphological structures and height of the villi were well-preserved in the gut, compared to the vehicle (Fig. 2A). Using semi-quantitative histological evaluation, the injury score of the SRT1720 treatment group was reduced by 51% in comparison with the vehicle group (Fig. 2B). We also examined the degree of apoptosis in the gut after I/R. By conducting the TUNEL assay on the gut tissue sections, we observed a significant increase in TUNEL-positive cells (green fluorescence) in the vehicle group, to 19.0 ± 1.3 apoptotic cells per villus (Fig. 3A–B). However, there was a significant decrease in the number of apoptotic cells in the intestine of the SRT1720 treatment group, compared to vehicle (Fig. 3A–B).
Figure 2. Effect of SRT1720 on gut architecture after intestinal I/R.
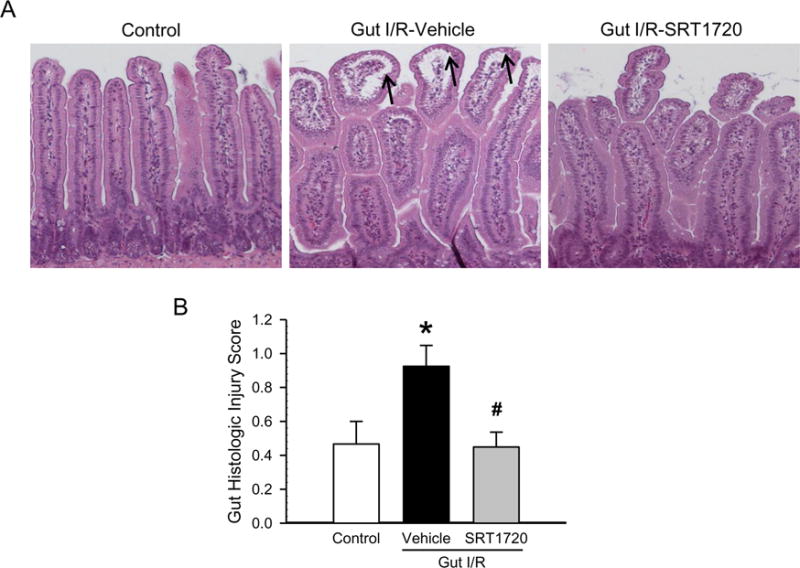
Sections of proximal jejunum from control, vehicle and SRT1720 treatment groups were collected 4 h after intestinal I/R and stained with hematoxylin-eosin. (A) Representative photomicrographs are at 100× magnification. Arrows indicate lifting of mucosal epithelium. (B) The semiquantitative histologic injury was scored as described in Materials and Methods. Data are presented as mean ± SE (n= 3/group) and compared by one-way ANOVA and SNK. *P < 0.05 vs. control; #P < 0.05 vs. vehicle.
Figure 3. Effect of SRT1720 on gut apoptosis after intestinal I/R.
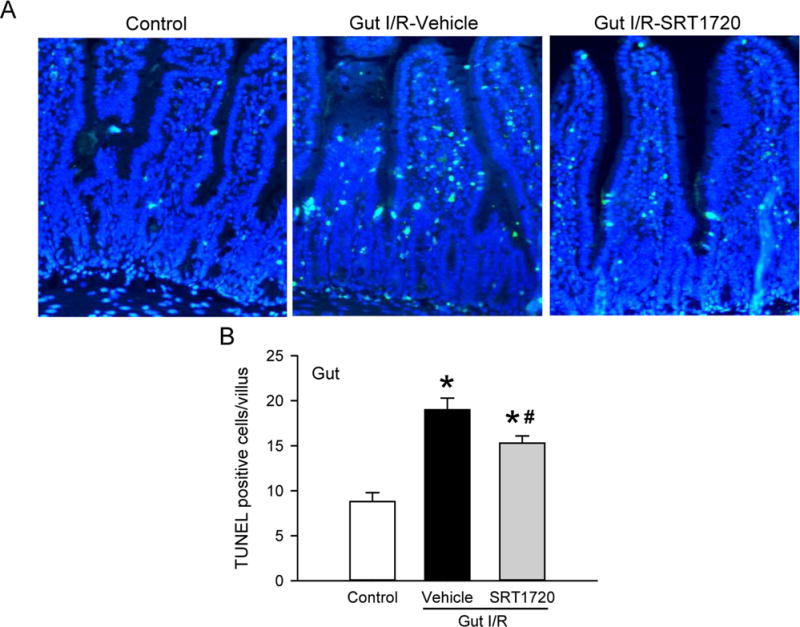
Proximal jejunum from control, vehicle, and SRT1720 treatment groups were collected 4 h after intestinal I/R and subjected to TUNEL assay. (A) Representative photomicrographs show TUNEL-positive (green) and DAPI (blue) staining for the nucleus at 200× magnification. (B) A graphical representation of TUNEL-positive staining cells averaged over 10 villi/animal. Data are presented as mean ± SE (n= 4/group) and compared by one-way ANOVA and SNK. *P < 0.05 vs. control; #P < 0.05 vs. vehicle.
