Abstract
Introduction
Bariatric surgery is the most effective therapeutic option to reduce weight in morbidly obese individuals, but it results in a number of mineral and vitamin deficiencies. Clinical Practice Guidelines (CPGs) attempt to balance those benefits and harms to provide guidance to physicians and patients.
Objectives
We compare and evaluate the quality of the evidence and of the development process of current CPGs that provide recommendations on vitamin D replacement in patients undergoing bariatric surgery, using a validated tool.
Methods
We searched 4 databases, with no time restriction, to identify relevant and current CPGs. Two reviewers assessed eligibility and abstracted data, in duplicate. They evaluated the quality of CPGs development process using the Appraisal of Guidelines, Research, and Evaluation II (AGREE II) tool that consists of 6 domains. A content expert verified those assessments.
Results
We identified 3 eligible CPGs: (1) the Endocrine Society (ES) guidelines (2010); (2) the American Association of Clinical Endocrinologists (AACE), The Obesity Society (TOS), and the American Society for Metabolic & Bariatric Surgery (ASBMS) guidelines (update 2013); and (3) the Interdisciplinary European (IE) guidelines on Metabolic and Bariatric Surgery (latest update 2014).
The ES and the AACE/TOS/ASBMS guidelines recommended high doses of vitamin D, varying from 3,000 IU daily to 50,000 IU 1-3 times weekly. Vitamin D doses were not mentioned in the IE guidelines. The recommendations were based on a low quality of evidence, if any, or limited to a single high quality trial, for some outcomes. In terms of quality, only the IE guidelines described their search methodology but none of the CPGs provided details on evidence selection and appraisal. None of the three CPGs rigorously assessed the preferences of the target population, resource implications, and the applicability of these guidelines. According to the AGREE II tool, we rated the ES guidelines as average in quality, and the other two as low in quality.
Conclusion
Current CPGs recommendations on vitamin D supplementation in bariatric surgery differ between societies. They do not fulfill criteria for optimal guideline development, in part possibly due to limited resources, and are based on expert opinion. Thus, the pressing need for high quality randomized trials to inform CPGs, to be developed based on recommended standards.
Keywords: bariatric surgery, Clinical Practice Guidelines, vitamin D replacement, quality of evidence, appraisal tool
1. Introduction
Obesity is a rapidly growing global health problem, contributing to a major increase in non-communicable diseases, especially metabolic and cardiovascular diseases [1, 2]. Bariatric surgery is considered the most effective therapy for sustained weight loss in obese patients [3]. While bariatric surgery substantially reduces the metabolic risk factors in this population, it is associated with several short and long term complications [3]. Vitamin and mineral deficiencies have been widely described following bariatric surgeries [3, 4]. In particular, hypovitaminosis D remains a major problem, not only pre-operatively but also post operatively, regardless of the type of the procedure and the supplementation dose [5, 6]. In a recent systematic review of 51 observational studies assessing 25(OH)D status in obese patients undergoing bariatric surgery (follow up range: 3 months to 11 years post-operatively), mean 25-hydroxyvitamin D (25(OH)D) level was less than 30 ng/ml, before and after bariatric surgery, despite various vitamin D supplementation regimens [7]. Furthermore, 25(OH)D level was less than 20 ng/ml in half of the studies that were identified [7].
Although there are several guidelines on nutritional replacement post bariatric surgery, clinical practice varies widely, as shown in recently conducted surveys in the UK and US [8, 9].
The Institute Of Medicine (IOM)1 recognizes the crucial role of Clinical Practice Guidelines (CPGs) in medical care, and has set 8 standards for the development of “trustworthy guidelines” [10]. These include: transparency, resolution of conflict of interest, explicitly defining guideline development group, the use of well conducted systematic reviews as a foundation of the recommendations, explanation of the rationale behind each recommendation, rating the strength of evidence, clear formulation of the recommendations, external review and guidelines update [10]. To assess the quality of published guidelines, forty different CPGs assessment tools have been identified in a systematic review by Siering et al [11]. These tools differ by the number of dimensions covered, the appraisers involved and the validation studies [11]. The Appraisal of Guidelines, Research, and Evaluation - AGREE - (English) and the Deutsches Instrument Zurmethodischen Leitlinien-Bewertung - DELBI - (German) tools were shown to be the most comprehensive tools, each covering thirteen quality dimensions and more than 20 items [11, 12]. The AGREE tool is a validated instrument, developed by a group of international guideline developers and researchers, to assess guidelines quality, methodological strategy and transparency [13]. It was initially published in 2003 [13], and updated into a more reliable version in 2009, allowing documentation of more details regarding the quality of each dimension assessed [14]. The guidelines quality appraisal using AGREE II tool is defined as “the confidence that the potential biases of guideline development have been addressed adequately and that the recommendations are both internally and externally valid, and are feasible for practice” [14]. Several dimensions of the AGREE II tool have been adopted by guidelines developing societies in the US and Europe, such as the Institute of Medicine [10], National Health Services (NHS) [15] and the Guidelines International Network (GIN) [16].
2. Objectives
The objectives of this paper are to compare and evaluate the quality of the evidence and of the development process of current CPGs, that provide recommendations on vitamin D replacement in patients undergoing bariatric surgery.
3. Methods
3.1. Identification of the CPGs
3.1.1. Search strategy
We conducted a search for English language CPGs on vitamin D replacement in bariatric surgery patients in December 2013, and updated it in April 2015, without any time restrictions. The following MeSH terms were used: vitamin D, vitamin D deficiency, bariatric, bariatric surgery, and guideline. Keywords included: cholecalciferol, ergocalciferol, hydroxyvitamin D, bilio-pancreatic diversion, Roux-En-Y gastric bypass, gastric sleeve, duodenal switch, laparoscopic gastric banding, and recommendation. We conducted the search in the following databases: Medline, PubMed, Embase and the National Guideline Clearinghouse. In addition, the references of recently published reviews on the topic were checked. For full details on the search methodology see Appendix A.
3.1.2. Eligibility criteria
Inclusion criteria
We included the latest update of CPGs discussing vitamin D replacement, separately or as part of other nutritional supplementations, in patients undergoing bariatric surgery.
Exclusion criteria
We excluded the older versions of the CPGs, if issued by the same organization. We also excluded review articles on the topic.
3.1.3. CPGs identification and data abstraction
Two reviewers (MC and NN) reviewed all references (title and abstract screening) in duplicate and independently. Similarly, they reviewed the full text of eligible articles and abstracted relevant data from the CPGs.
3.2. Appraisal of the Clinical Practice Guidelines
3.2.1. The AGREE II tool
The AGREE II tool includes 6 domains: scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability and editorial independence [14]. Each domain consists of 2-4 items, with the exception of the domain that discusses the rigor of development of the CPGs that consists of 8 items, as detailed in Appendix B. Reviewers rate each item using a score of 1-7. A score of 1 indicates that specific details relevant to the item assessed were absent or very poorly discussed [14]. A score of 7 indicates that the reporting was of high quality and all the items specified in the AGREE II tool user's manual were detailed [14]. A score between 2 and 6 indicates that the reporting of the AGREE II item was not complete [14].
3.2.2. Appraisal of the CPGs
Two reviewers with experience in research methods (MC and NN) abstracted information relevant to each item of the AGREE II domains and rated the identified CPGs independently, using the AGREE II tool, with oversight by the content expert (GEHF). Corresponding authors of the identified CPGs were contacted by email by the senior author (GEHF) for queries about the availability of additional relevant information needed for the scoring of the CPGs, that may have been available but not included in the published CPGs document.
