Abstract
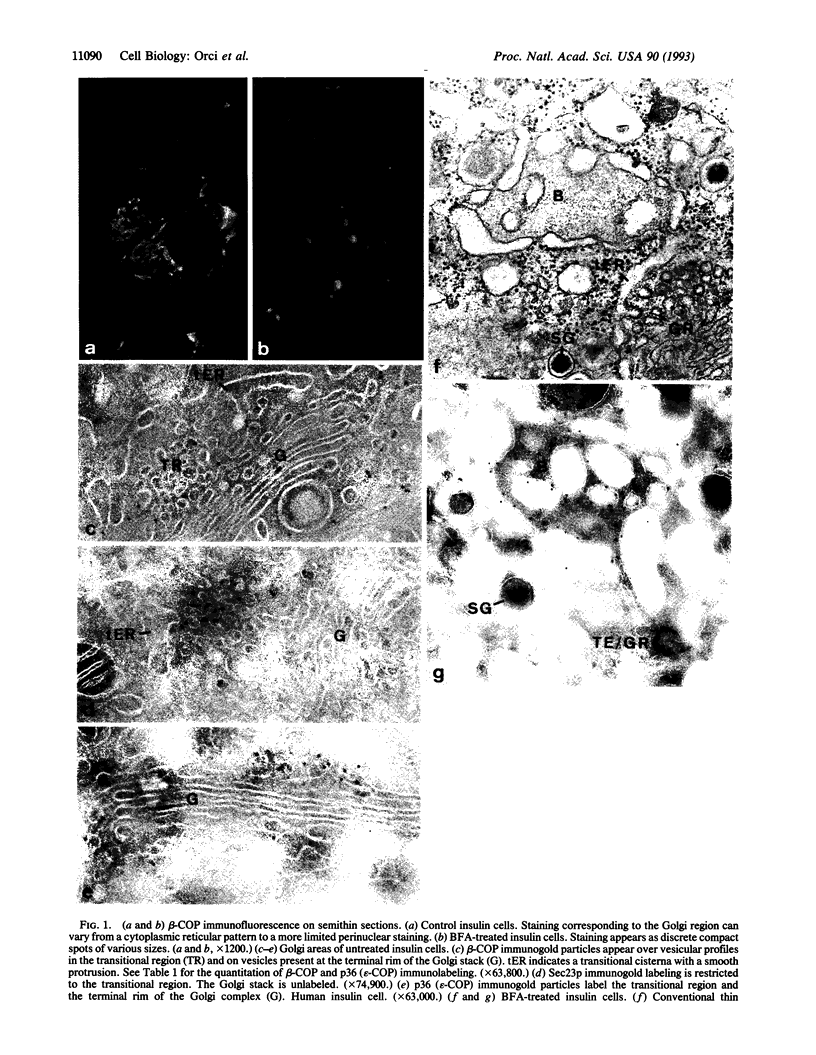
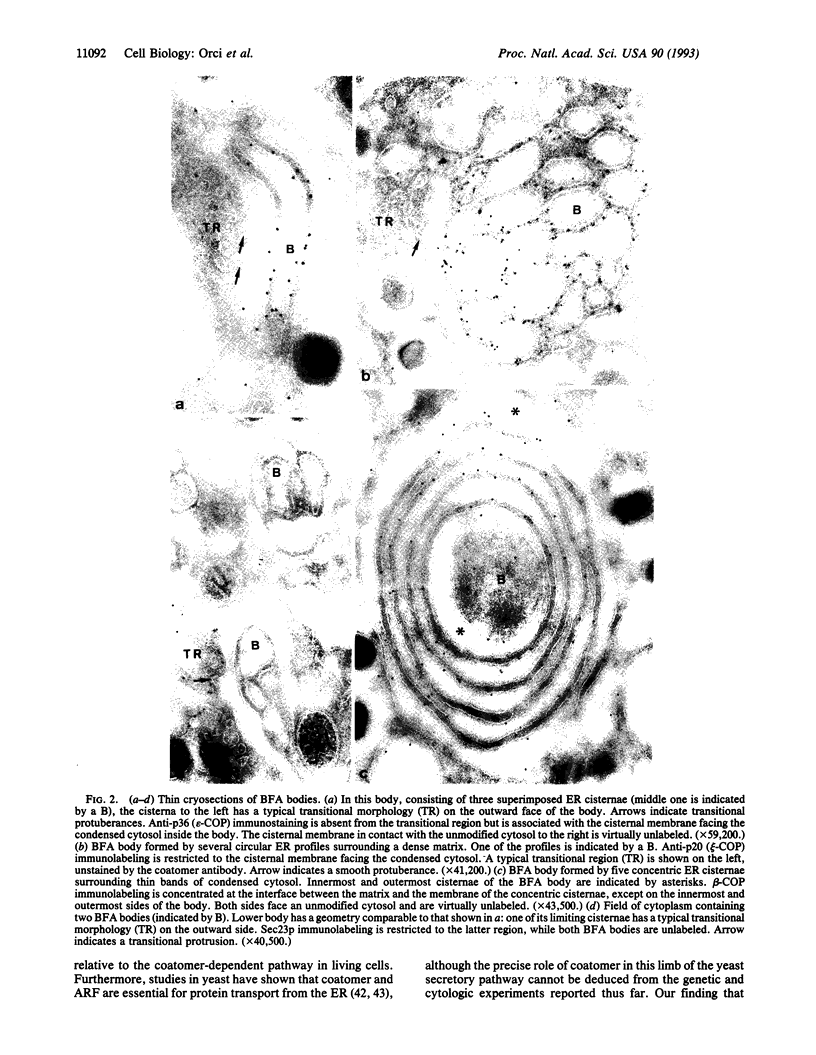
A specialized region of the endoplasmic reticulum--the BFA body--is defined by the site of accumulation of coatomer when nonclathrin coat protein (COP)-coated vesicle assembly is prevented by the drug brefeldin A (BFA). BFA bodies are formed by part smooth, part rough domains of endoplasmic reticulum that are cis to the classical transitional endoplasmic reticulum and to BFA-induced Golgi remnants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlowe C., Schekman R. SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature. 1993 Sep 23;365(6444):347–349. doi: 10.1038/365347a0. [DOI] [PubMed] [Google Scholar]
- De Lemos-Chiarandini C., Ivessa N. E., Black V. H., Tsao Y. S., Gumper I., Kreibich G. A Golgi-related structure remains after the brefeldin A-induced formation of an ER-Golgi hybrid compartment. Eur J Cell Biol. 1992 Aug;58(2):187–201. [PubMed] [Google Scholar]
- Donaldson J. G., Cassel D., Kahn R. A., Klausner R. D. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-COP to Golgi membranes. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J. G., Finazzi D., Klausner R. D. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992 Nov 26;360(6402):350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Lippincott-Schwartz J., Bloom G. S., Kreis T. E., Klausner R. D. Dissociation of a 110-kD peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J Cell Biol. 1990 Dec;111(6 Pt 1):2295–2306. doi: 10.1083/jcb.111.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duden R., Griffiths G., Frank R., Argos P., Kreis T. E. Beta-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to beta-adaptin. Cell. 1991 Feb 8;64(3):649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Oda K., Yokota S., Takatsuki A., Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988 Dec 5;263(34):18545–18552. [PubMed] [Google Scholar]
- Helms J. B., Rothman J. E. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992 Nov 26;360(6402):352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- Hendricks L. C., McCaffery M., Palade G. E., Farquhar M. G. Disruption of endoplasmic reticulum to Golgi transport leads to the accumulation of large aggregates containing beta-COP in pancreatic acinar cells. Mol Biol Cell. 1993 Apr;4(4):413–424. doi: 10.1091/mbc.4.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks L. C., McClanahan S. L., McCaffery M., Palade G. E., Farquhar M. G. Golgi proteins persist in the tubulovesicular remnants found in brefeldin A-treated pancreatic acinar cells. Eur J Cell Biol. 1992 Aug;58(2):202–213. [PubMed] [Google Scholar]
- Hidalgo J., Garcia-Navarro R., Gracia-Navarro F., Perez-Vilar J., Velasco A. Presence of Golgi remnant membranes in the cytoplasm of brefeldin A-treated cells. Eur J Cell Biol. 1992 Aug;58(2):214–227. [PubMed] [Google Scholar]
- Hobman T. C., Woodward L., Farquhar M. G. The rubella virus E1 glycoprotein is arrested in a novel post-ER, pre-Golgi compartment. J Cell Biol. 1992 Aug;118(4):795–811. doi: 10.1083/jcb.118.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosobuchi M., Kreis T., Schekman R. SEC21 is a gene required for ER to Golgi protein transport that encodes a subunit of a yeast coatomer. Nature. 1992 Dec 10;360(6404):603–605. doi: 10.1038/360603a0. [DOI] [PubMed] [Google Scholar]