SRT1720 reduces gut inflammation and oxidative stress after intestinal I/R
Induction of excessive inflammation and production of reactive oxygen species (ROS) are both important causes of tissue damage in I/R. To determine the local inflammatory response to intestinal I/R, tumor necrosis factor-α (TNF-α) levels were measured by RT-PCR in gut tissue. The TNF-α mRNA level was increased by 6.6-fold at 4 h after intestinal I/R, compared to control mice (Fig. 4A). However, when mice were treated with SRT1720, TNF-α levels were reduced by 60% compared to vehicle mice (Fig. 4A). Inducible nitric oxide synthase (iNOS) is an enzyme responsible for the generation of nitric oxide (NO), one form of ROS. The level of iNOS mRNA expression in the gut was increased by 144% as a result of intestinal I/R (Fig. 4B). However, treatment with SRT1720 inhibited the elevation of iNOS mRNA expression induced by I/R to the level of the control (Fig. 4B).
Figure 4. Effect of SRT1720 on gut inflammation and oxidative stress intestinal I/R.
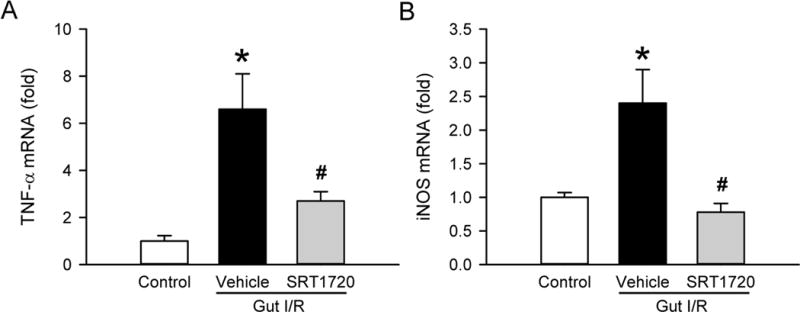
Gut tissue samples from control, vehicle, and SRT1720 treatment groups were collected 4 h after intestinal I/R to measure the expression of (A) TNF-α and (B) iNOS mRNA by RT-PCR. Their expression levels were normalized to β-actin. The value in the control group was designated as 1 for comparison. Data are presented as mean ± SE (n=4–7/group) and compared by one-way ANOVA and SNK. *P < 0.05 vs. control; #P < 0.05 vs. vehicle.
SRT1720 inhibits the systemic inflammatory response after intestinal I/R
Intestinal I/R is associated with a significant amount of systemic inflammation compared to I/R of other organs. We therefore collected blood 4 h after intestinal I/R and measured serum levels of pro-inflammatory cytokine interleukin 6 (IL-6) by ELISA to assess the extent of systemic inflammation. The serum IL-6 level was undetectable in the control, but increased to 1.56 ±0.27 ng/mL in the vehicle (Fig. 5). Treatment with SRT1720 resulted in a 43% reduction in serum IL-6 level, compared to the vehicle (Fig. 5). This result indicated that the systemic inflammatory response induced by intestinal I/R was attenuated by SRT1720 treatment.
Figure 5. Effect of SRT1720 on systemic inflammation after intestinal I/R.