3.3. Statistical analysis
For each domain of the AGREE II tool, the “obtained score” is calculated as the sum of all the scores given by raters for all the items included in this domain. The “scaled domain score” is calculated as a standardized score using the following formula: (Obtained Score –Minimum possible score)/ (Maximum possible score – Minimum possible score) [14]. The maximum score for each domain is derived by multiplying the number of items in this domain by the number of raters, multiplied by 7 (which corresponds to “strongly agree”). The minimum score is derived by multiplying the number of items in this domain by the number of raters, multiplied by 1 (which corresponds to “strongly disagree”) [14].
The agreement between the two raters is determined by the intra-class correlation coefficient (ICC) and the 95% Confidence Interval (CI), for the 23 items of the AGREE II tool in the identified CPGs. The degree of agreement is considered slight if ICC is between 0.01 and 0.2, fair if ICC is between 0.21 and 0.40, moderate if ICC is between 0.41 and 0.60, substantial if ICC is between 0.61 to 0.80, and perfect if ICC is between 0.81 and 1.00 [17].
The analysis was performed using SPSS version 22.
4. Results
The search strategy identified 514 citations. Figure 1 represents the flow diagram for CPGs selection. Although several papers have discussed recommendations on nutritional supplements replacement following bariatric surgery [18-20], we identified only three eligible CPGs developed by the following organizations (Table 1):
Figure 1. Flow diagram for study selection (search conducted in April 2015).
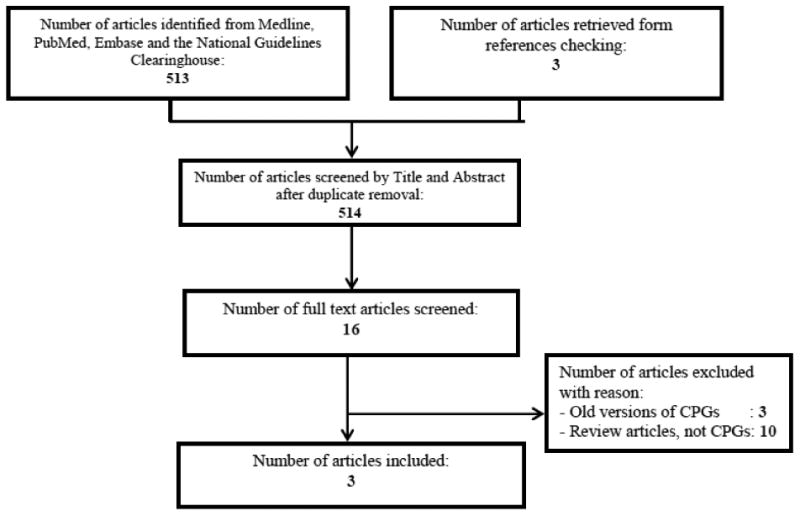
The search strategy yielded 3 CPGs that were included in this review. CPGs: Clinical Practice Guidelines
Table 1. Vitamin D replacement recommendations in Clinical Practice Guidelines.
| Guidelines | Target population | Vitamin D replacement doses* | Case of severe malabsorption* |
|---|---|---|---|
| Endocrine Society Clinical Practice Guideline 2010 | Bariatric surgery(type of surgery unspecified) | “First phase (weeks 1-2, liquids): oral vitamin D 50,000 IU daily.” No grading.“ Second phase (weeks 3-6, soft food): Calcitriol D 1,000 IU daily.” No grading“ Vitamin D can be provided with ergocalciferol, 50,000IU one to three times per week.” No grading |
“50.000 IU vitamin D 1-3 times daily.” No grading. |
| Malabsorptive surgical procedures | “Vitamin D supplementation is recommended postoperatively for malabsorptive obesity surgical procedures and the doses be adjusted by a qualified medical professional based on serum markers and measures of bone density.” Strong recommendation with moderate quality of evidence. | ||
| GRADE definition*: “Level of evidence: Evidence based on randomized controlled trials begins as high quality evidence, but our confidence in the evidence may be decreased for several reasons: study limitations, inconsistency of results, indirectness, imprecision, bias. High quality— Further research is very unlikely to change our confidence in the estimate of effect; Moderate quality— Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low quality— Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very low quality— Any estimate of effect is very uncertain.Strength of the recommendations: -Strong recommendation: the desirable effects of an intervention clearly outweigh the undesirable effects, or clearly do not; -Weak recommendation: when the trade-offs are less certain—either because of low quality evidence or because evidence suggests that desirable and undesirable effects are closely balanced.” | |||
| American Association of Clinical Endocrinologists (AACE) The Obesity Society (TOS) American Society for Metabolic & Bariatric Surgery (ASMBS) 2013 | Roux-en-Y gastric bypass Laparoscopic sleeve gastrectomy | “Vitamin D, at least 3,000 IU daily, titrate to >30 ng/ml.” Grade A†, BEL 1. | “Oral D2 or D3 may need to be as high as 50,000 units 1 to 3 times weekly to daily, more recalcitrant cases may require concurrent oral calcitriol (1,25(OH)2 D).” Grade D. |
| Laparoscopic adjustable gastric banding | “At least 3,000 IU of vitamin D daily (titrated to therapeutic 25-dihydroxy vitamin D levels)”. No grading. | ||
| Roux-en-Y gastric bypass Bilio-pancreatic diversion Bilio-pancreatic diversion and duodenal switch | “In patients who have undergone RYGB, BPD or BPD/DS, treatment with oral calcium citrate and vitamin D2 or D3 is indicated to prevent or minimize secondary hyperparathyroidism without inducing frank hypercalciuria.” Grade C, BEL 3. | ||
| AACE Protocol for Standardized Production of Clinical Practice Guidelines grading*:“ Best Evidence Level (BEL): BEL 1: meta-analysis and RCTs; BEL 2: non randomized and observational trials; BEL 3: Surveys and Case Series; BEL 4: No evidence. Grade A: ≥1 conclusive level 1 publications demonstrating benefit ≫ risk; Grade B: No conclusive level 1 publication OR ≥1 conclusive level 2 publications demonstrating benefit ≫ risk; Grade C: No conclusive level 1 or 2 publication; ≥1 conclusive level 3 publications demonstrating benefit ≫ risk; No risk at all and no benefit at all; Grade D: No conclusive level 1, 2, or 3 publication demonstrating benefit ≫risk; | |||
| Interdisciplinary European Guidelines on Surgery of Severe Obesity 2014 | Adjustable gastric banding Roux-en-Y gastric bypass | “Vitamin and micronutrient supplements (oral) should routinely be prescribed to compensate for their possible reduced intake and absorption.” No grading. | Not available |
| Bilio-pancreatic diversion | “Lifelong daily vitamin and micronutrient supplementation (vitamins should be administered in a water-soluble form): Vitamins A, D, E and K”. No grading. | ||
Information was taken verbatim from the guidelines or the guidelines grading documents and was included between quotations.
Grade A recommendation was based on:
- One pilot randomized controlled trial by Goldner et al. (2009) evaluating the effect of 3 doses of vitamin D supplementation 800, 2,000, and 5,000 IU/day in RYGBP patients.
-Endocrine Society Guidelines on vitamin D deficiency (2011) that did not target specifically bariatric surgery patients.
The Endocrine Society (ES) CPGs published in 2010 [21].