- Kahn R. A., Randazzo P., Serafini T., Weiss O., Rulka C., Clark J., Amherdt M., Roller P., Orci L., Rothman J. E. The amino terminus of ADP-ribosylation factor (ARF) is a critical determinant of ARF activities and is a potent and specific inhibitor of protein transport. J Biol Chem. 1992 Jun 25;267(18):13039–13046. [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell M. H. Two rapid and simple methods used for the removal of resins from 1.0 micron thick epoxy sections. J Microsc. 1978 Mar;112(2):253–255. doi: 10.1111/j.1365-2818.1978.tb01174.x. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Misumi Y., Miki K., Takatsuki A., Tamura G., Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986 Aug 25;261(24):11398–11403. [PubMed] [Google Scholar]
- Oda K., Hirose S., Takami N., Misumi Y., Takatsuki A., Ikehara Y. Brefeldin A arrests the intracellular transport of a precursor of complement C3 before its conversion site in rat hepatocytes. FEBS Lett. 1987 Apr 6;214(1):135–138. doi: 10.1016/0014-5793(87)80028-5. [DOI] [PubMed] [Google Scholar]
- Oprins A., Duden R., Kreis T. E., Geuze H. J., Slot J. W. Beta-COP localizes mainly to the cis-Golgi side in exocrine pancreas. J Cell Biol. 1993 Apr;121(1):49–59. doi: 10.1083/jcb.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L. A portrait of the pancreatic B-cell. The Minkowski Award Lecture delivered on July 19, 1973, during the 8th Congress of the International Diabetes Federation, held in Brussels, Belgium. Diabetologia. 1974 Jun;10(3):163–187. doi: 10.1007/BF00423031. [DOI] [PubMed] [Google Scholar]
- Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982 Jun;31(6 Pt 1):538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- Orci L., Palmer D. J., Ravazzola M., Perrelet A., Amherdt M., Rothman J. E. Budding from Golgi membranes requires the coatomer complex of non-clathrin coat proteins. Nature. 1993 Apr 15;362(6421):648–652. doi: 10.1038/362648a0. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Louvard D., Perrelet A. Clathrin-immunoreactive sites in the Golgi apparatus are concentrated at the trans pole in polypeptide hormone-secreting cells. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5385–5389. doi: 10.1073/pnas.82.16.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Meda P., Holcomb C., Moore H. P., Hicke L., Schekman R. Mammalian Sec23p homologue is restricted to the endoplasmic reticulum transitional cytoplasm. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8611–8615. doi: 10.1073/pnas.88.19.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Tagaya M., Amherdt M., Perrelet A., Donaldson J. G., Lippincott-Schwartz J., Klausner R. D., Rothman J. E. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991 Mar 22;64(6):1183–1195. doi: 10.1016/0092-8674(91)90273-2. [DOI] [PubMed] [Google Scholar]
- Orcl L., Palmer D. J., Amherdt M., Rothman J. E. Coated vesicle assembly in the Golgi requires only coatomer and ARF proteins from the cytosol. Nature. 1993 Aug 19;364(6439):732–734. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Palmer D. J., Helms J. B., Beckers C. J., Orci L., Rothman J. E. Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J Biol Chem. 1993 Jun 5;268(16):12083–12089. [PubMed] [Google Scholar]