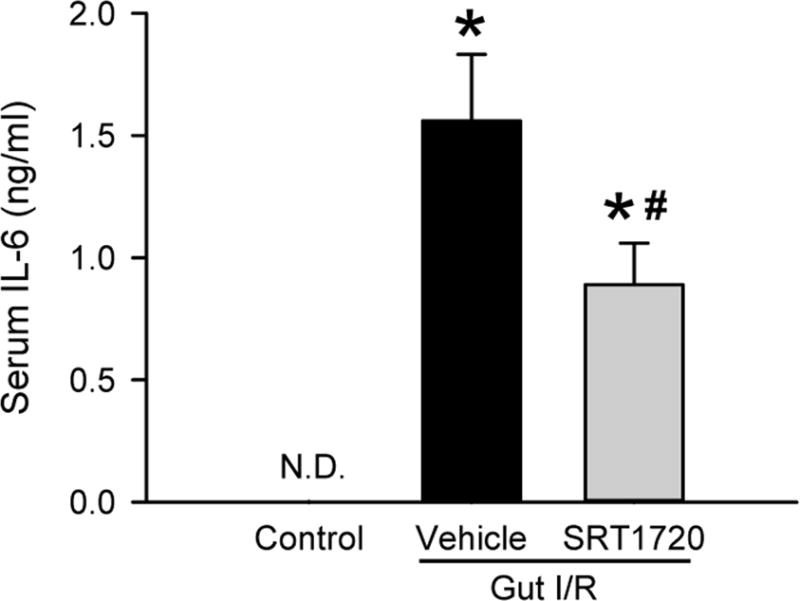
Serum samples from control, vehicle, and SRT1720 treatment groups were collected 4 h after intestinal I/R to measure the levels of IL-6 by ELISA. Data are presented as mean ± SE (n=4–7/group) and compared by one-way ANOVA and SNK. *P < 0.05 vs. control; #P < 0.05 vs. vehicle. N.D.: Not Detectable.
SRT1720 attenuates lung injury after intestinal I/R
The lungs are very sensitive to systemic inflammation. We examined the effect of SRT1720 treatment on lung injury induced by intestinal I/R. At 4 h after intestinal I/R, lung tissue demonstrated alveolar collapse, increased alveolar wall thickness and interstitial leukocyte infiltration, while these alterations were significantly reduced in the lungs of mice treated with SRT1720 at reperfusion (Fig. 6A). Quantification of the lung histologic injury showed a 3-fold increase in injury severity, compared to control animals, and a significant reduction in injury by 23% after treatment with SRT1720 (Fig. 6B). After observing the immune cells infiltrated in the lung tissue, we then assessed MPO activity, an enzyme predominantly produced by neutrophils. The MPO activity in the lungs of the vehicle group was increased by 11.5-fold, compared to the control (Fig. 6C). After treatment with SRT1720, there was a 39% decrease in MPO activity in the lung in comparison with the vehicle group (Fig. 6C).
Figure 6. Effect of SRT1720 on lung architecture and neutrophil infiltration after intestinal I/R.
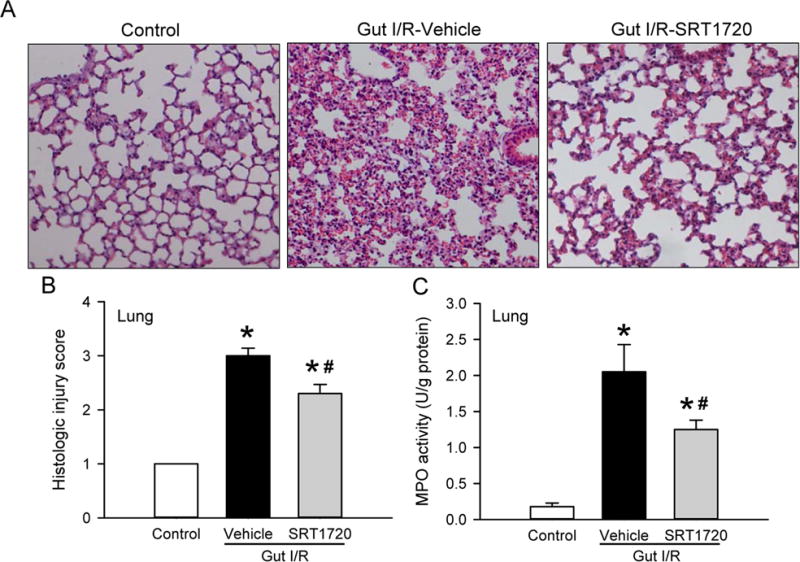
Lung tissue samples from control, vehicle, and SRT1720 treatment groups were collected 4 h after intestinal I/R. (A) Representative photomicrographs of the lung sections stained with hematoxylin-eosin are at 100× magnification. (B) The semiquantitative histologic injury of the lung tissues was scored as described in Materials and Methods. (C) Lung tissue myeloperoxidase (MPO) activity was determined spectrophotometrically. Data are presented as mean ± SE (n= 4–7/group) and compared by one-way ANOVA and SNK. *P < 0.05 vs. control; #P < 0.05 vs. vehicle.