The American Association of Clinical Endocrinologists, The Obesity Society, and the American Society for Metabolic & Bariatric Surgery (AACE/TOS/ASBMS) guidelines initially published in 2008 [22] and updated in 2013 [23].
The Interdisciplinary European (IE) guidelines initially published in 2007 [24], updated in 2013 [25], and in 2014 [26].
All three CPGs addressed vitamin D requirements, as part of guidelines for other nutritional supplementation.
4.1. Comparison of CPGs recommendations on vitamin D supplementation following bariatric surgery (Table 1)
The ES 2010 CPGs addressed the endocrine and nutritional management of patients following bariatric surgery [21]. They recommended vitamin D replacement to patients undergoing a malabsorptive procedure [21]. Suggestions were provided for doses to be used, i.e. ergocalciferol at doses ranging from 50,000 IU 1-3 times weekly, increasing to 50,000 IU 1-3 times daily in cases of severe vitamin D malabsorption. In the event of symptomatic severe malabsorption, oral or parenteral calcitriol were suggested. These CPGs also specified that secondary hyperparathyroidism may be treated with weekly parenteral ergocalciferol 100,000 IU, until the target 25-hydroxyvitamin D (25(OH)D) level ≥ 30 ng/ml (75 nmol/l) is achieved, and that active vitamin D (calcitriol) may be required [21].
The AACE/TOS/ASBMS guidelines addressed perioperative nutritional, metabolic and non-surgical support post-bariatric surgery [23]. They targeted Roux-en-Y Gastric Bypass (RYGBP), Laparoscopic Sleeve Gastrectomy (LSG) and Laparoscopic adjustable Gastric Banding (LAGB) surgeries [23]. The guidelines recommended at least 3,000 IU of vitamin D daily and specified that the dose is to be titrated to reach 25(OH)D levels ≥ 30 ng/ml (75 nmol/l) and may reach up to 6,000 IU daily; supplementation may start pre -operatively [23]. In case of severe malabsorption, a vitamin D dose of 50,000 IU 1 to 3 times weekly to daily was recommended; concomitant oral active vitamin D and calcitriol may be required [23].
The IE guidelines in 2014, similar to previous versions, recommended vitamin supplements to Adjustable Gastric Banding (AGB) and RYGBP, without mentioning specifically vitamin D. In Bilio-Pancreatic Diversion (BPD) patients, vitamin supplementation was recommended including vitamins A, D, E, K, without specifying dosing nor target 25(OH)D levels required [26].
4.2. Evaluation of the quality of evidence supporting recommendations on vitamin D supplementation in current CPGs (Table 1)
The ES recommendations on vitamin D supplementation following bariatric surgery was considered as a strong recommendation with moderate quality of evidence, while the doses of vitamin D suggested were not graded. The evidence directly supporting these recommendations was not readily evident; several observational studies on the prevalence of vitamin D deficiency following malabsorptive bariatric surgery were cited. No evidence was provided for the suggested vitamin D doses [21].
The AACE/TOS/ASBMS guidelines recommendation of a vitamin D dose of 3,000 IU daily was graded as “Grade A” [23]. This grade was determined according to the AACE Protocol for Standardized Production of Clinical Practice Guidelines, namely the presence of “≥1 conclusive level 1 publications demonstrating benefit ≫ risk” [27]. This recommendation was based on one randomized controlled pilot (N=45) trial by Goldner et al [28], and on the 2011 ES CPGs on the evaluation, treatment and prevention of vitamin D deficiency [29]. The former study compared 3 vitamin D doses: 800 IU, 2,000 IU and 5,000 IU daily, and showed that, starting from a baseline 25(OH)D level of 15-23 ng/ml (37-57 nmol/l), at one year following Roux-En-Y gastric bypass, 70-75% of the individuals reached the target 25(OH)D level of 30 ng/ml (75 nmol/l) in the intermediate and high dose arms, and only 44% in the low dose arm [28]. The 2011 ES CPGs suggested in obese patients and those with malabsorption a vitamin D dose of 6,000-10,000 IU daily as treatment, followed by a maintenance dose of 3,000-6,000 IU daily. This was considered a weak recommendation with a high quality of evidence. It was based on one observational and one interventional studies conducted in obese patients (not undergoing bariatric surgery) [30, 31]. The observational study was cross-sectional, conducted on 410 healthy women (BMI 17-30 kg/m2) and showed that body fat was correlated significantly with 25(OH)D level, although the correlation was very small (R2 0.02) [30]. The interventional study compared the response in normal weight versus obese individuals to phototherapy (whole body radiation) (N=13 per arm) or a single dose of vitamin D2 50.000 IU (N=11 per arm). In both interventions, the response in obese was attenuated [30,31]. Noteworthy, the recommended maintenance dose of 3,000 IU daily does not reflect the aforementioned doses in the pilot trial nor in the ES CPGs on vitamin D deficiency. The other two recommendations on vitamin D supplementation were to prevent hyperparathyroidism and for cases of severe malabsorption, and were graded as Grade C and D, respectively. These grades reflect the poor quality or lack of evidence, as recognized by the guidelines development group.
The IE guidelines recommended vitamin D supplementation in BPD patients. However, they did not specify the recommended doses of vitamin D supplementation nor the evidence behind such recommendation. In addition, grading was not provided [26].
4.3. Appraisal of guidelines development process using the AGREE II tool
A rigorous systematic assessment of the three aforementioned CPGs is detailed below. The agreement between the authors who rated these CPGs was considered “perfect”, with an ICC 0.904, and a 95% CI 0.735 - 0.955, as per classification of raters agreement [17].
4.3.1. Overview of results
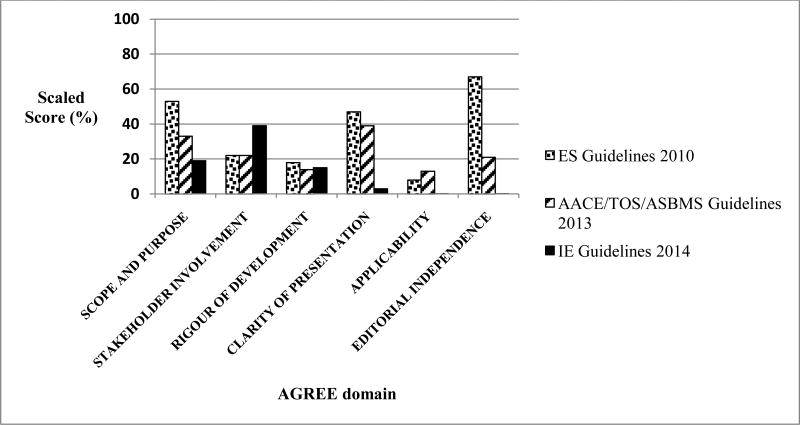
The systematic appraisal reveals relatively low scores for the three available CPGs, scores that were below 50%, for almost all domains of the AGREE II instrument. The only exceptions were for the ES CPGs that scored 53% for Scope and Purpose, and 67% for Editorial Independence (Figure 2). The other scores were comparable for some but not all domains between the three CPGs. They varied between 14-18% for Rigor of Development, 22-39% for Stakeholder Involvement, 3-47% for Clarity of Presentation, and 0-13% for Applicability (Figure 2, Appendix C). An in depth assessment of the scaled scores and their justification is provided below and in Appendix B.
Figure 2. Quality assessment of the Clinical Practice Guidelines (CPGs) regarding vitamin D supplementation based on the Appraisal of Guidelines, Research, and Evaluation II (AGREE II) instrument.