- Pelham H. R. Recycling of proteins between the endoplasmic reticulum and Golgi complex. Curr Opin Cell Biol. 1991 Aug;3(4):585–591. doi: 10.1016/0955-0674(91)90027-v. [DOI] [PubMed] [Google Scholar]
- Robinson M. S., Kreis T. E. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell. 1992 Apr 3;69(1):129–138. doi: 10.1016/0092-8674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Prydz K., Hansen S. H., van Deurs B. Ricin transport in brefeldin A-treated cells: correlation between Golgi structure and toxic effect. J Cell Biol. 1991 Nov;115(4):971–981. doi: 10.1083/jcb.115.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A., Fransen J. A., Matter K., Kreis T. E., Ginsel L., Hauri H. P. Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus. Eur J Cell Biol. 1990 Dec;53(2):185–196. [PubMed] [Google Scholar]
- Schweizer A., Matter K., Ketcham C. M., Hauri H. P. The isolated ER-Golgi intermediate compartment exhibits properties that are different from ER and cis-Golgi. J Cell Biol. 1991 Apr;113(1):45–54. doi: 10.1083/jcb.113.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T., Orci L., Amherdt M., Brunner M., Kahn R. A., Rothman J. E. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991 Oct 18;67(2):239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- Serafini T., Stenbeck G., Brecht A., Lottspeich F., Orci L., Rothman J. E., Wieland F. T. A coat subunit of Golgi-derived non-clathrin-coated vesicles with homology to the clathrin-coated vesicle coat protein beta-adaptin. Nature. 1991 Jan 17;349(6306):215–220. doi: 10.1038/349215a0. [DOI] [PubMed] [Google Scholar]
- Stearns T., Willingham M. C., Botstein D., Kahn R. A. ADP-ribosylation factor is functionally and physically associated with the Golgi complex. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1238–1242. doi: 10.1073/pnas.87.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenbeck G., Harter C., Brecht A., Herrmann D., Lottspeich F., Orci L., Wieland F. T. beta'-COP, a novel subunit of coatomer. EMBO J. 1993 Jul;12(7):2841–2845. doi: 10.1002/j.1460-2075.1993.tb05945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenbeck G., Schreiner R., Herrmann D., Auerbach S., Lottspeich F., Rothman J. E., Wieland F. T. Gamma-COP, a coat subunit of non-clathrin-coated vesicles with homology to Sec21p. FEBS Lett. 1992 Dec 14;314(2):195–198. doi: 10.1016/0014-5793(92)80973-k. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Application of cryoultramicrotomy to immunocytochemistry. J Microsc. 1986 Aug;143(Pt 2):139–149. doi: 10.1111/j.1365-2818.1986.tb02772.x. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Waters M. G., Serafini T., Rothman J. E. 'Coatomer': a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991 Jan 17;349(6306):248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]
- Yoshihisa T., Barlowe C., Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science. 1993 Mar 5;259(5100):1466–1468. doi: 10.1126/science.8451644. [DOI] [PubMed] [Google Scholar]