SRT1720 limits lung apoptosis after intestinal I/R
We then examined the effect of SRT1720 treatment on the induction of apoptosis in the lung by measuring the level of the cleaved caspase-3, the active form of the enzyme that executes apoptosis. At 4 h after intestinal I/R, the level of cleaved caspase-3 in the lung was significantly increased by 64% in the vehicle compared to the control group, as determined by Western blot (Fig. 7). Treatment with SRT1720 at reperfusion significantly reduced caspase-3 cleavage to control levels (Fig. 7).
Figure 7. Effect of SRT1720 on lung apoptosis after intestinal I/R.
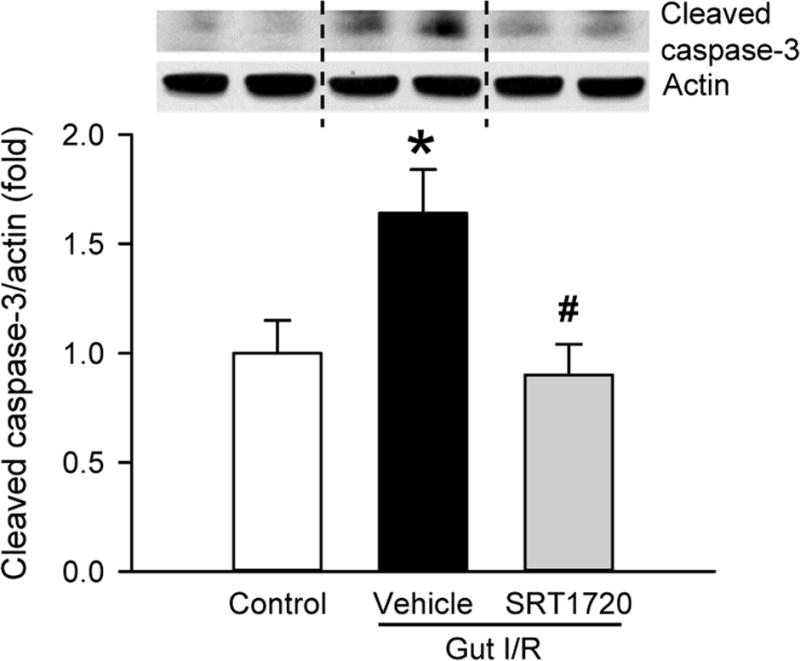
Lung tissues from control, vehicle, and SRT1720 treatment groups were collected 4 h after intestinal I/R to measure the expression of cleaved caspase-3 by Western blot. Band intensity was quantified with densitometry and normalized to β-actin. Representative Western blots are shown. Data are presented as mean ± SE (n= 4–7/group) and compared by one-way ANOVA and SNK. *P < 0.05 vs. control; #P < 0.05 vs. vehicle.
SRT1720 attenuates organ injury after intestinal I/R
In addition to remote organ injury in the lungs, we evaluated intestinal I/R-induced overall organ damage by measuring several serum organ injury markers. At 4 h after intestinal I/R, serum levels of AST, ALT, and LDH were increased by 4.8-, 6.5- and 11.2-fold, respectively, compared to the control (Fig. 8). Treatment with SRT1720 at reperfusion significantly reduced the levels of these organ injury markers by 23%, 24% and 26%, respectively, in comparison with the vehicle (Fig. 8).
Figure 8. Effect of SRT1720 on organ injury after intestinal I/R.
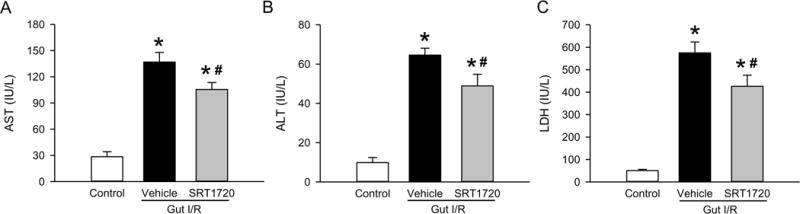
Serum samples from control, vehicle, and SRT1720 treatment groups were collected 4 h after intestinal I/R to measure (A) AST, (B) ALT and (C) LDH. Data are presented as mean ± SE (n=4–7/group) and compared by one-way ANOVA and SNK. *P < 0.05 vs. control; #P < 0.05 vs. vehicle.