The “Obtained score” for each domain is the sum of scores given by the raters for the items included in that domain.
The “Minimum possible score” for each domain is the number of items in this domain multiplied by 1, multiplied by the number of raters.
The “Maximum possible score” for each domain is the number of items in this domain multiplied by 7, multiplied by the number of raters.
For full details, see Appendix B.
AACE/TOS/ASBMS: American Association of Clinical Endocrinologists; The Obesity Society, and the American Society for Metabolic & Bariatric Surgery; CPG: Clinical Practice Guidelines; ES: Endocrine Society; IE: Interdisciplinary European.
4.3.2. Justification for the calculated scaled scores
Scope and Purpose
This domain assesses the overall aims of the guidelines, the health questions that are being discussed, and the specific population that the guidelines target [14] (Appendix B).
The ES guidelines clearly defined their health intent which was the “nutritional and endocrine management” of a target population consisting of “adults after bariatric surgery”, and the expected benefit were described as the prevention of complications, weight gain and progression of obesity-associated comorbidities [21]. The vitamin D recommendations targeted malabsorptive obesity surgical procedures and sleeve gastrectomy, and included specific recommendations for cases of secondary hyperparathyroidism and severe malabsorption. However, the CPGs did not provide details regarding the comparator used, the outcomes and the health care setting considered [21].
The AACE/TOS/ASBMS guidelines targeted bariatric surgery patients, without any details relevant to gender, age or type of surgical procedure [23]. Vitamin D recommendations targeted specific bariatric surgery types. In addition, recommendations for cases of severe malabsorption were provided. However, they did not discuss the health intents, expected benefits, outcomes, comparators and context of the guidelines [23].
The IE guidelines mentioned their target population, namely patients undergoing specific surgical procedures, such as gastric banding, RYGB and BPD. No further details regarding the overall objective of the guidelines and the health questions covered were provided [26].
Stakeholder Involvement
This domain describes the professional groups involved in the development of the guidelines, their affiliation, and defines the target users of these guidelines. In addition, it describes the views and preferences of the target population, collected from interviews of stakeholders or from literature review [14] (Appendix B).
All of the three CPGs were prepared by a group of individuals from various relevant disciplines, such as endocrinology, obesity, nutrition, bariatric surgery, gastroenterology. Only the ES guidelines panel included a methodologist [21]. The guidelines document mentioned the authors' names and affiliation, but did not define the exact contribution of each to the guidelines. Only the IE Guidelines defined their target users as “physicians, health care practitioners, health care policy makers and health care providers, and insurance companies” [26]. None of the guidelines sought the views and preferences of the target population.
Rigor of Development
This domain describes the search methodology to retrieve the evidence needed, the appraisal of the body of evidence, the methods used to formulate and update the recommendations, and considerations for the health benefits, risks and side effects of such recommendations [14] (Appendix B).
The ES guidelines did not include any details regarding the search methodology, the process for evidence selection, the strengths and limitations of the evidence [19]. They did, however, follow the approach recommended by the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) group to assess the quality of evidence, and to define the strength of recommendation [21]. The GRADE system classifies the quality of evidence based on study design and incorporates several considerations including study limitations, bias, indirectness and inconsistency of results and imprecision. According to GRADE, the strength of a recommendation depends not only on the quality of evidence but also on the balance of harms and benefits, patient's values and the available resources [32]. In the ES CPGs document, the benefits and risks of the intervention were not discussed in depth. The guidelines were reviewed by external experts but no details were provided to explain the methods used to complete the external review (i.e. rating scale, or questionnaire…), the summary of their findings, and how such review was used to inform the guidelines.
The AACE/TOS/ASBMS guidelines followed the AACE Protocol for Standardized Production of Clinical Practice Guidelines—2010 Update [27]. This protocol is being continuously updated, latest in 2014, as the AACE recognizes the importance of the standardization in the assessment and development of guidelines strategies [33]. This protocol balances the contribution of evidence-based medicine (EBM) methods and subjective factors in practice guidelines. Therefore, CPG are the product of a four-step process: first, evidence rating based on research methodology, using the 2004 AACE protocol; second, evidence analysis and identification of strengths and weaknesses; third, phrasing and grading of the recommendation, fourth step incorporation of qualifiers such as cost-effectiveness, risk-benefit analysis, resources availability, cultural factors [27]. However, the AACE/TOS/ASBMS guidelines document did not include information regarding the systematic search methodology, the criteria for selecting the evidence, its strengths and limitations. In addition, some of the recommendations were explicitly linked to specific studies while others were not [23].
The IE guidelines adopted the Oxford Center for Evidence-Based Medicine (OCEBM) classification system [34]. The latter system evaluates the quality of evidence based on study design but takes into consideration other factors including study quality, imprecision and indirectness. In addition to the quality of evidence, patients' values and treatment benefits and harms are incorporated in decision making [34]. In contrast to other evidence-based methodologies, the OCEBM system refrains from making recommendations. The IE guidelines document included a description of the search methods, including databases searched, time period and search terms. However, no further description of the evidence selection process, the evidence assessment and the harm benefit ratio was provided. Similarly, external reviewers were not mentioned [26].
None of the guidelines included an explicit statement regarding guidelines update, although the AACE/TOS/ASBMS and the IE guidelines were updated, in 5 years for the former, and in 1-5 years for the latter.
Clarity of presentation
This fourth domain assesses the presentation of the recommendations and whether they were made “easily identifiable” to the reader [14] (Appendix B).
The ES guidelines presented an easily identifiable summary of the recommendations. These recommendations specified the target population, but not the doses of vitamin D nor the purpose/outcome of the recommended action [21].
The AACE/TOS/ASBMS guidelines also provided an executive summary of the recommendations in the first pages of the document. However, the key recommendations were not easily identifiable [23].
The IE guidelines mentioned the need for vitamin D supplementation, specifically in BPD patients. For the other surgical procedures, vitamin supplementation (not specifically vitamin D) was deemed necessary; the dosing regimen, form of vitamin D and target 25(OH)D level were not specified [26].
Applicability
This domain describes tools to implement the recommendations in practice, indicating the facilitators, the drawbacks and the resources needed [14] (Appendix B).
None of the three CPGs described the facilitators, the barriers or the resources to be considered in their implementation. The ES and the AACE/TOS/ASBMS guidelines provided an executive summary, which is considered a criterion for applicability [14]. Similarly, both guidelines provided tables to monitor for various nutrients following bariatric surgery [21, 23]. The IE guidelines did not provide any discussion relevant to the applicability of the guidelines [26].
Editorial Independence
This domain assesses the influence of the funding agency and the impact of competing interests of the guideline development group members on the guidelines content [14] (Appendix B).
The ES guidelines mentioned explicitly “No corporate funding or remuneration” [21]. In addition, they described the competing interests of all members of the guidelines development group. However, they did not provide details related to the methods applied to identify these competing interests nor how they could have affected the CPGs content [21]. The AACE/TOS/ASBMS guidelines described only the competing interests without any further details [23]. The IE guidelines did not provide any details related to funding agencies and conflict of interests [26].
5. Discussion
The ES and the AACE/TOS/ASBMS guidelines recommended high doses of vitamin D supplementation following bariatric surgery, ranging from 3,000 IU daily to 50,000 IU 1-3 times weekly, and increasing to 50,000 IU 1-3 times daily in case of severe malabsorption [21, 23]. The IE guidelines recommended vitamin supplementation for AGB and RYGB and specified the need for vitamin D supplementation in BPD, without dose specification [26]. The evidence behind these recommendations was for the most part lacking, or limited to a single high quality trial (i.e. a single randomized controlled trial). If studies were cited, they did not necessarily reflect the CPGs recommended doses. Therefore, these guidelines were mostly based on expert opinion and professional judgment. The quality of the guidelines was rated as low for the AACE/TOS/ASMBS [23] and the IE Guidelines [26], and average for the ES Guidelines [21] (Figure 2).
The above CPGs have several limitations, based on the rigorous systematic assessment recommended by the AGREE II appraisal instrument [14]. With increasing awareness of the use of appropriate methodologies in the process of guidelines development, several scientific societies used the AGREE II tool principles during the guidelines development process. The Interdisciplinary Section for Antibiotic Resistance Control (ISKRA) of the Croatian Ministry of Health and Social Welfare and the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) have used the AGREE II tool fundamentals during their guidelines development process [35, 36]. The guidelines to support midwifery led care in labor and the European Hernia Society guidelines used the AGREE II tool at the peer review stage, before guidelines publication and dissemination [37, 38]. Similarly, the WHO assessed their reproductive health guidelines using this instrument and showed that the lowest scores were registered in the domains of applicability and editorial independence [39].
Several recently published papers have assessed the quality of clinical guidelines, addressing various topics from different specialties, using the AGREE II tool [40-52], Table 2. Low scoring was most consistently reported for the following domains: stakeholder involvement (specifically very restricted patient participation and involvement in the guideline development)[44,48,50], rigor of development (guidelines were mostly based on expert opinion rather than evidence) [41,44,49], applicability [41,43,44,46,48-51], editorial independence [41,44,49]. These findings are comparable to those we obtained while assessing the CPGs on vitamin D replacement in patients undergoing bariatric surgery. (Figure 2).
Table 2. Assessment of guidelines from various specialties using the AGREE II tool, with the individual scores of its various domains*.
| Author year | Guidelines | Guidelines assessed N | Major organizations | Scope and purpose Median/ Range or mean(SD) | Stakeholder involvement Median/ Range or mean (SD) | Rigor of development Median/ Range or mean (SD) | Clarity of presentation Median/ Range or mean (SD) | Applicability Median/ Range or mean (SD) | Editorial independence Median/ Range or mean (SD) |
|---|---|---|---|---|---|---|---|---|---|
| Chakhtoura 2015 | Peri-operative care of bariatric surgery | 3 | AACE, ES, IEG | 19-53 | 22-39 | 14-18 | 3-31 | 0-13 | 0-67 |
| Seron 2015 [43] | Cardiac rehabilitation | 9 | ACP, NICE, NZGG SIGN 1 | 80 (18) | 60 (32) | 56 (30) | 85 (12) | 49 (33) | 57 (35) |
| Guo 2014 [44] | Multiple Sclerosis | 27 | AAN, MSCT, EFNS 1 | 59 (16) | 29 (18) | 31 (21) | 60 (13) | 27 (18) | 29 (22) |
| Jarosova 2014 [47] | Prevention of intra-vascular catheter infection | 3 | CDC, RNAO, SARI | 86-96 | 85-89 | 36-91 | 61-99 | 28-84 | 0-58 |
| Pak 2014 [40] | Acute hypertension | 3 | ACEP, ESH/ESC, NHLBI | 74 | 52 | 67 | 93 | 52 | 53 |
| Sabharwal 2014 [51] | Musculoskeletal and orthopedic guidelines | 14 | AAOSb | 94(4) | 83(4) | 94(1) | 92(4) | 48(8) | 79(3) |
| Sanclemente 2014 [48] | Acne Vulgaris | 6 | GIN, ACP, AHRQ 1 | 72(37) | 46(26) | 58(26) | 93(11) | 23(31) | 64(45) |
| Acuña-Izcaray 2013 [42] | Asthma | 18 | GIA, SIGN1 | 10-57 | 4-66 | 8-64 | 17-85 | 3-53 | 0-58 |
| Holmer 2013 [49] | Diabetes mellitus control | 24 | AACE, ACP ADA, IDF, IDC JDC, NICE, SIGN, KDOQIb | 64 (6–94) | 52(6–94) | 48(0–81) | 81(61–94) | 43(21–83) | 26(0–75) |
| Rios 2013 [50] | Esophageal and gastric variceal bleed | 10 | AASLD, AGA, ASGE, NICE, SIGN, WGO 1 | 78 (20) | 47(29) | 52 (22) | 87 (10) | 26 (29) | 64 (22) |
| Sabharwal2 2013 [41] | Cardiac clinical practice | 101 | AHA, ACC, ESC, ESCS, NICE, STS | 85(8) | 58(7) | 46(16) | 82(10) | 22(11) | 29(24) |
| Stacey 2013 [45] | Cancer treatment induced symptoms | 12 | BCCA, ONS CCO 1 | 31 - 83 | 10 - 65 | 8 - 86 | 65 - 94 | 8 - 36 | 4-92 |
| Santos 2012 [46] | Anti-depressants during pregnancy | 19 | APA, ICSI NICE, SIGN, WFSBP 1 | 84 (12) | 67 (30) | 69(20) | 83 (17) | 44 (37) | 62(30) |
| Don Wauchpoe 2012 [52] | NACB guidelines | 11 | NACBb | 31-89 | 11-100 | 18-85 | 19-94 | 6-67 | 8-100 |
The reference range of scores for each domain is 0-100%.
These guidelines were identified by searching the AGREE website (http://www.agreetrust.org/resource-centre/agree-related-publications/), Google Scholar and PubMed, for the period 2010-2015, as the AGREE II tool was developed in 2009; Jacob et al., 2014 was not included in the table as it did not assess all the AGREE items. For consistency and comparability, guidelines that did not use the 2009 version of the AGREE tool were also excluded.
Not all organizations issuing guidelines were included as they were not considered as major organizations.
Domains scores of individual guidelines were not provided.
Abbreviations: AACE: American Association of Clinical Endocrinologists; AAN: American Academy of Neurology; AAOS: American Academy of Orthopedic Surgeons; AASLD: American Association for the Study of Liver Diseases; ACC: American College of Cardiology; ACEP: American College of Emergency Physicians; ACP: American College of Physicians; ADA: American Diabetes Association ; AHA: American Heart Association; AHRQ: Agency for Healthcare Research and Quality; ASGE American Society for Gastrointestinal Endoscopy; BCCA: British Columbia Cancer Agency; CDC: Centers for Disease Control and Prevention; CCO: Cancer Care Ontario; EFNS: European Federation of Neurological Societies; ES: Endocrine Society; ESC: European Society of Cardiology; ESH: European Society of Hypertension; ESCS: European Society of Cardiothoracic Surgeons; GIA: Global Initiative for Asthma; GIN: Guidelines International Network; ICSI: Institute for Clinical Systems Improvement; IDC: International Diabetes Center; IDF: International Diabetes Federation, IEG: Interdisciplinary European Guidelines; IPDMS: International Panel on the Diagnosis of Multiple Sclerosis; JDC: Joslin Diabetes Center; KDOQI: National Kidney Foundation Kidney Disease Outcomes Quality Initiative; MSCT: Multiple Sclerosis Cell Transplant group; NACB: National Academy of Clinical Biochemistry; NHLBI: National heart, Lung and Blood Institute; NZGG: New Zealand Guideline Group; STS: Society of Thoracic Surgeons; WGO: Word Gastroenterology Organization.