DISCUSSION
Intestinal I/R is major contributor to the morbidity and mortality associated with mesenteric artery occlusion, aortic surgery, small bowel transplant, and hemorrhagic shock (1, 16). In I/R injury, the ischemic phase causes loss of mitochondrial mass and activity resulting in cellular apoptosis and necrosis (10). Reactive oxygen species are generated upon reperfusion, which are then circulated along with pro-inflammatory cytokines and damage associated molecular patterns to distant organs (7). The remote organ injury and systemic inflammation of intestinal I/R makes this disease unique and particularly morbid (4). Influencing energy metabolism is showing promise in treating I/R (13, 17, 18). However, the effects of improved mitochondrial function in intestinal I/R on local organ injury, systemic inflammation, and remote organ injury are not well understood. In this study, we show that activation of Sirt1 by SRT1720 improves local and remote organ injury after intestinal I/R.
We find that treatment with SRT1720 in the setting of intestinal I/R improves intestinal mitochondrial mass and biogenesis. There is an associated protection against gut injury and apoptosis, and a reduction in local proinflammatory cytokine production. SRT1720 treatment also improves systemic inflammation. This in turn is associated with reduced lung morphological injury, decreased apoptosis in lung tissue, and improved lung inflammation.
Mitochondrial dysfunction is a consistent finding in I/R injury. Intestinal I/R is associated with mitochondrial injury which manifests as the development of mitochondrial swelling and a marked decrease in mitochondrial activity (9, 10). A decrease in mitochondrial mass is described after I/R injury (13, 18). Sirt1 is a known modulator of mitochondrial biogenesis through its interaction with peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (11). In fact, treatment with SRT1720 is known to stimulate the mitochondrial biogenesis pathway and increase mitochondrial mass in the setting of I/R and trauma-hemorrhage (13, 17, 19). SDH is a mitochondrial protein, part of complex II of the respiratory chain, the protein levels of which are used as a surrogate for mitochondrial mass (20). Herein, we show a significant decrease in SDH after intestinal I/R, which is restored by SRT1720 treatment. In addition, treatment with SRT1720 is associated with a significant increase in TFAM expression after I/R. TFAM is a high mobility group (HMG)-box mitochondrial transcription factor which is responsible for mitochondrial DNA transcription and replication (21). The increase in TFAM expression after SRT1720 suggests an enhancement in mitochondrial biogenesis as the mechanism of the observed increase in mitochondrial mass. In support of this, the protective effects of TFAM over-expression are associated with increased mitochondrial mass and activity after myocardial I/R (22).
Apoptosis is a major cell death pathway after intestinal I/R injury. In our study, we show that treatment with SRT1720 is associated with a significant decrease in TUNEL-positive nuclei after intestinal I/R. SRT1720 is also known to have anti-apoptotic effects in the setting of renal I/R (13, 17). Our observed effect may have been mediated through the direct action of Sirt1 on P53 (17). The use of caspase inhibitors to block apoptosis is protective against intestinal I/R injury (23). Inhibition of apoptosis is linked to an overall decrease in gut injury, and systemic and local inflammation after intestinal I/R (24).
Oxidative stress resulting from intestinal I/R plays a major role in the resulting gut tissue injury. Pretreatment with curcumin, a molecule with several biologic properties including antioxidant activity, improves intestinal histologic injury and increases superoxide dismutase and glutathione peroxidase activities in gut tissue after I/R (25). In this study, we find that gut iNOS expression is increased after I/R and is reduced with SRT1720 treatment. Previous research shows that inhibition of iNOS by aminoguanidine in LPS-challenged rats reduces bacterial translocation and intestinal permeability (26). This suggests that iNOS and excessive production of nitric oxide (NO) is detrimental to intestinal integrity. Activation of Sirt1 is linked to reduced iNOS in intestinal I/R. Similarly, oral reservatrol (a redox scavenger and Sirt1 activator) pretreatment in the setting of intestinal I/R is associated with reduced iNOS expression and NO generation (27).