Conversely, for several organizations developing CPGs, one or more of their guidelines scored high (>70%) on all the AGREE domains, using the 2003 or 2009 AGREE tool versions. These organizations include the National Institute for Health and Clinical Excellence (NICE) [46,50,53,54], the Scottish Intercollegiate Guidelines Network (SIGN) [53,55], the New Zealand Guidelines Group [43], the Royal College of Physicians of London [55], and the Canadian Task Force on Prevention Health Care group [55]. The American College of Chest Physicians (ACCP) evidence-based CPGs on anti-thrombotic therapy and prevention of thrombosis, rigorously addressed several of the aforementioned caveats encountered in many CPGs [56-59].
Since the AGREE II tool allows only the assessment of CPGs after their development, a comprehensive checklist was recently developed to guide CPGs developers during the process of development, implementation and evaluation of guidelines [60]. It is based on the guideline development manuals and methodology reports of various national and international organizations from Europe, North and Latin America, and Australia [60].
A scrutiny of the domains and items within each domain of the AGREE II tool that enter into the calculation of the scaled score for each domain reveals the laborious exercise that CPGs would have to follow. We acknowledge the challenges of developing high quality evidence based guidelines, a process that is quite taxing, in terms of human and financial resources; resources that often are not available. In fact, the best quality guidelines are those that are supported by governmental resources, as it has been shown for oncology guidelines [61].
The challenges in the management of the obese patient undergoing bariatric surgery are multiple. First, the post-operative care requires a multi-disciplinary approach [4]. Therefore, CPG would require input from various health care professional groups. This was not clearly achieved in the three CPGs. Second, vitamin and mineral deficiencies are a common threat following surgery and recommendations regarding replacement of each nutrient should be separately and rigorously evaluated. Finally, several uncertainties regarding vitamin D supplementation dosing and outcomes, in the general population [62], in patients undergoing surgery [63], and in the bariatric surgery population specifically [5], need to be addressed in large randomized controlled trials. Indeed, large variability in BMI, co-morbidities, fat mass and sun exposure, physical activity and lifestyle post-operatively, are all interfering factors that affect vitamin D status and response to supplementation, and render difficult recommending a single vitamin D dose that might be suitable to all patients undergoing bariatric surgery. An individualized approach, based on evidence based recommendations, but taking into account all these aforementioned predictors, would be ideal.
Strengths and weaknesses
This is the first review presenting a critical evaluation of the vitamin D replacement guidelines in bariatric surgery patients, based on the AGREE II tool. It sheds light on several caveats in the recently published evidence-based CPGs, and identifies important areas for improvement in guidelines development. The reviewers had a very high agreement in their rating of CPGs quality and our team included an expert in guidelines methodology, leading us to consider that our results and conclusions are accurate.
However, our review has several limitations. It includes only English published CPGs, and we may have missed CPGs published in other languages. Other limitations are related to the AGREE II tool per se. This tool does not define a score threshold to qualify a CPG as high or low in quality [14]. Furthermore, AGREE II is a methodological tool that does not evaluate the content and the clinical implications of CPGs [64]; accordingly, even when CPGs are based on low quality of evidence, they still may score high on the AGREE II tool, if the methods of their development abided by their predefined standards.
6. Conclusion
Prevention and treatment of hypovitaminosis D in patients undergoing bariatric surgery is crucial in order to prevent skeletal and possibly other complications. Current CPGs recommendations on vitamin D supplementation in bariatric surgery differ between societies, and these guidelines were mostly based on expert opinion and suffer from several limitations. They do not fulfill criteria for optimal guideline development, possibly due to the limited resources, and the lack of sufficient randomized trials at the time of their development to support their recommendations. To-date, the optimal dose of vitamin D following bariatric surgery remains unclear. Thus, the pressing need to develop CPGs using data from high quality randomized trials, some of which are ideally developed based on commonly accepted standards. A multidisciplinary approach incorporating evidence, in addition to other important considerations, such as clarity of presentation, scope and purpose, involvement of stakeholders and consideration of their views and needs, applicability issues including tools to implement the guidelines, cost and resource considerations, would help better define and achieve standards of clinical care in this specific population. Such undertaking is becoming more achievable considering the additional resources that have become available, provided it is planned for ahead of time, and the needed financial support is secured.
Supplementary Material
Acknowledgments
Funding: This work was supported by a grant from the Medical Resource Plan at the American University of Beirut, and made possible thanks to the Scholars in HeAlth Research Program (SHARP).
The authors would like to thank Miss Aida Farha, Medical Information Specialist, Saab Medical Library, for her advice and assistance in designing comprehensive and complex searches of the various medical literature resources and for the provision of select articles.
The authors would like also to thank the authors of the included CPG, Dr David Heber (ES guidelines), Dr Pauline Camacho and Dr Jeffrey Mechanick (AACE/TOS/ASBMS guidelines),and Dr Martin Fried (IE guidelines), for their replies on queries about the availability of additional relevant information, needed for the CPGs scoring using the AGREE II tool.
Footnotes
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; AACE, American Association of Clinical Endocrinologists; AGB, Adjustable Gastric Banding; AGREE, Appraisal of Guidelines, Research, and Evaluation; ASBMS, American Society for Metabolic & Bariatric Surgery; BMI, body mass index; BPD, Bilio-pancreatic Diversion; CPGs, Clinical Practice Guidelines; ES, Endocrine society; G-I-N, Guidelines International Network; IOM, Institute Of Medicine; LAGB: Laparoscopic Adjustable Gastric Banding; LSG, Laparoscopic Sleeve Gastrectomy; NHS, National Health Services; TOS, The Obesity Society; RYGBP, Roux-en-Y Gastric Bypass.
Authors' contribution: Study conception and design: Dr Marlene Chakhtoura, Dr Ghada El Hajj Fuleihan. Title and abstract screening: Dr Marlene Chakhtoura, Dr Nancy Nakhoul. Full text screening: Dr Marlene Chakhtoura, Dr Nancy Nakhoul. Data abstraction: Dr Marlene Chakhtoura and Dr Nancy Nakhoul. Data analysis and interpretation: Dr Marlene Chakhtoura, Dr Nancy Nakhoul, Dr Ghada El Hajj Fuleihan, Dr Elie Akl, and Dr Christos Mantzoros. Drafting the manuscript: all authors. Revising the manuscript content and approving the final version of the manuscript: all authors.