The cell death and injury resulting from intestinal I/R leads to the inflammatory reaction observed in the gut after such an insult (2). In fact, enhancing the clearance of apoptotic cells after intestinal I/R is promoted as a potential therapeutic strategy against intestinal I/R mediated local and systemic inflammation (28). We find that gut TNF-α mRNA is increased at 4 hours after intestinal I/R and is reduced by Sirt1 activation at reperfusion. Although we sought to measure TNF-α levels in serum, they were undetectable 4 hours after I/R. This is consistent with evidence that serum TNF-α levels increase shortly after reperfusion in intestinal I/R, but they decrease to baseline within 60 minutes (7). Inhibition of the pro-inflammatory cytokine TNF-α is promising in reducing local injury from intestinal I/R. In an intestinal I/R model, pretreatment with an anti-TNF antibody reduces intestinal microvascular injury (6). In a similar study by Guzel et al., pretreatment with infliximab, a monoclonal antibody against TNF-α, attenuates the severity of intestinal I/R injury (5). Expression of IL-6 and TNF-α can be inhibited by the activity of Sirt1 on NF-κB (29). Other studies in intestinal I/R show that nonspecific activation of Sirt1 with icariin pretreatment improves gut tissue injury and reduces serum TNF-α and IL-6 production (30).
Systemic release of TNF-α is associated with the development of acute lung injury (6, 7). Anti-TNF antibodies decrease lung injury after intestinal I/R. In one study by Caty et al., this improvement is independent of neutrophil infiltration (7). In contrast, Koksoy et al. shows improvement in both lung injury and neutrophil infiltration with anti-TNF pretreatment in a rat intestinal I/R model (6). We show that Sirt1 activation decreases lung neutrophil infiltration after intestinal I/R, which correlates with the decrease in TNF-α expression in the gut of treated mice. This supports previous research showing that inhibition of neutrophil infiltration after intestinal I/R reduces associated lung injury (4). The end result of the secondary inflammatory response in the lungs is the induction of cellular apoptosis which significantly contributes to lung injury (31). Our results indicate a significant decrease in lung apoptosis in SRT1720 treated mice after I/R, which is associated with a decrease in histologic lung injury.
Lung injury is a consistent and frequently studied consequence of intestinal I/R (4–7, 32). The effects of intestinal I/R on other remote organs vary from organ to organ, with the greatest evidence of injury and inflammation in the lung, less in renal and cardiac tissue, and minimal effect in the liver (32). In our study, the lung showed the greatest evidence of injury and recovery with SRT1720 treatment at 4 hours after I/R, in contrast to general organ injury or systemic inflammation. This may be due to drug dosing or time-point limitations, or due to inherent differences in other remote organs and their responses to intestinal I/R injury and SRT1720 treatment. We focused on the early immune and metabolic changes to intestinal I/R-induced injury at the 4 hour time-point. Further investigation is needed to determine whether the intestine or other organs require a longer time to recover from I/R injury after Sirt1 activation and whether there is a long-term beneficial effect of SRT1720 treatment.
In this study, we did not measure gut ATP because of the technical challenge of attaining accurate levels in heavily contaminated tissue. However, a correlation between mitochondrial mass and ATP levels in renal I/R has been previously established (13, 18). In addition, examining the effect of SRT1720 on bacterial translocation may give insight into the role of SRT1720 on gut barrier function and further explain the observed effects on lung injury (33). The decreases in the local and systemic injury markers were not optimal, which may have been due to dosing limitations. SRT1720 dosing was limited in part by its hydrophobicity, which necessitated DMSO in the solution for adequate solubility and we were not able to increase the dose of SRT1720 beyond 20mg/kg for this reason. According to Montaguti et al., there is no observed toxicity at 1.0ml/kg DMSO and in this study we used 0.8ml/kg DMSO (10% DMSO in 200μl) (34). Additionally, 10% DMSO is a commonly used solvent to dissolve hydrophobic compounds in animal studies (35). Unfortunately, there is no inactive small molecule isotype for SRT1720 to use as an additional control. In addition, delineating a dose response, therapeutic window, and effect on survival would likely shed light on the clinical utility of SRT1720 in the setting of intestinal I/R.
In summary, we show that post-treatment pharmacologic activation of Sirt1 in the setting of intestinal I/R improves mitochondrial mass and biogenesis in the gut, decreases oxidative stress in the gut, reduces gut and lung injury and apoptosis, and reduces local and systemic inflammation. Given that sirtuin activating compounds are moving into the clinical arena, activation of Sirt1 may be a promising treatment in intestinal I/R.
Acknowledgments
The authors thank Drs. Mahendar Ochani and Zhimin Wang for their assistance with the animal experiments and histology.
This study was supported by the National Institutes of Health (NIH) grants HL076179 and GM053008 (PW).
Footnotes
Conflicts of Interest and Source of Funding: All authors report no financial interests or potential conflicts of interest.
This work was presented for The Shock Society New Investigator Award (First Place) at the 38th Annual Conference on Shock, Denver, Colorado, June 6 to 9, 2015.