Declaration of interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Imes CC, Burke LE. The Obesity Epidemic: The USA as a Cautionary Tale for the Rest of the World. Curr Epidemiol Rep. 2014;1(2):82–8. doi: 10.1007/s40471-014-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McTigue KM, Harris R, Hemphill B, Lux L, Sutton S, Bunton AJ, et al. Screening and interventions for obesity in adults: summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2003;139(11):933–49. doi: 10.7326/0003-4819-139-11-200312020-00013. [DOI] [PubMed] [Google Scholar]
- 3.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Library. 2014;8:CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawaya RA, Jaffe J, Friedenberg L, Friedenberg FK. Vitamin, mineral, and drug absorption following bariatric surgery. Curr Drug Metab. 2012;13(9):1345. doi: 10.2174/138920012803341339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole AJ, Beckman LM, Earthman CP. Vitamin D Status Following Bariatric Surgery: Implications and Recommendations. Nutr Clin Pract. 2014;29(6):751–8. doi: 10.1177/0884533614546888. [DOI] [PubMed] [Google Scholar]
- 6.Bacci V, Silecchia G. Vitamin D status and supplementation in morbid obesity before and after bariatric surgery. Expert Rev Gastroenterol Hepatol. 2010;4(6):781–94. doi: 10.1586/egh.10.69. [DOI] [PubMed] [Google Scholar]
- 7.Chakhtoura M, Nakhoul N, Shawwa K, et al. Hypovitaminosis D in bariatric surgery: a systematic review of observational studies. Metabolism. 2015 doi: 10.1016/j.metabol.2015.12.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunstan MJ, Molena EJ, Ratnasingham K, Kamocka A, Smith NC, Humadi S, et al. Variations in oral vitamin and mineral supplementation following bariatric gastric bypass surgery: A national survey. Obes Surg. 2015;25(4):648–55. doi: 10.1007/s11695-014-1425-5. [DOI] [PubMed] [Google Scholar]
- 9.Brolin RE, Leung M. Survey of vitamin and mineral supplementation after gastric bypass and biliopancreatic diversion for morbid obesity. Obes Surg. 1999;9(2):150–4. doi: 10.1381/096089299765553395. [DOI] [PubMed] [Google Scholar]
- 10.Clinical Practice Guidelines We Can Trust (Institute Of Medicine Report 2011) [Accessed in Novemeber 2015]; Available from: https://www.iom.edu/∼/media/Files/Report%20Files/2011/Clinical-Practice-Guidelines-We-Can-Trust/Clinical%20Practice%20Guidelines%202011%20Insert.pdf.
- 11.Siering U, Eikermann M, Hausner E, Hoffmann-Eβer W, Neugebauer EA. Appraisal Tools for Clinical Practice Guidelines: A Systematic Review. PLoS One. 2013;8(12):e82915. doi: 10.1371/journal.pone.0082915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlayen J, Aertgeerts B, Hannes K, Sermeus W, Ramaekers D. A systematic review of appraisal tools for clinical practice guidelines: multiple similarities and one common deficit. Int J Qual Health Care. 2005;17(3):235–242. doi: 10.1093/intqhc/mzi027. [DOI] [PubMed] [Google Scholar]
- 13.Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12(1):18–23. doi: 10.1136/qhc.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Consortium ANS. Appraisal of Guidelines for Research & Evaluation II. AGREE II Instrument. [Accessed in November 2015];The Agree Research Trust. 2009 Available from: http://www.agreetrust.org/wp-content/uploads/2013/10/AGREE-II-Users-Manual-and-23-item-Instrument_2009_UPDATE_2013.pdf.
- 15.What makes a good clinical guideline? [Accessed in November 2015]; Available from: http://www.medicine.ox.ac.uk/bandolier/painres/download/whatis/whatareclinguide.pdf.
- 16.Qaseem A, Forland F, Macbeth F, OllenschlÃI(x00304)ger G, Phillips S, Van der Wees P. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156(7):525–31. doi: 10.7326/0003-4819-156-7-201204030-00009. [DOI] [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 18.Isom KA, Andromalos L, Ariagno M, Hartman K, Mogensen KM, Stephanides K, et al. Nutrition and Metabolic Support Recommendations for the Bariatric Patient. Nutr Clin Pract. 2014;29(6):718–39. doi: 10.1177/0884533614552850. [DOI] [PubMed] [Google Scholar]
- 19.Dewey M, Heuberger R. Vitamin D and calcium status and appropriate recommendations in bariatric surgery patients. Gastroenterol Nurs. 2011;34(5):367–74. doi: 10.1097/SGA.0b013e318229bcd0. [DOI] [PubMed] [Google Scholar]
- 20.Ziegler O, Sirveaux MA, Brunaud L, Reibel N, Quilliot D. Medical follow up after bariatric surgery: nutritional and drug issues. General recommendations for the prevention and treatment of nutritional deficiencies Diabetes Metab. 2009;35(6 Pt 2):544–57. doi: 10.1016/S1262-3636(09)73464-0. [DOI] [PubMed] [Google Scholar]
- 21.Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2010;95(11):4823–43. doi: 10.1210/jc.2009-2128. [DOI] [PubMed] [Google Scholar]
- 22.Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, Guven S, et al. Executive Summary of the Recommendations of the American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery Medical Guidelines for Clinical Practice for the Perioperative Nutritional, Metabolic, and Nonsurgical Support of the Bariatric Surgery Patient. Endocr Pract. 2008;14(3):318–36. doi: 10.4158/EP.14.3.318. [DOI] [PubMed] [Google Scholar]
- 23.Mechanick JI, Youdim A, Jones DB, Timothy Garvey W, Hurley DL, Molly McMahon M, Heinberg LJ, Kushner R, Adams TD, Shikora S, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient-2013 update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic &. Bariatric Surgery Endocr Pract. 2013;19(2):337–372. doi: 10.4158/EP12437.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fried M, Hainer V, Basdevant A, Buchwald H, Deitel M, Finer N, et al. Interdisciplinary European guidelines for surgery for severe (morbid) obesity. Obes Surg. 2007;17(2):260–70. doi: 10.1007/s11695-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 25.Fried M, Yumuk V, Oppert J-M, Scopinaro N, Torres AJ, Weiner R, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Facts. 2013;6(5):449–68. doi: 10.1159/000355480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fried M, Yumuk V, Oppert J, Scopinaro N, Torres A, Weiner R, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. 2014;24(1):42–55. doi: 10.1007/s11695-013-1079-8. [DOI] [PubMed] [Google Scholar]
- 27.Mechanick JI, Camacho PM, Cobin RH, Garber AJ, Garber JR, Gharib H, et al. American Association of Clinical Endocrinologists protocol for standardized production of clinical practice guidelines—2010 update. Endocr Pract. 2010;16(2):270–83. doi: 10.4158/EP.16.2.270. [DOI] [PubMed] [Google Scholar]
- 28.Goldner WS, Stoner JA, Lyden E, Thompson J, Taylor K, Larson L, et al. Finding the optimal dose of vitamin D following Roux-en-Y gastric bypass: a prospective, randomized pilot clinical trial. Obes Surg. 2009;19(2):173–9. doi: 10.1007/s11695-008-9680-y. [DOI] [PubMed] [Google Scholar]
- 29.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 30.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88(1):157–61. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 31.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr 26;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mechanick JI, Camacho PM, Garber AJ, Garber JR, Pessah-Pollack R, Petak SM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Protocol for Standardized Production of Clinical Practice Guidelines, Algorithms, and Checklists-2014 Update and the AACE G4G Program. Endocr Pract. 2014;20(7):692–702. doi: 10.4158/EP14166.PS. [DOI] [PubMed] [Google Scholar]
- 34.OCEBM Levels of evidence. [Accessed in November 2015]; Available from: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/
- 35.Andrasevic AT, Baudoin T, Vukelic D, Mimica S, Matanovic DB, Puzevski D, et al. ISKRA guidelines on sore throat: diagnostic and therapeutic approach–Croatian national guidelines. Europe PMC Plus. 2009;131(7-8):181–91. [PubMed] [Google Scholar]
- 36.Rosenfeld RM, Shiffman RN, Robertson P. Clinical Practice Guideline Development Manual, A Quality-Driven Approach for Translating Evidence into Action. Otolaryngol Head Neck Surg. 2013;148(1 suppl):S1–S55. doi: 10.1177/0194599812467004. [DOI] [PubMed] [Google Scholar]
- 37.Spiby H, Munro J. The development and peer review of evidence-based guidelines to support midwifery led care in labour. Midwifery. 2009;25(2):163–71. doi: 10.1016/j.midw.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Simons M, Aufenacker T, Bay-Nielsen M, Bouillot J, Campanelli G, Conze J, et al. European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia. 2009;13(4):343–403. doi: 10.1007/s10029-009-0529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polus S, Lerberg P, Vogel J, Watananirun K, Souza JP, Mathai M, et al. Appraisal of WHO guidelines in maternal health using the AGREE II assessment tool. PloS one. 2012;7(8):e38891. doi: 10.1371/journal.pone.0038891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pak KJ, Hu T, Fee C, Wang R, Smith M, Bazzano LA. Acute Hypertension: A Systematic Review and Appraisal of Guidelines. The Ochsner J. 2014;14(4):655–63. [PMC free article] [PubMed] [Google Scholar]
- 41.Sabharwal S, Patel V, Nijjer SS, Kirresh A, Darzi A, Chambers JC, et al. Guidelines in cardiac clinical practice: evaluation of their methodological quality using the AGREE II instrument. J R Soc Med. 2013;106(8):315–22. doi: 10.1177/0141076813486261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acuna-Izcaray A, Sanchez-Angarita E, Plaza V, Rodrigo G, Montes De Oca M, Gich I, et al. Quality assessment of asthma clinical practice guidelines: a systematic appraisal. Chest. 2013;144(2):390–7. doi: 10.1378/chest.12-2005. [DOI] [PubMed] [Google Scholar]
- 43.Serón P, Lanas F, Ríos E, Bonfill X, Alonso-Coello P. Evaluation of the Quality of Clinical Guidelines for Cardiac Rehabilitation: a critical review. J Cardiopulm Rehabil Prev. 2015;35(1):1–12. doi: 10.1097/HCR.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 44.Guo J, Cheng C, Yan W, Xu G, Feng J, Wang T, et al. Systematic Review of Clinical Practice Guidelines Related to Multiple Sclerosis. PLoS One. 2014;9(10):e106762. doi: 10.1371/journal.pone.0106762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stacey D, Macartney G, Carley M, Harrison MB. Development and evaluation of evidence-informed clinical nursing protocols for remote assessment, triage and support of cancer treatment-induced symptoms. Nurs Res Pract. 2013;2013:171872. doi: 10.1155/2013/171872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santos F, Sola I, Rigau D, Arevalo-Rodriguez I, Seron P, Alonso-Coello P, et al. Quality assessment of clinical practice guidelines for the prescription of antidepressant drugs during pregnancy. Curr Clin Pharmacol. 2012;7(1):7–14. doi: 10.2174/157488412799218842. [DOI] [PubMed] [Google Scholar]
- 47.Jarosova D, Zitnikova P. Assessing the methodological quality of clinical practice guidelines for preventing intravascular catheter-related infections. [Accessed in November 2015];2014 Available from: http://periodika.osu.cz/cejnm/dok/2014-04/6-jarosova-zitnikova.pdf.
- 48.Sanclemente G, Acosta J-L, Tamayo M-E, Bonfill X, Alonso-Coello P. Clinical practice guidelines for treatment of acne vulgaris: a critical appraisal using the AGREE II instrument. Arch Dermatol Res. 2014;306(3):269–77. doi: 10.1007/s00403-013-1394-x. [DOI] [PubMed] [Google Scholar]
- 49.Holmer HK, Ogden LA, Burda BU, Norris SL. Quality of clinical practice guidelines for glycemic control in type 2 diabetes mellitus. PloS One. 2013;8(4):e58625. doi: 10.1371/journal.pone.0058625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rios E, Seron P, Lanas F, Bonfill X, Quigley EM, Alonso-Coello P. Evaluation of the quality of clinical practice guidelines for the management of esophageal or gastric variceal bleeding. Eur J Gastroenterol Hepatol. 2014;26(4):422–31. doi: 10.1097/MEG.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 51.Sabharwal S, Patel NK, Gauher S, Holloway I, Athansiou T. High methodologic quality but poor applicability: assessment of the AAOS guidelines using the AGREE II instrument. Clin Orthop Relat Res. 2014;472(6):1982–8. doi: 10.1007/s11999-014-3530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Don-Wauchope AC, Sievenpiper JL, Hill SA, Iorio A. Applicability of the AGREE II instrument in evaluating the development process and quality of current National Academy of Clinical Biochemistry guidelines. Clin Chem. 2012;58(10):1426–37. doi: 10.1373/clinchem.2012.185850. [DOI] [PubMed] [Google Scholar]
- 53.Barajas-Nava L, Sola I, Delgado-Noguera M, Gich I, Villagran CO, Bonfill X, et al. Quality assessment of clinical practice guidelines in perioperative care: a systematic appraisal. Qual Saf Health Care. 2010;19(6):e50. doi: 10.1136/qshc.2009.038653. e. [DOI] [PubMed] [Google Scholar]
- 54.Delgado-Noguera M, Tort S, Bonfill X, Gich I, Alonso-Coello P. Quality assessment of clinical practice guidelines for the prevention and treatment of childhood overweight and obesity. Eur J Pediatr. 2009;168(7):789–99. doi: 10.1007/s00431-008-0836-5. [DOI] [PubMed] [Google Scholar]
- 55.Errez Ibarluzea IG, Ruiz OG, Alvarez FM, Gomez R, Herreros REP, Dominguez AR. Analysis of the quality of clinical practice guidelines on established ischemic stroke. Int J Technol Assess Health Care. 2008;24(3):333–41. doi: 10.1017/S0266462308080446. [DOI] [PubMed] [Google Scholar]
- 56.Guyatt GH, Norris SL, Schulman S, Hirsh J, Eckman MH, Akl EA, et al. Methodology for the development of antithrombotic therapy and prevention of thrombosis guidelines: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2_suppl):53S–70S. doi: 10.1378/chest.11-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacLean S, Mulla S, Akl EA, Jankowski M, Vandvik PO, Ebrahim S, et al. Patient Values and Preferences in Decision Making for Antithrombotic Therapy: A Systematic Review: Antithrombotic Therapy and Prevention of Thrombosis, 9th edition: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2_suppl):e1S–e23S. doi: 10.1378/chest.11-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ. Executive summary: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2_suppl):7S–47S. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, et al. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e152S. doi: 10.1378/chest.11-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schünemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, et al. Guidelines 2. 0: systematic development of a comprehensive checklist for a successful guideline enterprise CMAJ. 2014;186(3):E123–E42. doi: 10.1503/cmaj.131237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burgers J, Fervers B, Haugh M, Brouwers M, Browman G, Philip T, et al. International assessment of the quality of clinical practice guidelines in oncology using the Appraisal of Guidelines and Research and Evaluation Instrument. J Clin Oncol. 2004;22(10):2000–7. doi: 10.1200/JCO.2004.06.157. [DOI] [PubMed] [Google Scholar]
- 62.Meyer HE, Holvik K, Lips P. Should vitamin D supplements be recommended to prevent chronic diseases? BMJ. 2015;350:h321. doi: 10.1136/bmj.h321. [DOI] [PubMed] [Google Scholar]
- 63.Iglar PJ, Hogan KJ. Vitamin D status and surgical outcomes: a systematic review. Patient Saf Surg. 2015;9(1):1–10. doi: 10.1186/s13037-015-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al-Ansary LA, Alkhenizan A. Towards evidence-based clinical practice guidelines in Saudi Arabia. Saudi Med J. 2004;25(11):1555–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.