References
- 1.Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin Vasc Surg. 2010;23(1):4–8. doi: 10.1053/j.semvascsurg.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94(6):1133–8. doi: 10.1097/00000542-200106000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Turnage RH, Guice KS, Oldham KT. The effects of hypovolemia on multiple organ injury following intestinal reperfusion. Shock. 1994;1(6):408–12. doi: 10.1097/00024382-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo S, Yang WL, Aziz M, Jacob A, Wang P. Cyclic arginine-glycine-aspartate attenuates acute lung injury in mice after intestinal ischemia/reperfusion. Crit Care. 2013;17(1):R19. doi: 10.1186/cc12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzel A, Kanter M, Pergel A, Erboga M. Anti-inflammatory and antioxidant effects of infliximab on acute lung injury in a rat model of intestinal ischemia/reperfusion. J Mol Histol. 2012;43(3):361–9. doi: 10.1007/s10735-012-9396-0. [DOI] [PubMed] [Google Scholar]
- 6.Koksoy C, Kuzu MA, Kuzu I, Ergun H, Gurhan I. Role of tumour necrosis factor in lung injury caused by intestinal ischaemia-reperfusion. Br J Surg. 2001;88(3):464–8. doi: 10.1046/j.1365-2168.2001.01737.x. [DOI] [PubMed] [Google Scholar]
- 7.Caty MG, Guice KS, Oldham KT, Remick DG, Kunkel SI. Evidence for tumor necrosis factor-induced pulmonary microvascular injury after intestinal ischemia-reperfusion injury. Ann Surg. 1990;212(6):694–700. doi: 10.1097/00000658-199012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greer R. The temporal evolution of acute respiratory distress syndrome following shock. Eur J Anaesthesiol. 2010;27(3):226–32. doi: 10.1097/EJA.0b013e3283308e7f. [DOI] [PubMed] [Google Scholar]
- 9.Madesh M, Ramachandran A, Pulimood A, Vadranam M, Balasubramanian KA. Attenuation of intestinal ischemia/reperfusion injury with sodium nitroprusside: studies on mitochondrial function and lipid changes. Biochim Biophys Acta. 2000;1500(2):204–16. doi: 10.1016/s0925-4439(99)00107-6. [DOI] [PubMed] [Google Scholar]
- 10.Madesh M, Bhaskar L, Balasubramanian KA. Enterocyte viability and mitochondrial function after graded intestinal ischemia and reperfusion in rats. Mol Cell Biochem. 1997;167(1–2):81–7. doi: 10.1023/a:1006871622049. [DOI] [PubMed] [Google Scholar]
- 11.Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschop MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92(3):1479–514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan H, Yang HC, You L, Wang YY, He WJ, Hao CM. The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2013;83(3):404–13. doi: 10.1038/ki.2012.394. [DOI] [PubMed] [Google Scholar]
- 13.Khader A, Yang WL, Kuncewitch M, Jacob A, Prince JM, Asirvatham JR, Nicastro JM, Coppa GF, Wang P. Sirtuin 1 activation stimulates mitochondrial biogenesis and attenuates renal injury after ischemia-reperfusion. Transplantation. 2014;98(2):148–56. doi: 10.1097/TP.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 14.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bernis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450(7170):712–6. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baksi A, Kraydashenko O, Zalevkaya A, Stets R, Elliott P, Haddad J, Hoffmann E, Vlasuk GP, Jacobson EW. A phase II, randomized, placebo-controlled, double-blind, multi-dose study of SRT2104, a SIRT1 activator, in subjects with type 2 diabetes. Br J Clin Pharmacol. 2014;78(1):69–77. doi: 10.1111/bcp.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Block TA, Acosta S, Bjorck M. Endovascular and open surgery for acute occlusion of the superior mesenteric artery. J Vasc Surg. 2010;52(4):959–66. doi: 10.1016/j.jvs.2010.05.084. [DOI] [PubMed] [Google Scholar]
- 17.Funk JA, Schnellmann RG. Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1alpha activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol. 2013;273(2):345–54. doi: 10.1016/j.taap.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasbach KA, Schnellmann RG. PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun. 2007;355(3):734–9. doi: 10.1016/j.bbrc.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Luciano JA, Kautza B, Darwiche S, Martinez S, Stratimirovic S, Waltz P, Sperry J, Rosengart M, Shiva S, Zuckerbraun BS. Sirtuin 1 Agonist Minimizes Injury and Improves The Immune Response Following Traumatic Shock. Shock. 2015 doi: 10.1097/SHK.0000000000000412. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297(1):H13–20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci U S A. 1994;91(4):1309–13. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeuchi M, Matsusaka H, Kang D, Matsushima S, Ide T, Kubota T, Fujiwara T, Hamasaki N, Takeshita A, Sunagawa K, Tsutsui H. Overexpression of mitochondrial transcription factor a ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation. 2005;112(5):683–90. doi: 10.1161/CIRCULATIONAHA.104.524835. [DOI] [PubMed] [Google Scholar]
- 23.Farber A, Connors JP, Friedlander RM, Wagner RJ, Powell RJ, Cronenwett JL. A specific inhibitor of apoptosis decreases tissue injury after intestinal ischemia-reperfusion in mice. J Vasc Surg. 1999;30(4):752–60. doi: 10.1016/s0741-5214(99)70115-1. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Wang G, Zhai X, Hu Y, Gao D, Ma L, Yao J, Tian X. Selective inhibition of protein kinase C beta2 attenuates the adaptor P66 Shc-mediated intestinal ischemia-reperfusion injury. Cell Death Dis. 2014;5:e1164. doi: 10.1038/cddis.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yucel AF, Kanter M, Pergel A, Erboga M, Guzel A. The role of curcumin on intestinal oxidative stress, cell proliferation and apoptosis after ischemia/reperfusion injury in rats. J Mol Histol. 2011;42(6):579–87. doi: 10.1007/s10735-011-9364-0. [DOI] [PubMed] [Google Scholar]
- 26.Unno N, Wang H, Menconi MJ, Tytgat SH, Larkin V, Smith M, Morin MJ, Chavez A, Hodin RA, Fink MP. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology. 1997;113(4):1246–57. doi: 10.1053/gast.1997.v113.pm9322519. [DOI] [PubMed] [Google Scholar]
- 27.Dong W, Li F, Pan Z, Liu S, Yu H, Wang X, Bi S, Zhang W. Resveratrol ameliorates subacute intestinal ischemia-reperfusion injury. J Surg Res. 2013;185(1):182–9. doi: 10.1016/j.jss.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Wu R, Dong W, Wang Z, Jacob A, Cui T, Wang P. Enhancing apoptotic cell clearance mitigates bacterial translocation and promotes tissue repair after gut ischemia-reperfusion injury. Int J Mol Med. 2012;30(3):593–8. doi: 10.3892/ijmm.2012.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie J, Zhang X, Zhang L. Negative regulation of inflammation by SIRT1. Pharmacol Res. 2013;67(1):60–7. doi: 10.1016/j.phrs.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F, Hu Y, Xu X, Zhai X, Wang G, Ning S, Yao J, Tian X. Icariin protects against intestinal ischemia-reperfusion injury. J Surg Res. 2015;194(1):127–38. doi: 10.1016/j.jss.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Martin TR, Nakamura M, Matute-Bello G. The role of apoptosis in acute lung injury. Crit Care Med. 2003;31(4 Suppl):S184–8. doi: 10.1097/01.CCM.0000057841.33876.B1. [DOI] [PubMed] [Google Scholar]
- 32.Mura M, Andrade CF, Han B, Seth R, Zhang Y, Bai XH, Waddell TK, Hwang D, Keshavjee S, Liu M. Intestinal ischemia-reperfusion-induced acute lung injury and oncotic cell death in multiple organs. Shock. 2007;28(2):227–38. doi: 10.1097/01.shk.0000278497.47041.e3. [DOI] [PubMed] [Google Scholar]
- 33.Sun Z, Wang X, Deng X, Borjesson A, Wallen R, Hallberg E, Andersson R. Phagocytic and intestinal endothelial and epithelial barrier function during the early stage of small intestinal ischemia and reperfusion injury. Shock. 2000;13(3):209–16. doi: 10.1097/00024382-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Montaguti P, Melloni E, Cavalletti E. Acute intravenous toxicity of dimethyl sulfoxide, polyethylene glycol 400, dimethylformamide, absolute ethanol, and benzyl alcohol in inbred mouse strains. Arzneimittelforschung. 1994;44(4):566–70. [PubMed] [Google Scholar]
- 35.Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Toldo S, Kukreja RC. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation. 2009;120(11 Suppl):S31–6. doi: 10.1161/CIRCULATIONAHA.108.843979. [DOI] [PMC free article] [PubMed] [Google Scholar]


